Abstract
Mild hypothermia is neuroprotective, but the reasons are not well known. Inflammation contributes to ischemic damage; therefore, we examined whether the protection by hypothermia may be attributable to alterations in the inflammation. We examined whether hypothermia might alter the inflammatory cell-associated inducible nitric oxide synthase (iNOS) and subsequent nitric oxide (NO) and peroxynitrite generation in experimental stroke and inflammation. Rats underwent 2 hr of middle cerebral artery occlusion (MCAO). Brain inflammation was modeled by intravenous lipopolysaccharide (LPS) (2 mg/kg) injection. Temperature was maintained at 33°C for 2 hr immediately after MCAO and LPS injection, delayed 2 hr after MCAO or maintained at 38°C. Cultured microglia were activated with LPS and then incubated at 33 or 37°C. Both intraischemic and delayed mild hypothermia attenuated infarct size by 40% (p < 0.05). Immunohistochemistry was performed to identify cell type, iNOS, and peroxynitrite. The majority of iNOS- and peroxynitrite-positive cells were activated microglia–macrophages, and mild hypothermia significantly decreased the numbers of immunoreactive cells at 72 hr by >50% (p < 0.05). After ischemia, mild hypothermia decreased NO production by 40%. Similarly, hypothermia attenuated NO and iNOS in LPS-injected rats, as well as in cultured microglia. Aminoguanidine, an iNOS inhibitor, also attenuated infarct size and NO in ischemic and inflammation models. We conclude that mild hypothermia significantly inhibits the inflammatory response by affecting microglial iNOS–NO generation. Therapies directed against microglia or their activation may be useful in treating stroke.
Keywords: focal cerebral ischemia, mild hypothermia, inducible nitric oxide synthase, microglia, peroxynitrite, lipopolysaccharide, inflammation
There has been renewed interest in mild hypothermia as a method to protect brain in cerebral ischemia (Ginsberg et al., 1992; Karibe et al., 1994a,b; Maier et al., 1998,2001). Its neuroprotective effects have often been attributed to a decrease in cerebral blood flow and metabolic requirement for oxygen (Karibe et al., 1994b) and alteration in neurotransmitter release (Ginsberg et al., 1992; Huang et al., 1998). More recently, there have been reports that hypothermia may attenuate the inflammatory response to cerebral ischemia, especially when cooling is delayed. Several studies have provided evidence that postischemic hypothermia protects the brain from cerebral ischemia (Karibe et al., 1994a,b; Maier et al., 1998, 2001; Kawai et al., 2000) when energy stores have already been depleted and glutamate has been released. The observation that delayed cooling still resulted in cerebral protection suggests that mild hypothermia affects some of the injury mechanisms that occur later in the ischemic cascade.
Inflammation plays a central role in the pathogenesis of cerebral ischemia and secondary damage (Barone and Feuerstein, 1999). Inflammation is thought to contribute to the genesis of secondary damage and develops as a consequence of activation of microglia and resident perivascular and parenchymal macrophages and infiltration of peripheral inflammatory cells (Garcia et al., 1994; Toyoda et al., 1996; del Zoppo et al., 2000). Inflammatory cells generate potentially damaging nitric oxide (NO), oxygen free radicals, and cytokines. Cytokines activate microglia and stimulate expression of adhesion molecules leading to leukocyte infiltration. Activated microglia also potentiate the inflammatory response and generate reactive oxygen species and NO. Inflammatory cells also express more cytokines, leading to more glial cell activation and damage. In the context of brain ischemia, the activity of neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) is broadly deleterious (Eliasson et al., 1999; del Zoppo et al., 2000), and their inhibition is neuroprotective (Iadecola et al., 1995b; Cockroft et al., 1996;Zhang and Iadecola, 1998). NO and superoxide are themselves highly reactive but can also combine to form peroxynitrite, a particularly damaging reactive species. The toxicity of the free radicals and peroxynitrite results from their modification of macromolecules, especially DNA (Love, 1999). We and others have shown that mild hypothermia decreases tissue neutrophils (Toyoda et al., 1996; Maier et al., 1998). Mild hypothermia also attenuates downstream effects of inflammation by consumption of endogenous antioxidants (Kil et al., 1996), blood–brain barrier disruption, and cerebral edema (Karibe et al., 1994b).
Although it is well established that inflammation contributes to cerebral ischemic injury and that mild hypothermia is an effective neuroprotectant, whether and how inflammatory processes are altered to achieve hypothermic protection have not been extensively studied. In this study, we planned to determine whether mild hypothermia affects expression of iNOS and production of NO–peroxynitrite mediated by activated microglia–macrophages in an experimental model of stroke. Given that any observed differences could be related to the neuroprotective effects of mild hypothermia, we also evaluated the effect of hypothermia in a model of brain inflammation that does not result in cell death.
MATERIALS AND METHODS
Experiments were performed according to the guidelines for the animal care and use of laboratory animal protocols approved by the Stanford University Administrative Panel on Laboratory Animal Care. Animals were housed with food and water available ad libitumunder diurnal lighting conditions and a temperature-controlled environment until the day of experiment.
Focal cerebral ischemia model of rat. Male Sprague Dawley rats weighing between 290 and 320 gm were anesthetized with halothane and maintained during surgical procedures. A femoral artery was cannulated for the continuous monitoring of arterial blood pressure and blood sampling. Physiological parameters were monitored and maintained in the normal range. Blood gases were measured with an automatic pH/blood gas analyzer (model 178; Ciba Corning Diagnostics Corp., Medfield, MA). Ischemia was induced using an occluding intraluminal suture (Maier et al., 1998; Yenari et al., 2000). An uncoated 30-mm-long segment of 3–0 nylon monofilament suture with the tip rounded by flame was inserted into the stump of the common carotid artery and advanced into the internal carotid artery ∼19–20 mm from the bifurcation to occlude the ostium of middle cerebral artery (MCAO). At the end of the ischemic period, the suture was removed, and the animal was allowed to recover. Sham-operated animals were treated in the same manner as the ischemic animals, but no ischemia was applied. During surgery, rectal temperature was maintained between 37 and 38°C, corresponding to brain temperature of 38–39°C (Yenari et al., 2000). Mild hypothermia (33°C of rectal temperature, corresponding to brain temperature of 33°C) was achieved as described previously (Maier et al., 1998, 2001; Yenari et al., 2000). Cooling began on ischemia onset (intraischemic hypothermia) or was delayed by 2 hr (delayed hypothermia), cooling begins immediately after the suture is removed). Cooling was maintained for 2 hr, and these conditions were associated with neuroprotection in our hands. At the completion of the experiment, the animals were killed with a halothane overdose and prepared for additional analysis (described subsequently).
