Abstract
The molecular mechanisms underlying the contact behavior of Schwann cells (SCs) and SC–axon association are poorly understood. SC–SC and SC–axon interactions were studied using purified adult rat SCs and cocultures of SCs with embryonic dorsal root ganglion neurons. After contact of SCs with axons, SCs start to extend processes in alignment with axons. This unique alignment was quantitated using a new assay. SC–axon alignment and SC–SC band formation were disrupted in medium containing low extracellular calcium, indicating the involvement of calcium-dependent adhesion molecules. N-cadherin expression was strong in developing rat sciatic nerves but weak in adult sciatic nerves. In purified adult-derived rat SCs, N-cadherin expression was increased by mitogens (neuregulins) and decreased by high cell density. High-resolution confocal images show intense N-cadherin signals in SC process tips. Subcellular N-cadherin was accumulated in bands at intercellular junctions between SCs and was clustered at axon–SC contact sites. Blocking antibodies (rabbit and guinea pig IgG directed against the first extracellular domain of N-cadherin) and cyclic pentapeptides (including the HAV motif) were used to perturb N-cadherin function. All blocking agents reduced the number of N-cadherin-positive SC–SC junctions and perturbed axon-aligned growth of SC processes. Averaging over all N-cadherin-perturbation experiments, in controls 67–86% of SCs exhibited axon-aligned process growth, whereas in treated cultures only 41% of the SCs aligned with axons. These results are evidence that in mammals N-cadherin is important for formation of SC–SC junctions and SC process growth in alignment with axons.
Keywords: cell adhesion molecule, N-cadherin, neuregulin, axon–glia interaction, subcellular localization, function-blocking agents, Schwann cell–DRG neuron coculture
A defining property of Schwann cells (SCs) is their unique association with axons. At an early stage of nerve formation, developing SCs associate with axons, creating an interface of glial membrane in close apposition with axonal membrane (Peters and Muir, 1959; Prestige and Wilson, 1980; Ziskind-Conheim, 1988; Jessen et al., 1994). This close apposition of axonal and SC membranes allows bi-directional signaling between neurons and SCs (Salzer et al., 1980a,b). As nerve development proceeds, the SC exists in a highly dynamic relationship with axons as SCs proliferate, extend, and retract their processes. Eventually, SCs are segregated into mature SC–axon units (Martin and Webster, 1973; Webster et al., 1973). Throughout this process, association with axons is always maintained. If axon–SC association is disrupted, for example by injury, it must be reestablished after contact of denervated SCs with the regenerating axons.
Despite the advance in our knowledge of SC biology in recent years, our understanding of the molecular mechanisms underlying the association of SCs with axons remains deficient. In rodents, one candidate for mediating SC–axon attachment is L1 (Seilheimer et al., 1989; Wood et al., 1990). However, recent evidence from studies on L1 knock-out mice suggest that L1 cannot be the only molecule involved in initiating and maintaining this association (Dahme et al., 1997; Haney et al., 1999). In addition, antibodies to L1 did not block SC–axon interactions in chicken cells (Letourneau et al., 1990). Evidence for a role for the calcium-dependent adhesion molecule N-cadherin in SC–axon interaction has been reported in cultured chicken dorsal root ganglia (DRGs) (Letourneau et al., 1990, 1991). N-cadherin has also been implicated in promoting neuronal outgrowth of chick ciliary ganglion neurons on SCs, but its role in SC process outgrowth and alignment with axons was not addressed (Bixby et al., 1988; Seilheimer and Schachner, 1988; Bixby and Zhang, 1990). The molecular basis of mammalian SC behavior on their first contact with axons has received little attention.
The experiments reported here were designed to assess the role of N-cadherin in the formation of contacts between SCs and in the establishment of SC–axon association, characterized by assaying SC orientation in alignment with axons. Dissociated, purified, and mitogen-treated adult rat SCs were added to cultures of purified embryonic rat DRG neurons and studied over a 2 d period. N-cadherin was localized at the axon–SC interface and at contact areas of neighboring SCs. Using a quantitative alignment assay, we analyzed SC association with axons showing that SC processes were prevented from associating with axons both by lowering calcium levels in the medium and by digesting cadherins from SC surfaces. Treating cultures with N-cadherin blocking antibodies and specific N-cadherin binding peptides prevented N-cadherin band formation in SCs and thus their organization of networks. In addition, the treatments reduced the percentage of SCs associating with axons. These data provide evidence that N-cadherin mediates SC–SC interaction and the initial growth of SC processes in alignment with axons, an association that is prerequisite for contact-dependent signaling in SC proliferation and differentiation (Salzer et al., 1980a,b).
