Abstract
P2X receptors are ATP-gated cation channels that are widely expressed in the brain. The extracellular domains of all seven P2X receptors contain 10 conserved cysteines, which could form disulfide bonds or binding sites for transition metals that modulate P2X receptors. To test whether these cysteines are critical for receptor function, we studied wild-type rat P2X2 receptors and 10 mutant P2X2 receptors, each containing an alanine substituted for a cysteine. Nine mutants were functional but had reduced maximum currents compared with wild-type P2X2expressed in either Xenopus oocytes or human embryonic kidney (HEK) 293 cells. The 10th mutant (C224A) did not respond to ATP when expressed in oocytes and gave very small currents in HEK 293 cells. Seven mutants (C113A, C124A, C130A, C147A, C158A, C164A, and C214A) showed rightward shifts (9- to 30-fold) in their ATP concentration–response relationships and very little potentiation by zinc. In contrast, C258A and C267A had EC50values similar to those of wild-type P2X2 and were potentiated by zinc. Acidic pH potentiated wild-type and all mutant receptor currents. Despite the loss of zinc potentiation in seven mutants, these cysteines are unlikely to be exposed in the zinc-binding site, because [2-(trimethylammonium)ethyl] methanethiosulfonate bromide did not prevent zinc potentiation of wild-type receptor currents. On the basis of correlations in the maximum current, EC50, zinc potentiation, and pH potentiation, we suggest that the following cysteine pairs form disulfide bonds: C113–C164, C214–C224, and C258–C267. We also suggest that C124, C130, C147, and C158 form two disulfide bonds, but we are unable to assign specific cysteine pairs to these two bonds.
Keywords: P2X, purinergic, mutagenesis, disulfide bonds, DTT, zinc, pH
In both the CNS and peripheral nervous system, ATP acts as a neurotransmitter and causes fast excitatory responses by direct activation of a class of ligand-gated ion channels called P2X receptors (Barnard et al., 1997; Burnstock, 1997, 1999; Khakh, 2001). Over the past decade, seven P2X subtypes (P2X1–7) have been cloned from rats, each with homologs in mice and humans (Brake and Julius, 1996; North and Surprenant, 2000). With the exception of P2X6, all subtypes readily form homomeric receptors when expressed in heterologous cells (Lewis et al., 1995; Torres et al., 1998b, 1999; Le et al., 1999; King et al., 2000) and most likely assemble as trimers (Nicke et al., 1998; Stoop et al., 1999). Each subunit contains two transmembrane domains separated by a large extracellular domain (Newbolt et al., 1998; Torres et al., 1998a). The extracellular domain contains 10 cysteines conserved in all cloned P2X receptors, but the functional significance of these conserved cysteines is unknown.
These conserved cysteines could form up to five disulfide bonds, which would stabilize the conformation of the receptor (Hansen et al., 1997). Another common role for cysteines in proteins is the binding of transition metals (Christianson, 1991; Vallee and Falchuk, 1993). For example, three cysteines in the human skeletal muscle chloride channel are necessary for zinc inhibition (Kurz et al., 1999). Low concentrations of zinc (<100 μm) potentiate homomeric P2X2, P2X3, and P2X4 receptor currents (Seguela et al., 1996;Garcia-Guzman et al., 1997; Nakazawa and Ohno, 1997; Nakazawa et al., 1997; Le et al., 1998; Miller et al., 1998; Wildman et al., 1998,1999a,b; Xiong et al., 1999; Acuna-Castillo et al., 2000; Zhong et al., 2000). Thus, some of the conserved cysteines of P2X receptors might be necessary for zinc binding rather than disulfide bond formation.
In this study, we used site-directed mutagenesis to replace each of the conserved cysteines with an alanine to test whether these cysteines are necessary to form functional receptors and for the receptors to respond to zinc. We chose to study homomeric P2X2receptors because they exhibit slow desensitization to ATP and are highly potentiated by zinc. Our results suggest that none of the conserved cysteines are directly involved in zinc binding. Furthermore, by assuming that mutating either cysteine of a disulfide bond should produce similar changes in receptor properties, we have been able to make some specific predictions for which cysteine pairs are disulfide bonded.
MATERIALS AND METHODS
Mutagenesis. Rat P2X2 cDNA in pcDNA1 was obtained from Dr. D. Julius (University of California, San Francisco, CA) (Brake et al., 1994). Mutagenic oligonucleotides were obtained from Operon Technologies, Inc. (Alameda, CA) and contained the base changes necessary for a single amino acid substitution and for the introduction or deletion of a restriction enzyme recognition site. Most mutant P2X2receptors were made using the Kunkel mutagenesis method (Kunkel, 1985;Kunkel et al., 1987). Briefly, a uridine-containing P2X2 template was made by growing the plasmid in BW313 cells in medium supplemented with 0.4 μg/ml uridine. These cells were subsequently infected with M13K07 helper phage to make a single-stranded template. Mutagenic oligonucleotides were phosphorylated with T4 polynucleotide kinase, annealed to the single-stranded uridine-containing P2X2 template, and extended using Sequenase version 2.0 T7 polymerase (United States Biochemicals/Amersham, Piscataway, NJ). Transformation of the mutagenic reaction into DH5α cells removed uridine-containing DNA. Putative mutants were first screened by restriction enzyme digestion and subsequently confirmed by DNA sequencing (University of Michigan DNA Sequencing Core, Ann Arbor, MI). For some mutations, we used overlap extension by PCR (Ho et al., 1989; Vallejo et al., 1995). Each mutant is referred to by the original amino acid (one-letter code) followed by the residue number and the substituted amino acid (one-letter code). The oligonucleotides sequences and the diagnostic enzymes introduced or deleted were as follows: C113A, 5′-CCTTGGGAACAGCGCCAGAGAGCATG-3′ (addsHaeII); C124A, 5′-CACAGCTCTACCGCGCATTCAGACGACG-3′ (adds BsMI); C130A, 5′-GACGACGACGCCATTGCCGGACAG-3′ (addsBsaHI); C147A, 5′-TGGGATTCGCACAGGTCATGCGGTACCCTATTACCAT-3′ (adds KpnI); C158A, 5′-GACTCCAAGACCGCCGAGGTGTC-3′ (adds AciI); C164A, 5′-GTGTCAGCCTGGGCTCCGGTGGAG-3′ (addsBsaWI); C214A, 5′-CCTCAAGCATGCCACATTTGATCAGGAC-3′ (addsSphI); C224A, 5′-CTCTGACCCATATGCTCCCATCTTCAGG-3′ (addsNdeI); C224S, 5′-CTCTGACCCATACAGTCCAATCTTCAGGCT-3′ (removesXcmI); C258A, 5′-CAACTGGAATGCTGACCTGGACTTGTCTGA-3′ (addsBsMI); and C267A, 5′-CTGGACTTGTCTGAAAGTGAGGCCAACCCCAAATATTCTTTCCGGAGGC-3′ (removesTfiI).