In vivo brain inflammation model. Animals were anesthetized, and 2 mg/kg lipopolysaccharide (LPS) (Escherichia coliserotype 055:B5; Sigma, St. Louis, MO) was administered into the jugular vein. Control animals were given sterile normal saline. LPS-treated animals were kept at 37–38°C of rectal temperature (normothermia) or cooled to 32°C (hypothermia) immediately after injection. Hypothermia was maintained for 2 hr.
Microglial cultures and activation. Murine microglial cultures were prepared from astrocyte cultures as described previously (Yenari and Giffard, 2001). Whole brains from postnatal days 1–3 Swiss Webster mice were plated in 75 cm2 coated flasks at a density of two brains per flask in Eagle's Minimal Essential Minimum supplemented with 10% equine serum, 10% fetal bovine serum, epidermal growth factor (1 mg/100 ml), glutamine (2 mm), glucose (21 mm), and bicarbonate (26 mm) (plating media), plus penicillin (100 U/ml) and streptomycin (100 mg/ml). The cultures were maintained in a 37°C humidified incubator with a 5% CO2 atmosphere. Media was changed every 2–3 d for the first 10 d. Microglia were subcultured using methods described previously (Giulian and Baker, 1986; Sasaki et al., 1989;Smith, 1993) and modified slightly. After 10–14 d in vitro, flasks were inspected for microglia growing on top of a confluent cell layer. Microglia containing flasks were shaken at 160 rpm for 30 min at 37°C. The supernatant was collected and spun for 5 min at 800 ×g. The resulting pellet was resuspended in plating media and plated at a density of 2–3 × 105cells/ml in uncoated 24-well plates. The plates were returned to the incubator for 1 hr to allow the microglia to attach and then washed and returned to the incubator with fresh media and antibiotics. Cultures were used for experiments 24 hr after plating. Histochemical staining with Griffonia simplicifolia B4-isolectin (IB4; Sigma) confirmed that the majority of these cells were indeed microglia. Experiments were repeated four times using cells isolated from two to three different dissections. Microglia were activated by exposure to LPS (10 μg/ml) and phorbol 12-myristate 13-acetate (PMA) (1 nmin DMSO; a protein kinase C activator; Sigma). Cultures were washed three times in LPS- or PMA- containing media and then returned to the incubator. Control cultures were washed in only plating media. This dose of LPS or PMA was chosen because it was the lowest concentration in pilot experiments that consistently transformed resting microglia into the activated, amoeboid form. To perform hypothermia, cells were kept in an incubator, with the temperature set at 33°C during LPS or PMA treatment.
iNOS inhibition. Aminoguanidine, a relatively selective iNOS inhibitor, was administered intraperitoneally to rats after MCAO and LPS treatment. Ischemic rats received aminoguanidine hemisulfate (100 mg/kg in 1 ml of saline; A7009; Sigma) (n = 6) immediately after and 8, 24, 32, 48, and 52 hr after MCAO. The dose of aminoguanidine used was found to selectively inhibit iNOS activity (Zhang and Iadecola, 1998). The other set of rats received aminoguanidine hemisulfate (n = 6) immediately after LPS injection and 8, 24, 32, 48, and 52 hr later. For primary microglial cultures, aminoguanidine was dissolved in culture media (200 μm) and applied to LPS-stimulated cells.
Infarct analysis. Animals were perfused with heparinized saline and were fixed in 3% paraformaldehyde plus 20% sucrose. After fixation, the brains were cut into 3-mm-thick coronal slices: level 1 was from 4 mm anterior to bregma to 1 mm anterior to the bregma; level 2 was to 2 mm posterior to bregma; level 3 was to 5 mm posterior to bregma; and level 4 was to 8 mm posterior to bregma. Cryosections (30 μm) were prepared from each slice, placed on Superfrost Plus slides (Fischer Scientific, Pittsburgh, PA), air dried for 2 hr, frozen, and stored at −80°C until use. To assess ischemic injury, brain sections from four different slices of the brain were stained with cresyl violet. Areas of infarction were measured using an image analysis system described previously (Maier et al., 1998; Yenari et al., 1998). Briefly, infarct was evaluated by light microscopy in a blinded manner. Infarct areas as delineated by areas of nonstaining were measured with an image analysis system (MCID; Imaging Research Inc., Ontario, Canada) and were expressed as a percentage of the total area of ipsilateral hemisphere. Infarct areas from four coronal slices at different levels were summed and expressed as a percentage of the total area of ischemic hemispheres.
Immunohistochemistry. Cryosections were treated for endogenous peroxidases with 0.03% H2O2, blocked in 5% normal serum, and then incubated with primary antibody, followed by the secondary antibody (Vector Laboratories, Burlingame, CA). Antibodies were detected using the Elite Vectastain ABC kit (Vector Laboratories) and colorized with diaminobenzidine (Vector Laboratories). For double labeling, the primary antibodies were detected with Cy3- or FITC-conjugated secondary antibody (1:200; Jackson ImmunoResearch, West Grove, PA). The following primary antibodies were used: ED1 (1:200; T3003X; Research Diagnostics, Flanders, NJ) to detect activated microglia; anti-nitrotyrosine antibody (1:50; 06284; Upstate Biotechnology, Lake Placid, NY) to detect peroxynitrite; anti-iNOS antibody (1:500; 482728; Calbiochem, San Diego, CA); anti-microtubule-associated protein-2 (MAP-2) antibody (1:300; M4403; Sigma) to detect neurons; and anti-GFAP antibody (1:100; 556330; PharMingen, San Diego, CA) to detect astrocytes. Counterstain was performed using the nuclear marker 4′,6′-diamidino-2-phenylindole (DAPI). The number of immunopositive cells was counted under microscope and normalized to infarct size.
SDS-PAGE and immunoblotting. Animals were perfused with saline, and then brains were removed and sectioned into four coronal slices in the same manner described in histology. Slices containing maximal ischemic damage were chosen. Each slice was dissected into four parts: ischemic cortex, ischemic subcortex, contralateral cortex, and contralateral subcortex. Brain tissue was homogenized in Laemmli's lysis buffer plus protease inhibitors. Protein concentrations of each sample solution were determined using the BCA protein assay kit (Pierce, Rockford, IL), and the samples were stored in at −80°C until use. Aliquots containing 50 μg of protein were subjected to 7.5% SDS-PAGE. Protein bands were transferred to polyvinylidinene fluoride membrane (IPVH00010; Millipore, Bedford, MA) and probed for the iNOS by incubating in the primary antibody (1:2500; N32020; Transduction Laboratories, Lexington, KY), followed by a horseradish peroxidase-conjugated secondary antibody (sc-2005; Santa Cruz Biotechnology, Santa Cruz, CA). To determine the specificity of the iNOS primary antibody, we used iNOS antibody preabsorbed with anti-iNOS blocking peptide (482729; Calbiochem) instead of primary antibody. Blots were visualized using the ECL system (Amersham Biosciences, Piscataway, NJ) according to the directions of the manufacturer and exposed to x-ray film. Equal protein loading was confirmed by measuring β-actin. Membranes were stripped and probed for β-actin (1:5000; anti-β-actin; A5441; Sigma). Densitometric measurements were made from the film using a GS-700 imaging densitometer (Bio-Rad, Hercules, CA) and then quantified using Multi-Analyst (Bio-Rad). For quantification of relative protein expression, the optical density of the protein band of interest was normalized to the optical density of sham animal brain sample run in an adjacent lane on the same gel. Western blots were repeated two to three times using samples prepared from three different animals or cultures for each experimental condition studied.