MATERIALS AND METHODS
Schwann cell purification and culture
Segments of adult rat sciatic nerves were explanted in 35 mm culture dishes in D10 medium [DMEM (Invitrogen) plus 10% heat inactivated fetal bovine serum (FBS; Hyclone)] and re-explanted on new dishes until migrating cells consisted mostly of SCs. After 2 weeks, explants were incubated in 0.25% dispase (Roche) and 0.05% collagenase (Worthington) in DMEM. Explants were dissociated by trituration, and the cells were plated on poly-l-lysine substrate (PLL; 200 μg/ml; Invitrogen) in D10 medium supplemented with a mixture of mitogens consisting of 2 μm forskolin (Sigma), 20 μg/ml bovine pituitary extract (Biomedical Technologies), and 2.5 nm heregulin (Genentech Inc.). After 1 week, cells were digested in trypsin, and the cell suspension was incubated for 30 min with conditioned medium from mouse hybridoma cells (TIB-103, American Type Culture Collection) containing anti-Thy 1.1 and subsequently with rabbit complement (ICN) to remove remaining fibroblasts. The highly purified SCs were cultured on PLL-coated Petri dishes in D10 medium with mitogen mixture (Kleitman et al., 1998).
Purified dorsal root ganglion primary cultures
DRGs were dissected from rat embryos on the 15th day of gestation, considering the day of conception as day zero, and then incubated for 45 min in 0.25% trypsin (Worthington) in calcium- and magnesium-free HBSS (Invitrogen) at 37°C. The DRGs were then dissociated by gentle trituration and subsequently seeded on six-well glass slides (Cel-Line/Erie Scientific Co.). The six 10 mm glass wells (78 mm2) were pretreated with 200 μg/ml PLL and coated with 4 μl of dialyzed collagen (prepared from rat tail) in the presence of ammonia vapors to polymerize the collagen. The DRG cell suspension was seeded at a concentration of 5,000–10,000 cells per 20 μl in the center of each 78 mm2 well. Alternatively, pretreated 1 inch (500 mm2) dishes made from fluorocarbon Aclar (33C, Allied Fibers and Plastics) were coated with collagen and ammoniated, and subsequently 25,000 cells were seeded in the middle of the Aclar dish. All cultures were kept in neurobasal medium with B27 supplement (Invitrogen) and 10 ng/ml nerve growth factor (extracted from mouse submaxillary glands and partially purified using Sephadex G100 chromatography). Within the first 3 weeks, the DRG cell cultures received three treatments of 10 μm 5-fluoro2′deoxyuridine (Sigma) to produce pure neuronal cultures (DRG neuron cultures) (Kleitman et al., 1998). During this treatment period, a network of DRG axons was produced. The density of axons varied depending on the number of cells seeded (∼5,000 to ∼10,000 cells per 20 μl in the center of each 10 mm glass well).
Cocultures of Schwann cells and DRG neurons and function-blocking experiments
Live labeling of cultured SCs. Living SCs were stained with 6.5 μm Cell Tracker Green (5-chloro-methylfluorescein diacetate, Molecular Probes) in DMEM containing 0.05% Pluronic (Molecular Probes). The labeled SCs were removed from the culture dish by trypsin digestion (see below).
SC harvest protocols. SCs were digested for 3 min with 0.05% trypsin (Invitrogen) in calcium- and magnesium-free HBSS containing 0.02% ETDA at room temperature (RT). Alternatively, SCs were incubated for 10–15 min at 37°C with 0.05% trypsin (Worthington) in HBSS (Invitrogen) in the presence of 1 mm Ca2+ and 1.88 mm Mg2+(TCa2+). This harvesting protocol protects cadherins on the cell surface (Volk and Geiger, 1986; Takeichi, 1988) and was used for observing SC–axon interaction within the first hours of coculturing (time-lapse imaging). For experiments performed to analyze cadherin replenishment in SCs and SC adhesion in the presence or absence of cadherins on the cell surface, SCs were harvested by digestion with 0.05% trypsin and 0.02% ETDA in calcium- and magnesium-free HBSS (TE) for 10 min at 37°C (removing cadherins from cell surface) or in TCa2+ (protecting cadherins on cell surface, see above). Protein lysates were prepared from the differently harvested SC suspensions immediately and after 4 and 24 hr of culturing.
SC–SC and SC–axon association in normal and low calcium. For analyzing SC–SC association, TE- and TCa2+-treated SC suspensions were plated at a density of ∼10,000 SCs per 78 mm2on ammoniated collagen-coated slides in defined modified N2 medium (Bottenstein et al., 1979) using MEM/F12 (1:1; Invitrogen) with 1% heat-inactivated FBS. The calcium concentration in this medium was 1.22 mm Ca2+. For SC–axon cocultures, ∼3000 labeled SCs were seeded on DRG neuron cultures, which had developed an extensive axonal network within 3–4 weeks in vitro. The cocultures were kept in N2 with or without adding 1% FBS. Low calcium N2 medium was obtained using S-MEM (no calcium added; Invitrogen)/F12 (1:1). The calcium concentration was 0.15 mm; when supplemented with 1% FBS, the concentration was 0.22 mmCa2+. Approximately twofold as many SCs as DRG neurons were seeded, varying between 1.6- and 3.2-fold. This variation did not change the percentage of SCs aligned to axons. SC–DRG cocultures were analyzed for N-cadherin presence and process outgrowth in alignment with axons (see below).
