Abstract
We examined the role of SNAPs, soluble proteins that attachN-ethylmaleimide-sensitive factor (NSF), in regulating exocytosis in single rat adrenal chromaffin cells. Whole-cell dialysis of Ca2+-buffered solution or photolysis of caged-Ca2+ was used to manipulate cytosolic Ca2+ concentration ([Ca2+]i), whereas exocytosis was measured via carbon fiber amperometry or membrane capacitance. Buffering [Ca2+]i to ∼170 nm produced a mean rate of exocytosis of approximately one amperometric event per minute. Including α-SNAP (60 or 500 nm) in the intracellular solution dramatically increased the mean rate of exocytosis. The stimulatory action of α-SNAP requires ATP hydrolysis mediated via NSF, because this action was blocked by intracellular dialysis of ATP-γ-S (2 mm) and could not be mimicked by a mutant α-SNAP that does not stimulate the ATPase activity of NSF. This action of α-SNAP was significant only at [Ca2+]i between 100 and 300 nm and was not shared by β-SNAP (500 nm), suggesting that α-SNAP enhanced a component of exocytosis that is regulated by a high-affinity Ca2+ sensor. In cells dialyzed with both α- and β-SNAP, the rate of exocytosis was smaller than that produced by α-SNAP alone, suggesting that α- and β-SNAP interact competitively. Although only α-SNAP stimulated exocytosis at [Ca2+]i between 100 and 300 nm, both α- and β-SNAP isoforms equally slowed the time-dependent rundown of the exocytic response. Our results indicate that α- and β-SNAP have different actions in exocytosis. Thus, the ratio of different isoforms of SNAPs can determine release probability at the levels of [Ca2+]ithat are involved in regulation of exocytosis.
Keywords: SNAREs, catecholamine release, calcium dependence, whole-cell dialysis, flash photolysis, caged-calcium, amperometry, chromaffin cell
SNARE proteins play important roles in neurotransmitter release and other forms of membrane fusion (Rothman, 1994; Hanson et al., 1997; Robinson and Martin, 1998;Tokumaru and Augustine, 2002). Recent studies suggest that SNARE proteins expressed on two different membranes bring these membranes into close apposition and facilitate membrane fusion when SNARE proteins bind and “zip up” in a parallel orientation relative to their transmembrane anchor. At some point in the cycle of vesicle traffic, these SNARE complexes must be disassembled before a new SNARE complex can be assembled (Hanson et al., 1997; Xu et al., 1998). Thus, it is important to define how and when SNARE complexes dissociate.
In vitro experiments have shown that solubleN-ethylmaleimide-sensitive factor attachment proteins (SNAPs) stimulate the ATPase activity of NSF to disassemble the SNARE complex (Sollner et al., 1993a). It has been well established that SNAPs function in the intracellular trafficking of vesicles (Clary et al., 1990; Sollner et al., 1993b) and enhance Ca2+-dependent exocytosis in various cell types. Studies on chromaffin cells also suggest that SNAPs mediate a priming step of exocytosis via stimulation of the ATPase activity of NSF (Chamberlain et al., 1995; Barnard et al., 1997). Exogenous SNAPs increase the size of the most readily releasable pools of chromaffin granules without affecting the kinetics or Ca2+ dependence of exocytosis (Xu et al., 1999). These studies suggest that SNAPs play a role in priming vesicles but not in modifying the triggering of fusion of granules from the readily releasable pool.
Three different isoforms of SNAP (α, β, and γ) have been identified. Although α- and γ-SNAP are found in all cells, β-SNAP is expressed only in neurons (Whiteheart et al., 1993). Despite their differential distribution, it is not yet clear whether these SNAP isoforms serve different roles. The primary structures of these proteins are similar, and it has been suggested that they act synergistically in intra-Golgi transport (Whiteheart et al., 1993). Consistent with the possible functional equivalence of the SNAPs, the maximal stimulatory effect of α- and γ-SNAPs (Morgan and Burgoyne, 1995) or α- and β-SNAPs (Sudlow et al., 1996) on exocytosis in permeabilized chromaffin cells is less than the sum of the effect of each SNAP isoform alone. On the other hand, certain functions of α-SNAP are not supported by other SNAP isoforms (Clary et al., 1990;Sollner et al., 1993a; Schiavo et al., 1995). Thus, it is possible that the isoforms of SNAPs serve different functions in exocytosis. We have examined this possibility by comparing the effects of α- and β-SNAP in rat adrenal chromaffin cells. Although both α- and β-SNAPs are comparable in their ability to slow the rundown of exocytic responses during whole-cell dialysis, only α-SNAP induces, within minutes of whole-cell dialysis, a component of exocytosis that has a relatively high affinity for Ca2+ (essentially saturated at 500 nm). Thus, SNAPs influence exocytosis via two distinct molecular actions, only one of which is shared by the neuron-specific isoform β-SNAP.
MATERIALS AND METHODS
Cell preparation. Rat adrenal chromaffin cells were isolated and prepared as described previously (Xu and Tse, 1999). Briefly, male Sprague Dawley rats (200–250 gm) were euthanized with halothane in accordance with the standards of the Canadian Council on Animal Care. The adrenal medullas were dissociated enzymatically in a modified Hank's solution containing collagenase type I (3.0 mg/ml), hyaluronidase type I-S (2.4 mg/ml), deoxyribonuclease type I (0.2 mg/ml) for 30 min, followed by trypsin I (0.5 mg/ml) for 10 min at 37°C. Single chromaffin cells were plated in experimental chambers with a glass coverslip bottom that was previously coated with 0.25 mg/ml poly-l-lysine. The cells were maintained in standard culture (37°C; 5% CO2) in a DMEM medium supplemented with 10% horse serum, 50 U/ml penicillin G, and 50 μg/ml streptomycin (all from Life Technologies, Grand Island, NY). Recordings were performed at room temperature (21–24°C) on cells maintained in culture for 1–3 d.
Chemicals and solutions. Indo-1 was purchased from Teflabs (Austin, TX); BAPTA and ATP-γ-S were from Calbiochem (San Diego, CA). α-SNAP (wild type and L294A mutant) and β-SNAP were prepared as His-tagged fusion proteins as described in DeBello et al. (1995). The photolabile Ca2+ chelator, dimethoxynitrophenyl-EGTA-4 (DMNPE-4), was synthesized as described inEllis-Davies (1998). All other chemicals were purchased from Sigma-Aldrich (Oakville, Ontario, Canada).
The standard bath solutions contained (in mm): 150 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 8 glucose, and 10 Na-HEPES, pH 7.4. In experiments in which [Ca2+]i was controlled by intracellular diffusion of Ca2+-buffered solution via the whole-cell pipette, the composition of the different pipette solutions was as described in Table 1. For experiments in which [Ca2+]i was buffered to subnanomolar levels, all extracellular CaCl2 was also replaced by MgCl2 and 2 mm EGTA. In experiments in which [Ca2+]iwas elevated rapidly via flash photolysis of caged Ca2+, the pipette solution contained (in mm): 70 Cs-aspartate, 40 Cs-HEPES, 20 tetraethylammonium (TEA)-Cl, 3 MgCl2, 2 Na2-ATP, 0.3 GTP, 0.1 indo-1, 5 DMNPE-4 (∼75% saturated with Ca2+), pH 7.4. In experiments involving depolarization-triggered exocytosis, the pipette solution contained (in mm): 135 Cs-aspartate, 10 TEA-Cl, 20 HEPES, 3 MgCl2, 2 Na2-ATP, 0.3 GTP, and 0.1 indo-1, pH 7.4. In these experiments, the CaCl2 in the standard bath solution was increased to 10 mm, and tetrodotoxin (0.5 μm) and apamin (0.4 μm) were added to block the voltage-gated Na+ currents and the small-conductance Ca2+-activated K+ currents, respectively.
Table 1.
Compositions of pipette solutions for dialysis
| Calculated [Ca2+] | ∼0.1 nm | 50 nm | 100 nm | 170 nm | 300 nm | 500 nm | 170 nm (ATP-γ-S) |
|---|---|---|---|---|---|---|---|
| KCl | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| KAsp | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| HEPES | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| GTP | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Na2ATP | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | |
| Li4ATP-γ-S | 2.0 | ||||||
| MgCl2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| EGTA | 20 | 20 | 20 | 20 | 20 | 20 | |
| BAPTA | 10 | ||||||
| CaCl2 | 0 | 8 | 12 | 14 | 16 | 17 | 14 |
| Indo-1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| pCa | ∼10 | 7.3 | 7.0 | 6.8 | 6.5 | 6.3 | 6.8 |
The concentration of all ingredients is listed in millimolar. All solutions were adjusted to pH 7.4 by KOH.
