Abstract
The mechanisms by which trophic factors bring about spinal motor neuron (MN) survival and regulate their number during development are not well understood. We have developed an organotypic slice culture model for the in vitro study of the trophic requirements and cell death pathways in MNs of postnatal day 1–2 mice. Both lateral motor column (LMC) and medial motor column (MMC) neurons died within 72 hr when grown in serum-free medium without trophic factors. Brain-derived neurotrophic factor, ciliary neurotrophic factor, and 8-(4-chlorophenylthio)-cAMP promoted the survival of a proportion of the neurons, but glial cell line-derived neurotrophic factor (GDNF) was the most effective trophic factor, supporting ∼60% of MNs for 1 week in culture. Homozygous deficiency for bax, a proapoptotic member of the Bcl-2 family, saved the same proportion of neurons as GDNF, suggesting that GDNF alone was sufficient to maintain all “rescuable” MNs for at least 1 week. Analysis of MN survival inGFRα-1−/− mice demonstrated that the trophic effect of GDNF was completely mediated by its preferred coreceptor, GDNF family receptor α-1 (GFRα-1). None of the other GDNF family ligands supported significant MN survival, suggesting that there is little ligand–coreceptor cross talk within the slice preparation. Although MN subtypes can be clearly defined by both anatomical distribution and ontogenetic specification, the pattern of trophic factor responsiveness of neurons from the MMC was indistinguishable from that seen in the LMC. Thus, in contrast to all other factors and drugs studied to date, GDNF is likely to be a critical trophic agent for all early postnatal MN populations.
Keywords: subpopulation, trophic factor, apoptosis, neuronal death, Bax, organotypic
Our understanding of the trophic requirements of spinal motor neurons (MNs) comes from four principal experimental approaches: (1) rescue from axotomy, (2) prevention of naturally occurring neuronal death, (3) cultures of dissociated embryonic neurons, and (4) transgenic animals. Members of all the important neurotrophic factor families support MN survival, including neurotrophins, neurocytokines, and glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs). However, in no instance has a single trophic factor been shown to promote the long-term survival of all MNs. This might reflect the more “complex” trophic requirements of CNS neurons, which could depend on multiple trophic signals (Snider, 1994), possibly explaining why MN numbers are remarkably normal in most transgenic animals lacking individual trophic factors or receptors. Alternatively, it has been proposed that subgroups of MNs have different trophic requirements (de Lapeyriere and Henderson, 1997). For instance, mice lacking ciliary neurotrophic factor receptor α, GDNF, or GDNF receptor components have significantly fewer MNs at birth (de Chiara et al., 1995; Garces et al., 2000), which might reflect the loss of specific subpopulations during the period of programmed cell death. Unfortunately, these particular animals die in the perinatal period, so nothing is known about the postnatal fate of their MNs.
Somatic MNs are subdivided on anatomical grounds into a lateral motor column (LMC) and medial motor column (MMC), which innervate limb and axial musculature, respectively (Landmesser, 1980). More is known about the survival requirements of LMC neurons, which can be rescued by growth factors from death after peripheral nerve axotomy (Elliott and Snider, 1999). LMC neurons that have emerged from the period of programmed cell death remain vulnerable to axotomy after birth until they lose their “target dependence” at approximately postnatal day 7 (P7)–P10 (Lowrie and Vrbova, 1992). The trophic requirements of MMC neurons are difficult to address directly, because their axons are not accessible to axotomy, and they cannot be distinguished from other MNs in dissociated cultures.
The aim of the present study was to develop an in vitroparadigm to investigate the trophic requirements of MNs at an age when they are still target-dependent for survival in vivo. Dissociated cultures, described in embryonic MNs, have not been successful in postnatal neurons and do not allow the differentiation of MMC and LMC populations (Camu and Henderson, 1992). Organotypic slice cultures of rodent (predominantly rat) spinal cord allow P8 MNs to be maintained long-term in the presence of serum but without additional growth factors, probably because at this age the neurons are target-independent for survival (Corse and Rothstein, 1995). We have developed a slice preparation of mouse spinal cord taken at a younger age (P0–P2) to examine the trophic responsiveness of target-dependent MNs in wild-type and transgenic animals. The organotypic organization of the slice has permitted the direct comparison of LMC and MMC neuron trophic requirements. In addition, we have been able to study MN survival beyond the normal life span of GDNF receptor knock-out animals. Our findings indicate that GDNF is an extremely potent trophic factor that promotes the long-term survival of the majority of spinal MNs in the early postnatal period.
MATERIALS AND METHODS
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated.
Preparation of spinal cord slices. P1–P2 C3H mice were obtained from Harlan (Indianapolis, IN); the breeding and genotyping ofBax- and GFRα-1-deficient (P0–P1) mice (both on a C57BL/6 background) have been described previously (Knudson et al., 1995; Enomoto et al., 1998). Spinal cord slices were prepared on the basis of adaptations of a slice preparation of embryonic mouse brain (Sheppard et al., 1995; Brunstrom et al., 1997). The spinal cords were extracted under aseptic conditions and kept in ice-cold artificial CSF (ACSF) containing 175 mmsucrose, 37 mm d-glucose, 10 mm MgSO4, 2 mm CaCl2, 25 HEPES, 20 nmdl-α-tocopherol, and 20 nmdl-α-tocopherol acetate. The spinal cords were embedded in low-melting point agarose (2.5% in PBS; type VII agarose, A9045; Sigma), mounted with epoxy resin adhesive onto a UV-sterilized Teflon vibratome stage, and submersed in a bath of ice-cold ACSF. Two hundred fifty to 300 μm transverse slices through the lumbar enlargement were cut with a vibratome (Vibraslice NVSML1; Campden Instruments, Sileby, UK) and collected (five or six slices per spinal cord) at the bottom of the bath until all of the spinal cords had been cut.
