Abstract
Recent evidence suggests that internal calcium stores and calcium-induced calcium release (CICR) provide an important source of calcium that drives short-term presynaptic plasticity at central synapses. Here we tested for the involvement of CICR in short-term presynaptic plasticity at six excitatory synapses in acute rat hippocampal and cerebellar brain slices. Depletion of internal calcium stores with thapsigargin and prevention of CICR with ryanodine have no effect on paired-pulse facilitation, delayed release of neurotransmitter, or calcium-dependent recovery from depression. Fluorometric calcium measurements also show that these drugs have no effect on the residual calcium signal that underlies these forms of short-term presynaptic plasticity. Finally, although caffeine causes CICR in Purkinje cell bodies and dendrites, it does not elicit CICR in parallel fiber inputs to these cells. Taken together, these results indicate that for the excitatory synapses studied here, internal calcium stores and CICR do not contribute to short-term presynaptic plasticity on the milliseconds-to-seconds time scale. Instead, this plasticity is driven by the residual calcium signal arising from calcium entry through voltage-gated calcium channels.
Keywords: hippocampus, cerebellum, internal calcium stores, calcium-induced calcium release, ryanodine, thapsigargin, short-term presynaptic plasticity, presynaptic residual calcium
Short-term presynaptic plasticity on the milliseconds-to-seconds time scale allows synapses to continually modulate neurotransmitter release in response to presynaptic activity (Magleby, 1987; Zucker, 1989, 1999; Regehr and Stevens, 2001). Widespread forms of this plasticity include paired-pulse facilitation (PPF), Ca-dependent recovery from depression (CDR) and delayed release of neurotransmitter (DR). PPF is prominent at synapses with a low initial probability of release and is characterized by increased release in response to sequential presynaptic action potentials (Katz and Miledi, 1968; Atluri and Regehr, 1996). In contrast, depression predominates at synapses with a high initial probability of release (Eccles et al., 1941; Feng, 1941), and early recovery from this depression is accelerated by CDR (Dittman and Regehr, 1998; Wang and Kaczmarek, 1998). Finally, DR is found at many synapses and represents an increase in neurotransmitter release for hundreds of milliseconds after presynaptic activity (Barrett and Stevens, 1972; Rahamimoff and Yaari, 1973; Zengel and Magleby, 1981; Zucker and Lara-Estrella, 1983;Cohen and Van der Kloot, 1986; Goda and Stevens, 1994; Van der Kloot and Molgo, 1994; Atluri and Regehr, 1998). Each of these short-term plasticities is driven by the residual Ca signal that persists in the terminal after presynaptic activity.
There is currently debate over the contribution of different Ca sources to the residual Ca signal and short-term presynaptic plasticity at excitatory central synapses. Voltage-gated Ca channels are one important source of Ca (Katz and Miledi, 1967; Dunlap et al., 1995;Mintz et al., 1995). Recent studies indicate that Ca-induced Ca release (CICR) from internal Ca stores may be another important Ca source (Peng, 1996; Smith and Cunnane, 1996; Mothet et al., 1998; Narita et al., 1998; Krizaj et al., 1999; Llano et al., 2000; Narita et al., 2000; Emptage et al., 2001). Ca binding to ryanodine receptors located on internal Ca stores gates the opening of these receptors and triggers Ca release into the cytosol (Sitsapesan et al., 1995; Berridge, 1998). At peripheral synapses, extended trains of presynaptic activity can elicit CICR, which can in turn regulate neurotransmitter release (Peng, 1996; Smith and Cunnane, 1996; Narita et al., 1998, 2000). Presynaptic internal Ca stores and ryanodine receptors are present at some inhibitory central synapses, and CICR can influence spontaneous release rates and even elicit multivesicular release at inhibitory synapses onto cerebellar Purkinje cells (Llano et al., 2000). Although there have been few studies at excitatory central synapses, recent results at the associational–commissural (AC) synapse in the CA3 region of the hippocampus suggest that CICR contributes to both the residual Ca signal and PPF (Emptage et al., 2001). These results suggest that internal Ca stores and CICR may be an important Ca source contributing to short-term presynaptic plasticity at excitatory central synapses.
Here we survey the importance of internal Ca stores and CICR at multiple excitatory central synapses in acute hippocampal and cerebellar brain slices from young rats. Using caffeine to release Ca via ryanodine receptors, we show that significant CICR occurs in Purkinje cells but not at the presynaptic parallel fibers. Furthermore, using whole-cell voltage-clamp recordings and fluorometric Ca measurements, we show that depleting internal Ca stores or blocking ryanodine receptors has no effect on PPF, DR, CDR, or the residual Ca signals that drive these plasticities. These results indicate that at central excitatory synapses, internal Ca stores and CICR do not generally make important contributions to either the residual Ca signal or short-term presynaptic plasticity.
MATERIALS AND METHODS
Slices were cut from postnatal day 10–22 Sprague Dawley rats using standard procedures. Animals were anesthetized with halothane and decapitated, and their brains were rapidly removed and placed in ice-cold dissection solution equilibrated with 95% O2 and 5% CO2. For experiments using cerebellar slices, the dissection solution was the artificial CSF (ACSF; in mm: 125 NaCl, 26 NaHCO3, 25 Glucose, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2 and 2 CaCl2). For experiments using hippocampal slices, the dissection solution contained (in mm): 79 NaCl, 68 sucrose, 24 NaHCO3, 23 glucose, 2.3 KCl, 1.14 NaH2PO4, 6.4 MgCl2, and 0.46 CaCl2. Transverse cerebellar slices were prepared as described by Atluri and Regehr (1996); sagittal cerebellar slices were prepared as described byKreitzer and Regehr (2000); and hippocampal slices were prepared as described by Vogt and Regehr (2001). After preparation, hippocampal slices were held at 32°C for 20 min and then transferred to ACSF (2 mm MgCl2 and 3 mmCaCl2). All slices were held at room temperature (22–24°C) after 1 hr at 32°C. All experiments were performed at room temperature with 20 μm bicuculline in the ACSF. The perfusion tubing and recording chamber were either replaced or washed with ethanol before and after experiments using ryanodine, thapsigargin or AM 251. Caffeine, ryanodine, thapsigargin, baclofen, and bicuculline were purchased from Sigma (St Louis, MO); CNQX, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), and AM 251 were purchased from Tocris (Bristol, UK).
Electrophysiology. Whole-cell voltage-clamp recordings were obtained under visual control, using pipettes filled with an internal solution containing (in mm): 100 CsCl, 35 CsF, 10 EGTA, 10 HEPES, and 0.1 (±)-methoxyverapamil hydrochloride, pH 7.4. Pipette resistances were 2–3 MΩ for CA1 and CA3 pyramidal cells, 1–1.5 MΩ for Purkinje cells, and 2–3 MΩ for stellate cells. Access resistances were 5–15 MΩ for CA1 and CA3 pyramidal cells, 2–5 MΩ for Purkinje cells, and 5–10 MΩ for stellate cells. Access resistance and leak current were continually monitored, and experiments were discarded if either changed appreciably. Cells were voltage-clamped at −60 mV for CA1 and CA3 pyramidal cells, −40 mV for Purkinje cells, and −70 mV for stellate cells. Extracellular glass stimulus electrodes were filled with ACSF and placed in the afferent fiber tract. Stimulation of AC and mossy fiber (MF) synapses was as described by Vogt and Regehr (2001). Square pulses (5–20 μA) of 0.2 msec duration were used to evoke EPSCs. In some cases, a second stimulus electrode was placed nearby to minimize stimulus artifacts. For experiments studying AC and MF synapses, 0.1 μm NBQX was used to prevent contributions from recurrent excitation (Salin et al., 1996), and we confirmed MF synapses using 10 μm(2S,1′S,2′S)-2-(carboxycyclopropyl)glycine (Kamiya et al., 1996; Vogt and Regehr, 2001).
