Abstract
The diagnosis of a mycosis is often established through a biopsy, which allows to differentiate invasive and non-invasive lesions, and also to identify hyaline and dematiaceous fungi. However, pigmented fungal elements that do not correspond to dematiaceous fungi, which we have called pseudodematiaceous, can occasionally be present in biopsies. Herein, we present 2 cases of mycosis caused by pseudodematiaceous fungi in rhinosinusal biopsies. A new classification for fungi identified in biopsies is proposed, dividing them into 3 groups: hyaline, dematiaceous, and pseudodematiaceous.
Keywords: Pseudodematiaceous fungi, dematiaceous fungi, hyaline fungi, classification, biopsy
Introduction
The diagnosis of a mycosis is frequently made through histological examination.1,2 Fungal infections of geographic distribution and opportunistic fungal infections usually present clinical manifestations that mimic neoplastic diseases. Therefore, it is almost routine to undergo biopsy for the patients who need a correct diagnosis. Moreover, the increment in immunosuppressed patients and the populations who migrate from tropical or subtropical zones with endemic mycoses also contribute to the increase of individuals who will potentially require a biopsy due to this pathology.3-8 The foregoing supports the relevant role played by the modern pathology in the diagnosis of many infectious diseases, especially mycoses. The 2 most important questions that the biopsy should resolve in the case of a mycosis are as follows: (1) Is there invasion of the tissue? and (2) What type of fungus is present in the lesion? Regardless of the various and intricate fungal classifications, and the type of tissue reaction that can be found, the histological examination allows to classify, in a fast way, the fungal elements according to their shape and color.9-12 Although all fungi have pigments in their vegetative structures,13,14 only in some the coloration of their wall is striking and recognizable in histological routine sections stained with hematoxylin-eosin. These fungi are known as pigmented fungi or dematiaceous fungi, and they are associated with 3 types of basic anatomo-clinical entities: chromoblastomycosis, phaeohyphomycosis, and non-invasive forms. On the contrary, fungi that lack parietal pigmentation or are not recognizable using routine histological stains are known as hyaline fungi.3 This is a rather simple classification for fungi that are present in tissue samples, which facilitates the interpretation of the complementary studies and guides the definitive diagnosis. However, we have found cases of human mycosis with pigmented fungal elements in biopsies that do not fit to the classically described dematiaceous fungi, and therefore leading to a wrong diagnosis. Hence, we propose to call these fungi “pseudodematiaceous.”
Herein, we present 2 cases of this type of fungi, fully describing their light microscopy characteristics with ultrastructural correlation.
Cases
Case 1
A 33-year-old woman under treatment for acute lymphatic leukemia presented acute left periorbital rhinosinusitis and cellulitis. Biopsies were obtained from the nasal cavity, paranasal sinuses, and periorbital tissue. They were fixed in 10% neutral formalin, embedded in paraffin, and stained initially with hematoxylin-eosin, periodic acid-Schiff (PAS), Grocott, and Gram. The light microscopy examination showed a necro-hemorrhagic, tissular, and angio-invasive mycosis. The fungi consisted of irregular filamentous structures without septae, showing in several areas hyphae with granular brownish-yellowish cytoplasmic pigmentation, either in patchy or diffuse distribution. The mycotic wall did not present coloration (Figure 1); in some areas, non-pigmented, coenocytic hyphae predominated. Histochemical stains performed for melanin (Fontana-Masson) and hemosiderin (Prussian Blue) were negative.
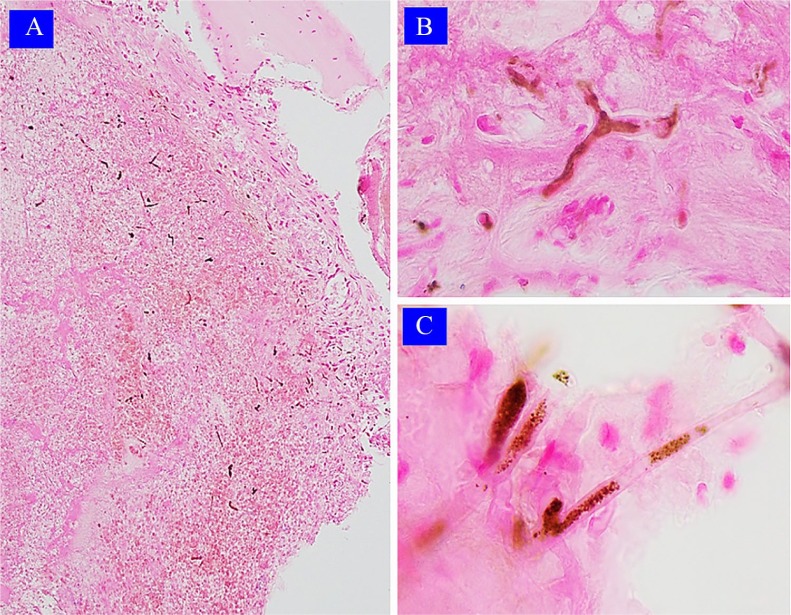
Figure 1.
