Abstract
Objective
We tested the hypothesis that systemic administration of an adenosine 2A receptor (A2AR) agonist will reduce multiorgan ischemia-reperfusion injury (IRI) in a porcine model of extracorporeal cardiopulmonary resusitation (ECPR).
Summary Background Data
Advances in ECPR have decreased mortality after cardiac arrest, however subsequent IRI contributes to late multisystem organ failure. Attenuation of IRI has been reported with use of an A2AR agonist.
Methods
Adult swine underwent 20 minutes of circulatory arrest, induced by ventricular fibrillation, followed by six hours of reperfusion with ECPR. Animals were randomized to vehicle control, low-dose A2AR agonist, or high-dose A2AR agonist. A perfusion specialist using a goal-directed resuscitation protocol managed all animals during the reperfusion period. Hourly blood, urine, and tissue samples were collected. Biochemical and microarray analyses were performed to identify differential inflammatory markers and gene expression between groups.
Results
Both treatment groups demonstrated significantly higher percent reduction from peak lactate after reperfusion compared to vehicle controls. Control animals required significantly more fluid, epinephrine, and higher final pump flow while having lower urine output than both treatment groups. The treatment groups had lower urine NGAL, an early marker of kidney injury (p=0.01), lower plasma aspartate aminotransferase, and reduced rate of troponin rise (p=0.01). Pro-inflammatory cytokines were lower while anti-inflammatory cytokines were significantly higher in the treatment groups.
Conclusions
Using a novel and clinically relevant porcine model of circulatory arrest and ECPR, we demonstrated that a selective A2AR agonist significantly attenuated systemic IRI and warrants clinical investigation.
Keywords: Extracorporeal Membrane Oxygenation, ECPR, Ischemia Reperfusion, Adenosine 2a, Cardiac Arrest, Porcine Model, gene expression microarray
Mini Abstract
This randomized, double-blinded, placebo controlled large animal study of swine undergoing 20-minutes of circulatory arrest, induced by ventricular fibrillation, followed by 6-hour reperfusion with ECPR. This novel and clinically relevant study demonstrated that addition of a selective A2AR agonist during ECPR significantly attenuated systemic IRI and warrants clinical investigation.
Introduction
Cardiac arrest is one of the leading causes of death worldwide, and extracorporeal cardiopulmonary resusitation (ECPR) has emerged as a therapeutic option for refractory cardiac arrest, which was reported in almost 4,000 patients worldwide in 20171-3. Despite advances in resuscitation technology, more than half of patients who experience cardiac arrest die without the return of spontaneous circulation3, 4. Even if the restoration of circulation occurs, survivors have a poor prognosis with less than one-third surviving to discharge3, 4. Global ischemia during cardiac arrest triggers several pathologic pathways that worsen after reperfusion5. Ischemia-reperfusion injury (IRI) after cardiac arrest leads to the development of post-arrest reperfusion syndrome, which is responsible for enhanced tissue damage, often with fatal outcomes6. Extensive efforts have been made to prevent the development and progression of post-cardiac arrest syndrome. To date, the only protective intervention proven to reduce this deadly syndrome and improve clinical outcomes is targeted temperature management, which includes induction of mild hypothermia or controlled normothemia5.
Post-arrest reperfusion injury begins immediately upon reperfusion involving oxidative stress and rapid activation of innate immune cells (lymphocytes, macrophages and neutrophils) with additional contribution from other cells (endothelial and epithelial cells). This phenomenon is well established within the first few hours of reperfusion7–12. In the setting of IRI, adenosine is a retaliatory metabolite, which serves largely as a protective agent with anti-inflammatory effects13–15. Adenosine mediates its effects through four different G protein-coupled receptors (A1R, A2AR, A2BR and A3R)16. Adenosine receptors have been studied extensively, and use of selective adenosine receptor agonists and antagonists have contributed to a better understanding of adenosine signaling in the setting of IRI17–21. It is well established that the activity of certain cells during acute inflammation, including lymphocytes, macrophages, monocytes, platelets, and neutrophils, is inhibited by A2AR activation9, 22, 23. Resulting in reduction of pro-inflammatory mediators, reduced endothelial adhesion molecule expression, less transmigration of circulating inflammatory cells into tissue, and thus less end-organ damage 13. The cellular responses are largely mediated through cAMP and result in inhibition of oxidative burst in neutrophils, reduced cytokine release, and inhibition of leukocyte activation24. Through the use of selective A2AR agonists, our lab has demonstrated decreased cellular inflammation with resultant amelioration of IRI in a lung transplant model by LaPar et al. and a cardiac model by Yang et al. 10, 25, 26. Additionally, systemically administered, A2AR agonists have been used very successfully in experimental models to reduce tissue injury in the setting of IRI in liver, kidney, bowel, heart, skin, and lung27–29.
The role of A2AR in post-cardiac arrest reperfusion injury has not been reported. In the current study, we sought to demonstrate the use of a selective A2AR agonist (ATL1223) in a novel, clinically relevant, porcine model of ECPR to attenuate global IRI. We hypothesized that infusion of ATL1223 upon initiation of circulatory support in a porcine model of ECPR would attenuate inflammatory responses and reduce tissue injury in multiple organs (heart, kidney and liver).
Materials and Methods
Animals and Monitoring
The current study was approved by the University of Virginia Animal Care and Use Committee and complied with the 1996 Guide for the Care and Use of Laboratory Animals as recommended by the US National Institutes of Health (NIH). Domestic swine of both sexes (35–45 kg) were used for this protocol.