NO generation measurement. NO production was evaluated measuring the nitrite, the stable metabolite of NO, content of the tissue homogenates or culture media with the Griess reaction (Salter et al., 1996). Ischemic cortex from the ipsilateral hemispheres or cortex from LPS-treated brains was dissected. Culture media were collected from the microglial cultures. Fresh brain tissue was homogenized in PBS with protease inhibitors at 4°C and then centrifuged at 12,000 × g for 1 hr at 4°C. After centrifugation, supernatant was collected and kept at −80°C until use. Duplicates of 100 μl of supernatant or culture media were added to 96-well microtiter plates and mixed with 100 μl of modified Griess reagent (G4410; Sigma). The plate was then read on a microtiter plate reader using a 540 nm filter. A standard curve with increasing concentrations of sodium nitrite was done in parallel and used for quantitation.
Statistical analysis. Data are given as means ± SEM. Comparisons between groups were performed using standard statistical methods using SigmaStat (SPSS, (Chicago, IL). The data were analyzed by one-way ANOVA, Kruskal–Wallis one-way ANOVA on ranks, or unpaired t test. Statistical significance was determined at the p < 0.05 level.
RESULTS
The physiologic variables of the rats studied are presented in Table 1. Differences in all parameters were not statistically significant between groups, except for temperature.
Table 1.
Physiological parameters
| N (n = 6) | HI (n = 6) | HD (n = 6) | |
|---|---|---|---|
| Arterial pH | 7.38 ± 0.02 | 7.34 ± 0.02 | 7.37 ± 0.01 |
| Arterial PCO2(mmHg) | 37.4 ± 3 | 38.5 ± 6 | 43.3 ± 6 |
| Arterial PO2 (mmHg) | 161.7 ± 8 | 159.4 ± 11 | 171.6 ± 10 |
| Hematocrit (%) | 41 ± 3 | 42 ± 4 | 39 ± 3 |
| Blood glucose (mg/dl) | 136 ± 7 | 123 ± 9 | 145 ± 9 |
| MAP (mmHg) | 128 ± 6 | 133 ± 9 | 136 ± 5 |
| Heart rate (beat/min) | 219 ± 17 | 178 ± 11 | 199 ± 14 |
| Temperature (intraischemic) | 37.0 ± 0.1 | 32.9 ± 0.1* | 37.0 ± 0.1 |
| Temperature (postischemic) | 37.0 ± 0.1 | 37.0 ± 0.1 | 33.0 ± 0.1* |
Measurements were performed at 1 hr of MCAO and 20 min after reperfusion. Data were analyzed using ANOVA, followed by Tukey's test. Data are mean ± SEM. N, Normothermia; HI, intraischemic hypothermia; HD, delayed hypothermia; PCO2, blood CO2 pressure; PO2, blood O2pressure; MAP, mean arterial pressure.
p < 0.05 versus normothermic ischemia.
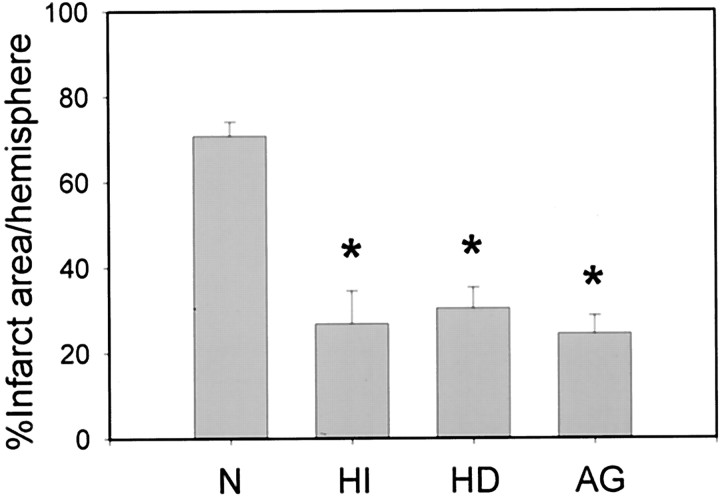
As we found previously (Maier et al., 1998, 2001; Yenari et al., 2000), mild hypothermia, maintained for 2 hr during the ischemic insult, was significantly neuroprotective. Cooling was still effective when onset was delayed up to 2 hr after ischemia onset. Infarct sizes 72 hr after ischemia onset were 70.8 ± 3.3% of ischemic hemisphere after normothermic ischemia, 26.8 ± 7.7% after intraischemic hypothermia, and 30.4 ± 4.8% after postischemic hypothermia (Fig. 1) (p < 0.05). To evaluate the damaging role of iNOS in our experimental model, we treated a separate group of ischemic animals with the relatively selective iNOS inhibitor aminoguanidine. Infarct size was similarly decreased by aminoguanidine treatment. (Fig. 1)
Fig. 1.
Mild hypothermia protects against experimental stroke. The extent of protection is similar to pharmacologic iNOS inhibition by aminoguanidine. Infarct size was measured on cresyl violet-stained coronal sections 72 hr after MCAO. The experimental groups are as follows: normothermia (N), 70.8 ± 3.3% of ipsilateral hemisphere, n = 6; intraischemic hypothermia (HI), 26.8 ± 7.7%, n = 6; delayed hypothermia (HD), 30.4 ± 4.8%, n = 6; aminoguanidine-treated (AG), 24.4 ± 4.2%,n = 4. *p < 0.05 versus normothermic ischemia. Kruskal–Wallis one-way ANOVA on ranks, followed by a multiple comparisons procedure (Dunn's test).