[Ca2+]i measurement and flash photolysis of caged Ca2+.[Ca2+]i was measured fluorometrically using the Ca2+indicator, indo-1, which was dialyzed into the cell via the whole-cell patch pipette. Details of the instrumentation and [Ca2+]imeasurement procedures are as described previously (Tse and Tse, 1999,2000). Indo-1 fluorescence was measured at 405 and 500 nm by photomultiplier tubes, and the ratio of fluorescence (405/500) was used to calculate [Ca2+]i according to the equation of Grynkiewicz et al., (1985): [Ca2+]i =K*(R −Rmin)/(Rmax− R).
The three calibration constants, Rmin,Rmax, andK*, were calibrated in situ by dialyzing various solutions of known Ca2+ concentration into cells as described previously (Tse and Tse, 1999; Tse et al., 1997). In all experiments except those involving flash photolysis of caged-Ca2+, the values forRmin,Rmax, andK* were 0.28, 3.20, and 2.53 μm.
For experiments involving photolysis of DMNPE-4, a flash of UV light (from a modified XF-10 xenon flash lamp; Hi-Tech Ltd., Salisbury, UK) was delivered to the cell as described previously (Tse and Tse, 2000;Tse et al., 1997). To boost photolysis and maintain a more sustained elevation of [Ca2+]i, the cell was continuously illuminated with UV light from a mercury lamp (used for indo-1 excitation) for at least 10s of seconds after the UV flash. In comparison with previous photolysis experiments with DM-nitrophen and a higher output UV flash lamp (Tse and Tse, 2000), this method caused [Ca2+]i to rise slower and the maximum rate of exocytosis to occur during the plateau of [Ca2+]i, which lasted at least a few seconds.
Electrophysiological recording. Single rat chromaffin cells were voltage clamped at a DC holding potential of −80 mV (corrected for junction potential) with the whole-cell gigaseal technique (Hamill et al., 1981). Recording pipettes were made from hematocrit glass (VWR Scientific, London, Ontario, Canada) and had resistances of 2.5–3.5 MΩ when filled with the pipette solution. In experiments involving depolarization-triggered exocytosis, exocytosis was measured as increases in membrane capacitance (ΔCm) resulting from the addition of granule membrane to cell membrane (Neher and Marty, 1982; Lindau and Neher, 1985). ΔCm was measured with a software-based phase-sensitive detector [Pulse Control; Herrington and Bookman (1994)] as described in a previous study (Xu and Tse, 1999). Briefly, a 50 mV (peak to peak), 822 Hz sinusoidal wave was superimposed onto the holding potential. The resulting current signal at two orthogonal phase angles was analyzed to generate an output for ΔCm and another output, ΔG, which reflects changes in membrane conductance, electrode seal, and series resistance.
In experiments involving detection of quantal catecholamine release, carbon fiber amperometry (Wightman et al., 1991; Chow and von Ruden, 1995; Zhou and Misler, 1995) was used as described previously (Xu and Tse, 1999). Briefly, +700 mV was applied to the carbon fiber electrode (tip diameter of 7 μm) using a VA-10 Voltammeter (NPI Electronic, Tamm, Germany). Amperometric current was stored without filtering onto videocassette recorder tapes with a NeuroData PCM recorder at 22 or 44 kHz (Neuro Data Corporation, New York, NY). For analysis, currents were filtered at 1 kHz and digitized at 10 kHz.
Statistical analysis. All treatments with either α- or β-SNAP were compared with control cells from the same cultures, with recordings performed alternately on SNAP-dialyzed and control cells. Only recordings that met the following criteria were included in our analysis. (1) The access resistance remained <20 MΩ, and the holding current was stable (<30 pA) throughout the experiment. (2) Cells dialyzed with <500 nm[Ca2+]i had a rate of exocytosis <0.1/sec during the first 2 min after establishment of whole-cell configuration. (3) When the pipette solution included EGTA or BAPTA to buffer Ca2+, the [Ca2+]i reported by indo-1 was within 20% of the calculated [Ca2+] of the pipette solution. (4) When depolarization trains were used to stimulate Ca2+ entry, the basal [Ca2+]i was <350 nm.
Unless indicated otherwise, all mean values in the text and figures are given as mean ± SEM. A Student's t test for two independent populations was used for statistical comparisons between data from SNAP-treated cells and those from control cells. Any difference with p < 0.05 was considered statistically significant and is marked with an asterisk in the figures.
RESULTS
α-SNAP enhances quantal release at low [Ca2+]i
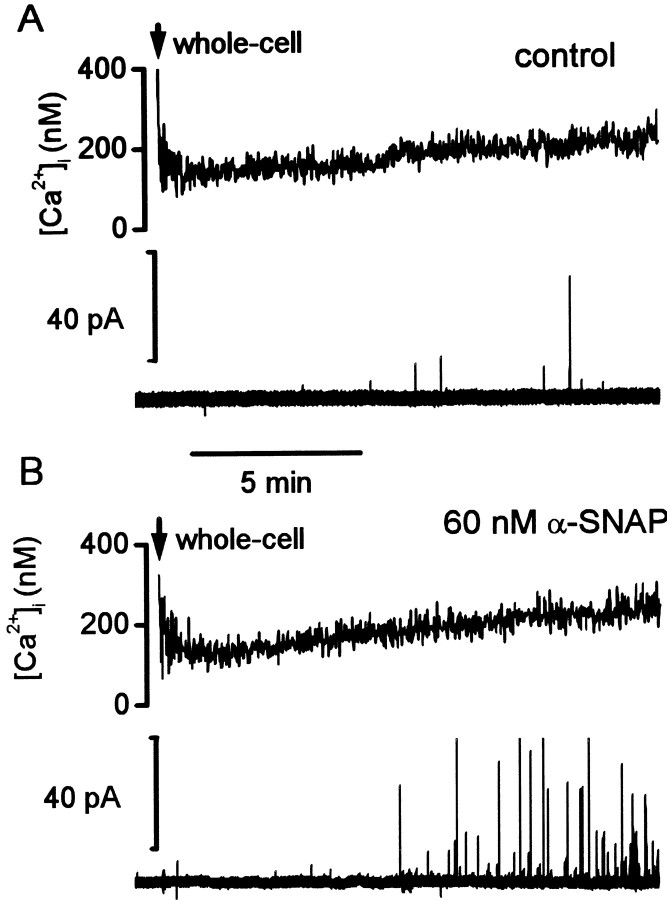
To examine whether α-SNAP affects exocytosis, we used amperometry to measure quantal release of catecholamine while buffering [Ca2+]i via intracellular dialysis of a Ca-EGTA solution. Figure1A shows an example of a recording from a control cell. In this case, within 10 sec after establishing the whole-cell configuration, sufficient Ca-EGTA solution entered the cell to overcome the endogenous Ca2+ buffer, and [Ca2+]i reached a stable level near 170 nm. Amperometric events reflecting the quantal release of catecholamine from individual granules were infrequent throughout the entire duration of the recording. In contrast, when α-SNAP (60 nm) was included in the intracellular solution, the frequency of the amperometric events increased dramatically (Fig.1B).
Fig. 1.
α-SNAP increased the rate of quantal release of catecholamine at ∼170 nm[Ca2+]i. A, Simultaneous recording of [Ca2+]i and amperometric events from a control cell. The cell was voltage clamped at −80 mV, and [Ca2+]i was buffered to ∼170 nm by diffusion of Ca2+-buffer solution via the whole-cell pipette. B, [Ca2+]i and amperometric events from a cell recorded with 60 nm α-SNAP in the pipette solution. In B, the frequency of amperometric events obviously increased after ∼6 min of whole-cell dialysis.