Cardiac arrest
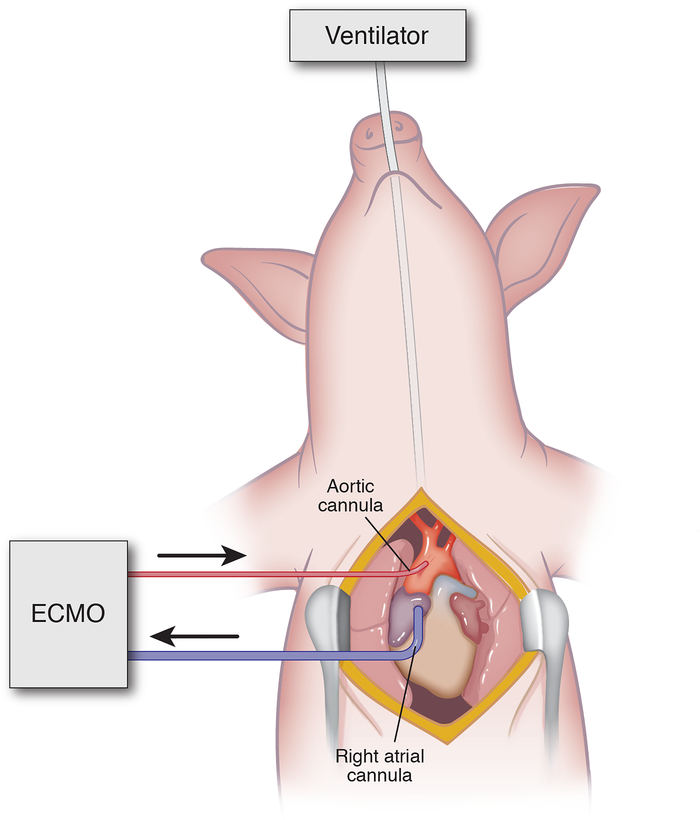
Baseline blood samples, arterial blood gas, hemodynamics, pulmonary mechanics, liver biopsy and urine samples were collected at the start of the experiment. First, a median sternotomy was performed. After administration of 100 U/kg of intravenous heparin (Hospira Inc., Lake Forest, IL), 18F arterial and 32F venous cannulae (Medtronic Inc, Minneapolis, MN) were placed in the ascending aorta and right atrium respectively (Figure 1). Prior to initiating circulatory support, cardiac arrest was induced with direct cardiac fibrillation using a 9V battery (Energizer Brands, LLC, St. Louis MO) and confirmed by direct cardiac visualization and electrocardiography. 20-minutes after cardiac arrest, repeat blood and tissue samples were obtained.
Figure 1.
Porcine Model of ECPR. Median Sternotomy with central ECMO cannulation from right atrium to ascending aorta.
Extracorporeal Cardiopulmonary Resuscitation
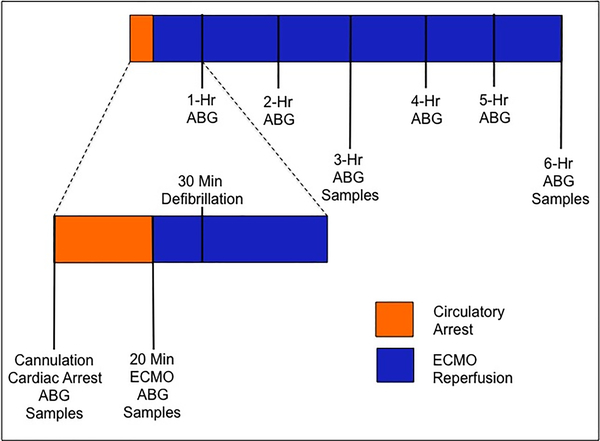
After 20 minutes of circulatory arrest, ECMO was initiated at a flow rate of 25 mL/kg/min. Over a five-minute interval, the ECMO flow rate was slowly titrated upward to ≧ 50 mL/kg/min with a goal mean arterial pressure (MAP) of 65 mmHg. Ten minutes after initiating ECMO support and administering electrolytes, defibrillation was performed with 10 J of direct energy using internal cardiac paddles (Figure 2).
Figure 2.
Experimental methodology describing timing of interventions and data collection. Samples include blood, urine, and liver biopsy. Arterial Blood Gas (ABG) includes lactate, PaO2, PaCO2, bicarbonate, and pH.
Treatment Groups and ECMO Maintenance
This was a randomized, double-blinded placebo controlled large animal study. Low-dose ATL1223 (0.3 ng/kg/min), high-dose ATL1223 (0.6 ng/kg/min) or DMSO vehicle (3 mL/hr, DMSO) was administered via continuous infusion through the arterial limb of the ECMO circuit upon initiation of flow. A clinical ECMO specialist managed the circuit with specific criteria for goal-directed resuscitation. Tissue, blood, and urine samples were collected at baseline, time of reperfusion (20 minutes) and each subsequent hour for the 6-hour reperfusion period (Figure 2).
Statistical Analysis
All data were reported as mean ± standard error of the mean. Data between all three groups were compared using ANOVA with Tukey’s correction for multiple comparisons to determine statistical significance. Each treatment group was compared to the control group using unpaired Student’s t-test. Rate of rise of troponin was calculated using linear regression and comparison of best-fit line. Prism 7 (GraphPad Software Inc., La Jolla, CA) was used to perform all statistical calculations. A threshold of p<0.05 was used for statistical significance.
Results
Novel Clinically Relevant Large Animal Model of ECPR
Fifteen consecutive animals underwent ventricular fibrillation with 20-minute circulatory arrest and were randomized to initiation of ECPR with DMSO, low-dose ATL1223 or high-dose ATL1223 (n=5/group). All animals were successfully defibrillated to sinus rhythm on first or second attempt after 10 minutes of ECMO and infusion of calcium, magnesium and bicarbonate, with no differences between the groups (Figure 2). All animals were maintained on ECMO support for 6 hours following the previously described protocol to maintain MAP (65–75mmHg), pH (7.2–7.4), and PCO2 (35–50mmHg), which did not differ between groups. There was no difference in heart rate, pulmonary artery pressure or blood pressure between groups and no adverse drug effects were observed. However, the ATL-treated pigs required significantly less support to maintain the hemodynamic goals.