Expression of P2X2 receptors. For most experiments, P2X2 receptors were expressed in defolliculated stage V–VI Xenopus laevis oocytes. Oocytes were harvested by procedures approved by the University of Michigan Committee on the Use and Care of Vertebrate Animals and have been described in detail previously (Zhou and Hume, 1998). P2X2 and mutant receptor RNA were synthesized using the T7 mRNA message machine kit from Ambion (Austin, TX), and 50 nl of RNA (5–100 ng/μl) was injected into each oocyte. Two-electrode voltage-clamp experiments were performed 1–5 d after RNA injection.
For some experiments, we used whole-cell recording of transiently transfected human embryonic kidney (HEK) 293 cells. Cells plated on 60 mm dishes were cotransfected with 1 μg of pcDNA3–enhanced green fluorescent protein (EGFP), 3 μg of the P2X2 mutant or wild-type plasmid, and 12 μl of LipofectAMINE (Invitrogen, San Diego, CA), split the following day, and recorded from 1 or 2 d after splitting.
Solutions. Our oocyte external recording solution contained (in mm): 90 NaCl, 1 KCl, 1.3 MgCl2, and 10 HEPES, pH 7.5, and our pipette solution contained 3 m KCl and 0.4m EGTA, pH 7.5. For whole-cell recording from HEK 293 cells, our external solution contained (in mm): 150 NaCl, 2 KCl, 1.5 MgCl2, 10 HEPES, and 10 glucose, pH 7.5, and our internal recording solution contained (in mm): 145 CsCl, 1.3 MgCl2, 5 K2EGTA, 10 HEPES, and 10 glucose, pH 7.5. Disodium ATP (Sigma-Aldrich, St. Louis, MO) was dissolved at 100 mm in double-distilled H2O, divided into aliquots, and stored at −20°C for 1–3 months. The 10 mm stock of ZnCl2 was dissolved in acidic double-distilled H2O to prevent precipitation. For recording, ATP stock solutions were diluted in external recording solution with the required zinc and used within 48 hr. To compensate for the chelation of Mg2+ by ATP, we added MgCl2 to our solutions such that all solutions contained 1 mm free Mg2+, as determined by the program Bound and Determined (Brooks and Storey, 1992). After the pH was adjusted to the required level, the ATP concentrations of all solutions were verified by taking spectroscopic measurements at 259 nm. Dithiothreitol (DTT) was obtained from Invitrogen, and solutions were made fresh on the day of recording. [2-(Trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET) was obtained from Toronto Research Chemicals (Toronto, Ontario, Canada), and MTSET solutions were made within 30 min of use.
Electrophysiological recordings and data analysis.P2X2 and mutant receptors expressed inXenopus oocytes were investigated by two-electrode voltage clamp with either an npi TurboTec 3 (npi Electronics, Tamm, Germany) or an Axon Instruments Geneclamp 500B (Axon Instruments, Foster City, CA). The holding potential was −50 mV. The rate of P2X2 receptor current desensitization is slow and concentration dependent, varies between batches of oocytes, and recovers slowly with a time constant of ∼4 hr (Zhou et al., 1998). Thus, obtaining concentration–response relationships for P2X2 receptors by sequentially applying higher concentrations of ATP to the same oocytes is likely to cause significant accumulation of desensitization. The resulting concentration–response relationship will be that for a mixture of desensitized and nondesensitized P2X2 receptors, which will underestimate the EC50, because desensitized receptors have a higher affinity for ATP. To minimize the effects of desensitization on the concentration–response relationship, our strategy was to expose each oocyte to only two concentrations of ATP: a small nondesensitizing concentration of ATP (approximately the EC10), immediately followed by a test concentration of ATP. For each test concentration of ATP, the ratio between these current amplitudes was subsequently averaged among five to seven oocytes (∼40 oocytes per construct). The plot of these average ratios was subsequently fitted using the Hill equation. For graphical display, the ratios were renormalized to a maximum response of 100%. This strategy makes no assumption about the recovery rate from desensitization and more accurately represents the response of the nondesensitized receptors. Indeed, the EC50 we obtained for wild-type P2X2 using this method was 130% of that obtained by estimating the EC50with sequential 30 sec applications of ATP to single oocytes.
Because this protocol does not average the concentration–response relationships of individual oocytes, the EC50 and Hill coefficient are reported in Table 1as the mean value and SE of the regression from Sigma Plot 7.0. To test whether the fits to the concentration–response relationships of two constructs were statistically different, we performed an Ftest. This test compares the residuals derived from fitting the average ratios to two separate Hill equations with the residuals derived from fitting the ratios to a single Hill equation. A p value of < 0.01 was considered to be significant. This statistical approach does not independently test whether the significant differences were a result of a change in EC50 values, Hill coefficients, or both. To test whether the EC50 values of mutants were significantly different from those of wild-type P2X2, we studied the responses of a series of oocytes expressing mutant or wild-type receptors to sequential application of increasing concentrations of ATP. Although, as noted above, this method underestimates the true EC50, it has the advantage that it yields an independent estimate of the EC50 and Hill coefficient from each oocyte and thus allowed a t test to be used to compare the mutants with the wild type. The conclusion reached from this analysis was that in all cases in which a significant difference between a mutant and the wild type was indicated by the F test, the cause was a change in the EC50. To avoid the confusion of having two different EC50 values and Hill coefficients quoted in Results for each mutant, these data are not shown. However, because of these results, the asterisks and number signs indicating significant differences in Table 1 are placed in the column for EC50 values, rather than in the column for Hill coefficients.
Table 1.
