Abstract
The lateral superior olive (LSO) is one of the most peripheral auditory nuclei receiving inputs from both ears, and LSO neurons are sensitive to interaural level differences (ILDs), one of the primary acoustical cues for sound location. We used the virtual space (VS) technique to present over earphones broadband stimuli containing natural combinations of localization cues as a function of azimuth while recording extracellular responses from single LSO cells. The responses of LSO cells exhibited spatial receptive fields (SRFs) in azimuth consonant with their sensitivity to ILDs of stimuli presented dichotically: high discharge rates for ipsilateral azimuths where stimulus amplitude to the excitatory ear exceeded that to the inhibitory ear, rapidly declining rates near the midline, and low rates for contralateral azimuths where the amplitude to the inhibitory ear exceeded that to the excitatory ear. Relative to binaural stimulation, presentations of the VS stimuli to the ipsilateral ear alone yielded increased rates, particularly in the contralateral field, confirming that the binaural SRFs were shaped by contralateral inhibition. Our finding that LSO neurons respond to azimuth consistent with their ILD sensitivity supports the long-held hypothesis that LSO neurons compute a correlate of the ILD present in free-field stimuli. Only weak correlations between the properties of pure-tone ILD functions and the SRFs were found, indicating that ILD sensitivity measured at only one sound level is not sufficient to predict sensitivity to azimuth. Sensitivity to spatial location was also retained over a wide range of stimulus levels under binaural, but not monaural, conditions.
Keywords: lateral superior olive, sound localization, binaural, inferior colliculus, cat, interaural level difference
Because the ear has no mechanism to sense sound location directly, it must be computed centrally, and localization in the horizontal dimension depends on the comparison of small differences in the sounds arriving at the two ears. The superior olivary complex (SOC) consists of several nuclei suited to separately encode the binaural cues to location: interaural time differences (ITDs), and interaural level differences (ILDs). The importance of the SOC nuclei for localization has been revealed in behavioral studies where lesions of their primary afferents (Masterton et al., 1967; Moore et al., 1974; Casseday and Neff, 1975; Thompson and Masterton, 1978; Jenkins and Masterton, 1982) or cell bodies (Kavanagh and Kelly, 1992) disrupt behavioral localization performance. Two SOC nuclei, the medial superior olive (MSO) and lateral superior olive (LSO), represent the major peripheral sites in the auditory pathway to receive converging inputs from both ears (Irvine, 1986). The MSO is thought to encode ITDs (Goldberg and Brown, 1969; Yin and Chan, 1990), whereas the LSO, the focus of the experiments here, has been hypothesized to encode ILDs (Boudreau and Tsuchitani, 1968).
LSO cells receive excitatory input from the glutamatergic spherical bushy cells of the ipsilateral anteroventral cochlear nucleus (AVCN) (Warr, 1966; Glendenning et al., 1985; Shneiderman and Henkel, 1985;Cant and Casseday, 1986; Smith et al., 1993) and inhibitory inputs indirectly from the contralateral VCN globular bushy cells via the ipsilateral medial nucleus of the trapezoid body (MNTB) (Morest, 1968;Warr, 1972; Tolbert et al., 1982; Glendenning et al., 1985; Smith et al., 1991, 1998). The projection of MNTB to LSO is tonotopic (Elverland, 1978; Glendenning et al., 1985; Spangler et al., 1985;Smith et al., 1998), matching the tonotopic arrangement of LSO cells (Tsuchitani and Boudreau, 1966; Guinan et al., 1972), and is glycinergic (Moore and Caspary, 1983). Consequently, LSO cells are ILD-sensitive because ipsilateral sound-evoked excitatory (E) responses can be inhibited (I) by sound at the contralateral ear; we call these IE cells to distinguish them from EI cells found in supraolivary nuclei. The contralateral inhibition depends not only on the intensity but also the onset time of the contralateral relative to the ipsilateral stimulus (Galambos et al., 1959; Boudreau and Tsuchitani, 1968; Caird and Klinke, 1983; Sanes and Rubel, 1988; Joris and Yin, 1995; Park et al., 1996; Batra et al., 1997).
It has been widely believed that the functional role of the LSO is to encode a correlate of the ILDs present in free-field sounds. Yet, the actual spatial-location coding ability of LSO cells has never been investigated. To overcome the difficulties of recording from the LSO while presenting free-field stimuli, we used a hybrid approach, the virtual acoustic space (VS) technique (Wightman and Kistler, 1989a;Brugge et al., 1994), to present over earphones precisely controlled stimuli containing all the acoustical cues to location in their natural combinations. Here we test the long-standing hypothesis that single LSO cells respond to variations in sound source azimuth in a manner consistent with their IE binaural nature.
Preliminary results have appeared (Tollin and Yin, 1999).
MATERIALS AND METHODS
General. Adult cats with clean external ears were initially anesthetized with ketamine hydrochloride (20 mg/kg) along with acepromazine (0.1 mg/kg). Atropine sulfate (0.05 mg/kg) was also given to reduce mucous secretions. A venous cannula was implanted in the femoral vein through which supplemental doses of sodium pentobarbital (3–5 mg/kg) were administered as needed to maintain areflexia. The cat's temperature was continuously monitored with a rectal thermometer and maintained with a heating pad at 37°C, and a tracheal cannula was inserted. Both pinnae were cut transversely, removed, and tight-fitting hollow earpieces were fitted snugly into the external auditory meati. Polyethylene tubing (30 cm, 0.9 mm inner diameter) was glued into a small hole made in each bulla to maintain normal middle ear pressure.
A ventral transpharyngeal approach was used, and the LSO was accessed by drilling small holes into the basioccipital bone. Small slits were then made in the dura through which parylene-coated tungsten microelectrodes (1–2 MΩ; Microprobe, Clarksburg, MD) were advanced ventromedially to dorsolaterally at an angle of 26–30° into the brainstem by a hydraulic microdrive affixed to a micromanipulator that could be remotely advanced outside the double-walled sound-attenuating recording chamber. Electrical activity was amplified and filtered (300–3000 Hz). Unit responses were discriminated with a BAK Electronics Inc. (Germantown, MD) amplitude-time window discriminator, and spike times were stored at a precision of 1 μsec. Several basic physiological response properties were measured for each single fiber or cell encountered. After the excitatory ear was determined, the characteristic frequency (CF), spontaneous activity, and threshold were measured using an automated threshold tracking routine. Poststimulus time, interval, and period histograms, and rate and synchronization measures were then obtained for CF tones at different sound pressure levels (SPLs) in 5–10 dB steps and displayed on-line.
Histology. In many experiments, electrolytic DC lesions were made to differentiate electrode tracks, mark locations of interest, and assist in estimating tissue shrinkage after histological processing. At the conclusion of each experiment, the brain was fixed in formaline by immersion or perfusion through the heart. The fixed tissue was cut into 50 μm frozen sections and stained with cresyl violet so that electrode tracts and lesions made during the recordings could be seen.
Stimuli. All stimuli were generated digitally at 16-bit resolution and converted to analog at a rate of 100 kHz. Overall stimulus level was controlled using custom-built programmable attenuators. The conditioned output of the digital-to-analog (D/A) converter was sent to an acoustic assembly (one for each ear) comprising an electrodynamic speaker (Realistic 40–1377), a calibrated probe-tube microphone (Bruel and Kjaer ½ in), and a hollow earpiece that was fit snugly into the cut end of the auditory meatus and sealed with Audilin. The hollow earpiece accommodated the small probe-tube microphone by which the sound delivery system to each ear was calibrated for tones between 50 Hz and 40 kHz in 50 Hz steps. The calibration data were used to compute digital filters that equalized the responses of the acoustical system and typically yielded flat frequency responses within ±2 dB for frequencies <25 kHz.
Tone bursts of varying frequency were used as search stimuli with the SPL of the tone to the ipsilateral ear being 5–10 dB higher than the tone to the contralateral ear so that the IE cells of the LSO would not be missed. Once a single unit was isolated, its CF and threshold level were estimated. Rate level functions were measured by presenting 200 repetitions of a 50 msec tone pip at CF (3.9 msec rise–fall times) every 100 msec from which the resulting peristimulus time (PST) histograms were examined. A tonic response with “chopping,” or multiple modes unrelated to the frequency of the stimulus is characteristic of most LSO cells (Tsuchitani, 1982). To quantify the regularity, the coefficient of variation (CV), which is the ratio of SD of interspike intervals to the mean interval, was computed from the intervals occurring during the first 25 msec of the CF tone (Young et al., 1988). The bin width was 0.2 msec. To determine the presence and nature of any binaural interaction, a CF tone or broadband noise (300 msec duration presented every 500 msec with a rise–fall time of 4 msec) was presented to the ipsilateral ear at 10–20 dB above the threshold level while the level of a CF tone or noise presented to the contralateral ear was varied. This procedure reveals whether ipsilaterally evoked neural responses can be inhibited by contralateral stimulation, another hallmark of LSO cells.
Virtual space stimuli. Sound source azimuth was manipulated in these experiments through the use of VS sounds. With VS stimuli, we are able to reproduce over earphones the sound pressure waveforms that would be produced in the ears by free-field sounds (Wightman and Kistler, 1989a,b). The method of synthesizing the VS stimuli was similar to that used in the human psychophysical experiments of Wightman and Kistler (1989a,b) and the physiological studies of Poon and Brugge (1993), Brugge et al. (1994), and Delgutte et al. (1999). Here, we used a single token of broadband, Gaussian noise of 200 or 300 msec in duration (4 msec rise–fall times) repeated 20 times every 300–500 msec, respectively. The same token of noise was used for all experiments. Before being delivered, the noise token was equalized digitally by the calibration filters appropriate for each ear, and then delivered either directly to the acoustical system in the case of non-VS stimuli or preprocessed through digital filters constructed from the head-related transfer function (HRTF) measurements made in one cat from the recordings of Musicant et al. (1990). HRTFs capture the frequency- and direction-dependent interactions with the head and pinna that a broadband sound undergoes as it propagates from the source to the eardrum so that a left- and right-ear pair of HRTFs for a given spatial position embodies all the static acoustical cues to location available from that particular position (Wightman and Kistler, 1989a,b). Thus, for any spatial location, the conditioned noise waveforms presented to the cats in these experiments were the same as those that would have been produced from the same noise token being presented in the free field from that particular location. The VS stimuli were synthesized for azimuths ranging from −90° to +90° in the horizontal plane in 4.5° steps. Positive azimuths correspond to azimuths contralateral to the recording site.
Two aspects concerning the synthesis of the VS stimuli required attention. First, the stimuli were bandpass filtered between 2 and 35 kHz because this is the frequency range where the HRTF recordings ofMusicant et al. (1990) were most reliable. Therefore, the VS stimuli contained little energy at <2 kHz. Second, the frequency response of the acoustic delivery system in some cats showed a rapid roll-off at high frequencies, and as a consequence, attempts to digitally equalize the roll-off resulted in poor signal-to-noise ratios because a large portion of the amplitude coding range of D/A converter was allocated to boosting the high-frequency components. For these cats the stimuli were low-pass filtered at 30 kHz to avoid this problem.
Rationale for using virtual space stimulation. It is important to address at this point our rationale for using VS rather than free-field stimulation. First, despite the pivotal role the LSO is hypothesized to play in localization, there have been only a few studies on binaural interaction at this site. And surprisingly, not one of these has investigated the actual spatial-location coding ability of LSO cells. Investigation of location coding in general is limited by difficulties such as placing the positions of the pinnae in a “normal” orientation, generating signals and controlling their direction over a wide range of space at high spatial resolution and knowing precisely the acoustic signals at the two ears. The latter is essential to relate any measured neuronal response to the direction-dependent acoustical cues. With free-field sounds, it can also be difficult to separate spatial sensitivity caused by the amplification effects of the pinna at any one ear from binaural influences (Semple et al., 1983). Previous studies of LSO cells avoided these complications by presenting tones or broadband noise over earphones, so the relative temporal and intensive differences between the signals to the two ears could be precisely defined. But although earphone delivery of signals has been necessary because it afforded independent control over ITD and ILD and has proven useful for studying neural mechanisms of localization, such studies have not addressed the contributions of spectral cues or natural combinations of cues. Clearly, neither traditional free-field nor earphone approaches allow a complete investigation of the role each acoustical cue to spatial location plays under natural conditions in shaping the neural responses of spatially sensitive cells.