N-cadherin function-blocking experiments. N-cadherin function-perturbing agents, including two different N-cadherin blocking antibodies and cyclic pentapeptides interfering with N-cadherin binding, were used in SC–SC adhesion and SC–axon alignment assays as described below. Cyclic pentapeptides CHAVC and CHGVC were received from Adherex Technologies (Ottawa, Canada) (Williams et al., 2000). CHAVC includes the CAR sequence histidine–alanine–valine (HAV); the presumptive N-cadherin interaction region, CHGVC, is a nonbinding control peptide (HGV) (Blaschuk et al., 1990). HAV was shown to block N-cadherin-mediated adhesion in a dose-dependent manner with half-maximal inhibition at 0.32 mm (Williams et al., 2000). HAV and HGV were used at concentrations between 0.5 and 0.75 mg/ml, respectively.
A rabbit polyclonal serum raised against the HAV sequence (L7) and a control serum raised against the cytoplasmic domain of N-cadherin (L4) were obtained from Adherex Technologies (Alexander et al., 1993). L7 has been shown to block N-cadherin function in endothelial and glial cells (Alexander et al., 1993; Wilby et al., 1999; Schnädelbach et al., 2000). IgG fractions were purified from the rabbit sera by chromatography using protein A-loaded Sepharose affinity columns (HiTrap, Amersham Biosciences) at pH 4.5 and subsequently desalted by exchange centrifugation (Centricons YM-30, Millipore). Purified IgGs had a concentration of ∼7 mg IgG/ml 3 mmNaHPO4, 17 mmNa2HPO4, pH 7. The potency of the purified IgGs was tested using the aggregation of N-cadherin-transfected L-cells (NC+L-cells; provided by D. Colman, Mt. Sinai, NY) (Shan et al., 2000). A guinea pig antibody, gp1260, which was raised against the crystallized N-terminal fragment of N-cadherin, and a guinea pig preimmune serum were kindly provided by G. Huntley (Mt. Sinai, NY) and D. Colman (Fannon and Colman, 1996). This antibody was shown to effectively block the aggregation of NC+L-cells and inhibited the function of synaptic N-cadherin in the late phase of long-term potentiation in hippocampal slices (Bozdagi et al., 2000).
DRG cultures were kept overnight in low calcium N2 before SCs were added together with the function-blocking agents in normal calcium N2. SC–SC cultures and SC–DRG cocultures were incubated for 4 hr in the presence of 0.5–0.75 mg of HAV or HGV peptides per milliliter of N2. The cultures were treated for 24 hr with function-blocking antibodies. Guinea pig IgG, gp1260, and its preimmune serum were diluted 1:100; L7 or L4 IgG was used at dilutions of 1:50 to 1:100 (final concentration of ∼70–140 μg IgG per milliliter N2 medium), respectively.
Immunofluorescence
Cultures were fixed for 25 min in 4% paraformaldehyde in Tris-buffered saline (TBS; 1 mmCa2+), permeabilized for 5 min using 0.3% Triton X-100 (Sigma), and blocked in 10% normal donkey serum (NDS;Jackson ImmunoResearch) in TBS. Subsequently, cultures were incubated overnight in mouse monoclonal antibody raised against the cytoplasmic domain of N-cadherin (1:500; BD Transduction) together with rabbit anti-neurofilament M (1:1000 R13; gift by G. Shaw, University of Florida, Gainesville, FL) or rabbit anti-S100 (1:700; Dako). After rinsing three times for 10 min in TBS, cultures were incubated with secondary antibodies: donkey anti-mouse-Cy3 (1:500 in 10% NDS-TBS) and donkey anti-rabbit-Cy5 (1:100; both from Jackson ImmunoResearch) for 45 min at RT. After three rinses in TBS and a final rinse in H2O, cultures were dried and mounted in Vectashield (H-100, Vector).
Quantification and densitometry
Quantification of SC-alignment with axons. SC–DRG cocultures were analyzed by counting axon-aligned SCs. A SC with two or more processes aligned to axons over the entire length of their extensions was scored as an axon-aligned SC. Assessing alignment of SCs to axons was done in two ways. (1) Axon-aligned SCs were counted on an IX70 inverted microscope (Olympus) with a 40× (0.6 numerical aperture) objective using a broad bandpass filter (BP) permissive to green (SCs) and red (axons) fluorescence. (2) Green (SCs) and red (axons) images were captured separately using a CCD camera (DEI-750, Optronics) and narrow bandpass filters for each fluorescence channel, assuring that selection of fields was not biased for SC–axon alignment. At least 10 sets of green and red images were overlaid to count axon-aligned SCs in each culture; four cultures for each condition were analyzed, blinded toward treatment. Between 750 and 1000 SCs were scored for alignment under each condition analyzed. The results were expressed as percentages of axon-aligned SCs in each culture and statistically analyzed using t test and multiple comparisons a posteriori according to Tukey and Kramer (GraphPad InStat).
