Abstract
The functional expression of large-conductance (BK-type) Ca2+-activated K+(KCa) channels was examined in developing chick lumbar motoneurons (LMNs) between embryonic day 6 (E6) and E13 using patch-clamp recording techniques. The macroscopic KCacurrent of E13 LMNs is inhibited by iberiotoxin and resistant to apamin. The average macroscopic KCa density was low before E8 and increased 3.3-fold by E11, with an additional 1.8-fold increase occurring by E13. BK-type KCa channels could not be detected in inside-out patches from E8 LMNs but were readily detected at E11. The density of voltage-activated Ca2+currents did not change between E8 and E11. Surgical ablation of target tissues at E5 caused a significant reduction in average KCadensity in LMNs measured at E11. Conversely, chronic in ovo administration of d-tubocurarine, which causes an increase in motoneuron branching on the surface of the muscle target tissue, evoked a 1.8-fold increase in average LMN KCadensity measured at E11. Electrical activity also contributed to developmental regulation of LMN KCa density. A significant reduction in E11 KCa density was found after chronicin ovo treatment with the neuronal nicotinic antagonist mecamylamine or the GABA receptor agonist muscimol, agents that reduce activation of LMNs in ovo. Moreover, 3 d exposure to depolarizing concentrations of external K+ to LMNs cultured at E8 caused an increase in KCa expression. Conversely, tetrodotoxin caused a decrease in KCaexpression in cultured E8 LMNs developing for 3 d in the presence of neurotrophic factors that promote neuronal survival in the absence of target tissues.
Keywords: motoneuron, development, Ca2+-activated K+ channels, slowpoke, electrical activity, trophic factors
The expression of a specific electrophysiological phenotype in vertebrate neurons is developmentally regulated (McCobb et al., 1990; Spitzer, 1991; Dryer, 1998;Messengill et al., 1997). A critical factor underlying the intrinsic electrophysiological phenotype of neurons is the ensemble and distribution of ionic channels expressed in the plasma membrane. Ion channel expression changes throughout development to accommodate new demands on the cell, particularly around the time of synapse formation with target tissues (Dryer, 1998; Martin-Caraballo and Greer, 2000). Developmental changes in ion channel expression lead to robust changes in action potential waveform and firing pattern during embryonic development, including in spinal motoneurons (McCobb et al., 1990;Spitzer, 1991; Martin-Caraballo and Greer, 2000).
Inductive cell–cell interactions regulate the functional expression of at least some ion channels in developing neurons. For example, early interactions with target tissues mediated by soluble target-derived factors control maturation of K+ channel expression in avian parasympathetic and sympathetic neurons (Dourado et al., 1994; Raucher and Dryer, 1995; Subramony et al., 1996; Cameron et al., 1998). In contrast, less is known about regulation of ion channel expression in CNS cells. Here we examine the role of extrinsic factors in regulation of Ca2+-activated K+ (KCa) channels in chick lumbar motoneurons (LMNs). LMNs are born around embryonic day 2 (E2) and begin sending axons toward hindlimb muscles by E4. LMNs are functionally active by E6, when spontaneous bursts of electrical activity can be recorded at the ventral roots (O'Donovan and Landmesser, 1987). Between E6 and E11, LMNs undergo a period of programmed apoptotic cell death that results in an ∼50% reduction in the number of cells within the motoneuron pool (Chu-Wang and Oppenheim, 1978). Differentiation of the hindlimb neuromuscular system is virtually complete by E11, by which time contractile muscles and functional synapses capable of generating spontaneous contractions are present.
During the course of neuromuscular differentiation, LMNs are exposed to a host of central and peripheral environmental influences (Qin-Wei et al., 1994; Caldero et al., 1998). Central influences arise as the result of network interactions between motoneurons, interneurons, and descending supraspinal fibers. Peripheral environmental influences arise in part from LMN interactions with hindlimb target tissues. Target innervation and target-derived neurotrophic factors are critical factors determining LMN survival in vivo (Qin-Wei et al., 1994; Caldero et al., 1998). However, the role of target innervation and electrical activity in regulating ion channel expression in motoneurons has not been investigated.
KCa channels play a critical role in regulating excitability by modulating action potential waveform and firing frequency (Sah and Bekkers, 1996; Martin-Caraballo and Greer, 2000). Large-conductance (BK) KCa channels contribute to spike repolarization and the early phases of the afterhyperpolarization, whereas small-conductance (SK) KCa channels contribute to later phases of afterhyperpolarizing potentials. Here we describe developmental changes in BK-type KCa channel expression and its regulation by target interaction and electrical activity in chick LMNs. We demonstrate that between E8 and E13 there is a sharp increase in KCa and that this change is mediated by a combination of interactions with target tissues and electrical activity.
MATERIALS AND METHODS
Motoneuron isolation and culture. Labeling, dissociation and culture of chick LMNs were performed as described byMcCobb et al. (1989, 1990). Chick LMNs were retrogradely labeledin ovo with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, 1 mg/ml in 20% ethanol and 80% saline). Dye injection into muscles of the thigh and foreleg was performed 1–2 d before spinal cord dissociation. To study the expression of ionic currents in acutely dissociated LMNs, recordings were made 3–4 hr after spinal cord dissociation. The potential influence of target myotubes and various culture conditions on KCa expression was studied in cells isolated at E8 and cultured for 72 hr before recording. Spinal cords were excised into a Ca2+- and Mg2+-free solution and mildly trypsinized (E6, 0.1% for 20 min; E8, 0.2% for 30 min; E11, 0.4% for 40 min; and E13, 0.45% for 45 min), dissociated by trituration, and plated onto poly-d-lysine-coated glass coverslips. Basal culture medium consisted of Eagle's minimal essential medium (BioWhittaker, Walkersville, MA), supplemented with 10% heat-inactivated horse serum, 2 mm glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. For experiments involving nerve–muscle cocultures, E11 hindlimb muscles were dissected and cleaned of connective tissue in a Ca2+- and Mg2+-free solution. After incubation for 15 min with 0.05% type II collagenase, tissue was dissociated by trituration through a series of fire-polished Pasteur pipettes. Myotubes were plated onto poly-d-lysine-coated glass coverslips for 45 min, and an excess of medium was then added. Myotube cultures were maintained for 2 d before adding dissociated LMNs.
In ovo manipulations of embryonic development. DiI was injected into the hindlimb at E5, followed by drug application onto the vascularized chorioallantoic membrane ∼18 hr later. The following drugs were applied daily until E10: d-tubocurarine (2 mg/d), mecamylamine (0.28 mg/d), and muscimol (0.1 mg, twice per day). The doses of d-tubocurarine and muscimol used here are reported to optimally inhibit spontaneous motility of the hindlimbin ovo (Usiak and Landmesser, 1999). The neuronal nicotinic antagonist mecamylamine has been used previously at this dose to examine the role of synaptic activity in the regulation of apoptosis and KCa expression in chick ciliary ganglia (Subramony and Dryer, 1996). Drugs were prepared in a physiological saline containing (in mm): NaCl (139), KCl (3), MgCl2 (1), CaCl2 (3), and NaHCO3 (17). The survival rate varied among the different treatments: for d-tubocurarine, 3 of 13 treated embryos survived to E11; for mecamylamine, 5 of 11; and for muscimol, 4 of 6. The motility of surviving embryos was determined as the number of hindlimb kicks in a 3 min observation period on E10. Motility rates (movements every 3 min) were 34 ± 2 in control embryos (n = 5), 0 ind-tubocurarine-treated embryos (n= 3), 7 ± 3 with mecamylamine (n = 5), and 4 ± 1 with muscimol (n = 4).
Removal of the hindlimb was also performed 18 hr after DiI injection on E5. This was done by pulling the leg through a hole in the amnion and cutting at the level of the thigh with a pair of spring scissors or a battery-operated electrocautery unit (Harvard Apparatus, South Natick, MA). The survival rate after limb removal was between 40 and 50% for all operated embryos.