Case 1: Light microscopy findings. (A) Necrotic and hemorrhagic tissue with abundant pigmented hyphae (hematoxylin-eosin 100×, original magnification). (B) Hyphae with pigmented cytoplasm (hematoxylin-eosin 400×, original magnification). (C) Coenocytic hyphae with hyaline and granular pigmented cytoplasm forms; note the absence of pigmentation of its cell wall (hematoxylin-eosin 400×, original magnification).
For the ultrastructural analysis of the pigment, the tissue was retrieved from the paraffin block and went under routine process to obtain Epon blocks for semi-thin and ultra-thin sections. The ultrastructural analysis revealed hyphae with granular-floccular and slightly electron-dense cytoplasm. They also revealed a multilamellar cell wall with variable electron density, but being mostly highly electron-dense (Figure 2). A granular, external layer of melanin-type granules was not found in the cell wall of the hyphae. Despite the presence of pigmented hyphae in the biopsy, the histopathological diagnosis was an invasive mucormycosis. The culture was positive for Rhizopus spp.
Figure 2.
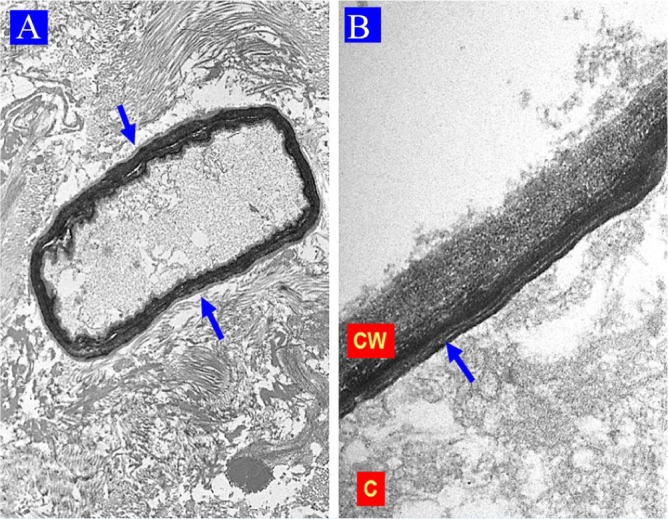
Case 1: Electron microscopy findings. (A) Necrotic tissue with a coenocytic hyphae (blue arrows) showing a floccular and slightly granular electron-dense cytoplasm (uranyl acetate, lead citrate 6000×, original magnification). (B) Coenocytic hyphae, note the cytoplasm (C), the plasmatic membrane (red arrow), and the multilayered cell wall (CW) with intense and variable electron density (uranyl acetate, lead citrate 43 000×, original magnification).
Case 2
A 74-year-old man was diagnosed with chronic fungal rhinosinusitis. Due to the suspicion of a right maxillary fungal ball, the patient underwent sinus biopsies, which were fixed in 10% neutral formalin, embedded in paraffin and stained initially with hematoxylin-eosin, PAS, Grocott, and Gram. The tissue examination demonstrated a fungal ball composed of randomly arranged, regular filamentous fungi with septate, from 3.3 to 3.6 µm of thickness and around 180 µm of length. They revealed a pigmented, yellowish-brown cell wall (Figure 3). Deeper histological sections were performed; they showed that some hyphae ended in a spherical vesicle with phialides and pigmented conidia. Subsequently, the melanin stain was positive in the hyphae cell wall and in the spores. In addition, immunohistochemical technique was performed for Aspergillus genus in the biopsy, which was positive. No tissue invasion was found. For the ultrastructural study of the pigment, the sample was retrieved from the paraffin block and reprocessed routinely for transmission electron microscopy. This examination revealed hyphae with regular, trilaminar cell wall in which the most external layer, very thin and granular, was consistent with granular melanin (Figure 4). Despite the presence of pigmented hyphae, the pathological conclusion was an aspergillar-fungal ball (aspergilloma).