Presynaptic labeling and Ca measurements. Presynaptic fiber tracts were labeled with AM esters of either Magnesium Green or Oregon Green 488 BAPTA-1 (Molecular Probes, Eugene, OR), as described previously (Regehr and Tank, 1991; Regehr and Atluri, 1995). AC and MF fiber tracts were labeled as described by Vogt and Regehr (2001), parallel fiber (PF) tracts were labeled as described by Regehr and Atluri (1995), and individual climbing fibers were labeled usingin vivo injection of Fluo-4 Dextran (Molecular Probes) as described by Kreitzer et al. (2000). Slices were placed on an upright microscope (Olympus Optical, Tokyo, Japan; or Zeiss, Thornwood, NY) and visualized with either a 40× or 60× water immersion objective. Stimulus electrodes were placed as for electrophysiology experiments. A small region of labeled fibers was illuminated, and fluorescence signals were measured with a photodiode (Regehr and Atluri, 1995;Kreitzer et al., 2000; Vogt and Regehr, 2001). The Magnesium Green, Oregon Green 488 BAPTA-1, and Fluo-4 Dextran filter set was 450–490 excitation, 510 dichroic, and 520 emission. With increasing Ca concentrations, Magnesium Green, Oregon Green 488 BAPTA-1, and Fluo-4 Dextran fluorescence increases.
Postsynaptic labeling and Ca measurements. Whole-cell voltage-clamp recordings of Purkinje cells were obtained using pipettes filled with an internal solution containing (in mm): 130 CsGlu, 20 CsCl, 2 MgCl2, 0.2 EGTA, 10 HEPES, 4 Na2ATP, 0.4 NaGTP, and 0.2 Oregon Green 488 BAPTA-1, pH 7.4. Pipette resistance was 2–3 MΩ; access resistance was 5–15 MΩ; and Purkinje cells were voltage-clamped at −60 mV. After obtaining whole-cell recordings, Purkinje cells were allowed to fill with 200 μm Oregon Green 488 BAPTA-1 for 5–10 min. Fluorescence signals from the Purkinje cell soma and proximal dendrites were measured with a photodiode. In some experiments, Purkinje cells were depolarized between trials to elicit action potentials that replenished internal Ca stores and allowed stable caffeine-evoked CICR. During these experiments, 10 μm NBQX and 1 μm TTX were present in the ACSF.
Focal application of drugs. Drugs were loaded into glass micropipettes with a tip diameter of 2–5 μm. Pipettes were attached to a pneumatic injection system (PV820; World Precision Instruments, Sarasota, FL), and pressure pulses at 3–5 psi for 5 sec duration were used to eject drugs into the ACSF. The system was calibrated with a solution containing fast green to detect leakage of pipette solution or back-filling of pipettes with ACSF. Pipettes were placed ∼10–20 μm above the slice and ∼10–20 μm upstream from the recording site. Although pipettes contained high concentrations of drugs, the concentration reaching the cell was considerably diluted.
Data acquisition and analysis. Outputs from both the photodiode and the AxoPatch 200A or 200B amplifiers were digitized with a 16-bit digital-to-analog converter (Instrutech, Port Washington, NY), Pulse Control software (Herrington and Bookman, 1995), and a Macintosh computer (Apple, Cupertino, CA). Analysis was done on- and off-line using Igor Pro software (Wavemetrics, Lake Oswego, OR). Whole-cell recordings were filtered at 2–5 kHz with an eight-pole Bessel filter. Photodiode recordings of stimulus-evoked fluorescence signals were digitally filtered at 500 or 200 Hz with a four-pole Bessel filter. Photodiode recordings of puff-evoked fluorescence signals in Figure 1were digitally filtered at 10 Hz with a four-pole Bessel filter. Data are reported as average ± SEM.
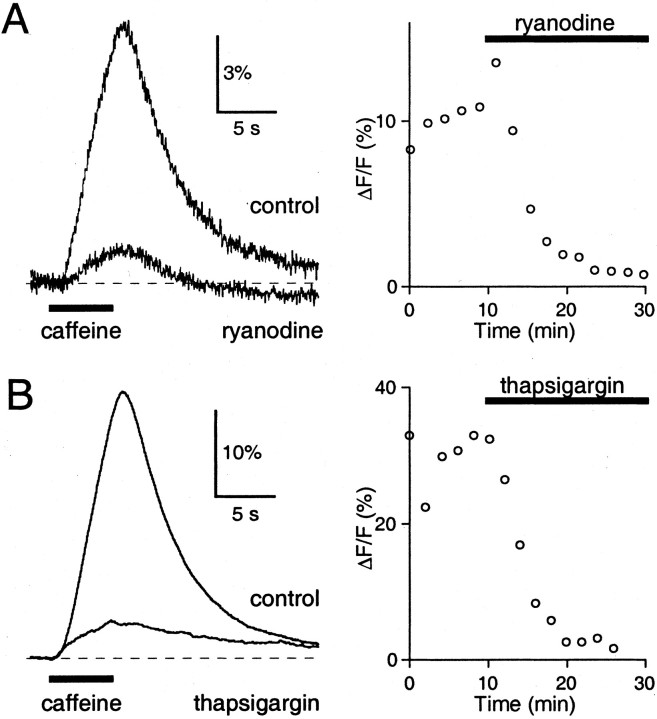
Fig. 1.
Ryanodine and thapsigargin abolish caffeine-evoked CICR in Purkinje cells. A Purkinje cell was filled with 200 μm Oregon Green 488 BAPTA-1 via a whole-cell recording pipette, and 40 mm caffeine was applied using a 5 sec pressure puff from a nearby micropipette. Fluorescence measurements were restricted to a small area that included the Purkinje cell soma and proximal dendrites. A, In control conditions, a ΔF/F signal was recorded (left) in response to caffeine application (solid bar). Bath application of 10 μm ryanodine abolished this ΔF/F signal. The time course is shown on the right, with the solid bar indicating ryanodine application. B, Similar results were found for bath application of 10 μmthapsigargin. Representative traces are averages of four or five trials.
RESULTS
We examined the role of internal calcium stores and CICR in short-term presynaptic plasticity and presynaptic residual calcium signals at six excitatory central synapses in acute hippocampal and cerebellar brain slices of young rats. These synapses were studied because they exhibit many forms of short-term presynaptic plasticity and are amenable to presynaptic Ca measurements. Internal Ca stores were depleted with thapsigargin (Treiman et al., 1998), which inhibits the Ca-ATPases that load these stores. CICR was blocked with high concentrations of ryanodine (Sitsapesan et al., 1995).