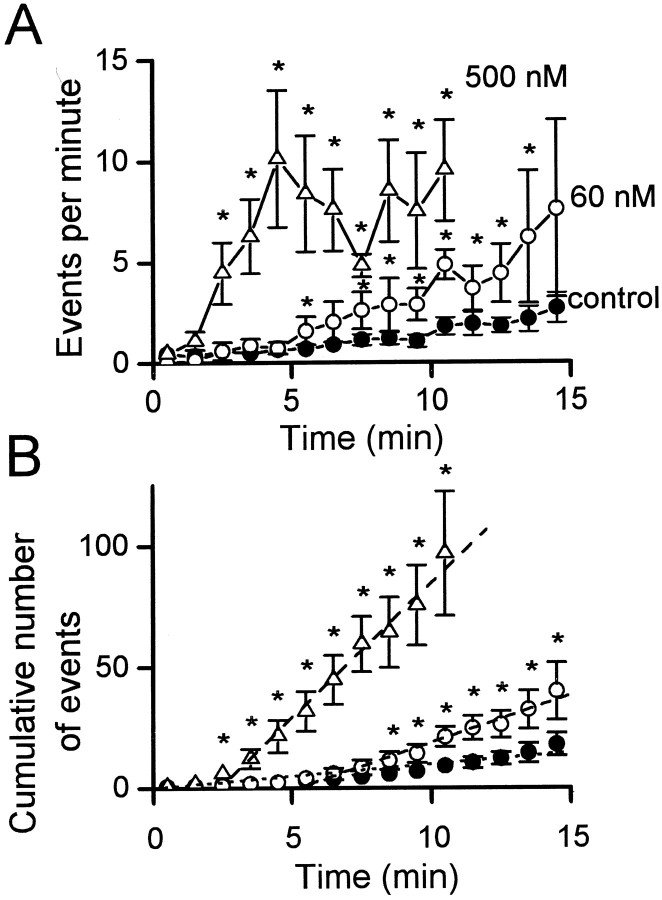
To quantify this stimulatory effect of α-SNAP, we compared the mean rate of amperometric events in control experiments and in experiments in which α-SNAP was dialyzed into the cells (Fig.2A). For both conditions, the rate of exocytosis varied over time. In control cells, the mean frequency of amperometric events was typically approximately one per minute after 2–10 min of whole-cell dialysis. When α-SNAP (60 nm) was included in the recording pipette, the frequency of the amperometric events was similar to the control cells during the first 5 min of whole-cell dialysis. However, 6–10 min after beginning whole-cell dialysis, the mean frequency of amperometric events was several-fold higher than in control cells (Fig.2A). This delay in the effect of α-SNAP presumably reflects the time required for dialysis of the protein into the cytoplasm (Pusch and Neher, 1988).
Fig. 2.
The effect of different concentrations of α-SNAP. A, Plot of the mean number of amperometric events per minute from individual cells dialyzed with control solution (●, n = 18–34), with 60 nm α-SNAP (○, n = 5–7), or with 500 nmα-SNAP (▵, n = 7–13). The time refers to the duration of whole-cell dialysis. [Ca2+]i was clamped near 170 nm by intracellular diffusion of Ca2+-buffer solution. The asterisksindicate the times when data points for α-SNAP are statistically different from controls. B, Plot of the data inA as the mean of the cumulative number of amperometric events from individual cells at different durations of whole-cell dialysis. The rate of exocytosis in control cells and cells recorded with α-SNAP was estimated from linear regression (lines shown). For control cells, all the data points were included (r = 0.98). For cells dialyzed with α-SNAP, each linear regression started at the time when the comparison with control cells became significantly different inA. For cells dialyzed with 60 mm α-SNAP, the linear regression included the data between the 6th and 15th minute of whole-cell dialysis (r = 0.98). For cells dialyzed with 500 nm α-SNAP, the linear regression included the data between the 3rd and 15th minute of whole-cell dialysis (r = 0.99).
The magnitude of the effect of α-SNAP was concentration dependent: when 500 nm α-SNAP was included in the pipette solution, the increase in the frequency of amperometric events was more dramatic than when the pipette contained 60 nm α-SNAP (Fig.2A). The time of onset of the α-SNAP effect also was more rapid when the concentration of α-SNAP was increased: the frequency of the amperometric events was significantly higher than that of the control cells by the third minute of dialysis of 500 nm α-SNAP. For these three treatments, we estimated the mean rate of exocytosis by plotting the cumulative number of amperometric events occurring after whole-cell dialysis (Fig.2B). The slopes of these functions, which indicate the mean rate of amperometric events, varied for each treatment. Because the exogenous α-SNAP will enter the cell gradually, we estimated the slope of the data from cells dialyzed with α-SNAP only at times after the treatment groups became significantly different from control (Fig. 2A,B,asterisks). In control cells, the rate of exocytosis was 0.98 events per minute, whereas the rate increased fourfold, to 3.9 events per minute, when 60 nmα-SNAP was in the pipette. With 500 nm α-SNAP the rate of exocytosis was 11.1 events per minute, which was 11-fold that of control (Fig. 2B).
Ca2+ dependence of the α-SNAP effect
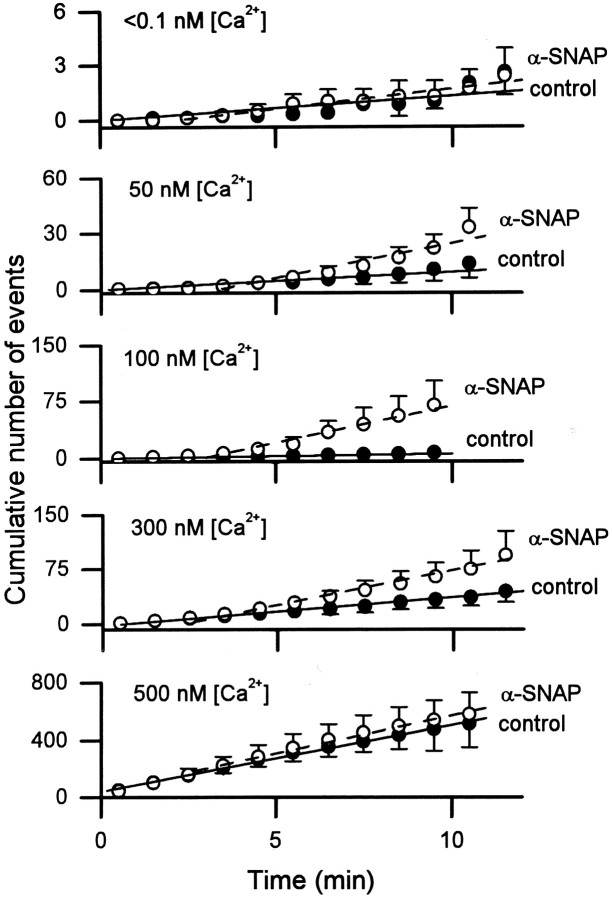
The above results show that increasing the cytoplasmic concentration of α-SNAP dramatically increases the rate of quantal catecholamine release from rat chromaffin cells when [Ca2+]i is ∼170 nm. We next characterized the influence of [Ca2+]i on the action of α-SNAP by examining the effect of α-SNAP (500 nm) at [Ca2+]i ranging from subnanomolar to ∼3 μm. In the first set of experiments, [Ca2+]i was set at various levels by diffusion of buffered Ca2+ solutions (Table1) from the recording pipette to the interior of the cell (Augustine and Neher, 1992). Subnanomolar [Ca2+]i was achieved by dialyzing the cells with a solution containing a high concentration of a fast Ca2+ chelator, BAPTA (10 mm), with no free Ca2+ added. The [Ca2+] of this solution was calculated to be <0.1 nm. To set [Ca2+]i at values up to 500 nm, we used dialysis solutions containing a mixture of EGTA and Ca2+ (as described in Table 1). Over this range of [Ca2+]i, the rate of exocytosis was slow (Fig. 3), and the rate of exocytosis was determined from the slope of plots of the cumulative number of amperometric events (as in Fig.2B). Remarkably, α-SNAP significantly increased the rate of exocytosis at [Ca2+]i between 100 and 300 nm but had no significant effect at higher or lower [Ca2+]i. The stimulatory effect of α-SNAP that occurs in this range of [Ca2+]i was observed for at least 10 min after beginning whole-cell dialysis (Fig.3).
Fig. 3.
Plot of the mean cumulative number of amperometric events from individual cells after whole-cell dialysis with solutions buffered to different [Ca2+]i, with or without 500 nm α-SNAP. As in Figure 2B, the linear regression of control cells (filled symbols) included all data points; the linear regression for cells dialyzed with α-SNAP (open symbols) began at the third minute of whole-cell dialysis.
