Abstract
The tonic influence of dopamine D1 and D2receptors on the activity of striatal neurons in vivowas investigated by performing intracellular recordings concurrently with reverse microdialysis in chloral hydrate-anesthetized rats. Striatal neurons were recorded in the vicinity of the microdialysis probe to assess their activity during infusions of artificial CSF (aCSF), the D1 receptor antagonist SCH 23390 (10 μm), or the D2 receptor antagonist eticlopride (20 μm). SCH 23390 perfusion decreased the excitability of striatal neurons exhibiting electrophysiological characteristics of spiny projection cells as evidenced by a decrease in the maximal depolarized membrane potential, a decrease in the amplitude of up-state events, and an increase in the intracellular current injection amplitude required to elicit an action potential. Conversely, a marked depolarization of up- and down-state membrane potential modes, a decrease in the amplitude of intracellular current injection required to elicit an action potential, and an increase in the number of spikes evoked by depolarizing current steps were observed in striatal neurons after local eticlopride infusion. A significant increase in maximal EPSP amplitude evoked by electrical stimulation of the prefrontal cortex was also observed during local eticlopride but not SCH 23390 infusion. These results indicate that in intact systems, ongoing dopaminergic neurotransmission exerts a powerful tonic modulatory influence on the up- and down-state membrane properties of striatal neurons and controls their excitability differentially via both D1- and D2-like receptors. Moreover, a significant component of D2 receptor-mediated inhibition of striatal neuron activity in vivo occurs via suppression of excitatory synaptic transmission.
Keywords: dopamine, D1 receptor, D2receptor, striatum, striatal neurons, electrophysiology, in vivo intracellular recording, reverse microdialysis, rat
It is well established that corticostriatal glutamatergic and nigrostriatal dopaminergic (DAergic) systems are critically involved in the integration of motor information by striatal medium spiny projection neurons (for review, see Onn et al., 2000). A considerable body of data implicates dysfunction of these systems in movement disorders such as Parkinson's disease and Tourette's syndrome. Although it is known that the activation of convergent corticostriatal glutamatergic inputs depolarizes striatal neurons and drives their activity, the complex influence of DA on the activity states of striatal neurons and its interaction with glutamatergic neurotransmission remains a matter of controversy.
It is known that striatal spiny projection neurons generally exhibit a characteristic shift in membrane potential that consists of “up” and “down” states representing the depolarized and hyperpolarized condition of the membrane, respectively (Wilson, 1993; O'Donnell and Grace, 1995; Wilson and Kawaguchi, 1996; Stern et al., 1997; Onn and Grace, 1999, 2000). In the dorsal striatum and nucleus accumbens, the up state is believed to be driven primarily by excitatory glutamatergic inputs and is not observed after mechanical or pharmacological disruption of afferent inputs (Wilson, 1993; O'Donnell and Grace, 1995). Given that the up state is also not present in vitro(Calabresi et al., 2000), the precise influence of D1 and D2 receptor activation on the probability that a spiny neuron will reach the up state and fire action potentials is not known.
Nonetheless, recent voltage-clamp studies have generated predictions as to the impact of DA on neuronal excitability during up- and down-state membrane potentials (Nicola et al., 2000). It has been shown that D1 receptor activation inhibits evoked activity at hyperpolarized membrane potentials (Calabresi et al., 1987;Hernández-López et al., 1997) and facilitates spike activity when the cell is clamped at a depolarized membrane potential (Hernández-López et al., 1997). On the other hand, D2 receptor activation attenuates spike activity when the membrane potential is held at relatively depolarized levels mimicking the up state (Hernández-López et al., 2000). These studies predict that in the intact animal, DA modulates the excitability of striatal neurons differentially in a manner dependent on the steady-state membrane potential set by afferent drive and the DA receptor subtype involved in modulating membrane activity (Nicola et al., 2000). Although intriguing, this model is predicated on the proposition that a prolonged depolarized condition induced by intracellular current injection into the soma can approximate the naturally occurring up state driven by glutamatergic afferents in vivo. However, because the DA receptor-dependent modulation of the glutamatergic afferent-driven depolarization in vivo is likely to occur in the distal dendrites, this model needs to be tested using a preparation in which the glutamatergic and DAergic inputs are preserved and behaving naturally.
Thus, the aim of the current study was to examine the influence of endogenous DA and local DA D1 and D2 receptor activation on the membrane activity of striatal spiny neurons without compromising the integrity of the neuronal network or altering the natural activity of the recorded neuron. To this end, we have performed in vivo intracellular recordings on neurons located proximal to a microdialysis probe and have used the reverse dialysis method as a means to deliver D1 and D2 receptor antagonists locally in the vicinity of the recorded neuron. The current studies reveal that locally infused DA D1 and D2 receptor antagonists exert opposite influences on the membrane properties of individual striatal neurons exhibiting neuronal activity characteristic of spiny projection cells.
Some of the results of these studies have been published previously in abstract form (West and Grace, 2000b).
MATERIALS AND METHODS
Drugs. Chloral hydrate, PBS, eticlopride hydrochloride, and SCH 23390 hydrochloride were purchased from Sigma (St. Louis, MO). All other reagents were of the highest grade commercially available.
Subjects and surgery. Intracellular recordings of striatal neurons were obtained in vivo from male Sprague Dawley rats (Hilltop, Scottdale, PA) weighing 275–450 gm. Before experimentation, animals were housed two per cage under conditions of constant temperature (21–23°C) and maintained on a 12 hr light/dark cycle with food and water available ad libitum. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and adhere to the Guide for the Care and Use of Laboratory Animals published by the United States Public Health Service. Before surgery, animals were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus (Narishige, Tokyo, Japan). The level of anesthesia was periodically verified (every 10–15 min) via testing for the hindlimb compression reflex and maintained using supplemental administration of chloral hydrate (80 mg/ml) via a lateral tail vein (∼0.2 ml/0.5 hr). Temperature was monitored using a rectal probe and maintained at 37°C with a heating pad.
In vivo intracellular recording. Intracellular recording electrodes were manufactured from 1.0 mm outer diameter borosilicate glass tubing (World Precision Instruments, Sarasota, FL) using a Flaming-Brown P-80/PC electrode puller. Microelectrodes were filled with a potassium acetate (3 m) solution containing 2% biocytin using a nonmetallic Microfil syringe needle. Intracellular electrode impedances typically ranged from 30 to 100 MΩ as measuredin situ. After a burr hole (∼2–3 mm in diameter) was drilled over the dorsal striatum (coordinates: −0.5–2.0 mm anterior from bregma, 2.0–3.5 mm lateral from the midline, 3–6 mm ventral from brain surface), the dura was resected, and the electrode was lowered into the striatum using a Narishige micromanipulator. All coordinates were derived from a rat brain stereotaxic atlas (Paxinos and Watson, 1986). Electrode potentials were amplified via a headstage connected to a Neurodata IR-183 intracellular preamplifier (Cygnus Technology, Delaware Water Gap, PA). Intracellular current was injected via an active bridge circuit integral to the preamplifier, and the amplitude of this current was monitored on a Philips PM3337 storage oscilloscope (Fluke, Eindhoven, The Netherlands), and any variation in electrode balance was immediately compensated by adjusting the bridge. Cell penetrations were defined as stable when the cell exhibited a resting membrane potential of at least −55 mV, fired action potentials having an amplitude of at least 50 mV (range, 52–78 mV) with a positive overshoot, and fired a train of spikes after membrane depolarization. Data were collected for cells that had been defined as stable when these electrophysiological properties were maintained for a minimum period of 5 min. Because some striatal cells were observed to hyperpolarize considerably during the first 5–20 min of recording and sometimes stop firing, neuronal membrane fluctuations were monitored over 5 min baseline periods and allowed to stabilize before the experimental testing phase (i.e., current injection or synaptic activation). In within-subjects experiments, pre-drug measurements of basal activity were always taken during a time period immediately before the drug infusion (generally at least 10 min after the stabilization of the neuron). During this pre-drug time period no change in the membrane potential fluctuations were observed over several minutes. After experimental manipulations, striatal cells were injected with biocytin (∼10–60 min) via application of depolarizing pulses (∼0.5 nA, 300 msec) through the recording electrode. After the electrode was withdrawn from the cell, the extracellular electrode tip potential was recorded, and membrane potential measurements were corrected accordingly.
Data analysis. Electrophysiological data obtained during intracellular recordings were digitized by a NeuroData Neurocorder (DR-390) and stored on VHS videotapes. Data were analyzed off-line using Neuroscope software applications developed in our laboratory using an Intel-based microcomputer with a data acquisition board interface (Microstar Laboratories, Bellevue, WA). Basal neuronal activity and the influence of local drug infusions were determined by comparing the membrane potential and spike activity recorded during the last 30–60 sec of the 5 min aCSF (control) infusion period with similar recordings made during drug infusions (see below). The existence of bistable membrane activity was determined as described previously (O'Donnell and Grace, 1995). Briefly, the bistable state was operationally defined as the presence of a membrane potential that is maintained at a steady-state depolarized and hyperpolarized membrane potential, without regard as to whether this was a property of the cell membrane or the result of the system of interconnections within the CNS. The presence of an up event was defined as a rapid transition in membrane potential to a depolarized plateau potential exhibiting an amplitude ≥8 mV (range, 8–42 mV) that was maintained for at least 100 msec. In all cases, time interval plots of membrane potential activity (30–60 sec recordings sampled at 10 kHz) recorded from neurons exhibiting bistable activity could be fitted to a dual Gaussian distribution with a confidence of ≥0.95 using Origin 6.1 (Microcal Corporation). From these plots, the maximal depolarized and hyperpolarized membrane potentials within the distribution, the up and down state modes (the membrane potential at which the neuron spends the most time in each state), and the area under both modal distributions (time spent in each state) could be determined. The amplitude of up events was measured from the beginning of the rising phase to the peak of the depolarization plateau. Additionally, the duration of up events was measured from the beginning of the rising phase to the point where the falling phase returned to the initial baseline membrane potential. The frequency of up events per 30 sec sample was also determined. The input resistance in up and down states was determined for all striatal cells by injecting a series of hyperpolarizing current pulses (150 msec, 0.1–1.5 nA) and plotting the resulting membrane potential deflections against the amplitude of the current pulse (see Fig.1D). For each neuron, the membrane potential was measured immediately before current injection, and the membrane potential state (up or down) was determined via comparisons with time interval plots of membrane potential activity fitted to dual Gaussian distributions as described above. The linear portion of the resulting data points was then fitted to a least-squares regression line, and the input resistance was estimated from the slope of the lines. The statistical significance of drug-induced changes in measures of cell activity in within-subjects experiments was determined using a pairedt test. The statistical significance of drug-induced changes in measures of cell activity in between-subjects experiments was determined using a one-way ANOVA.