Prominent caffeine-evoked CICR at Purkinje cells
We assessed the efficacy of ryanodine and thapsigargin by testing their ability to disrupt caffeine-evoked Ca signals in Purkinje cells (Llano et al., 1994; Kano et al., 1995). Purkinje cells were loaded with the Ca indicator Oregon Green 488 BAPTA-1, and fluorescence signals were monitored from the soma and proximal dendrites. Pressure application of caffeine from a nearby extracellular micropipette led to CICR in Purkinje cells, which caused a Ca-evoked fluorescence increase (Fig. 1), measured as a change in fluorescence over background fluorescence (ΔF/F) signal. After obtaining a stable ΔF/F signal in response to caffeine application at 2 min intervals, we washed ryanodine or thapsigargin into the bath. For the representative experiments shown in Figure 1, the peak ΔF/F signal was reduced to 11.3% of control for 10 μmryanodine (Fig. 1A) and 10.1% of control for 10 μm thapsigargin (Fig. 1B). In general, the peak ΔF/F signal was reduced to 7.5 ± 5.8% of control for 100 μmryanodine (n = 3), 9.4 ± 4.7% of control for 10 μm ryanodine (Fig. 1A;n = 5), and −1.4 ± 3.6% of control for 10 μm thapsigargin (Fig. 1B;n = 6). In these experiments, a small ΔF/F signal often persisted even after prolonged exposure to ryanodine or thapsigargin. This signal may reflect residual CICR not blocked by ryanodine or thapsigargin, or may reflect a mechanical artifact that can also be observed with puff application of external solution alone. High concentrations of caffeine can also directly affect the properties of Ca indicators via nonspecific, hydrophobic interactions with the fluorophore (Muschol et al., 1999), and this could also contribute to the remaining ΔF/F signal. These experiments demonstrate that either ryanodine or thapsigargin effectively prevents CICR in Purkinje cells.
Lack of caffeine-evoked CICR at parallel fibers
We next used pressure application of caffeine to directly test for CICR at the parallel fiber presynaptic inputs to Purkinje cells (Fig.2). Parallel fibers were loaded with the high-affinity Ca indicator Oregon Green 488 BAPTA-1 AM. Fluorescence signals were monitored from a region of parallel fibers 300–500 μm from the loading site, and parallel fibers were stimulated with an extracellular electrode. Drugs were pressure-applied from an extracellular micropipette near the recording site.
Fig. 2.
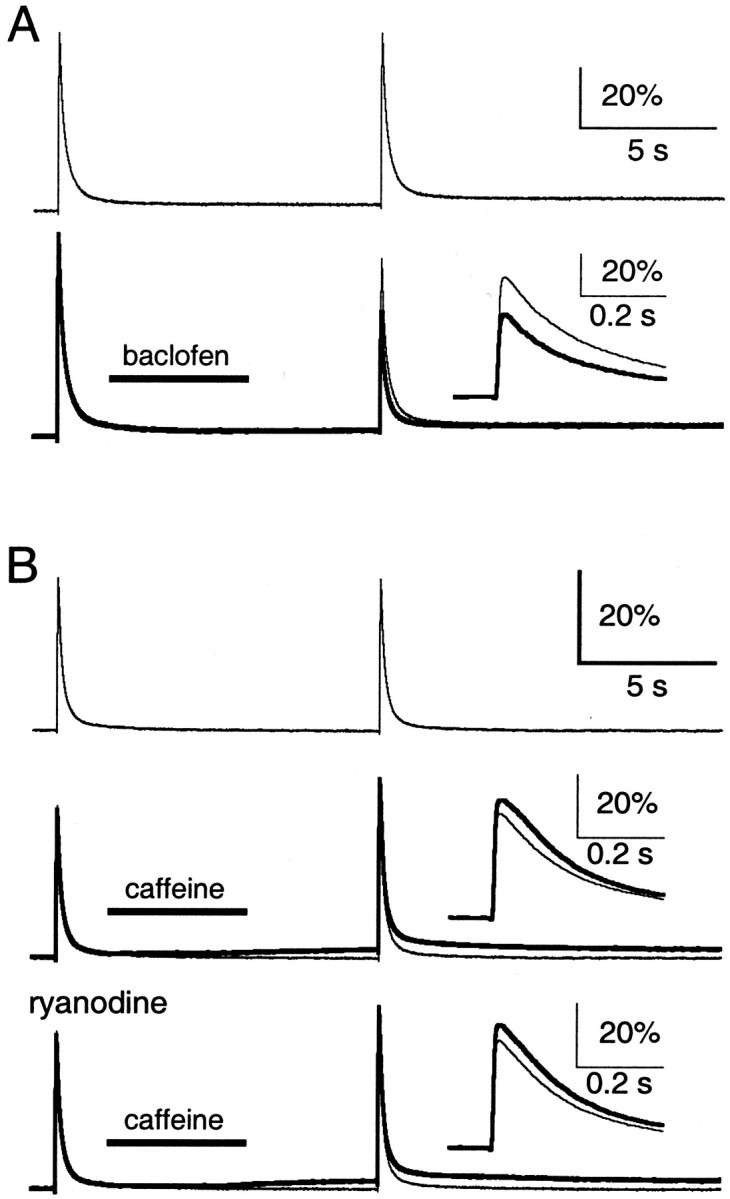
Lack of caffeine-evoked CICR at the parallel fibers. Parallel fibers were filled with Oregon Green 488 BAPTA-1 AM. In each trial, parallel fibers were stimulated with a control and test stimulus separated by 10 sec. A, ΔF/F signals in the absence of drug application (top, bottom, light traces) and with puff application of 500 μm baclofen (bottom, bold trace).Inset, Test ΔF/F signals with baclofen (bold trace) or without (light trace) on an expanded time scale. B, ΔF/F signals in the absence of drug application (top, middle, bottom, light trace) and with puff application of 40 mm caffeine, in the absence (middle, bold trace) and presence (bottom, bold trace) of 10 μm ryanodine. Insets, Test ΔF/F signals with caffeine (bold trace) or without (light trace) on an expanded time scale. Representative traces are averages of three to five trials. For insets in B, the slow ΔF/F signal has been subtracted.
The efficacy of pressure application was tested with baclofen, which inhibits presynaptic Ca channels by activating GABAB receptors (Mintz and Bean, 1993; Dittman and Regehr, 1996). As shown in a representative experiment, baclofen (500 μm) greatly reduced the test stimulus-evoked ΔF/F signal (Fig. 2A). In five such experiments, baclofen reduced the peak of this signal to 52.9 ± 5.6% of control.
In contrast, caffeine had small effects on the ΔF/F signals. Caffeine (40 mm) produced a slow ΔF/Fsignal that was much smaller than the stimulus-evoked ΔF/F signal (Fig. 2B) and was often in an opposite direction from the mechanical artifact. In 11 such experiments, the slow ΔF/F signal was 13.9 ± 2.5% of the control stimulus-evoked ΔF/Fsignal. Caffeine also produced a slight increase in the test stimulus-evoked ΔF/F signal (Fig.2B). In 11 such experiments, caffeine increased the peak of this signal by 12.3 ± 3.4%.
