Abstract
Chronic pain, the most common complication of diabetes, is treated with medication often to no avail. Our study aimed to compare the use of mindfulness meditation and progressive relaxation to reduce chronic pain in older females with diabetes. Methods The 105 study participants were divided randomly into 3 groups: Group MM (mindfulness meditation), Group CM (control meditation), and Group PM (progressive relaxation meditation). Assessment of analgesic effectiveness required changes in average daily pain Brief Pain Inventory (BPI) modified for painful diabetic peripheral neuropathy and Patient Global Impression of Change using descriptive statistics, Student’s t test, and analysis of variance where applicable. Results Both Groups MM and PM experienced significant (P < .05) reduction in average daily pain in last 24 hours at study end compared to baseline (28.7% and 39.7%, respectively). Group MM had more significant (P < .01) reduction of pain compared to control, a score of 5.2 ± 1.2 dropped to 3.0 ± 1.1 by week 12 of treatment. Groups MM and PM showed significant improvement in patients’ impression at study end, 75 ± 5.1% (n = 36) and 61 ± 6.5% (n = 32), respectively. In Group MM, patient satisfaction scores increased significantly (P < .05) to 3.8 ± 1.9 by week 12. Conclusion Integrative therapies such as mindfulness meditation can be part of a comprehensive pain management plan. Benefits include reduction of pain-related medication consumption, better treatment outcomes, improvement in comorbid conditions such as anxiety and depression as well as no risk of addiction or abuse.
Keywords: meditation, mindfulness meditation, progressive relaxation meditation, type 2 diabetes mellitus, chronic pain, diabetes, older females, Pakistan
Introduction
Diabetes is a chronic disease characterized by impaired insulin secretion, insulin resistance, or both.1 The prevalence of diabetes in South Asia is higher when compared to worldwide statistics, and Pakistan’s prevalence is recorded as 7.1%.2 Pakistan is categorized as a low-income country or a frontier market. There is a large proportion of the population who have low household incomes and have low illiteracy rates. There are notably wide gaps in financial resources between different people and inadequate access to health care. A particularly vulnerable group in the population are elderly females.3,4
Diabetes is often accompanied by a host of complications. Adequate glycemic control is the cornerstone of reducing the risk of diabetic complications and their impact on patients’ lives.5 Achieving target ranges of glycemic control in diabetes requires persistent management of lifestyle aspects such as diet, exercise, medication, and glucose monitoring.
Diabetic peripheral neuropathy (DPN) is the most common microvascular complication and affects up to half of the diabetic population. Most DPN sufferers experience chronic pain. Chronic pain in diabetes reduces the quality of life for an individual, has higher rates of health care costs, and involves loss of productivity.4,6,7
Considering the uniqueness of chronic ill health that South Asian females face at an earlier age, a gender-specific study is vital is ascertaining effective approaches to help females in countries such as Pakistan deal with chronic pain.4,8
Meditation is an alternative option that has several benefits.9There are several types of meditation; mindfulness-based cognitive therapy and progressive relaxation meditation (PRM) are some of the most common.10
Mindfulness-based cognitive therapy is an 8-week protocolized group therapy program that combines meditation exercises with elements of cognitive therapy. The central theme in mindfulness is directing one’s attention to the experience at hand while accepting that thoughts and feelings are transitory.11,12
PRM is another popular method of meditation that is used often to target the reduction of pain. It consists entirely of focusing on a muscle group, tensing, and then relaxing that particular muscle group till the entire body has undergone this process by the end of the session.13,14
The aim of our study was to compare the use of mindfulness meditation and progressive relaxation in reducing chronic pain for elderly females with diabetes. We hypothesized that elderly diabetic females who are using mindfulness meditation regularly will have a reduction in chronic pain when compared to those individuals who use progressive relaxation meditation.
Materials and Methods
Study Participants
Inclusion criteria were elderly females diagnosed with type 1 or type 2 diabetes >12 months and age >55 years.
Exclusion criteria were the presence of psychosis, major psychiatric or neurocognitive disorder, dementia, untreated attention-deficit hyperactivity disorder, use of psychotherapy, the presence of diabetic foot ulcers, deformed/contracted foot, or peripheral vascular disease.
Instruments and Measures
Demographic Details and Chronic Pain Status
Demographic measures including age, gender, annual household income (dollars), education (years), marital status (yes/no), and type of diabetes were similar between the 3 groups. Chronic pain associated with diabetes included median duration of pain (years) and baseline average daily pain score; n (SD) were similar between the 3 groups. Demographic details are summarized in Table 1.