Whole-cell and single-channel recordings. LMNs were identified during patch-clamp recordings using an Olympus Optical (Tokyo, Japan) IX70 inverted stage microscope equipped with epifluorescent optics and rhodamine filters. All LMNs selected for recording showed a punctate fluorescent staining pattern because of retrograde transport of DiI from its site of injection in the hindlimb. Recordings were performed at room temperature (22–24°C). All external recording solutions contained 600 nm tetrodotoxin (TTX) to block inward Na+ currents during whole-cell recordings. Recording electrodes were made from thin-wall borosilicate glass (3–4 MΩ). To measure KCa or Ca2+ currents, a 25 msec depolarizing step to +30 mV was applied from a holding potential of −40 mV in normal external saline and after a 3 min incubation in Ca2+-free external saline, and net current amplitude was obtained by digital subtraction (control, Ca2+-free). Voltage commands and data acquisition and analysis were performed with an AxoPatch 1D amplifier and pClamp software (Axon Instruments, Foster City, CA). For quantitative analyses, we normalized for cell size by dividing current amplitudes by cell capacitance. Cell capacitance was determined by integration of the current transient evoked by a 10 mV voltage step from a holding potential of −60 mV. Average values for cell capacitance were as follows: E6, 25.1 ± 0.7 pF (n= 15); E8, 25.9 ± 2.7 pF (n = 10); E11, 28. 0 ± 1.4 pF (n = 23); and E13, 44.6 ± 2.3* pF (n = 9) (*p < 0.05 vs E6, E8, or E11).
Single-channel analysis was performed as described previously (Cameron et al., 1998; Lhuillier and Dryer, 1999). Briefly, patches were excised in Ca2+-free saline containing 10 mm EGTA. KCa channel activity was stimulated by bath application of a saline solution containing 5 μm free Ca2+. Single-channel data were filtered at 2 kHz with a four-pole Bessel filter and stored on magnetic videotape for off-line digitization (10 kHz) and analysis using pClamp software. Throughout, all data values are presented as mean ± SEM; n represents the number of LMNs from which a particular measurement was made. Significant differences were calculated by using Student's unpaired t test when single comparisons were made. Differences between multiple groups were tested using one-way ANOVA followed by post hoc analysis using Tukey's honest significant difference test for unequal n(Statistica software, Tulsa, OK).
Intracellular and extracellular solutions. The composition of the Ca2+- and Mg2+-free solution was (in mm): NaCl (137), KCl (2.7), glucose (25), and HEPES-NaOH (25), pH 7.4. For whole-cell recordings of KCa, the external saline solution was (in mm): NaCl (145), KCl (5.4), MgCl2 (0.8), CaCl2(5.4), glucose (5), and HEPES (13), pH 7.4 (with NaOH). Pipette saline solution was (in mm): KCl (120), MgCl2 (2), HEPES-KOH (10), and EGTA (10), pH 7.4. For whole-cell recordings of voltage-activated Ca2+ currents, the external saline solution was (in mm): tetraethylammonium chloride (145), CaCl2 (10), glucose (5), and HEPES (10), pH 7.4 (with tetraethylammonium hydroxide). Pipette saline solution was (in mm): Cs-aspartate (140), MgCl2(5), HEPES-CsOH (10), EGTA (10), MgATP (1), and NaGTP (0.1), pH 7.4. For all extracellular Ca2+-free solutions, CaCl2 was replaced by an equimolar concentration of MgCl2. For single-channel recordings, the external Ca2+-free saline consisted of (in mm): KCl (150), EGTA (10), and HEPES-KOH (5), pH 7.2. The pipette solution for single-channel recordings consisted of (in mm): NaCl (112.5), KCl (37.5), EGTA (10), and HEPES-NaOH (10), pH 7.4. Under these conditions the calculated free Ca2+ concentration is 10−11m. The 5 μm Ca2+-free solution used for recording single-channel activity had the following composition (in mm): KCl (150), EGTA (1), CaCl2(0.97), and HEPES-KOH (5), pH 7.2. The composition of the Ca2+-EGTA buffer was calculated using chelate software written by Dr. R. A. Steinhardt (University of California, Berkeley, CA) and the equilibrium constants reported bySteinhardt et al. (1977).
Chemicals and drugs. 8-(4-Chlorophenylthio)-cAMP (CPT-cAMP),d-tubocurarine, mecamylamine, muscimol, neurotrophin-4 (NT4), tetrodotoxin, trypsin, and collagenase were from Sigma (St. Louis, MO); ciliary neurotrophic factor (CNTF) was obtained from R & D Systems (Minneapolis, MN). Culture supplements and serum were from BioWhittaker.
RESULTS
Properties of KCa channels in E11 LMNs
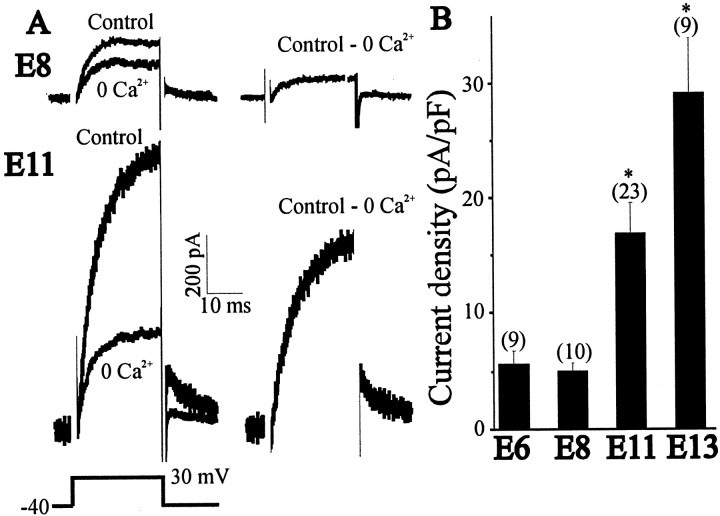
The functional characteristics of KCachannels in chick LMNs have not been described previously. Therefore, whole-cell outward K+ currents were recorded in control and Ca2+-free saline after 25 msec depolarizing steps to +30 mV from a holding potential of −40 mV, and net Ca2+-dependent outward currents were obtained by digital subtraction (Fig.1A). This procedure eliminated contributions from other Ca2+-independent, voltage-activated K+ currents expressed at this stage of development (McCobb et al., 1990). Typical current traces from acutely isolated E11 LMNs, the first stage at which a robust KCa could be detected, are shown in Figure1A. Maximal conductance was observed by step pulses to +40 mV, and a gradual fall in outward conductance occurred as test pulses exceeded +50 mV (Fig. 1B). In a few recordings, KCa was evoked from more negative holding potentials (−80 mV). This did not result in an increase in the amplitude of KCa, indicating that inactivating components of KCa are not expressed in LMNs.
Fig. 1.