Unlike the caffeine-evoked ΔF/F signal seen in Purkinje cells (Fig. 1), bath application of ryanodine or thapsigargin did not affect the ΔF/F signals seen in parallel fibers (Fig. 2B). The slow ΔF/F signal was still 11.9 ± 3.0% (n = 3) of the control stimulus-evoked ΔF/F signal in 10 μmryanodine and 11.9 ± 4.7% (n = 5) of this signal in 10 μm thapsigargin. Moreover, caffeine continued to increase the peak of the test stimulus-evoked ΔF/F signal by 13.9 ± 4.5% (n = 3) in ryanodine and 9.5 ± 3.2% (n = 5) in thapsigargin. The slow ΔF/F signal and the small increase in the test stimulus-evoked ΔF/F signal thus likely reflect a direct interaction of caffeine with the Ca indicator (Muschol et al., 1999). These results suggest that CICR may not be important for presynaptic Ca signaling at parallel fiber synapses. We thus proceeded to test the role of Ca stores and CICR on short-term presynaptic plasticity and the presynaptic residual Ca signal at this and other excitatory central synapses.
Paired-pulse facilitation
We next used ryanodine and thapsigargin to test for the involvement of internal Ca stores in PPF. These studies were conducted at 4 different excitatory synapses: the cerebellar parallel fiber to Purkinje cell (PF→PC) synapse, the hippocampal AC synapse between CA3 pyramidal cells, the hippocampal MF synapse between dentate gyrus granule cells and CA3 pyramidal cells, and the hippocampal Schaffer collateral (SC) synapse between CA3 and CA1 pyramidal cells. EPSCs were monitored with whole-cell voltage-clamp recordings. Synaptic inputs were stimulated with pairs of pulses separated by 25 or 75 msec. PPF is defined as A2/A1, where A1 andA2 are the amplitudes of the EPSCs evoked by the first and second pulses, respectively. PPF25 and PPF75 indicate the PPF amplitude for the two interpulse intervals. PPF25 and PPF75 were prominent at all these synapses, with values of 2.67 ± 0.14 and 2.09 ± 0.09 (n = 8) at the PF→PC synapse, 2.08 ± 0.11 and 1.88 ± 0.09 (n = 12) at the AC synapse, 3.48 ± 0.39 and 2.53 ± 0.34 (n = 4) at the MF synapse, and 1.92 ± 0.27 and 1.66 ± 0.14 (n= 5) at the SC synapse.
Neither thapsigargin nor ryanodine affected PPF at these synapses. A representative experiment is shown for the PF→PC synapse in Figure3A, in which thapsigargin did not affect the EPSC, PPF25, or PPF75. The percent changes for PPF25 and PPF75 relative to that seen in control conditions were −4.2 ± 5.1 and 4.9 ± 5.1%, respectively, for 10 μm thapsigargin (n = 4), −2.9 ± 6.3 and 2.0 ± 4.8% for 100 μm ryanodine (n = 4), and −3.5 ± 3.7 and 3.4 ± 3.3% for pooled thapsigargin and ryanodine experiments (n = 8). Hereafter, data from thapsigargin and ryanodine experiments are pooled. As shown in representative experiments, similar results were obtained using 10 μm ryanodine at the AC (Fig. 3B) and MF (Fig. 3C) synapses, and 100 μmryanodine at the SC synapse (Fig. 3D). The overall percent changes for PPF25 and PPF75 in thapsigargin or ryanodine were 2.7 ± 4.3 and 4.7 ± 5.0% (n = 12) at the AC synapse, −9.4 ± 13.6 and 11.9 ± 11.4% (n= 4) at the MF synapse, and −0.1 ± 19.0 and 2.5 ± 11.1% (n = 5) at the SC synapse. In some experiments, although PPF25 and PPF75 remained unchanged, 100 μm ryanodine decreased the peak EPSC. This may reflect a decrease in fiber excitability, because the prespike amplitude was also reduced by this high concentration of ryanodine (data not shown). Overall, these results indicate that internal Ca stores and CICR are not involved in PPF at these four synapses.
Fig. 3.
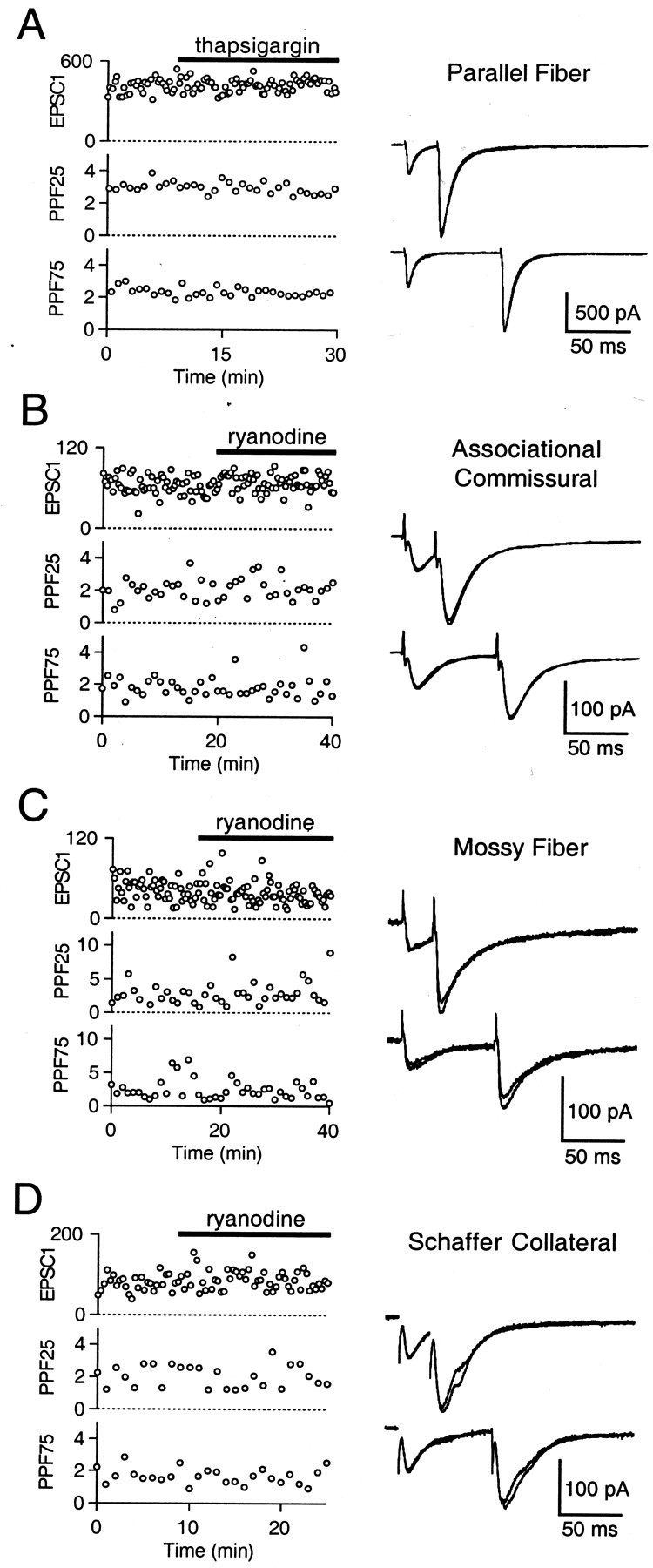
Disrupting CICR has no effect on paired-pulse facilitation at four excitatory synapses. A, At the PF→PC synapse, peak EPSC (EPSC1, picoamperes), PPF25, and PPF75 remain unchanged after bath application of 10 μm thapsigargin (solid bar). Representative traces (right) are superimposed averages of five trials before and after thapsigargin application. Similar results were found using 10 μmryanodine at the AC (B) and MF (C) synapses, and 100 μm ryanodine at the SC synapse (D).