Table 1.
Population Characteristics of the Patients Undergoing Different Types of Meditation (N = 105)a.
| Variable | Group MM (% or SD); N = 36 | Group PM (% or SD); N = 32 | Group CM (% or SD); N = 37 | Total (% or SD); Total N = 105 | P b |
|---|---|---|---|---|---|
| Age | 62.9 ± 12.0 | 64.4 ± 11.0 | 64.1 ± 16.0 | 63.8 ± 13.0 | .08 |
| BMI (kg/m2), mean ± SD | 28.9 ± 4.2 | 30.1 ± 5.1 | 30.0 ± 5.3 | 29.7 ± 5.4 | .53 |
| Education completed | |||||
| No education | 5 ± 1.1 | 3 ± 1.8 | 7 ± 1.3 | 15 ± 1.4 | |
| 1st to 11th grade | 12 ± 1.3 | 17 ± 2.3 | 19 ± 3.9 | 48 ± 2.5 | .48 |
| High school graduate | 9 ± 1.2 | 7 ± 1.9 | 4 ± 1.9 | 20 ± 1.6 | |
| Vocational training | 7 ± 2.6 | 4 ± 1.1 | 3 ± 1.2 | 14 ± 1.6 | |
| College degree | 3 ± 1.1 | 1 ± 1.5 | 4 ± 2.1 | 8 ± 1.5 | |
| Annual income of household | |||||
| $10 000 or less | 32 ± 8.6 | 28 ± 4.6 | 35 ± 4.0 | 95 ± 5.7 | .89 |
| $10 001-$20 000 | 3 ± 1.1 | 2 ± 1.5 | 1 ± 1.3 | 6 ± 1.3 | |
| Did not answer | 1 ± 1.4 | 2 ± 1.1 | 1 ± 1.9 | 4 ± 1.9 | |
| Duration of diabetes in years | 12.9 ± 1.3 | 12.8 ± 1.1 | 13.5 ± 1.5 | 13.0 ± 1.3 | .98 |
| HbA1c values (%) | 8.3 ± 2.1 | 9.5 ± 1.1 | 7.2 ± 1.2 | 8.3 ± 1.4 | .47 |
| Median duration of pain, years | 11 ± 1.9 | 8 ± 2.0 | 9 ± 1.4 | 28 ± 1.7 | .10 |
| Mean number of concurrent medications, n (SD) | 4 ± 1.2 | 5 ± 1.5 | 4 ± 1.7 | 4.3 ± 1.4 | .63 |
| Average daily pain in last 24 hours (BPI Q4) (SD) | 5.2 ± 1.2 | 5.0 ± 1.5 | 5.4 ± 1.9 | 5.2 ± 1.5 | .54 |
| Satisfaction scores | 2.0 ± 1.0 | 2.1 ± 1.1 | 2.2 ± 1.5 | 2.1 ± 1.2 | .82 |
Abbreviations: SD, standard deviation; BMI, body mass index; Group MM; mindfulness meditation; Group PM, progressive relaxation meditation; Group CM, control meditation; HbA1c, hemoglobin A1c; BPI, Brief Pain Inventory.
a Data are reported as mean ± SD.
b P < .05 are significant.
Assessment of Analgesic Effectiveness
This was assessed by observing any change in the average daily pain Brief Pain Inventory (BPI) modified for painful diabetic peripheral neuropathy (BPI-DPN Q4) and the Patient Global Impression of Change (PGIC).15 These 2 questionnaires are best conducted together to get a clearer picture of the analgesic’s effectiveness of the treatment.
The comprehensive validated instrument BPI-DPN Q4 was used during the patient visits to assess the pain severity on the day of the treatment.16 This particular question states, “Please rate your pain due to diabetes by circling the one number that best describes your pain on the average.” The numerical rating scale ranges from 0 (no pain) to 10 (pain as bad as you can imagine). This single question is very similar to how physicians evaluate their patients’ pain in most clinical settings and it has adequate clinical relevance.
The PGIC measures the patient’s impression of change on a 7-point scale (very much improved to very much worse). The PGIC is patient-reported and asks the subject to “indicate how you feel now, compared to how you felt before receiving treatment in this study” on the 7-point scale that ranges from −3 (very much worse), 0 (no change), to +3 (very much improved). This rating scale permits a global evaluation of the patient’s impression of change in their condition. The PGIC was completed at all of the 3 regular scheduled visits (weeks 4, 8, and 12).17
Patient satisfaction with the treatment was also measured on a 4-point rating scale (0 = poor to 4 = excellent) in response to the question, “How would you rate the use of meditation for your pain?”