Quantification of SC network formation. The blockage of SC network formation was quantified in SC-only cultures by counting single SCs and SCs contacting neighboring SCs (SCs in groups). The number of N-cadherin-positive bands between SCs arranged in groups was counted in four experiments, by an observer blinded toward culture treatment, and divided by the number of SCs found in groups: this ratio is referred to as “adhesion factor” (see Table 1). Means were compared using Tukey–Kramer multiple comparisons (GraphPad InStat).
Table 1.
SC adhesion and network formation are perturbed when N-cadherin function is blocked
| Culturen (experiment) | Condition | Number of single SCs per culture | Number of SCs in groups per culture | Number of N-cadherin contacts in SCs in groups | Adhesion factor N-cadherin contacts per SCs in groups |
|---|---|---|---|---|---|
| 4 (1) | N2 | 225 | 466 | 229 | 0.49 ± 0.09 |
| 3 (1) | Low Ca2+ | 201 | 77 | 5 | 0.08 ± 0.07 |
| 3 (1) | gp1260 | 168 | 82 | 26 | 0.33 ± 0.03 |
| 3 (1) | gp preim. | 187 | 331 | 183 | 0.46 ± 0.05 |
| 2 (2) | N2 | 213 | 732 | 347 | 0.47 ± 0.07 |
| 2 (2) | Low Ca2+ | 402 | 162 | 11 | 0.07 ± 0.01 |
| 2 (2) | L4 | 110 | 337 | 167 | 0.5 ± 0.05 |
| 3 (2) | Rabbit IgG | 109 | 575 | 254 | 0.44 ± 0.02 |
| 3 (2) | L7 | 159 | 647 | 131 | 0.22 ± 0.06 |
SCs were counted as single cells or as cells in groups, and the number of N-cadherin positive cell–cell contacts among grouped SCs was determined. SC cultures were treated for 24 hr with L7 IgG, L4 IgG, gp1260, and preimmune serum (gp preim.), and control cultures (N2) as well as low calcium (low Ca2+) treated cultures were included. The “adhesion factor” was determined by dividing the number of N-cadherin-positive cell–cell contacts by the number of SCs in groups. Shown are means with SDs. The adhesion factor was significantly different in low calcium-, gp1260-, and L7-treated cultures compared with controls (N2, gp preim., L4, rabbit IgG;p < 0.01, Tukey–Kramer, multiple comparisons). SC cultures in low calcium had more single SCs than controls.
Densitometry. Western blots were analyzed using the Fluo-S Multi-Imager system (Bio-Rad). Relative N-cadherin protein amounts were determined by measuring the background-corrected N-cadherin signal value (optical density multiplied by area of band) divided by the background-corrected signal of the β-actin band detected in the same lane.
SDS-PAGE and Western blotting
All samples were homogenized in Lämmeli sample buffer (2% SDS, 10% glycerol, 0.1% bromophenol blue, 1 mmCaCl2, 125 mm Tris-HCl, pH 6.8), boiled for 5 min, and then frozen at −80°C. Protein concentration was measured using DC Protein assay (Bio-Rad). Positive controls were protein lysates of epidermoid carcinoma cell line A 431 (E-cadherin), rat brain (N-cadherin, R-cadherin), mouse neonate (M-cadherin) (all from BD Transduction), and Madin-Darby canine kidney (MDCK) epithelial cells (K-cadherin). Samples of 15–20 μg of protein per lane were loaded under denaturing conditions (1% β mercaptoethanol, 0.1% SDS) and separated on 7.5% acrylamide gels for 1.25 hr at 140 V. The proteins were blotted on nitrocellulose (Hybond C, Amersham Biosciences) and visualized by staining in 0.1% Ponceau S in 5% acetic acid. Membranes were blocked for 1 hr using 10% dry milk (Carnation) in TBS containing 0.05% Tween 20 (Fisher; TBST) and then incubated overnight at 4°C in primary antibodies diluted in 10% dry milk/TBST. Monoclonal antibodies were anti N-cadherin (1:3500), anti M-cadherin (1:200), anti E-cadherin (1:3000), anti R-cadherin (1:1000) (all from BD Transduction), and anti-β-actin (1:50,000; Sigma). A goat polyclonal antibody was used against K-cadherin (1:100; Santa Cruz Biotechnology). After rinsing, blots were incubated for 1 hr at RT in anti-mouse-HRP (1:10,000; Promega) or rabbit anti-goat-HRP (1:20,000; Pierce) in 10% dry milk/TBST. After further rinses, the Western blot chemiluminescence reagent (Renaissance, NEN) was incubated for 1 min at RT, and signals were detected by 1–2 min exposures of X-OMAT-LS films (Kodak) developed in an X-OMAT 2000 processor (Kodak).