Recent results demonstrate that presynaptic Ca signals and PPF at the PF→PC synapse can be modulated by retrograde signaling via Ca-dependent cannabinoid release from Purkinje cells (Kreitzer and Regehr, 2001). We tested the possibility that disrupting postsynaptic internal Ca stores in Purkinje cells can modify retrograde signaling to occlude any effects of disrupting presynaptic internal Ca stores. In the presence of 10 μm AM 251, an antagonist of CB1 receptors that blocks retrograde signaling, the percent changes for PPF25 and PPF75 in thapsigargin were −5.3 ± 5.0 and −8.0 ± 1.1% (n = 3), indicating that retrograde signaling does not occlude any presynaptic effect of thapsigargin.
Delayed release
DR is the continued release of neurotransmitter for hundreds of milliseconds after a presynaptic action potential. This phenomenon is driven by the residual Ca signal and is eliminated by chelators of presynaptic Ca (Cummings et al., 1996; Atluri and Regehr, 1998). The effects of ryanodine and thapsigargin on DR were assessed at the parallel fiber to stellate cell synapse. We recorded from stellate cells using whole-cell voltage clamp and examined synaptic inputs after a single pulse to the parallel fibers (Atluri and Regehr, 1998). As shown in a representative experiment, spontaneous quantal events before stimulation are rare in these cells, but stimulation produces prominent DR (Fig. 4Ai,top). After recording stable synaptic inputs for 10 min, 10 μm ryanodine was bath-applied for 25 min. The prominent DR was still apparent after prolonged wash-in of ryanodine (Fig. 4Ai, bottom). This is illustrated using a raster plot of quantal events recorded throughout the experiment (Fig. 4Aii). In general, we found that DR was unchanged by either ryanodine (10 μm,n = 4) or thapsigargin (10 μm,n = 4). This is shown using average poststimulus time histogram (PSTH) plots of quantal event frequency compiled 10 min before and after complete wash-in of ryanodine (Fig. 4B, top) or thapsigargin (Fig. 4B, bottom). These results indicate that CICR does not make a prominent contribution to the Ca signal that drives DR at this synapse.
Fig. 4.
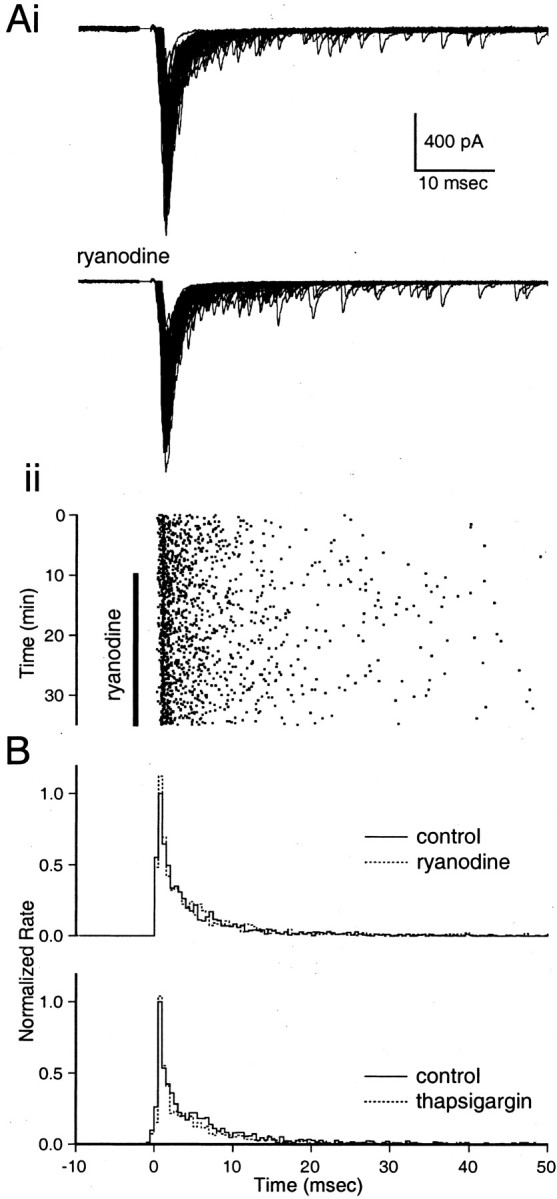
Disrupting CICR has no effect on delayed release at the parallel fiber to stellate cell synapse. Ai, Seventy consecutive traces before (top) and after (bottom) bath application of 10 μm ryanodine. Aii, Raster plot of quantal events, with the vertical bar indicating the time of ryanodine application. B, Average PSTH plots of quantal events before (solid line) and after (dashed line) 10 μm ryanodine (B, top;n = 4) or 10 μm thapsigargin (B, bottom; n = 4). PSTH plots for each experiment were made for 10 min periods in both control conditions and after 10 min drug application. These PSTH plots were then normalized with respect to the peak rate in control conditions. Normalized PSTH plots from the different experiments were then averaged.
Ca-dependent recovery from depression
The climbing fiber to Purkinje cell (CF→PC) synapse exhibits profound paired-pulse depression (PPD), characterized by decreased release in response to sequential presynaptic action potentials (Eccles et al., 1966; Dittman and Regehr, 1998; Hashimoto and Kano, 1998;Silver et al., 1998). The rapid phase of recovery from depression is driven by increases in presynaptic Ca, and is known as CDR (Dittman and Regehr, 1998; Wang and Kaczmarek, 1998). Ryanodine was used to test for the importance of CICR in CDR. We recorded from Purkinje cells using whole-cell voltage clamp and stimulated climbing fibers with pairs of pulses separated by varying interstimulus intervals. As shown in a representative experiment, 100 μm ryanodine had no effect on the initial EPSC amplitude (Fig.5A) or the recovery from depression (Fig. 5B). PPD is defined as (A1 − A2)/A1, and curves of PPD at different interstimulus intervals indicate the time course of recovery from depression (Fig. 5C). We fit these curves with a function of the form Ao +A1exp(−t/τ1) +A2exp(−t/τ2), with A1 and τ1corresponding to the CDR component. In control conditions the parameters {Ao,A1, τ1,A2, and τ2} were {7%, 35%, 57 msec, 57%, and 1.5 sec} (n = 17), and in the presence of ryanodine they were {4%, 45%, 65 msec, 50%, and 1.7 sec} (n = 5). For three cells, PPD curves were obtained both in control conditions {4%, 46%, 59 msec, 49%, and 1.9 sec} and in the presence of ryanodine {5%, 47%, 67 msec, 48%, and 1.6 sec}. The similarity of the amplitude and time course of the fast component of recovery from depression in ryanodine and control conditions suggests that CICR does not contribute to CDR at this synapse.
Fig. 5.