Urdu is the national language of Pakistan, and the 2 questionnaires used in the current study were translated using the back-translation method. The questionnaires were translated to Urdu by one qualified translator and then translated back into English by an independent qualified translator who was blinded to the original questionnaires. The 2 source language versions were compared and a consensus was then reached.
Procedure
Trained research staff used the diabetic clinic roster to identify eligible patients and then talked to them in the clinic waiting room about the study.
Elderly females were recruited from the diabetic clinic of Punjab Care Hospital, Lahore, Pakistan, during the time period July 2018 to January 2019. Initially pamphlets in Urdu (national language of Pakistan) were used to announce the opportunity to participate in the research. These were placed at prominent locations in and around the particular clinic.
Interested individuals who provided informed consent were taken to a private room in the clinic to complete the study instruments. The study participants who were unable to read the self-administered surveys were interviewed instead by trained research staff so as to fulfil all the study measures. Patients were then informed that they would be required to come to the clinic 2 times every week for a total of 16 sessions over an 8-week time period. Each visit would entail a 30-minute session on meditation. All patients were randomly allocated, using computer software, into the 3 meditation groups. All meditation sessions were conducted simultaneously in 3 separate rooms of the hospital. None of the patients had ever meditated before.
The sessions were conducted mainly by 3 fully trained and experienced meditation instructors. All instructors had completed a training program in mindfulness meditation, progressive relaxation meditation, and yoga, respectively. The instructors had no relationship or contact with the study participants other than at the meditation sessions held at the clinic.
The study participants were randomly placed into 3 groups based on their treatment regimens: Group MM (mindfulness meditation), Group CM (control meditation), and Group PM (progressive relaxation meditation).
Group PM underwent 16 sessions in progressive muscle relaxation meditation. The sessions consisted of 5 minutes of sitting quietly, 23 minutes of progressive muscle relaxation, and 2 to 3 minutes of awakening.
Group MM underwent mindfulness-based cognitive therapy. The 16 sessions combined meditation exercises with elements of cognitive therapy.
Group CM underwent 16 sessions of 15 minutes of discussion followed by 20 minutes of sitting quietly and were told to relax as best as possible.
Out of the 200 patients invited, 119 agreed to participate in the study. By the time the study was initiated and during the course of the 8-week regimen, 14 patients had dropped out for reasons that were unrelated to the study, such as lack of transport and family support. So we finally had a total of 105 individuals from which all data included in this study is derived from.
Data Analyses
For statistical analysis we used Package for Social Science (SPSS) Version 23 (SPSS Inc, Chicago IL, USA). We used Student’s t test to compare the tested variable between the groups. The baseline visits and the follow-up visits were compared using repeated-measures analysis of variance with a Greenhouse-Geisser correction. The χ2 test was used to compare the presence and absence of pain/symptoms in the control and test groups. The calculated P value was deemed significant at <.05. To describe the absolute values and the changes from baseline of average daily pain scores, BPI-DPN Q4 and PGIC scores, we utilized descriptive statistics.
The study participants were randomly placed into 3 groups based on their treatment regimens: Group MM (N = 36), Group CM (N = 32), and Group PM (N = 37).
Baseline, 4-week, 8-week, and 12-week follow-up measures of chronic pain and patient impression of change were assessed.
Results
Demographic and Diabetic Status Details
Of the 105 study participants, the mean (SD) age was 62.9 (12) years. The average duration of diabetes was 13.0 ± 1.3 years. Population characteristics and differences among study participants with diabetes undergoing the different forms of meditation are summarized in Table 1.
Sample Characteristics
Most of the study participants had an annual household income of $10 000 or less (89, 79.4%) and education below high school (77, 68.75%). The duration of diabetes in years (average 13.0 ± 1.3) and concurrent medications did not differ significantly between the 2 groups. No adverse effects were reported in any group for the duration of the study.