Culture monitoring and confocal imaging
Live time-lapse imaging was performed on an enclosed Nikon microscope connected to a Paultek CCD camera and the SUN station image capturing system. TCa2+-treated SCs were seeded on axons of DRG neurons and observed at 37°C in 1:1 neurobasal medium and HBSS (containing 10 mm HEPES, pH 7.4) over the first hours of coculturing.
Confocal microscopy was performed on Olympus Fluoview 2.1.39 and ZeissLSM 510 microscopes. The recording parameters on the Olympus were as follows. For fluorescein, an argon laser (488 nm) and a BP filter (450–515 nm) were used; for Cy3, a krypton laser (568 nm) and a longpass filter (LP; 510 nm) were used. On the Zeiss confocal microscope, for Cell Tracker Green, an argon laser (488 nm) and BP 500–550 nm were used; for fluorochrome Cy3, a helium laser (543 nm) and BP 565–615 nm were used; and for Cy5, a helium laser (633 nm) and an LP (650 nm) were used. For most images the pinhole opening was <100 μm (Airie units 1), and the confocal scans were <1 μm thickness. The image resolution was ∼0.28 μm for horizontal scanning (XY plane) and ∼0.8 μm for orthogonal scanning (XZ plane) when using a 100× oil-immersion objective with 1.4 numerical aperture.
RESULTS
Steps of early association of Schwann cells with axons
The early stages of SC–axon association were observed by time-lapse monitoring and are represented in Figure1. Within the first hour after seeding on DRG neurons, most SCs adhered to axons and remained in contact with axons and acquired spindle-shaped, bipolar morphology. Some SCs adhered to the collagen substrate, migrated randomly and acquiring irregular shapes, and on contact with an axon bundle, these SCs changed their conformation and shortened their processes, as their cell bodies were drawn close to the axons (Fig. 1A). Immediately after axon contact, all SCs started to extend new processes possessing fan-shaped endings oriented along the axons (Fig. 1B,arrowheads). Then, most SCs acquired spindle-shaped morphology by growing two short processes along axons, with the SC soma slightly flattened and closely adjoined to the axon bundle (Fig.1C). However, SCs that encountered a network of axons going in different directions initially interacted with several axons by extending multiple short processes (Fig. 1D). Finally, within 24 hr SCs became embedded in the axon bundle, which sometimes appeared locally defasciculated. The SC morphology changed from displaying multiple processes contacting several axon bundles to the typical bipolar spindle-shape with flattened cell body and very long processes in alignment with axons. To better understand the molecular mechanism underlying the aligned growth of SC processes along axons, the role of the calcium-dependent adhesion molecule N-cadherin was studied. In the following experiments a new assay was used to analyze the role of N-cadherin by measuring quantitatively SC alignment along axons as an expression of SC–axon association.
Fig. 1.
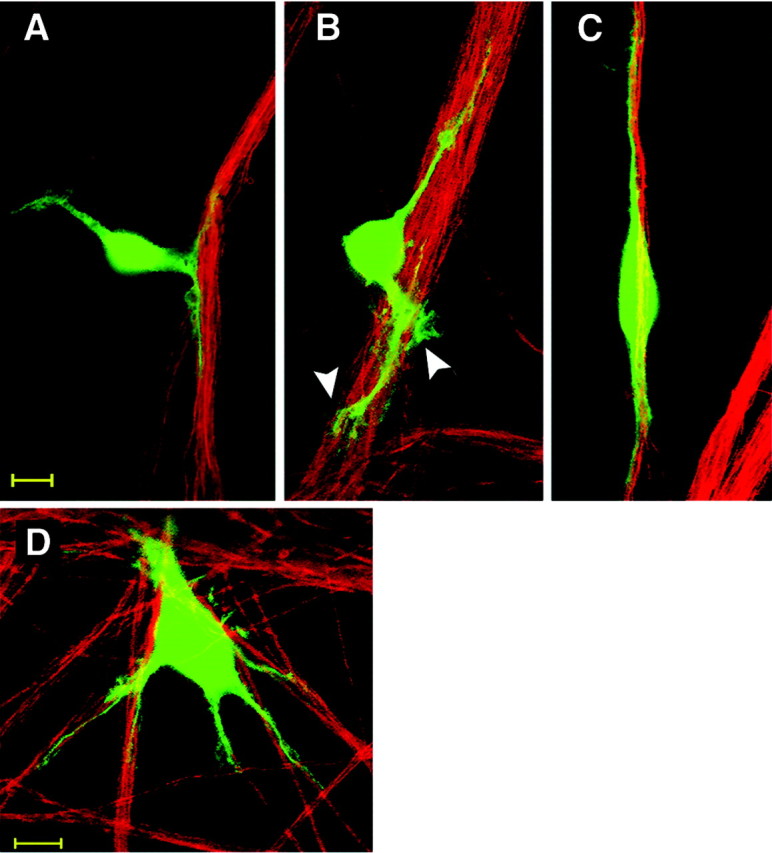
Schwann cell alignment with axons is achieved by oriented growth of SC processes. The displayed confocal images show early steps of SC–axon interaction that occur during the first hour of coculturing SCs with the axonal network of purified DRG neurons. Cell Tracker-labeled SCs (green) contact axons (immunostained for neurofilament M; red) by extending short processes (A). SC processes show fan-shaped lamellipodia (B, arrowheads). A spindle-shaped bipolar SC is shown with both processes aligned to axons (C). During these early stages, some SCs show multiple processes contacting various axon bundles (D); however, most cells display bipolar orientation within 1–2 hr in the presence of axons. Scale bar, 10 μm.