Cells of monocytic lineage (identified by ED1 immunoreactivity) appeared 24 hr after ischemia and increased in number at 72 hr, especially within the peri-infarct area. No ED1-positive cells were observed in sham-operated animals. Mild hypothermia decreased the number of ED1-positive cells (Fig.2A), whether hypothermia was applied during or 2 hr after ischemia onset. iNOS-positive cells appeared 24 hr after ischemia in the ischemic hemisphere and increased in number by 72 hr. No iNOS-positive cells were observed in sham-operated animals or contralateral hemispheres of ischemic brains. Like ED1-labeled cells, iNOS-positive cells were also observed mainly in the peri-infarct regions. Mild hypothermia decreased the number of iNOS-positive cells (Figs. 2B,3A). The numbers of ED1-positive cells and iNOS-positive cells in the adjacent sections were counted. Hypothermia decreased the number of ED1-positive cells to 44.8 ± 6.0% (intraischemic hypothermia) and 47.8 ± 4.7% (delayed hypothermia) of normothermia. The ratios of iNOS/ED1-positive cells were 0.79 ± 0.06 (normothermic ischemia), 0.83 ± 0.05 (intraischemic hypothermia), and 0.71 ± 0.11 (delayed hypothermia). Although there were no significant differences between the groups, the intensity of the iNOS stain appeared decreased in hypothermic sections compared with normothermic sections (Fig.2B). We could not observe any difference in the intensity in aminoguanidine-treated sections.
Fig. 2.

Mild hypothermia attenuates microglia–monocytes, iNOS, and peroxynitrite after MCAO. Immunohistochemical stains of ED1 (to identify activated microglia–monocytes), iNOS, and nitrotyrosine (to identify peroxynitrite) 72 hr after MCAO. Within the infarct, ED1 immunoreactivity is seen mainly in the cortex (A). The majority iNOS-positive cells reside in peri-infarct area of the ischemic hemisphere (B). The presence of nitrotyrosine immunoreactivity, a nitration product of tyrosine in proteins by peroxynitrite, suggests active NO production in the ischemic area (C). The densities of ED1-, iNOS-, and nitrotyrosine-positive cells are decreased by mild hypothermia (A–C, Hypothermia, respectively). Arrowheads show immunoreactive cells. Images are taken from the normothermic and delayed hypothermic brain sections. Scale bar, 50 μm.
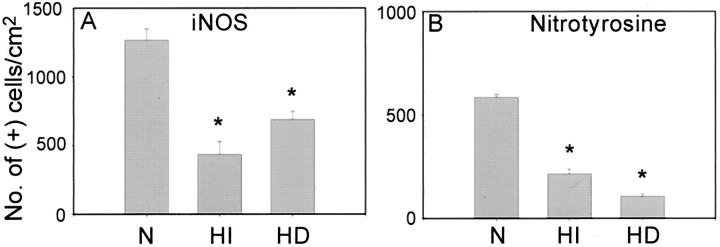
Fig. 3.
Mild hypothermia decreases iNOS and nitrotyrosine immunoreactivity in ischemic brain. Mild hypothermia reduced the number of iNOS-positive cells within ischemic brain 72 hr after ischemia.A, The experimental groups are as follows: normothermic ischemia (N), 1265 ± 83 cells/cm2, n = 6; intraischemic hypothermia (HI), 435 ± 93 cells/cm2, n = 6; delayed hypothermia (HD), 688 ± 59 cells/cm2, n = 6. Mild hypothermia reduced densities of peroxynitrite-positive cells 72 hr after ischemia. B, The experimental groups are as follows: normothermic ischemia (N), 585 ± 14 cells/cm2, n = 6; intraischemic hypothermia (HI), 214 ± 23 cells/cm2, n = 6; delayed hypothermia (HD), 106 ± 12 cells/cm2, n = 6. *p < 0.05 versus normothermia. ANOVA, followed by Tukey's test.
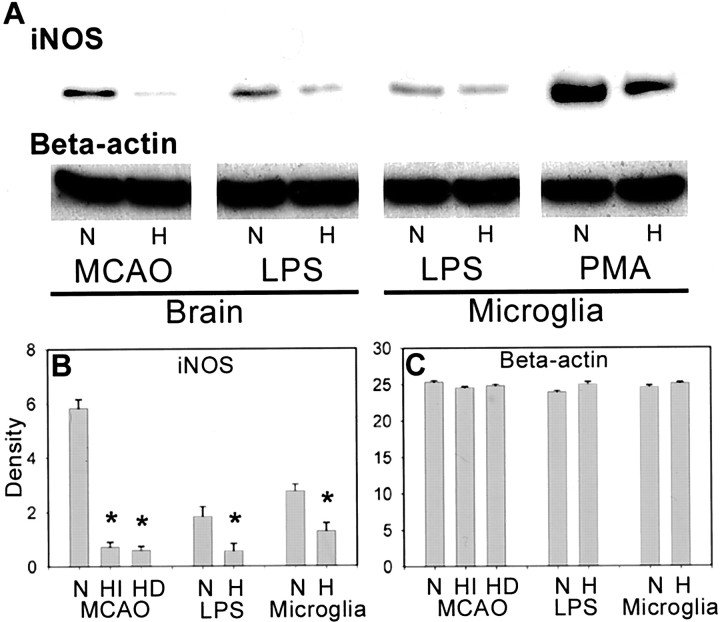
Quantitative measurement of iNOS protein in whole brain lysates was performed using Western blot analysis. We detected iNOS protein bands in samples prepared from peri-infarct areas of the ischemic brains at 72 hr after MCAO. iNOS was not detectable in sham-operated animal brains. Both intraischemic and delayed hypothermia decreased iNOS levels in ischemic brain compared with normothermic ischemic brain (Fig. 4, MCAO).
Fig. 4.
Mild hypothermia reduces iNOS in the brain and within microglia. Protein samples taken from ischemic brains 72 hr after MCAO or LPS treatment and cultured microglia 72 hr after LPS or PMA treatment were subjected to SDS-PAGE and probed for iNOS. iNOS was detected in ischemic brain (MCAO), LPS-treated brain (LPS), cultured microglia treated with LPS (Microglia), or PMA (PMA) under normothermic conditions (N) but was not detectable in sham animals or inactive cultured microglia. Intraischemic (HI) and delayed hypothermia (HD) in MCAO brains, hypothermia (H) in LPS brains, and cultured microglia show decreased iNOS expression. Representative Western blots of iNOS and β-actin (A). Optical densities of iNOS and β-actin bands in B and C, respectively. *p < 0.05 versus normothermia. ANOVA, followed by Tukey's test or unpaired t test.
In the ischemic hemisphere, nitrotyrosine-positive cells were detected 72 hr after insult. Rare cells stained with anti-nitrotyrosine antibody were detected in sham-operated animals. Within ischemic brain, nitrotyrosine was seen within cells, as well as extracellularly, consistent with previous reports (Gursoy-Ozdemir et al., 2000). Intraischemic and delayed mild hypothermia decreased densities of nitrotyrosine-positive cells compared with normothermia (Figs.2C, 3B).