Average Daily Pain
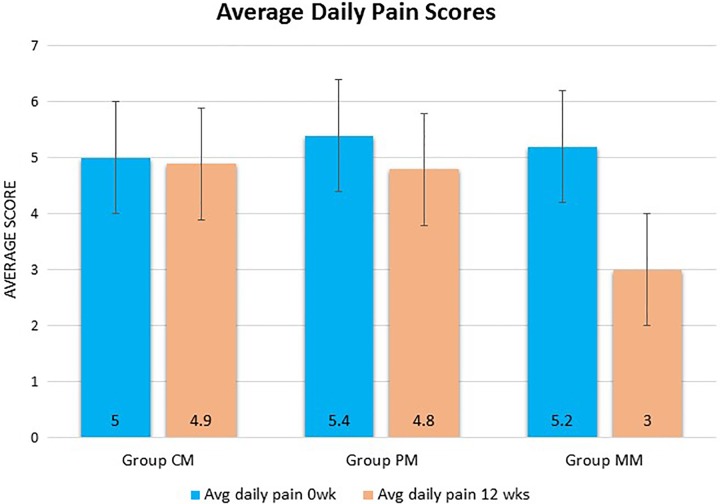
Groups MM and PM experienced a significant (P < .05) reduction in average daily pain in the last 24 hours at the study end when compared to baseline (28.7% and 39.7%, respectively).
In Group PM, the baseline BPI-DPN Q4 score for average daily pain was 5.4 ± 1.9, which dropped to 4.8 ± 1.0 by week 12 (P < .05). The overall change in mean daily pain intensity was 0.6 ± 0.1 (95% confidence interval [CI] = −0.85, −0.10).
Group MM showed a more significant (P < .01) reduction of pain when compared to the control group. A baseline score of 5.2 ± 1.2 dropped to 3.0 ± 1.1 by week 12 of treatment. The overall change in mean daily pain intensity was 2.2 ± 0.1 (95% CI = −2.50, −1.80).
In Group CM, there was a nonsignificant (P > .05) reduction in the scores. The baseline scores of 5.0 ± 1.9 reduced to 4.9 ± 1.0 at week 12. The overall change in the mean daily pain intensity was −0.5 ± 0.2 (95% CI = −0.59, −0.20). These results are shown in Figure 1.
Figure 1.
Average daily pain scores of Groups PM, CM, and MM taken at the beginning of treatment and end of the treatment (12 weeks). Group PM (n = 32), Group CM (n = 37), and Group MM (n = 36). Each histogram bar represents the mean ± SD. P < .05 was deemed significant. **P < .01, *P < .05.
Patient Global Impression of Change
Groups MM and PM both showed a significant improvement in patients’ impression at the study end, 75 ± 5.1% (n = 36) and 61 ± 6.5% (n = 32), respectively. The improvement in Group MM was highly significant (P = .01), while Group PM also had significant improvement (P = .04). However, Group CM showed no significant change.
Patient Satisfaction
The patient baseline satisfaction scores of 2.1 ± 1.1 in Group PM increased to 3.0 ± 1.1 by week 12 of treatment. In Group CM, patient satisfaction scores increased from 2.2 ± 1.5 to 2.7 ± 1.5 by week 12. In Group MM, patient satisfaction scores were 2.0 ± 1.0, increasing significantly (P < .05) to 3.8 ± 1.9 by week 12 of treatment.
Discussion
There is a lack of gender-specific studies on the 2 most common types of meditation and their impact on chronic pain experienced by elderly female diabetics in South Asia.18 To the best of our knowledge, this is the first study of its type to be conducted in Pakistan.
Difficulties faced by the population in frontier markets such as Pakistan are highlighted in our study, as indicated by the income and educational level results we obtained. The socioeconomic status of an individual has an impact on the awareness of therapeutic options for chronic diseases such as diabetes mellitus.19 The regional uniqueness of cultural and socioeconomic circumstances in South Asia negatively affects elderly females in particular.20,21
Glycemic control is the cornerstone of preventing and decreasing the severity of the ensuing complications of diabetes.22,23 Diabetic peripheral neuropathy is the most common complication of diabetes and is often accompanied by chronic pain.6,7 This affects how diabetic individuals participate in self-care activities. Chronic pain is also associated with anxiety and depression, which in turn is connected to higher HbA1c levels and severity of diabetic complications.24–26