Fig. 1.
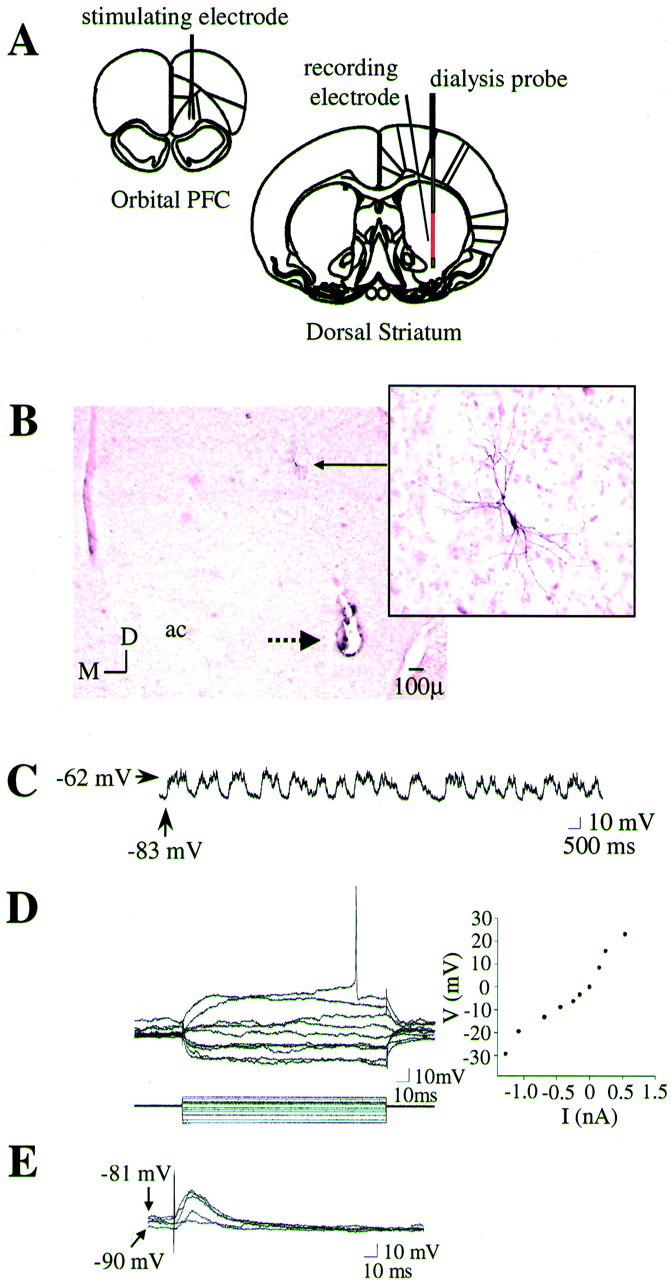
Intracellular recordings from striatal neurons located proximal to the microdialysis probe. A, Positioning of implants. Electrodes and microdialysis probes were stereotaxically implanted using a micromanipulator (see Materials and Methods). All coordinates were derived from the stereotaxic atlas ofPaxinos and Watson (1986). The corticostriatal pathway from the orbital PFC to the central striatum was activated in some experiments via electrical stimulation. B, Left, Coronal section (2.5×) depicting a photomontage of a striatal neuron (solid arrow) labeled after intracellular biocytin injection (enlarged to 20× on the right). Note that the neuron was located proximal to the active zone of the microdialysis probe (extends dorsally 4 mm from the termination point of the probe track indicated by the dashed arrow). ac, Anterior commissure. C, Intracellular recordings from the striatal neuron labeled in B revealed that this cell did not fire spontaneously but did exhibit membrane activity characterized by rapid and spontaneous transitions from a hyperpolarized state to a depolarized plateau. D,Left, In the same cell, intracellular injection of 150 msec duration constant current pulses (bottom traces) induced deflections in the membrane potential (top traces). Right, A plot of the steady-state voltage deflections against the current pulse amplitudes derived from recordings shown on the left. The input resistance of this neuron (as well as the mean input resistance of all neurons recorded proximal to the dialysis probe) was very similar to that observed in intact animals. E, Single pulses of electrical stimulation delivered to the PFC evoked short-latency EPSPs in this same striatal neuron in a stimulus amplitude-dependent manner.
Electrical stimulation. In each experiment, twisted-pair bipolar stimulating electrodes (Plastics One) were implanted into the orbital prefrontal cortex (PFC) (coordinates: 3.7–4.7 mm anterior to bregma, 0.2–2.3 mm lateral to midline, 2.5–4.0 mm ventral to brain surface) ipsilateral to the recording electrode (see Fig. 1). Stimulation sites in the medial, ventral, and ventrolateral orbital PFC were selected on the basis of results of striatal retrograde and anterograde tracing (Deniau et al., 1996) and electrophysiological studies (Nakamura et al., 1979; West and Grace, 1999, 2000a). Single pulses of electrical stimuli with durations of 200–250 μsec and intensities between 0.1 and 3.0 mA were generated using a Grass stimulator (S88) and photoelectric constant current/stimulus isolation unit (PSIU6F, Grass Instruments, Quincy, MA) and delivered at a frequency of 0.2 Hz.
Simultaneous microdialysis and intracellular recording. Concentric microdialysis probes (Bioanalytical Systems, West Lafayette, IN) having 3–4 mm of exposed membrane (320 μm diameter, ∼6000 Da permeability) were implanted into the dorsal striatum (coordinates: 0.1–0.7 mm anterior to bregma, 2.0–3.5 mm lateral to midline, 5.5–6.5 mm ventral to brain surface) over a 25–30 min period (3–4 μm/sec) using a micromanipulator (Narishige). Probes were then attached using dental cement (Kerr, Romulus, MI) to a screw positioned in the skull near the burr hole. After implantation, probes were perfused with aCSF containing (in mm): 147 NaCl, 3.0 KCl, 0.8 MgCl2, 1.2 CaCl2, 2.0 NaH2PO4, and 2.0 Na2HPO4 at a rate of 2 μl/min using a Bioanalytical Systems Baby Bee microperfusion pump as described previously (West and Galloway, 1996, 1997). We have also shown previously (Moore et al., 2000) that the implantation and perfusion of the microdialysis probe does not alter the membrane properties of viable striatal neurons recorded proximal to the probein vivo. Electrophysiological recordings were initiated ∼3–4 hr after probe implantation. Electrodes were positioned to enter the brain surface ∼1 mm lateral to the probe and lowered at a 10° angle (see Fig. 1). The distance between the recording electrode at the surface of the brain and its final position near the center of the exposed length of the dialyzing membrane was estimated to be ∼4.6 mm. In within-subjects experiments, after isolating a stable cell and recording basal activity for at least 5 min, the effects of intracellular current injection were observed, and drugs were infused intrastriatally via reverse dialysis. Typical recordings lasted ∼20–30 min (range, 10–86 min). The conversion from aCSF to drug infusion during the microdialysis procedure was accomplished using a liquid switch (Carnegie Medicine/BAS, West Lafayette, IN). Once the drug was administered, basal activity and the effects of intracellular current injection were recorded in the presence of drug. It is estimated that the time elapsed between the switch from aCSF to drug and the beginning of drug infusion into the brain was ∼4 min (taking into account the dead space in the microdialysis inlet tubing). To ensure that drug was being delivered into the brain during a given recording period, the dialysis tubing dead space (8 μl) and perfusate flow rate (2 μl/min) were taken into account, and syringes containing drug were switched 4 min before the initiation of basal activity assessment. All drugs were soluble in aCSF. Effective doses of eticlopride and SCH 23390 were derived from previous in vitro receptor binding (Hall et al., 1985) and in vivomicrodialysis (Bean and Roth, 1991; Wolf and Chang-Jiang, 1998) studies. To offset factors such as the permeability of dialysis probe membrane to drug, perfusion flow rate, and drug diffusion in the brain, which are known to limit the amount of drug reaching the site of action (Benveniste and Hüttemeier, 1990), drug concentrations used in the current study were of necessity higher than the reported DA receptor affinity in striatal membrane preparations.
Histology. After experimentation, animals were deeply anesthetized and perfused transcardially with ice-cold saline followed by 4% paraformaldehyde in 0.1 m PBS. Brains were then removed and post-fixed in 4% paraformaldehyde/PBS for at least 1 week. After this period, brains were immersed in PBS/sucrose solution (25%) until saturated. The tissue was sectioned into 60 μm coronal slices, mounted, and stained with cresyl violet to enable histological determination of stimulating and recording electrode sites. In cases in which cells were injected with biocytin through the recording electrode, tissue sections were processed for biocytin immunoreactivity as described previously (Onn and Grace, 1999).