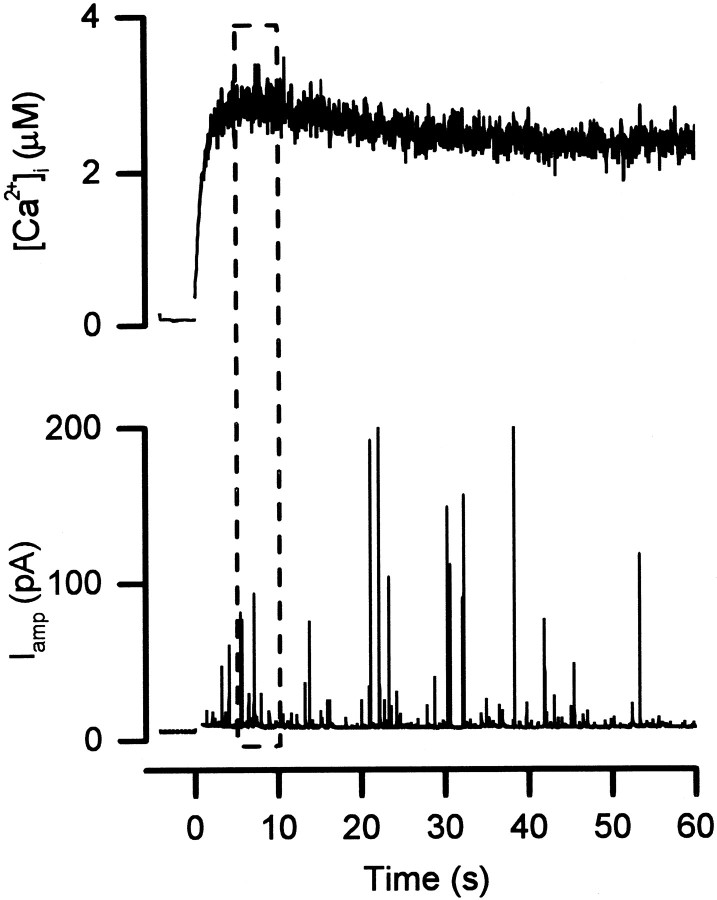
When [Ca2+]i was elevated to the micromolar range, the rate of triggered exocytosis was high. This could cause the pool of readily releasable granules to be depleted before α-SNAP could even enter the cell. To circumvent this possible complication, a caged Ca2+compound (DMNPE-4) was included in the pipette solution. A flash of UV light was then delivered, 3–5 min after beginning whole-cell dialysis, to photolyze the caged-Ca2+ compound and increase [Ca2+]iuniformly and much more rapidly than whole-cell dialysis (Neher and Zucker, 1993; Thomas et al., 1993; Tse and Tse, 2000; Tse et al., 1997). Figure 4 shows an example of such an experiment in a control cell. Before the delivery of the flash of UV light (at time 0), the caged Ca2+ compound buffered [Ca2+]ito a very low level. In this condition, no amperometric events were detected for the first 3–4 min of whole-cell recording in 17 of 20 cells (including the cell shown in Fig. 4), and only one amperometric event was recorded from each of the remaining three cells (two control and one dialyzed with α-SNAP). However, amperometric signals were readily detected after a UV light flash that caused [Ca2+]i to rise to ∼2.5 μm (Fig. 4). With our method of photolysis, [Ca2+]i reached a plateau in a few seconds, and the rate of exocytosis peaked during this plateau. Therefore we estimated from the mean number of events occurring during the 5 sec plateau of the [Ca2+]i elevation. α-SNAP had no significant effect on the rate of amperometric signals under such conditions, comparable to what was observed when [Ca2+]i was elevated to 0.5 μm by dialysis of the mixture of EGTA and Ca2+ (Fig. 3, bottom).
Fig. 4.
Simultaneous measurements of [Ca2+]i and amperometric events during flash photolysis of caged Ca2+. A UV flash was delivered at time 0 to photolyze the caged Ca2+. The rate of exocytosis was estimated from the number of amperometric events during a 5 sec interval (indicated by the dashed-line box) when [Ca2+]i was at its plateau.
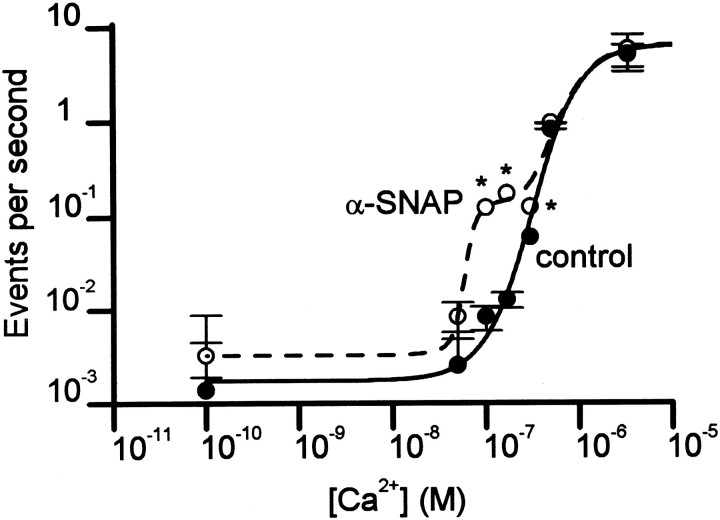
To quantify the influence of [Ca2+]i on the effect of α-SNAP, we determined the relationship between [Ca2+]i and the rate of exocytosis. In control cells, this relationship was roughly sigmoidal when plotted on logarithmic coordinates (Fig.5). Similar results were reported in a previous study on bovine chromaffin cells, which also used whole-cell dialysis to manipulate [Ca2+]i but measured the rate of exocytosis via increases in membrane capacitance (Augustine and Neher, 1992). Including α-SNAP (500 nm) in the dialysis solution caused a change in the relationship, but only at [Ca2+]i between 100 and 300 nm. Thus, α-SNAP appears to preferentially enhance a component of exocytosis that is triggered at relatively low [Ca2+]i.
Fig. 5.
The Ca2+ dependence of the enhancing effect of α-SNAP. For [Ca2+]i up to 500 nm, [Ca2+]i was controlled via whole-cell dialysis of Ca2+-buffered solution, and the rate of exocytosis (events per second) was measured as described in Figures2B and 3. For higher [Ca2+]i, flash photolysis of caged-Ca2+ (similar to Fig. 4) was used. Data from control cells (filled symbols) were fitted to and sigmoidal function was centered at pCa 6.51, which corresponds to aKd of 1.3 μm[Ca2+]i and a Hill coefficient of 3.5. Data from cells dialyzed with 500 nm α-SNAP (open symbols) were fitted to the sum of two sigmoidal functions, one identical to that for the control data, the other centered at pCa 7.25, which corresponds to a Kd of 80 nm [Ca2+]i and a Hill coefficient of 6.5.
α-SNAP action requires the ATPase activity of NSF
α-SNAP attaches the ATPase, NSF, to the SNARE protein complex (Sollner et al., 1993a,b). Such an action has been proposed to underlie previous observations that ATP is required for the stimulatory effect of α-SNAP on exocytosis in permeabilized adrenal chromaffin cells (Morgan and Burgoyne, 1995; Sudlow et al., 1996). To determine whether the increase in exocytosis caused by α-SNAP in our experimental conditions was also dependent on ATP, intracellular ATP was replaced by a nonhydrolyzable analog, ATP-γ-S (Table 1). Because these nucleotides (∼0.5 kDa) are 70-fold smaller in mass than α-SNAP (35 kDa), the time required for diffusion of ATP into (or out of) the cell should be approximately fourfold faster than for diffusion of α-SNAP (Pusch and Neher, 1988). Indeed, it has been reported that intracellular ATP exchanges within 4 min of whole-cell dialysis (Parsons et al., 1995). Thus, the nucleotide concentration should reach equilibrium well before the concentration of α-SNAP.