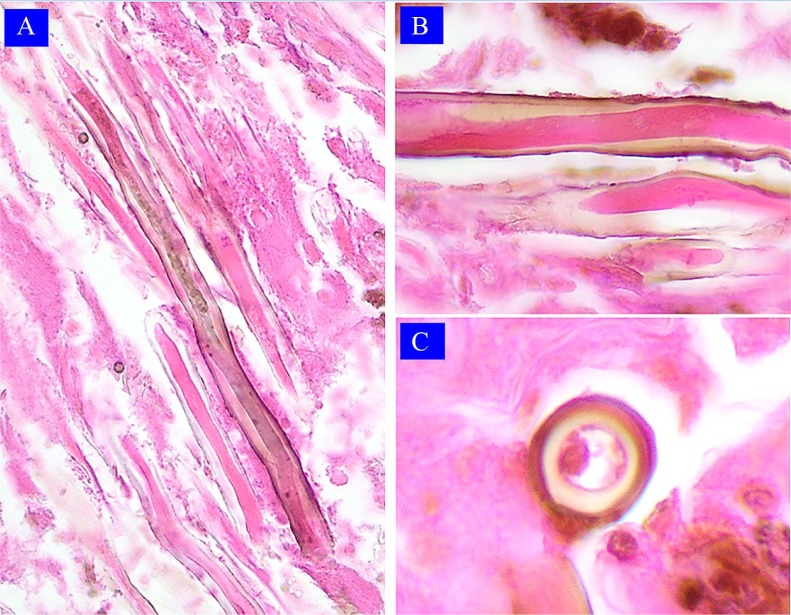
Figure 3.
Case 2: Light microscopy findings. (A) Thick, regular hyphae, some slightly pigmented (hematoxylin-eosin 400×, original magnification). (B) Regular thick hyphae and pigmented cell wall in longitudinal section (hematoxylin-eosin 100×, original magnification). (C) Thick pigmented cell wall hyphae in cross-section (hematoxylin-eosin 100×, original magnification).
Figure 4.
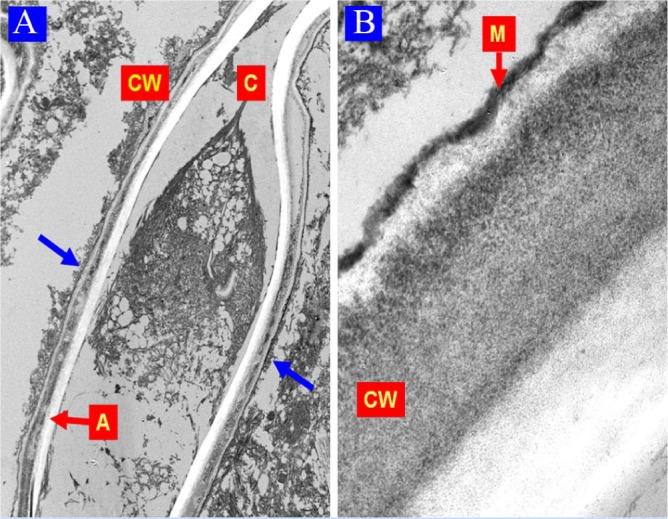
Case 2: Electron microscopy findings. (A) Well-defined hyphae (blue arrows); note its cytoplasm (C), cell wall (CW), and a clear central artifactual band (A) (uranyl acetate, lead citrate 6000×; original magnification). (B) Hyphae CW with trilaminar architecture; the external is thinner and highly electron-dense and corresponds to melanin granules (M) (uranyl acetate, lead citrate 43 000×; original magnification).
For electron microscopy analysis of both cases, the tissue embedded in paraffin blocks was carefully extracted under stereo microscopic observation with a scalpel and retrieved using histoclear solution (2 changes; 20 min each). Thereafter, the tissue was set in 100% ethyl alcohol (3 changes; 15 min each) followed by 2 steps of 15 min placing the tissue in lower volume ethyl alcohol solutions (80% and 50%, respectively). After these hydration steps, the sample was placed in distilled water (50 min) and then immersed in glutaraldehyde 8% overnight. Following these retrieval steps, the block embedding, staining, and sectioning were those routinely performed for most of the tissue submitted for ultrastructural analysis. They included Veronal buffer wash, post-fixation and stain with osmium tetroxide 1% (1 h), distilled water wash, and immersion in aqueous uranyl acetate (1 h). The following steps were dehydration with increasing acetone volume (20%, 35%, 50%, 70%, 85%, and 95%; 10 min each and 100% 3 times). The tissue was embedded in Epon-acetone 1:1 overnight followed next day by placing the tissue in silicone molds which were filled up with resin Epon 812. The polymerization of the resin was obtained after 48 h, always under controlled temperature in stove. The blocks obtained were submitted for semi-thin sections (1-1.4 µm) and stained with toluidine blue. After choosing the required zone for analysis, ultra-thin sections (50 nm) were performed; they were placed in copper grids and stained with uranyl acetate 4% (90 s) and lead citrate (10 min). The ultra-thin stained sections were analyzed in a Philips Tecnai 120 kV transmission electron microscope.