Although azimuthal location was manipulated here by filtering noise through HRTFs measured in only one cat, we believe our use of VS stimulation and of nonindividualized HRTFs does not pose any serious problems for the conclusions reached in this paper for several reasons. First, psychophysical studies have shown that the perception of location by humans (Wightman and Kistler, 1989b) and owls (Poganiatz et al., 2001) is similar whether stimuli are presented in the free-field or over earphones using the observers' individual HRTFs. Second, although HRTFs in different cats can exhibit differences in the magnitudes of the localization cues (Musicant et al., 1990; Rice et al., 1992; Xu and Middlebrooks, 2000), simply changing pinnae position can also lead to changes in HRTFs (Middlebrooks and Knudsen, 1987;Young et al., 1996) and in the spatial receptive fields (SRFs) of units in the inferior and superior colliculus and auditory cortex (Middlebrooks and Pettigrew, 1981; Middlebrooks and Knudsen, 1987; Sun and Jen, 1987). So in animals with mobile pinnae, any measurements of HRTFs will provide just a “snap-shot” of the possible set of HRTFs. On the other hand, variations in the SRFs of inferior colliculus (Keller et al., 1998) and auditory cortical cells (Reale et al., 1996) using HRTFs from different animals were relatively small, and the general features of the SRFs were preserved, provided the HRTFs were similar. Indeed, measurements have shown that some characteristics like ITDs and ILDs can be fairly stable across adult cats (Roth et al., 1980; Irvine, 1987; Martin and Webster, 1989; Rice et al., 1992).
Here, we used HRTFs to present sounds containing cues that would be present for noise stimuli presented at azimuths in the frontal hemisphere of an adult cat. Moreover, the monaural and binaural relationships between these cues varied naturally with changes in sound source azimuth, a condition that has not been met in previous studies of the LSO. Our main concern about using nonindividualized HRTFs here is whether single units in the LSO are somehow able to “recognize” that the spectral pattern arriving at each ear is different than what might be expected based on the cats' own HRTFs. Clearly, such a process would somehow have to separate the spectral characteristics of the acoustic signal from the spectral characteristics imposed by the HRTFs. It seems unlikely at this early stage that specializations sufficient to permit recognition of the broadband spectral patterns of the HRTFs would be present given the narrow range of frequencies over which LSO cells respond.
Recording protocol. Because recording time with each unit was limited, an experimental protocol was followed to maximize the probability of getting useful information from each cell. Generally, after determining the CF and threshold for each cell and measuring a monaural rate-level function for ipsilateral tones at CF, a tone ILD function was measured as described above to determine the binaural nature of the cell. Cells with physiological signatures consistent with LSO (e.g., ILD sensitivity, chopping PST histograms) were then further studied with the VS stimuli. First, a rate-level function was measured for the VS stimulus located at the midline directly in front (0° azimuth, 0° elevation) delivered to the excitatory ipsilateral ear only. Responses to VS stimuli were then measured under both binaural and monaural conditions as a function of azimuth with the stimulus sound level chosen to be 10–20 dB above the ipsi-ear only threshold determined from the rate-level function to the (0°, 0°) stimuli. Discharge rate was averaged over the entire 200 msec stimulus duration. Some rate-azimuth functions were smoothed by a three-point triangular filter. We then explored the importance of each localization cue by manipulating them digitally, the results of which are presented in our companion paper (Tollin and Yin, 2002). If time permitted, the experiments were repeated with higher stimulus levels. All statistical analyses used nonparametric tests.
RESULTS
Our results are based on detailed recordings of 28 single LSO units in nine cats. The 28 units had CFs >3 kHz and exhibited physiological signatures consistent with those reported in previous studies of LSO cells: low-spontaneous rates (in spikes per second: mean, 3.9; median, 0.0; SD, 8.89), chopping PST histograms to short tone pips (mean CV, 0.46; median, 0.46; SD, 0.12; range, 0.25–0.69), and all were inhibited by contralateral stimulation. Histology was available for eight of nine cats, allowing us to verify that 25 of the 28 units were located in the LSO; the three remaining units were from the cat for which histology was not available. The localization of these units as a function of CF was in general agreement with the tonotopic organization of LSO (Tsuchitani and Boudreau, 1966; Guinan et al., 1972).
LSO units are sensitive to interaural level differences
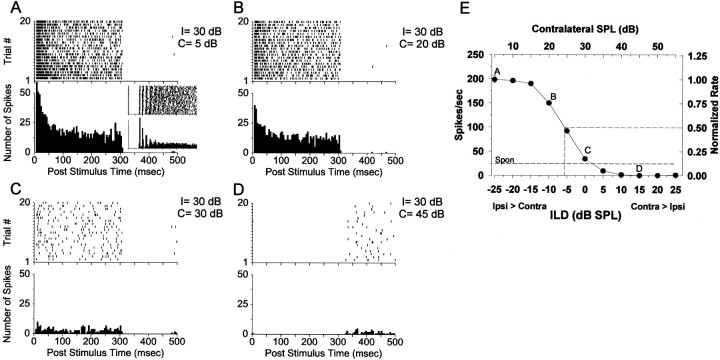
All units in this study were sensitive to manipulations of ILDs, consistent with IE binaural interaction. Figure1 demonstrates the ILD sensitivity of one unit that had a CF of 16 kHz and a threshold of 8 dB SPL. Although the spontaneous activity of this cell was higher than average at 24 spikes/sec, its binaural response characteristics were representative of our population of cells. The stimulus delivered to the ipsilateral, excitatory ear was held fixed at 30 dB SPL. Panels A–D show the temporal discharge pattern of the unit, displayed as dot rasters, and the associated poststimulus time histograms (PSTHs) for 20 repetitions of the stimulus as the level of the tone to the contralateral ear was increased to the levels indicated. The inset in panel A, which shows the first 40 msec of the response to short tone pips (50 msec duration) presented to the excitatory ipsilateral ear alone at 22 dB above threshold, demonstrates the characteristic chopping response (CV, 0.28) exhibited by most of our cells. The data points in panel E show the mean discharge rate and ± 1 SEM as the level of the tone delivered to the contralateral ear was varied from 5–55 dB SPL (top abscissa). The decreasing responses with increasing level at the contralateral ear reflect the inhibitory effect of that input. The lower abscissa shows the corresponding ILD (contralateral minus ipsilateral level in dB). The rasters and PSTHs reveal that the ipsilateral sound-evoked activity of the unit decreased as the level of the stimulus to the contralateral ear was increased above 15 dB SPL. Like most units in our sample, at large positive ILDs the ipsi-evoked activity was completely inhibited and also exhibited offset responses, indicating a release from inhibition (Fig. 1D). For this unit, inhibition of the discharge rate below spontaneous levels did not occur until ILD = +5 dB.
Fig. 1.
Responses of an LSO cell to variations in the ILD of a CF tone. A–D, Dot rasters and PST histograms in response to a 300 msec ipsilateral tone at 30 dB SPL as a function of the level of a tone at the contralateral ear, as shown in the top right of each panel. Theinset in A shows the first 40 msec of the response to short tone pips at CF presented monaurally to the ipsilateral ear only, demonstrating the characteristic chopping response exhibited by most of our cells (bin width, 400 μsec and the top tic on the ordinate, 150 spikes). E, Mean discharge rate ± 1 SEM versus ILD (in decibels: SPL). (In this and all subsequent figures, where the error bars are not present, the SEM is less than the height of the data point.) The top abscissaindicates the level of the tone at the contralateral ear, and theright ordinate shows the rate normalized to the maximum. Thedashed horizontal line shows the spontaneous rate of the unit.
All units in this study exhibited contralateral inhibition similar to that shown in Figure 1, although the ILD at which the discharge rate began to decrease and the slope of the ILD function varied from unit to unit. We took as one measure of the effectiveness of the contralateral inhibition relative to ipsilateral excitation the ILD at which the discharge rate was reduced to one-half the maximum, yielding the half-maximal ILD (see right-hand ordinate of Fig.1E). The half-maximal ILD is near the location along the ILD axis of the steepest portion of the ILD tuning function. The half-maximal ILD for the unit in Figure 1 was −5.5 dB. The median half-maximal ILD for the cells included in this study was −6.0 dB (mean, −4.15 dB; SD, 11.06 dB; n = 20). Consistent with the observations from other studies (Boudreau and Tsuchitani, 1968; Sanes and Rubel, 1988; Joris and Yin, 1995; Park et al., 1996), these data indicate that the bulk of the sensitivity to ILD in LSO cells as determined dichotically with pure tones at CF occurs for stimulus conditions for which the sound level at the ipsilateral ear is greater than that at the contralateral ear.
Contralateral inhibition shapes spatial receptive fields in azimuth
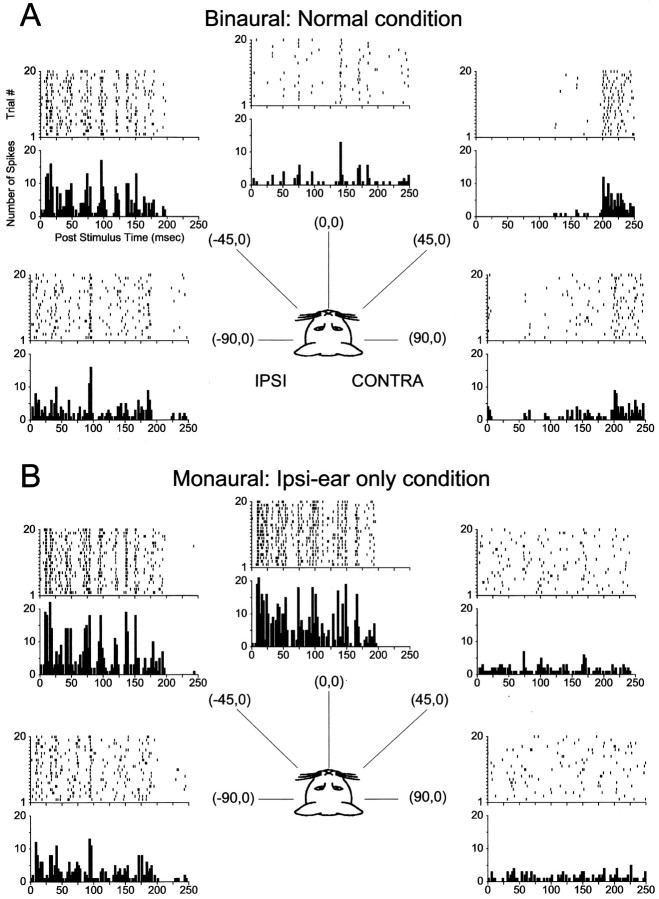
To the extent that the IE nature of the binaural interaction of LSO cells actually governs the sensitivity to variations in sound source azimuth, we expected that as we varied sound source azimuth cells would discharge at most azimuths in the ipsilateral hemisphere, but would be inhibited at large contralateral azimuths. Figure2 shows an example of the temporal discharge patterns and associated PSTHs for noise stimuli presented from five different virtual space positions on the horizontal plane for the same unit as Figure 1.
Fig. 2.
Responses of the same cell in Figure 1 to 200 msec broadband noise presented from five different azimuths along the horizontal plane under binaural (A, normal) and monaural ipsilateral-ear only (B, ipsi-only) conditions. Negative azimuths indicate sound sources in the ipsilateral sound field. The stimulus was presented at ∼20 dB above threshold at (0°,0°) in the ipsi-only condition. The top andbottom figures in each panel show the dot rasters and PST histograms for 20 presentations of the stimuli at each azimuth.
Figure 2A shows the responses in azimuth for the condition in which the stimuli were presented binaurally to both ears (normal). The normal condition approximates traditional free-field presentation of sounds. For sounds located in the ipsilateral hemifield, this unit responded throughout the duration of the stimuli. However, as the source was moved toward the midline, there was a decrease in the response relative to the response to more ipsilateral sources. For sources contralateral to the midline, there were virtually no responses when the stimuli were presented, but there were clear offset responses occurring shortly after the stimulus had been turned off at 200 msec. Offset responses that exceeded the level of spontaneous activity were seen in 9 of 25 cells tested in the normal binaural condition; offset responses were never seen in the normal condition for sources in the ipsilateral sound field or in the ipsi-only condition. The robust discharge for sounds in the ipsilateral field coupled with both the absence of responses during and the offset responses after stimulus presentation for sounds in the contralateral field are indicative of the IE nature of this cell. Thus, increasing the level of the stimulus to the contralateral ear relative to that at the ipsilateral ear by placing the sound sources at more lateral azimuths in the contralateral field inhibits the cell.