Slice culture, fixing, and processing. One micrometer polyethylene terephthalate (PET) membrane inserts (Falcon 3102; Becton Dickinson, Franklin Lakes, NJ) were presoaked in defined medium consisting of EOL1 (Annis et al., 1990; lacking ethanolamine) with the addition of (in gm/l): 3.4 glucose, 1.2 NaHCO3, 59.1 leucine, and 56.4 glycine. Four to six randomly selected slices were placed on each insert; the excess medium was aspirated; and the inserts were placed in organ culture dishes (Falcon) over 1.6 ml of defined medium with added growth factors or drugs. The dishes were placed in a humidified incubator with 5% CO2 at 37°C, and the slices were maintained for up to 1 week with a daily 50% medium change. Artemin (ARTN) was made as described previously (Baloh et al., 1998). GDNF, neurturin (NRTN), and persephin (PSPN) were provided by Genentech (San Francisco, CA). CNTF was a gift from Cephalon (Westchester, CA). Brain-derived neurotrophic factor (BDNF) was purchased from Sigma. After testing a range of concentrations (see Results), GDNF and the other GFLs were used at a concentration of 200 ng/ml, whereas BDNF and CNTF were used at 500 ng/ml.
At the end of the experiment, slices were fixed in 2% paraformaldehyde in PBS at 4°C (15 min), cryoprotected (30% sucrose overnight at 4°C), embedded in OCT (Sakura Finetek USA, Inc., Torrance, CA), and frozen in liquid nitrogen. Serial cryostat sections (12 μm) cut parallel to the plane of the slice were collected in strict rotation on three Vectabond-coated slides (Vector Laboratories, Burlingame, CA). Thus, each slide contained sections taken at 24 μm intervals through a slice. Sections immediately adjacent to each other were on different slides and could therefore be processed with different staining techniques. The slides were dried and stored at −20°C.
Counting motor neurons in sections of spinal cord slices.One of the three slides from each slice culture was selected at random for Nissl staining with cresyl violet (0.5% w/v in 20% ethanol), whereas the others were reserved for selective immunostaining. A cultured slice is not uniform throughout its thickness, because there is overgrowth of glia on the top surface, whereas the central part of the bottom 50–80 μm of the slice dies, possibly because of insufficient oxygenation. For this reason, all quantification was performed on two nonadjacent sections taken between 12 and 84 μm from the top surface of the slice. Nissl-stained MNs in the LMC and MMC were identified and counted according to recognized criteria (Clarke and Oppenheim, 1995): a large (>25 μm) soma, a clear nucleus with an intact nuclear membrane, and one clump of nucleolar material; in addition, counting was confined to cells in the gray matter of the ventral horn of the spinal cord. These criteria allowed unambiguous MN identification in a high-power field with excellent interobserver correlation. To ensure that no systematic bias was introduced into counts from different conditions (Clarke and Oppenheim, 1995; Guillery and Herrup, 1997), we ascertained that the size of the MN nucleoli did not change significantly between fresh tissue and slices maintained in GDNF for 6 d. Counts from the right and left halves of the spinal cord were considered separately to allow for alignment problems and sections damaged during sectioning. MN counts in each half were expressed as a percentage of the mean number of MNs present in the lateral or medial motor column in 12 μm sections of three fresh cords. Four to six slices per condition contributed 8–12 counts to each data point, allowing the calculation of a mean ± SD percent survival. Statistical significance was tested using Student'st test. Each figure is representative of the results of two to four independent experiments.
Immunohistochemistry. Slides were thawed, postfixed in 1% paraformaldehyde in PBS (5 min at room temperature), permeabilized with 0.2% Triton X-100 (Sigma; 60 min at room temperature), and blocked in 2% normal goat serum in PBS (Sigma; 60 min at room temperature). Primary antibodies (applied overnight at 4°C or 60 min at room temperature) included rabbit anti-Ret (1:200; Immuno-Biological Laboratories) and goat anti-GDNF family receptor α-1 (GFRα-1) (1:100; R & D Systems, Minneapolis, MN). The specificity of antibody staining was confirmed by the complete absence of staining in the dorsal root ganglion ofRet−/− andGFRα-1−/− mice (H. Enomoto, personal communication). Secondary antibodies fromJackson ImmunoResearch (West Grove, PA) were applied for 1 hr at room temperature (Cy3 goat anti-rabbit, 1:1000; and HRP anti-goat, 1:750). A tyramide signal amplification step (PerkinElmer Life Sciences, Boston, MA) was used to increase detection of GFRα-1. All antibodies were diluted in 1–2% normal goat serum in PBS. Immunolabeled sections were counterstained with Hoechst 33342 (bisbenzimide) and then coverslipped with Vectashield (Vector Laboratories). Fluorescence images were viewed on a Zeiss (Thornwood, NY) Axiovert 100M UV microscope and imaged with a CCD camera and Slidebook 3.0.2.12 software (Intelligent Imaging Innovations, Denver, CO).
RESULTS
GDNF supports the prolonged survival of motor neurons in slice culture
To date, the main in vitro rodent MN culture paradigms have used purified embryonic neuronal cultures or slices taken from postnatal spinal cord (Camu and Henderson, 1992; Corse and Rothstein, 1995). Metrizamide density gradient centrifugation, usually combined with immunopanning, has been extremely useful in the study of embryonic MNs in serum-free medium (Hanson et al., 1998) but has not been applied successfully to postnatal neurons, and the anatomical origin of the MNs is difficult to establish. Spinal cord slice cultures (mainly rat) can be harvested at P8 and MNs maintained for months in the presence of serum without additional trophic support (Corse and Rothstein, 1995), probably because the neurons are largely target-independent at the time of harvesting (Lowrie and Vrbova, 1992). However, this renders P8 cultures unsuitable for studying the trophic regulation of neuronal death. Embedding the fragile tissue in 2.5% agarose allowed us to prepare slices from much younger (P0–P2) mouse spinal cord and to directly compare the trophic requirements of well defined subpopulations of MNs at a time when they are still target-dependent for survival. MNs were counted on Nissl-stained sections as described in Materials and Methods (Fig.1A,C). In preliminary experiments, MN identity in the cultured slices was confirmed by positive staining for Ret, the signaling component of the GDNF receptor complex (Fig. 1B,D), the p75 neurotrophin receptor; the vesicular acetylcholine transporter; and phosphorylated neurofilaments (data not shown). Hoechst 33342 (bisbenzimide) counterstaining demonstrated that the MNs had a large, healthy-appearing nucleus with no evidence of DNA condensation (Fig.1D). A comparison of MN numbers in Nissl- and Ret-stained adjacent sections showed no significant difference, indicating that the vast majority of MNs present in the slice after 6 d in culture express Ret. The variable staining intensity of adjacent MNs in a single section (compare Fig. 6B) and the inability to clearly identify nucleoli limit the usefulness of immunohistochemistry in the counting of these large neurons. Thus, in accordance with established practice and criteria (Clarke and Oppenheim, 1995), routine MN counts were performed in a high-power field on Nissl-stained sections with excellent interobserver correlation.