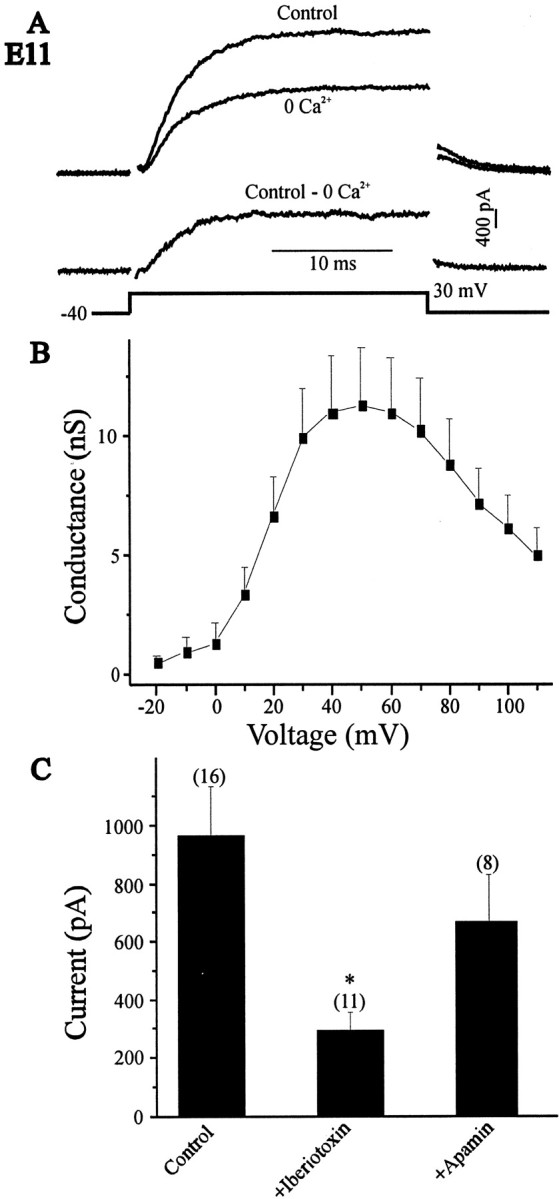
Properties of KCa currents in E11 LMNs. A, Outward currents were evoked in control and Ca2+-free saline (left traces) by 25 msec depolarizing pulses to +30 mV from a holding potential of −40 mV (bottom left). Net macroscopic KCa was obtained after digital subtraction of raw traces (right trace). B, Mean macroscopic KCaconductance as a function of voltage in 13 LMNs. A decline in conductance at command potentials positive to +50 is predicted for a Ca2+-dependent current. C, Effect of iberiotoxin (200 nm) and apamin (1 μm) on macroscopic KCa currents. Dissociated E11 LMNs were treated with these toxins for at least 30 min before whole-cell recordings. Control LMNs were not exposed to toxins before recording.
To determine the nature of the KCa channels generating the Ca2+-dependent outward current in E11 LMNs, we tested the effects of the BK channel blocker iberiotoxin and the SK channel blocker apamin. Both iberiotoxin (200 nm) and apamin (1 μm) were applied for at least 30 min before whole-cell recordings and compared with control cells (no channel blocker applied). Application of iberiotoxin caused a significant (p < 0.05) reduction in mean KCa amplitude compared with controls. In contrast, apamin had no significant effect on the mean amplitude of KCa (Fig. 1C). These results suggest that BK channels mediate most of the Ca2+-dependent outward current expressed by LMNs.
Tail current analysis and inside-out patch recordings were used to provide additional characterization of the KCachannels of E11 chick LMNs. Ca2+-dependent outward tail currents approach null asymptotically as the command potential approaches EK (−80 mV under the conditions of these recordings; Fig.2B). To analyze the deactivation kinetics of KCa channels, we measured the decay time constant of tail current over the voltage range relevant for action potential repolarization (−60–0 mV). A single-exponential curve provided excellent fits to the tail currents, and no significant improvement was obtained by adding extra terms. The decay time constants of KCa tail currents were nearly voltage-independent between −60 and 0 mV, suggesting a weak voltage dependence for KCa channel deactivation (Fig. 2C). Moreover, the tail currents decay relatively quickly compared with other neuronal cell types (Cameron et al., 2000;Ramanathan et al., 2000).
Fig. 2.
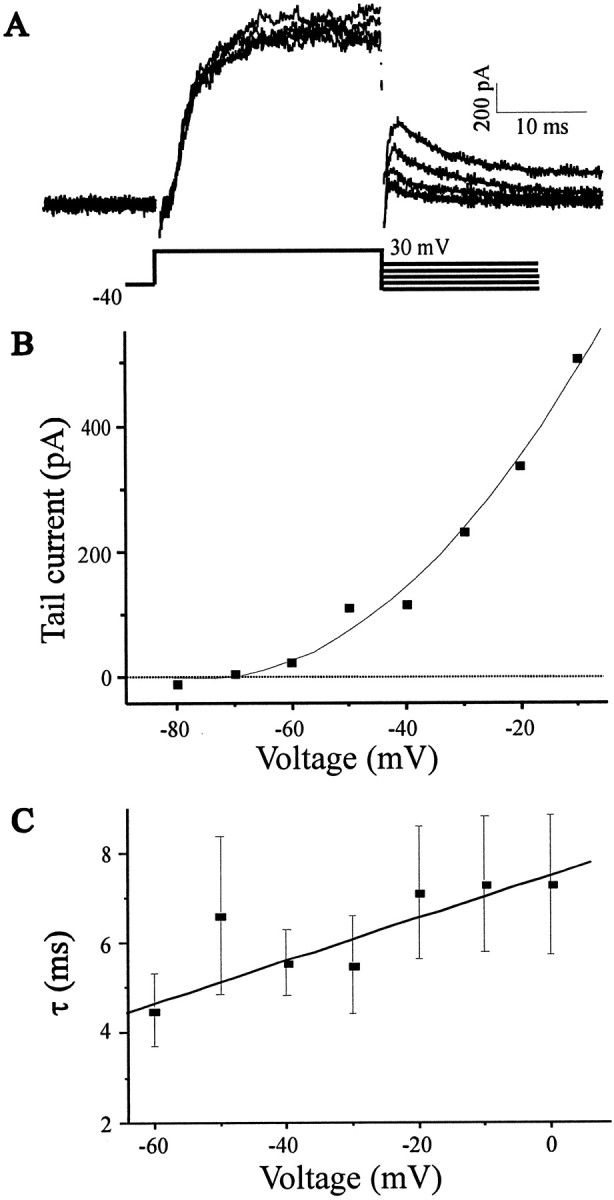
Tail currents and analysis of KCadeactivation kinetics in LMNs. A, Tail currents from the same neuron represented in Figure 1 evoked by the voltage-clamp protocol are shown below the current traces. The decay phases of the tail currents were fitted with single-exponential curves.B, Plot of Ca2+-dependent tail current amplitude as a function of voltage. The tail currents become undetectable at −70 mV, close to the calculatedEK of −78 mV. C, Plot of mean tail current decay time constant as a function of voltage showing that deactivation kinetics are only weakly voltage-dependent over this range of test potentials (n = 7 cells).
For recordings of single-channel activity in inside-out patches, E11 LMNs were bathed in a Ca2+-free solution during patch excision (Fig. 3). In 7 of 14 patches excised from the soma, outward channel activity was minimal in Ca2+-free solution, but the activity of BK channels increased after application of an external solution containing 5 μm free Ca2+(Fig. 3A). None of the patches appeared to contain more than one functional high-conductance channel, on the basis of several minutes of monitoring maximal current amplitudes at 0 mV in 5 μm free Ca2+. The interpolated reversal potential of unitary currents was close to the calculated EK (−35 mV under the conditions of these recordings; Fig. 3B), and the unitary conductance was determined from all-point histograms (Fig.3C). The mean unitary conductance in seven patches examined was 115 ± 10 pS with internal [K] of 37.5 mm and external [K] of 150 mm, identical to the BK-type KCa channels of chick ciliary ganglion neurons under the same ionic conditions (Cameron et al., 1998; Cameron and Dryer, 2000). Open time distributions were constructed from digitized data obtained in 5 μmCa2+ at 0 mV, ignoring transitions of <0.1 msec duration. Single exponential curves provided good fits to the open-time distributions, and the mean open τ was 1.0 ± 0.2 msec (n = 7; Fig. 3D). Mean open channel probability under these conditions was 0.26 ± 0.08 (n = 7; Fig. 3E), less than KCa channels of ciliary ganglion neurons observed under the same conditions (Cameron and Dryer, 2000). We also examined 13 inside-out patches excided from E8 LMNs. We did not observe large-conductance BK-type KCa channels evoked by 5 μm free Ca2+ in any of the patches excised at that developmental stage. However, in five patches we observed an intermediate-conductance (IK) KCa channel with a mean unitary conductance of 50 ± 6 pS and gating properties similar to those of an intermediate conductance channel that we have described in chick ciliary ganglion cells (Dryer et al., 1991; Lhuillier and Dryer, 1999). As noted below, macroscopic measurements at different stages are consistent with this observation.