A2AR Agonism Attenuates Systemic Reperfusion Injury
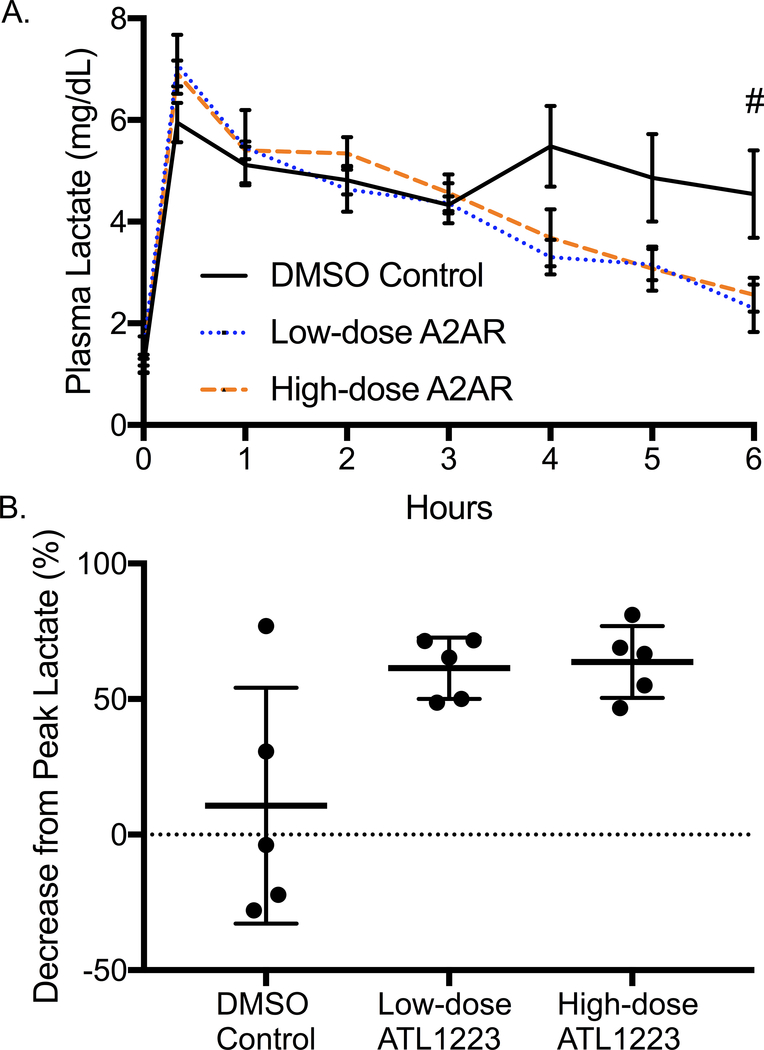
After 20 minutes of circulatory arrest and initiation of resuscitation with ECPR, all animals demonstrated significant rise in blood lactate with no statistical difference among the groups (DMSO: 6.27 ± 2.4 mg/dL, low-dose ATL1223: 6.74 ± 1.9 mg/dL, high-dose ATL1223: 6.57 ± 1.3 mg/dL, p=0.56). Over the 6-hour reperfusion period, both low and high-dose ATL1223 groups demonstrated significantly lower final blood lactate (Figure 3A). While the DMSO control group had a small decrease in lactate over the 6-hour period (10.7 ± 19.5%), both treatment arms demonstrated better lactate clearance (low-dose ATL1123: 61.4% ± 5.1%, p=0.04; high-dose ATL1223: 63.7% ± 5.9%, p=0.03, Figure 3B).
Figure 3.
A: Plasma lactate trends from baseline to 6-hours of ECPR reperfusion (# p=0.04). B: Final percent decrease in plasma lactate (p=0.01) after 6-hours of ECPR reperfusion. Both high dose and low dose A2AR agonism mitigates lactate expression in the plasma.
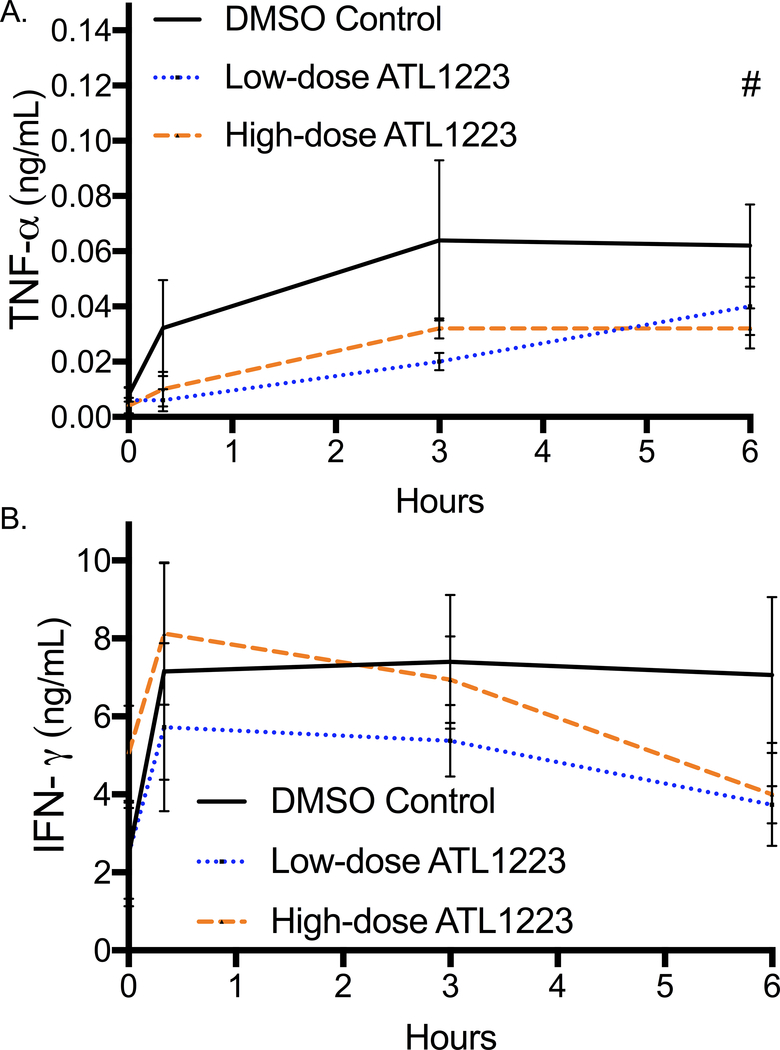
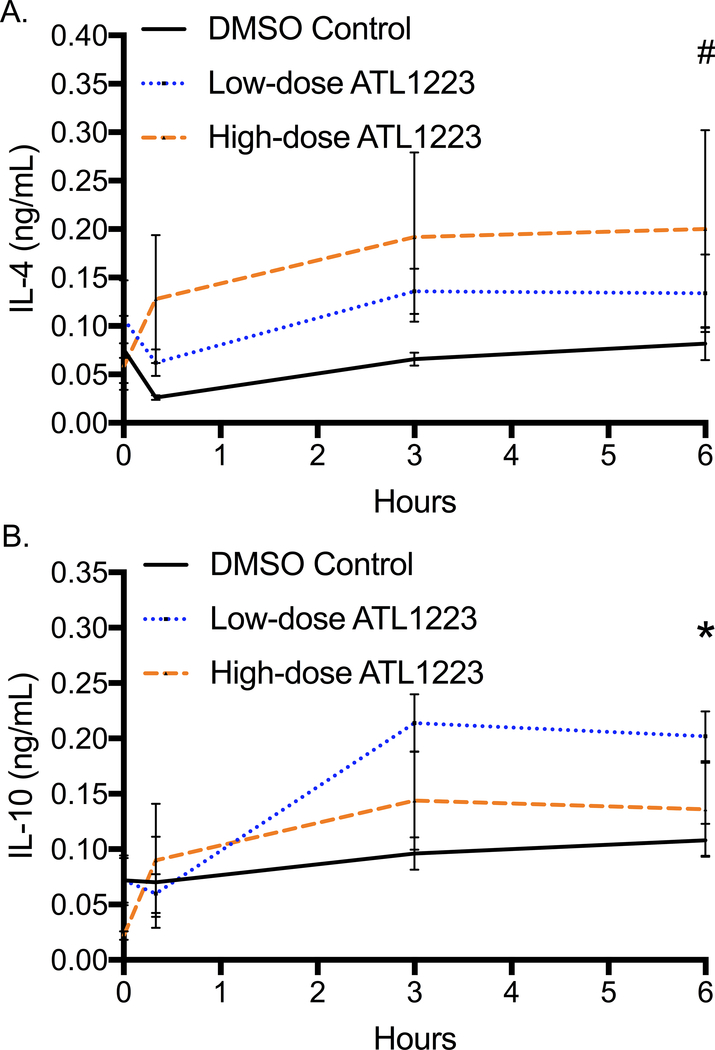
Plasma concentration of tumor necrosis factor alpha (TNF-α) was significantly different at the end of 6 hours of reperfusion with the highest levels in the control group (DMSO: 0.06 ± 0.005 ng/mL, low-dose ATL1223: 0.04 ± 0.01 ng/mL, high-dose ATL1223: 0.028 ± 0.004 ng/mL, p=0.04, Figure 4A). The final plasma concentration of TNF-α was significantly lower in the high-dose ATL1223 group compared to DMSO control (p=0.001). Final plasma interferon gamma (INF-γ) concentration at 6 hours of reperfusion was not statistically different between groups (p=0.22, Figure 4B). However, the percent increase of INF-γ from baseline was significantly different between groups (DMSO: 225%, low-dose ATL1223: 31.1%, high-dose ATL1223: 9.1%, p=0.01) with DMSO controls being almost ten times higher. The final plasma concentration of anti-inflammatory cytokine interleukin-4 (IL-4) was significantly different between groups (DMSO: 0.04 ± 0.01 ng/mL, low-dose ATL1223: 0.13 ± 0.04 ng/mL, high-dose ATL1223: 0.26 ± 0.08 ng/mL, p=0.04, Figure 5A) with DMSO controls being the lowest. The high-dose ATL1223 group had significantly higher expression of plasma IL-4 at 6 hours of reperfusion compared to DMSO controls (p=0.03). Finally, interleukin-10 (IL-10), another anti-inflammatory cytokine, was also significantly increased in the ATL1223 treatment groups after 6 hours of reperfusion (DMSO: 0.11 ± 0.01 ng/mL, low-dose ATL1223: 0.202 ± 0.02 ng/mL, high-dose ATL1223: 0.12 ± 0.03 ng/mL, p=0.01, Figure 5B). The low-dose ATL1223 group had significantly higher expression of IL-10 at the end of 6 hours of reperfusion compared to DMSO controls (p=0.01).