N-cadherin expression is strong in embryonic and weak in adult peripheral nerves
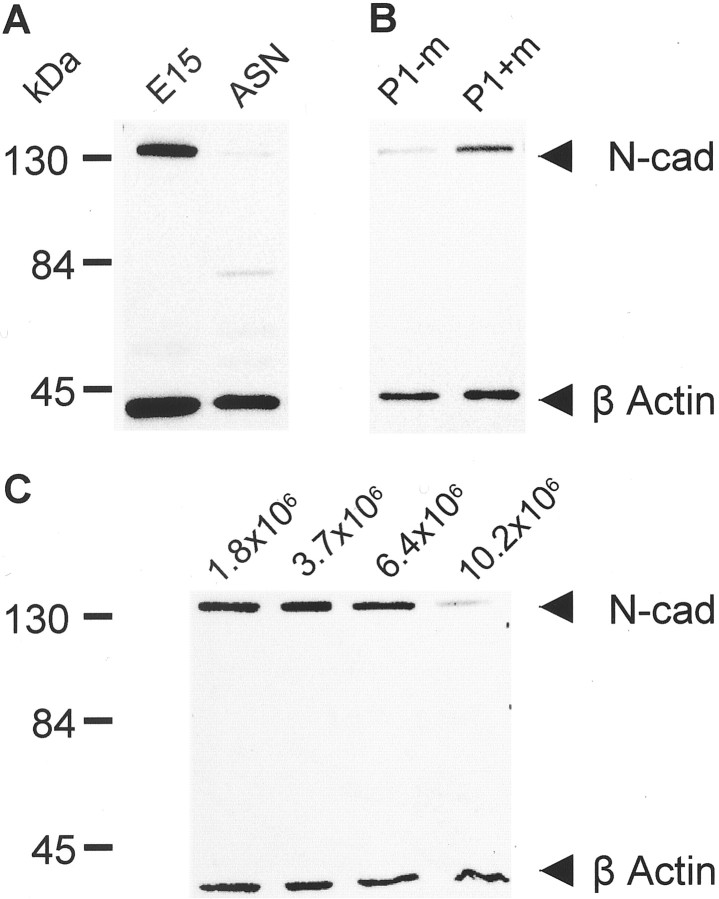
N-cadherin amounts in developing and adult rat peripheral nerves were compared by immunoblotting using protein samples from homogenized limbs of embryonic day (E) 15 rats and from adult sciatic nerves. Immunocytochemical staining of E15 frontlimb buds revealed that N-cadherin staining was restricted to peripheral nerve structures (I. Wanner, K. Jessen, P. Wood, unpublished data). The Western blots show a strong N-cadherin band in E15 limbs and a severalfold weaker band in adult sciatic nerves (Fig.2A).
Fig. 2.
N-cadherin is present in developing peripheral nerves and SCs cultured in the presence of mitogens. A, N-cadherin expression was analyzed in embryonic and adult peripheral nerves. N-cadherin is detected as a strong band at 130 kDa in embryonic limb (E15), but a much weaker signal is seen in adult sciatic nerve (ASN). B, N-cadherin protein amounts increase after culturing adult sciatic nerve-derived SCs in the presence of mitogens. Dissociated SCs from adult sciatic nerve explants (P1) were cultured for 3 d in the absence (P1-m) or presence (P1+m) of mitogens (forskolin, pituitary extract, and heregulin β1). The Western blots show weak N-cadherin immunoreactivity in SCs in the absence of mitogens (P1-m). Normalized values measured in four different autoradiograms using background-corrected ratios of N-cadherin divided by β-actin show a mean 1.3-fold increase in N-cadherin protein amounts after mitogen treatment (P1+m; p < 0.05; Tukey–Kramer, multiple comparisons) compared with SCs cultured without mitogens (P1-m). C, Cell density regulates N-cadherin protein amount in cultured rat SCs. Equal amounts of protein (15 μg per lane) from purified SCs of the same sciatic nerve preparation and passage number (P3) but cultured at various cell densities (1.8 × 106 SCs/100 mm2, subconfluent; 3.7 × 106 SCs/100 mm2, pattern forming; 6.4 × 106 SCs/100 mm2, confluent; and 10.5 × 106 SCs/100 mm2, over-confluent) in the presence of mitogens were analyzed by Western blotting. Strong N-cadherin signals are detected in samples of 1.8, 3.7, and 6.4 × 106SCs/100 mm2, but a weak signal is seen in cells grown at a density of 10.5 × 106 cells/100 mm2. Desitometric analysis shows that N-cadherin protein amount drops more then threefold in over-confluent versus confluent or subconfluent SC cultures.