Fig. 1.

Postnatal MNs can be grown in slice cultures of mouse spinal cord. Photomicrographs are shown of 12 μm sections of a single slice, taken from the lumbar enlargement of a P1 mouse, after 6 d of culture in the presence of GDNF. A, Staining for Nissl substance with cresyl violet allows the identification of MNs in the LMC and MMC in the ventral horn of the gray matter.B, Immunohistochemistry for Ret, the signaling component of the GDNF receptor complex, in an adjacent section demonstrates staining confined to MNs (red). C, A high-power view of the Nissl-stained section shows characteristic MN morphology in the LMC. D, A Hoechst 33342 (bisbenzimide) counterstain demonstrates that the nuclei (blue) of the surviving MNs appear healthy. Scale bar, 40 μm.
Fig. 6.
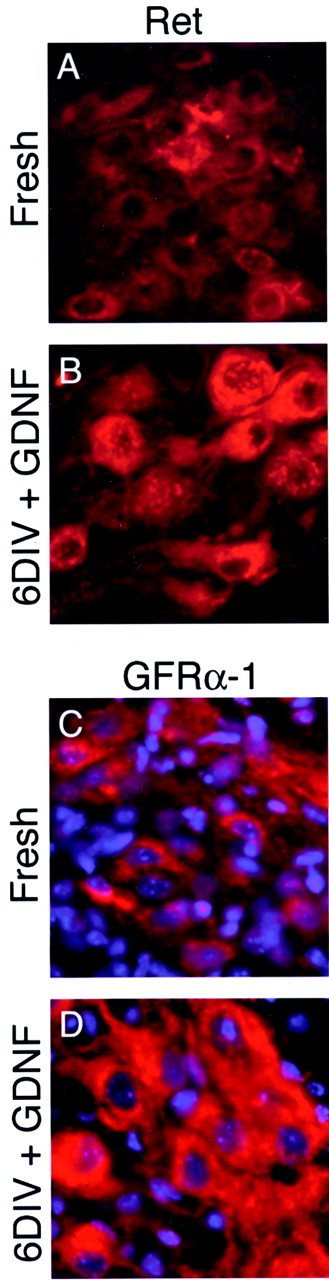
Expression of Ret and GFRα-1 increases in culture. Sections of fresh cord and of slices cultured for 6 d in the presence of GDNF were stained for the signaling component of the GDNF receptor complex Ret (A, B) and for the preferred GDNF coreceptor GFRα-1 (C, D). A, C, Both Ret and GFRα-1 are clearly expressed in fresh cord, but staining is of variable intensity. B, D, By 6 DIV, in the presence of GDNF both receptor components are clearly upregulated in the surviving MNs. Antibody specificity was confirmed by an absence of staining in Ret−/− and GFRα-1−/− mice (data not shown).
Initial observations were confined to MNs of the LMC because the timing and extent of their loss during development and their postaxotomy survival requirements have been well characterized (Elliott and Snider, 1999; Oppenheim et al., 2000). Although a large number of growth factors are recognized as MN survival factors, we selected GDNF for the initial characterization of this new slice culture system because of its well described robust survival-promoting effects on MNs in variousin vitro and in vivo settings (Henderson et al., 1994; Oppenheim et al., 1995). In preliminary experiments, we found that concentrations of GDNF that are supramaximal for the survival of dissociated rat sympathetic neurons (10–50 ng/ml) supported the survival of very few MNs in the slices, but higher concentrations were effective, consistent with studies of growth factors in other slice culture preparations (Brunstrom et al., 1997; Bilak et al., 1999). To determine the optimal concentration of GDNF for MN survival, parallel cultures were treated with 0–500 ng/ml GDNF for 6 d (Fig.2A). There was a robust response to increasing concentrations of GDNF. The difference in survival between 200 and 500 ng/ml was not statistically significant, suggesting the response was no longer limited by trophic factor concentration. Thus, subsequent positive control cultures were maintained in 200 ng/ml GDNF.
Fig. 2.

GDNF supports the long-term survival of MNs in slice culture. A, Slices were cultured for 6 d in increasing concentrations of GDNF. Nissl-stained LMC motor neurons were counted in 12 μm frozen sections of the slices as described in Materials and Methods. MN survival is expressed as a percentage of the mean number present in 12 μm sections of fresh cord.B, In a separate experiment, slices were cultured for 1, 3, or 7 d in the presence (filled squares) or absence (filled circles) of GDNF (200 ng/ml). *p < 0.05; **p < 0.001.
To follow the time course of MN death in the presence and absence of trophic support, cultures were established and fixed at 1, 3, and 7 d after slicing (Fig. 2B). MNs died rapidly in the absence of trophic factors, with the majority of the death occurring in the first 72 hr. By contrast, in the presence of GDNF ∼50% of MNs survived for at least 7 d (p< 0.0001). MN death in the presence of GDNF occurred only during the first 24 hr, and this was at a rate indistinguishable from that seen in the absence of growth factor. The difference in survival between the two conditions was accounted for by the continued death of MNs deprived of trophic support between 24 and 72 hr, whereas numbers remained constant in GDNF-treated slices. Thus, GDNF promotes the sustained (at least 1 week) survival of P1 MNs in slice culture.