Figure 4.
Plasma pro-inflammatory cytokine expression A: tumor necrosis factor alpha (TNF-α) and B: interferon gamma (INF-γ) decreases from baseline to 6-hours of ECPR reperfusion after A2AAR agonist treatment. # p=0.01.
Figure 5.
Plasma anti-inflammatory cytokine expression A: interleukin-4 (IL-4), B: interleukin-10 (IL-10) increases from baseline to 6-hours of ECPR reperfusion. # p=0.04, * p=0.01.
A2AR Activation Reduces Fluid Requirements and Improves Hemodynamics
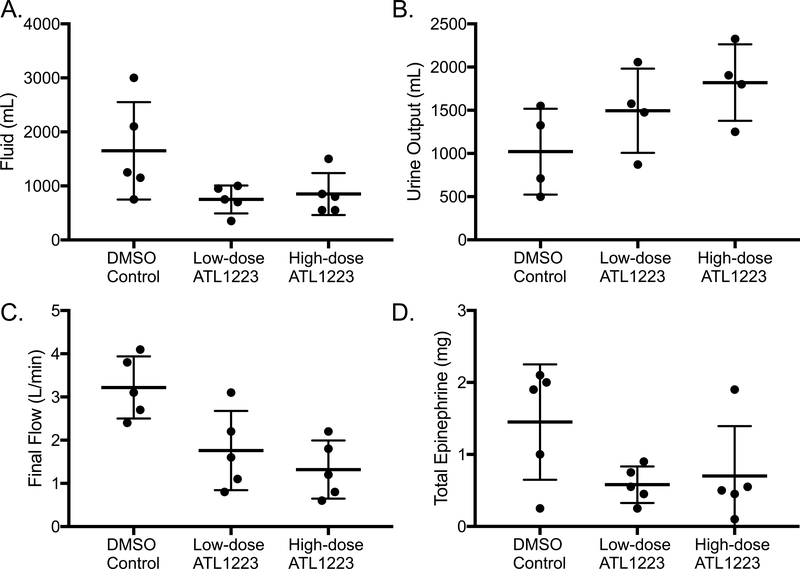
Total fluid requirements trended toward a significant difference between groups (DMSO: 1650 ± 402.8 mL, low-dose ATL1223: 750 ± 115.1 mL, high-dose ATL1223: 850 ± 173.9 mL, p=0.06, Figure 6A) with the control group requiring almost double the volume to maintain hemodynamic parameters. Treated animals (low and high-dose ATL1223 combined) received less total fluid than the DMSO controls (p=0.02). Similarly, total urine output trended toward a dose dependent response to ATL1223 (DMSO: 1022 ± 248.3 mL, low-dose ATL1223: 1495 ± 243.4 mL, high-dose ATL1223: 1821 ± 221.3 mL, p=0.11, Figure 6B) with control animals having the lowest urine output. One DMSO control animal was anuric for the last 4 hours of reperfusion.
Figure 6.
Fluids and Hemodynamics. A. Intravenous fluid requirement (p=0.06) was lower in the treatment animals B. Urine output (p=0.11) was higher in the treatment animals C. Final flow (p=0.01) was lower in the treatment animals D. Total epinephrine dose (p=0.17) was lower in the treatment groups after 20 minutes of circulatory arrest and 6-hours of ECPR reperfusion.
The ability to wean off ECMO was significantly different between groups as evidenced by significantly higher final pump flow requirement in the DMSO control group (DMSO: 3.22 ± 0.3 L/min, low-dose ATL1223: 1.76 ± 0.4 L/min, high-dose ATL1223: 1.32 ± 0.3 L/min, p=0.01, Figure 6C). The DMSO control animals required almost twice as much support as the treated animals. Similarly, total vasopressor requirements trended toward a statistical difference between groups (DMSO: 1.5 ± 0.4 mg, low-dose ATL1223: 0.6 ± 0.1 mg, high-dose ATL1223: 0.7 ± 0.3 mg, p=0.17, Figure 6D) with DMSO control animals requiring on average more than twice as much epinephrine over the 6-hour perfusion period. Treated animals (low and high-dose ATL1223 combined) received less total epinephrine than the DMSO controls (p=0.03).