Fig. 3.
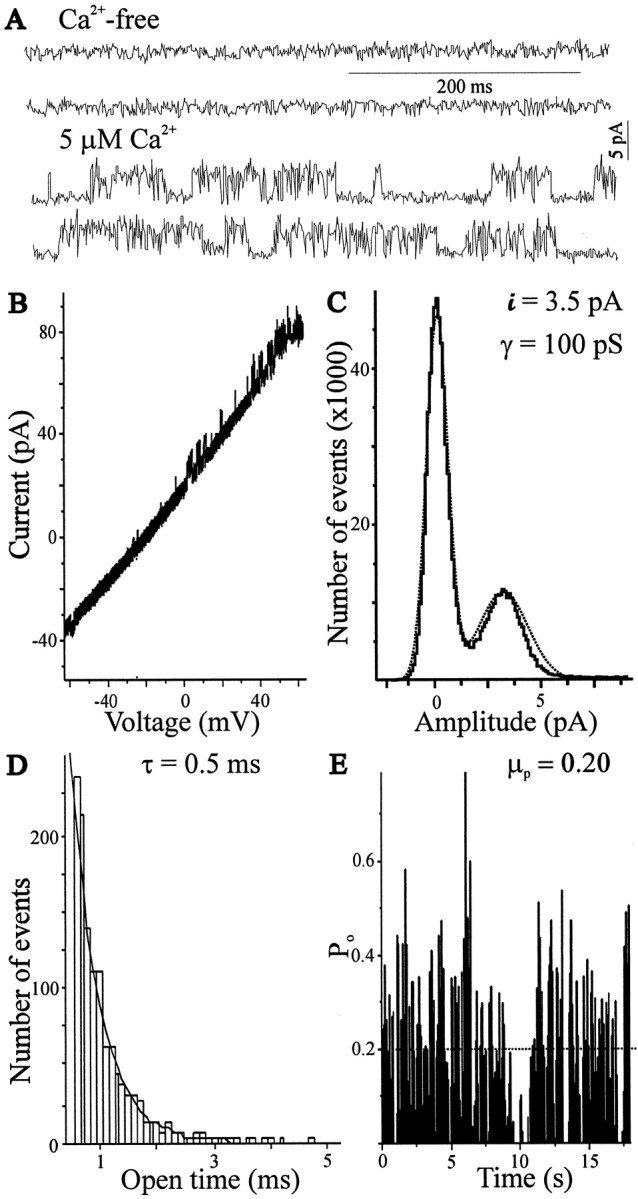
Biophysical properties of large-conductance Ca2+-dependent K+ channels recorded in E11 LMNs. A, In a typical patch held at 0 mV, ion channels are quiescent in Ca2+-free medium but become active after bath application of saline containing 5 μm free Ca2+. B, Current–voltage relationship for KCa channels in LMNs. The reversal potential of unitary currents was determined by a voltage ramp (from −60 to 60 mV at 0.6 V/sec). Unitary currents reversed close to the EK (−35 mV) calculated for these ionic conditions. C, All-point histograms from the patch shown in A fitted as the sum of two Gaussian functions (dotted line). Unitary current was determined as the difference in the peaks of the all-point histogram. Data from all of the patches analyzed in this way (n = 7) yielded a mean unitary conductance of 115 pS under these ionic conditions.D, Open-time histogram (bin width, 0.1 msec) with a superimposed fitted single-exponential curve (dotted line) with a time constant of 0.5 msec. E, Probability of KCa channel opening (po) over time in the presence of 5 μm-free Ca2+and 0 mV. The averagepo for this patch (dotted line) was 0.20 (po epoch interval, 50 msec). Recordings were filtered at 2 kHz, and data were digitized at 10 kHz before analysis.
Developmental changes in the functional expression of KCa
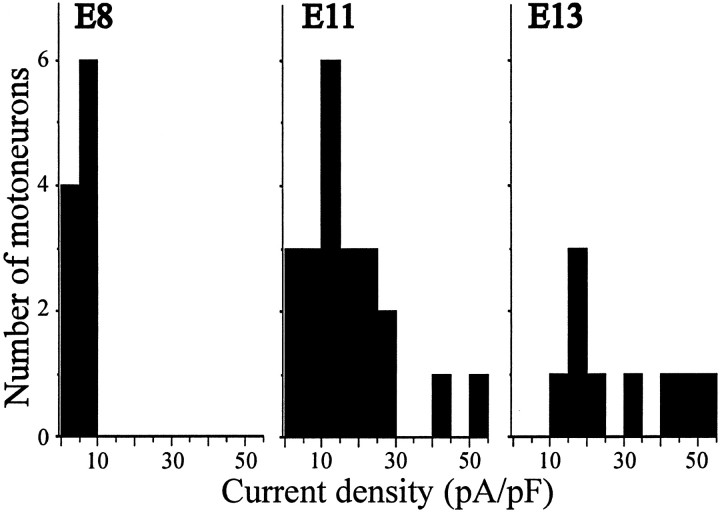
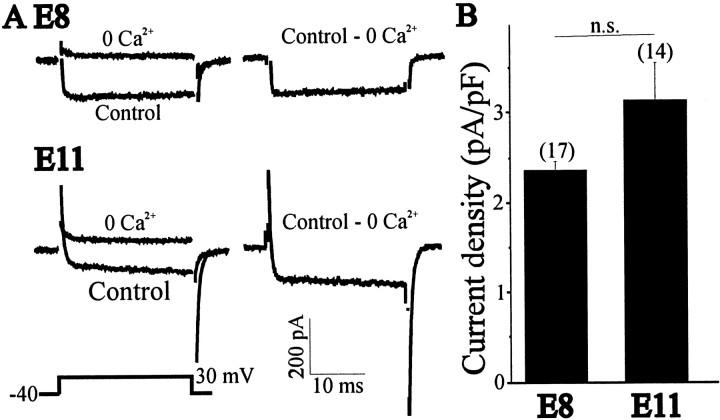
To study the development of KCa, LMNs were acutely isolated at various stages of development, and the functional expression of KCa was determined by whole-cell recordings. Between E8 and E11, there is a 4.7-fold increase in the amplitude of the net Ca2+-dependent outward current from an average of 135 ± 29 pA (n = 10) to 754 ± 97 pA (n = 35) (Fig.4A). To compensate for changes in cell size that occur throughout these developmental stages, whole-cell currents in each cell were normalized to cell capacitance (see Materials and Methods). Between E6 and E8, there was no significant change in KCa density. Between E8 and E11, KCa density increased 3.3-fold, with an additional 1.8-fold increase observed between E11 and E13, the last stage recorded (Fig. 4B). This age-dependent increase in KCa density can be seen as a rightward shift in current density histograms constructed for LMNs (Fig.5). These increases in KCa density cannot be attributed to developmental changes in voltage-evoked Ca2+ influx. To address this question, we recorded Ca2+currents in E8 and E11 LMNs using CsCl-filled electrodes (Fig.6A). Whole-cell recordings in LMNs indicate that Ca2+current density did not change significantly between E8 and E11 (Fig.6B), as observed in a previous study by McCobb et al. (1989). Therefore, the change in macroscopic KCadensity is most likely caused by changes in the number of functional BK-type KCa channels in the plasma membrane, which is consistent with the results of single-channel recordings noted above.
Fig. 4.