GDNF promotes the survival of an equivalent number of motor neurons as bax deficiency
The neurons that die in the first 24 hr even in the presence of high concentrations of GDNF might require a different trophic factor for survival or could represent cells that die as a result of the harvesting procedure. To help distinguish between these two possibilities, we examined spinal cord slice cultures prepared frombax−/− animals (Fig.3). Bax is a proapoptotic member of the Bcl-2 family that plays a central role in neuronal programmed cell death; sympathetic and cerebellar granule neurons from bax−/− mice fail to undergo apoptosis in response to classical death triggers, including trophic factor withdrawal (Deckwerth et al., 1996; Miller et al., 1997). Similarly,bax−/− MNs in the facial nucleus and lumbar spine are protected from death during development and after axotomy (Deckwerth et al., 1996; White et al., 1998). To take into account the effect of bax deletion on developmental MN death, we compared sections of fresh P1 spinal cord frombax−/− animals and theirbax+/+ littermates and found that thebax−/− lumbar LMC contained more than twice as many MNs (26.8 ± 6 vs 11.6 ± 3 neurons per section; p < 0.0001). Comparing initially absolute MN numbers in the slice cultures (Fig. 3A),bax+/+ MNs showed an absolute dependence on trophic support for survival [7 ± 2 (GDNF) vs 0.5 ± 1 (no trophic factor) neurons per section; p < 0.05]. In contrast, the presence or absence of GDNF did not affect the number of surviving bax−/− MNs after 6 d of culture (15 ± 5 vs 14.5 ± 3 neurons per section; NS). The lack of increased MN death inbax−/− slices cultured in the absence of GDNF suggested that, similar to primary cultures of sensory and sympathetic neurons, bax deficiency prevents MN programmed death caused by trophic factor deprivation. Importantly, the addition of GDNF to bax−/−cultures did not result in a further increase in MN survival, demonstrating that GDNF is not able to promote survival beyond that resulting from bax deficiency.
Fig. 3.

GDNF promotes the survival of an equivalent number of MNs as bax deficiency. A, Slices were cultured for 6 d in the presence or absence of GDNF (200 ng/ml) from animals homozygous for a deletion in the proapoptotic Bcl-2 family member bax (bax−/−) and cultured alongside slices from wild-type littermates. MNs from bax−/− cords survived 6 DIV in the absence of GDNF. The addition of GDNF did not further increase the number of MNs surviving in the bax−/− cords. Absolute unilateral LMC counts are presented for each genotype. B, Expressed as a percentage of the neurons in fresh cord, the same proportion of wild-type MNs survived in the presence of GDNF as bax−/− MNs both in the presence and the absence of trophic factor. No TF, No trophic factor. **p < 0.001.
To determine the relative magnitude of the saving effects of GDNF and of bax deficiency, we compared MN survival in GDNF-treatedbax+/+ slices and inbax−/− slices grown without trophic factor. Given the above-described differences in baseline MN counts in the two genotypes, MN survival at 6 d in vitro (DIV) was expressed as a percentage of the number of neurons in the intact spinal cord of the respective genotype at the time of harvesting (Fig. 3B). The proportion of MNs surviving at 6 DIV in bax−/− slices without trophic support was not significantly different from that seen inbax+/+ slices cultured in the presence of GDNF (54 ± 6 vs 59 ± 9%, respectively; NS). Thus, not only does the addition of GDNF fail to promote the survival of more MNs thanbax deficiency alone, but the same proportion of neurons is also saved by both bax deficiency and GDNF. We conclude that a single polypeptide trophic factor, GDNF, is able to support the long-term (1 week) survival of the majority of MNs capable of being rescued.
The GDNF and bax−/−observations allowed two phases of MN death to be distinguished over the time course of this slice culture paradigm (Fig.2B): an initial period (0–24 hr) of rapid death in which neither GDNF nor bax deficiency affected survival (trophic factor-independent death) and a second period (24–72 hr) of slower death that could be prevented by both interventions (trophic factor-dependent death). To further characterize the regulation of MN death, slices were grown in medium without GDNF and treated for 6 d with either cycloheximide (CHX), an inhibitor of protein synthesis, or Boc-aspartyl (O-methyl) fluoromethylketone (BAF), a pan-caspase inhibitor, both of which inhibit apoptosis in trophic factor-deprived sympathetic neurons (Martin et al., 1988; Deshmukh et al., 1996) (Fig. 4). At a time (6 DIV) when most of the MNs deprived of trophic support were dead, both CHX (Fig. 4A) and BAF (Fig. 4B) rescued >50% of the number of MNs maintained by GDNF. Thus, MN death in the slice cultures bears the hallmarks of neuronal apoptosis.
Fig. 4.

Inhibitors of protein synthesis and caspases prevent MN death in the absence of trophic support. Slices were grown in increasing concentrations of CHX, an inhibitor of protein synthesis (A), or BAF, a pan-caspase inhibitor (B). Positive control cultures were grown in GDNF (200 ng/ml). *p < 0.05; **p < 0.01; ***p < 0.001.
BDNF, CNTF, and cAMP support the survival of only a proportion of motor neurons
To extend the observations of trophic factor responsiveness in this system, BDNF, CNTF, and insulin-like growth factor 1 (IGF-1) were selected as representatives of the major classes of factors that are known to promote MN survival (Elliott and Snider, 1999). Given the need for higher concentrations of GDNF in slices compared with dissociated cultures, all factors were tested at one low concentration (50 ng/ml) and one high concentration (500 ng/ml). Low concentrations of the factors were ineffective (data not shown), but high concentrations of BDNF and CNTF supported the survival of 20–30% of MNs (Fig.5A). IGF-1 was not significantly protective at any concentration (data not shown). Survival in the presence of BDNF combined with CNTF (53 ± 25%) was significantly greater than with either BDNF (34 ± 19%;p < 0.05) or CNTF (25 ± 7%; p< 0.005) alone but did not exceed the effect of GDNF. Furthermore, the response to GDNF could not be improved by the addition of either BDNF or CNTF (Fig. 5B).