Although there were indeed hallmarks of the IE binaural nature of this cell in the responses to the VS stimuli, if given only the responses in Figure 2A we could not be sure whether the contralateral inhibition actually contributed to the responses. This is because sounds in the field contralateral to one ear have lower sound levels than those in the ipsilateral field, so a drop in discharge rate is expected for cells with monotonic rate-level functions even for monaural cells lacking binaural interactions. To test the hypothesis that the binaural SRFs were shaped by contralateral inhibition, we repeated the measurement of the SRF, but this time presenting the stimuli to only the ipsilateral ear.
Figure 2B shows the responses as a function of azimuth for the monaural ipsilateral ear only (“ipsi-only”) condition. The unit in the ipsi-only condition responds robustly and tonically for stimuli not only in the ipsilateral field but also, to a lesser degree, in the contralateral field, with no offset responses. Because the only difference between the normal and ipsi-only condition was the presence of the stimuli at the contralateral ear, this clearly demonstrates the contribution of the contralateral inhibition on the spatial sensitivity of the cell. Hence, IE binaural interaction governs at least some of the selectivity of this cell to spatial location in azimuth.
Note that both the dot rasters and PSTHs in Figure 2 show temporal synchrony in the responses to the noise stimuli as evidenced by the vertical column of dots in the rasters and peaks in the PSTHs. This synchrony likely results from the synchronization of the responses to the envelope of the same token of the noise stimulus (“frozen noise”), filtered by the HRTF of each spatial location (Keller and Takahashi, 2000).
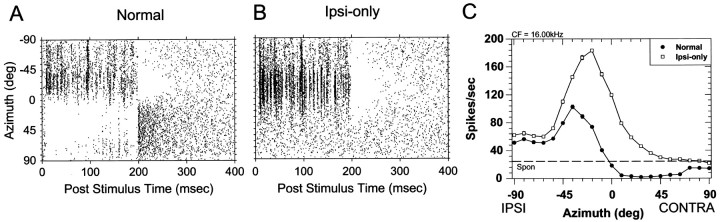
Finally, to reveal more completely the binaural interactions exhibited by this unit as a function of the azimuth of the sound, Figure3 shows the temporal discharge patterns and average discharge rates for azimuths spanning ±90°. Figure3B shows the dot rasters of responses to the stimuli at 21 different azimuths in the ipsi-only condition displayed continuously from −90° in the ipsilateral field to 90° in the contralateral field. When the data are presented in this way, it is clear that the unit responds tonically for stimuli presented at virtually every azimuth, but especially for those stimuli presented in the ipsilateral hemifield. Figure 3A shows the raster for the normal condition where offset responses are seen at virtually all azimuths in the contralateral hemifield while no offset responses are seen in the ipsi-only condition. Figure 3C plots the mean discharge rate (±SEM) for the two stimulus configurations. We shall call these the ipsi-only and normal azimuthal SRFs. Relative to the ipsi-only condition, the mean discharge rates for the normal condition are suppressed at all azimuths. As predicted from the tone ILD function in Figure 1E, the response of the cell in the normal condition was suppressed below the spontaneous rate at all contralateral azimuths where the stimuli to the contralateral inhibitory ear would be expected to exceed the stimulus level at the ipsilateral ear. All cells presented in this paper responded to the VS stimuli in a manner similar to that shown in Figures 1-3, so the data will be presented in terms of the azimuthal SRFs of each cell, as depicted in Figure 3C.
Fig. 3.
A,B, Temporal discharge patterns for the same cell in Figure 2 plotted continuously over the 20 stimulus presentations as a function of each of 21 azimuths for the normal (A) and ipsi-only (B) conditions. Each tic mark on theordinate of A and B denotes a different azimuthal position. C, The spatial receptive fields in azimuth for the ipsi-only and normal stimulus conditions. Each data point plots the mean discharge rate of the cell averaged over the 200 msec duration of the noise stimuli and 20 presentations. Thehorizontal line indicates the spontaneous rate.
Spatial receptive field shape depends on unit CF
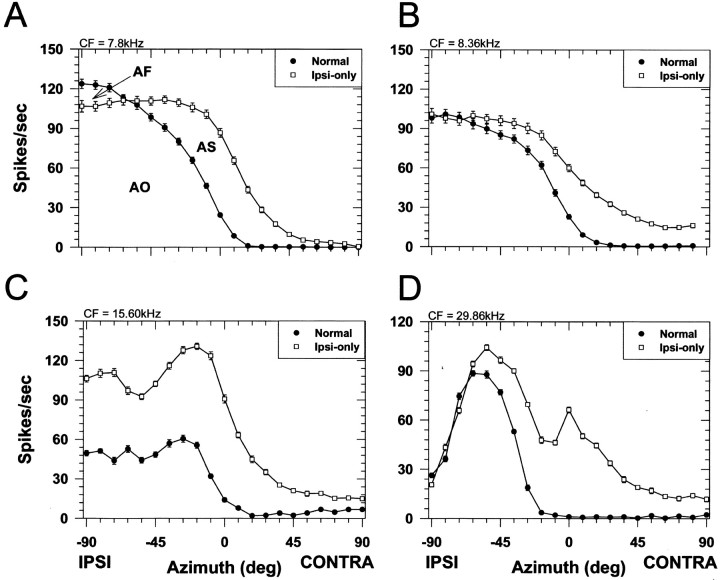
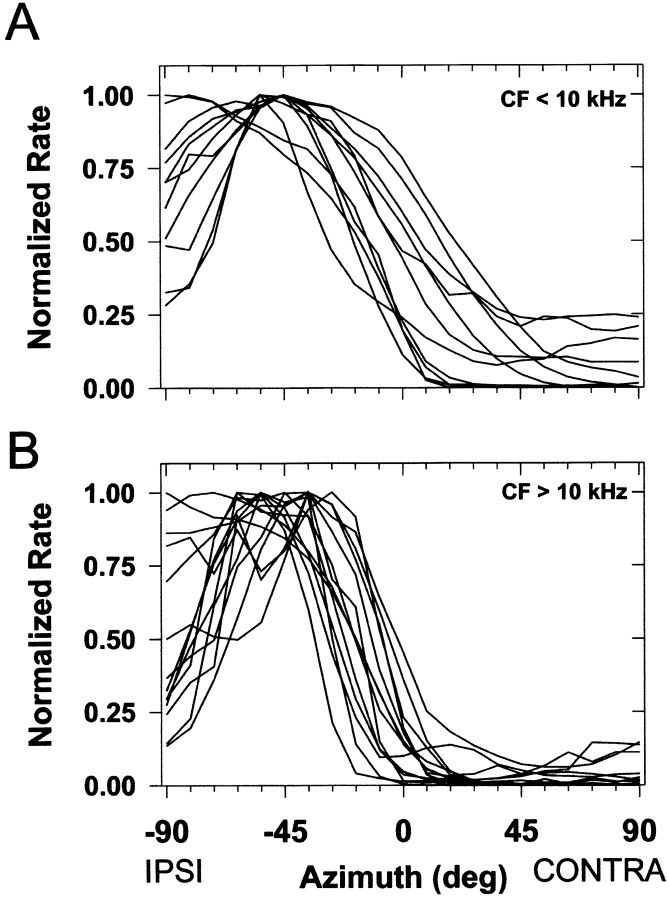
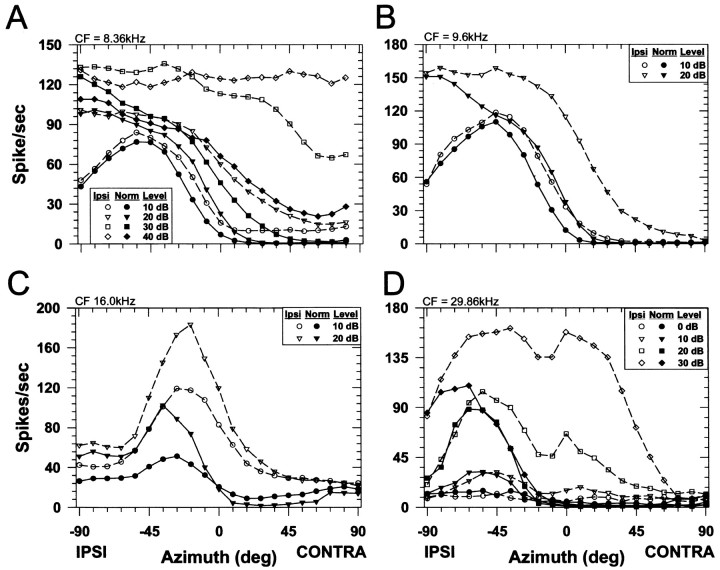
Figure 4 shows representative ipsi-only and normal SRFs for four cells that had CFs spanning 7.8 to nearly 30 kHz. It is in this frequency region where ILDs are most prominent and the monaural, spectral cues are believed to be present in the cat (Musicant et al., 1990; Rice et al., 1992). Like the representative unit shown earlier, each unit shown in Figure 4responded tonically to the stimuli and was clearly modulated by spatial location with high discharge rates in the ipsilateral sound field, a segment near the midline of rapidly declining rates, and low rates for contralateral sounds. There were two general shapes to the SRFs: (1) sigmoidal (Fig. 4A,B) and (2) complex (Fig.4C,D). Regardless of the shape, at these sound levels, for each unit the binaural normal SRFs generally had shapes similar to the monaural ipsi-only SRFs. However, consistent with IE interaction, the binaural responses at most azimuths in the frontal hemisphere were inhibited relative to the monaural responses at the same azimuths, particularly in the contralateral hemisphere. Note that the net effect of the contralateral inhibition for each unit was to “push” the SRF into the ipsilateral hemifield.
Fig. 4.
Spatial receptive fields of four LSO cells. The CF of each cell is indicated in the top left of each figure. Same format as in Figure 3C.
Population characteristics of spatial receptive fields
Figure 5 shows the normal SRFs for the 25 LSO units tested in this condition; each SRF has been normalized by the maximum discharge rate. Figure 5A shows that the normalized SRFs for most of the units (8/11) with CFs less than ∼10 kHz had smoothly rising sigmoidally shaped normal SRFs with the response decreasing slightly for ipsilateral azimuths nearing −90°. Figure 5B shows that most of the units (9/14) with CFs >10 kHz typically had more complex SRFs with the presence of peaks and dips and a much more pronounced decrease (by >50% of maximum) in rate for ipsilateral azimuths nearing −90°. In addition the slopes of the SRFs near ∼0° azimuth appear to be steeper and tend to fall more toward the ipsilateral sound field for the high frequency cells. To quantify these differences, the properties of the monaural and binaural SRFs for each cell were summarized in three different ways, each of which provides evidence that contralateral inhibition contributes to the SRFs of LSO cells. Ipsi-only SRFs were measured in 21 cells, and normal SRFs were measured in 25 cells; both ipsi-only and normal SRFs were measured in 18 cells.
Fig. 5.
Spatial receptive field shape depends on CF.A, Normalized SRFs for LSO cells with CFs <10 kHz.B, Normalized SRFs for cells with CFs >10 kHz.
In the first metric, we determined the azimuth at which the discharge rate fell to 50% of the maximum rate, the half-maximal azimuth (Delgutte et al., 1999). The half-maximal azimuth indicates the spatial location of the medial border of the SRFs where it has the steepest slope and therefore indicates the azimuth around which the SRF has the greatest spatial resolving power. As an estimate of the slope of the SRFs, we determined the range of azimuths between 25 and 75% maximum discharge.