EC50, Hill coefficients, and maximal currents
| Xenopus oocytes | HEK 293 cells | |||
|---|---|---|---|---|
| EC50 (μm) | Hill coefficient | Maximum current (μA) | Maximum current (pA) | |
| P2X2 | 32.2 ± 4.2 | 2.03 ± 0.40 | −31.4 ± 2.0 | −2202 ± 493 |
| (n = 18) | (n = 6) | |||
| C113A | 990 ± 1491-a,1-b | 1.23 ± 0.09 | −4.6 ± 0.71-a | −393 ± 921-a |
| (n = 8) | (n = 6) | |||
| C124A | 462 ± 1021-a | 1.33 ± 0.22 | −9.4 ± 1.01-a | −413 ± 1461-a |
| (n = 8) | (n = 7) | |||
| C130A | 359 ± 171-a | 1.93 ± 0.12 | −3.5 ± 0.51-a | −477 ± 2511-a |
| (n = 8) | (n = 5) | |||
| C147A | 459 ± 491-a | 1.51 ± 0.15 | −8.7 ± 1.91-a | −507 ± 3291-a |
| (n = 8) | (n = 6) | |||
| C158A | 411 ± 61-a | 1.72 ± 0.03 | −13.8 ± 1.81-a | −475 ± 1481-a |
| (n = 6) | (n = 6) | |||
| C164A | 869 ± 2041-a,1-b | 1.14 ± 0.12 | −11.0 ± 1.21-a | −633 ± 2571-a |
| (n = 8) | (n = 5) | |||
| C214A | 303 ± 371-a | 1.32 ± 0.14 | −0.43 ± 0.071-a | −44 ± 81-a |
| (n = 13) | (n = 4) | |||
| C224A | No response | No response | No response | −42 ± 271-a |
| (n = 20) | (n = 5) | |||
| C258A | 30.2 ± 2.1 | 1.49 ± 0.10 | −8.3 ± 1.71-a | −100 ± 381-a |
| (n = 6) | (n = 5) | |||
| C267A | 30.2 ± 1.7 | 1.74 ± 0.12 | −0.09 ± 0.011-a | −77 ± 151-a |
| (n = 7) | (n = 5) | |||
The half-maximal effective concentrations (EC50), Hill coefficients, and maximal currents of wild-type and mutant receptors to ATP were determined as described in Materials and Methods. EC50 and Hill coefficients are given as the mean ± SE of the fit from Sigma Plot 7.0. The maximum current amplitudes are given as the mean ± SEM of the responses to 2 mm ATP when receptors were expressed in Xenopus oocytes or 5 mm ATP when receptors were expressed in HEK 293 cells.
Values significantly different from wild type (p < 0.01).
Two mutants that had their EC50 significantly right-shifted (p< 0.01) compared with wild type and the seven other mutants that were characterized.
To compare the maximum response of each mutant in oocytes, we examined the response of oocytes injected with a saturating amount of RNA (5 ng per oocyte) after 2 d of incubation and measured the current amplitude in response to 2 mm ATP. All data shown are from the same batch of oocytes. To compare the maximum response of each mutant in HEK 293 cells, we measured the response of cells to 5 mm ATP 2 d after transfection. To control for varying transfection efficiencies between dishes and among cells, we recorded only from cells that were brightly fluorescent because of the EGFP marker that was cotransfected. As judged by eye, the intensity of the EGFP was similar for all of the mutants tested.
For experiments in which the modulation of receptors was examined, the zinc potentiation ratio reported is the ratio of the current amplitude in the presence of ATP and zinc to the amplitude in the presence of ATP alone. Similarly, the pH potentiation ratio reported is the ratio of the current amplitude in the presence of ATP at a pH of 6.5 to that at a pH of 7.5. Zinc potentiation and pH potentiation are both allosteric regulatory mechanisms that left-shift the concentration–response relationship to ATP but have little if any effect on the maximum current (Wildman et al., 1998; Xiong et al., 1999). For instance, 10 μm ATP is approximately the EC10 at a pH of 7.5 in the absence of zinc, but it is approximately the EC80 in the presence of 20 μm zinc or at a pH of 6.5 (Clyne et al., 2002). Because the maximum response does not change, the potentiation ratio decreases as the concentration of ATP increases. To compare the magnitude of potentiation between mutants, it is therefore necessary to select a concentration of ATP that produces a small and equivalent response in the absence of the potentiating substance. In all experiments, we used a concentration of ATP that approximated the EC10 at a pH of 7.5 with no zinc (estimated from the parameters shown in Table 1) as our basal condition. Student's t tests were used to determine whether population means differed, with significance taken to bep < 0.01. All data are expressed as the mean ± SEM, unless otherwise specified.
RESULTS
Effect of DTT and MTSET on the response of P2X2receptors to ATP and zinc
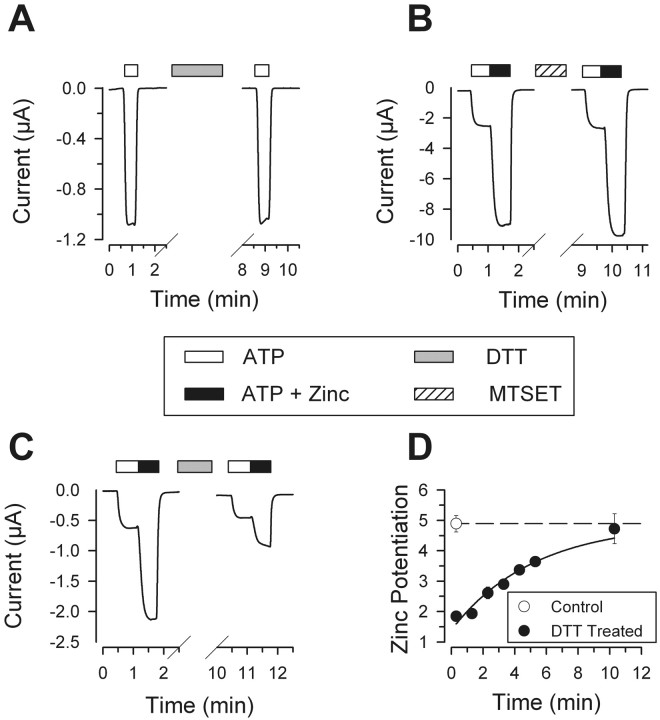
As an initial test of whether P2X2 receptors contain disulfide bonds necessary for receptor function, we examined the effects of a reducing agent, DTT, on P2X2receptors expressed in Xenopus oocytes. In agreement with previous reports (Li et al., 1997; Rassendren et al., 1997), a 5–10 min incubation in 10 mm DTT had no effect on the current amplitudes of P2X2 receptors in response to 10 μm ATP (mean current amplitude after DTT was 104 ± 8% of the current amplitudes before treatment;n = 9) (Fig.1A). Thus, P2X2 receptors do not contain DTT-susceptible disulfide bonds that significantly affect the response to ATP.
Fig. 1.
Effect of DTT and MTSET on P2X2receptor currents. A, A 5 min incubation with 10 mm DTT did not affect the current responses of oocytes expressing P2X2 to 10 μm ATP.B, The response of P2X2 receptors to 10 μm ATP was potentiated fourfold by 5 μmzinc, and a 5 min incubation with 1 mm MTSET did not affect the potentiation of P2X2 receptor currents by 5 μm zinc. C, Potentiation of P2X2 receptor currents by 5 μm zinc was reduced but not eliminated by a 5 min incubation with 10 mmDTT followed by a 2 min wash. D, Recovery of zinc potentiation after treatment with DTT. The closed circles show the potentiation of P2X2 receptor responses to 10 μm ATP by 5 μm zinc after a 5 min incubation with 10 mm DTT and variable wash times (n = 5–8 per time point; the error bars were smaller than the size of the circles). For comparison, the average zinc potentiation of P2X2 receptors is shown as an open circle at the first time point and extrapolated as a dashed line for other time points (n = 7).