Fig. 5.
MN survival is promoted by BDNF, CNTF, and raised levels of intracellular cAMP. A, Slices were grown for 6 d with the indicated trophic factors GDNF (200 ng/ml), BDNF (500 ng/ml), and CNTF (500 ng/ml). BDNF and CNTF both promoted the survival of a proportion of the MNs. BDNF and CNTF in combination supported the survival of more MNs than either trophic factor alone and were able to match but not to exceed the effect of GDNF. B, The concomitant addition of BDNF or CNTF did not further increase the survival response of MNs to GDNF. C, Elevating intracellular levels of cAMP using the membrane-permeable analog CPT-cAMP also promoted the survival of a proportion of the MNS.D, Treatment of the slices with CPT-cAMP (200 μm) in addition to trophic factor did not further increase the survival response to BDNF or CNTF. CPT, CPT-cAMP. *p < 0.05; **p < 0.01; ***p < 0.001.
Raising intracellular levels of cAMP has been reported to promote both the survival and responsiveness to trophic factors of dissociated neurons (Rydel and Greene, 1988; Meyer-Franke et al., 1995; Hanson et al., 1998). We therefore determined whether the relative or absolute failure of other trophic factors to match the potency of GDNF could be the result of low intracellular levels of cAMP. To address this issue, we used the membrane-permeable cAMP analog 8-(4-chlorophenylthio)-cAMP (CPT-cAMP). In contrast to dissociated embryonic MNs, in which raising intracellular cAMP has short-term survival effects equivalent to those of any trophic factor given in isolation (Hanson et al., 1998), CPT-cAMP promoted only modest survival of MNs in slice culture (Fig.5C). Additionally, the coadministration of CPT-cAMP with either BDNF or CNTF did not significantly increase MN survival after 6 d of treatment (Fig. 5D). Raising intracellular levels of cAMP also failed to significantly increase the survival response to GDNF after 3 d of treatment (58 ± 31% in GDNF and CPT-cAMP vs 50 ± 10% in GDNF alone; NS). Thus, in contrast to dissociated embryonic MNs, raising intracellular levels of cAMP had only a modest effect on postnatal MN survival in slice culture and did not increase their responsiveness to trophic factors.
GDNF survival signaling is mediated by the GFRα-1 coreceptor
Given the robust MN survival response to GDNF, the expression patterns of the GDNF receptor complex components Ret and GFRα-1 were examined. Ret staining was of variable intensity in fresh spinal cord (Fig. 6A) but was more intense after 6 DIV in the presence of GDNF (Fig.6B). Staining was particularly strong in the soma but also extended into neuronal processes (data not shown). Staining for the GFRα-1 subunit was difficult to detect in fresh spinal cord even with tyramide signal amplification (Fig. 6C). GFRα-1 expression also dramatically increased after 6 DIV in the presence of GDNF (Fig. 6D).
GDNF was the first identified member of the GFLs, which also includes NRTN, ARTN, and PSPN. All GFLs support MN survival in embryonicin vitro or postnatal in vivo axotomy paradigms (Klein et al., 1997; Milbrandt et al., 1998; Soler et al., 1999). For this reason, the relative effectiveness of the different GFLs in promoting MN survival was tested (Fig.7A). There was significant variability in the number of surviving MNs 6 d after GFL treatment in slices cultured in the presence of NRTN (2–19%) and ARTN (2–17%). Taken over three to five independent experiments testing each condition, there was a tendency for more neurons to be found in cultures containing NRTN and ARTN (9 ± 5 and 12 ± 5%, respectively, mean ± SEM) but this was not statistically different from survival in the absence of trophic support (4 ± 3%). The suggestion of a small effect of NRTN and ARTN alone raised the possibility of the existence of responsive MNs that are present in numbers that are below the sensitivity of the assay. For this reason, we added NRTN and ARTN in combination to see whether any additive effect could be demonstrated (Fig. 7B), but survival was not significantly greater than in medium alone (2 ± 3 vs 8 ± 7%; NS). Furthermore, the combination of all four GFLs did not promote the survival of more MNs than GDNF alone (62 ± 15 vs 64 ± 10%; NS).
Fig. 7.

GDNF is the exclusive physiological ligand for GFRα-1 in P1 MNs in slice culture. A, Slices were cultured for 6 d in the presence of 200 ng/ml GDNF, NRTN, ARTN, or PSPN. The mean ± SEM of three to five independent experiments for each condition is shown. B, In a separate experiment, a combination of NRTN and ARTN did not increase MN survival over baseline. The addition of NRTN, ARTN, and PSPN to GDNF did not significantly change the survival response to GDNF alone. C, Slice cultures prepared from mice null for GFRα-1 and wild-type littermates were treated with GDNF alone. GDNF survival signaling was completely abolished in GFRα-1−/− MNs. ***p < 0.001.
To examine the physiological importance of GFRα-1 for GDNF-dependent MN survival, slice cultures were prepared fromGFRα-1−/− mice and cultured in the presence of GDNF or no trophic factor. The survival response to GDNF was completely abolished inGFRα-1−/−neurons (Fig. 7C). Thus, the trophic effect of GDNF on MNs is completely mediated by the GFRα-1 coreceptor. Furthermore, despitein vitro evidence in cell lines of potential cross talk between different GFL members and their respective receptors (Creedon et al., 1997; Jing et al., 1997), in this paradigm only GDNF induces a significant MN survival signal through GFRα-1. The absence of a detrimental effect of the other GFLs when coadministered with GDNF suggests that they do not compete with GDNF for receptor binding.