Amelioration of Renal, Hepatic and Cardiac Injury with A2AR Agonism
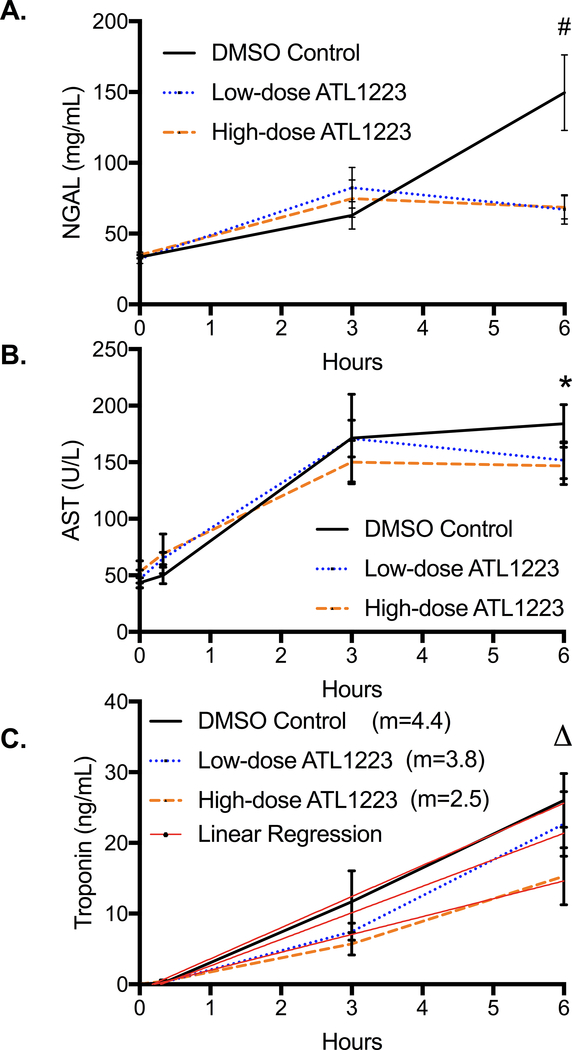
Urine concentration of NGAL, an early marker of acute kidney injury (AKI), was significantly higher in the DMSO control group at 6 hours of reperfusion versus ATL1223-treated groups (DMSO: 149.6 ± 26.6 mg/dL, low-dose ATL1223: 67.1 ± 10.3 mg/dL, high-dose ATL1223: 68.5 ± 8.3 mg/dL, p=0.01, Figure 7A) with the control groups being more than double compared with treated groups. Both low-dose (p=0.02) and high-dose (p=0.04) ATL1223 groups demonstrated significantly lower urinary expression of NGAL compared to the DMSO controls. This biochemical difference correlates with the finding of reduced urine output in the DMSO controls as mentioned above.
Figure 7.
Plasma biochemical markers of injury in A: Kidney - Urine NE gelatinase136 associated lipocalin (NGAL) B: Liver - Aspartate Aminotransferase (AST) were lower in the treatment groups from baseline to 6-hours of ECPR reperfusion. C: Troponin (m=slope calculated by Linear Regression, p=0.01) had a lower rate of rise from baseline to 6-hours of ECPR reperfusion in the treatment groups. # p=0.001, * p=0.09, Δ p=0.17.
Hepatic injury was assessed with measurement of plasma aspartate aminotransferase (AST), which peaked by 3 hours in the ATL1223 treatment groups but continued to rise in the DMSO control group (Figure 7B). There was no statistical difference at 6 hours after reperfusion between groups (DMSO: 184.0 ± 16.9 U/L, low-dose ATL1223: 151.8 ± 16.3 U/L, high-dose ATL1223: 146.8 ± 16.6 U/L, p=0.09, Figure 7B). However the difference was significant when the DMSO control group was compared to the combined low and high-dose ATL1223 treatment groups at 6 hours of reperfusion (p=0.048).
Troponin I, a measure of cardiac injury, was elevated and higher in the DMSO control group at 3 and 6 hours of reperfusion, but there was no statistical difference at 6 hours of reperfusion between the groups (DMSO: 26.0 ± 3.8 ng/mL, low-dose ATL1223: 22.7 ± 4.6 ng/mL, high-dose ATL1223: 15.3 ± 4.1 ng/mL, p=0.17, Figure 7C). However, plasma troponin I demonstrated a more rapid rate of rise (m=slope) in the DMSO control animals compared to the ATL groups (DMSO: m= 4.4 ± 0.19, low-dose ATL1223: m=3.8 ± 0.47, high-dose ATL1223: m=2.5 ± 0.24, p=0.01, Figure 7C).
Discussion
Administration of a selective A2AR agonist in a clinically-relevant, large animal model of ECPR resulted in better clinical outcomes compared with ECPR alone as evidenced by lower required pump flows, decreased epinephrine doses, and less fluid administration required to obtain adequate hemodynamics after 6 hours of reperfusion. Reproducible injury was achieved with circulatory arrest and 20 minutes of ischemia, followed by defibrillation and 6 hours of reperfusion with standardized ECMO support. The cardiac arrest and subsequent global reperfusion triggered an extensive inflammatory response and induced multiorgan injury. A2AR agonism attenuated tissue injury as evidenced by reduced plasma lactate, AST, and urine NGAL. The protective effect of A2AR agonist for post-arrest global injury is through inhibition of inflammatory responses and enhancement of organ/tissue survival pathways.
The large animal ECPR model used in the research presented here is clinically relevant and provided reproducible injury. We standardized our approach with pre-arrest central cannulation, which is more durable then strategies used in other porcine models30, 31. Different from 30 or 60 minutes of circulatory arrest as used by Rojas and colleagues, we chose a 20-min period due to its clinical relevance and high success rate of electric defibrillation back to sinus rhythm after induction of ECPR5, 31. The duration of ECMO support in the present study is longer than that reported by other groups in an effort to capture a more complete assessment of IRI after cardiac arrest and ECPR5. The median duration of support for patients undergoing ECPR in clinical practice ranges from 54–130 hours32. Furthermore, to fully assess the effects of A2AR agonism in this model we evaluated both standard dosing (0.3 ng/kg/min) and a high-dose of ATL1223 (0.6 ng/kg/min) to determine if there was a dose-dependent effect. The standard dosing was based on prior work in our porcine lung transplant model providing treatment to transplant recipient animals during reperfusion, which was subsequently translated into a clinical trial19, 25.