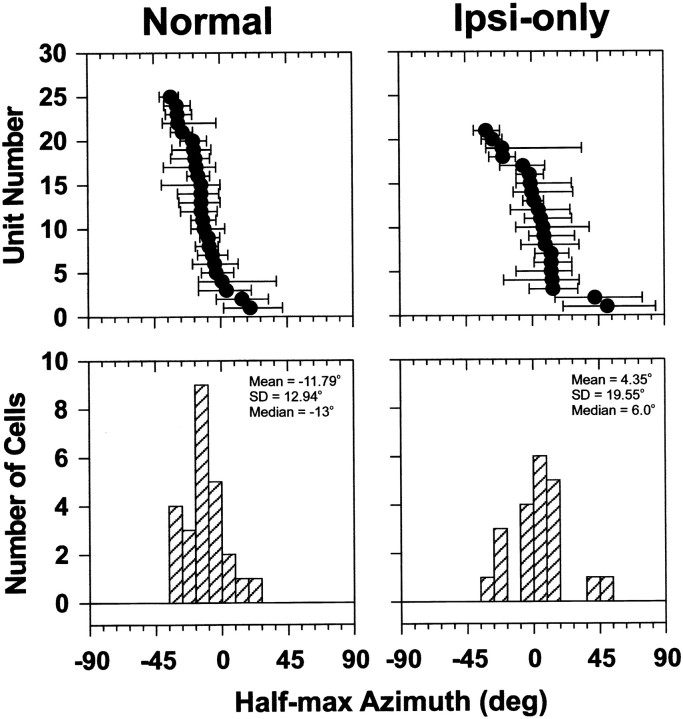
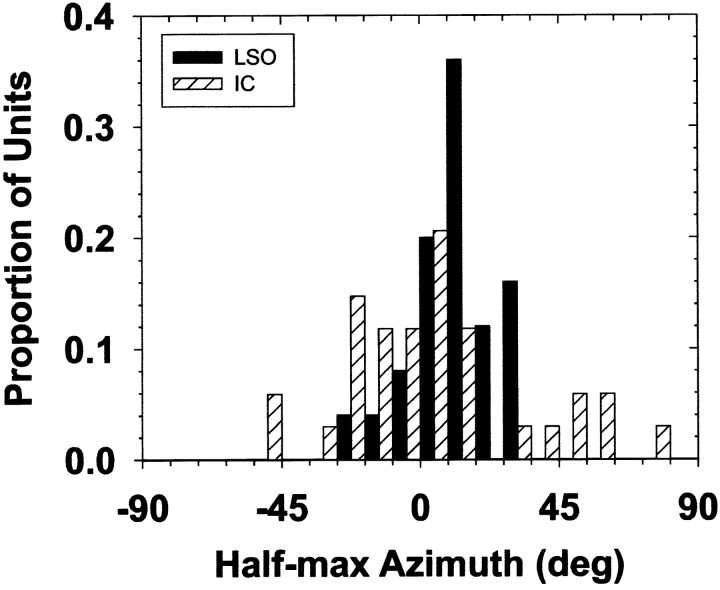
The top panels in Figure 6 show the population of half-maximal azimuths and associated 25–75% ranges for both the normal and ipsi-only SRFs. The bottom panels of Figure 6 show histograms of the half-maximal azimuths over the population of cells. The population data show that the normal SRFs were located more into the ipsilateral sound field than the ipsi-only SRFs and also had smaller 25–75% ranges, and therefore steeper slopes, than the ipsi-only SRFs. This can be seen by comparing the widths of the bars of the normal to those on the ipsi-only half-maximal azimuths in Figure 6 (top panels). The median half-maximal azimuth for the ipsi-only SRFs was 6.0°, whereas the median for the normal SRFs was −13o. The median range of the normal SRFs was 25° (mean, 26.0°; SD, 10.3°) whereas that of the ipsi-only was 33° (mean, 36.6°; SD, 16.8°). The Mann–WhitneyU test for 21 ipsi-only and 25 normal SRFs indicates a significant difference between the ipsi-only and normal half-maximal azimuths (U = 127; p = 0.003) and their ranges (U = 162; p = 0.027). These differences between the normal and ipsi-only SRFs did not depend on differences in the CFs of the cells because there were no significant differences in the CFs of the 21 cells for which ipsi-only SRFs were measured and the 25 cells for which normal SRFs were measured (Mann–Whitney U = 291; p = 0.529).
Fig. 6.
The top panels plot the distribution of half-maximal azimuths (filled circles) and the ranges of azimuth corresponding to 25–75% of maximum discharge (bars). Data for the normal and ipsi-only conditions are shown in the left andright columns, respectively. Note that the data for each cell is plotted as a function of increasing half-maximal azimuth toward the ipsilateral sound field for both the normal and ipsi-only configurations. As a result, the same cell number in each plot does not necessarily designate the same cell. The bottom panelsshow histograms of the normal and ipsi-only half-maximal azimuths. The normal binaural spatial receptive fields are located predominantly in the ipsilateral sound field, whereas the ipsi-only receptive fields are located more into the contralateral field.
Second, the binaural nature of these cells was further assessed by calculating the modulation index (MI) = (Rmax −Rmin)/Rmax, where Rmax andRmin are the maximum and minimum discharge rates, respectively (Delgutte et al., 1999). The MI varies between 0 and 1 with a value of 0 indicating that the discharge rate of a cell was not modulated at all with changes in sound source azimuth, whereas an MI of 1 indicates that the discharge rate varied between its maximal rate and a rate of 0 spikes/sec. Because of the IE nature of the LSO cells, it was expected that most cells should have binaural MI near 1 because the contralateral inhibition would be expected to suppress even spontaneous discharges (Figs. 1-3). A Mann–WhitneyU test revealed significant differences in the MI between the populations of ipsi-only (mean, 0.935; SD, 0.068; median, 0.969) and normal (mean, 0.977; SD, 0.047; median, 0.997) SRFs (U = 374; p = 0.012). Thus, relative to the monaural condition, the discharge rate of the cells was modulated more completely with changes in azimuth in the binaural condition, consistent with IE interaction. Note, however, that the ipsi-only SRFs in Figure 4 clearly show that, at the sound levels used here [10–20 dB above threshold for a stimulus presented at (0°, 0°) in the ipsi-only condition], the cells can still be sensitive to azimuth based simply on monaural cues. Presumably, the monaural sensitivity reflects jointly the way the spectral features of the HRTFs change with azimuth and the degree to which the frequency tuning characteristics of these cells resolve these changes.
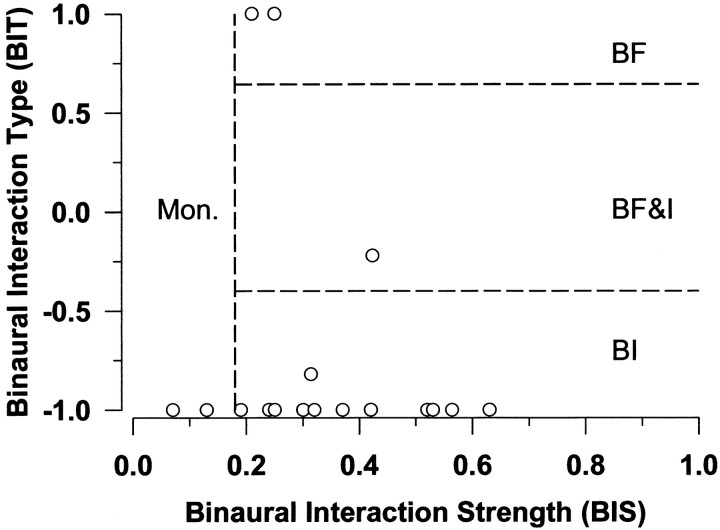
As a final characterization of the SRFs, two quantitative measures defined by Delgutte et al. (1999), the binaural interaction strength (BIS) and the binaural interaction type (BIT), were computed. As shown in Figure 4A, when the normal and ipsi-only responses are plotted on the same abscissa, the curves can potentially define three regions. The BIS is given by (AF + AS)/(A0 + AF + AS) where AF indicates an area of facilitation where the normal binaural response is greater than the ipsi-only monaural response, AS is an area of suppression where the normal response is less than the ipsi-only, and finally A0, an area common to both the normal and ipsi-only responses. The BIS varies between 0 and 1; a BIS of 0 indicates that the normal and ipsi-only responses are identical at all azimuths, whereas a value of 1 indicates that either the normal or ipsi-only response is considerably different than the other one for all azimuths indicating the presence of binaural interaction. The BIT indicates the nature of the binaural interaction and is given by (AF − AS)/(AF + AS) and takes values between −1 and 1; a BIT of −1 indicates that the nature of the binaural interaction is purely inhibitory (IE or EI binaural interaction), whereas a value of 1 indicates a purely facilitatory interaction (EE binaural interaction). Based on the values of the BIS and BIT indices, Delgutte et al. (1999) classified cells as exhibiting monaural (Mon), binaural facilitation (BF), binaural inhibition (BI), or binaural facilitation and inhibition (BF&I) types of responses. Figure 7 shows the BIT and BIS values of the 18 units for which both ipsi-only and normal SRFs were measured; the dashed lines delineate the classification regions of Delgutte et al. (1999). The data show that 13 units had responses consistent with the BI classification (two units had values of BIS = 0.3 and BIT = −1 so that their data points overlapped in Fig. 7) with two more with BI equal to −1.0, although their BIS values placed them in the Mon category. The BI classification is expected for units exhibiting purely IE binaural interaction.
Fig. 7.
Plot of the BIT versus the BIS for all LSO units. The dotted lines indicate the boundaries suggested byDelgutte et al. (1999) to classify units into four separate categories of binaural interaction: monaural (Mon.), binaural facilitation (BF), binaural inhibition (BI), and mixed facilitatory–inhibitory interactions (BF&I).
Three other units were not classified as BI. One unit was classified as BF&I; this unit had ipsi-only and normal SRFs similar to the unit in Figure 4A where at large lateral angles in the ipsilateral field, the response to the normal condition exceeded the ipsi-only response, yet the primary binaural interaction over the rest of the field, and to dichotic pure-tone stimulation, was consistent with the BI classification. The remaining two units were classified as BF units because the discharge rate in the normal condition for some azimuths in the ipsilateral field was higher than those for the ipsi-only condition. Although not reflected in the SRFs, both units were clearly IE as assessed from their sensitivity to ILDs measured dichotically with tones at CF. However, the tone ILD function for one of these units indicated that there was little to no effect of the contralateral inhibition on the ipsilaterally evoked responses until the level of the contralateral stimulus greatly exceeded the level of the ipsilateral stimulus. Therefore, the effective ILDs provided by the VS stimuli as a function of azimuth as seen through the frequency selectivity of this unit may not have been great enough to observe the effects of the contralateral inhibition resulting in an SRF that was governed predominantly by monaural influences. For the other unit, it was not clear why the SRF did not show a BI interaction since both the tone ILD and broadband noise ILD functions indicated a clear IE interaction. In summary, almost all of the cells in our sample were classified as BI using the scheme suggested by Delgutte et al. (1999)although there were a few isolated exceptions.
The determinants of the SRFs
It should be clear that LSO cells are not encoding spatial location per se since they are modulated by changes in azimuth not only binaurally, but also monaurally. Furthermore, as we show below, the discharge rate is also modulated by overall SPL. Rather, the responses of these cells are likely to reflect jointly the absolute sensitivity of each cell to binaural and/or monaural localization cues and the way the magnitudes of the actual localization cues change as a function of azimuth with the VS stimuli as “seen” through the frequency and level selectivity of the cell. For example, one of our LSO cells discussed in the previous section was clearly sensitive to ILD as assessed with tones, but was not as sensitive to changes in azimuth because the range of ILDs over which it was responsive was outside the range of ILDs provided by the VS stimuli as a function of azimuth; hence, its SRFs were determined primarily by monaural level cues at the ipsilateral excitatory ear. It is precisely because LSO cells, or any spatially sensitive neurons in the ascending auditory pathway, respond to a variety of stimulus features that makes it difficult to determine which cue or combinations of cues determines the SRF of the cells. Although we address this question more directly in the companion paper (Tollin and Yin, 2002) in which we manipulate directly the ILDs in the VS sounds themselves, it is instructive to look at the other potential “clues” to the determinants of the SRFs in the LSO cell responses.
First, given the IE nature of LSO cells, it is reasonable to expect that their response to changes in stimulus azimuth would reflect the changes in ILD as the azimuth is changed. What is the evidence that these units are being modulated with changes in azimuth specifically by the ILD presented in the virtual space sounds? And can we eliminate the possibility that the SRFs for LSO cells are attributable to the other main binaural cue to sound location, ITDs? After all, several studies have shown that high-CF LSO cells are sensitive to the ongoing ITDs of low-frequency envelopes of amplitude modulated tones but not ongoing ITDs of the carrier itself (Caird and Klinke, 1983; Joris and Yin, 1995; Batra et al., 1997). Additionally, LSO cells are also sensitive to onset time differences in transient sounds (Wu and Kelly, 1992;Sanes, 1990; Joris and Yin, 1995; Park et al., 1996).