RESULTS
In the current within-subject studies, one cell was recorded per rat (n = 11). Additionally, in vivointracellular recordings were made from 39 striatal neurons recorded in 34 rats in the between-subjects studies. From the above groups, seven biocytin-stained neurons (14%) were recovered and localized to the dorsal striatum. Most of these neurons were estimated to lie within a distance of ∼500 μm from the microdialysis probe track (Fig.1B). In several cases biocytin-immunoreactive processes were observed to lie in close proximity to the microdialysis probe track (<25 μm).
Electrode and microdialysis probe placement
In cells responding to synaptic activation, all stimulating electrode tips implanted into the cortex were confirmed to lie in the PFC between ∼3.4 and 4.2 mm anterior to bregma, 0.5 and 2.0 mm lateral to the midline, and 2.5 and 4.7 mm ventral to the dural surface (Paxinos and Watson, 1986). All dialysis probe tips were confirmed to lie within the dorsal striatum between ∼0.3 mm posterior and 1.4 mm anterior to bregma, 2.0 and 4.8 mm lateral to the midline, and 4.5 and 7.7 mm ventral to the dural surface (Paxinos and Watson, 1986). In cases in which the recording electrode tracks could be identified, they were observed to lie within the striatal coordinates reported for the above probe tip placements in the vicinity of the dialysis probe track (<1.0 mm).
Within-subjects studies: effects of local dopamine antagonist infusions
To examine the influence of local tonic DA D1 and D2 receptor activation on the basal activity of striatal neurons in the intact system, recordings were made from the same neurons (n = 11) during aCSF infusion and after intrastriatal infusion of the DA receptor antagonists (5–20 min). Comparisons of time histograms of the membrane potential constructed from the same neuron recorded during separate periods of aCSF and SCH 23390 infusion revealed a decrease in the maximal depolarized membrane potential, which was observed as a leftward shift in the time spent at a given membrane potential (Fig.2A,B, Table1). During aCSF infusion, 50% (three of six) of striatal neurons were spontaneously active (Fig.2C). After SCH 23390 infusion, none of these neurons (zero of six) exhibited spontaneous action potential discharge, and the average amplitude of up events was significantly reduced (Fig.2A, Table 1). Moreover, there was a significant increase in the average minimal amplitude of intracellular current injection required to elicit action potential discharge (rheobase) in these same neurons (n = 6; p < 0.005; paired t test) in the absence of a significant effect on membrane potential before current injection (aCSF control = −70.4 mV; SCH 23390 = −79.8 mV; p > 0.05; pairedt test), demonstrating that the excitability of these neurons was reduced (Figs. 2C,3). As reported previously (Wilson and Kawaguchi, 1996), the input resistance measured with hyperpolarizing pulses was lower in the down state (23.8 ± 4.3 mΩ) than in the up state (36.4 ± 7.3 mΩ; p < 0.05). Local SCH 23390 infusion did not significantly alter the mean input resistance in the up or down states (Table 1) (p > 0.05) or the spike threshold (aCSF control = −43.4 ± 1.5 mV; SCH 23390 = −43.9 ± 1.5 mV; p > 0.05) of these neurons.
Fig. 2.
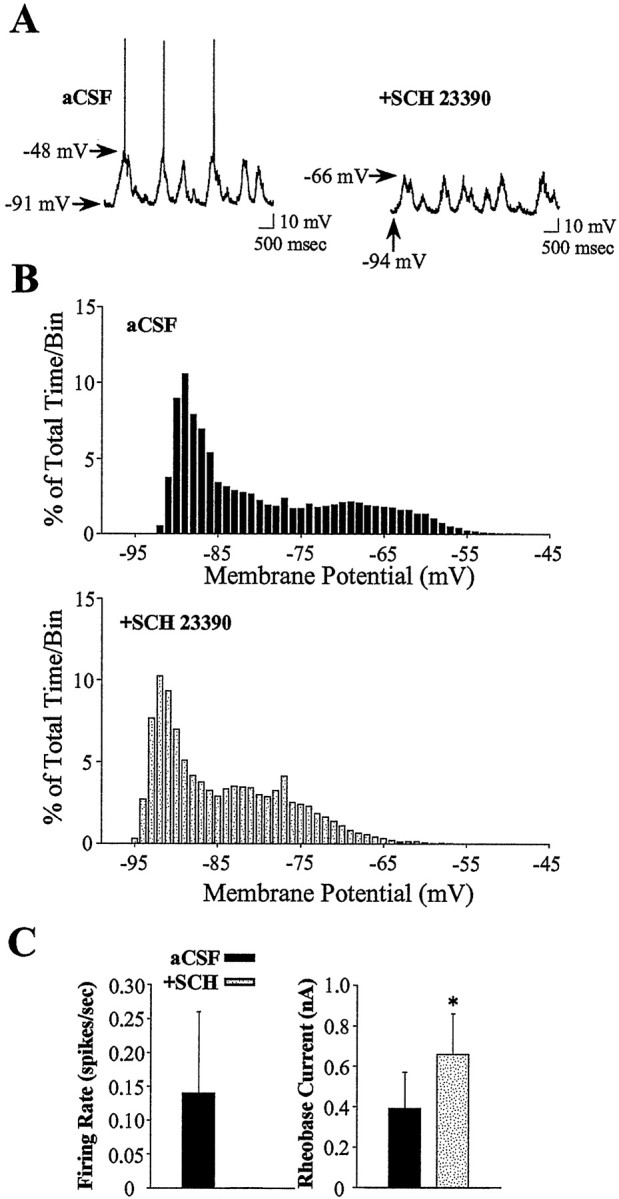
Intrastriatal SCH 23390 infusion attenuates the excitability of striatal neurons. A,Left, During aCSF (vehicle) infusion, this striatal neuron exhibits rapid spontaneous shifts in steady-state membrane potential and spontaneous spike discharge. Right, During local SCH 23390 infusion (10 μm, 10 min), this same cell exhibits a hyperpolarization of the membrane and cessation of action potential discharge. Arrows indicate the membrane potential at its maximal depolarized and hyperpolarized levels.B, Comparisons of time histograms of the membrane potential (1 mV bins) constructed from the same neuron recorded during separate periods of aCSF (top, black bars) and SCH 23390 (bottom, gray bars) infusion revealed a decrease in the maximal depolarized membrane potential and an overall hyperpolarizing shift in the time spent at a given membrane potential. C, The mean ± SEM firing rate and rheobase current were determined in striatal neurons recorded during intrastriatal aCSF and again after SCH 23390 (n = 6) infusion (5–20 min). Left, A cessation of action potential discharge was observed after local SCH infusion. Right, The average minimal current amplitude required to reach threshold (rheobase) was significantly increased after intrastriatal SCH 23390 infusion (*p < 0.005; paired t test). SCH 23390 infusion did not significantly affect the membrane potential recorded before current injection (aCSF control = −70.4 mV; SCH 23390 = −79.8 mV;p > 0.05, paired t test).
Table 1.
Effects of intrastriatal SCH 23390 infusion on the membrane properties of striatal neurons
| ACSF-control | + SCH 23390 | |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Maximal depolarized MP1-a | −55.3 ± 4.8 mV | −63.8 ± 3.1 mV* |
| (p < 0.05) | ||
| Maximal hyperpolarized MP | −89.7 ± 1.3 mV | −90.7 ± 1.5 mV |
| Up-state frequency | 0.94 ± 0.03 Hz | 1.01 ± 0.10 Hz |
| % Time in up state | 67.1 ± 3.1 | 65.2 ± 1.8 |
| Up-state amplitude | 21.7 ± 2.9 mV | 17.1 ± 2.0 mV* |
| (p < 0.05) | ||
| Up-state duration | 716.5 ± 48.5 msec | 668.6 ± 55.1 msec |
| Up-state MP mode | −72.5 ± 3.0 mV | −78.8 ± 2.1 mV |
| Up-state input resistance | 36.4 ± 7.3 mΩ | 28.4 ± 3.6 mΩ |
| % Time in down state | 33.0 ± 3.0 | 34.8 ± 1.8 |
| Down-state MP mode | −83.2 ± 2.6 mV | −84.7 ± 2.7 mV |
| Down-state input resistance | 23.8 ± 4.3 mΩ | 24.5 ± 3.0 mΩ |
Statistical significance was determined by comparing SCH 23390 values with pre-drug aCSF control values using a pairedt test. All values represent data averaged fromn = 6 neurons. Activity recorded during aCSF and SCH 23390 infusion was analyzed by comparing time interval plots of membrane potential activity fitted to a dual Gaussian distribution using Origin 6.1 (Microcal Corp.). The maximal (most depolarized) and the minimal (most hyperpolarized) membrane potential within the distribution, the up- and down-state modes (the membrane potential at which the neuron spends the most time in each state), and the area under both modal distributions (time spent in each state) were determined. The amplitude, duration, and frequency of up events and the input resistance of the membrane in up and down states were determined as described in Materials and Methods. MP, Membrane potential.
This calculation does not include membrane potential fluctuations contributed by action potentials.
Fig. 3.