P2X2 receptor currents are potentiated by the binding of zinc to the extracellular domain (Nakazawa and Ohno, 1997;Nakazawa et al., 1997; Miller et al., 1998; Wildman et al., 1998; Xiong et al., 1999). One way that cysteines might play a role in zinc potentiation is by acting as part of the zinc-binding site. We tested this hypothesis by examining whether the sulfhydryl reagent MTSET could block zinc potentiation. Limiting the amount of desensitization was essential in these experiments, because they required repeated applications of ATP to single oocytes. We therefore used 5 μm zinc, a concentration that gave submaximal potentiation but caused little desensitization (Fig.1B). When 10 μm ATP was used, 5 μm zinc potentiated the current amplitudes of P2X2 receptors by threefold to fivefold (mean fold potentiation, 4.01 ± 0.1; n = 46). Maximal potentiation was achieved with 20 μm zinc (mean fold potentiation, 11.2 ± 1.1; n = 35), but these responses showed substantial desensitization (Fig.2). Zinc potentiation to 5 μm ATP was not reduced after incubation in 1 mm MTSET for 5 min (Fig. 1B). As a positive control for the effectiveness of MTSET, we used a mutant P2X2 receptor containing a cysteine substitution at I328. As reported previously (Rassendren et al., 1997; Egan et al., 1998; Stoop et al., 1999), incubation in 1 mm MTSET for 5 min was sufficient to cause a 57 ± 3% reduction of I328C receptor currents in response to 1 μm ATP (n = 6). Thus, either free cysteines are not part of the zinc binding site or MTSET cannot gain access to the zinc binding site.
Fig. 2.
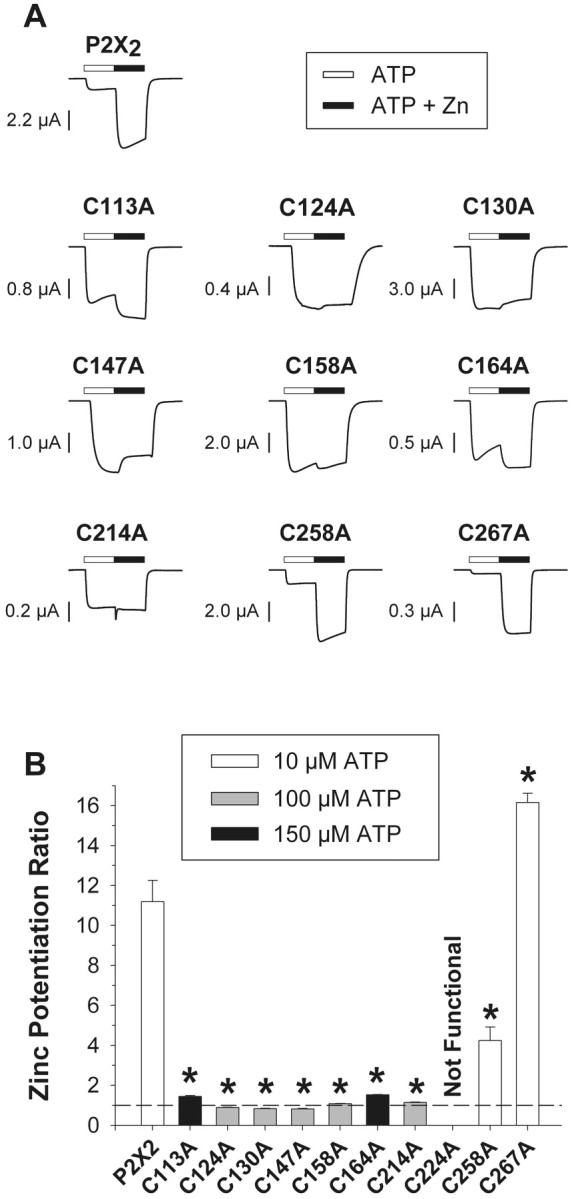
Zinc potentiation was greatly reduced or eliminated in seven cysteine mutants. A, Zinc potentiation of ATP-induced currents of single oocytes expressing wild-type and mutant receptors. The ATP concentration used for each mutant (∼EC10) is indicated in B. The zinc concentration was 20 μm. B, Mean zinc potentiation ratio of 6–19 oocytes per construct. The zinc potentiation ratio is the ratio of the current amplitude in the presence of ATP and 20 μm zinc to the current amplitude of ATP alone. The dashed line indicates no potentiation. Asterisks indicate values significantly different from wild type (p < 0.01).
A third potential role for the conserved cysteines is to form disulfide bonds essential for modulatory actions but not for the basic response to ATP. We therefore examined zinc potentiation of P2X2 receptor currents after treatment with DTT. Exposure of P2X2 receptors to 10 mmDTT for 5 min, followed by a 2 min wash, reduced the zinc potentiation ratio by 40% to 2.6 ± 0.2 (n = 8) (Fig.1C). Shorter wash times also reduced but never eliminated zinc potentiation (Fig. 1D). Because DTT has a high affinity for zinc (Kd = 10−10.3) (Cornell and Crivaro, 1972), the decreased potentiation to zinc immediately after DTT treatment might result from residual DTT chelating some of the zinc in the test solutions. However, the time course of recovery of zinc potentiation (τ = 5.2 ± 1.2 min) was much slower than our bath exchange speed (τ ∼ 5 sec), suggesting that some of the effects of DTT on zinc potentiation might be attributable to the disruption of one or more disulfide bonds, with the slow time course of recovery representing the time course of re-formation of these bonds. To test this idea, we added MTSET shortly after DTT treatment, because cysteines bound with MTSET cannot re-form disulfide bonds. After exposing P2X2 receptors to 10 mm DTT for 5 min, we washed the oocytes for 1 min (so that residual DTT did not cleave MTSET from any cysteines it encountered) before exposing them to 1 mm MTSET for 5 min. We subsequently allowed for an additional minute of wash before testing for zinc potentiation to ensure that residual free MTSET did not chelate zinc. The zinc potentiation ratio of these receptors exposed to DTT and then MTSET was 77 ± 5% (n = 5) of the initial zinc potentiation ratio before treatment, equivalent to the percentage of recovery (77 ± 3%; n = 5) of receptors treated with DTT followed by a 7 min wash in bath recording solution. Thus, if the decrease in zinc potentiation after DTT treatment is attributable to disruption of a disulfide bond and recovery is attributable to re-formation of this bond, then the resulting free cysteines must be buried and inaccessible to MTSET. Perhaps a simpler explanation is that this effect of DTT does not act through changes in disulfide bonds.