Clinically, there is an increasing use of ECPR for post arrest treatment32. However, only 35–63% of patients who require ECPR survive to hospital discharge4, 32. Several retrospective analyses have demonstrated weaning off ECMO support is the strongest predictor for long-term survival4, 33. Our data established animals treated with a selective A2AR agonist required significantly lower pump flow rates, vasopressors and fluid resuscitation to maintain normal hemodynamic parameters (Figure 6). Seventy percent of animals in the high or low-dose treatment groups were on 2 L/min flow or less by the end of the 6-hour perfusion period and were likely stable enough to tolerate ECMO decannulation. All five animals in the DMSO control group required significantly higher ECMO flow for hemodynamic support with an average final flow over 3.2 L/min. Despite a theoretical risk of bradycardia and hypotension with higher levels of A2AR agonism, these effects were not seen in the present study. There was no difference in heart rate or pulmonary artery pressure between the groups and the DMSO control animals required the highest dosages of vasopressor, fluid and pump support to maintain a MAP 65–85 mmHg. The low and high-dose study group design provides evidence indicating ATL1223 does not induce hemodynamic compromise in this model. Finally, there were no other adverse medication effects identified during this study.
Plasma lactate is a sensitive marker of tissue ischemia and injury that is easily measured and trended in porcine models34. Several retrospective studies of clinical ECPR have demonstrated longer time for plasma lactate to return to baseline predicts reduced survival and poor outcomes2, 35. In the present study, all animals demonstrated a significant rise in plasma lactate after circulatory arrest with no difference in the peak between groups. Importantly, animals treated with a selective A2AR agonist demonstrated significantly greater reduction in lactate compared to controls, with most treatment animals returning to the normal range by the end of the 6-hour study period (Figure 3). Given the major clinical implications of lactate trends during ECPR following cardiac arrest, these results strongly support the benefit of A2AR agonism in attenuation of post-arrest reperfusion injury.
The improved clinical outcomes observed with A2AR agonist treatment were in part driven by attenuation of the inflammatory response during the post-arrest reperfusion. These data demonstrated a significant reduction in TNF-α expression (Figure 4A), which is a downstream pro-inflammatory cytokine in the nuclear factor kappa beta (NF-κB) pathway induced by IRI36. NF-κB has been identified as a critical mediator of cellular inflammation after IRI and correlates with poor outcomes in animal models37. Additionally, INF-γ trended towards significantly lower levels in the A2AR agonist treatment groups (Figure 4B). This cytokine is largely produced by T cells and is an important early signal in IRI38. Recent work by Borg et al demonstrated adenosine pathway dependent inhibition of INF-γ orchestrates cardiac wound healing after myocardial infarction, which likely contributed to the attenuation of cardiac injury observed in our model (Figure 7)18.
The administration of A2AR agonist increased expression of the anti-inflammatory cytokine IL-4 (Figure 5A). This important signaling molecule has been implicated in the role of polarization of macrophages into an M2 phenotype to stimulate recovery after IRI in kidney and neurologic tissue39, 40. IL-10 expression also differed between groups (Figure 5B) and has previously been implicated in hepatic response to IRI through a Kupffer cell dependent mechanism41. Our data show that A2AR agonism reduces global IRI and promotes an anti-inflammatory state, as evidenced by increased levels of IL-4 and IL-10. However, future studies are needed to identify the mechanisms by which A2AR signaling leads to the up regulation of these anti-inflammatory cytokines.
Renal injury after cardiac arrest and ECPR is a common complication with almost 60% of patients experiencing acute renal failure requiring renal replacement therapy32. This is a major problem considering the high morbidity, mortality and healthcare costs associated with renal replacement therapy. Clinically, decreased urine output is an early marker of renal injury with rising plasma creatinine lagging 24–36 hours behind33. Even with careful protocol driven fluid administration, we demonstrated increased urine output with A2AR agonist treatment (Figure 6B). Furthermore, several studies have correlated higher urine NGAL concentration with increased incidence of renal injury in ICU and cardiac surgery patients42, 43. Our data confirm significantly lower urine NGAL in the A2AR agonist treatment groups (Figure 7A). Finally, microarray gene expression analysis identified A2AR-dependent pathways leading to attenuation of renal necrosis (Supplemental Figure 1). In particular, NRF2-mediated oxidative stress response and tight junction signaling represent areas for future research to identify targeted therapies to attenuate renal IRI.
Selective A2AR agonists have been previously demonstrated to attenuate cardiac injury in animal models of ischemia reperfusion26. Despite limited translation into human studies, this protective effect may provide significant benefit for patients undergoing ECPR resulting in earlier cardiac recovery and weaning of ECMO support. In the present study, 6 hours did not capture peak troponin levels and the final values were not statistically different between groups. However, we demonstrate that administration of an A2AR agonist reduces the rate of troponin increase in a dose-dependent fashion as measured by linear regression (Figure 7C). Previous studies have suggested peak troponin is a surrogate of total cardiac injury and the rate of rise correlates with the severity of injury44. These data are further corroborated in our study by increased pump flow and vasopressor requirements in the control group. Recent work by Woods and colleagues characterized post-cardiac arrest myocardial dysfunction as a calcium/calmodulin dependent phenomenon having profound effects on recovery45. These mechanisms likely play an important role in weaning from ECMO support and overall prognosis. Future research will explore the role of these pathways in our ECPR model.
Ischemic hepatitis resulting in acute liver failure in patients undergoing ECPR is one of the most moribund complications following cardiac arrest46. Despite recent advances in extracorporeal liver support, limited options exist for treatment of fulminant ischemic hepatitis secondary to IRI after cardiac arrest. Therefore, efforts must focus on prevention and attenuation of primary injury to prevent this deadly complication. Our data support the hypothesis that a selective A2AR agonist reduces hepatic injury after ECPR. AST, one of the earliest clinically relevant markers of liver injury was found to be significantly elevated in the DMSO control animals compared to animals receiving A2AR agonist treatment (Figure 7B). Furthermore, we identified genes in pathways for apoptosis, cell death, and organismal death that showed trends toward down-regulation with A2AR agonist treatment. Similarly, genes affecting cell viability and cell survival showed trends for up regulation in animals receiving A2AR treatment (Supplemental Figure 3). These pathways corroborate our biochemical data demonstrating less liver IRI with A2AR agonist therapy. Future work in liver IRI and ECPR should focus on elucidation of these pathways and identify which genes are regulated in an A2AR-dependent mechanism and which pathways result from attenuation of secondary injury. These mechanisms may lead to other targeted therapies to prevent this severe complication.