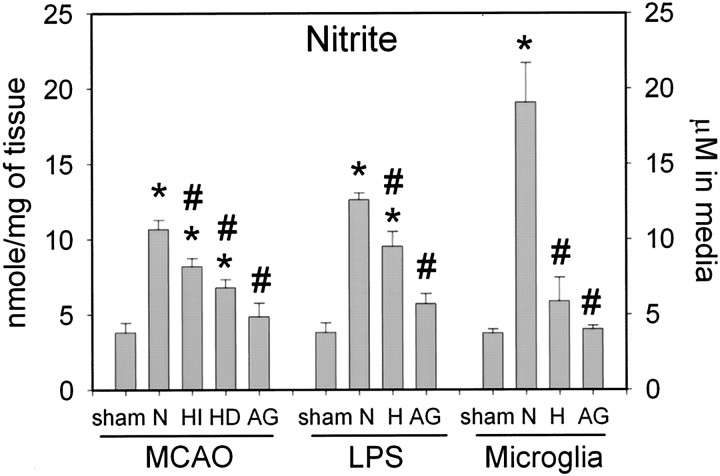
To determine whether hypothermia attenuates NO production in ischemia, we measured nitrite content in the ischemic brain using the Griess reaction. Like iNOS, NO production was significantly increased at 72 hr after MCAO. Compared with normothermia, both intraischemic and delayed hypothermia attenuated NO production at 72 hr (see Fig. 6,MCAO). NO production was decreased by aminoguanidine treatment to a similar extent as hypothermia (see Fig. 6,MCAO).
Fig. 6.
Mild hypothermia decreases NO generation in brain and microglia. Seventy-two hours after exposure, NO production was measured by determining nitrite content in ischemic brain (MCAO) and after systemic LPS administration (LPS) and in cultured microglia exposed to LPS (Microglia). In ischemic brains, intraischemic (HI; n = 4) and delayed (HD; n = 4) hypothermia decreased brain nitrite levels compared with normothermia (N;n = 4). Aminoguanidine (AG;n = 4) also decreased nitrite levels. In a model of pure brain inflammation, LPS-induced NO generation in the brain was decreased by mild hypothermia (H; n= 5) and aminoguanidine treatment (AG;n = 6) compared with the normothermic group (N; n = 5). Cultured microglia exposed to 10 μg/ml LPS generated NO at 37°C (N;n = 6), but NO generation was significantly decreased at 33°C (H; n = 4). Aminoguanidine (AG; n = 4) also decreased NO production in cultured microglia. *p< 0.05 versus sham; #p < 0.05 versus normothermia. ANOVA, followed by Tukey's test.
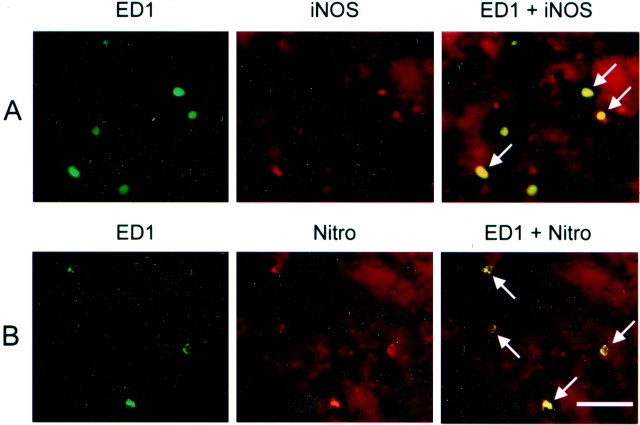
To identify the cells that expressed iNOS and nitrotyrosine, double immunofluorescent labeling was performed. Cell type markers to identify neurons (MAP-2), astrocytes (GFAP), and activated microglia–macrophages (ED1) were used. MAP-2 and GFAP failed to colocalize with iNOS, but the majority of ED1-positive cells were iNOS positive (Fig. 5A). To evaluate the effect of hypothermia on the proportion of ED1-positive, iNOS-expressing cells, the numbers of iNOS/ED1-colabeled cells and ED1-positive cells in the same sections were counted. The proportion of colabeled iNOS/ED1-positive cells among all ED1-positive cells was 0.73 ± 0.04 (normothermic ischemia), 0.74 ± 0.09 (intraischemic hypothermia), 0.63 ± 0.11 (delayed hypothermia), and 0.81 ± 0.05 (aminoguanidine-treated group). There were no significant differences between the groups.
Fig. 5.
Colocalization of iNOS- and nitrotyrosine (Nitro)-producing cells in brains 72 hr after MCAO. Double immunofluorescent staining was performed for a microglial–monocyte marker (ED1, green), iNOS (A, red), and nitrotyrosine (B, red). The majority of ED1-positive cells colocalized with iNOS (A, ED1 + iNOS, arrows). Similarly, the majority of ED1-positive cells also colocalized with nitrotyrosine (B, ED1 + Nitro, arrows). Scale bar, 40 μm.
Nitrotyrosine-labeled cells were also colocalized using the same cell type markers. Similarly, the majority of the ED1-positive cells were nitrotyrosine positive, although extracellular staining was also observed, and is consistent with the diffusible nature of NO or peroxynitrite (Fig. 5B). Mild hypothermia decreased the overall numbers of colabeled cells, but the proportion of colabeled cells was unchanged from normothermia. We used DAPI staining to identify the nuclei of the stained cells. Several cells that were not labeled with DAPI or appeared pyknotic were also nitrotyrosine positive. These cells were also MAP-2 positive. Most of the cells with MAP-2 and nitrotyrosine label appeared dysmorphic with pyknotic DAPI-stained nuclei, suggesting that these cells were damaged.
To further confirm the significance of hypothermia on the inflammatory response, we also studied a separate set of animals using a model of pure brain inflammation to determine whether any of the observed changes were not influenced by the smaller infarcts seen with mild hypothermia. To test the hypothesis that brain temperature independently altered the inflammatory response, we administered LPS to rats. LPS elicits an inflammatory response, characterized by infiltration of leukocytes, activation of microglia, expression of adhesion molecules, and iNOS. Notable in this model was that these histological changes are not associated with neuronal death up to 72 hr after LPS injection. NO production significantly increased from basal to peak levels 72 hr after LPS treatment in both whole brain and cultured microglia. Compared with normothermia, hypothermia decreased iNOS and NO production in LPS-treated brains (Figs. 4,6, LPS).
Because we noted reduced iNOS staining in the cells of monocyte lineage (Fig. 2B) but no alteration in the proportion of iNOS-positive cells, we assessed the contribution of temperature to microglial activation by studying pure culture. In these experiments, hypothermia reduced iNOS generation (Fig. 4, Microglia) and subsequent NO production (Fig. 6, Microglia) after stimulation by LPS. Cultured microglia were also activated using another stimulator, PMA. PMA-stimulated microglia markedly induced iNOS expression. iNOS induction was suppressed by hypothermia (Fig. 4,Microglia).
NO production was completely blocked by aminoguanidine treatment, suggesting that NO production is mainly attributable to LPS-induced iNOS expression (Fig. 6, Microglia).