Two points indicate that ITDs play only a minor role in shaping the SRFs of LSO units measured with long-duration broadband noise. First, confirming the findings of Joris and Yin (1995), we found in both units tested that LSO cells were not modulated in any systematic way by ITDs of broadband noises (Fig.8A). Although there was some modulation of the response at very large ITDs, there was virtually no modulation in the range of ITDs expected for the VS stimuli used in these experiments (±300 μsec; Musicant et al., 1990). Second, Joris and Yin (1995) found that for LSO cells to be modulated by changes in the ITDs of the envelopes of narrowband stimuli, the noise stimuli, and hence the envelopes, of the signals presented to the two ears must be correlated. As a test of the hypothesis, in one unit we measured SRFs with both correlated and uncorrelated noise stimuli. If ITDs in the envelopes of the noise stimuli at the two ears contributed to the azimuthal SRFs for this unit, then the spatial selectivity of the unit should be abolished when envelope ITDs are rendered useless by decorrelating the noise stimuli presented to the two ears. Instead, the shapes of the SRFs are nearly identical for the correlated and uncorrelated conditions (Fig. 8B), although there are differences in terms of discharge rate (part of which is attributable to the fact that the root mean square levels of the two stimuli differed by ∼1 dB and favored the contralateral ear). Together, our observations here along with those of Joris and Yin (1995) support the hypothesis that the major determinant of the SRFs for LSO cells is ILDs.
Fig. 8.
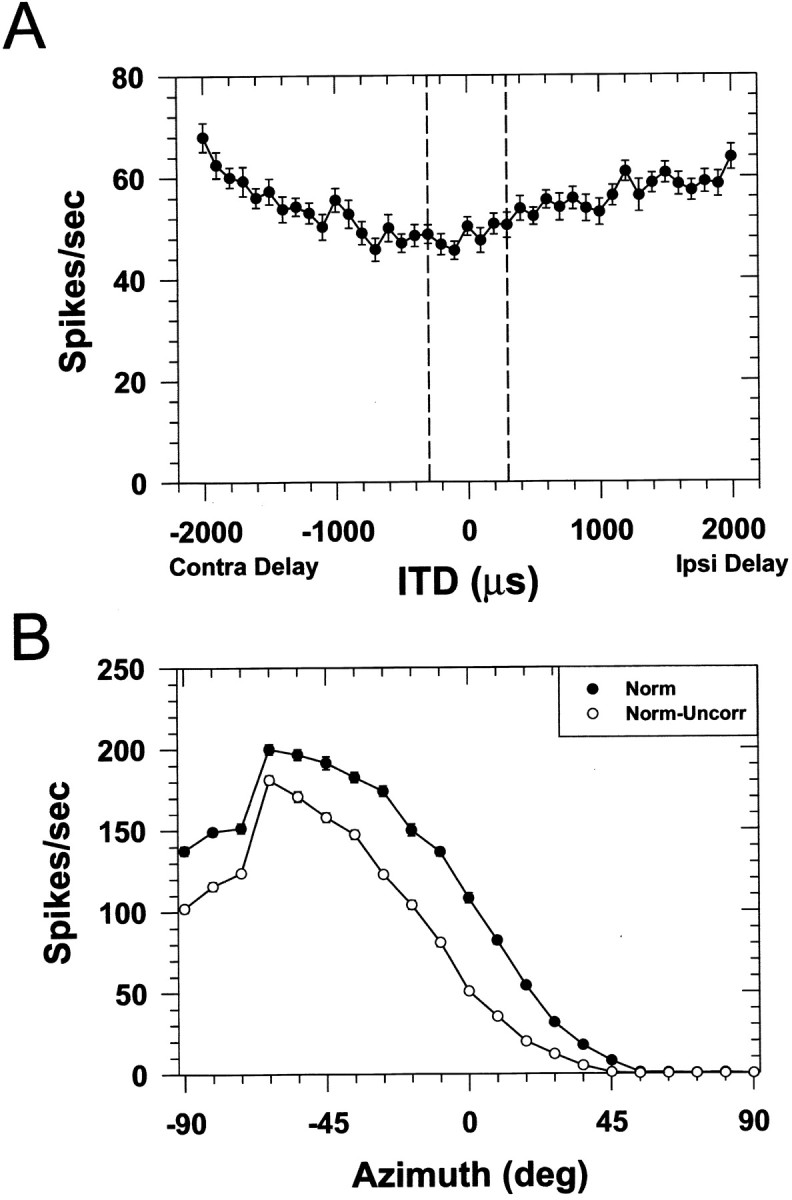
A, Responses of one LSO cell to broadband noise as a function of the interaural time difference. Data points plot the mean discharge rate ± 1 SEM. Negative delays indicate that the onset of the noise to the contralateral ear was delayed with respect to that at the ipsilateral ear. Thevertical dashed lines indicate the range of ITDs expected for the average adult cat. The response of the cell is not modulated by ongoing ITDs in broadband noise over this range of ITDs.B, Normal binaural SRFs of another LSO cell under conditions in which the broadband noises presented to the two ears were identical (filled circles) or uncorrelated (open circles).
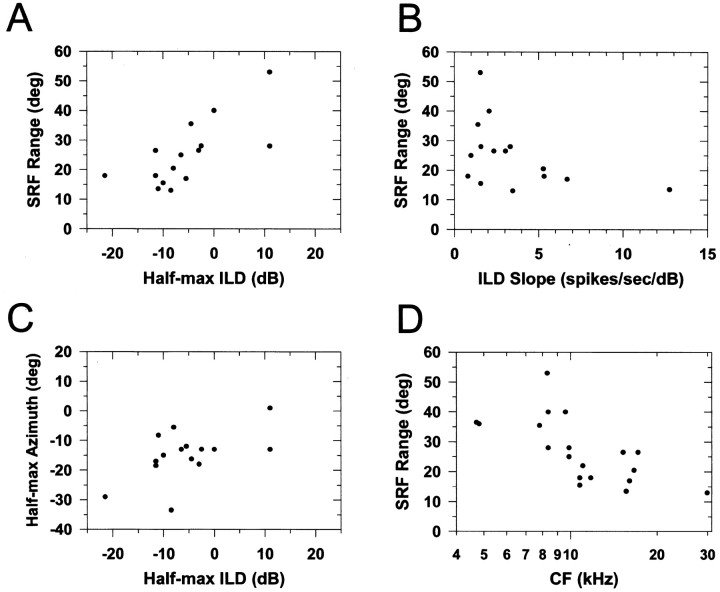
Next, to what extent does the ILD selectivity of LSO cells to CF tones determine the SRF properties? To address this question, we examined for each cell the relationship between the half-maximal azimuths, ranges, and MIs of the normal SRFs and the half-maximal ILDs and slopes of the tone ILD functions (Fig. 1E) by computing the Spearman rank correlations between these properties. Recall that tone ILD functions were measured by presenting CF tones to the ipsilateral ear at a level held fixed at ∼20 dB above the threshold of the units and varying the level of a CF tone presented to the contralateral ear. The analysis presented here was based on the responses of 15 of the 25 cells tested in the normal condition; of the 10 cells not included in this analysis, six had incomplete ILD functions and four had normal SRFs that were not predominantly IE in nature, at least at the sound levels used in the experiments (Fig. 7). Figure9A shows the significant correlation between the range of the SRF and the half-maximal ILD (r = 0.72; p = 0.003). Several relationships were apparent, but just missed significance: Figure9B shows the SRF range and the slope of the tone ILD function (r = −0.46; p = 0.084); the SRF modulation index and the dynamic range of the ILD function (r = 0.51; p = 0.055); and Figure9C shows the half-maximal azimuth of the SRF and the half-maximal ILD (r = 0.48; p = 0.068). Not enough noise ILD functions were measured for a corresponding analysis with noise.
Fig. 9.
Scatterplots of the properties of the azimuthal SRFs and the properties of the ILD functions measured with tones at CF.A, The SRF range versus the half-maximal ILD.B, The SRF range versus the slope of the ILD function.C, The half-maximal azimuth of the SRFs versus the half-maximal ILD. D, The SRF range versus the CF of the unit.
Here we briefly probe further whether the selectivity to tone ILDs of the cells can be used to determine directly the SRF properties. First, we measured the ILDs actually present in the HRTFs of Musicant et al. (1990) used to generate the VS stimuli for this study through 1/6-octave filters centered on the CFs of the same 15 cells. For these CFs, which ranged from 7.8 to 29.86 kHz, we found a nearly linear relationship between azimuth and ILD for −45° to +45°, and the mean rate of change of ILD with azimuth over this range for these frequencies was 0.39 dB/o. The mean half-maximal ILD for these 15 LSO cells as determined dichotically was −5.6 dB (SD, 8.41 dB). Using the half-maximal ILD of each cell and the rate of change of ILD for the VS stimuli at its CF, we predict a population half-maximal azimuth of −14.5° (SD, 20.4°) that did not differ significantly from the value of −14.9° (SD, 8.4) observed empirically (Wilcoxon t = 310; p > 0.95). But although the ensemble half-maximal azimuths could be predicted, the individual empirical half-maximal azimuth of each cell could not be (Spearman's r = 0.4; p = 0.138). This, along with the analyses above indicates that ILD selectivity of each LSO cell as measured dichotically with tones at the CF of each cell and at only one overall sound level cannot be readily generalized to azimuthal spatial selectivity; that is, the properties of the tone ILD functions cannot always predict the SRF properties.
Our results also confirm ILD measurements of Irvine (1987) and Martin and Webster (1989), who reported that ILDs for pure tones in cats vary with azimuth monotonically for frequencies up to ∼8–10 kHz. And above ∼10 kHz, ILDs are nonmonotonic generally increasing with azimuth to nearly 45° from the midline, but then declining. The nonmonotonicity in the ILD azimuth functions in those studies is qualitatively similar to the nonmonotonic shapes of the SRFs for high-CF units we observed (Fig. 4C,D). They also showed that, in general, the rate at which ILD changes with changes in azimuth from the midline increased as the frequency of the tone increased. If these LSO cells are encoding ILD, then we should observe a dependence of the range of the SRFs on the CF of the cells. Confirming the above hypothesis, for the normal SRFs, a significant relation between the CF of the cells and their SRF ranges was found (Fig. 9D) (Spearman's r = −0.72; p = 0.002). Paralleling this finding, there was a significant correlation between the rate of change of ILD as computed above for the 15 cells and their CFs (r = −0.57; p = 0.024).
In addition, we also computed the rate of change of the sound level at one ear through 1/6 octave filters centered at the CFs of the 21 cells tested in the ipsi-only condition. The mean monaural rate of change was 0.18 dB/o, approximately half that of the ILD. Although there was a significant relation between the monaural rate of change of level and CF (r = −0.57;p = 0.007) similar to that above for ILD, no such relation existed between CF and ipsi-only SRF range (r= −0.07; p = 0.75). This analysis reveals that the acoustical cues provided by the VS stimuli are in accordance with the differences in the ranges of the SRFs observed under the ipsi-only and normal stimulus conditions and provides evidence that the SRFs in the normal binaural condition were determined predominantly by ILDs.
It has not escaped our notice that across-frequency effects may have contributed to findings that the properties of the tone ILD functions cannot always predict the SRF properties. Although there is evidence of LSO IE units with ipsilateral off-CF inhibitory sidebands (Brownell et al., 1979; Caird and Klinke, 1983), we believe that, for the most part, the effect of such influences in our sample of units was generally quite small. One important difference between our preparation and that of Brownell et al. (1979) is that we used a barbiturate anesthetic, whereas they used an unanesthetized decerebrate preparation. And in both units tested, Brownell et al. (1979) found that ipsilateral inhibitory sidebands disappeared after the administration of a barbiturate anesthetic so it is possible that the barbiturate anesthetic used here weakened the influence of any ipsilateral off-CF inhibition in most units. We did not routinely assess the presence of inhibitory side bands, but such ipsilateral sidebands might have been the cause of the BF response types we observed in two LSO cells (Fig.7).
Effect of sound level on spatial receptive fields
In nine cells, the effect of changing the stimulus level on the properties of the SRFs was measured. Figure10 shows how the monaural ipsi-only SRFs and the binaural normal SRFs were affected by increases in sound level in four units with CFs spanning from 8.4 to ∼30 kHz. Sound levels shown in the figure are relative to the threshold sound level for the condition in which the stimuli were presented monaurally to the ipsilateral ear at (0°, 0°). As the level was increased, there were marked changes in the ipsi-only SRFs with the discharge rates increasing substantially at virtually all azimuths, particularly in the contralateral field. The discharge rate of the normal SRFs also increased, but nearly always only in the ipsilateral field, not in the contralateral. Thus, the monaural SRFs were not as robust to increases in level and often saturated at high levels even for locations in the contralateral sound field, and LSO cells are able to code information about sound source azimuth over a wider range of stimulus levels when stimulated binaurally.
Fig. 10.
The effect of overall sound level on the SRFs of four LSO cells. Open and filled symbolsindicate the ipsi-only and normal stimulus conditions, respectively. The parameter is the overall stimulus level above the threshold level for the ipsi-only condition measured at (0°, 0°). The SEM is not shown in this figure.