Effect of cysteine mutations on the concentration–response relationships to ATP
To directly test the necessity of the 10 cysteines in the extracellular domain of P2X receptors, we constructed P2X2 mutant receptors, each of which contained an alanine substituted for one of the 10 conserved cysteines. Nine of the 10 mutants were functional in oocytes, and all 10 were functional in HEK 293 cells. To compare the levels of expression, we studied a group of oocytes or HEK 293 cells in which all of the cells were from a single batch and all recordings were made at a fixed time (2 d after RNA injection or DNA transfection). In both expression systems, the maximum current that could be obtained from the mutant receptors in response to a high concentration of ATP was smaller than for the wild type (Table 1). Six of the mutants (C113A, C124A, C130A, C147A, C158A, and C164A) gave currents that were ∼25% of wild type (range 11–42%) in both expression systems. One mutant (C258A) gave small currents (∼5% of wild type) when expressed in HEK 293 cells but somewhat larger currents (∼25% of wild type) when expressed in oocytes. The remaining three mutants (C214A, C224A, and C267A) gave very small or undetectable currents (<4% of wild type) in both expression systems. Indeed, for ATP concentrations up to 5 mm, we never detected any currents from oocytes expressing two independent isolates of the C224A mutation or from the more conservative C224S mutation. It should be noted that when high concentrations of RNA were used and >2 d was allowed for receptor expression in oocytes, the maximum current continued to increase beyond the amplitudes shown in Table 1, and that for some of the oocytes illustrated in Figures 2 and 3, the maximal currents were greater than −50 μA.
Fig. 3.
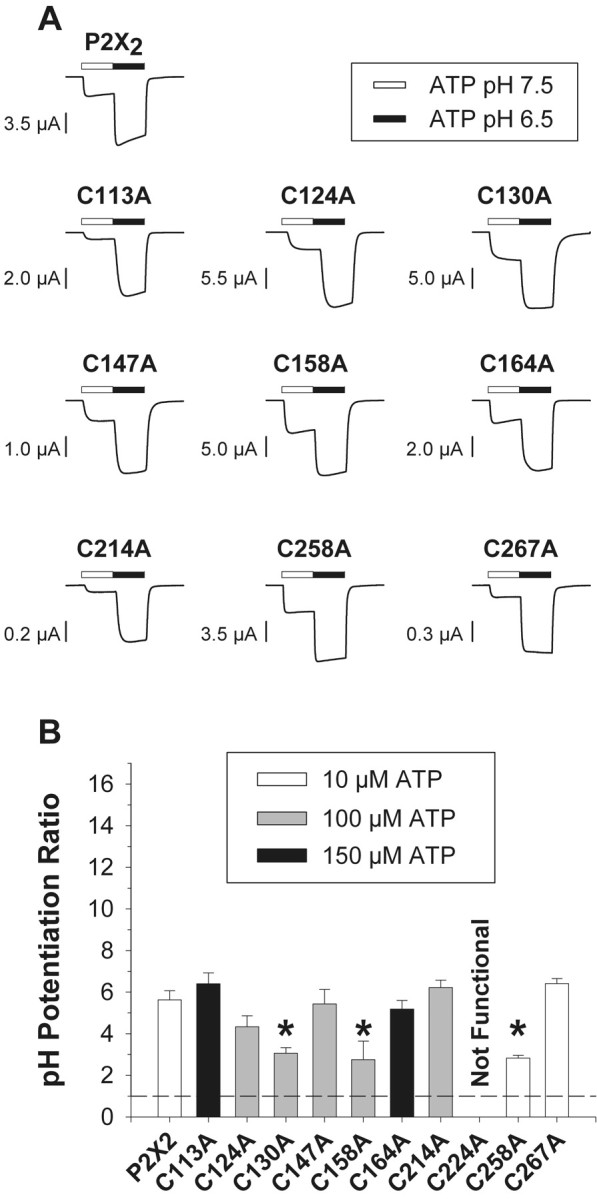
Zinc-resistant mutants were significantly potentiated by acidic pH (a pH of 6.5). A, Potentiation of ATP-induced currents of single oocytes expressing wild-type and mutant receptors by acidic pH. The ATP concentration used for each mutant (∼EC10) is indicated in Band for each mutant was the same concentration as used to test zinc potentiation in Figure 2. B, Mean potentiation of 6–19 oocytes per construct. The pH potentiation ratio is the ratio of the current amplitude in the presence of ATP at a pH of 6.5 to the current amplitude in the presence of ATP at a pH of 7.5. The dashed line indicates no potentiation. Asterisksindicate potentiation ratios significantly different from wild type (p < 0.01).
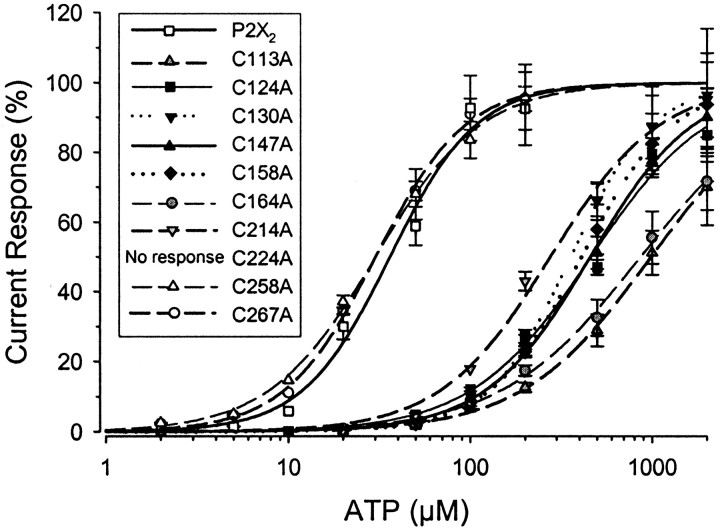
We decided to perform a more detailed characterization of mutant receptor properties in oocytes, because the currents from the C224A mutant were too small to perform accurate studies in either system, and the currents from the other mutants gave a better signal-to-noise ratio in the oocyte recordings. The concentration–response relationships of the nine mutant receptors characterized in oocytes (Fig.4, Table 1) fell into three groups: (1) two mutations (C258A and C267A) did not significantly alter the EC50 from that of wild type, (2) five mutations (C124A, C130A, C147A, C158A, and C214A) significantly increased the EC50 (by ∼12-fold), and (3) two mutations (C113A and C164A) caused a significantly larger increase in the EC50 (∼25-fold) than the other seven mutations. Thus, these mutations differentially affected the response of the receptors to ATP. We subsequently examined whether these mutations also had varying effects on the modulation of receptors.
Fig. 4.