Developmental changes in the expression of macroscopic KCa in acutely isolated LMNs. A, Representative currents in E8 and E11 LMNs recorded in control and Ca2+-free saline. B, Mean KCa density between E6 and E13. In this and subsequent figures, error bars represent SEM, and the number of cells recorded is given above each bar. Note the significant increase in mean current density between E8 and E11, with an additional increase at E13.
Fig. 5.
Histograms of KCa current densities in E8, E11, and E13 LMNs. Note the rightward shift in the number of LMNs expressing higher current densities with increasing developmental stage.
Fig. 6.
Voltage-activated Ca2+ currents in E8 and E11 LMNs. A, Representative current traces in control and Ca2+-free saline. Total Ca2+ currents were obtained by digital subtraction (control, Ca2+-free), with representative examples shown on the right. Currents were evoked after a 250 msec step to +30 mV from a holding potential of −40 mV (left, bottom trace). B, Data compiled from many cells indicate no change in mean Ca2+ current density between E8 and E11.
Regulation of KCa channel expressionin vitro
Changes in the functional expression of KCain LMNs coincide with a period of significant maturation of the hindlimb neuromuscular system. By E11, neurons of the LMN pool have undergone considerable transformation because of interactions with target muscle (Qin-Wei et al., 1994; Caldero et al., 1998), and this may play a role in the expression of LMN KCachannels, as it does in autonomic neurons (Dourado et al., 1994;Raucher and Dryer, 1995). To determine whether epigenetic factors play a role in regulating KCa expression, LMNs were isolated at E8, when KCa is expressed at low current density, and maintained for 3 d in culture under several growth conditions. One group of LMNs was cocultured in the presence of hindlimb myotubes. Other LMNs were cultured in media containing depolarizing concentrations of KCl (50 mm) or in normal cultured media supplemented with 40 ng/ml CNTF, 10 ng/ml NT4 or 1 μm CPT-cAMP (a membrane-permeable analog of cAMP). These later conditions were chosen because previous studies have shown that they can enhance the survival and differentiation of LMNs developingin vitro (Arakawa et al., 1990; Becker et al., 1998; Hanson et al., 1998; Soler et al., 1998), possibly by mimicking in vivo conditions induced by electrical activity, target tissue interactions, or both. After 3 hr to 3 d in cell culture, whole-cell recordings were performed as described above. We observed that some culture conditions could support the normal developmental expression of macroscopic KCa, but that others could not, indicating regulation by epigenetic factors.
The density of macroscopic KCa in E8 LMNs cocultured for 3 d with hindlimb myotubes was 5-fold greater than that of E8 LMNs cultured for 3 hr with muscle cells (Fig.7A). These culture conditions therefore support developmental changes in the functional expression of KCa similar to those observed during normalin vivo development. Addition of TTX (1 μm) to the culture medium did not affect KCa expression in LMNs that were cocultured with muscle. Thus, in the presence of a normal target tissue, KCa expression in cultured LMNs can occur in the absence of ongoing spike activity.
Fig. 7.
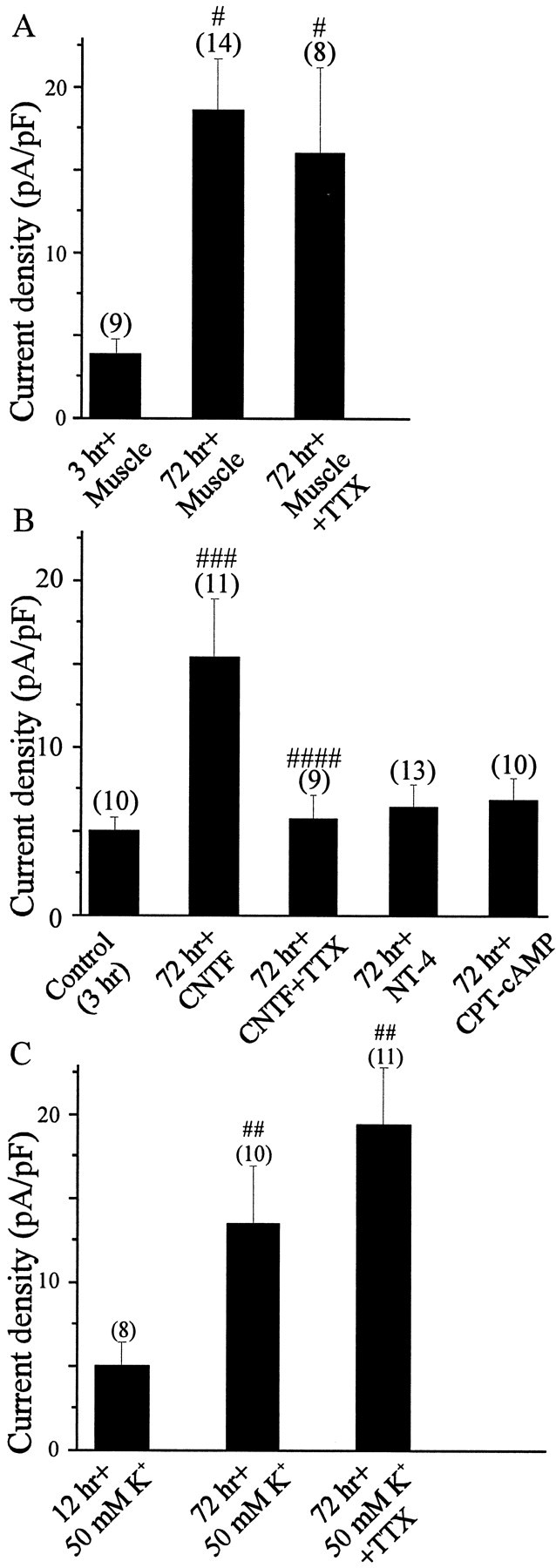
Effect of growth conditions on the expression of KCain vitro. LMNs were dissociated on E8 and maintained in culture for 72 hr in the presence of hindlimb myotubes (A), the survival factors CNTF (40 ng/ml), NT4 (10 ng/ml), and CPT-cAMP (1 μm) (B), or 50 mm extracellular K+ ions (C). To examine the role of electrical activity on KCaexpression, we added 1 μm TTX to culture media. Control neurons were cultured for 3 hr or overnight before whole-cell recordings. #p < 0.05 versus 3 hr with muscle;##p < 0.05 versus 12 hr in 50 mm K+;###p < 0.05 versus 3 hr in CNTF; ####p < 0.05 versus 72 hr in CNTF.
On the other hand, there are culture conditions in which spike activity does play a role in the regulation of macroscopic KCa. For example, LMNs cultured for 3 d in the presence of 40 ng/ml CNTF expressed KCa at high density (Fig. 7B). This trophic factor is one of several that can support survival of cultured LMNs, which form active networks under these growth conditions. In contrast to the effects of target tissues, the stimulatory effects of CNTF on KCa expression were abolished by adding 1 μm TTX to the culture medium (Fig.7B). Thus, spike activity can regulate expression of KCa channels in LMNs under some conditions. The density of macroscopic KCa was also examined in LMNs cultured for 3 d in depolarizing conditions (i.e., in media containing 50 mm K+) but in the absence of target tissues or target-derived trophic factors. These conditions have long been known to support survival of LMNs in the absence of trophic factors, and they produce a marked elevation in intracellular free Ca2+ thought to mimic that produced by ongoing spike activity (Soler et al., 1998). The density of macroscopic KCa in E8 LMNs cultured for 3 d in media containing 50 mmK+ was 2.9-fold greater than that of E8 LMNs cultured for 12 hr in 50 mmK+ (Fig. 7C). In other words, chronic depolarization can sustain normal or near-normal developmental changes in KCa, even in the absence of target tissues. The effect of depolarization was gradual, because 12 hr depolarization of E8 LMNs with 50 mmK+ did not induce any significant change in KCa current density versus age-paired, dissociated E8 LMNs cultured for 12 hr in normal media containing 5.4 mm K+ (data not shown). Moreover, adding Ca2+ channel blockers such as nimodipine to the culture media completely inhibited the effect of 50 mmK+ solutions on LMN KCa expression (data not shown).