The effect of stimulus level on the SRFs was quantified by computing the half-maximal azimuth for the normal and ipsi-only SRFs at each sound level tested. For seven of nine units, only small changes (mean, 0.73°/dB) in half-maximal azimuth were found in the normal SRFs for changes in sound level over a 10–30 dB range, with the half-maximal azimuths expanding into the contralateral sound field. The two remaining units had half-maximal azimuths that moved slightly into the ipsilateral field with increasing sound level (less than −0.8°/dB). The ipsi-only SRFs were much more sensitive to stimulus level with discharge rates often saturating at many azimuths. The change in half-maximal azimuth with level was always greater in the ipsi-only condition than the normal condition; on average, the effect of level on ipsi-only half-maximal azimuth was 5.0 times greater than in the normal. The 5:1 ratio is clearly an underestimate because for several cells, half-maximal azimuth for the ipsi-only condition could not be determined at large sound levels because its responses never dropped to <50% of the maximum discharge rate. Increasing level generally increased the BIS of the cell but did not change the BIT. Hence, our classification of the two units shown in bottom left of Figure 7 as monaural may be attributable to our having presented the VS stimuli at a relatively low sound level.
We also examined the effect of stimulus level on the other characteristics of the SRFs. For all but one unit, the normal SRF range increased with stimulus level but by <2o/dB (mean, 0.81°/dB;N = 9). The range of the remaining unit decreased by −0.6°/dB; it was one of the units whose SRFs moved slightly into the ipsilateral field with increasing level. The MI remained fixed at a value of 1.0 for 3 units, increased in 5 units, and decreased in 1 unit. The latter unit was not one of the two units whose SRF properties changed differently.
These findings are consistent with those of Boudreau and Tsuchitani (1968), who found that the LSO generally encodes a correlate of the relative difference in level between the stimuli at the two ears (i.e., ILD). But they also showed that ILD sensitivity was affected by the overall level of the stimuli presented to the two ears. In six of the cells included in this study, we measured CF tone or noise ILD functions at two or more levels. The normalized ILD functions for each of these cells were shifted toward more negative ILDs as the base level of the stimulus to the ipsilateral ear was increased, but by less than would be expected if the cells were simply computing a fixed ILD. Consistent with our observations, Tsuchitani and Boudreau (1969)also showed that, given a fixed ILD, LSO cells can also be sensitive to the overall sound level of the two stimuli with discharge rates generally decreasing with increasing overall level. These data suggest that as overall stimulus level increases the strength of the contralateral inhibition generally increases relative to the ipsilateral excitation. This is opposite to what we observed when the overall levels of the VS sounds were increased; half-maximal azimuth moved toward the contralateral field as the level of the stimuli was increased. Again, it appears that pure-tone ILD sensitivity is not always sufficient to generalize to spatial location sensitivity.
Can sensitivity to azimuth in LSO IE units account for sensitivity to azimuth in IC EI units?
Although the LSO projects bilaterally to the dorsal nucleus of the lateral lemniscus and the inferior colliculus, anatomical studies have revealed a large excitatory projection from the middle and median limbs of the LSO to the contralateral IC (Roth et al., 1978; Glendenning and Masterton, 1983; Saint Marie et al., 1989; Glendenning et al., 1992;Oliver et al., 1995; Oliver, 2000). To the extent that these cells govern the responses of the IC cells to which they project, the spatial tuning of cells in the LSO could account for the spatial tuning in the contralateral IC. Delgutte et al. (1999) recently measured spatial sensitivity of cells in the IC of the cat using VS stimuli synthesized from the same HRTFs used here. Approximately one-third (35 of 102) of the units in that study exhibited response types that were consistent with purely EI binaural interaction; that is, responses that were generally inhibited under binaural stimulation relative to stimulation of the contralateral excitatory ear in isolation. Figure11 shows a histogram of the half-maximal azimuths of the SRFs from our population of LSO units (which all exhibited IE binaural interaction) and the half-maximal azimuths from a subgroup of the population of IC cells from the study of Delgutte et al. (1999) that exhibited EI interaction, that is, the IC units that exhibited the BI binaural interaction response type. One of these 35 IC units was omitted because its response did not fall to <50% of maximum response. To account for the midline-crossing projection of the LSO to the contralateral IC, the half-maximal azimuths for the population of LSO units in Figure 11 have been reflected around the midline to account for the EI nature of the IC units. The comparison reveals that the overall range of half-maximal azimuths encompassed by the LSO SRFs accounts for 74% of the half-maximal azimuths obtained for the IC units.
Fig. 11.
Histogram of half-maximal azimuths of SRFs measured using similar stimuli in LSO and the 34 IC cells from the study of Delgutte et al. (1999) exhibiting predominantly EI binaural interaction. The half-maximal azimuths of the LSO cells have been reflected about the midline.
If a population of LSO units like that here provides direct input to EI cells in the contralateral IC, then the properties of the SRFs should also be similar. A Mann–Whitney U test revealed that, although there were no significant differences in the SRF range parameters between these two populations (N = 59;U = 462; p = 0.57), the differences in the half-maximal azimuths reached significance (N = 59;U = 289; p = 0.037). The data in Figure11 show that many cells in the IC have SRF half-maximal azimuths well into the contralateral sound field, and no cells in the LSO had comparable half-maximal azimuths in the ipsilateral field. Thus, although the spatial response properties of the IE LSO cells can account for much, but not all, of the spatial selectivity of the EI cells in the contralateral IC, other supraolivary mechanisms must play a role as well.
DISCUSSION
Contralateral inhibition shapes LSO spatial receptive fields
This is the first characterization of the sensitivity of single LSO units of any species to changes in the azimuth of sounds. All units were modulated by variations in azimuth with larger responses for ipsilateral and poorer responses for contralateral azimuths. For nearly all units, for the sound levels used here, the ipsi-only SRF was similar in shape to the normal SRF, which is not unexpected, because the stimulus level to the ipsilateral ear is higher for ipsilateral azimuths than for contralateral. But binaurally, the effect of the contralateral inhibition was clear, as demonstrated by the increased suppression of responses to frontal and contralateral azimuths and could be measured quantitatively by movement of the half-maximal azimuths toward the ipsilateral field, larger modulation indices, and smaller SRF ranges in the normal SRFs as compared with ipsi-only SRFs. The SRFs of nearly all cells exhibited a binaural inhibition response type. Two supplemental experiments suggested that ILDs and not ITDs were the binaural cues shaping the SRFs in azimuth. Finally, sensitivity to azimuth was retained over a large range of sound levels under the binaural, but not the monaural ipsi-only, conditions. Together, the data support the long-standing but heretofore untested hypothesis that when presented with long-duration broadband stimuli containing all of the monaural and binaural cues to location, high-CF (>3 kHz) LSO units respond to azimuthal variations consistent with their IE nature as determined dichotically (Galambos et al., 1959;Boudreau and Tsuchitani, 1968; Caird and Klinke, 1983; Sanes and Rubel, 1988; Joris and Yin, 1995). In other words, LSO cells are encoding a correlate of the ILD present in the stimuli as a function of azimuth.
We found few strong relationships between the normal SRF properties and those of the tone ILD functions. There are several likely reasons for this. First, physiologically, Tsuchitani and Boudreau (1969) showed that ILD sensitivity in LSO is not only a function of the ILD itself, but also the overall sound level. Second, acoustically, as the azimuth of the VS stimuli was changed, the intensity of the sound changed at both ears, creating ILDs by increases in level at the ipsilateral ear caused by amplification effects of the pinna and decreases at the contralateral ear caused by the acoustic shadowing effect (Irvine, 1987). Here, we measured ILD functions using tones at one fixed level at the ipsilateral ear and did not routinely vary the level at both ears as would be the case under free-field conditions. Finally,Wenstrup et al. (1988) have shown that only with more detailed recordings of the binaural and monaural response characteristics of spatially sensitive cells can the properties of SRFs be predicted. In the same IC cells, they measured both ILD functions dichotically at various overall levels and azimuthal SRFs for free-field sounds. Then using the ILD and overall stimulus level measured at the ears in free-field as a function of azimuth along with the ILD tuning functions, they could predict the SRFs. Their experiment showed that, although ILDs were important in shaping SRFs, so too was the overall level. Like our findings here, their SRFs could not be predicted from the ILD function at any one level alone.
Azimuthal sensitivity in LSO cells is robust to stimulus level
Psychophysical studies have shown that localization performance is relatively invariant over a range of stimulus levels (Altshuler and Comalli, 1975; Recanzone et al., 1998; Macpherson and Middlebrooks, 2000). The SRFs of LSO cells were also tolerant to increases in sound level so that under binaural, but not monaural, conditions LSO cells have the capacity to encode the cues to location robustly over a range of stimulus levels: the edges of the SRFs were changed five times more under ipsi-only than normal conditions and many half-maximal azimuths could not be measured in ipsi-only SRFs because of response saturation. That is, at high levels under monaural stimulation, LSO cells lose their ability to signal azimuth accurately. It is known that there is at least some ability to localize sounds monaurally, albeit with more error than binaurally (Wightman and Kistler, 1997), and monaural minimum audible angles (MAAs) (Mills, 1958) are substantially worse than binaural (Hausler et al., 1983). Our data predict that monaural, but not necessarily binaural, localization and MAAs in azimuth should deteriorate substantially as level is increased.
The role of the LSO in shaping the spatial selectivity observed at higher levels
Our data provide a missing link in the study of the neural processing of the location of sounds. It has long been known that cells in the dorsal nucleus of the lateral lemniscus (DNLL) (Brugge et al., 1970), IC (Semple et al., 1983; Moore et al., 1984; Aitkin and Martin, 1987), medial geniculate body (Aitkin and Jones, 1992; Barone et al., 1996), and auditory cortical areas (Middlebrooks and Pettigrew, 1981;Imig et al., 1990; Rajan et al., 1990; Middlebrooks et al., 1998;Recanzone et al., 2000) are often sensitive to changes in sound location, and most spatially sensitive cells respond preferentially to stimuli in the contralateral hemifield. It has generally been believed that these higher-order nuclei derive their “EI-like” spatial sensitivity in large part from the cells in the LSO, because it is known that LSO cells that are sensitive to high frequencies send projections predominantly to the contralateral DNLL and IC (Roth et al., 1978; Glendenning et al., 1981; Glendenning and Masterton, 1983;Shneiderman and Henkel, 1987; Shneiderman et al., 1988, 1999; Oliver et al., 1995; Oliver, 2000). Yet, until now, this notion has not been supported by direct measurements of SRFs in LSO cells.
In the cat, the projection from the LSO to the contralateral IC is predominantly excitatory (Saint Marie et al., 1989; Glendenning et al., 1992; Oliver et al., 1995), and the decussation of ascending LSO efferents effectively converts the IE responses of LSO to EI responses at supra-olivary levels. Here, we showed that LSO cells respond to sound source azimuth in a manner consistent with their IE binaural interaction, and to the extent to which these cells project contralaterally, we would expect to see analogous EI responses in higher-level auditory nuclei. However, the spatial selectivity of only ∼75% of the IC units displaying EI interaction from the study ofDelgutte et al. (1999) could be accounted for by the selectivity seen in our population of LSO units. The SRFs of LSO cells could not account for the large positive half-maximal azimuths displayed by some IC units, even after changing stimulus parameters that affect the properties of the SRFs, such as overall sound level (Fig. 10). Therefore, supraolivary mechanisms must be present to shape the spatial selectivity of IC units.
Instead of LSO providing all ILD sensitivity, there seems to be a progressive refinement of ILD coding both within and above the level of the LSO. It is likely that the SRFs of IC cells result from the convergence of excitatory and inhibitory inputs of both LSOs and DNLLs. Supporting this notion, EI binaural interaction is still evident in many units in the IC of the rat even when the cell bodies of the SOC have been destroyed by kainic acid (Sally and Kelly, 1992; Li and Kelly, 1992). Hutson et al. (1991) have indicated several decussating pathways above the SOC that could produce binaural interactions. Moreover, intracellular recordings in IC units show the existence of contralateral excitation and ipsilateral inhibition in the same cell implying bilateral inputs converging on single IC cells (Nelson and Erulkar, 1963; Covey et al., 1996; Kuwada et al., 1997), and in some IC cells the ipsilateral inhibitory synaptic potentials can be blocked by local application of the GABA-A antagonist bicuculline (Faingold et al., 1989). Park and Pollak (1994) have demonstrated that local inhibition in the IC, arising most likely from the GABAergic inputs from the DNLL (Faingold et al., 1993), plays an important role in ILD sensitivity and the formation of SRFs. The DNLL receives excitatory input from the contralateral LSO (Saint Marie et al., 1989; Saint Marie and Baker, 1990; Glendenning et al., 1992;Shneiderman et al., 1999), sends projections bilaterally to the IC (Shneiderman et al., 1988; Shneiderman and Oliver, 1989) and is comprised of predominantly GABAergic neurons (Adams and Mugnaini, 1984). In addition, glycinergic neurons of the LSO project to the ipsilateral IC (Glendenning et al., 1985, 1992; Saint Marie et al., 1989; Saint Marie and Baker, 1990) and likely exert an inhibitory effect (Kelly and Li, 1997). Hence, the excitatory input from the contralateral LSO and the inhibitory influences from the ipsilateral LSO and the contralateral DNLL likely contribute jointly to the binaural selectivity of IC units. Not surprisingly, ILD tuning in LSO and IC cells differs when tested with similar stimuli (Park, 1998).