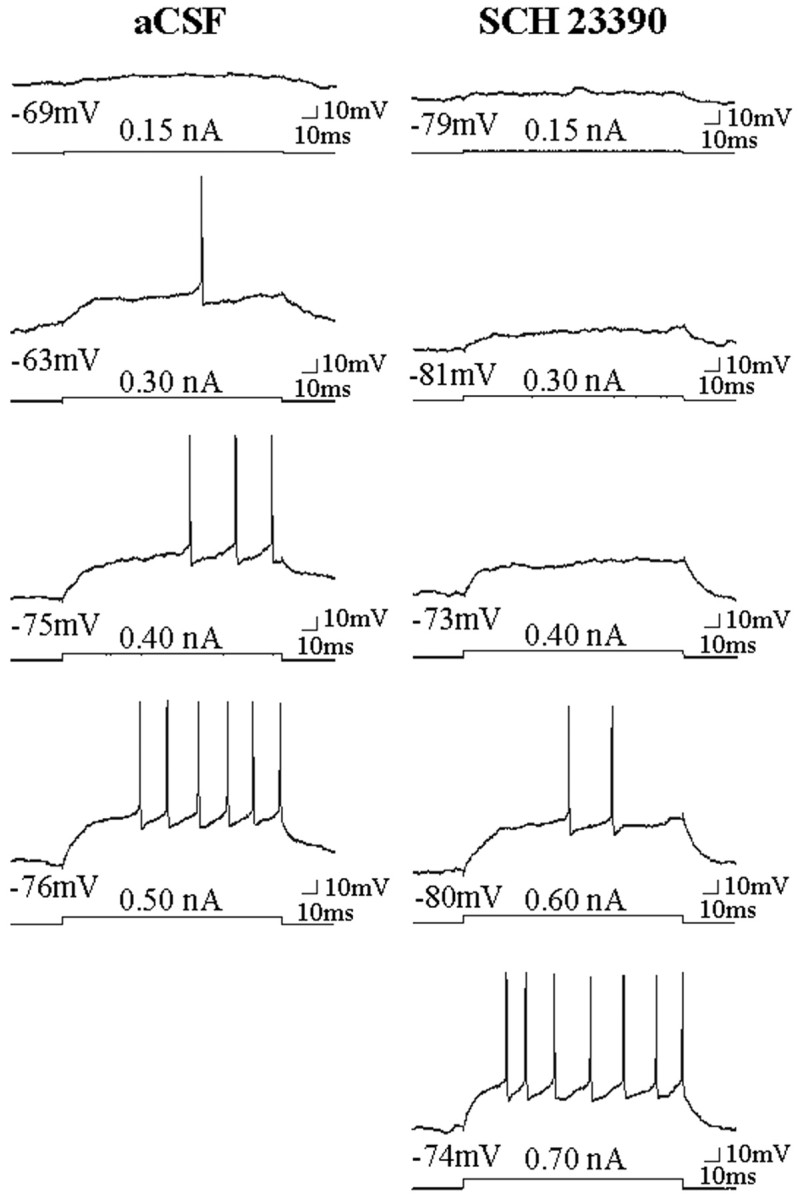
Intrastriatal SCH 23390 infusion decreases the responsiveness of single striatal neurons to intracellular current injection. Left column, Response of a single cell to increasing amplitudes of intracellular current injected during aCSF infusion. Right column, Response of the same cell to depolarizing current pulses injected during SCH 23390 infusion (∼6–8 min). Note that after SCH 23390 (10 μm) infusion, the responsiveness of this cell to intracellular current injection was decreased even when the membrane potential before the current pulse was more depolarized than control conditions (third trace, 0.4 nA). Bottom traces indicate current injection steps.Top traces indicate the voltage response. The membrane potential before current injection is indicated below the voltage trace. The current amplitude is indicated above the current step.
Intrastriatal infusion of the D2 receptor antagonist eticlopride (20 μm) induced a robust depolarization of the membrane potential of single striatal neurons and caused some cells to fire multiple action potentials (Figs.4A,5). Comparisons of membrane potential distributions plotted as time histograms from individual cells recorded during separate periods of aCSF and eticlopride infusion revealed an overall depolarizing shift in the maximal hyperpolarized membrane potential, revealed as a rightward shift in the time spent at a given membrane potential (Fig. 4B, Table2). During aCSF infusion, two of five striatal neurons were spontaneously active. After eticlopride infusion (5–20 min), the firing rate of two of five neurons was potently increased (Figs. 4C, 5). Additionally, the mean up and down state membrane potential modes were significantly more depolarized after eticlopride infusion (Table 2) (*p < 0.05; paired t test). Analysis of the effects of eticlopride on the responsiveness of these neurons to intracellular current injection was not performed because most of the neurons in this group were not held long enough to enable these tests to be carried out.
Fig. 4.
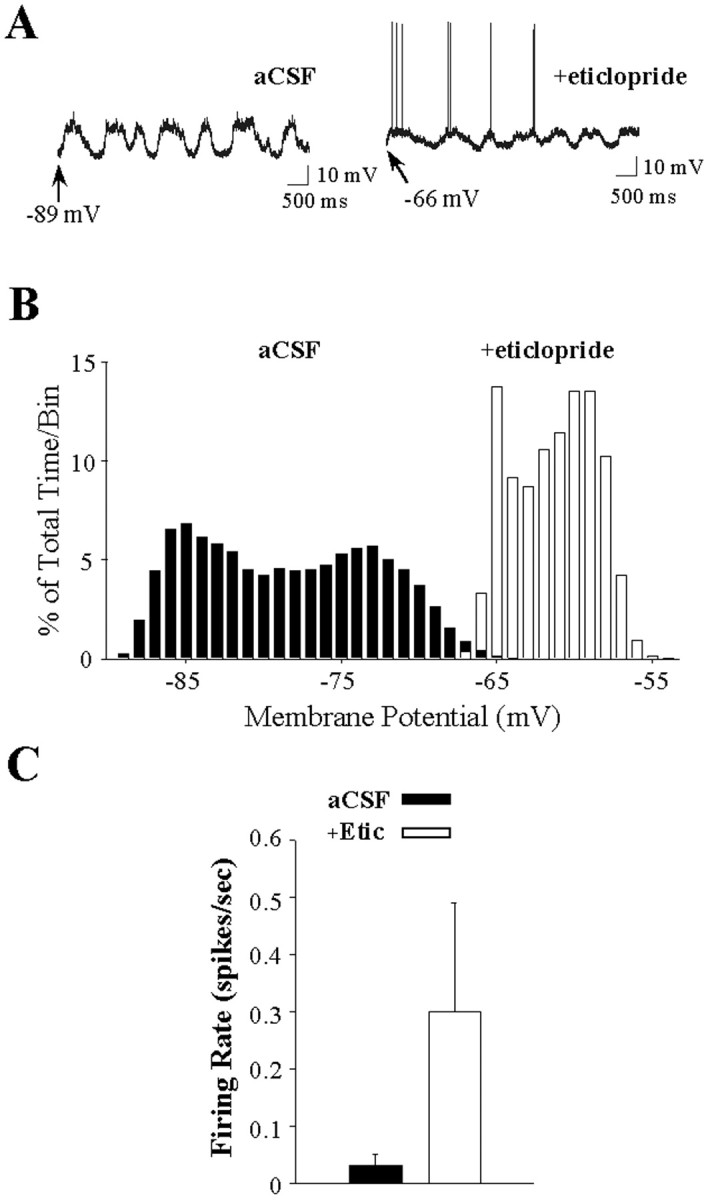
Intrastriatal eticlopride infusion increases the excitability of striatal neurons. A,Left, During aCSF (vehicle) infusion, this striatal neuron exhibits rapid spontaneous shifts in steady-state membrane potential but does not exhibit spontaneous spike discharge.Right, During local eticlopride infusion (20 μm, 4.5–5.5 min), the membrane potential of this same cell is depolarized, and the cell fires action potentials. Arrows indicate the membrane potential at its most depolarized and hyperpolarized levels. B, Comparisons of time histograms of the membrane potential constructed from the same neuron recorded during separate periods of aCSF (black bars) and eticlopride (white bars) infusion reveal a decrease in the maximal hyperpolarized membrane potential (1 mV bins), demonstrated by a rightward shift in the time spent at a given membrane potential.C, An increase in the mean ± SEM firing rate was observed in some neurons (2 of 5) recorded during local eticlopride infusion.
Fig. 5.
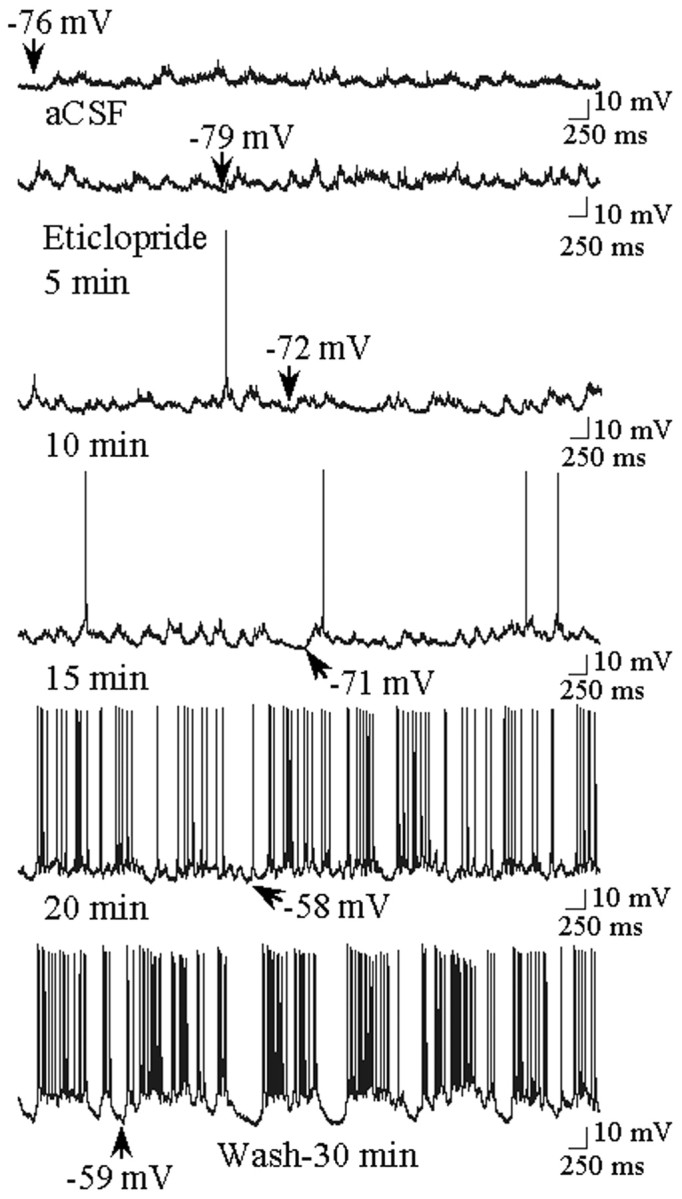
Time course of the excitatory effects of eticlopride on neuronal activity of a single striatal cell. During aCSF infusion the neuron is primarily hyperpolarized and is not firing action potentials (top trace). Approximately 5–10 min after eticlopride (20 μm) infusion, the neuron depolarizes and begins to fire action potentials. The neuron is robustly activated after 20 min of eticlopride infusion and remains activated 30 min after the discontinuation of eticlopride infusion (aCSF wash, bottom trace).