We identified at least two other culture conditions that support LMN survival but that do not allow for normal expression of macroscopic KCa. Thus, adding 1 μm CPT-cAMP or 10 ng/ml NT4 to culture media allowed E8 LMNs to be maintained in culture for 3 d, as reported previously (Hanson et al., 1998;Becker et al., 1998). Indeed, the motoneurons were large and healthy under these growth conditions, and exhibited extensive neuritic arborizations. However, these culture conditions did not allow for normal developmental expression of KCa channels in LMNs (Fig. 7B). In other words, the development of macroscopic KCa in LMNs is not simply a question of time in culture and appears to be regulated by multiple epigenetic factors. It should be noted that we were unable to culture E8 LMNs for 3 d in normal culture media in the absence of trophic factors or target tissues because of ongoing apoptotic cell death that has long been known to occur in spinal motoneurons developing in vitro (O'Brien and Fischbach, 1986).
In vivo regulation of KCa by electrical activity and target tissues
The data presented above are consistent with the hypothesis that skeletal muscle target tissues and electrical activity are both involved in the developmental expression of functional KCa channels in LMNs. However, there are limitations to what can be learned from tissue culture experiments. Therefore, we have manipulated the in vivo interactions of LMNs with target tissues, as well as motoneuron electrical activityin vivo, and have examined the consequences of these perturbations on the development of macroscopic KCa. Drug injections or target removal were performed starting 18 hr after DiI labeling of LMNs on E5. Whole-cell KCa density was then measured in acutely dissociated E11 LMNs.
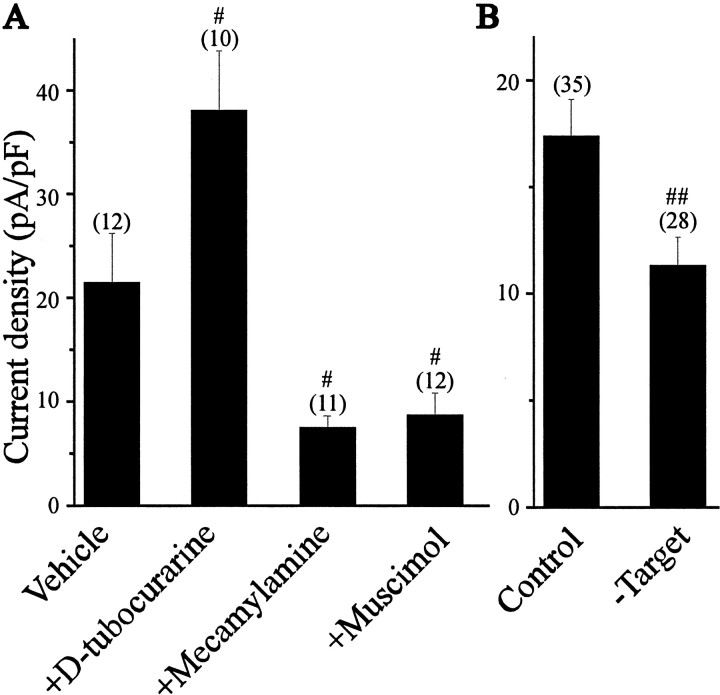
If electrical activity is a significant factor in stimulating KCa expression in LMNs, then it is reasonable to expect a significant reduction in KCa density after inhibition of spontaneous spinal motoneuron activity. This was accomplished by in ovo application of the GABAA receptor agonist muscimol or the neuronal nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine (Millner and Landmesser, 1999, Usiak and Landmesser, 1999). We observed that daily treatments with either of these drugs starting at E5 significantly reduced E11 KCa density compared with vehicle-treated controls (Fig.8A). Thus, in ovo application of mecamylamine produced a 2.8-fold reduction of KCa density, whereas muscimol induced a 2.4-fold reduction in KCa density compared with vehicle-treated controls. Voltage-activated Ca2+ currents recorded on E11 were unaffected by either of these treatments (data not shown). Both of these agents reduced spontaneous motility of the chick embryos, consistent with a decrease in LMN activity. An inhibitory effect of mecamylamine on muscle is unlikely at this dose, and in any case, dailyin ovo application of the muscle AChR antagonistd-tubocurarine caused an increase in KCa expression (see below). These data provide additional evidence that ongoing activity in LMNs has a stimulatory effect on the expression of KCa.
Fig. 8.
Effects of electrical activity and target tissue interactions on the functional expression of KCa in LMNs developing in vivo. A, Inhibition of LMNs by in ovo application of muscimol or mecamylamine decreases KCa density, suggesting a role for activity in regulation of KCa. In contrast, in ovotreatment with the neuromuscular blocker d-tubocurarine significantly increased KCa density, consistent with a role for target tissue interactions in KCa regulation.B, Removal of target tissues reduced KCadensity in LMNs compared with sham-operated controls.#p < 0.05 versus vehicle;##p < 0.05 versus control.
If interactions with target tissues are a significant factor in regulating LMN KCa channels, than perturbations that either decrease or increase contacts between LMNs and hindlimb muscle cells should produce corresponding changes in KCa. We have made two different perturbations to test this hypothesis. Previous studies have shown that chronic treatment with d-tubocurarine, a skeletal muscle nicotinic receptor antagonist, stimulates intramuscular branching of LMNs and thereby increases access to target-derived trophic factors (Tang and Landmesser, 1993; Oppenheim et al., 2000). We have observed that dailyin ovo treatment of chick embryos withd-tubocurarine between E5 and E10 evokes a 1.8-fold increase in average KCa current density compared with vehicle-injected controls measured at E11 (Fig.8A). This dose ofd-tubocurarine is unlikely to alter cholinergic synaptic activation of LMNs, and it again bears noting that the effect on KCa is the opposite of that produced by the nAChR antagonist mecamylamine. We also performed a more direct set of experiments in which the hindlimb target was unilaterally removed on E6. Removal of target tissues caused a 35% reduction in KCa current density in E11 LMNs compared with sham-operated controls. These data, together with the cell culture data presented previously, indicate that target tissues have a stimulatory effect on functional expression of KCa channels in developing LMNs.
DISCUSSION
In this study we have characterized the gating properties and developmental regulation of large-conductance KCachannels in embryonic chick LMNs. Three main conclusions can be drawn from these experiments. First, embryonic LMNs express a robust macroscopic KCa at E11–E13 that is mediated primarily by BK-type channels with relatively fast gating kinetics. Second, the largest developmental changes in the functional expression of large-conductance KCa channels in embryonic LMNs coincide with a period of significant neuromuscular maturation. Third, KCa expression in embryonic LMNs developing in vivo and in vitro is regulated by a combination of electrical activity and target tissue interactions.
Properties of large-conductance KCa channels in embryonic LMNs
Embryonic LMNs express BK-type KCa channels that give rise to robust macroscopic currents by E11. Approximately 70% of the Ca2+-dependent macroscopic current in E11 LMNs was blocked by iberiotoxin, a selective blocker of BK channels (Galvez et al., 1990), whereas apamin, an inhibitor of SK channels, had no significant effect on macroscopic KCa. On the other hand, the BK-type KCa channels of LMNs differ in some ways from the BK channels described previously in other neuronal cell types. For example, KCa deactivation kinetics did not show a substantial voltage dependence over a fairly wide range of membrane potentials. This feature is similar to ciliary cells of the chick ciliary ganglion but is different from choroid cells, which exhibit sharp voltage dependence over the same range of voltages (Cameron and Dryer, 2000). Second, the gating kinetics inferred from single-channel and tail current analyses were quite fast, considerably faster than those that we have observed in three classes of autonomic neurons at similar developmental stages (Raucher and Dryer, 1995; Cameron and Dryer, 2000). There is substantial evidence to indicate that protein products of the avian slo locus yield channels with different kinetic properties. These differences can emerge from alternative splicing of slo transcripts (Lagrutta et al., 1994; Tseng-Crank et al., 1994) and from coassembly with different auxiliary β-subunits of the channel (Dworetzky et al., 1996;Ramanathan et al., 2000), and it seems likely that one or both of these factors are responsible for the functional differences between KCa channels of chick LMNs and the various populations of chick autonomic neurons.