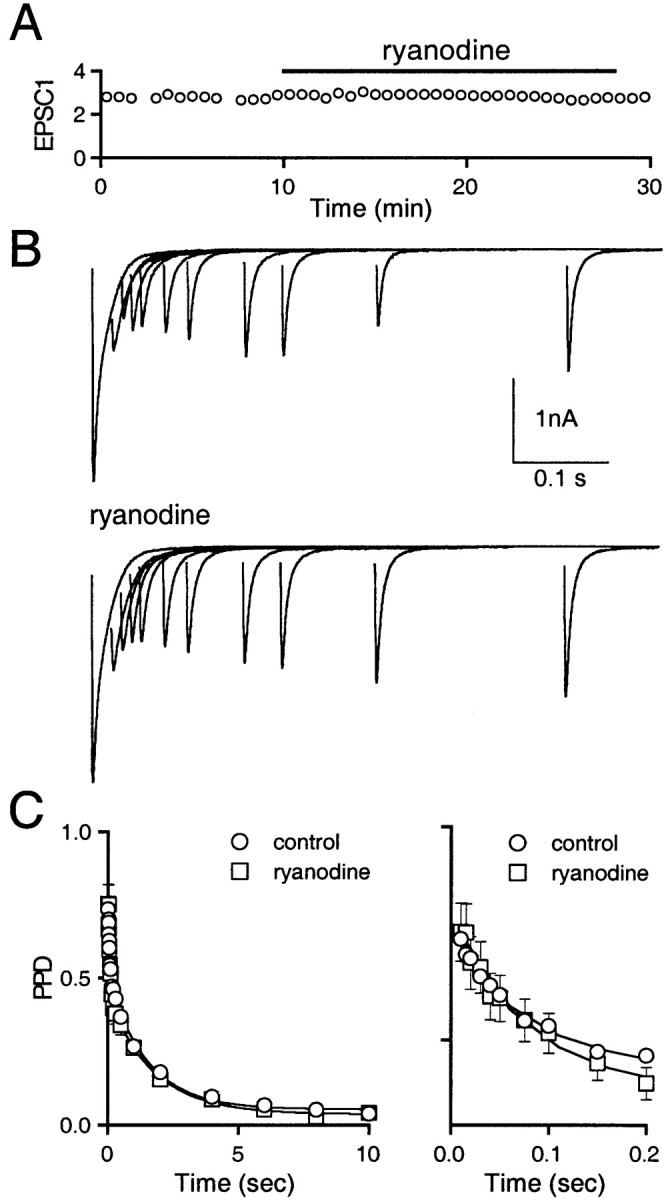
Disrupting CICR has no effect on calcium-dependent recovery from depression at the climbing fiber to Purkinje cell synapse. A, Initial EPSC amplitude (EPSC1, nanoamperes) remains unchanged after bath application of 100 μm ryanodine (solid bar). B, Representative tracesbefore (top) and after (bottom) bath application of ryanodine. C, PPD curves showing recovery from depression over 10 sec, with the first 200 msec expanded on theright. Representative traces inB are averages of two trials. PPD curves are averages ± SEM from 17 (control) or 5 (ryanodine) experiments.
Presynaptic residual Ca signal
We next used ryanodine and thapsigargin to test directly for the involvement of CICR in shaping the residual Ca signal at PF, AC, MF, and CF synapses. For these experiments, presynaptic fibers were labeled with low-affinity Ca indicators, which provide an accurate means of detecting changes in the amplitude and time course of residual Ca. Presynaptic fibers were activated with either single pulses or pairs of pulses separated by 25 or 75 msec, and the resulting ΔF/F signals were detected as described previously (Regehr and Atluri, 1995). A representative experiment for the effect of ryanodine on the residual Ca signal is shown for the PF synapse in Figure 6A. After recording stable peak ΔF/F responses for 10 min, ryanodine was bath-applied for 20 min. Ryanodine had no effect on either the peak or half-decay time of the ΔF/F signal (Fig. 6A). In general, the residual Ca signal at the PF synapse was unchanged by either ryanodine (100 μm,n = 2; 10 μm, n = 5) or thapsigargin (10 μm, n = 3). The overall percent changes for peak and half-decay time of the first ΔF/F signal in ryanodine or thapsigargin relative to that seen in control conditions were 5.2 ± 3.5 and −5.2 ± 2.4% (n = 10). The lack of effect of ryanodine or thapsigargin on Ca transients in parallel fibers evoked by single stimuli is consistent with the results of Sabatini and Regehr (1995). As shown for representative experiments, similar results were obtained using 10 μm ryanodine at the AC synapse (Fig. 6B), and 100 μmryanodine at the MF (Fig. 6C) and CF (Fig.6D) synapses. The overall percent changes for peak and half-decay time of the first ΔF/F signals in ryanodine or thapsigargin were −1.6 ± 4.4 and −0.2 ± 3.2% (n = 3) at the AC synapse, 0.8 ± 1.8 and −6.1 ± 2.7% (n = 3) at the MF synapse, and −7.1 ± 1.9 and 14.3 ± 6.3% (n = 5) at the CF synapse. These results indicate that CICR does not determine the size or time course of the residual Ca signal.
Fig. 6.
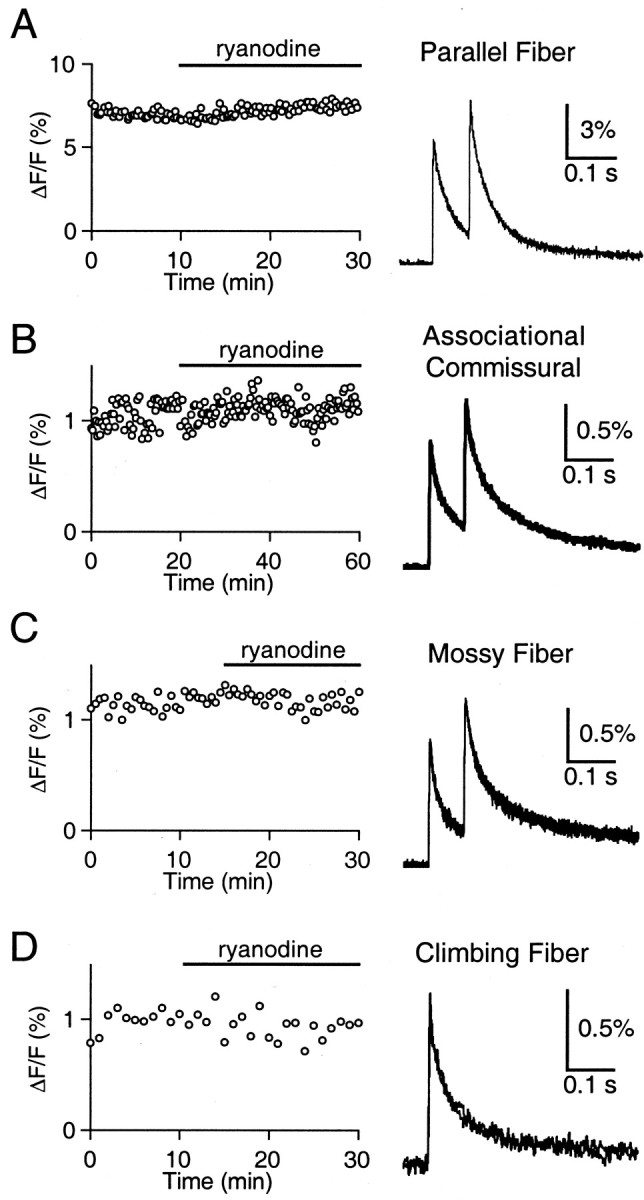
Disrupting CICR has no effect on the residual calcium signal at four excitatory synapses. A, At the PF synapse, peak ΔF/F signal (left) remains unchanged after bath application of 10 μm ryanodine (solid bar). Representativetraces (right) are superimposed averages of five trials before and after ryanodine application. Similar results were found using 10 μm ryanodine at the AC synapse (B), and 100 μm ryanodine at the MF (C) and CF (D) synapses.