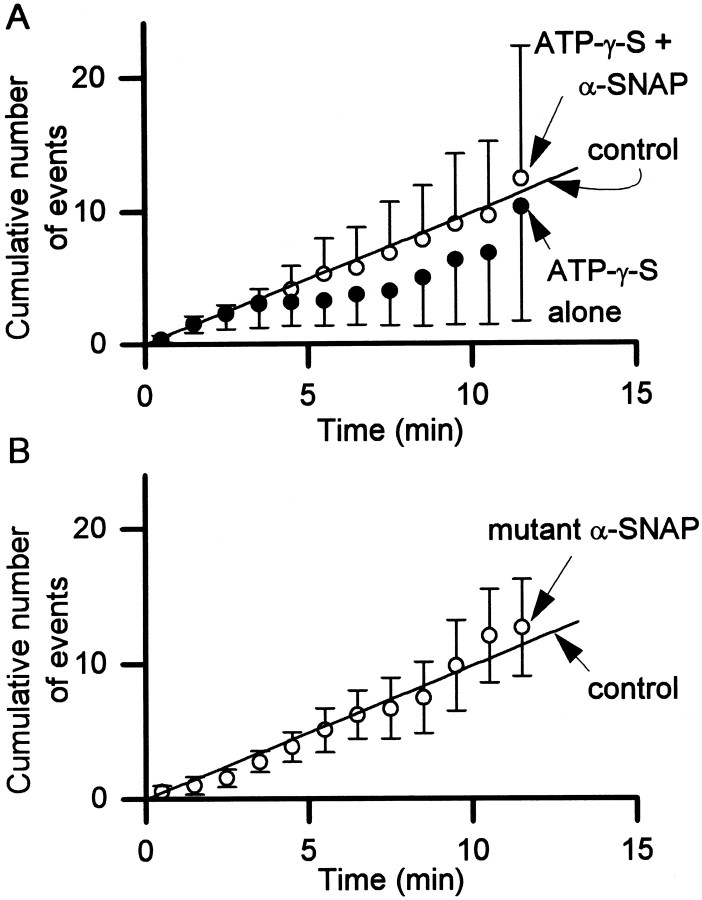
Because the effect of α-SNAP was most dramatic when [Ca2+]i was ∼170 nm, we examined the effect of ATP-γ-S at this [Ca2+]i. Addition of α-SNAP (500 nm) had no effect on the rate of exocytosis in the presence of ATP-γ-S (Fig.6A). Intracellular ATP-γ-S alone (without added α-SNAP) caused the rate of exocytosis to be insignificantly smaller than that measured with 2 mm ATP inside the cells (control conditions, as in Fig. 2B). Thus the action of α-SNAP to stimulate quantal release requires ATP hydrolysis.
Fig. 6.
The effect of α-SNAP on exocytosis required ATP hydrolysis and stimulation of the ATPase activity of NSF.A, Replacement of intracellular ATP with ATP-γ-S (2 mm) abolished the effect of α-SNAP (500 nm) but had no significant effect in the absence of exogenous α-SNAP.B, The rate of exocytosis was not affected by a mutant α-SNAP that does not stimulate the ATPase activity of NSF. In all experiments shown here, [Ca2+]i was buffered to ∼170 nm. For comparison, the regression line (from Fig. 2B) for control data obtained in the presence of intracellular ATP, but no exogenous α-SNAP, is also shown.
Binding of NSF to α-SNAP triggers the ATPase activity of NSF (Morgan et al., 1994). To determine whether the stimulatory effect of α-SNAP on exocytosis involves such an action, we examined the intracellular action of a mutant α-SNAP (L294A). This point mutation still permits α-SNAP to bind to NSF but fails to stimulate the ATPase activity of NSF (Barnard et al., 1997). Dialysis with this mutant form of α-SNAP (for up to 12 min, along with 2 mm ATP, at 170 nm[Ca2+]i) caused no change in the rate of exocytosis in comparison with controls (Fig.6B). The loss of activity after this point mutation indicates that the stimulatory effect of α-SNAP is mediated by stimulation of the ATPase activity of NSF [also see Graham and Burgoyne (2000)].
α-SNAP does not change quantal characteristics
To explore whether the vesicles undergoing exocytosis in the presence of α-SNAP differ from those that normally fuse, we compared the properties of quantal release events detected by carbon fiber amperometry (Chow and von Ruden, 1995). This method can provide information about the kinetics of catecholamine release from individual granules (as determined from the rise time and half-width of individual events) and the amount of catecholamine within each released quantum (represented by the total charge of each event).
Detection of the kinetics of catecholamine release is affected by the positioning of the carbon fiber electrode, which determines the path of diffusion of catecholamines from the surface of the cell to the electrode (Chow and von Ruden, 1995). We minimized such positioning effects by using each cell as its own control and comparing signals recorded from individual cells before and after α-SNAP treatment. With [Ca2+]ibuffered to ∼170 nm, the frequency of quantal release events increased dramatically after 3 min of whole-cell dialysis of 500 nm α-SNAP (Fig. 2). For the purpose of this analysis, we divided the release events into two groups: those detected before the α-SNAP effect was evident (within the first 2 min of dialysis) and those detected later (>3 min after beginning α-SNAP dialysis). However, on average only two events were recorded from each cell before the α-SNAP effect was evident, so that the total sample size was inadequate for statistical comparisons despite recording signals from 13 cells.
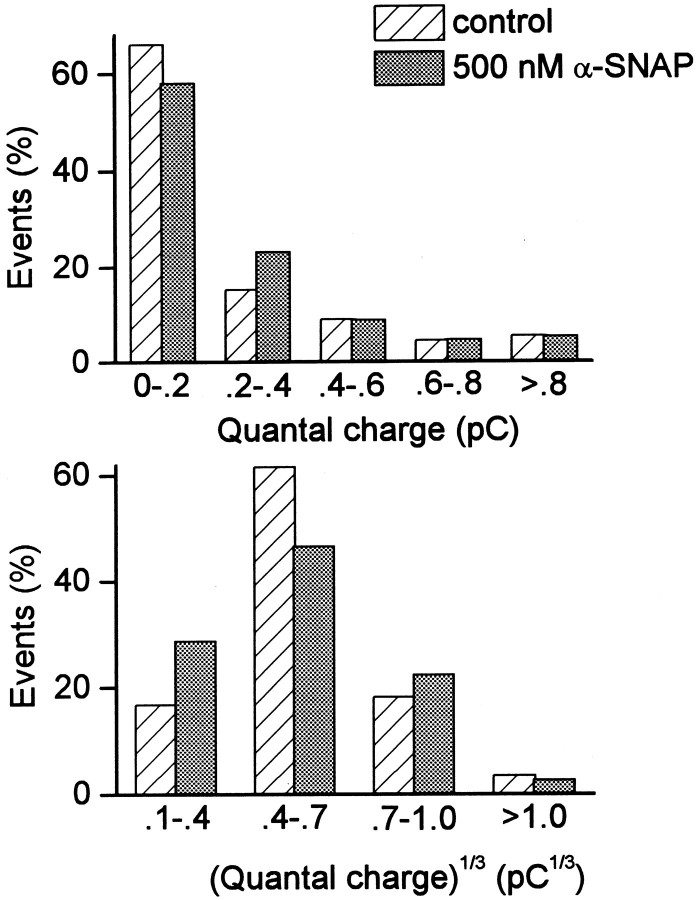
In comparison with the kinetics of amperometry signals, the measurement of the total charge in individual quanta is far less dependent on the positioning of the carbon fiber electrode (Chow and von Ruden, 1995), permitting comparisons between two separate groups of cells. For such purposes, we compared data from all control cells with those collected 3 min or later after the start of α-SNAP dialysis (500 nm), with [Ca2+]i buffered to ∼170 nm in both cases. The distribution of quantal charge values was unaffected by α-SNAP (Fig.7, top). Likewise, the cube root of quantal charge, which has a normal (Gaussian) distribution (Xu and Tse, 1999), was unchanged in the presence of α-SNAP (Fig. 7,bottom). The mean ± SD of the cube root of quantal charge was 0.56 ± 0.20 pC1/3 for control cells and 0.56 ± 0.21 pC1/3after >3 min of α-SNAP dialysis, which was not statistically different (p > 0.9). These values are also similar to measurement of quanta released from KCl-stimulated rat chromaffin cells (Xu and Tse, 1999). Overall, this analysis indicates that the amount of catecholamine released from vesicles in response to α-SNAP was indistinguishable from that released under control conditions. This suggests that α-SNAP acts on the same population of granules normally triggered to fuse by Ca2+ and that α-SNAP does not affect the loading of catecholamines into vesicles or discharge of catecholamines from fused vesicles.
Fig. 7.
The quantal characteristics of the catecholamine-containing granules were not altered by α-SNAP.A, Plot of the quantal charge (Q) of individual amperometric events. Q was calculated from the time integral of each signal. Each histogram compared the events from control cells with events from cells dialyzed for at least 4 min with 500 nm α-SNAP. In all experiments, [Ca2+]i was buffered to ∼170 nm. B, Plot of the cube root ofQ (Q1/3). α-SNAP caused no statistical difference in the distribution ofQ (Mann–Whitney U test) orQ1/3 (Student's ttest).