Regulation of LMN KCa channels by electrical activity and target tissue interactions
Whole-cell recordings indicate that the largest increase in macroscopic KCa density in LMNs occurred between E8 and E11, with an additional increase apparent by E13. This effect is probably not caused by changes in Ca2+dynamics, because there was no significant difference in the density of Ca2+ current during these same developmental stages. Single-channel recordings in E8 and E11 LMNs further indicate that this effect is associated with increased expression of BK channels in the plasma membrane, although we cannot exclude that a small portion of this increase could be attributable to SK- or IK-type KCa channels. Changes in expression of BK-type KCa channels in LMNs occur relatively late compared with the expression of most other ion channels in these neurons (McCobb et al., 1989, 1990), a pattern strikingly similar to that observed in autonomic neurons (Dourado and Dryer, 1992;Raucher and Dryer, 1995). Functional expression of KCa in LMNs coincides with a stage of significant maturation of the hindlimb neuromuscular system (O'Donovan and Landmesser, 1987). This is also similar to chick ciliary and sympathetic ganglion neurons, in which KCaexpression coincides precisely with synapse formation with target tissues (Dourado and Dryer, 1992; Dourado et al., 1994; Raucher and Dryer, 1995). This temporal correlation is probably not a coincidence because target innervation plays an active role in regulating KCa channel expression in developing LMNs and in ciliary neurons. Thus, treatments that evoke a decrease or increase in interactions between LMNs and hindlimb target tissues evoke corresponding changes in the expression of KCa in LMNs developing in vivo. Moreover, coculture of LMNs with target tissues supports robust in vitro expression of these channels. The effect of target tissue ablation on LMN KCa expression in vivo, although significant, is not large. However, it bears noting that target ablation causes a large increase in apoptotic cell death of LMNs, and it is possible that the remaining LMNs represent a subpopulation of cells that interact with other target tissues. The nature of this experimental design may therefore cause us to underestimate the extent to which target-derived factors regulate KCaexpression in LMNs in vivo. In any case, there is a precedent for this type of observation. Thus, target tissues also play an active role in regulation of KCa channels in large ciliary ganglion neurons developing in vitro orin vivo (Dourado et al., 1994; Subramony et al., 1996;Cameron et al., 1998), and this may be a phenomenon that occurs in many cell types (Raucher and Dryer, 1995; Dryer, 1998).
What is the target-derived factor involved in regulation of KCa channel expression in LMNs? At the present time there is no clear candidate. Although CNTF promotes LMN survival in culture (Hughes et al., 1993; Qin-Wei et al., 1994) and stimulates KCa expression in vitro, its role as a target-derived trophic molecule for motoneurons is controversial (Sendtner et al., 1994). There may be multiple factors that contribute not only to the survival but also to the electrophysiological differentiation of LMNs. It is important to note that factors that promote motoneuron survival do not necessarily increase KCa channel expression in LMNs, as indicated by the present results with a membrane-permeable cAMP analog and with NT4. In chick ciliary ganglion neurons, the target-derived factor regulating KCa expression is an ortholog of transforming growth factor β1 (TGFβ1) (Cameron et al., 1998). It is certainly possible that a target-derived member of the TGFβ superfamily (e.g., TGFβ1, bone morphogenetic proteins, glial-derived neurotrophic factor, and neurturin) may play a similar role for LMNs.
The present results indicate that ongoing electrical activity also plays a significant role in KCa channel regulation in LMNs. Thus, conditions that evoked depolarization of cultured LMNs (e.g., elevated external K+) increased expression of KCa, whereas treatments that reduced spontaneous activity (e.g., TTX) reduced KCa expression under certain conditions. Moreover, treatments that alter the in vivo activity of LMNs also affected macroscopic KCa density. Thus, application of agents that reduce the spontaneous hindlimb motility of chick embryos (e.g., the GABAA agonist muscimol or the nAChR antagonist mecamylamine) caused a marked decrease in KCa, probably because of direct inhibition of LMNs, their excitatory afferents, or both (Millner and Landmesser, 1999). This observation stands in contrast to developing chick ciliary ganglion neurons, which express KCa channels at normal density when afferent synaptic inputs are chronically blocked by mecamylamine in vivo (Subramony and Dryer, 1996). On the other hand, there is a precedent for regulation of KCa by activity, because rat cerebellar neurons developing in vitro exhibit increased KCa expression in response to treatments that cause chronic membrane depolarization (Muller et al., 1998). Perhaps this is a common feature in CNS as opposed to autonomic neurons. In any case, these data provide additional evidence that different variants of BK KCa channels are subjected to different modes of developmental regulation.
Significant changes in the expression of KCa in chick LMNs continue after the main wave of apoptotic LMN cell death is complete (Chu-Wang and Oppenheim, 1978; Williams et al., 1987). However, the largest changes in LMN KCaexpression coincide with the gradual elimination of polyneuronal innervation of fast-twitch muscle fibers in the chick (Phillips and Bennett, 1987a,b). Synapse elimination and indeed many other aspects of neuromuscular junction differentiation depend on a specific pattern of motoneuron activity (for review, see Buonanno and Fields, 1999; Sanes and Lichtman, 1999). There is now considerable evidence that large-conductance KCa channels regulate the action potential waveform and the temporal pattern of spike discharge in vertebrate neurons (Lang et al., 1997; Golding et al., 1999;Martin-Caraballo and Greer, 2000). Additional studies will determine whether age-dependent changes in KCa channel expression correlate with significant changes in action potential waveform and firing properties of developing LMNs. It is possible that the appearance and gradual increase in functional KCa channels in LMNs between E8 and E13 induce a refinement in their electrophysiological properties that contributes to proper activity-dependent maturation of neuromuscular junctions.
Footnotes
This work was supported by a Muscular Dystrophy Association research grant to S.E.D., by National Institutes of Health Grant NS32748 to S.E.D., and by an Alberta Heritage Foundation for Medical Research postdoctoral fellowship to M.M.-C.
Correspondence should be addressed to Dr. Stuart E. Dryer, Department of Biology and Biochemistry, University of Houston, Houston, TX 77204-5513. E-mail: sdryer@uh.edu.