Chronic pain, an aspect of neuropathic pain, leads to significant medical, social, and economic consequences.27 Pain is considered a multidimensional experience with sensory, cognitive, and affective factors. These include attention, distraction, suggestion, and other emotional states. The core 3 interacting dimensions of the pain experience include sensory-discriminative, motivational affective, and cognitive-interpretative. The gate control theory has suggested that activity in the cognitive and/or motivational modes can modulate the sensory dimension of the pain experience.28 This has led to an increase in interest toward alternatives to medication and the possibilities of designing adjunctive therapy for afflicted patients.29
A study by Sternbach suggested that psychological and behavioral strategies for the control of pain could provide a more effective long-term solution than medications for many patients.30 The response to pain therapy varies between patients experiencing neuropathic pain.31,32 Many patients experience inadequate relief and few patients have access to pain specialists.33–36 There is also evidence of dependence on pain medication and its subsequent negative consequences.37
The study by Nakata et al indicates that neuromodulatory interventions such as meditation can help alter brain processes involved in the experience of pain and thus provide relief for many patients. Pain-related neural activity is reduced in brain regions such as the insula, secondary somatosensory cortex, and the thalamus. The characteristics of the modulation of this activity may depend on the type of meditation and the duration of meditation practice.38 Novice meditators show an increase in activity in the areas involved in the cognitive regulation of nociceptive processing (the anterior cingulate cortex and anterior insula) and reduced activation in the primary somatosensory cortex. Expert meditators show decreased activation in the dorsolateral and ventrolateral prefrontal cortex regions and enhancements in the primary pain processing regions (the insula, somatosensory cortex, and thalamus).39
Two of the most common types of meditation are mindfulness meditation and progressive relaxation meditation. Mindfulness meditation has been receiving an increase in attention for its potential as part of an integrative therapy regimen to help patients deal with chronic pain.40 In mindfulness meditation, the attentional stance toward proprioception is that of detached observation. In terms of chronic pain, using cognitive reappraisal to separate the sensory dimension of the pain experience from the resultant evaluative alarm reaction can reduce suffering.32
Mindfulness meditation has several key aspects. See Figure 2 for aspects of mindfulness-based meditation.
Figure 2.
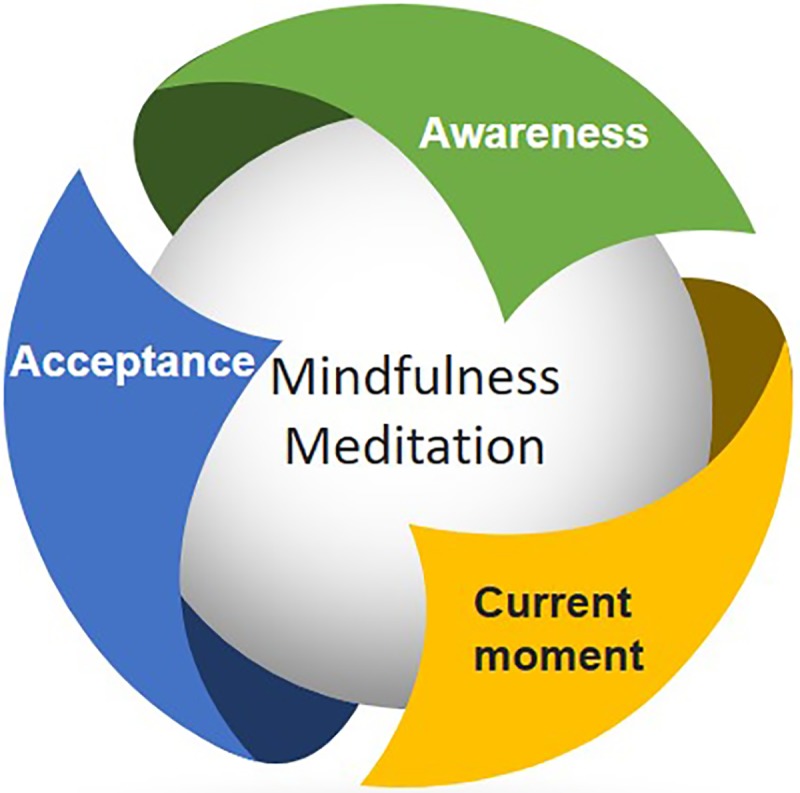
Various aspects of mindfulness-based meditation.