DISCUSSION
Our primary finding is that internal Ca stores and CICR do not contribute to short-term presynaptic plasticity or the residual Ca signal on the milliseconds-to-seconds time scale at a number of excitatory central synapses. Furthermore, although prominent caffeine-evoked CICR is present in Purkinje cells, it is not found in the parallel fiber synaptic inputs onto those cells. These results suggest that Ca influx through voltage-gated Ca channels generates the residual Ca signal that shapes short-term presynaptic plasticity at excitatory central synapses.
Role of internal Ca stores in presynaptic Ca signaling and short-term presynaptic plasticity
Previous studies provide insight into the source of Ca that gives rise to the presynaptic residual Ca signal. After a single stimulus, Ca influx coincident with the presynaptic action potential is confined to a period of several hundred microseconds (Sabatini and Regehr, 1998). This rapid influx can generate a large peak Ca signal, which equilibrates through the presynaptic terminal and gives rise to the residual Ca signal. The dependence of this residual Ca signal on the concentrations of external Ca and cadmium, as well as the additivity of the block by subtype-specific Ca channel toxins (Mintz et al., 1995), also suggests that Ca influx through voltage-gated Ca channels is sufficient to produce the residual Ca signal that drives short-term presynaptic plasticity.
Although previous studies implicate a role for internal Ca stores in some forms of synaptic plasticity, most studies suggest that CICR does not contribute to short-term presynaptic plasticity at central excitatory synapses. At peripheral synapses, internal Ca stores and CICR have, in some cases, been shown to shape the residual Ca signal and presynaptic plasticity (Peng, 1996; Smith and Cunnane, 1996; Narita et al., 1998, 2000). However, this contribution often takes place during trains of presynaptic activity, and short-term presynaptic plasticities such as PPF may remain unaltered (Narita et al., 2000). At central excitatory synapses, CICR in dendrites has been shown to contribute to postsynaptic responses and long-term postsynaptic plasticity (Obenaus et al., 1989; Alford et al., 1993; Wang et al., 1997; Emptage et al., 1999; Futatsugi et al., 1999). In contrast, evidence for the importance of CICR in presynaptic terminals for long-term plasticity has been indirect (Reyes and Stanton, 1996;Reyes-Harde et al., 1999) or absent. Furthermore, drugs that disrupt CICR have generally not been found to affect baseline synaptic strength or short-term presynaptic plasticity (Reyes and Stanton, 1996; Emptage et al., 1999; Reyes-Harde et al., 1999).
Our results contrast with a study suggesting an important role for CICR in mediating presynaptic Ca transients and PPF at the AC synapse (Emptage et al., 2001). These conflicting results may reflect several differences in our experimental conditions. Emptage at al. (2001) used organotypic slices, measured PPF with whole-cell current-clamp recordings, and measured Ca transients from individual boutons with single-photon confocal recordings. We used acute brain slices, which can have different properties from organotypic slices. This is illustrated by the differences in the magnitude of PPF in these preparations (the percent increase in PPF at 75 msec is 88% in acute slices, compared with 33% in organotypic slices). We also used whole-cell voltage-clamp recordings with low concentrations of NBQX to limit recurrent excitatory connections in the CA3 region. Finally, we measured the residual Ca signal from populations of presynaptic fibers and used low-intensity illumination to improve stability.
Complications associated with studying internal Ca stores
A number of complications can arise when studying internal Ca stores and CICR. First, although caffeine is often used to elicit CICR, it can directly interact with the fluorescence properties of Ca indicators (Muschol et al., 1999). This artifact is difficult to correct and can lead to false-positive results. Second, ryanodine is used to block CICR but at high concentrations may reduce EPSC size via a decrease in fiber excitability. Third, bath application of drugs used to study CICR may affect internal Ca stores in glia or postsynaptic neurons (Castonguay and Robitaille, 2001). Glia can release ATP or glutamate that can affect neurotransmitter release by activating presynaptic receptors (Araque et al., 1998, 2001; Haydon, 2001). Ca elevation in postsynaptic cells can evoke the release of retrograde messengers that can inhibit neurotransmitter release from presynaptic terminals (Kreitzer and Regehr, 2001; Wilson and Nicoll, 2001).
Potential role for internal Ca stores in presynaptic function and plasticity
Although our results indicate that CICR does not contribute to PPF, DR, or CDR at the synapses we studied, anatomical studies suggest that CICR could contribute to synaptic transmission at some excitatory central synapses. Although ryanodine receptors are generally expressed at high density in dendrites and somata, they may also be present at much lower density in the presynaptic terminals of some excitatory central synapses (Kuwajima et al., 1992; Sharp et al., 1993; Furuichi et al., 1994; Ouyang et al., 1997). Ryanodine receptors are also present in boutons of inhibitory cerebellar synapses, where they contribute to presynaptic Ca signaling and synaptic transmission (Llano et al., 2000). In some cells the expression of ryanodine receptors may change during development. This is the case for granule cells and their associated parallel fibers in the avian cerebellum, where ryanodine receptors are only prominent in mature animals (Ouyang et al., 1997) (It has not been possible to test this in rat cerebellar slices because of the difficulty of quantifying EPSCs in mature Purkinje cells.) Thus, the possibility remains that internal Ca stores may play a role in presynaptic function at the synapses we have studied, perhaps at a different developmental stage or after trains of presynaptic activity. However, our results suggest that, in general, Ca influx through voltage-gated Ca channels provides the primary source of Ca responsible for the residual Ca signal and multiple forms of short-term presynaptic plasticity at excitatory central synapses.
Footnotes
This work was supported by National Institutes of Health Grant R01-NS32405-01 to W.G.R. We thank Solange Brown, Dawn Blitz, John Decker, Alex Jackson, Anatol Kreitzer, and Matthew Xu-Friedman for comments on this manuscript.
Correspondence should be addressed to Wade G. Regehr, Department of Neurobiology, Harvard Medical School, 220 Longwood Avenue, Boston, MA 02115. E-mail: wade_regehr@hms.harvard.edu.