The medial and lateral motor columns have similar trophic response profiles
One of the proposed explanations for the partial responsiveness of MNs to so many trophic factors is the existence of subpopulations with different trophic requirements (de Lapeyriere and Henderson, 1997), possibly in a way that would be analogous to sensory neurons in the dorsal root ganglion (Snider and Silos-Santiago, 1996). The anatomical subdivision of MNs into lateral and medial motor columns is clearly preserved in an organotypic slice, thus providing an opportunity to compare the trophic requirements of the two populations. Whereas MNs of the LMC are found only in the brachial and lumbar enlargements, MMC neurons are present throughout the length of the spinal cord (Landmesser, 1980). Nevertheless, the distribution of MNs in the MMC was not completely uniform; MMC motor neuron numbers were particularly variable at the level of the lumbar enlargement, but larger and more consistent numbers were found at levels with no LMC. Therefore, slices were made of midthoracic cord (which contains MMC but no LMC) and cultured alongside slices of lumbar enlargement from the same animals in the presence of BDNF, CNTF, and GFLs (Fig.8). Neurons from the MMC showed a robust survival response to GDNF, with ∼60% surviving after 6 d in culture, which was not statistically significantly different from that of LMC neurons. The two populations also displayed very similar responses to BDNF and CNTF (Fig. 8A). The lack of a survival response in MMC neurons after 6 DIV to NRTN, ARTN, or PSPN was also similar to the situation in LMC neurons (Fig.8B). Thus, the profile of trophic factor responsiveness of MNs in the medial and lateral motor columns was indistinguishable in this experimental paradigm.
Fig. 8.

LMC and MMC motor neurons show similar patterns of trophic factor responsiveness. Slices were prepared from the lumbar enlargement and thoracic cord of the same mice to establish the respective trophic requirements of LMC and MMC neurons.A, Mirroring the situation in the LMC, BDNF, and CNTF supported the survival of a proportion of MMC motor neurons.B, Similarly, GDNF was the only GFL to exert a trophic effect on MMC neurons. Note that the absolute number of MNs in the thoracic MMC is significantly less than in the lumbar LMC (5.0 ± 0.8 vs 11.6 ± 0.8 neurons per section; p < 0.001). *p < 0.05; **p < 0.01; ***p < 0.001. 0, No trophic factor; G, GDNF; B, BDNF;C, CNTF; N, neurturin; A, artemin; P, persephin.
DISCUSSION
The trophic requirements of MNs and the mechanisms by which growth factors bring about their survival are incompletely understood.In vitro approaches are well suited to address these questions directly but are hindered by the fact that MNs constitute a minority of neurons within the spinal cord and are difficult to culture. In the present study, we have established long-lived slice cultures of P0–P1 mouse spinal cord, at a time when MNs have emerged from the period of naturally occurring cell death and yet remain trophic factor-dependent for survival. BDNF, CNTF, and cAMP support the survival of a proportion of the neurons, but GDNF on its own is able to maintain the majority of MNs that survive the harvesting procedure. The trophic effect of GDNF is absolutely dependent on the presence of its preferred coreceptor, GFRα-1. In the context of a slice, which may be more physiological than dissociated neurons, GFRα-1 is not able to mediate a survival response to the other GFLs. The organotypic nature of the spinal cord preparation further allowed us to demonstrate that trophic factor responsiveness is very similar in two anatomically distinct MN subpopulations.
GDNF supports the survival of the majority of early postnatal motor neurons in slice culture
A strong trophic effect of GDNF on early postnatal mouse MNs has been demonstrated in axotomy studies (Yan et al., 1995; Vejsada et al., 1998; Yuan et al., 2000). Furthermore,GDNF−/− andGFRα-1−/− mice are among the few trophic factor ligand or receptor transgenic animals to display a significant developmental loss of MNs (Baloh et al., 2000). Although postnatal MNs are clearly very responsive to GDNF (Nguyen et al., 1998), the importance of GDNF for their survival cannot be tested in knock-out animals, becauseGDNF−/−,GFRα-1−/−, and, indeed, Ret−/− mice die within 24 hr of birth. Our findings suggest that GDNF may be particularly effective at promoting MN survival during this early postnatal period.
The concentration of GDNF needed to produce a maximal effect (Fig.2A) was higher than that reported in dissociated embryonic MNs (Henderson et al., 1994). The nature of the slice preparation is probably a significant factor. The need for such high concentrations of GDNF has been reported in a spinal cord slice culture preparation to protect P8 MNs from excitoxic injury (Bilak et al., 1999). Similarly, high concentrations of other trophic factors are required to elicit changes in neuronal migration in slices of embryonic cortex (Brunstrom et al., 1997). Thus, although the concentration of GDNF used in the slice preparation is higher than in dissociated cultures, this is probably not an accurate measure of the concentration at its point of action.
The loss of MNs in the first 24 hr was indistinguishable between cultures grown with or without GDNF (Fig. 2B). This death also occurred in slices taken frombax−/− animals, irrespective of the presence or absence of GDNF (Fig. 3B). The number of MNs surviving in the presence of GDNF rapidly stabilized at ∼60%, and this number could not be augmented by other trophic factors (Figs.5B, 7B). Furthermore, a combination of BDNF and CNTF matched but did not exceed the trophic effect of GDNF (Fig.5A). The inability to exceed 60–70% MN survival regardless of molecular genetic, trophic, or pharmacological interventions suggested the presence of a ceiling effect, with the remaining neurons inevitably dying as a result of axotomy and trauma during the slice-harvesting procedure. There remains a formal possibility, however, that untested [e.g., hepatocyte growth factor (HGF)] or unidentified trophic factors could prevent a proportion of the death during the first 24 hr of culture. Continued trophic factor deprivation beyond 24 hr led to the death of all remaining MNs over 3–4 d. This second phase of death required bax expression and involved macromolecular synthesis and caspases, features characteristic of death in neurons deprived of trophic support (Martin et al., 1988; Deshmukh et al., 1996). Thus, we conclude that much of the MN death after 24 hr in our slices is apoptotic and can be completely suppressed by a single polypeptide trophic factor, GDNF.