Adult-derived SCs express N-cadherin, and the expression is regulated by mitogens and cell density
SCs obtained by dissociating cultured adult rat sciatic nerve explants also show low levels of N-cadherin (Fig.2B). In contrast, SCs cultured in the presence of a mixture of mitogens consisting of forskolin, pituitary extract, and recombinant heregulin β1 (177–244) and 10% bovine serum showed prominent N-cadherin expression. SCs were cultured directly after dissociation of sciatic nerve explants for 3 d in the presence or absence of mitogens to test their effect on N-cadherin expression. Protein extracts of these cultures were analyzed along with samples from SCs cultured for 3 weeks in the presence of mitogens. Protein samples from these three conditions were analyzed together with lysates of sciatic nerves from adult rats and probed for N-cadherin and β-actin. This analysis showed similar amounts of N-cadherin in adult sciatic nerves and SCs cultured without mitogens. However, the N-cadherin signal increased significantly in SCs cultured in the presence of mitogens after 3 d (Fig. 2B), and continued culturing with mitogens increased N-cadherin amounts up to twofold compared with SCs without mitogens (data not shown).
Schwann cells in culture organized themselves in networks forming a swirling pattern. Typically, this pattern appeared at a density of ∼3 × 106 cells in a 100 mm PLL-coated Petri dish. However, when the cell density reached 9 × 106 cells or more, the swirling pattern disappeared. Moreover, SCs that were harvested from such dense cultures and seeded together with DRG neurons showed impaired interactions with axons. SC processes were shorter and alignment with axons occurred later compared with low density-derived SCs (data not shown). When SCs derived from such dense cultures were dissociated and replated for 1 d at lower density, they exhibited low N-cadherin immunoreactivity (data not shown). Thus, the expression of N-cadherin in SCs cultured at different cell densities was assessed using Western blotting.
Cultures were defined as subconfluent if the SCs had not yet formed swirls (lysates were taken from cultures with ∼1.8 × 106 cells on a 100 mm PLL-coated Petri dish). Cultures were pattern-forming when swirls were seen (samples were taken from cultures containing 3.7 × 106 cells/100 mm dish). Cultures were considered confluent at a density of 5–6 × 106 cells (samples were harvested from a culture of 6.4 × 106 cells/100 mm dish), and cultures were over-confluent when the swirling pattern was lost but the cells had not detached from the substrate (samples were taken from cultures with 10.5 × 106cells/100 mm dish). In SC samples from over-confluent cultures, the N-cadherin amount (background-corrected ratios of N-cadherin to β-actin) was less than one-third of the amount in samples derived from subconfluent or confluent cultures (Fig. 2C). Further studies are needed to address the mechanisms underlying the mitogen-induced upregulation as well as the cell contact-dependent downregulation of N-cadherin protein amounts in rat SCs.
N-cadherin is localized at intercellular junctions between SCs and at SC–axon contact sites
The subcellular distribution of N-cadherin in SC and SC–neuron cultures was determined by high resolution confocal imaging. In SCs examined 6 hr (Fig. 3A) or 24 hr (Fig. 3B) after replating, N-cadherin was concentrated at SC membranes contacting neighboring cells, forming bands of attachment (Fig. 3A, arrow) or “button-shaped” junctions (Fig. 3B, arrowheads). The ultrastructural examination of N-cadherin-enriched “zipper”-like bands of apposing SC membranes showed multiple adherens junctions (our unpublished observation). In isolated SCs, N-cadherin accumulated at the process endings and in pseudopodia (Fig. 3A, arrowheads). These endings could also be stained with ezrin, a microvilli marker, which colocalized with N-cadherin (data not shown).
Fig. 3.