REFERENCES
- 1.Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol (Lond) 1993;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 4.Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atluri PP, Regehr WG. Delayed release of neurotransmitter from cerebellar granule cells. J Neurosci. 1998;18:8214–8227. doi: 10.1523/JNEUROSCI.18-20-08214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett EF, Stevens CF. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol (Lond) 1972;227:691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 8.Castonguay A, Robitaille R. Differential regulation of transmitter release by presynaptic and glial Ca2+ internal stores at the neuromuscular synapse. J Neurosci. 2001;21:1911–1922. doi: 10.1523/JNEUROSCI.21-06-01911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen IS, Van der Kloot W. Facilitation and delayed release at single frog neuromuscular junctions. J Neurosci. 1986;6:2366–2370. doi: 10.1523/JNEUROSCI.06-08-02366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings DD, Wilcox KS, Dichter MA. Calcium-dependent paired-pulse facilitation of miniature EPSC frequency accompanies depression of EPSCs at hippocampal synapses in culture. J Neurosci. 1996;16:5312–5323. doi: 10.1523/JNEUROSCI.16-17-05312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 14.Eccles JC, Katz B, Kuffler SW. Nature of the “endplate potential” in curarized muscle. J Physiol (Lond) 1941;124:574–585. [Google Scholar]
- 15.Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol (Lond) 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 17.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 18.Feng TP. Studies on the neuromuscular junction. Chin J Physiol. 1941;16:341–372. [Google Scholar]
- 19.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci. 1994;14:4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/s0896-6273(00)81123-x. [DOI] [PubMed] [Google Scholar]
- 21.Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto K, Kano M. Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. J Physiol (Lond) 1998;506:391–405. doi: 10.1111/j.1469-7793.1998.391bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 24.Herrington J, Bookman RJ. Pulse control V4.5: IGOR XOPs for patch clamp data acquisition. University of Miami; Miami: 1995. [Google Scholar]
- 25.Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol (Lond) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kano M, Garaschuk O, Verkhratsky A, Konnerth A. Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones. J Physiol (Lond) 1995;487:1–16. doi: 10.1113/jphysiol.1995.sp020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz B, Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol (Lond) 1967;189:535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol (Lond) 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 30.Kreitzer AC, Gee KR, Archer EA, Regehr WG. Monitoring presynaptic calcium dynamics in projection fibers by in vivo loading of a novel calcium indicator. Neuron. 2000;27:25–32. doi: 10.1016/s0896-6273(00)00006-4. [DOI] [PubMed] [Google Scholar]
- 31.Krizaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J Neurosci. 1999;19:7249–7261. doi: 10.1523/JNEUROSCI.19-17-07249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuwajima G, Futatsugi A, Niinobe M, Nakanishi S, Mikoshiba K. Two types of ryanodine receptors in mouse brain: skeletal muscle type exclusively in Purkinje cells and cardiac muscle type in various neurons. Neuron. 1992;9:1133–1142. doi: 10.1016/0896-6273(92)90071-k. [DOI] [PubMed] [Google Scholar]
- 33.Llano I, DiPolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994;12:663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 34.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 35.Magleby KL. Short-term changes in synaptic efficacy. In: Edelman GM, Gall WE, Cowan WM, editors. Synaptic function. Wiley; New York: 1987. pp. 21–56. [Google Scholar]
- 36.Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 37.Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 38.Mothet JP, Fossier P, Meunier FM, Stinnakre J, Tauc L, Baux G. Cyclic ADP-ribose and calcium-induced calcium release regulate neurotransmitter release at a cholinergic synapse of Aplysia. J Physiol (Lond) 1998;507:405–414. doi: 10.1111/j.1469-7793.1998.405bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muschol M, Dasgupta BR, Salzberg BM. Caffeine interaction with fluorescent calcium indicator dyes. Biophys J. 1999;77:577–586. doi: 10.1016/S0006-3495(99)76914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narita K, Akita T, Osanai M, Shirasaki T, Kijima H, Kuba K. A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals. J Gen Physiol. 1998;112:593–609. doi: 10.1085/jgp.112.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narita K, Akita T, Hachisuka J, Huang S, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity. J Gen Physiol. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obenaus A, Mody I, Baimbridge KG. Dantrolene-Na (Dantrium) blocks induction of long-term potentiation in hippocampal slices. Neurosci Lett. 1989;98:172–178. doi: 10.1016/0304-3940(89)90505-3. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang Y, Martone ME, Deerinck TJ, Airey JA, Sutko JL, Ellisman MH. Differential distribution and subcellular localization of ryanodine receptor isoforms in the chicken cerebellum during development. Brain Res. 1997;775:52–62. doi: 10.1016/s0006-8993(97)00840-8. [DOI] [PubMed] [Google Scholar]
- 44.Peng Y. Ryanodine-sensitive component of calcium transients evoked by nerve firing at presynaptic nerve terminals. J Neurosci. 1996;16:6703–6712. doi: 10.1523/JNEUROSCI.16-21-06703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahamimoff R, Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol (Lond) 1973;228:241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regehr WG, Atluri PP. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995;68:2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regehr WG, Stevens CF. Physiology of synaptic transmission and short-term plasticity. In: Cowan WM, Südhof TC, Stevens CF, editors. Synapses. Johns Hopkins University; Baltimore: 2001. pp. 135–176. [Google Scholar]
- 48.Regehr WG, Tank DW. Selective fura-2 loading of presynaptic terminals and nerve cell processes by local perfusion in mammalian brain slice. J Neurosci Methods. 1991;37:111–119. doi: 10.1016/0165-0270(91)90121-f. [DOI] [PubMed] [Google Scholar]
- 49.Reyes M, Stanton PK. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J Neurosci. 1996;16:5951–5960. doi: 10.1523/JNEUROSCI.16-19-05951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes-Harde M, Empson R, Potter BV, Galione A, Stanton PK. Evidence of a role for cyclic ADP-ribose in long-term synaptic depression in hippocampus. Proc Natl Acad Sci USA. 1999;96:4061–4066. doi: 10.1073/pnas.96.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabatini BL, Regehr WG. Detecting changes in calcium influx which contribute to synaptic modulation in mammalian brain slice. Neuropharmacology. 1995;34:1453–1467. doi: 10.1016/0028-3908(95)00129-t. [DOI] [PubMed] [Google Scholar]
- 52.Sabatini BL, Regehr WG. Optical measurement of presynaptic calcium currents. Biophys J. 1998;74:1549–1563. doi: 10.1016/S0006-3495(98)77867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silver RA, Momiyama A, Cull-Candy SG. Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses. J Physiol (Lond) 1998;510:881–902. doi: 10.1111/j.1469-7793.1998.881bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sitsapesan R, McGarry SJ, Williams AJ. Cyclic ADP-ribose, the ryanodine receptor and Ca2+ release. Trends Pharmacol Sci. 1995;16:386–391. doi: 10.1016/s0165-6147(00)89080-x. [DOI] [PubMed] [Google Scholar]
- 57.Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol (Lond) 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 59.Van der Kloot W, Molgo J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994;74:899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- 60.Vogt KE, Regehr WG. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. J Neurosci. 2001;21:75–83. doi: 10.1523/JNEUROSCI.21-01-00075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L-Y, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Rowan MJ, Anwyl R. Induction of LTD in the dentate gyrus in vitro is NMDA receptor independent, but dependent on Ca2+ influx via low-voltage-activated Ca2+ channels and release of Ca2+ from intracellular stores. J Neurophysiol. 1997;77:812–825. doi: 10.1152/jn.1997.77.2.812. [DOI] [PubMed] [Google Scholar]
- 63.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 64.Zengel JE, Magleby KL. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981;77:503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 66.Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- 67.Zucker RS, Lara-Estrella LO. Post-tetanic decay of evoked and spontaneous transmitter release and a residual-calcium model of synaptic facilitation at crayfish neuromuscular junctions. J Gen Physiol. 1983;81:355–372. doi: 10.1085/jgp.81.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]