GDNF is the exclusive physiological ligand for GFRα-1
The vast majority of MNs express both Ret and GFRα-1 (Glazner et al., 1998; Golden et al., 1998; Yu et al., 1998). The receptor for NRTN, GFRα-2, is found in a proportion of MNs in both the medial and lateral motor columns (Garces et al., 2000). Both GFLs support thein vitro survival of embryonic MNs (Henderson et al., 1994;Milbrandt et al., 1998; Soler et al., 1999; Garces et al., 2000).In vitro and in vivo experiments have shown that GFRα-1 and GFRα-2 have a high degree of binding specificity to GDNF and NRTN, respectively (Buj-Bello et al., 1997; Klein et al., 1997). In cell lines, however, GDNF and NRTN can both induce Ret phosphorylation through nonpreferred GFRα coreceptors (Baloh et al., 1997; Creedon et al., 1997; Jing et al., 1997). Indeed, in GFRα-2-deficient neuronsin vitro, NRTN is able to support the survival of both trigeminal sensory neurons and dissociated embryonic MNs (Rossi et al., 1999; Garces et al., 2000). The abolition of the survival response to GDNF inGFRα-1−/− MNs in the present study supports the model that GFRα-1 is absolutely necessary for GDNF signaling (Fig. 7C). In contrast to studies in dissociated embryonic MNs, NRTN did not significantly promote MN survival (Fig. 7A,B). In our slices, furthermore, the addition of NRTN, ARTN, and PSPN did not significantly affect the survival response to GDNF. Thus not only were none of these factors in isolation survival-promoting for MNs, but they also showed no evidence of competition with GDNF for receptor binding, supporting a model of a physiologically exclusive interaction between GDNF and GFRα-1 (Buj-Bello et al., 1997; Klein et al., 1997; Leitner et al., 1999).
GDNF is a potent trophic factor for both medial and lateral column motor neurons
One unresolved question in developmental neurobiology is why multiple factors promote MN survival but none of these is sufficient to maintain all neurons long-term (Oppenheim, 1996). One theory is that collaborative peptide signaling is required in the CNS, reflecting its greater complexity, compared with many PNS populations, in which a single trophic factor is sufficient (Snider, 1994). An alternative explanation is that several MN subpopulations are dependent on single trophic factors (de Lapeyriere and Henderson, 1997). More recently, it has been proposed that CNS neurons in culture lose trophic factor responsiveness secondary to a loss of connectivity (Goldberg and Barres, 2000), which can be mitigated in vitro either by depolarization or by raising intracellular levels of cAMP (Shen et al., 1999). The most striking findings of the present study (Figs. 5, 7) are the following: (1) relatively few trophic factors are able to promote robust MN survival; (2) the survival response to GDNF is much greater than to any other trophic factor; (3) the response to GDNF given in isolation is sustained; and (4) elevating intracellular levels of cAMP does not significantly increase trophic factor responsiveness. Two major differences between the slice cultures reported here and otherin vitro studies of trophic factor-dependent MNs are that an organotypic environment is preserved, and the neurons are harvested at a later age than has been studied to date. Thus, an intriguing possibility is that the growth factor responsiveness of MNs is context-dependent. For instance, interneuronal connections may be sufficiently preserved to render the further elevation of intracellular cAMP levels ineffective (Fig. 5C,D). In addition, cell–cell interactions, which would likely involve glia as well as neurons, and molecules in the extracellular matrix may not only provide direct trophic support but could also regulate responsiveness to trophic factors (Oppenheim, 1996). Alternatively, it is possible that MNs become progressively more dependent on GDNF after emerging from the period of naturally occurring neuronal death and before they become target-independent for survival.
The hypothesis that MN subpopulations require different trophic factors for survival has received support from three observations. First, HGF selectively promotes the survival of dissociated MNs from the lumbar spinal cord (Yamamoto et al., 1997; Novak et al., 2000). Second, small but distinct pools of LMC motor neurons have been observed to be missing before birth inGFRα-1−/− mice (Garces et al., 2000). Finally, in contrast to the situation in other populations, the number of MNs in the third and fourth cranial nerve nuclei is not affected by GDNF underexpression or overexpression (Oppenheim et al., 2000). Although the sensitivity of the slice culture assay may be insufficient to detect small changes in neuronal numbers, the similarity of the trophic factor response profiles of MMC and LMC motor neurons is of interest (Fig. 8). Although MN subpopulations can be distinguished both anatomically (Landmesser, 1980) and by combinatorial transcription factor expression (Tsuchida et al., 1994), these subdivisions may not be matched by correspondingly distinct trophic requirements. Our findings emphasize the preeminence of GDNF over other MN trophic factors and raise the possibility that GDNF is more important in maintaining the trophic status of postnatal MNs than would be predicted from findings in embryonic cultures.
Footnotes
This work was supported by grants from the Patrick Berthoud Trust, UK and National Institutes of Health Grants NS01856, NS40304 (J.E.B.), AG13729 (E.M.J.), NS39358, and AG13730 (J.M.). W.P.R. was supported by a fellowship from the Patrick Berthoud Trust. We thank Dr. William Snider for advice and input at the inception of this work and Dr. Hideki Enomoto, Girish Putcha, Emily Storch, and Charlie Harris for technical assistance. We also thank Dr. Alan Pearlman, Dr. Brian Tsui-Pierchala, Dr. Judy Golden, and Leo Wang for many thoughtful scientific discussions and critical reading of this manuscript.
Correspondence should be addressed to Dr. Eugene M. Johnson Jr, Department of Molecular Biology and Pharmacology, Washington University School of Medicine, 4566 Scott Avenue, Box 8103, St. Louis, MO 63110. E-mail: ejohnson@pcg.wustl.edu.