Discussion
Invasive and non-invasive mycoses by hyaline and pigmented fungi are emerging infections of major importance in these days, especially in immunosuppressed patients.3,7,8,15 Its precise diagnosis often depends on an adequate interpretation of the biopsy findings together with the results of currently available mycological and molecular biology tests.1,2,16 As many hyalohyphomycetes, zygomycetes, and phaeohyphomycetes are common contaminants in laboratory environments, the identification of the fungi in the biopsy represents an unequivocal proof of invasive or non-invasive fungal infection.1,12
The detection of pigment in the fungal agents found in the biopsy is the most important step to distinguish between the 2 main groups of fungi: hyalines and dematiaceous. The identification should be carried out with the current hematoxylin-eosin staining; because with histochemical special stains, the fungi can appear artificially pigmented. In this context, we must bear in mind some considerations: all fungi have endogenous pigments; there are several types of pigmented substances; the pigments can be located in diverse fungal structures; the reproduction elements of many fungi are pigmented; there are variations in the production of mycotic pigments in vivo and in vitro conditions; the endogenous pigmentation of the fungi can be diminished or it can be lost; pigment production can be induced in vitro in fungi that usually are not colored; and the pigmentation can be enhanced with special histochemical techniques.13,16
The main pigments produced by fungi are melanins, flavins, and carotenoids; the most important is melanin, which is located mainly in the cell wall and behaves as a factor of protection and virulence.14
In the context of the histological examination, we consider a dematiaceous fungus when its hyphae or vegetative-spherical fungal cells have a cell wall with brown or yellowish-brown pigmentation, and the pigment corresponds to melanin.
The presence of pigments in vegetative elements of non-dematiaceous fungi has already been described in vitro, including the communication of pigmentation by carotenoids in vegetative hyphae of zygomycetes and ascomycetes.17-19
In the first case we presented, the pigmentation of the hyphae corresponded to granular yellowish-brown non-melanic material located in the cytoplasm of the hyphae, and the agent was a filamentous coenocytic fungus of the order Mucorales, with a positive culture for Rhizopus spp. In the second case, although hyphae were found with melanic pigmentation of their cell wall, they were hyphae in an asexual reproduction state, specifically stipes or conidiophores of a hyalohyphomycete of the genus Aspergillus.
Considering the morphological and ultrastructural findings of our cases, and according to this discussion of the diagnostic criteria, we propose to add the “pseudodematiaceous” fungi type as a different category into the general morphological classification of fungi. Our proposal for this new category is mainly based on the analysis of tissue or cytological samples; hence, the morphological types would include hyaline, dematiaceous, and pseudodematiaceous fungi.
We consider pseudodematiaceous fungi that in tissue or cytology samples present 1 or more of the following characteristics: (1) non-melanic pigmentation, (2) non-parietal pigmentation, and (3) parietal melanic pigmentation only in reproductive elements, such as coniophores, sporogenic elements, and spores.
A precise diagnosis of fungi in tissue samples or cytologies is not always easy to perform, and it is not uncommon to make errors, because the pathologist usually has access in histological sections and smears only to vegetative fungal elements that are common to many other fungi. On the contrary, most of the pathologists are not familiarized with the morphological richness of the kingdom of the fungi and with the intricate mycological taxonomy.20
We consider that the evaluation of fungal pigmentation in biopsies and cytologies is a diagnostic key after the recognition of the fungal forms. We truly believe that our proposed morphological classification for fungi present in tissues and cytological samples can facilitate the labor of the general surgical pathologist and the infectious diseases pathologist, who usually are requested to make the diagnosis of mycoses in a fast and accurate manner, but always in concordance with the mycology and the molecular pathology tests. In the cases we studied, the transmission electron microscopy was the most useful complementary technique for the examination and search of pigments; therefore, we consider it as a very valuable diagnostic tool, especially when there are no cultures nor molecular techniques for mycological diagnosis.