Methods such as questionnaires for screening and assessment focus on the presence and quality of neuropathic pain. We used the validated clinical questionnaires for average daily pain (BPI-DPN Q4) and the PGIC.41
In Group PM, the baseline BPI-DPN Q4 score for average daily pain dropped from 5.4 ± 1.9 to 4.8 ± 1.0 by week 12 (P < .05), but this reduction was more significant in Group MM (P < .01) where the baseline score of 5.2 ± 1.2 dropped to 3.0 ± 1.1. This suggests mindfulness meditation compared to progressive relaxation was more effective at reducing the pain. Our results are in line with other studies indicating that practicing mindfulness meditation improves clinically relevant cognitive and health outcomes, including the reduction of chronic pain.39,42–44 The reduction of the pain experience of patients practicing mindfulness meditation suggests that increased awareness and sensitivity to the attributes of pain and to stress reactions in the moment can lead to the emergence of new cognitive and behavioral coping responses toward pain and stress. These can, in the long term, replace nonadaptive conditioned pain behaviors and stress reactions.32
Training in mindfulness meditation has shown improvement in many psychological aspects of patients afflicted with chronic pain such as anxiety, depression, emotional regulation, cognitive control, and mood.45–47 In our particular age group of interest, mindfulness-based meditation has been noted to reduce geriatric anxiety, which is often associated with chronic pain.48 With the integration of mindfulness meditation in a multidisciplinary pain management plan, clinicians can improve treatment outcomes and potentially decrease pain-related medication utilization specifically in vulnerable patient populations.49
The change in score for average daily pain was apparent in Group MM but also reached significance in Group PM. Progressive relaxation is another common method of meditation that is often employed to reduce chronic pain.50 It generates the “relaxation response,” which is the opposite of the stress or “fight or flight” response.51 Evidence suggests that the benefits of meditation including other types such as transcendental include reduction in inflammation, desensitization of central pain pathways, reduced emotional reactivity to pain, and enhancing the brain’s response to endorphins.52,53
A studies by Chen and Francis has suggested that progressive relaxation meditation can also benefit the management of chronic pain.54 This is especially useful for older adults.51 The difference between the results in Group PM are comparable to other studies that have compared progressive relaxation mediation to cupping and self-hypnosis. Progressive relaxation has shown to have benefits but less so in comparison.55,56
In terms of the patient impression of change and satisfaction scores, both Groups MM and PM showed a significant improvement in patients’ impression at the study end. However, this was again more prominent in Group MM, which is in line with the studies by Petersen et al57 and Ball et al,42 which showed the long-term benefits of using mindfulness meditation including reduction of chronic pain, improved quality of life, and satisfaction reporting.
Although evidence suggests that mindfulness-based interventions are effective for pain management, in the real world these might be difficult to achieve due to resource and time constraints. Studies have suggested that using brief mindfulness-based interventions to be more feasible and pragmatic for the safe treatment of pain.58
Conclusion
Although it is not often possible to completely eliminate chronic pain, many patients can benefit by learning skills that will help lead a productive life with less discomfort and disability.
When integrative therapies such as mindfulness meditation are involved in a comprehensive pain management plan, the consumption of pain-related medications and consequent addiction could be reduced, the interference of chronic pain with daily life activities would decrease, and the activity levels of the patients could improve. Moreover, mindfulness meditation can be used as a stand-alone treatment or in conjunction with other treatment modalities such as pharmacological and nonpharmacological measures to treat pain and enhance functioning. The advantages of mindfulness meditation include no risk of addiction or abuse, better treatment outcomes, and improvement in comorbid conditions of chronic pain such as anxiety and depression.37 Various mindfulness skills have a common goal, which is to achieve an acceptance of what is occurring within the phenomenological field and acceptance of it to reduce mental judgement.59
Gender-specific association between mindfulness meditation and its effect on chronic pain in the older females of South Asia requires the spotlight to be on tailor made treatment plans for specific patients in these clinical settings and warrants further extensive research.
While these findings help shed light on the gaps in knowledge about the impact of mindfulness meditation on chronic pain, there is a clear need for future studies to confirm these findings on a larger scale.
Ultimately, the primary goal for most patients is to experience the most relief possible from treatments that are effective yet have minimal adverse effects. As we have highlighted in our study, neuromodulatory treatments such as mindfulness meditation need to be conceptualized by health care providers as low-risk tools that can contribute to the overall benefit of the patient’s health.
Identifying individuals that are more amenable to the benefits of this particular type of meditation could pave the way for implementing diabetes educational programs that are specific to these patient’s unique needs and stress on the importance of integrative therapies to health care providers that the patients come across.
Limitations of the Study
There are several limitations to the study such as self-reported outcomes, lack of generalizability to other populations, and small sample size. Additionally, there is not much awareness in the literature about individual differences and how these can contribute to the different responses to mindfulness mediation. Factors that can influence differential training effects include temperamental, personality, or genetic differences between individuals, and group dynamics along with personality of the trainer can also influence training effects but these are not clearly understood.59
Acknowledgments
We thank Dr Fatima and Dr Zainab for their work and support.
Authors’ Note: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this article.