Table 2.
Effects of intrastriatal eticlopride infusion on the membrane properties of striatal neurons
| ACSF-control | + Eticlopride | |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Maximal depolarized MP2-a | −63.4 ± 4.4 mV | −55.4 ± 6.8 mV |
| Maximal hyperpolarized MP | −88.8 ± 3.0 mV | −74.8 ± 4.1 mV* |
| (p < 0.05) | ||
| Up-state frequency | 0.80 ± 0.06 Hz | 0.91 ± 0.18 Hz |
| % Time in up state | 65.6 ± 4.6 | 67.5 ± 2.4 |
| Up-state amplitude | 16.9 ± 3.1 mV | 11.6 ± 1.2 mV |
| Up-state duration | 833.7 ± 71.4 msec | 822.1 ± 105.9 msec |
| Up-state MP mode | −78.4 ± 2.8 mV | −67.1 ± 4.6 mV* |
| (p< 0.05) | ||
| % Time in down state | 34.4 ± 4.6 | 32.5 ± 2.4 |
| Down-state MP mode | −85.5 ± 3.2 mV | −71.2 ± 4.4 mV* |
| (p < 0.05) |
Statistical significance was determined by comparing eticlopride values with pre-drug aCSF control values using a pairedt test. All values represent data averaged fromn = 5 neurons. Activity recorded during aCSF and eticlopride infusion was analyzed by comparing time interval plots of membrane potential activity fitted to a dual Gaussian distribution using Origin 6.1 (Microcal Corp.). The maximal (most depolarized) and the minimal (most hyperpolarized) membrane potential within the distribution, the up- and down-state modes (the membrane potential at which the neuron spends the most time in each state), and the area under both modal distributions (time spent in each state) were determined. The amplitude, duration, and frequency of up events were determined as described in Materials and Methods. MP, Membrane potential.
This calculation does not include membrane potential fluctuations contributed by action potentials.
Between-subjects studies
To control for potential recording time effects on membrane activity, increase the likelihood of achieving a steady-state drug concentration at the recording site, and allow for a more thorough examination of the effects of DA antagonists on activity evoked by intracellular current injection and synaptic activation in a larger population of neurons, additional studies were performed using a between-subjects design, and comparisons between control and drug treatment groups were made across cells. Striatal neurons in both control and drug groups often exhibited spontaneous shifts in membrane potential from a hyperpolarized state to a depolarized plateau (Fig.6). Additionally, time histograms of the membrane potential of individual neurons plotted over a 30 sec baseline period revealed that the majority of cells from control and both drug groups exhibited bimodal distributions in membrane potential (Fig. 6,insets), which is a characteristic of neurons exhibiting a bistable pattern of activity. Comparisons of basal activity and up and down state characteristics were not performed in between-subjects groups because of the considerable variability in membrane properties observed across cells and the lack of statistical power associated with between-cell comparisons.
Fig. 6.
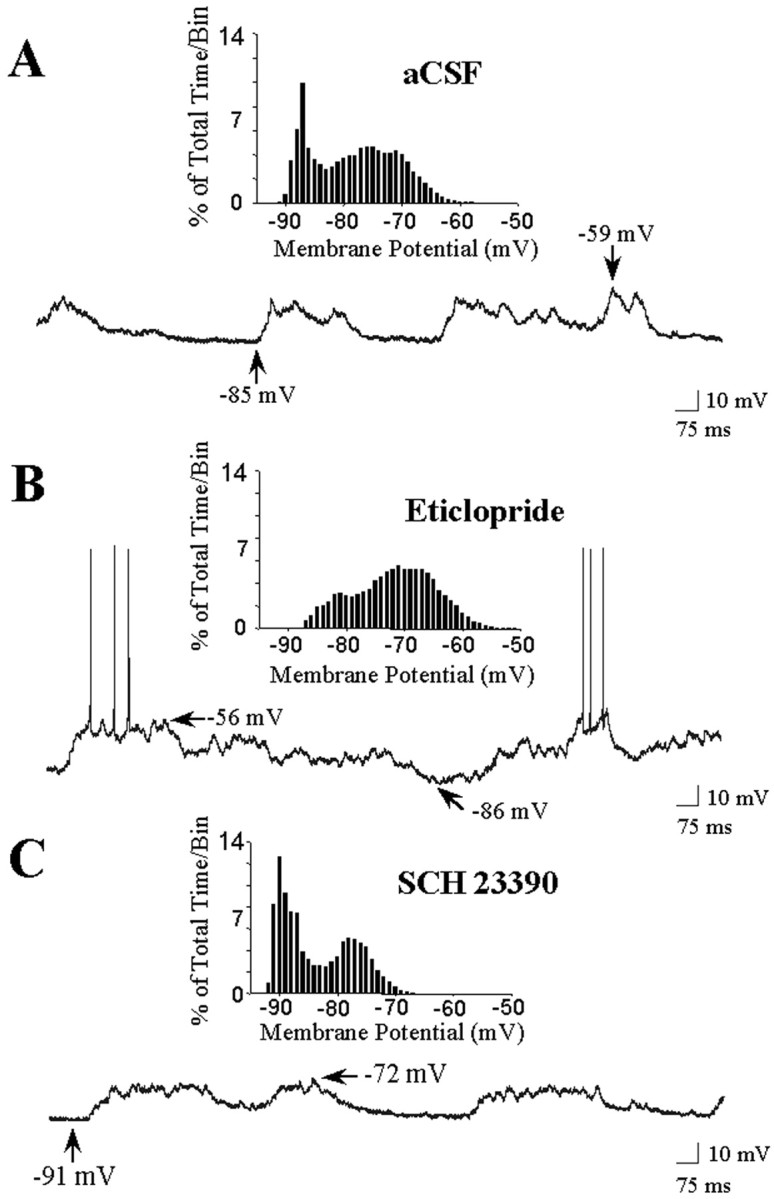
Effects of local D1 and D2antagonist infusion on spontaneous activity recorded across cells. Striatal neurons were recorded after the initiation (10–90 min) of intrastriatal aCSF, eticlopride, or SCH 23390 infusion.A, During aCSF (vehicle) infusion, this striatal neuron exhibits rapid spontaneous shifts in steady-state membrane potential but does not exhibit spontaneous spike discharge. B, During local eticlopride infusion (20 μm, 10–90 min), the majority of neurons exhibited up- and down-state activity, and 40% of the cells fired action potentials. C, During intrastriatal SCH 23390 infusion (10 μm, 10–90 min), the majority of neurons exhibited up- and down-state activity; however, a significant leftward shift in the maximal depolarized membrane potential was observed (seeinset). Insets show representative time histograms of the membrane potential of the same cells plotted over a 30 sec baseline period. The majority of neurons from all groups exhibited bimodal distributions in membrane potential characteristic of striatal projection cells. Arrows indicate the membrane potential at its maximal depolarized and hyperpolarized levels.
Between-subjects studies: intracellular current injection
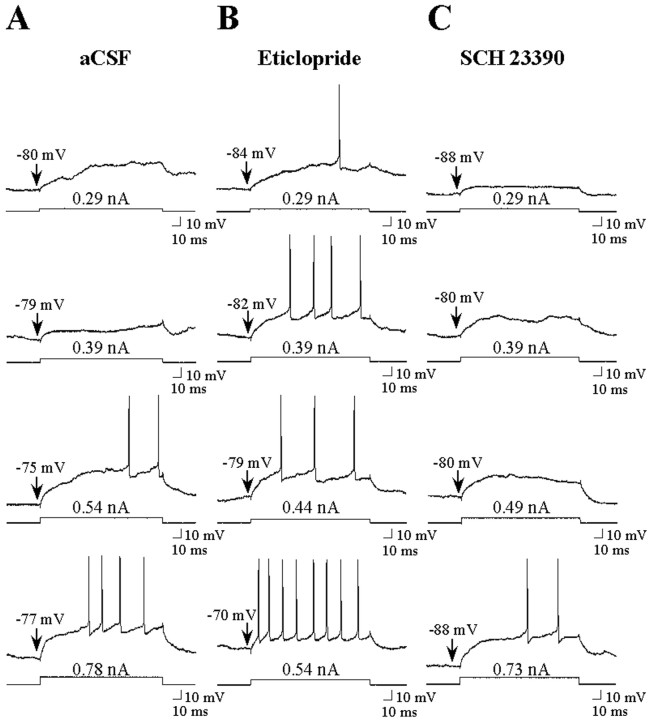
Similar to the within-subjects studies, the input resistance measured with hyperpolarizing pulses was lower in the down state than in the up state in all groups and was unaffected by drug infusion (data not shown). Action potentials could be evoked by depolarizing the membrane via intracellular current injection into neurons from both aCSF control and drug groups (Fig. 7). In cells from all groups, a gradual depolarization of the membrane preceded the action potential evoked by positive current injection, and a prominent afterhyperpolarization after the spike was typically observed (Fig. 7). No significant differences in the characteristics of spikes evoked by rheobase current (minimal amplitude of intracellular current injection required to elicit action potential discharge) were observed in neurons recorded in aCSF control, SCH 23390, and eticlopride groups (data not shown). Consistent with within-subjects studies, neurons recorded during SCH 23390 infusion exhibited an increase in the current amplitude required to reach threshold (Figs.7C, 8A) (Q = 1.7;#p < 0.05; ANOVA with Dunn's test) in the absence of a significant drug effect on the membrane potential recorded before current injection (p > 0.05; aCSF control = −77.4 ± 2.6 mV; SCH 23390 = −79.3 ± 2.9 mV; p > 0.05; ANOVA). Additionally, a decrease in the maximal depolarized membrane potential (leftward shift) was observed in neurons recorded during SCH 23390 infusion (aCSF maximal membrane potential = −57.1 ± 2.0 mV; SCH 23390 maximal membrane potential = −66.4 ± 2.8 mV; F = 5.24; p < 0.05; ANOVA with Dunnett's test).