The concentration–response relationships for ATP of the cysteine mutants. Concentration–response relationships were determined as described in Materials and Methods. Each point represents the mean ± SEM. The EC50 and Hill coefficients of these fits are summarized in Table 1.
Effect of cysteine mutations on the zinc potentiation of receptor currents
The failure of MTSET to block zinc potentiation argues that cysteines are unlikely to be directly involved in binding to zinc. Even so, some of the conserved cysteines might form disulfide bonds essential for the receptor to fold into a conformation that has a proper zinc-binding site. We therefore examined the effects of the C to A mutations on zinc potentiation. As explained in Materials and Methods, it was important that the concentration of ATP used in these experiments be at approximately the EC10 of the concentration–response relationship at a pH of 7.5 in the absence of zinc, which varied among the mutants. Because there was no need to minimize desensitization in these experiments, we used 20 μm zinc, which produced maximal potentiation in wild-type P2X2.
Two mutations (C267A and C258A) were similar to wild type in that they showed dramatic potentiation in response to 20 μm zinc. Two other mutations (C113A and C164A) gave currents that showed small but significant potentiation to zinc. The remaining cysteine mutations (C124A, C130A, C147A, C158A, and C214A) showed no zinc potentiation or slight inhibition by 20 μm zinc. In wild-type P2X2, a separate zinc-dependent inhibitory process dominates over potentiation at higher concentrations of zinc (Clyne et al., 2002). This inhibition of current in response to high concentrations of zinc (>100 μm) was also present in the C to A mutants. Therefore, it was not possible to determine whether the zinc site associated with potentiation had been eliminated in these mutants or simply shifted to a lower affinity. It seems likely that partial occupancy of the low-affinity site for zinc inhibition in the absence of a high-affinity site for zinc potentiation accounts for the observed inhibition of current by 20 μm zinc in the C124A, C130A, and C147A mutants.
Effect of cysteine mutations on the pH potentiation of receptor currents
To test whether these mutations differentially disrupt the ability of P2X2 receptors to respond to other modifiers of channel gating, we looked at the effects of extracellular pH (Fig.3). Lowering the pH of the extracellular solution potentiates P2X2 receptor currents (King et al., 1996, 1997;Nakazawa et al., 1997; Wildman et al., 1998). In our solutions, the relationship between pH and the response of P2X2receptors to 10 μm ATP had a pKa of 7.0 (data not shown), which is similar to the values reported by others (King et al., 1996, 1997; Nakazawa et al., 1997; Wildman et al., 1998). In agreement with other reports, we also found that a very acidic pH (≤5.5) inhibits P2X2 receptor currents (Wildman et al., 1998). We therefore used a pH of 6.5 for these studies, and used the same concentrations of ATP that were used to study zinc potentiation (∼EC10 at a pH of 7.5). In contrast to the loss of zinc potentiation in seven mutants, all nine functional mutants were significantly potentiated by an acidic pH (although C130A, C158A, and C258A showed slightly less potentiation than wild type). The ability to observe substantial pH potentiation in all mutants demonstrates that the failure to observe zinc potentiation in seven mutants was not because we chose an inappropriate concentration of ATP or had saturated our recording apparatus.
DISCUSSION
In this study, we used site-directed mutagenesis to investigate the necessity of the 10 cysteine residues in the extracellular domain of P2X2 receptors. Because these cysteines are conserved in all known P2X receptors, we suspected that they might play an essential role in receptor function. To our great surprise, all 10 alanine-substituted cysteine mutants produced functional receptors in HEK 293 cells, and nine were functional in Xenopus oocytes. Compared with wild-type P2X2, the ATP concentration–response relationships of nine mutants in oocytes were either right-shifted (C113A, C124A, C130A, C147A, C158A, C164A, and C214A) or unaltered (C258A and C267A). The currents for C224A in HEK 293 cells were too small to accurately determine the EC50, but because no currents were seen until the ATP concentration was >200 μm, the concentration–response relationship of this mutant was also greatly right-shifted.
Zinc-binding site of P2X2 receptors is unlikely to contain cysteine residues
The first indication that the zinc-binding site lacks free cysteines is that the sulfhydryl-modifying reagent MTSET failed to block potentiation by zinc. Given the coordination chemistry of zinc binding sites, an MTSET bound to a cysteine in the binding site would be incompatible with zinc binding, so either there are no free cysteines or any free cysteines are accessible to zinc but not MTSET. The latter is not simply a formal possibility but a very real one, because we have shown recently that two histidines of P2X2 are essential for zinc modulation, although treating these receptors with the histidyl-modifying reagent DEPC has no effect on zinc potentiation (Clyne et al., 2002). To clarify this issue, it was necessary to examine the effects of the cysteine mutations on zinc modulation.
One might expect that mutating a residue in the zinc-binding site would eliminate zinc potentiation without altering other receptor properties greatly. Indeed, we demonstrated recently that mutating either one of two histidines (H120 and H213) gave exactly this result (Clyne et al., 2002). However, all seven mutations that decreased or eliminated potentiation to 20 μm zinc (C113, C124, C130, C147, C158, C164, and C214) caused significant increases in the EC50 for ATP. We therefore believe that the alteration in zinc potentiation in these seven mutants is most likely secondary to more general structural changes in the mutant receptors, rather than attributable to a direct role of any of these cysteines in binding zinc.
Two mutations (C258A and C267A) had little effect on the ATP concentration–response relationships and thus may not cause as dramatic structural changes as the others. Although mutating C267 slightly increased zinc potentiation and mutating C258 slightly decreased zinc potentiation, neither mutation produced an effect similar to mutating the key histidines identified by Clyne et al. (2002). In summary, we present evidence that none of the cysteines are likely to be involved in zinc binding.
Potential assignment of disulfide bonds to specific cysteine pairs
The 10 conserved cysteines of P2X2 receptors have been proposed to form disulfide bonds, but this had not been tested previously. Presumably, these are intramolecular disulfide bonds, because P2X receptors can dissociate into monomers under nonreducing conditions (Nicke et al., 1998). If we assume that the dominant effect of a cysteine mutation is caused by the disruption of a disulfide bond, then mutating the other cysteine that shares this disulfide bond should produce a receptor with similar properties. Therefore, by comparing the similarity of the mutant receptors, we can tentatively assign disulfide bonds to specific cysteine pairs. This analysis, however, requires that breaking different disulfide bonds creates discernibly different effects.
The concentration–response relationships of C113A and C164A were much more right-shifted than those of the other seven functional cysteine mutants. In addition, C113A and C164A showed small but significant potentiation by 20 μm zinc, a property not shared by the other five right-shifted mutants. The amplitude of the maximal currents and the potentiation of C113A and C164A receptor currents by pH were similar to each other. On the basis of these criteria, we suggest that C113 and C164 may form a disulfide bond.