The limitations of this study include the inherent variability of injury and recovery in this large animal model. Furthermore, given the acute 6-hour duration, we were unable to confirm long-term benefit of A2AR agonist therapy. In further studies, an increased sample size, as well as gene expression analysis of samples procured from a longer reperfusion period, may allow for definitive molecular findings. However, given the findings of clinical and biochemical improvement as well as gene expression evidence of molecular injury attenuation, these results support our conclusion. The treatment groups were randomized and researchers were blinded to which animals receive high/low dose ATL1223 or DMSO to prevent bias. Additionally, interventions on the ECMO circuit were standardized with protocol driven management by a clinical perfusion specialist. Finally, neurologic recovery was not evaluated in this study, which is a major predictor of prognosis in patients who experience cardiac arrest and undergo ECPR.
In conclusion, this novel clinically relevant porcine model of ECPR demonstrates that a selective A2AR agonist attenuates systemic IRI. These data establish an improvement in both clinical and biochemical parameters in animals undergoing ECPR with addition of a selective A2AR agonist. Furthermore, we identified specific inflammatory and cell survival pathways in liver and kidney tissues that showed a trend of differential regulation by A2AR agonism in response to systemic IRI. Given the high rate of multi-organ failure and an in-hospital mortality rate approaching 60% in patients undergoing ECPR after cardiac arrest, translation of targeted therapy with a selective A2AR agonist may attenuate ischemia reperfusion injury and improve survival. These data indicate addition of an A2AR agonist to ECPR reduces reperfusion injury following global ischemia and warrants clinical investigation.
Supplementary Material
Supplemental Figure 1. Renal tissue microarray gene expression after 6-hours of ECPR reperfusion represents a trend of inhibition in pathways leading to necrosis.
Supplemental Figure 2. Hepatic Tissue Gene expression demonstrating baseline versus 6 hours post-reperfusion gene expression in treated and untreated groups, showing a trend of activation of immune pathways in the untreated group (few highlighted in red boxes).
Supplemental Figure 3. Hepatic tissue microarray gene expression after 6-hours of ECPR reperfusion, where A represents a trend of inhibition of pathways and B represents a trend in activation of pathways in the treated group.
Acknowledgements
Special thanks to Tony Herring, Cindy Dodson, and Sheila Hammond for their technical support and continued commitment to the completion of this project. Also, thanks to Brandon Cho for his assistance with tissue processing and sample isolation.
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers T32HL007849 (I.L.K.), UM1HL088925 (I.L.K.), R01HL119218 (I.L.K. and V.E.L.), R01 DK080074 (V.M), R01 DK109581 (V.M), R21 DK100678 (D.M) supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding for this project was provided by the European ELSO Society through an annual research grant. Finally, the University of Virginia Beller Cardiovascular Research Grant funded this research.
Disclosure
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers T32HL007849 (I.L.K.), UM1HL088925, R01HL119218 (I.L.K. and V.E.L.), R01 DK080074 (V.M), R01 DK109581 (V.M), R21 DK100678 (D.M) supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The European ELSO, through their annual research grant, provided additional funding for this project. The University of Virginia Beller Cardiovascular Research Grant provided funding for this research.
Abbreviations
- ECPR
Extracorporeal Cardiopulmonary Resuscitation
- ECMO
Extracorporeal Membrane Oxygenation
- IRI
Ischemia-Reperfusion Injury
- A2AR
Adenosine 2A Receptor
- MAP
Mean Arterial Pressure
- PEEP
Positive End-Expiratory Pressure
- ACT
Activated Clotting Time
- ELISA
Enzyme-Linked Immunosorbent Assay
- NGAL
NE Gelatinase136 Associated Lipocalin
- AKI
Acute Kidney Injury
- TNF-α
Tumor Necrosis Factor Alpha
- INF- γ
Interferon Gamma
- IL-4
Interleukin-4
- IL-10
Interleukin-10
- AST
Aspartate aminotransferase
- NIH
National Institutes of Health
Footnotes
There are no financial disclosures or conflicts of interest from any of the authors.
References
- 1.Amberman K, Shen I. Minimizing reperfusion injuries: successful resuscitation using eCPR after cardiac arrest on a post-operative Norwood patient. J Extra Corpor Technol 2010; 42(3):238–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Lasa JJ, Rogers RS, Localio R, et al. Extracorporeal Cardiopulmonary Resuscitation (E-CPR) During Pediatric In-Hospital Cardiopulmonary Arrest Is Associated With Improved Survival to Discharge: A Report from the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation 2016; 133(2):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ELSO Statistics[ELSO web site]. 2017. Available at: https://www.elso.org/Registry/Statistics.aspx. Accessed 11/26/17, 2017.