In summary, although the SRFs set up initially in the LSO are important, their spatial selectivity does not determine in a one-to-one manner the precise spatial sensitivity of cells higher in the ascending auditory pathway. Rather, the spatial sensitivity conferred by LSO cells provides the foundation on which the SRFs observed in higher-order auditory nuclei are constructed.
Footnotes
This work was supported by National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grants DC-00116 and DC-02840 (T.C.T.Y) and DC-00376 (D.J.T.). We thank Dr. Bertrand Delgutte and the reviewers for comments on earlier versions of this manuscript. We also acknowledge the support of R. Kochhar for software and I. Siggelkow for histology.
Correspondence should be addressed to Daniel J. Tollin, Department of Physiology, Room 290, Medical Sciences Building, University of Wisconsin-Madison, 1300 University Avenue, Madison, WI 53706. E-mail:tollin@physiology.wisc.edu.
REFERENCES
- 1.Adam TJ, Schwarz DW, Finlayson PG. Firing properties of chopper and delay neurons in the lateral superior olive of the rat. Exp Brain Res. 1999;124:489–502. doi: 10.1007/s002210050645. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain Res Bull. 1984;13:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 3.Aitkin L, Jones R. Azimuthal processing in the posterior auditory thalamus of cats. Neurosci Lett. 1992;142:81–84. doi: 10.1016/0304-3940(92)90625-h. [DOI] [PubMed] [Google Scholar]
- 4.Aitkin LM, Martin RL. The representation of stimulus azimuth by high best-frequency azimuth-selective neurons in the central nucleus of the inferior colliculus of the cat. J Neurophysiol. 1987;57:1185–1200. doi: 10.1152/jn.1987.57.4.1185. [DOI] [PubMed] [Google Scholar]
- 5.Altshuler MW, Comalli PE. Effect of stimulus intensity and frequency on median horizontal plane sound localization. J Aud Res. 1975;15:262–265. [Google Scholar]
- 6.Barone P, Clarey JC, Irons WA, Imig TJ. Cortical synthesis of azimuth-sensitive single-unit responses with nonmonotonic level tuning: a thalamocortical comparison in the cat. J Neurophysiol. 1996;75:1206–1220. doi: 10.1152/jn.1996.75.3.1206. [DOI] [PubMed] [Google Scholar]
- 7.Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. I. Heterogeneity of responses. J Neurophysiol. 1997;78:1222–1236. doi: 10.1152/jn.1997.78.3.1222. [DOI] [PubMed] [Google Scholar]
- 8.Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- 9.Brownell WE, Manis PB, Ritz LA. Ipsilateral inhibitory responses in the cat lateral superior olive. Brain Res. 1979;177:189–193. doi: 10.1016/0006-8993(79)90930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugge JF, Anderson DJ, Aitkin LM. Responses of neurons in the dorsal nucleus of the lateral lemniscus of cat to binaural tonal stimulation. J Neurophysiol. 1970;33:441–458. doi: 10.1152/jn.1970.33.3.441. [DOI] [PubMed] [Google Scholar]
- 11.Brugge JF, Reale RA, Hind JE, Chan JCK, Musicant AD, Poon PW. Simulation of free-field sound sources and its application to studies of cortical mechanisms of sound localization in the cat. Hear Res. 1994;73:67–84. doi: 10.1016/0378-5955(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 12.Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res. 1983;52:385–399. doi: 10.1007/BF00238032. [DOI] [PubMed] [Google Scholar]
- 13.Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol. 1986;247:457–476. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- 14.Casseday JH, Neff WD. Auditory localization: role of auditory pathways in brain stem of the cat. J Neurophysiol. 1975;38:842–858. doi: 10.1152/jn.1975.38.4.842. [DOI] [PubMed] [Google Scholar]
- 15.Covey E, Kauer JA, Casseday JH. Whole-cell patch-clamp recording reveals subthreshold sound-evoked postsynaptic currents in the inferior colliculus of awake bats. J Neurosci. 1996;16:3009–3018. doi: 10.1523/JNEUROSCI.16-09-03009.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgutte B, Joris PX, Litovsky RY, Yin TCT. Receptive fields and binaural interactions for virtual-space stimuli in the cat inferior colliculus. J Neurophysiol. 1999;81:2833–2851. doi: 10.1152/jn.1999.81.6.2833. [DOI] [PubMed] [Google Scholar]
- 17.Elverland HH. Ascending and intrinsic projections of the superior olivary complex in the cat. Exp Brain Res. 1978;32:117–134. doi: 10.1007/BF00237396. [DOI] [PubMed] [Google Scholar]
- 18.Faingold CL, Gehlbach G, Caspary DM. On the role of GABA as an inhibitory neurotransmitter in inferior colliculus neurons: iontophoretic studies. Brain Res. 1989;500:302–312. doi: 10.1016/0006-8993(89)90326-0. [DOI] [PubMed] [Google Scholar]
- 19.Faingold CL, Anderson CA, Randall ME. Stimulation or blockade of the dorsal nucleus of the lateral lemniscus alters binaural and tonic inhibition in contralateral inferior colliculus neurons. Hear Res. 1993;69:98–106. doi: 10.1016/0378-5955(93)90097-k. [DOI] [PubMed] [Google Scholar]
- 20.Galambos R, Schwartzkopf J, Rupert A. Microelectrode study of superior olivary nuclei. Am J Physiol. 1959;197:527–536. doi: 10.1152/ajplegacy.1959.197.3.527. [DOI] [PubMed] [Google Scholar]
- 21.Glendenning KK, Masterton RB. Acoustic chiasm: efferent projections of the lateral superior olive. J Neurosci. 1983;3:1521–1537. doi: 10.1523/JNEUROSCI.03-08-01521.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glendenning KK, Brunso-Bechtold JK, Thompson GC, Masterton RB. Ascending auditory afferents to the nuclei of the lateral lemniscus. J Comp Neurol. 1981;197:673–703. doi: 10.1002/cne.901970409. [DOI] [PubMed] [Google Scholar]
- 23.Glendenning KK, Hutson KA, Nudo RJ, Masterton RB. Acoustic chiasm II: anatomical basis of binaurality in lateral superior olive of cat. J Comp Neurol. 1985;232:261–285. doi: 10.1002/cne.902320210. [DOI] [PubMed] [Google Scholar]
- 24.Glendenning KK, Baker BN, Hutson KA, Masterton RB. Acoustic chiasm V: inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol. 1992;319:100–122. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- 26.Guinan JJ, Jr, Norris BE, Guinan SS. Single auditory units in the superior olivary complex. II: Location of unit categories and tonotopic organization. Int J Neurosci. 1972;4:147–166. [Google Scholar]
- 27.Hausler R, Colburn S, Marr E. Sound localization in subjects with impaired hearing. Spatial- discrimination and interaural-discrimination tests. Acta Otolaryngol Suppl. 1983;400:1–62. doi: 10.3109/00016488309105590. [DOI] [PubMed] [Google Scholar]
- 28.Hutson KA, Glendenning KK, Masterton RB. Acoustic chiasm. IV: eight midbrain decussations of the auditory system in the cat. J Comp Neurol. 1991;312:105–131. doi: 10.1002/cne.903120109. [DOI] [PubMed] [Google Scholar]
- 29.Imig TJ, Irons WA, Samson FR. Single-unit selectivity to azimuthal direction and sound pressure level of noise bursts in cat high-frequency primary auditory cortex. J Neurophysiol. 1990;63:1448–1466. doi: 10.1152/jn.1990.63.6.1448. [DOI] [PubMed] [Google Scholar]
- 30.Irvine DRF. The auditory brainstem: a review of the structure and function of auditory brainstem processing mechanisms. In: Ottoson D, editor. Progress in sensory physiology, Vol 7. Springer; Berlin: 1986. pp. 1–277. [Google Scholar]
- 31.Irvine DR. Interaural intensity differences in the cat: changes in sound pressure level at the two ears associated with azimuthal displacements in the frontal horizontal plane. Hear Res. 1987;26:267–286. doi: 10.1016/0378-5955(87)90063-3. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins WM, Masterton RB. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- 33.Joris PX, Yin TCT. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. J Neurophysiol. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh GL, Kelly JB. Midline and lateral field sound localization in the ferret (Mustela putorius): contribution of the superior olivary complex. J Neurophysiol. 1992;67:1643–1658. doi: 10.1152/jn.1992.67.6.1643. [DOI] [PubMed] [Google Scholar]
- 35.Keller CH, Takahashi TT. Representation of temporal features of complex sounds by the discharge patterns of neurons in the owl's inferior colliculus. J Neurophysiol. 2000;84:2638–2650. doi: 10.1152/jn.2000.84.5.2638. [DOI] [PubMed] [Google Scholar]
- 36.Keller CH, Hartung K, Takahashi TT. Head-related transfer functions of the barn owl: measurement and neural responses. Hear Res. 1998;118:13–34. doi: 10.1016/s0378-5955(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 37.Kelly JB, Li L. Two sources of inhibition affecting binaural evoked responses in the rat's inferior colliculus: the dorsal nucleus of the lateral lemniscus and the superior olivary complex. Hear Res. 1997;104:112–126. doi: 10.1016/s0378-5955(96)00182-7. [DOI] [PubMed] [Google Scholar]
- 38.Kuwada S, Batra R, Yin TCT, Oliver DL, Haberly LB, Stanford TR. Intracellular recordings in response to monaural and binaural stimulation of neurons in the inferior colliculus of the cat. J Neurosci. 1997;17:7565–7581. doi: 10.1523/JNEUROSCI.17-19-07565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Kelly JB. Binaural responses in rat inferior colliculus following kainic acid lesions of the superior olive: interaural intensity difference functions. Hear Res. 1992;61:73–85. doi: 10.1016/0378-5955(92)90038-o. [DOI] [PubMed] [Google Scholar]
- 40.Macpherson EA, Middlebrooks JC. Localization of brief sounds: effects of level and background noise. J Acoust Soc Am. 2000;108:1834–1849. doi: 10.1121/1.1310196. [DOI] [PubMed] [Google Scholar]
- 41.Martin RL, Webster WR. Interaural sound pressure level differences associated with sound- source locations in the frontal hemifield of the domestic cat. Hear Res. 1989;38:289–302. doi: 10.1016/0378-5955(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 42.Masterton B, Jane JA, Diamond IT. Role of brainstem auditory structures in sound localization. I. Trapezoid body, superior olive, and lateral lemniscus. J Neurophysiol. 1967;30:341–359. doi: 10.1152/jn.1967.30.2.341. [DOI] [PubMed] [Google Scholar]
- 43.Middlebrooks JC, Knudsen EI. Changes in external ear position modify the spatial tuning of auditory units in the cat's superior colliculus. J Neurophysiol. 1987;57:672–687. doi: 10.1152/jn.1987.57.3.672. [DOI] [PubMed] [Google Scholar]
- 44.Middlebrooks JC, Pettigrew JD. Functional classes of neurons in primary auditory cortex of the cat distinguished by sensitivity to sound location. J Neurosci. 1981;1:107–120. doi: 10.1523/JNEUROSCI.01-01-00107.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middlebrooks JC, Xu L, Eddins AC, Green DM. Codes for sound-source location in nontonotopic auditory cortex. J Neurophysiol. 1998;80:863–881. doi: 10.1152/jn.1998.80.2.863. [DOI] [PubMed] [Google Scholar]
- 46.Mills AW. On the minimum audible angle. J Acoust Soc Am. 1958;30:237–246. [Google Scholar]
- 47.Moore CN, Casseday JH, Neff WD. Sound localization: the role of the commissural pathways of the auditory system of the cat. Brain Res. 1974;82:13–26. doi: 10.1016/0006-8993(74)90889-0. [DOI] [PubMed] [Google Scholar]