Only two cysteine mutants (C258A and C267A) had ATP concentration–response relationships similar to those of wild-type P2X2. Although the maximum responses of these mutants differed from each other in oocytes, both had dramatically reduced currents in HEK 293 cells. C158A and C267A were both potentiated by pH and were the only two mutants with currents potentiated more than threefold by zinc. Thus, C258 and C267 are candidates to form a second disulfide bond.
Four cysteine mutants (C124A, C130A, C147A, and C158A) were quite similar in their level of expression, EC50, failure to potentiate to zinc, and potentiation in response to a shift to a pH of 6.5. These four residues are excellent candidates to form two more disulfide bonds, but there is no basis on which to suggest the specific pairing.
A striking feature that C214A and C224A shared was that both gave currents much smaller than the other mutants in both expression systems. Furthermore, C214A differed from the other four mutants with moderately right-shifted concentration–response relationships (C124A, C130A, C147A, and C158A) in that it had an EC50 closer to that of wild type. The currents from the C224A mutant were too small to characterize in detail in either system, but this mutant clearly had a concentration–response relationship that was substantially right-shifted. Therefore, C214 and C224 are potential candidates to form a fifth disulfide bond.
Reconciling the effects of DTT and single amino acid substitutions
The results described above suggest that disrupting any one of five disulfide bonds significantly destabilizes the receptor. If this is correct, then the finding that DTT had no effect on the ATP-induced current amplitudes of wild-type receptors in this study and others (Li et al., 1997; Rassendren et al., 1997) might be interpreted as meaning that in the mature receptor, these disulfides are inaccessible to DTT. A similar inaccessibility of DTT has been suggested for the disulfide bonds in the inwardly rectifying K+channel Kir 2.1 (Cho et al., 2000). This is also consistent with the generalization that disulfides are often accessible to reducing agents only after exposure to denaturing agents (Creighton, 1988).
Another possibility is that the structural changes that result from disrupting the accessible disulfide bonds in the mature folded receptor are less severe than the changes that result when the receptor folds in the absence of a bond, as occurs for the mutant receptors. For example, the greatly reduced maximum response of C214A and C224A might indicate that they form a disulfide bond necessary to stabilize a folding intermediate that allows other disulfide bonds to form or other posttranslational modifications (e.g., glycosylation) to occur. Furthermore, we cannot exclude the possibility that disulfide bonds in P2X2 receptors rearrange during protein folding and that the above cysteine pairs represent these transient disulfide bonds, which might be more critical than the final bonds of the mature receptor.
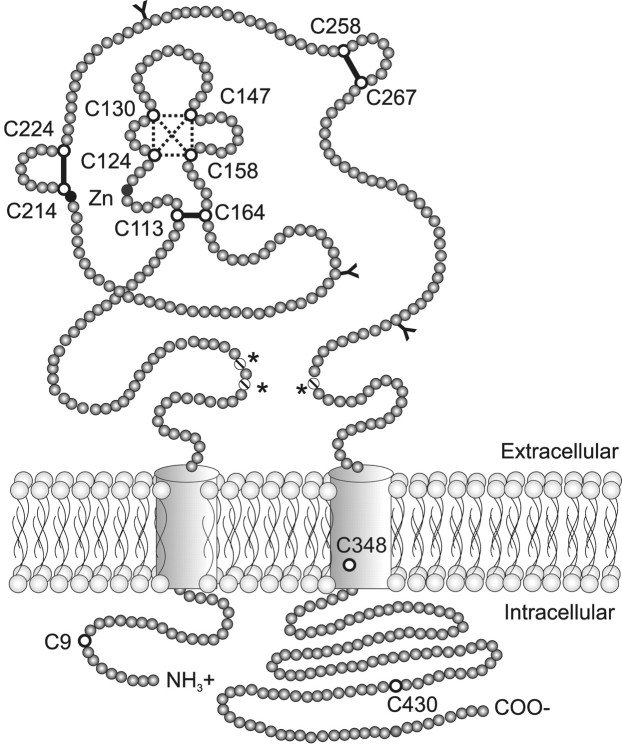
In summary, our results suggest that the 10 conserved cysteines in P2X2 receptors have no direct role in zinc or pH potentiation but form five intramolecular disulfide bonds. By assuming that mutating either cysteine of a disulfide bond should have a similar effect on the receptor, we propose that the rat P2X2 receptor contains disulfide bonds between C113–C164, C258–C267, and C214–C224 and that two additional bonds exist among C124, C130, C147, and C158 (Fig.5). Gratifyingly, the same cysteine pairings were recently proposed for the human P2X1 receptor in an article published while this article was in press (Ennion and Evans, 2002). Although these cysteine pairs may represent the cysteine pairs present during protein processing, the simplest interpretation is that these are the final cysteine pairings in the mature protein. If so, then we can now identify a number of discontinuous regions in the primary sequence of P2X receptors that are close to each other in the three-dimensional structure. In addition to the five disulfide bonds suggested here, our recent work on zinc modulation by histidines suggests that H120 and H213 both contribute to the zinc binding site and so must be close together (Clyne et al., 2002), and the work of Jiang et al. (2000) andEnnion et al. (2000) suggests that positively charged lysine residues at positions 69, 71, and 308 bind to the phosphates of ATP. These predictions await additional tests by biochemical, biophysical, or structural approaches.
Fig. 5.
Model of the P2X2 receptor. All of the cysteines are shown as open circles. The solid lines indicate three proposed disulfide bonds. Thedotted box connects four cysteines that are proposed to form two additional disulfide bonds but among which we were unable to predict the pattern of pairing. The black circlesindicate two histidines (120 and 213) that are suggested by Clyne et al. (2002) to form part of a site that binds zinc (Zn). Thehatched circles indicate three lysines (69, 71, and 308) suggested to bind to the phosphates of ATP (asterisks) (Jiang et al., 2000; Ennion et al., 2002). The transmembrane domains (residues F31 to V51 and I331 to L353) are represented ascylinders, all other amino acids are indicated asgray circles, and the threeN-glycosylation sites of P2X2 are shown asY shapes.
Footnotes
This work was supported by National Institutes of Health Grant NS-039196. We thank Richard Evans for sharing data reported by Ennion et al. (2002) before publication and thank the members of the Hume laboratory for comments on this manuscript.
Correspondence should be addressed to Richard I. Hume, Department of Molecular, Cellular, and Developmental Biology, 3095 Natural Science Building, 830 North University Avenue, Ann Arbor, MI 48109. E-mail:rhume@umich.edu.