Fig. 7.
Intrastriatal dopamine antagonist infusion alters the responsiveness of single striatal neurons to intracellular current injection. A, Typical response of a single cell to gradually increasing amplitudes of intracellular current injected during aCSF infusion. B, Typical response of a cell to similar depolarizing current pulses injected during eticlopride (20 μm) infusion (∼10–90 min). Note that after eticlopride infusion, the responsiveness of this cell to intracellular current injection was increased relative to across-cell aCSF controls. C, Typical response of a cell to similar depolarizing current pulses injected during SCH 23390 (10 μm) infusion (∼10–90 min). Note that after SCH 23390 infusion, the responsiveness of this cell to intracellular current injection was decreased relative to across-cell aCSF controls.Bottom traces indicate current injection steps.Top traces indicate the voltage response. The membrane potential before current injection is indicated above the voltage trace. The current amplitude is indicated above the current step.
Fig. 8.
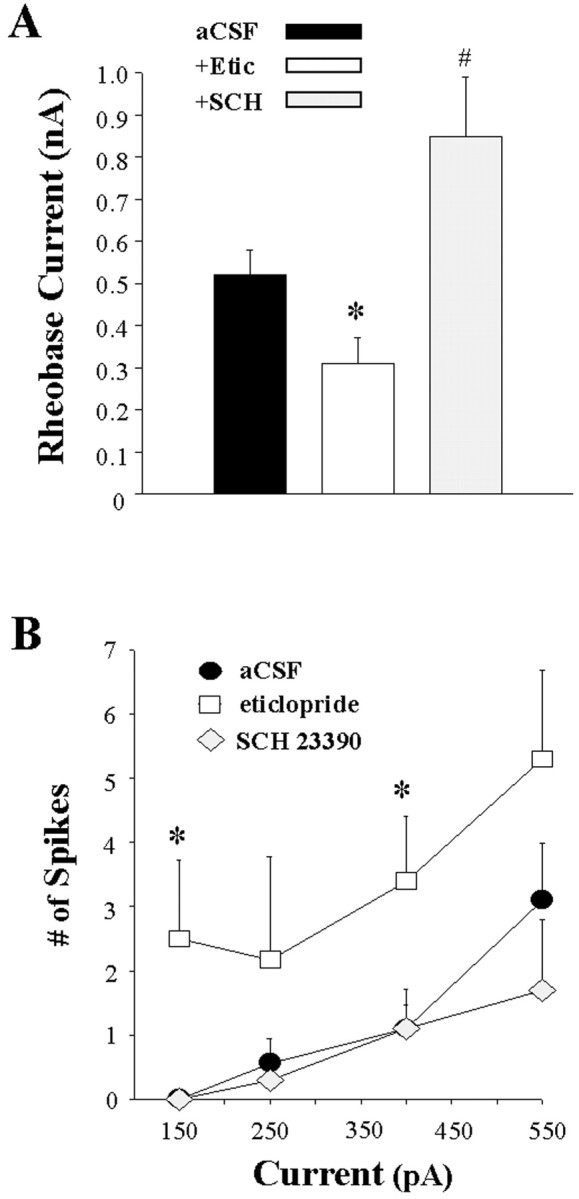
Opposite effects of local D1 and D2 antagonist infusion on activity evoked by intracellular injection of depolarizing current. The mean ± SEM rheobase current and number of spikes evoked by current steps of increasing intensity were determined in separate populations of striatal neurons recorded during intrastriatal aCSF, eticlopride (20 μm), or SCH 23390 (10 μm) infusion (5–90 min). Comparisons of the above measures of neuronal activity were made among the aCSF control (n = 19 cells), eticlopride (n = 10 cells), and SCH 23390 (n = 10) groups using a one-way ANOVA.A, Eticlopride infusion induced a decrease in the minimal current amplitude required to reach threshold (*p < 0.05; ANOVA, Dunn's test), whereas SCH 23390 was observed to increase the rheobase current (#p < 0.05; ANOVA, Dunn's test).B, An overall increase in the number of spikes elicited for a given current intensity was observed after eticlopride infusion (*p < 0.05; ANOVA, Dunnett's test), whereas SCH 23390 was without effect (p > 0.05).
In neurons recorded during eticlopride infusion, there was a decrease in the mean amplitude of intracellular current injection required to elicit an action potential (Figs. 7B, 8A) (Q = 2.0; *p < 0.05; ANOVA with Dunn's test). Additionally, the membrane potential of striatal neurons recorded before intracellular current injection was significantly depolarized during eticlopride infusion (aCSF control = −77.4 ± 2.6 mV; eticlopride = −67.5 ± 3.4 mV;F = 3.77; p < 0.05; ANOVA with Dunnett's test). The number of spikes evoked by single pulses of suprathreshold levels of current was also significantly greater in neurons recorded during local eticlopride infusion (Fig.8B) (F = 7.7; *p < 0.05; ANOVA with Dunnett's test).
Between-subjects studies: prefrontal cortex stimulation
In a subpopulation of striatal neurons recorded in control (n = 8), eticlopride (n = 6), and SCH 23390 (n = 5) groups (across cells), EPSPs and occasionally spikes could be evoked by single pulses of electrical stimuli delivered to the orbital PFC (Fig. 9). To compare the effects of PFC stimulation on cells from control and drug groups, a series of single pulses of electrical stimuli (200 μsec, 0.2 Hz) were delivered at gradually increasing stimulus intensities (0.2–3.0 mA). At higher stimulus intensities (1.0–3.0 mA), EPSPs exhibiting rapid onset latencies (∼3–5 msec) were observed that typically reached maximal amplitude and did not increase further when higher intensity pulses were delivered. For all cells, responsiveness to PFC stimulation was assessed by analyzing the onset latency, duration, and amplitude of the EPSP evoked at the lowest stimulus intensity required to produce a response of maximal amplitude. EPSP amplitudes were measured from the beginning of the rising phase to the peak of the depolarization. EPSP duration was measured from the beginning of the rising phase to the point where the falling phase returned to the initial baseline membrane potential. Analyses of EPSP characteristics revealed no significant differences in the maximal EPSP evoked by electrical stimulation of the PFC between the aCSF control and SCH 23390 groups (p > 0.05; ANOVA). However, a marked increase in the maximal EPSP amplitude was observed in cells recorded during local eticlopride infusion as compared with aCSF controls (Fig. 9A,B) (F = 3.63; p < 0.05; ANOVA with Dunnett's test). There were no significant differences in the average membrane potential before electrical stimulation in control (-89.1 ± 2.3 mV) and eticlopride (-86.3 ± 2.8 mV) groups (p > 0.05; t test). Moreover, the mean ± SEM EPSP onset latency (control = 5.6 ± 0.8 msec; eticlopride = 4.5 ± 1.1 msec; SCH 23390 = 4.4 ± 0.3 msec), EPSP duration (control = 52.3 ± 3.0 msec; eticlopride = 58.7 ± 3.4 msec; SCH 23390 = 47.4 ± 4.6 msec), and current intensity required to evoke an EPSP of maximal amplitude (control = 2.2 ± 0.23 mA; eticlopride = 2.3 ± 0.37 mA; SCH 23390 = 2.4 ± 0.29 mA) were not significantly different in cells recorded from control, eticlopride, and SCH 23390 groups (p > 0.05; ANOVA).
Fig. 9.
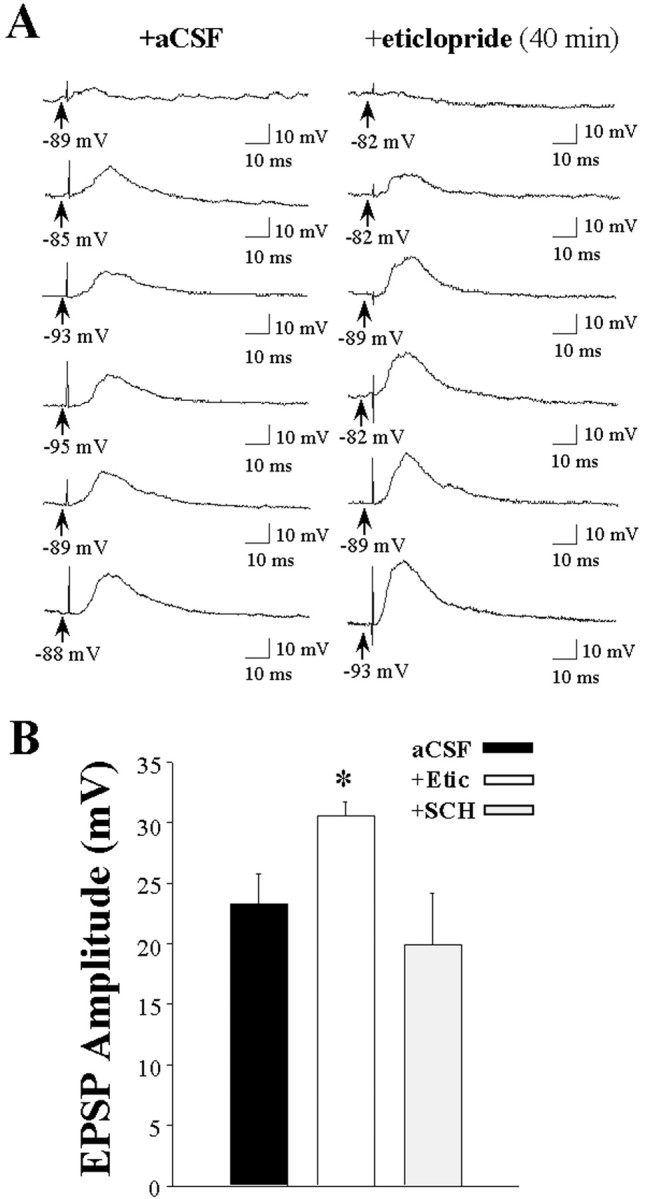
Intrastriatal eticlopride infusion increases the responsiveness of striatal neurons to electrical stimulation of the prefrontal cortex. A series of single pulses (0.2 Hz) of electrical stimuli were delivered at increasing stimulus intensities (0.2–3.0 mA) to striatal neurons recorded during intrastriatal aCSF, eticlopride (20 μm), or SCH 23390 (10 μm) infusion. A, Representative traces of EPSPs evoked by an increasing series of stimulus intensities in separate single striatal neurons during aCSF (left) and eticlopride (right) infusion. B, Comparisons of mean maximal EPSP amplitudes in control (23.3 ± 2.5 mV), eticlopride (30.6 ± 1.1 mV), and SCH 23390 (19.9 ± 4.3) groups revealed that the response evoked in striatal neurons after electrical stimulation of the PFC is increased during intrastriatal eticlopride (*p < 0.05; ANOVA, Dunnett's test) but not SCH 23390 (p > 0.05) infusion.