DISCUSSION
In this study, we show that mild hypothermia inhibits microglia–monocytes activation and infiltration. Furthermore, these inflammatory cells appear to be the primary source of iNOS, NO, and peroxynitrite, which are inhibited by mild hypothermia. Such effects were observed when cooling was applied during or 2 hr after ischemia. Thus, inhibition of iNOS induction by microglia–macrophages presumably contributed to the robust protective effect of mild hypothermia against stroke injury. We further showed in in vivo and in vitro models of LPS-induced inflammation that mild hypothermia inhibited the inflammatory response in a similar manner. Ischemic damage and increased NO production was inhibited by iNOS inhibitor. All of these results suggest that mild hypothermia directly inhibits the inflammatory response and iNOS induction.
After ischemia, endothelial cells upregulate adhesion molecules (Okada et al., 1994; Zhang et al., 1995), allowing entry of peripheral leukocytes, which then release reactive oxygen species (Traystman et al., 1991), cyclooxygenase products, NO, and cytokines (del Zoppo et al., 2000), which contribute to secondary injury. Several studies have shown that inhibition of leukocyte infiltration by blocking various adhesion molecules reduced the infarction (Bowes et al., 1995; Goussev et al., 1998; Yenari et al., 1998). Furthermore, mild hypothermia has been shown to decrease adhesion molecule expression and inflammatory cell infiltration (Maier et al., 1998; Inamasu et al., 2000, 2001;Kawai et al., 2000).
Microglial activation has been observed as early as 6 hr after insult (Lyons et al., 2000), and macrophages–microglia increased in number for several days before reaching a plateau (Garcia et al., 1994;Schroeter et al., 1994; Stoll et al., 1998; Barone and Feuerstein, 1999). Inhibition of microglial activation can protect against stroke (Yrjanheikki et al., 1999). After ischemia, microglial activation results in a series of functional and morphological modifications that involve proliferation (Kato and Wood, 1998). Although microglia play an important role in ischemia, there are few studies that investigate the effect of hypothermia on microglial action, especially in the transient MCAO model. The hypothermic inhibition of microglial activation in global ischemia was shown previously (Kumar and Evans, 1997; Abraham and Lazar, 2000). Inamasu et al. (2000) suggested the possibility that postischemic hypothermia might delay microglial activation.
This study demonstrates the importance of the inflammation in hypothermic neuroprotection. Not only did mild hypothermia decrease densities of microglia–monocytes, but these cells also generated less damaging substances. This was the case even when cooling was delayed by 2 hr, a time when energy stores are already depleted (Hoehn-Berlage et al., 1995) and glutamate is already released (Graham et al., 1990;Huang et al., 1998). Therefore, the protective effect of mild hypothermia may be attributable to other downstream factors, including inflammation. Furthermore, we show that mild hypothermia also decreased microglial–monocyte generation of iNOS, NO, and peroxynitrite after LPS treatment. Therefore, the neuroprotective effect of mild hypothermia may be primarily attributable to suppression of inflammatory cell activation and infiltration. Decreased ischemic damage and NO production by aminoguanidine suggest that NO plays important roles in inflammation after ischemia.
There has been interest in the exact mechanism and regulation of NO and NOS in ischemic damage (Zhang and Iadecola, 1998; De Alba et al., 1999;Forster et al., 1999; Loihl et al., 1999; Fassbender et al., 2000;Hirabayashi et al., 2000). Recently, it is known that ischemia causes a surge in nNOS activity, followed later by increases in iNOS in a range of cells, including infiltrating neutrophils and macrophages, activated microglia, and astrocytes (Love, 1999). The effects of ischemia on the activity of nNOS are thought to be secondary to the activation of NMDA receptors. However, iNOS upregulation and activity is mediated by transcriptional inducers (Love, 1999). In contrast to nNOS, which generates NO early after ischemia onset (Iadecola, 1997; Eliasson et al., 1999), iNOS appears somewhat later in inflammatory cells and contributes to the evolution of the brain injury (Love, 1999). In fact, NO produced by iNOS is a major mechanism of cytotoxicity in models of inflammation (MacMicking et al., 1997). Others have shown that iNOS null mice had smaller infarcts and better neurological outcome than wild-type littermates (Iadecola et al., 1997; Zhao et al., 2000). Treatment with antisense oligodeoxynucleotide to iNOS protected against ischemia-induced brain injury (Parmentier-Batteur et al., 2001). Administration of iNOS inhibitors reduced infarct volume (Iadecola et al., 1995b; Nagayama et al., 1998; Zhang and Iadecola, 1998).
Although the protective effects of mild hypothermia against ischemic brain injury have been studied in the past, there have been very few reports on the interaction between mild hypothermia and induction of NOS in brain injury. Mild hypothermia inhibited total NO synthesis in cerebral ischemia models (Kader et al., 1994; Kumura et al., 1996;Fabian Loidl et al., 1997). However, all of these studies focused on the nNOS activity, and there have been no reports to our knowledge on the effect of mild hypothermia on iNOS expression by microglia in focal cerebral ischemia. One study showed that mild hypothermia attenuated astroglial iNOS activity in global ischemia (Nomura, 1998).Chatzipanteli et al. (1999) reported that hypothermia decreased early constitutive NOS activation and prevented the delayed induction of iNOS in the traumatic brain injury model. One study using microglial cultures suggested that hypothermia inhibited proliferation, superoxide, and nitric oxide production (Si et al., 1997). We show here that mild hypothermia decreases microglial expression of iNOS and NO–peroxynitrite production by cultured microglia. Our results are consistent with those of Si and colleagues in that hypothermia inhibited microglial generation of reactive nitrogen species.
From the brain tissue sections, we found that the majority of microglia–monocytes and iNOS expression occurred in the peri-infarct area. Hypothermic protection was observed mainly in the cortical regions but not in the subcortical regions, as we described previously (Maier et al., 1998, 2001). This suggests that inhibition of microglial iNOS expression may be an important mechanism of hypothermic protection. To our knowledge, this is the first report to directly show that mild hypothermia inhibits expression of iNOS and reactive nitrogen species by microglia in cerebral ischemia.
Hypothermia inhibited microglial expression of iNOS and reactive nitrogen species at 3 d after ischemia, although cooling occurred at earlier time points. It is possible that hypothermia interferes with iNOS regulation during or shortly after ischemia. Nuclear factor κB is known to regulate the expression of iNOS and other inflammatory mediators; therefore, mild hypothermia may be exerting its anti-inflammatory effects by interfering with this mechanism. This deserves additional investigation.
Whether mild hypothermia inhibited microglia or peripheral blood monocytes cannot be inferred from the results presented here. To our knowledge, there are no specific antibodies or other markers to reliably differentiate between activated microglia and peripheral monocytes–macrophages. Given that the functions of both cell populations are very similar, we do not believe that this will affect our interpretation of the results.