- 4.Haneya A, Philipp A, Diez C, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation 2012; 83(11):1331–7. [DOI] [PubMed] [Google Scholar]
- 5.Psotova H, Ostadal P, Mlcek M, et al. Ischemic Postconditioning and Nitric Oxide Administration Failed to Confer Protective Effects in a Porcine Model of Extracorporeal Cardiopulmonary Resuscitation. Artif Organs 2015. [DOI] [PubMed] [Google Scholar]
- 6.Kagawa E, Dote K, Kato M, et al. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation 2012; 126(13):1605–13. [DOI] [PubMed] [Google Scholar]
- 7.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 2013; 12(4):265–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma AK, LaPar DJ, Zhao Y, et al. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med 2011; 183(11):1539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma AK, Laubach VE, Ramos SI, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2010; 139(2):474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Day YJ, Toufektsian MC, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 2005; 111(17):2190–7. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Harrison SM, Steele DS. ATP-dependent effects of halothane on SR Ca2+ regulation in permeabilized atrial myocytes. Cardiovasc Res 2005; 65(1):167–76. [DOI] [PubMed] [Google Scholar]
- 12.Hamad EA, Zhu W, Chan TO, et al. Cardioprotection of controlled and cardiac-specific over-expression of A(2A)-adenosine receptor in the pressure overload. PLoS One 2012; 7(7):e39919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day YJ, Li Y, Rieger JM, et al. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol 2005; 174(8):5040–6. [DOI] [PubMed] [Google Scholar]
- 14.Reece TB, Ellman PI, Maxey TS, et al. Adenosine A2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J Thorac Cardiovasc Surg 2005; 129(5):1137–43. [DOI] [PubMed] [Google Scholar]
- 15.Rivo J, Zeira E, Galun E, et al. Attenuation of reperfusion lung injury and apoptosis by A2A adenosine receptor activation is associated with modulation of Bcl-2 and Bax expression and activation of extracellular signal-regulated kinases. Shock 2007; 27(3):266–73. [DOI] [PubMed] [Google Scholar]
- 16.Ross SD, Tribble CG, Linden J, et al. Selective adenosine-A2A activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant 1999; 18(10):994–1002. [DOI] [PubMed] [Google Scholar]
- 17.Link AA, Kino T, Worth JA, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol 2000; 164(1):436–42. [DOI] [PubMed] [Google Scholar]
- 18.Borg N, Alter C, Gorldt N, et al. CD73 on T-Cells Orchestrates Cardiac Wound Healing After Myocardial Infarction by Purinergic Metabolic Reprogramming. Circulation 2017. [DOI] [PubMed] [Google Scholar]
- 19.Wagner CE, Pope NH, Charles EJ, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charles EJ, Mehaffey JH, Sharma AK, et al. Lungs donated after circulatory death and prolonged warm ischemia are transplanted successfully after enhanced ex vivo lung perfusion using adenosine A2B receptor antagonism. J Thorac Cardiovasc Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huerter ME, Sharma AK, Zhao Y, et al. Attenuation of Pulmonary Ischemia-Reperfusion Injury by Adenosine A2B Receptor Antagonism. Ann Thorac Surg 2016; 102(2):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chhabra P, Linden J, Lobo P, et al. The immunosuppressive role of adenosine A2A receptors in ischemia reperfusion injury and islet transplantation. Curr Diabetes Rev 2012; 8(6):419–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarek PE, Huang CT, Lutz ER, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008; 111(1):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasko G, Xu DZ, Lu Q, et al. Adenosine A2A receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med 2006; 34(4):1119–25. [DOI] [PubMed] [Google Scholar]
- 25.LaPar DJ, Laubach VE, Emaminia A, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg 2011; 142(4):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Day YJ, Toufektsian MC, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 2006; 114(19):2056–64. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Shiba H, Fung JJ, et al. The role of the A2a receptor agonist, regadenoson, in modulating hepatic artery flow in the porcine small-for-size liver graft. J Surg Res 2012; 174(1):e37–45. [DOI] [PubMed] [Google Scholar]
- 28.Field JJ, Nathan DG, Linden J. The role of adenosine signaling in sickle cell therapeutics. Hematol Oncol Clin North Am 2014; 28(2):287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma AK, LaPar DJ, Stone ML, et al. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am J Transplant 2013; 13(9):2255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni L, Chen Q, Zhu K, et al. The influence of extracorporeal membrane oxygenation therapy on intestinal mucosal barrier in a porcine model for post-traumatic acute respiratory distress syndrome. J Cardiothorac Surg 2015; 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rojas A, Chen L, Bartlett RH, et al. Assessment of liver function during extracorporeal membrane oxygenation in the non-heart beating donor swine. Transplant Proc 2004; 36(5):1268–70. [DOI] [PubMed] [Google Scholar]
- 32.Singal RK, Singal D, Bednarczyk J, et al. Current and Future Status of Extracorporeal Cardiopulmonary Resuscitation for In-Hospital Cardiac Arrest. Can J Cardiol 2017; 33(1):51–60. [DOI] [PubMed] [Google Scholar]
- 33.Brivet FG, Kleinknecht DJ, Loirat P, et al. Acute renal failure in intensive care units--causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med 1996; 24(2):192–8. [DOI] [PubMed] [Google Scholar]
- 34.Albrecht M, Meybohm P, Broch O, et al. Evaluation of remote ischaemic post-conditioning in a pig model of cardiac arrest: A pilot study. Resuscitation 2015; 93:89–95. [DOI] [PubMed] [Google Scholar]
- 35.Mazzeffi MA, Sanchez PG, Herr D, et al. Outcomes of extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest in adult cardiac surgery patients. J Thorac Cardiovasc Surg 2016; 152(4):1133–9. [DOI] [PubMed] [Google Scholar]
- 36.Lu C, Ren D, Wang X, et al. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochim Biophys Acta 2014; 1842(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh CH, Chen TP, Wang YC, et al. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J Surg Res 2009; 155(1):147–56. [DOI] [PubMed] [Google Scholar]
- 38.Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol 2006; 176(5):3108–14. [DOI] [PubMed] [Google Scholar]
- 39.Zhang MZ, Wang X, Wang Y, et al. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int 2017; 91(2):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Liu J, Zhao S, et al. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke 2016; 47(2):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellett JD, Atkinson C, Evans ZP, et al. Murine Kupffer cells are protective in total hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J Immunol 2010; 184(10):5849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yegenaga I, Kamis F, Baydemir C, et al. ANNALS EXPRESS: Neutrophil gelatinase-associated lipocalin is a better biomarker than cystatin C for predicting imminent acute kidney injury in critically ill patients. Ann Clin Biochem 2017:4563217694051. [DOI] [PubMed] [Google Scholar]
- 43.Kamis F, Yegenaga I, Musul M, et al. Neutrophil gelatinase-associated lipocalin levels during the first 48 hours of intensive care may indicate upcoming acute kidney injury. J Crit Care 2016; 34:89–94. [DOI] [PubMed] [Google Scholar]
- 44.Panteghini M, Pagani F, Bonetti G. The sensitivity of cardiac markers: an evidence-based approach. Clin Chem Lab Med 1999; 37(11–12):1097–106. [DOI] [PubMed] [Google Scholar]
- 45.Woods CE, Shang C, Taghavi F, et al. In Vivo Post-Cardiac Arrest Myocardial Dysfunction Is Supported by Ca2+/Calmodulin-Dependent Protein Kinase II-Mediated Calcium Long-Term Potentiation and Mitigated by Alda-1, an Agonist of Aldehyde Dehydrogenase Type 2. Circulation 2016; 134(13):961–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raurich JM, Llompart-Pou JA, Ferreruela M, et al. Hypoxic hepatitis in critically ill patients: incidence, etiology and risk factors for mortality. J Anesth 2011; 25(1):50–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Renal tissue microarray gene expression after 6-hours of ECPR reperfusion represents a trend of inhibition in pathways leading to necrosis.
Supplemental Figure 2. Hepatic Tissue Gene expression demonstrating baseline versus 6 hours post-reperfusion gene expression in treated and untreated groups, showing a trend of activation of immune pathways in the untreated group (few highlighted in red boxes).
Supplemental Figure 3. Hepatic tissue microarray gene expression after 6-hours of ECPR reperfusion, where A represents a trend of inhibition of pathways and B represents a trend in activation of pathways in the treated group.