REFERENCES
- 1.Acuna-Castillo C, Morales B, Huidobro-Toro JP. Zinc and copper modulate differentially the P2X4 receptor. J Neurochem. 2000;74:1529–1537. doi: 10.1046/j.1471-4159.2000.0741529.x. [DOI] [PubMed] [Google Scholar]
- 2.Barnard EA, Simon J, Webb TE. Nucleotide receptors in the nervous system: an abundant component using diverse transduction mechanisms. Mol Neurobiol. 1997;15:103–129. doi: 10.1007/BF02740631. [DOI] [PubMed] [Google Scholar]
- 3.Brake AJ, Julius D. Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- 4.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 5.Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Current status of purinergic signalling in the nervous system. Prog Brain Res. 1999;120:3–10. doi: 10.1016/s0079-6123(08)63541-4. [DOI] [PubMed] [Google Scholar]
- 8.Cho HC, Tsushima RG, Nguyen TT, Guy HR, Backx PH. Two critical cysteine residues implicated in disulfide bond formation and proper folding of Kir2. Biochemistry. 2000;39:4649–4657. doi: 10.1021/bi992469g. [DOI] [PubMed] [Google Scholar]
- 9.Christianson DW. Structural biology of zinc. Adv Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- 10.Clyne JD, LaPointe LD, Hume RI. The role of histidine residues in modulation of the rat P2X2 receptor by zinc and pH. J Physiol (Lond) 2002;539:347–359. doi: 10.1113/jphysiol.2001.013244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornell NW, Crivaro KE. Stability constant for the zinc-dithiothreitol complex. Anal Biochem. 1972;47:203–208. doi: 10.1016/0003-2697(72)90293-x. [DOI] [PubMed] [Google Scholar]
- 12.Creighton TE. Disulphide bonds and protein stability. BioEssays. 1988;8:57–63. doi: 10.1002/bies.950080204. [DOI] [PubMed] [Google Scholar]
- 13.Egan TM, Haines WR, Voigt MM. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J Neurosci. 1998;18:2350–2359. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ennion SJ, Evans RJ. Conserved cysteine residues in the extracellular loop of the human P2X1 receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol Pharmacol. 2002;61:1–10. doi: 10.1124/mol.61.2.303. [DOI] [PubMed] [Google Scholar]
- 15.Ennion S, Hagan S, Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem. 2000;275:29361–29367. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund P-E, Stuhmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- 17.Hansen MA, Barden JA, Balcar VJ, Keay KA, Bennett MR. Structural motif and characteristics of the extracellular domain of P2X receptors. Biochem Biophys Res Commun. 1997;236:670–675. doi: 10.1006/bbrc.1997.6815. [DOI] [PubMed] [Google Scholar]
- 18.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 19.Jiang LH, Rassendren F, Surprenant A, North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J Biol Chem. 2000;275:34190–34196. doi: 10.1074/jbc.M005481200. [DOI] [PubMed] [Google Scholar]
- 20.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Neurosci Rev. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 21.King BF, Ziganshina LE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br J Pharmacol. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br J Pharmacol. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King BF, Townsend-Nicholson A, Wildman SS, Thomas T, Spyer KM, Burnstock G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J Neurosci. 2000;20:4871–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 26.Kurz LL, Klink H, Jakob I, Kuchenbecker M, Benz S, Lehmann-Horn F, Rudel R. Identification of three cysteines as targets for the Zn2+ blockade of the human skeletal muscle chloride channel. J Biol Chem. 1999;274:11687–11692. doi: 10.1074/jbc.274.17.11687. [DOI] [PubMed] [Google Scholar]
- 27.Le K-T, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le K-T, Boue-Grabot E, Archambault V, Seguela P. Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J Biol Chem. 1999;274:15415–15419. doi: 10.1074/jbc.274.22.15415. [DOI] [PubMed] [Google Scholar]
- 29.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Peoples RW, Weight FF. Enhancement of ATP-activated current by protons in dorsal root ganglion neurons. Pflügers Arch. 1997;433:446–454. doi: 10.1007/s004240050299. [DOI] [PubMed] [Google Scholar]
- 31.Miller KJ, Michel AD, Chessell IP, Humphrey PP. Cibacron blue allosterically modulates the rat P2X4 receptor. Neuropharmacology. 1998;37:1579–1586. doi: 10.1016/s0028-3908(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 32.Nakazawa K, Ohno Y. Effects of neuroamines and divalent cations on cloned and mutated ATP-gated channels. Eur J Pharmacol. 1997;325:101–108. doi: 10.1016/s0014-2999(97)00107-6. [DOI] [PubMed] [Google Scholar]
- 33.Nakazawa K, Liu M, Inoue K, Ohno Y. pH dependence of facilitation by neurotransmitters and divalent cations of P2X2 purinoceptor/channels. Eur J Pharmacol. 1997;337:309–314. doi: 10.1016/s0014-2999(97)01293-4. [DOI] [PubMed] [Google Scholar]
- 34.Newbolt A, Stoop R, Virginio C, Surprenant A, North RA, Buell G, Rassendren F. Membrane topology of an ATP-gated ion channel (P2X receptor). J Biol Chem. 1998;273:15177–15182. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- 35.Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 37.Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 1997;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seguela P, Haghighi A, Soghomonian J-J, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North RA. Contribution of individual subunits to the multimeric P2X2 receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- 40. Torres GE, Egan TM, Voigt MM. Topological analysis of the ATP-gated ionotropic [correction of ionotrophic] P2X2 receptor subunit. FEBS Lett 425 1998a. 19 23[Erratum (1998) 427:152]. [DOI] [PubMed] [Google Scholar]
- 41.Torres GE, Haines WR, Egan TM, Voigt MM. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Cell Biol. 1998b;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- 42.Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits: specificities exist with regard to possible partners. J Biol Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- 43.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 44.Vallejo AN, Pogulis RJ, Pease LR. Mutagenesis and synthesis of novel recombinant genes using PCR. In: Dieffenbach CW, Dveksler GS, editors. PCR primer: a laboratory manual. Cold Spring Harbor Laboratory; Plainview, NY: 1995. pp. 603–612. [Google Scholar]
- 45.Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wildman SS, King BF, Burnstock G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. Br J Pharmacol. 1999a;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br J Pharmacol. 1999b;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- 49.Zhong Y, Dunn PM, Burnstock G. Pharmacological comparison of P2X receptors on rat coeliac, mouse coeliac and mouse pelvic ganglion neurons. Neuropharmacology. 2000;39:172–180. doi: 10.1016/s0028-3908(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z, Hume RI. Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. J Physiol (Lond) 1998;507:353–364. doi: 10.1111/j.1469-7793.1998.353bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Z, Monsma LR, Hume RI. Identification of a site that modifies desensitization of P2X2 receptors. Biochem Biophys Res Commun. 1998;252:541–545. doi: 10.1006/bbrc.1998.9689. [DOI] [PubMed] [Google Scholar]