- 48.Moore DR, Hutchings ME, Addison PD, Semple MN, Aitkin LM. Properties of spatial receptive fields in the central nucleus of the cat inferior colliculus. II. Stimulus intensity effects. Hear Res. 1984;13:175–188. doi: 10.1016/0378-5955(84)90107-2. [DOI] [PubMed] [Google Scholar]
- 49.Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci. 1983;3:237–242. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morest DK. The collateral system of the medial nucleus of the trapezoid body of the cat, its neuronal architecture and relation to the olivo-cochlear bundle. Brain Res. 1968;9:288–311. doi: 10.1016/0006-8993(68)90235-7. [DOI] [PubMed] [Google Scholar]
- 51.Musicant AD, Chan JCK, Hind JE. Direction-dependent spectral properties of cat external ear: new data and cross-species comparisons. J Acoust Soc Am. 1990;87:757–781. doi: 10.1121/1.399545. [DOI] [PubMed] [Google Scholar]
- 52.Nelson PG, Erulkar SD. Synaptic mechanisms of excitation and inhibition in the central auditory pathway. J Neurophysiol. 1963;26:908–923. doi: 10.1152/jn.1963.26.6.908. [DOI] [PubMed] [Google Scholar]
- 53.Oliver DL. Ascending efferent projections of the superior olivary complex. Microsc Res Tech. 2000;51:355–363. doi: 10.1002/1097-0029(20001115)51:4<355::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 54.Oliver DL, Beckius GE, Shneiderman A. Axonal projections from the lateral and medial superior olive to the inferior colliculus of the cat: a study using electron microscopic autoradiography. J Comp Neurol. 1995;360:17–32. doi: 10.1002/cne.903600103. [DOI] [PubMed] [Google Scholar]
- 55.Park TJ. IID sensitivity differs between two principal centers in the interaural intensity difference pathway: the LSO and the IC. J Neurophysiol. 1998;79:2416–2431. doi: 10.1152/jn.1998.79.5.2416. [DOI] [PubMed] [Google Scholar]
- 56.Park TJ, Pollak GD. Azimuthal receptive fields are shaped by GABAergic inhibition in the inferior colliculus of the mustache bat. J Neurophysiol. 1994;72:1080–1102. doi: 10.1152/jn.1994.72.3.1080. [DOI] [PubMed] [Google Scholar]
- 57.Park TJ, Grothe B, Pollak GD, Schuller G, Koch U. Neural delays shape selectivity to interaural intensity differences in the lateral superior olive. J Neurosci. 1996;16:6554–6566. doi: 10.1523/JNEUROSCI.16-20-06554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poganiatz I, Nelken I, Wagner H. Sound-localization experiments with barn owls in virtual space: influence of interaural time difference on head-turning behavior. J Assoc Res Otolaryngol. 2001;1:1–21. doi: 10.1007/s101620010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poon PW, Brugge JF. Virtual-space receptive fields of single auditory nerve fibers. J Neurophysiol. 1993;70:667–676. doi: 10.1152/jn.1993.70.2.667. [DOI] [PubMed] [Google Scholar]
- 60.Rajan R, Aitkin LM, Irvine DR, McKay J. Azimuthal sensitivity of neurons in primary auditory cortex of cats. I. Types of sensitivity and the effects of variations in stimulus parameters. J Neurophysiol. 1990;64:872–887. doi: 10.1152/jn.1990.64.3.872. [DOI] [PubMed] [Google Scholar]
- 61.Reale RA, Chen J, Hind JE, Brugge JF. An implementation of virtual acoustic space for neurophysiological studies of directional hearing. In: Carlile S, editor. Virtual auditory space. Springer; Heidelberg: 1996. pp. 153–183. [Google Scholar]
- 62.Recanzone GH, Makhamra SD, Guard DC. Comparison of relative and absolute sound localization ability in humans. J Acoust Soc Am. 1998;103:1085–1097. doi: 10.1121/1.421222. [DOI] [PubMed] [Google Scholar]
- 63.Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol. 2000;83:2723–2739. doi: 10.1152/jn.2000.83.5.2723. [DOI] [PubMed] [Google Scholar]
- 64.Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hear Res. 1992;58:132–152. doi: 10.1016/0378-5955(92)90123-5. [DOI] [PubMed] [Google Scholar]
- 65.Roth GL, Aitkin LM, Andersen RA, Merzenich MM. Some features of the spatial organization of the central nucleus of the inferior colliculus of the cat. J Comp Neurol. 1978;182:661–680. doi: 10.1002/cne.901820407. [DOI] [PubMed] [Google Scholar]
- 66.Roth GL, Kochhar RK, Hind JE. Interaural time differences: implications regarding the neurophysiology of sound localization. J Acoust Soc Am. 1980;68:1643–1651. doi: 10.1121/1.385196. [DOI] [PubMed] [Google Scholar]
- 67.Saint Marie RL, Baker RA. Neurotransmitter-specific uptake and retrograde transport of [3H]glycine from the inferior colliculus by ipsilateral projections of the superior olivary complex and nuclei of the lateral lemniscus. Brain Res. 1990;524:244–253. doi: 10.1016/0006-8993(90)90698-b. [DOI] [PubMed] [Google Scholar]
- 68.Saint Marie RL, Ostapoff E-M, Morest DK, Wenthold RJ. Glycine immunoreactive projection of the cat lateral superior olive: possible role in midbrain ear dominance. J Comp Neurol. 1989;279:382–396. doi: 10.1002/cne.902790305. [DOI] [PubMed] [Google Scholar]
- 69.Sally SL, Kelly JB. Effects of superior olivary complex lesions on binaural responses in rat inferior colliculus. Brain Res. 1992;572:5–18. doi: 10.1016/0006-8993(92)90444-e. [DOI] [PubMed] [Google Scholar]
- 70.Sanes DH. An in vitro analysis of sound localization mechanisms in the gerbil lateral superior olive. J Neurosci. 1990;10:3494–3506. doi: 10.1523/JNEUROSCI.10-11-03494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Semple MN, Aitkin LM, Calford MB, Pettigrew JD, Phillips DP. Spatial receptive fields in the cat inferior colliculus. Hear Res. 1983;10:203–215. doi: 10.1016/0378-5955(83)90054-0. [DOI] [PubMed] [Google Scholar]
- 73.Shneiderman A, Henkel CK. Evidence of collateral axonal projections to the superior olivary complex. Hear Res. 1985;19:199–205. doi: 10.1016/0378-5955(85)90139-x. [DOI] [PubMed] [Google Scholar]
- 74.Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- 75.Shneiderman A, Oliver DL. EM autoradiographic study of the projections from the dorsal nucleus of the lateral lemniscus: a possible source of inhibitory inputs to the inferior colliculus. J Comp Neurol. 1989;286:28–47. doi: 10.1002/cne.902860103. [DOI] [PubMed] [Google Scholar]
- 76.Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- 77.Shneiderman A, Stanforth DA, Henkel CK, Saint Marie RL. Input-output relationships of the dorsal nucleus of the lateral lemniscus: possible substrate for the processing of dynamic spatial cues. J Comp Neurol. 1999;410:265–276. [PubMed] [Google Scholar]
- 78.Smith PH, Joris PX, Carney LH, Yin TCT. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- 79.Smith PH, Joris PX, Yin TCT. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol. 1993;331:245–260. doi: 10.1002/cne.903310208. [DOI] [PubMed] [Google Scholar]
- 80.Smith PH, Joris PX, Yin TCT. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol. 1998;79:3127–3142. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- 81.Spangler KM, Warr WB, Henkel CK. The projections of principal cells of the medial nucleus of the trapezoid body in the cat. J Comp Neurol. 1985;238:249–262. doi: 10.1002/cne.902380302. [DOI] [PubMed] [Google Scholar]
- 82.Sun XD, Jen PH. Pinna position affects the auditory space representation in the inferior colliculus of the FM bat, Eptesicus fuscus. Hear Res. 1987;27:207–219. doi: 10.1016/0378-5955(87)90002-5. [DOI] [PubMed] [Google Scholar]
- 83.Thompson GC, Masterton RB. Brain stem auditory pathways involved in reflexive head orientation to sound. J Neurophysiol. 1978;41:1183–1202. doi: 10.1152/jn.1978.41.5.1183. [DOI] [PubMed] [Google Scholar]
- 84.Tolbert L, Morest D, Yurgelun-Todd D. The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: horseradish peroxidase labelling of identified cell types. Neuroscience. 1982;7:3031–3052. doi: 10.1016/0306-4522(82)90228-7. [DOI] [PubMed] [Google Scholar]
- 85.Tollin DJ, Yin TCT. Spatial receptive fields of cells in the lateral superior olive of the cat. Soc Neurosci Abstr. 1999;29:267.13. [Google Scholar]
- 86.Tollin DJ, Yin TCT. The coding of spatial location by single units in the lateral superior olive of the cat. II. The determinants of spatial receptive fields in azimuth. J Neurosci. 2002;22:1468–1479. doi: 10.1523/JNEUROSCI.22-04-01468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsuchitani C. Discharge patterns of cat lateral superior olivary units to ipsilateral tone-burst stimuli. J Neurophysiol. 1982;47:479–500. doi: 10.1152/jn.1982.47.3.479. [DOI] [PubMed] [Google Scholar]
- 88.Tsuchitani C, Boudreau JC. Single unit analysis of cat superior olive S segment with tonal stimuli. J Neurophysiol. 1966;29:684–697. doi: 10.1152/jn.1966.29.4.684. [DOI] [PubMed] [Google Scholar]
- 89.Tsuchitani C, Boudreau JC. Stimulus level of dichotically presented tones and cat superior olive S-segment cell discharge. J Acoust Soc Am. 1969;46:979–988. doi: 10.1121/1.1911818. [DOI] [PubMed] [Google Scholar]
- 90.Warr WB. Fiber degeneration following lesions in the anterior ventral cochlear nucleus of the cat. Exp Neurol. 1966;14:453–474. doi: 10.1016/0014-4886(66)90130-0. [DOI] [PubMed] [Google Scholar]
- 91.Warr WB. Fiber degeneration following lesions in the multipolar and globular cell areas in the ventral cochlear nucleus of the cat. Brain Res. 1972;40:247–270. doi: 10.1016/0006-8993(72)90132-1. [DOI] [PubMed] [Google Scholar]
- 92.Wenstrup JJ, Fuzessery ZM, Pollak GD. Binaural neurons in the mustache bat's inferior colliculus. II. Determinants of spatial responses among 60-kHz EI units. J Neurophysiol. 1988;60:1384–1404. doi: 10.1152/jn.1988.60.4.1384. [DOI] [PubMed] [Google Scholar]
- 93.Wightman FL, Kistler DJ. Headphone simulation of free-field listening. I: Stimulus synthesis. J Acoust Soc Am. 1989a;85:858–867. doi: 10.1121/1.397557. [DOI] [PubMed] [Google Scholar]
- 94.Wightman FL, Kistler DJ. Headphone simulation of free-field listening. II: Psychophysical validation. J Acoust Soc Am. 1989b;85:868–878. doi: 10.1121/1.397558. [DOI] [PubMed] [Google Scholar]
- 95.Wightman FL, Kistler DJ. Monaural sound localization revisited. J Acoust Soc Am. 1997;101:1050–1063. doi: 10.1121/1.418029. [DOI] [PubMed] [Google Scholar]
- 96.Wu SH, Kelly JB. Binaural interaction in the lateral superior olive: time difference sensitivity studied in mouse brain slice. J Neurophysiol. 1992;68:1151–1159. doi: 10.1152/jn.1992.68.4.1151. [DOI] [PubMed] [Google Scholar]
- 97.Xu L, Middlebrooks JC. Individual differences in external-ear transfer functions of cats. J Acoust Soc Am. 2000;107:1451–1459. doi: 10.1121/1.428432. [DOI] [PubMed] [Google Scholar]
- 98.Yin TCT, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]
- 99.Young ED, Robert JM, Shofner WP. Regularity and latency of units in ventral cochlear nucleus: implications for unit classification and generation of response properties. J Neurophysiol. 1988;60:1–29. doi: 10.1152/jn.1988.60.1.1. [DOI] [PubMed] [Google Scholar]
- 100.Young ED, Rice JJ, Tong SC. Effects of pinna position on head-related transfer functions in the cat. J Acoust Soc Am. 1996;99:3064–3076. doi: 10.1121/1.414883. [DOI] [PubMed] [Google Scholar]