Differential actions of α- and β-SNAP
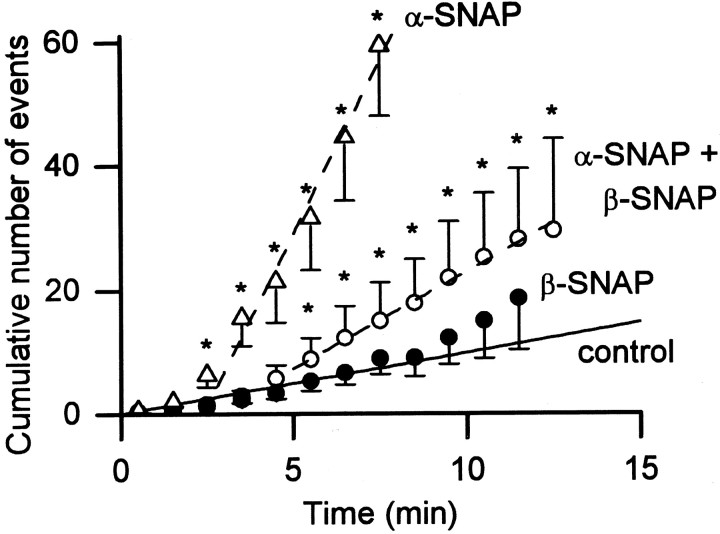
We compared the effects of α- and β-SNAP on quantal release from rat adrenal chromaffin cells when [Ca2+]i was buffered to ∼170 nm. Whole-cell dialysis with β-SNAP (500 nm) for 12 min did not cause any significant increase in the rate of exocytosis (Fig. 8, ●). In contrast, whole-cell dialysis of α-SNAP under similar conditions caused a 10-fold increase in the rate of exocytosis (Fig. 8, ▵). When α- and β-SNAP were dialyzed into the cell together (500 nm of each), the increase in the rate of exocytosis was only threefold (Fig. 8, ○). These data indicate that α- and β-SNAP differ in their ability to enhance quantal release of catecholamines at submicromolar [Ca2+]i. In fact, the two isoforms of SNAP apparently compete with each other at their exocytic site(s) of action.
Fig. 8.
Effect of β-SNAP. Plot of the mean of the cumulative number of amperometric events recorded in individual cells dialyzed with β-SNAP (500 nm) alone (●) or simultaneously with α-SNAP (500 nm, ○). The experimental conditions were similar to those in Figure 1. The data from cells dialyzed with 500 nm α-SNAP (▵ withdashed line; from Fig. 2D) and the linear regression for the data from control cells (with no α- or β-SNAP; solid line) were included for comparison. All data points for β-SNAP alone were not statistically different from control, but all data points between the 6th and 13th minute of dialysis of α- and β-SNAP were statistically different from control (the linear regression of these data points had a slope of 3.1;r = 0.99).
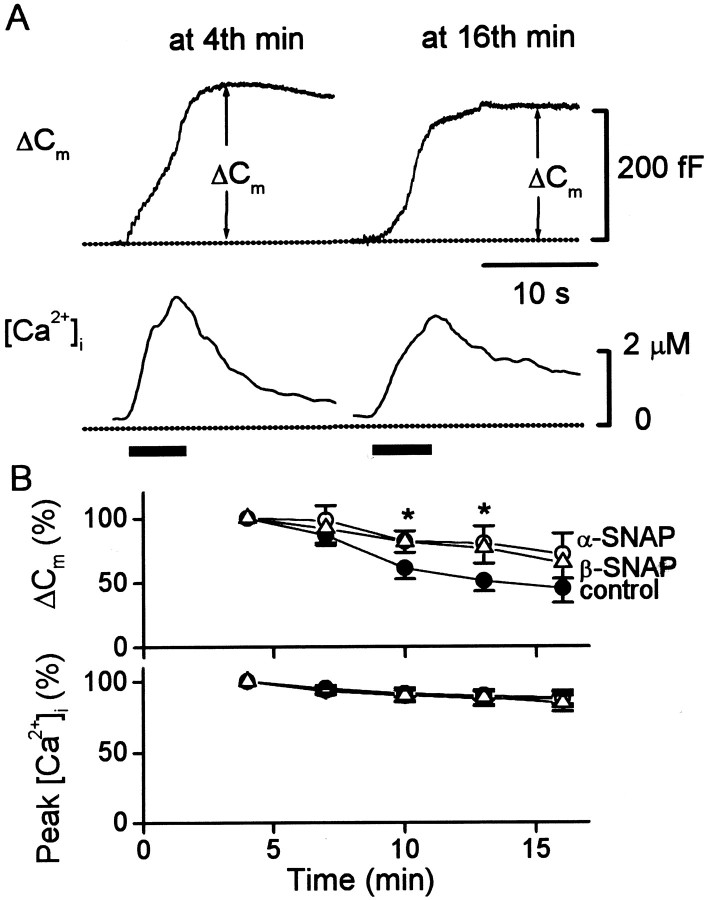
Given the previous reports that α- and β-SNAP may be equivalent in supporting exocytosis in chromaffin cells (Sudlow et al., 1996), we made further comparisons between these two isoforms. We next determined the actions of these proteins on exocytosis when [Ca2+]i was elevated by a train of depolarizations. Because of the high rate of exocytosis under such conditions, amperometric signals merge together and are difficult to resolve. Thus, the exocytic response was measured as an increase in membrane capacitance (ΔCm) (Neher and Marty, 1982; Augustine and Neher, 1992; Seward and Nowycky, 1996; Xu and Tse, 1999). Figure9A shows examples of capacitance responses elicited by depolarization of a cell dialyzed with β-SNAP (500 nm). The cell was voltage clamped at −80 mV, and trains of depolarizations (50 msec pulse duration, 15 pulses at 4 Hz) were applied at the times indicated by the bars below. Each train of depolarization caused [Ca2+]i to rise into the micromolar range (bottom traces) and caused exocytosis (ΔCm; top traces). For the control cells (n = 24), the average amplitude of ΔCm signals measured at the fourth minute of whole-cell dialysis was 6.2 ± 0.9% of the initial cell membrane capacitance. Similar values were obtained for cells dialyzed with either α- or β-SNAP (6.2 ± 0.6 and 6.4 ± 0.5%; n = 17 and 12, respectively), showing that these proteins had no immediate effect on the exocytic responses to depolarizations. Because the extracellular [Ca2+] was raised to 10 mm, the resting [Ca2+]i was slightly elevated to 216 ± 14 nm in these experiments (Fig. 9A). The relationship shown in Figure 5indicates that α-SNAP should produce a higher basal rate of exocytosis after a few minutes of whole-cell recording at this [Ca2+]i. However, this effect clearly caused no measurable change in the size of the vesicle pool that was triggered by depolarizations delivered at the fourth minute of whole-cell recording (Fig. 9B).
Fig. 9.
Effects of α- or β-SNAP on amplitude and time-dependent rundown of the exocytic response. A, Amplitude of the exocytic response (measured as increase in membrane capacitance, Cm) triggered by a train of 15 depolarizations (indicated by a bar below the traces) delivered at the 4th and 16th min after establishment of whole cell. The cell was recorded with 500 nm β-SNAP in the pipette solution. The train of depolarization delivered at 4 min of whole cell increased the membrane capacitance by 233 fF, corresponding to a 5.8% increase in cell membrane capacitance (initial cell capacitance = 4 pF). At 16 min of whole cell, the train of depolarization triggered a ∼25% smaller increase inCm, and the rise in [Ca2+]i was also slightly smaller.B, Plot of the exocytic response and peak [Ca2+]i triggered by trains of depolarization delivered at the 7th, 10th, 13th, and 16th minute of whole cell dialysis. The values of the exocytic response and peak [Ca2+]i were normalized to the values of the individual cell obtained at the fourth minute, which had no statistical difference between control cells (●) and cells dialyzed with 500 nm α-SNAP (○) or β-SNAP (▵). Theasterisks indicate that the data for α- or β-SNAP are statistically different from control cells at the same duration of dialysis. Note that exogenous α- or β-SNAP (500 nm) significantly reduced the time-dependent rundown in the exocytic response between the 10th and 13th minute but had no significant effect on the smaller rundown in peak [Ca2+]i. However, there is no significant difference between the data for α- and β-SNAP.
In control cells, the responses to depolarizations decreased gradually with time during whole-cell dialysis (Augustine and Neher, 1992; Seward and Nowycky, 1996). This rundown was observed when delivering stimuli at 3 min intervals while measuring [Ca2+]i andCm increases. After 13–16 min of whole-cell dialysis, the exocytic responses of control cells were reduced to <50% of the responses produced by the test stimulus (Fig.9B, top graph). This effect was unrelated to the amount of Ca2+ entering the cells via voltage-gated channels, because the peak magnitude of [Ca2+]i signals changed little over this time period (Fig. 9B, bottom graph). However, the responses evoked 10 and 13 min after beginning whole-cell dialysis were significantly larger than control in cells dialyzed with α-SNAP (500 nm) (Fig.9B, top). Rundown also was greatly reduced in cells dialyzed with an identical concentration of β-SNAP. Neither isoform of SNAP had significant effects on the peak magnitude of [Ca2+]i signals produced by the stimuli (Fig. 9B, bottom). Thus, although the two isoforms of SNAP differ in their ability to trigger exocytosis at submicromolar [Ca2+]i levels, they are both capable of retarding the rundown of exocytosis that occurs during prolonged dialysis with a patch pipette.