Author Contributions: NH conceptualized and supervised the study. ASAS contributed to the methodology and formal data analysis. NH and ASAS were responsible for the writing—original draft preparation, review, and editing.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study received the hospital research grant approved by the Punjab Care Hospital Research Council (IAC-0457). The funders did not have any role in the study design, execution, data collection, or analysis and in the manuscript preparation or editing.
ORCID iD: Nadia Hussain  https://orcid.org/0000-0001-6314-2485
https://orcid.org/0000-0001-6314-2485
Ethical Approval: This present study was a placebo controlled, parallel group study that was approved by the Punjab Care Hospital Review Board (Approval No. 38920), conducted in a single clinical center in agreement with the ethical guidelines of the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable regulatory requirement. Informed consent was obtained from all individual participants included in the study.
References
- 1. Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. [DOI] [PubMed] [Google Scholar]
- 2. Akhter J. The burden of diabetes in Pakistan: the national diabetes survey. J Pak Med Assoc. 1999;49:205–206. [PubMed] [Google Scholar]
- 3. UNESCO Institute for Statistics. Pakistan: http://uis.unesco.org/country/PK. Published 2016. Accessed August 29, 2019. [Google Scholar]
- 4. Bajaj S, Jawad F, Islam N, et al. South Asian women with diabetes: psychosocial challenges and management: consensus statement. Indian J Endocrinol Metab. 2013;17:548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group; Nathan DM, Zinman B, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med. 2009;169:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benbow SJ, Daousi C, MacFarlane IA. Diagnosing and managing chronic painful diabetic neuropathy. Diabet Foot. 2004;7:34–41. [Google Scholar]
- 7. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30:374–385. [DOI] [PubMed] [Google Scholar]
- 8. Bhurji N, Javer J, Gasevic D, Khan NA. Improving management of type 2 diabetes in South Asian patients: a systematic review of intervention studies. BMJ Open. 2016;6:e008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pokorski M, Suchorzynska A. Psychobehavioral effects of meditation. Adv Exp Med Biol. 2018;1023:85–91. [DOI] [PubMed] [Google Scholar]
- 10. Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57:63–69. [DOI] [PubMed] [Google Scholar]
- 11. Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin Psychol Rev. 2015;37:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Alsubaie M, Abbott R, Dunn B, et al. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: a systematic review. Clin Psychol Rev. 2017;55:74–91. [DOI] [PubMed] [Google Scholar]
- 13. Shapiro S, Lehrer PM. Psychophysiological effects of autogenic training and progressive relaxation. Biofeedback Self Regul. 1980;5:249–255. [DOI] [PubMed] [Google Scholar]
- 14. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269–277. [DOI] [PubMed] [Google Scholar]
- 15. Scott W, McCracken LM. Patients’ impression of change following treatment for chronic pain: global, specific, a single dimension, or many? J Pain. 2015;16:518–526. [DOI] [PubMed] [Google Scholar]
- 16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 17. van Nooten F, Trundell D, Staniewska D, Chen J, Davies EW, Revicki DA. Evaluating the measurement properties of the self-assessment of treatment version II, follow-up version, in patients with painful diabetic peripheral neuropathy. Pain Res Treat. 2017;2017:6080648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilbert CE, Shah SP, Jadoon MZ, et al. Poverty and blindness in Pakistan: results from the Pakistan national blindness and visual impairment survey. BMJ. 2008;336:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizvi A, Bhatti Z, Das JK, Bhutta ZA. Pakistan and the Millennium Development Goals for maternal and child health: progress and the way forward. Paediatr Int Child Health. 2015;35:287–297. [DOI] [PubMed] [Google Scholar]
- 21. Jayawardena R, Ranasinghe P, Byrne NM, Soares MJ, Katulanda P, Hills AP. Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health. 2012;12:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 23. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crispin-Trebejo B, Robles-Cuadros MC, Bernabe-Ortiz A. Association between depression and glycemic control among type 2 diabetes patients in Lima, Peru. Asia Pac Psychiatry. 2015;7:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dirmaier J, Watzke B, Koch U, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79:172–178. [DOI] [PubMed] [Google Scholar]
- 26. Krein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005;28:65–70. [DOI] [PubMed] [Google Scholar]
- 27. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. [DOI] [PubMed] [Google Scholar]