REFERENCES
- 1.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10:3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker E, Soler RM, Yuste VJ, Gine E, Sanz-Rodriguez C, Egea J, Martin-Zanca D, Comella JX. Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord. J Neurosci. 1998;18:7903–7911. doi: 10.1523/JNEUROSCI.18-19-07903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buonanno A, Fields RD. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Curr Opin Neurobiol. 1999;9:110–120. doi: 10.1016/s0959-4388(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 4.Caldero J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motor neurons in chick embryo. J Neurosci. 1998;18:356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron J, Dryer SE. BK-Type KCa channels in two parasympathetic cell types: differences in kinetic properties, developmental expression. J Neurophysiol. 2000;84:2767–2776. doi: 10.1152/jn.2000.84.6.2767. [DOI] [PubMed] [Google Scholar]
- 6.Cameron J, Lhuillier L, Subramony P, Dryer SE. Developmental regulation of neuronal K+ channels by target-derived TGFβ in vivo and in vitro. Neuron. 1998;21:1045–1053. doi: 10.1016/s0896-6273(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 7.Chu-Wang I-W, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord I: a light and electron microscopy study of naturally occurring and induced cell loss during development. J Comp Neurol. 1978;177:33–58. doi: 10.1002/cne.901770105. [DOI] [PubMed] [Google Scholar]
- 8.Dourado MM, Dryer SE. Changes in the electrical properties of chick ciliary ganglion neurones during embryonic development. J Physiol (Lond) 1992;449:411–428. doi: 10.1113/jphysiol.1992.sp019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dourado MM, Brunwell C, Wisgirda ME, Jacob MH, Dryer SE. Target tissues and innervation regulate the characteristics of K+ currents in chick ciliary ganglion neurons developing in situ. J Neurosci. 1994;14:3156–3165. doi: 10.1523/JNEUROSCI.14-05-03156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dryer SE. Role of cell-cell interactions in the developmental regulation of Ca2+-activated K+ currents in vertebrate neurons. J Neurobiol. 1998;37:23–36. doi: 10.1002/(sici)1097-4695(199810)37:1<23::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Dryer SE, Dourado MM, Wisgirda ME. Characteristics of multiple Ca2+-activated K+ channels in acutely dissociated chick ciliary-ganglion neurones. J Physiol (Lond) 1991;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworetzky S, Boissard CG, Lum-Raga JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang C-P, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSloβ subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci. 1996;16:4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- 14.Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson MG, Jr, Shen S, Wiemelt AP, McMorris FA, Barres BA. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–7371. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes RA, Sendtner M, Thoenen H. Members of several gene families influence survival of rat motoneurons in vitro and in vivo. J Neurosci Res. 1993;36:663–671. doi: 10.1002/jnr.490360607. [DOI] [PubMed] [Google Scholar]
- 17.Lagrutta A, Shen K-Z, North RA, Adelman JP. Functional differences among alternatively spliced variants of slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem. 1994;269:20347–20351. [PubMed] [Google Scholar]
- 18.Lang EJ, Sugihara I, Llinas R. Differential roles of apamin- and charybdotoxin-sensitive K+ conductances in the generation of inferior olive rhythmicity in vivo. J Neurosci. 1997;17:2825–2838. doi: 10.1523/JNEUROSCI.17-08-02825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lhuillier L, Dryer SE. TGFβ1 regulates the gating properties of intermediate-conductance KCa channels in developing sympathetic neurons. J Neurophysiol. 1999;82:1627–1631. doi: 10.1152/jn.1999.82.3.1627. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Caraballo M, Greer JJ. Development of potassium conductances in perinatal rat phrenic motoneurons. J Neurophysiol. 2000;83:3497–3508. doi: 10.1152/jn.2000.83.6.3497. [DOI] [PubMed] [Google Scholar]
- 21.McCobb DP, Best PM, Beam KG. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989;2:1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- 22.McCobb DP, Best PM, Beam KG. The differentiation of excitability in embryonic chick limb motoneurons. J Neurosci. 1990;10:2974–2984. doi: 10.1523/JNEUROSCI.10-09-02974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messengill JL, Smith MA, Son DI, O'Dowd DK. Differential expression of K4-ap currents and Kv3.1 potassium channels transcripts in cortical neurons that develop distinct firing phenotypes. J Neurosci. 1997;17:3136–3147. doi: 10.1523/JNEUROSCI.17-09-03136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller YL, Reitstetter R, Yool AJ. Regulation of Ca2+-dependent K+ channel expression in rat cerebellum during postnatal development. J Neurosci. 1998;18:16–25. doi: 10.1523/JNEUROSCI.18-01-00016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien RJ, Fischbach GD. Isolation of embryonic chick motoneurons and their survival in vitro. J Neurosci. 1986;6:3265–3274. doi: 10.1523/JNEUROSCI.06-11-03265.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donovan MJ, Landmesser LT. The development of hindlimb motor activity studied in the isolated spinal cord of the chick embryo. J Neurosci. 1987;7:3256–3264. doi: 10.1523/JNEUROSCI.07-10-03256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppenheim RW, Prevette D, D'Costa A, Wang S, Houenou LJ, McIntosh JM. Reduction of neuromuscular activity is required for the rescue of motoneurons from naturally occurring cell death by nicotinic-blocking agents. J Neurosci. 2000;20:6117–6124. doi: 10.1523/JNEUROSCI.20-16-06117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips WD, Bennett MR. Elimination of distributed acetylcholine receptor clusters from developing fast-twitch fibres in an avian muscle. J Neurocytol. 1987a;16:1–10. doi: 10.1007/BF02456693. [DOI] [PubMed] [Google Scholar]
- 30.Phillips WD, Bennett MR. Elimination of distributed synaptic acetylcholine receptor clusters on developing avian fast-twitch muscle fibres accompanies loss of polyneuronal innervation. J Neurocytol. 1987b;16:785–797. doi: 10.1007/BF01611986. [DOI] [PubMed] [Google Scholar]
- 31.Qin-Wei Y, Johnson J, Prevette D, Oppenheim RW. Cell death of spinal motoneurons in the chick embryo following deafferentation: rescue effects of target tissue extracts, soluble proteins, and trophic factors. J Neurosci. 1994;14:7629–7640. doi: 10.1523/JNEUROSCI.14-12-07629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramanathan K, Michael TH, Fuchs PA. β subunits modulate alternatively spliced, large conductance, calcium-activated potassium channels of avian hair cells. J Neurosci. 2000;20:1675–1684. doi: 10.1523/JNEUROSCI.20-05-01675.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raucher S, Dryer SE. Target-derived factors regulate the expression of Ca2+-activated K+ currents in developing chick sympathetic neurons. J Physiol (Lond) 1995;486:605–614. doi: 10.1113/jphysiol.1995.sp020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long term potentiation. J Neurosci. 1996;16:150–154. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 36.Sendtner M, Carroll P, Holtmann B, Hughes RA, Thoenen H. Ciliary neurotrophic factor. J Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- 37.Soler RM, Egea J, Mintenig GM, Sanz-Rodriguez C, Iglesias M, Comella JX. Calmodulin is involved in membrane depolarization-mediated survival of motoneurons by phosphatidylinositol-3 kinase- and MAPK-independent pathways. J Neurosci. 1998;18:1230–1239. doi: 10.1523/JNEUROSCI.18-04-01230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spitzer NC. A developmental handshake: neuronal control of ionic currents and their control of neuronal differentiation. J Neurobiol. 1991;22:659–673. doi: 10.1002/neu.480220702. [DOI] [PubMed] [Google Scholar]
- 39.Steinhardt RA, Zucker R, Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977;58:185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramony P, Dryer SE. The effects of innervation on the developmental expression of Ca2+-activated K+ currents in embryonic parasympathetic neurons are not activity-dependent. Dev Brain Res. 1996;91:149–152. doi: 10.1016/0165-3806(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 41.Subramony P, Raucher S, Dryer L, Dryer SE. Posttranslational regulation of Ca2+-activated K+ currents by a target-derived factor in developing parasympathetic neurons. Neuron. 1996;17:115–124. doi: 10.1016/s0896-6273(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 42.Tang J, Landmesser LT. Reduction of intramuscular nerve branching and synaptogenesis is correlated with decreased motoneuron survival. J Neurosci. 1993;13:3095–3103. doi: 10.1523/JNEUROSCI.13-07-03095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 44.Usiak MF, Landmesser LT. Neuromuscular activity blockade induced by muscimol and d-tubocurarine differently affects the survival of embryonic chick motoneurons. J Neurosci. 1999;19:7925–7939. doi: 10.1523/JNEUROSCI.19-18-07925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams C, Wohlenberg G, O'Donovan MJ. Regional variations in the extent and timing of motoneuron cell death in the lumbosacral spinal cord of the chick embryo. Brain Res. 1987;431:215–221. doi: 10.1016/0165-3806(87)90210-0. [DOI] [PubMed] [Google Scholar]