The protection by hypothermia, especially delayed hypothermia, is especially important because microglial activation is a delayed and long-lasting phenomenon after ischemia, which may be an attractive therapeutic target for human stroke. We show that intact microglia–monocytes generate iNOS and NO–peroxynitrite 3 d after ischemia and are inhibited by both intraischemic and postischemic hypothermia. An active response by microglia is believed to contribute to cerebral damage (Gonzalez-Scarano and Baltuch, 1999); therefore, it is possible that the potential neuroprotective mechanisms of hypothermia are mediated in part through the suppression of microglia–monocyte activation. A minority of other iNOS-positive cells were not labeled with ED1, GFAP, or MAP-2 and could represent other leukocyte populations. Neutrophil infiltration is also present in the ischemic brain at 3 d (Iadecola et al., 1995a; Maier et al., 1998). Therefore, these other iNOS-producing cells could be neutrophils. However, the predominant inflammatory cell population at 3 d after ischemia was microglia–monocytes rather than neutrophils. NO produced by the other inflammatory cells or neurons themselves (via nNOS) at earlier time points probably caused the tissue damage, leaving nitrotyrosine remnants in injured cells. However, at 3 d after ischemia, microglia plays major role in NO production.
Hypothermia may provide an approach to potentially reduce ongoing damage during reperfusion in stroke patients. Our studies showed that hypothermia that was initiated after 2 hr of ischemia and persisted during reperfusion significantly reduced the cortical infarct volume. These findings led us and others (Garcia et al., 1993; Iadecola et al., 1995a; Du et al., 1996) to the notion that focal ischemic injury is an ongoing process that persists into the postischemic period, and postischemic hypothermia can suppress the deleterious processes such as iNOS induction and reactive nitrogen species generation, even days after treatment.
In summary, we show that (1) mild hypothermia protects against experimental stroke when applied during ischemia and after 2 hr of delay, (2) mild hypothermia attenuates iNOS expression and NO–peroxynitrite production in experimental stroke, (3) mild hypothermia also attenuates NO production in a model of pure brain inflammation and in cultured microglia, and (4) microglia may be an important source of reactive nitrogen species production, and mild hypothermia appears to inhibit this. Hypothermic suppression of iNOS expression by activated microglia is a novel finding and provides insight into the mechanisms of such neuroprotection.
Footnotes
This project was funded in part by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (M.A.Y.), an American Heart Association Beginning grant-in-aid, Western Affiliate (M.A.Y.), and National Institutes of Health Grant R01 NS 40516 (M.A.Y.). We thank Guo Hua Sun and Danye Cheng for expert technical assistance and Beth Hoyte for assistance with the figures.
Correspondence should be addressed to Midori A. Yenari, 1201 Welch Road, Medical School Lab Surge Building, P304, Stanford, CA 94305-5487. E-mail: yenari@alum.mit.edu.
REFERENCES
- 1.Abraham H, Lazar G. Early microglial reaction following mild forebrain ischemia induced by common carotid artery occlusion in rats. Brain Res. 2000;862:63–73. doi: 10.1016/s0006-8993(00)02072-2. [DOI] [PubMed] [Google Scholar]
- 2.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- 4.Chatzipanteli K, Wada K, Busto R, Dietrich WD. Effects of moderate hypothermia on constitutive and inducible nitric oxide synthase activities after traumatic brain injury in the rat. J Neurochem. 1999;72:2047–2052. doi: 10.1046/j.1471-4159.1999.0722047.x. [DOI] [PubMed] [Google Scholar]
- 5.Cockroft KM, Meistrell M, III, Zimmerman GA, Risucci D, Bloom O, Cerami A, Tracey KJ. Cerebroprotective effects of aminoguanidine in a rodent model of stroke. Stroke. 1996;27:1393–1398. doi: 10.1161/01.str.27.8.1393. [DOI] [PubMed] [Google Scholar]
- 6.De Alba J, Cardenas A, Moro MA, Leza JC, Lorenzo P, Lizasoain I. Use of brain slices in the study of pathogenic role of inducible nitric oxide synthase in cerebral ischemia-reperfusion. Gen Pharmacol. 1999;32:577–581. doi: 10.1016/s0306-3623(98)00280-8. [DOI] [PubMed] [Google Scholar]
- 7.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Eliasson MJL, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabian Loidl C, Capani F, Lopez-Costa JJ, Selvin-Testa A, Lopez EM, Pecci-Saavedra J. Long term changes in NADPH-diaphorase reactivity in striatal and cortical neurons following experimental perinatal asphyxia: neuroprotective effects of hypothermia. Int J Neurosci. 1997;89:1–14. doi: 10.3109/00207459708988460. [DOI] [PubMed] [Google Scholar]
- 11.Fassbender K, Fatar M, Ragoschke A, Picard M, Bertsch T, Kuehl S, Hennerici M. Subacute but not acute generation of nitric oxide in focal cerebral ischemia. Stroke. 2000;31:2208–2211. doi: 10.1161/01.str.31.9.2208. [DOI] [PubMed] [Google Scholar]
- 12.Forster C, Clark HB, Ross ME, Iadecola C. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathologica. 1999;97:215–220. doi: 10.1007/s004010050977. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. 1992;4:189–225. [PubMed] [Google Scholar]
- 16.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 18.Goussev AV, Zhang Z, Anderson DC, Chopp M. P-selectin antibody reduces hemorrhage and infarct volume resulting from MCA occlusion in the rat. J Neurol Sci. 1998;161:16–22. doi: 10.1016/s0022-510x(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 19.Graham SH, Shiraishi K, Panter SS, Simon RP, Faden AI. Changes in extracellular amino acid neurotransmitters produced by focal cerebral ischemia. Neurosci Lett. 1990;110:124–130. doi: 10.1016/0304-3940(90)90799-f. [DOI] [PubMed] [Google Scholar]
- 20.Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974–1980. doi: 10.1161/01.str.31.8.1974. [DOI] [PubMed] [Google Scholar]
- 21.Hirabayashi H, Takizawa S, Fukuyama N, Nakazawa H, Shinohara Y. Nitrotyrosine generation via inducible nitric oxide synthase in vascular wall in focal ischemia-reperfusion. Brain Res. 2000;852:319–325. doi: 10.1016/s0006-8993(99)02117-4. [DOI] [PubMed] [Google Scholar]
- 22.Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab. 1995;15:1002–1011. doi: 10.1038/jcbfm.1995.126. [DOI] [PubMed] [Google Scholar]
- 23.Huang FP, Zhou LF, Yang GY. Effects of mild hypothermia on the release of regional glutamate and glycine during extended transient focal cerebral ischemia in rats. Neurochem Res. 1998;23:991–996. doi: 10.1023/a:1021088523137. [DOI] [PubMed] [Google Scholar]