REFERENCES
- 1.Annis CM, Edmond J, Robertson RT. A chemically-defined medium for organotypic slice cultures. J Neurosci Methods. 1990;32:63–70. doi: 10.1016/0165-0270(90)90072-n. [DOI] [PubMed] [Google Scholar]
- 2.Baloh RH, Tansey MG, Golden JP, Creedon DJ, Heuckeroth RO, Keck CL, Zimonjic DB, Popescu NC, Johnson EM, Jr, Milbrandt J. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18:793–802. doi: 10.1016/s0896-6273(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 3.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 4.Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors—implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 5.Bilak MM, Shifrin DA, Corse AM, Bilak SR, Kuncl RW. Neuroprotective utility and neurotrophic action of neurturin in postnatal motor neurons: comparison with GDNF and persephin. Mol Cell Neurosci. 1999;13:326–336. doi: 10.1006/mcne.1999.0756. [DOI] [PubMed] [Google Scholar]
- 6.Brunstrom JE, Gray-Swain MR, Osborne PA, Pearlman AL. Neuronal heterotopias in the developing cerebral cortex produced by neurotrophin-4. Neuron. 1997;18:505–517. doi: 10.1016/s0896-6273(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 7.Buj-Bello A, Adu J, Pinon LG, Horton A, Thompson J, Rosenthal A, Chinchetru M, Buchman VL, Davies AM. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997;387:721–724. doi: 10.1038/42729. [DOI] [PubMed] [Google Scholar]
- 8.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J Neurosci Methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 9.Clarke PGH, Oppenheim RW. Neuron death in vertebrate development: in vivo methods. Methods Cell Biol. 1995;46:277–321. [PubMed] [Google Scholar]
- 10.Corse AM, Rothstein JD. Organotypic spinal cord cultures and a model of chronic glutamate-mediated motor neuron degeneration. In: Ohnishi ST, Ohnishi T, editors. Central nervous system trauma: research techniques. CRC; Boca Raton, FL: 1995. pp. 341–351. [Google Scholar]
- 11.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Chiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 13.Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 14.de Lapeyriere O, Henderson CE. Motoneuron differentiation, survival and synaptogenesis. Curr Opin Genet Dev. 1997;7:642–650. doi: 10.1016/s0959-437x(97)80012-3. [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson EM., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol. 1996;135:1341–1354. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott JL, Snider WD. Axotomy-induced motor neuron death. In: Koliatsos VE, Ratan RR, editors. Cell death and diseases of the nervous system. Humana; Totowa, NJ: 1999. pp. 181–196. [Google Scholar]
- 17.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 18.Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, de Lapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20:4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glazner G, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 21.Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Hanson MG, Jr, Shen S, Wiemelt AP, McMorris FA, Barres BA. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–7371. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 25.Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, Delaney J, Schultz H, Zhou R, Fox GM. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J Biol Chem. 1997;272:33111–33117. doi: 10.1074/jbc.272.52.33111. [DOI] [PubMed] [Google Scholar]
- 26.Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, Vandlen R, Simmons L, Gu Q, Hongo JA, Devaux B, Poulsen K, Armanini M, Nozaki C, Asai N, Goddard A, Phillips H, Henderson CE, Takahashi M, Rosenthal A. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997;387:717–721. doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- 27.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 28.Landmesser LT. The generation of neuromuscular specificity. Annu Rev Neurosci. 1980;3:279–302. doi: 10.1146/annurev.ne.03.030180.001431. [DOI] [PubMed] [Google Scholar]
- 29.Leitner ML, Molliver DC, Osborne PA, Vejsada R, Golden JP, Lampe PA, Kato AC, Milbrandt J, Johnson EM., Jr Analysis of the retrograde transport of glial cell line-derived neurotrophic factor (GDNF), neurturin, and persephin suggests that in vivo signaling for the GDNF family is GFRα coreceptor-specific. J Neurosci. 1999;19:9322–9331. doi: 10.1523/JNEUROSCI.19-21-09322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowrie MB, Vrbova G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992;15:80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- 31.Martin DP, Schmidt RE, DiStefano PS, Lowry OH, Carter JG, Johnson EM., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988;106:829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 33.Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang LC, Vandlen R, Moffat B. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998;20:245–253. doi: 10.1016/s0896-6273(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 34.Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson EM., Jr Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 36.Novak KD, Prevette D, Wang S, Gould TW, Oppenheim RW. Hepatocyte growth factor/scatter factor is a neurotrophic survival factor for lumbar but not for other somatic motoneurons in the chick embryo. J Neurosci. 2000;20:326–337. doi: 10.1523/JNEUROSCI.20-01-00326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 39.Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, Arumae U, Pasternack M, Saarma M, Airaksinen MS. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron. 1999;22:243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 41.Rydel RE, Greene LA. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci USA. 1988;85:1257–1261. doi: 10.1073/pnas.85.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen S, Wiemelt AP, McMorris FA, Barres BA. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23:285–295. doi: 10.1016/s0896-6273(00)80780-1. [DOI] [PubMed] [Google Scholar]
- 43.Sheppard AM, Brunstrom JE, Thornton TN, Gerfen RW, Broekelmann TJ, McDonald JA, Pearlman AL. Neuronal production of fibronectin in the cerebral cortex during migration and layer formation is unique to specific cortical domains. Dev Biol. 1995;172:504–518. doi: 10.1006/dbio.1995.8034. [DOI] [PubMed] [Google Scholar]
- 44.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 45.Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos Trans R Soc Lond B Biol Sci. 1996;351:395–403. doi: 10.1098/rstb.1996.0034. [DOI] [PubMed] [Google Scholar]
- 46.Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 48.Vejsada R, Tseng JL, Lindsay RM, Acheson A, Aebischer P, Kato AC. Synergistic but transient rescue effects of BDNF and GDNF on axotomized neonatal motoneurons. Neuroscience. 1998;84:129–139. doi: 10.1016/s0306-4522(97)00497-1. [DOI] [PubMed] [Google Scholar]
- 49.White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto Y, Livet J, Pollock RA, Garces A, Arce V, de Lapeyriere O, Henderson CE. Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development. 1997;124:2903–2913. doi: 10.1242/dev.124.15.2903. [DOI] [PubMed] [Google Scholar]
- 51.Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 52.Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R. Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci. 1998;18:4684–4696. doi: 10.1523/JNEUROSCI.18-12-04684.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Q, Wu W, So KF, Cheung AL, Prevette DM, Oppenheim RW. Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. NeuroReport. 2000;11:2237–2241. doi: 10.1097/00001756-200007140-00035. [DOI] [PubMed] [Google Scholar]