DISCUSSION
Previous studies have shown that oversupply of SNAP enhanced exocytosis in various cell types, including the squid giant synapse (DeBello et al., 1995), bovine chromaffin cells (Morgan and Burgoyne, 1995; Kibble et al., 1996), the crayfish neuromuscular junction (He et al., 1999), and pancreatic β cells (Nagamatsu et al., 1999). Our experiments show that exogenous α-SNAP (500 nm) dramatically increases the rate of exocytosis in rat chromaffin cells. This effect of α-SNAP was significant only at [Ca2+]i between 100 and 300 nm and was attributable to the well documented ability of α-SNAP to interact with NSF. In contrast, this effect was not produced by β-SNAP, a closely related isoform (Whiteheart et al., 1993), although both isoforms of SNAP equally slowed the rate of rundown in the exocytic response during prolonged dialysis with a patch pipette. Thus, in rat chromaffin cells it appears that α-SNAP has two distinct actions, only one of which is shared by β-SNAP.
There is strong evidence from previous studies in chromaffin cells that one action of oversupplying α-SNAP is to increase the supply of releasable granules (Kibble et al., 1996; Xu et al., 1999). However, the preferential action of α-SNAP on exocytosis evoked by [Ca2+]i between 100 and 300 nm cannot be explained by an increase in the size of the readily releasable pool of chromaffin granules alone. If α-SNAP simply increased the size of this pool, the stimulation of exocytosis would be evident over a wider range of [Ca2+]i. Another argument against this possible mechanism is that α-SNAP failed to cause any increase (for the first 7 min of dialysis) in the amplitude of the exocytic response triggered by trains of depolarizations. Because such stimuli effectively exhaust granules in the “readily releasable pool” and mobilize additional granules to replenish this pool in chromaffin cells (von Ruden and Neher, 1993; Horrigan and Bookman, 1994; Engisch et al., 1997; Voets et al., 1999; Xu and Tse, 1999), the responses to these stimuli would be enhanced if α-SNAP were increasing the pool. The overall Ca2+dependence of the α-SNAP effect also rules out two other possible mechanisms. First, α-SNAP is unlikely to increase the sensitivity of a low Ca2+ affinity form of exocytosis. This component is typically half-saturated ∼10 μm in chromaffin cells when [Ca2+]i is raised rapidly (Heinemann et al., 1994), and a change in its sensitivity to Ca2+ would cause a parallel shift in the relationship between [Ca2+]i and exocytosis to lower [Ca2+]i levels, which was not observed (Fig. 5). Second, this effect cannot be explained by the stimulation of a Ca2+-independent “constitutive” exocytosis, because there was no enhancement of the rate of exocytosis at very low [Ca2+]i. Therefore, the most conservative interpretation is that α-SNAP works late in the cascade of events that leads to vesicle fusion by selectively enhancing a component of Ca2+-dependent exocytosis that has a relatively high affinity for Ca2+(essentially saturated at 500 nm[Ca2+]i).
The molecular mechanisms underlying this late action of α-SNAP in membrane fusion are not yet clear. NSF and α-SNAP preferentially break cis-SNARE complexes to increase the formation oftrans-SNARE complexes that mediate membrane fusion and are resistant to NSF (Weber et al., 2000). It is possible that such a mechanism underlies our observations. Specifically, α-SNAP may be working by dissociating cis-SNARE complexes and thereby promoting a subsequent [Ca2+]-induced formation of trans-SNARE complexes that lead to membrane fusion (Chen et al., 1999). α- and β-SNAP may have differing actions on the interaction of SNARE proteins with synaptotagmin, a protein implicated in Ca2+ regulation of exocytosis (DeBello et al., 1993; Littleton and Bellen, 1995; Sudhof and Rizo, 1996; Augustine et al., 1999). α-SNAP has been reported to displace synaptotagmin from the SNARE complex, whereas β-SNAP has the opposite effect (Sollner et al., 1993a; Schiavo et al., 1995). Thus another possible explanation for our observations is that exogenous α-SNAP promotes dissociation of synaptotagmin from the SNARE complex, thereby changing the Ca2+ requirement for triggering exocytosis. Although speculative, this mechanism could account for the ability of α-SNAP to generate a population of vesicles that can be triggered to undergo Ca2+-dependent exocytosis at much lower [Ca2+]i. It is particularly intriguing that β-SNAP, which does not enhance exocytosis at 100–300 nm[Ca2+]i, is preferentially expressed in the brain (Whiteheart et al., 1993). Perhaps β-SNAP plays a significant role in the low-affinity form of Ca2+-dependent exocytosis that is expressed at many synapses (Augustine, 2001).
At first glance, our results appear to be at odds with previous studies reporting that α-SNAP causes a significant enhancement of catecholamine secretion from bovine adrenal chromaffin cells only at 1–10 μm[Ca2+]i(Chamberlain et al., 1995; Morgan and Burgoyne, 1995) and that different isoforms of SNAP are interchangeable in the “priming” of releasable granules in these cells (Morgan and Burgoyne, 1995; Sudlow et al., 1996). However, the arguments presented above indicate that α-SNAP may regulate an additional, late step in exocytosis that is distinct from the previously documented role of SNAPs in ATP-dependent priming of releasable vesicles (Chamberlain et al., 1995; Sudlow et al., 1996; Barnard et al., 1997; Xu et al., 1998) (for review, seeRobinson and Martin, 1998). The effect of SNAPs on vesicle priming may be evident in our experiments on rat chromaffin cells as a slowing of rundown of depolarization-triggered exocytic responses; rundown was prevented equally well by α- and β-SNAP (Fig. 9B), as reported for the effects of SNAPs on vesicle priming (Morgan and Burgoyne, 1995). The effect of SNAPs on rundown of exocytosis took 10 min to develop (Fig. 9B), which is also consistent with a previous report (Xu et al., 1999) that the priming effect of α-SNAP occurs 8–10 min after beginning whole-cell dialysis of chromaffin cells (at 30–33°C).
Our data indicate that the ratio of the different isoforms of SNAP present in a cell significantly affects the rate of exocytosis at submicromolar [Ca2+]i. This range of [Ca2+]iis of physiological interest because it straddles the range of resting [Ca2+]i for constitutive exocytosis in all cell types, basal secretion in secretory cells, and spontaneous transmitter release from neurons. Thus, α-SNAP will influence the rate of exocytosis under such “resting” conditions. α-SNAP also will strengthen the influence that resting [Ca2+]i levels have on exocytosis resulting from stimulus-induced rises in [Ca2+]i. For example, consider the case in which a pool of releasable vesicles is examined by measuring exocytosis triggered by a sudden elevation of [Ca2+]i to micromolar levels. Dialysis of a cell for 5 min with a solution containing 500 nm α-SNAP will increase the rate of ATP-dependent vesicle priming. With a resting [Ca2+]i of 100–200 nm, α-SNAP might cause little or no increase in the pool of releasable vesicles because these vesicles will be exocytosed at an elevated rate as they are primed. Subsequent elevation of [Ca2+]i to the micromolar range might then produce an exocytic response indistinguishable from that of control conditions. In contrast, if resting [Ca2+]i is 50 nm, a level at which α-SNAP causes little increase in “basal” exocytosis, then α-SNAP might cause a larger increase in the pool of releasable vesicles because the releasable pool will not be tapped by the low resting [Ca2+]i. In this case, elevating [Ca2+]i to the micromolar range will yield a larger exocytic response than in conditions in which the α-SNAP was not added. Given that the submicromolar range of [Ca2+]i triggers various forms of presynaptic plasticity, such as augmentation and post-tetanic potentiation (Swandulla et al., 1991; Tank et al., 1995;Zucker 1999; Dittman et al., 2000), it is possible that SNAPs are involved in such forms of plasticity.
In summary, our experiments show that SNAPs have multiple roles in exocytosis in rat chromaffin cells. One role, supported by both α- and β-SNAP, is to prime chromaffin granules for fusion. The other role is to confer a high sensitivity to submicromolar [Ca2+] levels; this function is unique to α-SNAP and is antagonized by β-SNAP. Further studies will help to identify the molecular bases for these two actions of this important family of SNARE-related proteins.