DISCUSSION
The results of this study indicate that in the intact system where both the natural neuronal activity states and ongoing DAergic transmission are preserved, endogenous DA modulates the membrane activity of striatal spiny neurons differentially via local DA D1 and D2 receptor activation. In particular, tonic D1 receptor activation increases membrane excitability, whereas tonic D2 receptor activation decreases the excitability of striatal neurons in vivo. Moreover, the facilitatory and inhibitory influences of local D1 and D2 receptor activation, respectively, are exerted at both up- and down-state membrane potentials.
Technical considerations
Recordings were performed from striatal neurons that, on the basis of the termination of the recording electrode tracks and in some cases the location of the soma of biocytin-labeled neurons, were all estimated to lie within 500 μm of the microdialysis probe. The responsiveness of striatal neurons to local DA antagonist infusions also demonstrated that the soma or dendritic field of the recorded neuron came into contact with the infused drug. It is unlikely that the microdialysis procedure itself altered the activity of neurons recorded in this study, because we have observed previously that the effects of local microdialysis on ongoing synaptic activity, passive membrane properties, and evoked activity of striatal neurons are negligible (Moore et al., 2000). Additionally, the finding that DA antagonist administration elicited potent effects on striatal neuron activity argues against the proposition that the microdialysis procedure substantially depletes the extracellular pool of DA within the vicinity of the probe.
Overall, neurons recorded proximal to the microdialysis probe exhibited electrophysiological characteristics similar to those reported by other laboratories (Calabresi et al., 1990; Wilson, 1993; Wickens and Wilson, 1998; Mahon et al., 2000), except that they generally exhibited more hyperpolarized up and down states (Wilson and Kawaguchi, 1996; Stern et al., 1997; Wickens and Wilson, 1998; Reynolds and Wickens, 2000). Although part of this may have been caused by the partial destruction of excitatory inputs after probe implantation, the fact that (1) the modal membrane potential in both the up and down states was relatively hyperpolarized and (2) previous studies have shown that the down-state membrane potential is determined by an inwardly rectifying potassium current and not synaptic inputs (Wilson and Kawaguchi, 1996) make this explanation unlikely. It is more likely that methodological differences, such as pipette electrolyte concentrations or the type of anesthesia [because different anesthetics have variable effects on bistable activity patterns in corticostriatal and striatal output neurons in vivo (Mahon et al., 2001)], contributed to the observed differences between studies.
Influence of local D1 receptor antagonism on spontaneous activity and intracellular current-elicited firing frequency
Tonic stimulation of the D1 receptorin vivo appears to exert a primary facilitatory effect on spontaneous activity when the cell is in the up state. Thus, in both within- and between-subjects studies, intrastriatal infusion of the D1 antagonist SCH 23390 potently inhibited striatal neuron activity by causing a decrease in the maximal depolarized membrane potential. Additionally, in both within- and between-subjects studies, SCH 23390 infusion potently inhibited depolarization-evoked activity when the cell was in the down state. Local SCH 23390 infusion also decreased the amplitude of up events in within-subjects studies.
Consistent with our findings, recent studies using extracellular recordings have shown that electrical stimulation of the medial forebrain bundle enhances the activity of a subpopulation of striatal neurons, and this is blocked by SCH 23390 (Gonon, 1997). Additional studies using iontophoretic techniques in anesthetized (Hu and Wang, 1988; Hu and White, 1997) and freely moving (Kiyatkin and Rebec, 1996) rats have shown that DA applied at low ejection currents will facilitate the excitatory effects of glutamate on striatal neurons via a D1 receptor-dependent mechanism. DA D1 receptor activation has also been shown to enhance L-type Ca2+ currents and evoked spike discharge (Surmeier et al., 1995; Hernández-López et al., 1997) and NMDA receptor-mediated responses (Cepeda and Levine, 1998; Cepeda et al., 1998) and reduce GABAAreceptor currents in striatal neurons (Flores-Hernandez et al., 2000). Blockade of these D1 receptor-dependent effects would be expected to decrease the excitability of striatal neurons.
In contrast, multiple studies in vitro report inhibitory effects of D1 receptor activation on striatal neurons (for review, see Calabresi et al., 1987, 2000). Studies using voltage-clamp techniques also show that D1receptor activation decreases sodium currents and increases anomalous rectifier K+ currents in striatal spiny neurons (Surmeier and Kitai, 1993). These actions of D1 receptor signaling are believed to suppress evoked activity when the neuron is in the down state (Nicola et al., 2000). However, our results show that the D1antagonist SCH 23390 decreased the responsiveness of striatal neurons to depolarizing current, which was usually delivered at membrane potentials residing near the down state (e.g., −80 mV). These findings are not necessarily in conflict with the above in vitrostudies because, in the current in vivo studies, the antagonist perfusion is likely to have had direct effects on the recorded neuron as well as indirect affects on the surrounding network of GABAergic, nitrergic, and cholinergic interneurons believed to be potently modulated by DA (Calabresi et al. 2000). Because these interneurons receive considerably more active DAergic input in vivo, they are likely to play a significant role in modulating the activity of striatal projection cells in the intact animal. Thus, a disruption of D1 receptor signaling on the network level is likely to result in complex direct and indirect effects on the recorded neuron in vivo, which may not be relevant to in vitro preparations.
Influence of local D2 receptor antagonism on spontaneous and evoked activity
In contrast to the evidence for D1receptor-induced excitation of striatal neurons, tonic D2 receptor stimulation appears to exert the opposite effects. Thus, in within-subjects studies, reverse dialysis of the D2 antagonist eticlopride induced a rightward depolarizing shift in the maximal hyperpolarized membrane potential and up- and down-state membrane potential modes of striatal cells exhibiting bistable activity patterns. In between-subjects studies, eticlopride infusion decreased the amplitude of intracellular current injection required to elicit an action potential and increased the number of spikes evoked by suprathreshold levels of current injection delivered when the cell was in the down state.
In agreement with our findings, the majority of studies in vivo and in vitro have shown that D2 agonists generally induce a decrease in the excitability of striatal spiny neurons (for review, see Onn et al., 2000), although the physiological consequences of D2 receptor activation may not be consistent across all striatal neuron types (for review, see Nicola et al., 2000). It is possible that the depolarizing effects of eticlopride observed within both membrane potential states in the current within-subjects studies may be a consequence of the blockade of D2 receptor-mediated activation of a depolarization-activated K+ channel (Kitai and Surmeier, 1993). Pharmacological antagonism of tonic D2 receptor activation may also result in decreased suppression of Na+ channels in some neurons, which could act to increase the magnitude of spontaneous membrane depolarizations. A recent study has also demonstrated that D2 receptor activation decreases the activity of L-type Ca2+ channels and suppresses evoked activity in striatal spiny neurons (Hernández-López et al., 2000). Thus, although D2-like receptors can modulate membrane conductances of striatal neurons via a diverse array of signaling mechanisms, the primary effect of D2receptor stimulation in vivo is an inhibition of membrane excitability at both depolarized and hyperpolarized membrane potential states.
DA D2 receptors also appear to exert a tonic inhibitory influence over corticostriatal glutamatergic afferents. Thus, in a subpopulation of neurons that responded to electrical stimulation of the orbital PFC, a significant increase in the maximal EPSP amplitude was observed during local eticlopride infusion as compared with aCSF controls. This is the first report demonstrating that removal of tonic D2 receptor activation augments PFC stimulation-evoked EPSPs in striatal neurons recordedin vivo. These observations are consistent with previous studies using striatal brain slices demonstrating that bath-applied DA or D2 agonists decreased the amplitude of EPSPs evoked by electrical stimulation of corticostriatal pathways (O'Donnell and Grace, 1994; Hsu et al., 1995; Levine et al., 1996) and EPSCs evoked by local stimulation (Umemiya and Raymond, 1997). In the accumbens this D2 receptor-mediated suppression appears to also be tonic in nature, because D2antagonist administration will increase cortico-accumbens evoked responses (O'Donnell and Grace, 1994). Although it is currently not clear whether the regulatory action of DA on EPSPs in striatal neurons is occurring via a presynaptic or postsynaptic mechanism [however, seeGrace (2002)], the current study indicates that DA exerts a powerful tonic inhibitory influence over frontal-cortical afferent-evoked responses in the intact animal.