- 24.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 25.Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995a;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- 26.Iadecola C, Zhang FY, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995b;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- 27.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T. Post-ischemic hypothermia delayed neutrophil accumulation and microglial activation following transient focal ischemia in rats. J Neuroimmunol. 2000;109:66–74. doi: 10.1016/s0165-5728(00)00211-3. [DOI] [PubMed] [Google Scholar]
- 29.Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T. Intra-ischemic hypothermia attenuates intercellular adhesion molecule-1 (ICAM-1) and migration of neutrophil. Neurol Res. 2001;23:105–111. doi: 10.1179/016164101101198217. [DOI] [PubMed] [Google Scholar]
- 30.Kader A, Frazzini VI, Baker CJ, Solomon RA, Trifiletti RR. Effect of mild hypothermia on nitric oxide synthesis during focal cerebral ischemia. Neurosurgery. 1994;35:272–277. doi: 10.1227/00006123-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Karibe H, Chen SF, Zarow GJ, Gafni J, Graham SH, Chan PH, Weinstein PR. Mild intraischemic hypothermia suppresses consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res. 1994a;649:12–19. doi: 10.1016/0006-8993(94)91043-x. [DOI] [PubMed] [Google Scholar]
- 32.Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1994b;14:620–627. doi: 10.1038/jcbfm.1994.77. [DOI] [PubMed] [Google Scholar]
- 33.Kato H, Wood PL. Neuroinflammation: mechanisms and managements, pp91–107. Humana; Totowa, NJ: 1998. Inflammatory markers in stroke. [Google Scholar]
- 34.Kawai N, Okauchi M, Morisaki K, Nagao S. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke. 2000;31:1982–1989. doi: 10.1161/01.str.31.8.1982. [DOI] [PubMed] [Google Scholar]
- 35.Kil HY, Zhang J, Piantadosi CA. Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. J Cereb Blood Flow Metab. 1996;16:100–106. doi: 10.1097/00004647-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Kumar K, Evans AT. Effect of hypothermia on microglial reaction in ischemic brain. NeuroReport. 1997;8:947–950. doi: 10.1097/00001756-199703030-00026. [DOI] [PubMed] [Google Scholar]
- 37.Kumura E, Yoshimine T, Takaoka M, Hayakawa T, Shiga T, Kosaka H. Hypothermia suppresses nitric oxide elevation during reperfusion after focal cerebral ischemia in rats. Neurosci Lett. 1996;220:45–48. doi: 10.1016/s0304-3940(96)13238-9. [DOI] [PubMed] [Google Scholar]
- 38.Loihl AK, Asensio V, Campbell IL, Murphy S. Expression of nitric oxide synthase (NOS)-2 following permanent focal ischemia and the role of nitric oxide in infarct generation in male, female and NOS-2 gene-deficient mice. Brain Res. 1999;830:155–164. doi: 10.1016/s0006-8993(99)01388-8. [DOI] [PubMed] [Google Scholar]
- 39.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons SA, Pastor A, Ohlemeyer C, Kann O, Wiegand F, Prass K, Knapp F, Kettenmann H, Dirnagl U. Distinct physiologic properties of microglia and blood-borne cells in rat brain slices after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:1537–1549. doi: 10.1097/00004647-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 41.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 42.Maier CM, Ahern KV, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- 43.Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- 44.Nagayama M, Zhang F, Iadecola C. Delayed treatment with aminoguanidine decreases focal cerebral ischemic damage and enhances neurological recovery in rats. J Cereb Blood Flow Metab. 1998;18:1107–1113. doi: 10.1097/00004647-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Nomura Y. A transient brain ischemia- and bacterial endotoxin-induced glial iNOS expression and NO-induced neuronal apoptosis. Toxicol Lett. 1998;102–103:65–69. doi: 10.1016/s0378-4274(98)00286-0. [DOI] [PubMed] [Google Scholar]
- 46.Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 47.Parmentier-Batteur S, Bohme GA, Lerouet D, Zhou-Ding L, Beray V, Margaill I, Plotkine M. Antisense oligodeoxynucleotide to inducible nitric oxide synthase protects against transient focal cerebral ischemia-induced brain injury. J Cereb Blood Flow Metab. 2001;21:15–21. doi: 10.1097/00004647-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Salter M, Duffy C, Garthwaite J, Strijbos PJ. Ex vivo measurement of brain tissue nitrite and nitrate accurately reflects nitric oxide synthase activity in vivo. J Neurochem. 1996;66:1683–1690. doi: 10.1046/j.1471-4159.1996.66041683.x. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki A, Levison SW, Ting JP. Comparison and quantitation of Ia antigen expression on cultured macroglia and ameboid microglia from Lewis rat cerebral cortex: analyses and implications. J Neuroimmunol. 1989;25:63–74. doi: 10.1016/0165-5728(89)90087-8. [DOI] [PubMed] [Google Scholar]
- 50.Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 51.Si QS, Nakamura Y, Kataoka K. Hypothermic suppression of microglial activation in culture: inhibition of cell proliferation and production of nitric oxide and superoxide. Neuroscience. 1997;81:223–229. doi: 10.1016/s0306-4522(97)00172-3. [DOI] [PubMed] [Google Scholar]
- 52.Smith ME. Phagocytosis of myelin by microglia in vitro. J Neurosci Res. 1993;35:480–487. doi: 10.1002/jnr.490350504. [DOI] [PubMed] [Google Scholar]
- 53.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 54.Toyoda T, Suzuki S, Kassell NF, Lee KS. Intraischemic hypothermia attenuates neutrophil infiltration in the rat neocortex after focal ischemia-reperfusion injury. Neurosurgery. 1996;39:1200–1205. doi: 10.1097/00006123-199612000-00024. [DOI] [PubMed] [Google Scholar]
- 55.Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 56.Yenari MA, Giffard RG. Ischemic vulnerability of primary murine microglial cultures. Neurosci Lett. 2001;298:5–8. doi: 10.1016/s0304-3940(00)01724-9. [DOI] [PubMed] [Google Scholar]
- 57.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 58.Yenari MA, Onley D, Hedehus M, deCrespigny A, Sun GH, Moseley ME, Steinberg GK. Diffusion- and perfusion-weighted MRI of focal cerebral ischemia and cortical spreading depression under conditions of mild hypothermia. Brain Res. 2000;885:208–219. doi: 10.1016/s0006-8993(00)02942-5. [DOI] [PubMed] [Google Scholar]
- 59.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang F, Iadecola C. Temporal characteristics of the protective effect of aminoguanidine on cerebral ischemic damage. Brain Res. 1998;802:104–110. doi: 10.1016/s0006-8993(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 61.Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682:182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- 62.Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872:215–218. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]