The correct recognition of the type of fungi is critical for a precise diagnosis and a timely treatment of patients.
Acknowledgments
The authors thank Ms Kate Masterson for her support in the redaction and revision of the grammar of our manuscript.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: DO, DC, and GPM participated in the fieldwork, contributed to data analysis, and participated in the revision of the manuscript.
ORCID iD: David Oddó  https://orcid.org/0000-0002-3974-7393
https://orcid.org/0000-0002-3974-7393
References
- 1. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sangoi AR, Rogers WM, Longacre TA, Montoya JG, Baron EJ, Banaei N. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens. Am J Clin Pathol. 2009;131:364-375. [DOI] [PubMed] [Google Scholar]
- 3. Nucci M, Anaissie EJ. Hyalohyphomycosis. In: Anaissie EJ, McGinnis MR, Pfaller MAS, eds. Clinical Mycology. China: Churchill Livingstone; 2009:309-328. [Google Scholar]
- 4. Gómez LV, Cardona-Castro N. Phaeohyphomycosis, an emerging opportunistic fungal infection. Rev CES Med. 2016;30:66-67. [Google Scholar]
- 5. Molina-Morant D, Sánchez-Montalvá A, Salvador F, Sao-Aviles A, Molina I. Imported endemic mycoses in Spain: evolution of hospitalized cases, clinical characteristics and correlation with migratory movements, 1997-2014. PLoS Negl Trop Dis. 2018;12:e0006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017;17:e367-e377. [DOI] [PubMed] [Google Scholar]
- 7. Sutton DA. Rare and emerging agents of hyalohyphomycosis. Curr Fungal Infect Rep. 2008;2:134-142. [Google Scholar]
- 8. Ravikant KT, Gupte S, Kaur M. E review on emerging fungal infection and their significance. J Bacteriol Mycol Open Access. 2015;1:39-41. [Google Scholar]
- 9. Tarrand JJ, Lichterfeld M, Warraich I, et al. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119:854-858. [DOI] [PubMed] [Google Scholar]
- 10. Slavin M, van Hal S, Sorrell TC, et al. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect. 2015;21:490.e1-490.e10. [DOI] [PubMed] [Google Scholar]
- 11. Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayayo E. Histopathological diagnosis of mycoses. Rev Iberoam Micol. 2004;21:1-9. [PubMed] [Google Scholar]
- 13. Sunduram C, Shantveer GU, Umabala P, Lakshmi V. Diagnostic utility of melanin production by fungi: study on tissue sections and culture smears with Masson-Fontana stain. Indian J Pathol Microbiol. 2014;57:217-222. [DOI] [PubMed] [Google Scholar]
- 14. Belozerskaya TA, Gessler NN, Aver’yanov AA. Melanin pigments of fungi. In: Mérillon JM, Ramawat K, eds. Fungal Metabolites (Reference Series in Phytochemistry). Cham, UK: Springer; 2017:263-291. Website. 10.1007/978-3-319-25001-4_29. [DOI] [Google Scholar]
- 15. Pfaller MA, Diekema DJ. Unusual fungal and pseudo fungal infections of humans. J Clin Microbiol. 2005;43:1495-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shinozaki M, Tochigi N, Sadamoto S, Yamagata Murayama S, Wakayama M, Nemoto T. Histopathological diagnosis of invasive fungal infections in formalin-fixed and paraffin-embedded tissues in conjunction with molecular methods: comparison of reproducibility and reliability of histopathological evaluation, polymerase chain reaction, and in situ hybridization. Med Mycol J. 2018;59:E7-E18. [DOI] [PubMed] [Google Scholar]
- 17. Narsing Rao MP, Xiao M, Li WJ. Fungal and bacterial pigments: secondary metabolites with wide applications. Front Microbiol. 2017;8:1113. doi:10.3389/fmicb.2017.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avalos J, Carmen Limón M. Biological roles of fungal carotenoids. Curr Genet. 2015;61:309-324. [DOI] [PubMed] [Google Scholar]
- 19. Pombeiro-Sponchiado SR, Sousa GS, Andrade JCR, Lisboa HF, Gonçalves RCR. Production of melanin pigment by fungi and its biotechnological applications. Website. 10.5772/67375. [DOI]
- 20. Tedersoo L, Sánchez-Ramírez S, Koljalg U, et al. High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018;90:135-159. [Google Scholar]