- 28. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109:5–12. [DOI] [PubMed] [Google Scholar]
- 29. Chiesa A, Serretti A. Mindfulness-based interventions for chronic pain: a systematic review of the evidence. J Altern Complement Med. 2011;17:83–93. [DOI] [PubMed] [Google Scholar]
- 30. Sternbach RA. Pain: A Psychophysiological Analysis. New York, NY: Academic Press; 2013. [Google Scholar]
- 31. Wolfe GI, Trivedi JR. Painful peripheral neuropathy and its nonsurgical treatment. Muscle Nerve. 2004;30:3–19. [DOI] [PubMed] [Google Scholar]
- 32. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. [DOI] [PubMed] [Google Scholar]
- 33. Dureja GP, Jain PN, Shetty N, et al. Prevalence of chronic pain, impact on daily life, and treatment practices in India. Pain Pract. 2014;14:E51–E62. [DOI] [PubMed] [Google Scholar]
- 34. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 35. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. [DOI] [PubMed] [Google Scholar]
- 36. Jefferies K. Treatment of neuropathic pain. Semin Neurol. 2010;30:425–432. [DOI] [PubMed] [Google Scholar]
- 37. Majeed MH, Ali AA, Sudak DM. Psychotherapeutic interventions for chronic pain: evidence, rationale, and advantages. Int J Psychiatry Med. 2019;54:140–149. [DOI] [PubMed] [Google Scholar]
- 38. Nakata H, Sakamoto K, Kakigi R. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol. 2014;5:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–225. [DOI] [PubMed] [Google Scholar]
- 40. Khoo EL, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioural therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perrot S, Lanteri-Minet M. Patients’ global impression of change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain. 2019;23:1117–1128. [DOI] [PubMed] [Google Scholar]
- 42. Ball EF, Sharizan ENSM, Franklin G, Rogozinska E. Does mindfulness meditation improve chronic pain? A systematic review. Curr Opin Obstet Gynecol. 2017;29:359–366. [DOI] [PubMed] [Google Scholar]
- 43. Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- 44. Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Can J Psychiatry. 2012;57:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: a randomized controlled trial. Soc Cogn Affect Neurosci. 2015;10:1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barnhofer T, Crane C, Hargus E, Amarasinghe M, Winder R, Williams JM. Mindfulness-based cognitive therapy as a treatment for chronic depression: a preliminary study. Behav Res Ther. 2009;47:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeidan F, Martucci KT, Kraft RA, McHaffie JG, Coghill RC. Neural correlates of mindfulness meditation-related anxiety relief. Soc Cogn Affect Neurosci. 2014;9:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hazlett-Stevens H, Singer J, Chong A. Mindfulness-based stress reduction and mindfulness-based cognitive therapy with older adults: a qualitative review of randomized controlled outcome research. Clin Gerontol. 2019;42:347–358. [DOI] [PubMed] [Google Scholar]
- 49. Majeed MH, Ali AA, Sudak DM. Mindfulness-based interventions for chronic pain: evidence and applications. Asian J Psychiatr. 2018;32:79–83. [DOI] [PubMed] [Google Scholar]
- 50. Hassed C. Mind-body therapies—use in chronic pain management. Aust Fam Physician. 2013;42:112–117. [PubMed] [Google Scholar]
- 51. Middaugh SJ, Pawlick K. Biofeedback and behavioral treatment of persistent pain in the older adult: a review and a study. Appl Psychophysiol Biofeedback. 2002;27:185–202. [DOI] [PubMed] [Google Scholar]
- 52. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40:251–265. [DOI] [PubMed] [Google Scholar]
- 53. Elias AN, Wilson AF. Serum hormonal concentrations following transcendental meditation—potential role of gamma aminobutyric acid. Med Hypotheses. 1995;44:287–291. [DOI] [PubMed] [Google Scholar]
- 54. Chen YL, Francis AJ. Relaxation and imagery for chronic, nonmalignant pain: effects on pain symptoms, quality of life, and mental health. Pain Manag Nurs. 2010;11:159–168. [DOI] [PubMed] [Google Scholar]
- 55. Lauche R, Materdey S, Cramer H, et al. Effectiveness of home-based cupping massage compared to progressive muscle relaxation in patients with chronic neck pain—a randomized controlled trial. PLoS One. 2013;8:e65378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57:198–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: a randomized controlled trial. Pain Med. 2015;16:641–652. [DOI] [PubMed] [Google Scholar]
- 58. McClintock AS, McCarrick SM, Garland EL, Zeidan F, Zgierska AE. Brief mindfulness-based interventions for acute and chronic pain: a systematic review. J Altern Complement Med. 2019;25:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang YY, Posner MI. Tools of the trade: theory and method in mindfulness neuroscience. Soc Cogn Affect Neurosci. 2013;8:118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]