Dopamine receptor antagonism and direct and indirect striatal output pathways
Gene regulation studies have shown that DA differentially affects striatal projection neurons comprising the direct and indirect pathways because of their differential expression of D1 or D2 receptors, respectively (for review, seeGerfen, 2000). This issue of DA receptor segregation was not addressed in the current study, primarily because of difficulties related to the time required for effective drug wash out and/or recovery from long-term effects of the high-affinity antagonist infusion. However, in the studies in which the activity of the same neuron was monitored before and after drug infusion, all 11 neurons responded to DA antagonist infusion. Unfortunately, it is not possible to determine whether these responses were mediated via direct effects of drug on the recorded neuron or via circuit interactions using the current techniques.
Functional implications
Tonic DA D1 and D2receptor activation was found to exert potent effects on the synaptic efficacy and the excitability of striatal neurons. In this respect, DA appears to act as a gate, in which the integration of information arising from frontal and motor cortex inputs is dependent on the current activity state of the corticostriatal system. Thus, the tonic D2-mediated inhibition of synaptic efficacy may be important in suppressing striatal output when the PFC is relatively inactive and the animal is not engaged in goal-related behavior. Conversely, during a state of behavioral activation the PFC together with converging excitatory drive from the motor cortices can overcome the inhibitory D2 effects and drive the neuron into the up state, at which point D1 receptor activation is capable of depolarizing the membrane further and facilitating spike discharge. In agreement with this hypothesis, long-term depression of corticostriatal inputs is reversed by concurrent stimulation of the substantia nigra in controls but not in DA-depleted animals (Reynolds and Wickens, 2000). Interestingly, these DA-depleted animals also exhibit a decrease in the maximal depolarized membrane potential and a depression of up-state amplitude (Reynolds and Wickens, 2000), similar to that observed in the current study after local infusion of the D1 receptor antagonist. Thus, by controlling the excitability of striatal neurons via distinct effects on membrane activity and afferent drive, the DA system is positioned to exert a true modulatory influence over information processing within this highly integrative brain region.
Footnotes
This work was supported by United States Public Health Service Grants MH 45156, 57440 (A.A.G.), NS 10725, Tourette Syndrome Association, and National Alliance for Research on Schizophrenia and Depression (A.R.W.). We thank Nicole MacMurdo and Christy Wyant for their excellent technical assistance, Aline Pinto for help with the photomontage, and Brian Lowry for the development of software (Neuroscope) used in data acquisition and analysis. We also thank Drs. Stan B. Floresco and Holly Moore for their valuable comments and suggestions regarding this manuscript.
Correspondence should be addressed to Dr. Anthony R. West, Department of Neuroscience, 446 Crawford Hall, University of Pittsburgh, Pittsburgh, PA 15260. E-mail:West@brain.bns.pitt.edu.
REFERENCES
- 1.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bean AJ, Roth RH. Extracellular dopamine and neurotensin in rat prefrontal cortex in vivo: effects of median forebrain bundle stimulation frequency, stimulation pattern, and dopamine autoreceptors. J Neurosci. 1991;11:2694–2702. doi: 10.1523/JNEUROSCI.11-09-02694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste H, Hüttemeier PC. Microdialysis—theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Mercuri NB, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 5.Calabresi P, Mercuri NB, Stefani A, Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons I. An in vivo analysis. J Neurophysiol. 1990;63:651–652. doi: 10.1152/jn.1990.63.4.651. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, Bernardi G. Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol. 2000;61:231–265. doi: 10.1016/s0301-0082(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 7.Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- 8.Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- 9.Deniau JM, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73:761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- 10.Flores-Hernandez J, Hernandez S, Snyder G, Yan Z, Fienberg AA, Moss S, Greengard P, Surmeier DJ. D1 receptor activation reduces GABAA receptor currents in neostriatal neurons through a PKA/DARPP-32/PPI signaling cascade. J Neurophysiol. 2000;83:2996–3004. doi: 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- 11.Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:s64–s70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- 12.Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grace AA. Dopamine. Psychopharmacology, the fifth generation of progress Bloom FE, Kupfer DJ. 2002. Raven; New York, in press. [Google Scholar]
- 14.Hall H, Kohler C, Gawell L. Some in vitro binding properties of 3[H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. Eur J Pharmacol. 1985;111:191–199. doi: 10.1016/0014-2999(85)90756-3. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández-López S, Tkatch T, Perez-Garcia E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLCβ1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu K-S, Huang C-C, Yang C-H, Gean P-W. Presynaptic D2 dopaminergic receptors mediate inhibition of excitatory synaptic transmission in rat neostriatum. Brain Res. 1995;690:264–268. doi: 10.1016/0006-8993(95)00734-8. [DOI] [PubMed] [Google Scholar]
- 18.Hu XT, Wang RY. Comparison of effects of D1 and D2 receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. J Neurosci. 1988;8:4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu XT, White FJ. Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neurosci Lett. 1997;224:61–65. doi: 10.1016/s0304-3940(97)13443-7. [DOI] [PubMed] [Google Scholar]
- 20.Kitai ST, Surmeier DJ. Cholinergic and dopaminergic modulation of potassium conductances in neostriatal neurons. Adv Neurol. 1993;60:40–52. [PubMed] [Google Scholar]
- 21.Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- 22.Levine MS, Li Z, Cepeda C, Cromwell HC, Altemus K. Neuromodulatory actions of dopamine on synaptically-evoked neostriatal responses in slices. Synapse. 1996;24:65–78. doi: 10.1002/syn.890240102. [DOI] [PubMed] [Google Scholar]
- 23.Mahon S, Delord B, Deniau J-M, Charpier S. Intrinsic properties of rat striatal output neurones and time-dependent facilitation of cortical inputs in vivo. J Physiol (Lond) 2000;527.2:345–353. doi: 10.1111/j.1469-7793.2000.t01-1-00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahon S, Deniau J-M, Charpier S. Relationship between EEG potentials and intracellular activity of striatal and cortico-striatal neurons: an in vivo study under different anesthetics. Cereb Cortex. 2001;11:360–373. doi: 10.1093/cercor/11.4.360. [DOI] [PubMed] [Google Scholar]
- 25.Moore H, West AR, Grace AA. Effects of continuous microdialysis on the electrophysiological properties of rat cortical and striatal neurons recorded proximal to the probe. Soc Neurosci Abstr. 2000;26:1440. [Google Scholar]
- 26.Nakamura S, Iwatsubo K, Tsai CT, Iwama K. Cortically induced inhibition of neurons of rat substantia nigra (pars compacta). Jpn J Physiol. 1979;29:353–357. doi: 10.2170/jjphysiol.29.353. [DOI] [PubMed] [Google Scholar]
- 27.Nicola S, Surmeier DJ, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onn S-P, Grace AA. Alterations in electrophysiological activity and dye coupling of striatal spiny and aspiny neurons in dopamine-denervated rat striatum recorded in vivo. Synapse. 1999;33:1–15. doi: 10.1002/(SICI)1098-2396(199907)33:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Onn S-P, Grace AA. Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci. 2000;20:2332–2345. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onn S-P, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:s48–s56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds JNJ, Wickens JR. Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience. 2000;99:199–203. doi: 10.1016/s0306-4522(00)00273-6. [DOI] [PubMed] [Google Scholar]
- 35.Stern EA, Jaeger D, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- 36.Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. In: Arbuthnott GW, Emson PC, editors. Progress in brain research, Vol 99, Chemical signaling in the basal ganglia, Vol 99. Elsevier; Amsterdam: 1993. pp. 309–324. [DOI] [PubMed] [Google Scholar]
- 37.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 38.Umemiya M, Raymond LA. Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons. J Neurophysiol. 1997;78:1248–1255. doi: 10.1152/jn.1997.78.3.1248. [DOI] [PubMed] [Google Scholar]
- 39.West AR, Galloway MP. Intrastriatal infusion of (±)-S-nitroso-N-acetylpenicillamine releases vesicular dopamine via an ionotropic glutamate receptor-mediated mechanism: an in vivo microdialysis study in chloral hydrate-anesthetized rats. J Neurochem. 1996;66:1971–1980. doi: 10.1046/j.1471-4159.1996.66051971.x. [DOI] [PubMed] [Google Scholar]
- 40.West AR, Galloway MP. Endogenous nitric oxide facilitates striatal dopamine and glutamate efflux in vivo: role of ionotropic glutamate receptor-dependent mechanisms. Neuropharmology. 1997;36:1571–1581. doi: 10.1016/s0028-3908(97)00148-2. [DOI] [PubMed] [Google Scholar]
- 41.West AR, Grace AA. Nitric oxide modulates the basal activity and responsiveness of striatal neurons to electrical stimulation of the orbital prefrontal cortex. Soc Neurosci Abstr. 1999;25:173. [Google Scholar]
- 42.West AR, Grace AA. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J Neurophysiol. 2000a;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- 43.West AR, Grace AA. Intrastriatal infusion of the dopamine D2 receptor antagonist eticlopride increases the excitability of striatal neurons: a combined in vivo intracellular recording and microdialysis study. Soc Neurosci Abstr. 2000b;26:833. [Google Scholar]
- 44.Wickens JR, Wilson CJ. Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. J Neurophysiol. 1998;79:2358–2364. doi: 10.1152/jn.1998.79.5.2358. [DOI] [PubMed] [Google Scholar]
- 45.Wilson CJ. The generation of natural firing patterns in neostriatal neurons. In: Arbuthnott GW, Emson PC, editors. Progress in brain research, Vol 99, Chemical signaling in the basal ganglia. Elsevier; Amsterdam: 1993. pp. 277–297. [DOI] [PubMed] [Google Scholar]
- 46.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf ME, Chang-Jiang X. Amphetamine and D1 dopamine receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: modification by repeated amphetamine administration. J Neurochem. 1998;70:198–209. doi: 10.1046/j.1471-4159.1998.70010198.x. [DOI] [PubMed] [Google Scholar]