Footnotes
This work was supported by the Canadian Institutes of Health Research (Grant MOP-12070 to F.W.T.) and the Alberta Heritage Foundation for Medical Research (Senior Scholar Award to F.W.T.), as well as the National Institutes of Health (Grant NS-21624 to G.J.A. and Grant GM53395 to G.C.R.E.-D.). We thank A. Tse and W. F. Dryden for comments and J. Morgan for help with the α-SNAP mutagenesis.
Correspondence should be addressed to Frederick W. Tse, Department of Pharmacology, 9-70 Medical Science Building, University of Alberta, Edmonton, Alberta, Canada, T6G 2H7. E-mail:Fred.Tse@Ualberta.ca.
REFERENCES
- 1.Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 2.Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol (Lond) 1992;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustine GJ, Burns ME, DeBello WM, Hilfiker S, Morgan JR, Schweizer FE, Tokumaru H, Umayahara K. Proteins involved in synaptic vesicle trafficking. J Physiol (Lond) 1999;520:33–41. doi: 10.1111/j.1469-7793.1999.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard RJO, Morgan A, Burgoyne RD. Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J Cell Biol. 1997;139:875–873. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain LH, Roth D, Morgan A, Burgoyne RD. Distinct effects of alpha-SNAP, 4–2-2 proteins, and calmodulin on priming and triggering of regulated exocytosis. J Biol Chem. 1995;130:1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YA, Scales SJ, Patel SM, Doung Y-C, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 7.Chow RH, von Ruden L. Electrochemical detection of secretion from single cells. In: Sakmann B, Neher E, editors. Single-channel recording. Plenum; New York: 1995. pp. 245–276. [Google Scholar]
- 8.Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- 9.DeBello WE, Betz H, Augustine GJ. Synaptotagmin and neurotransmitter release. Cell. 1993;74:947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- 10.DeBello WE, O'Connor V, Dresbach T, Whiteheart SW, Wang SS-H, Schweizer FE, Betz H, Rothman JE, Augustine GJ. SNAP mediated protein-protein interactions essential for neurotransmitter release. Nature. 1995;373:626–630. doi: 10.1038/373626a0. [DOI] [PubMed] [Google Scholar]
- 11.Dittman JS, Krietzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis-Davies GCR. Synthesis of photosensitive EGTA derivatives. Tetrahedron Lett. 1998;39:953–956. [Google Scholar]
- 13.Engisch KL, Chernevskaya NI, Nowycky MC. Short-term changes in the Ca2+–exocytosis relationship during repetitive pulse protocols in bovine adrenal chromaffin cells. J Neurosci. 1997;17:9010–9025. doi: 10.1523/JNEUROSCI.17-23-09010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham ME, Burgoyne RD. Comparison of cysteine string protein (Csp) and mutant α-SNAP overexpression reveals a role of Csp in late steps of membrane fusion in dense-core exocytosis in adrenal chromaffin cells. J Neurosci. 2000;20:1281–1289. doi: 10.1523/JNEUROSCI.20-04-01281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 16.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;397:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Hanson PI, Heuser JE, Jahn R. Neurotransmitter release—four years of SNARE complexes. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 18.He P, Southhard RC, Chen D, Whiteheart SW, Cooper RL. Role of α-SNAP in promoting efficient neurotransmission at the crayfish neuromuscular junction. J Neurophysiol. 1999;82:3406–3416. doi: 10.1152/jn.1999.82.6.3406. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann C, Chow RH, Neher E, Zucker RS. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994;67:2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrinton J, Bookman RJ. Pulse control V: Igor XOPs for patch clamp data acquisition. University of Miami; Miami: 1994. [Google Scholar]
- 21.Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 22.Kibble AV, Barnard RJO, Burgoyne RD. Patch-clamp capacitance analysis of α-SNAP on exocytosis in adrenal chromaffin cells. J Cell Sci. 1996;109:2417–2422. doi: 10.1242/jcs.109.9.2417. [DOI] [PubMed] [Google Scholar]
- 23.Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Arch. 1985;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- 24.Littleton JT, Bellen HJ. Synaptotagmin controls and modulates synaptic-vesicle fusion in a Ca2+-dependent manner. Trends Neurosci. 1995;18:177–183. doi: 10.1016/0166-2236(95)93898-8. [DOI] [PubMed] [Google Scholar]
- 25.Morgan A, Burgoyne RD. A role for soluble NSF attachment proteins (SNAPs) in regulated exocytosis in adrenal chromaffin cells. EMBO J. 1995;14:232–239. doi: 10.1002/j.1460-2075.1995.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan A, Dimaline R, Burgoyne RD. The ATPase activity of N-ethylmaleimide-sensitive fusion protein (NSF) is regulated by soluble NSF attachment proteins. J Biol Chem. 1994;269:29347–29350. [PubMed] [Google Scholar]
- 27.Nagamatsu S, Watanabe T, Nakamichi Y, Yamamura C, Tsuzuki K, Matsushima S. α-Soluble N-ethylmaleimide-sensitive factor attachment protein is expressed in pancreatic β cells and functions in insulin but not γ-aminobutyric acid secretion. J Biol Chem. 1999;274:8053–8060. doi: 10.1074/jbc.274.12.8053. [DOI] [PubMed] [Google Scholar]
- 28.Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- 30.Parsons TD, Coorssen JR, Horstman H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 31.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- 32.Robinson LJ, Martin TFJ. Docking and fusion in neurosecretion. Curr Opin Cell Biol. 1998;10:483–492. doi: 10.1016/s0955-0674(98)80063-x. [DOI] [PubMed] [Google Scholar]
- 33.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 34.Schiavo G, Gmachl MJS, Stenbeck G, Sollner TH, Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 35.Seward EP, Nowycky MC. Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells in response to trains of depolarizing pulses. J Neurosci. 1996;16:553–562. doi: 10.1523/JNEUROSCI.16-02-00553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 37.Sollner T, Whiteheart SW, Brunner M, Erdjumnet-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 38.Steyer JA, Horstmann H, Almers W. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- 39.Sudlow AW, McFerran BW, Bodhill H, Barnard RJO, Morgan A, Burgonyne RD. Similar effects of α- and β-SNAP on Ca2+-regulated exocytosis. FEBS Lett. 1996;393:185–188. doi: 10.1016/0014-5793(96)00880-0. [DOI] [PubMed] [Google Scholar]
- 40.Sudhof TC, Rizo J. Synaptotagmins: C2 domain proteins that regulate vesicle traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 41.Swandulla D, Hans M, Zipser K, Augustine GJ. Role of residual calcium in synaptic depression and post-tetanic potentiation: fast and slow calcium signal in nerve terminals. Neuron. 1991;7:915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]
- 42.Tank DW, Regehr WG, Delaney KR. A quantitative analysis of presynaptic calcium dynamics that contribute to short-term enhancement. J Neurosci. 1995;15:7940–7952. doi: 10.1523/JNEUROSCI.15-12-07940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas P, Wong JG, Lee AK, Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993;11:93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- 44.Tokumaru H, Augustine GJ (2002) SNARE proteins in neurotransmitter release. Trends Neurosci, in press.
- 45.Tse FW, Tse A. α-Latrotoxin stimulates inward current, rise in cytosolic calcium concentration, and exocytosis in pituitary gonadotrophs. Endocrinology. 1999;140:3025–3033. doi: 10.1210/endo.140.7.6849. [DOI] [PubMed] [Google Scholar]
- 46.Tse FW, Tse A. Stimulation of Ca2+-independent exocytosis in rat pituitary gonadotrophs by G-protein. J Physiol (Lond) 2000;526:99–108. doi: 10.1111/j.1469-7793.2000.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tse FW, Tse A, Hille B, Horstmann H, Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- 48.Voets T, Neher E, Mosaer T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- 49.von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- 50.Weber T, Parlati F, McNew JA, Johnston RJ, Westermann B, Sollner TH, Rothman JE. SNARE pins are functionally resistant to disruption by NSF and α SNAP. J Cell Biol. 2000;149:1063–1072. doi: 10.1083/jcb.149.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiteheart SW, Griff IC, Brunner M, Clary DO, Mayer T, Buhrow SA, Rothman JE. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- 52.Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DD, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Tse FW. Brefeldin A increases the quantal size and alters the kinetics of catecholamine release from rat adrenal chromaffin cells. J Biol Chem. 1999;274:19095–19102. doi: 10.1074/jbc.274.27.19095. [DOI] [PubMed] [Google Scholar]
- 54.Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- 55.Xu T, Ashery U, Burgoyne RD, Neher E. Early requirement for α-SNAP and NSF in the secretory cascade in chromaffin cells. EMBO J. 1999;18:3293–3304. doi: 10.1093/emboj/18.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
- 57.Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]