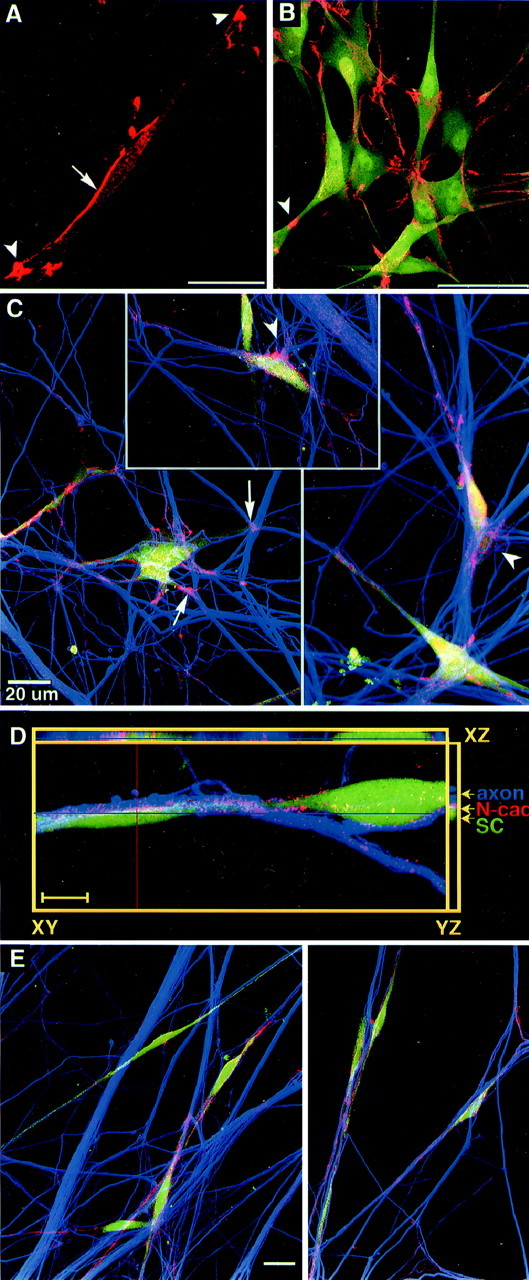
N-cadherin is localized at regions of SC and SC–axon contacts. A, B, Confocal images show SCs cultured 6 hr (A) and 24 hr (B) on ammoniated collagen that were immunostained for N-cadherin (red) and S100 (green). An intense band of N-cadherin is seen at intercellular junctions between SCs (A,arrow). Strong N-cadherin signals are detected in process tips of SCs (A, arrowheads; scale bar, 25 μm) and at cell–cell contact areas (B, arrowhead; scale bar, 50 μm). C, N-cadherin localization is shown in SC–DRG cocultures 24 hr after seeding SCs onto DRG neurons. Confocal images show vitally stained SCs (Cell Tracker,green), immunostained axons (anti-neurofilament M, Cy5; color coded in blue), and N-cadherin (red/pink/yellow). N-cadherin is accumulated at sites of contact with axons (arrows) and at filopodia and lamellipodia of SC processes (arrowheads). D, N-cadherin (red) is shown in an SC (green) associated with a small axon bundle (blue) after 24 hr of coculturing with DRG neurons. Out of a series of five optical sections, one confocal section of 0.4 μm is shown (XY,center). Orthogonal sectioning through the stack of all five images (depth 1.6 μm) is shown in horizontal (XZ) and vertical (YZ) planes. In all three planes, intense accumulation of N-cadherin (red) between SC process (green) and axons (blue,arrowheads) is seen in all areas of contact. Scale bar, 10 μm. E, SCs are in alignment with axons after 4 d of coculturing with DRG neurons. Confocal images show triple labeling using Cell Tracker to stain SCs (green) and anti-neurofilament M antibody to label axons (blue) and anti-N-cadherin (red). N-cadherin immunofluorescence is weaker and more evenly distributed than after 24 hr. This image was recorded using higher detector voltage compared with Cto increase signal intensity for N-cadherin. Scale bar, 20 μm.
During the early stage of contact with axons, SCs had strong N-cadherin staining (Fig. 3C). N-cadherin was accumulated at sites of contact with axons in fan-shaped cytoplasma protrusions and lamellipodia (Fig. 3C, arrowheads). At 24 hr, when SCs were aligned with axons, N-cadherin was localized at axon–SC contact sites displayed by high resolution (∼200 nm) horizontal (xy-axis) and vertical confocal sectioning through the culture (xz-axis) (Fig. 3D). N-cadherin did not appear to be colocalized with the SC marker (Cell Tracker,green) or neurofilament intermediate (blue), because these markers were localized to the cytoplasm of SCs and axons, whereas N-cadherin was membrane associated. For the same reason it was impossible to determine whether N-cadherin staining was of neuronal or SC origin; presumably N-cadherin from both membranes was interacting in a homophilic manner. In 1 d SC–DRG cocultures, adherens junctions were found at juxtaposed SC–axon membranes (our unpublished data).
After 2–4 d of coculture, N-cadherin staining of SCs appeared to be less intense, and the brightly stained foci disappeared; N-cadherin appeared to be more evenly distributed on membranes contacting axons (Fig. 3E). The 4 d coculture shown in Figure3E shows immunostaining performed in parallel to 1 d cocultures depicted in Figure 3C.
These confocal images show that N-cadherin was present at cell–cell contact zones between SCs and between SCs and axons during early stages of association. However, coincidentally with the morphological alterations of SCs, the subcellular distribution of N-cadherin changed from intensely clustered to sparsely distributed. The results suggest that N-cadherin may be important for SC network formation and could play a role in early SC–axon interaction.
Calcium is required for SC network formation and for the alignment of SCs with axons
Cadherin-mediated cell–cell adhesion can be inhibited by lowering the extracellular calcium concentration (Takeichi, 1988; Tamura et al., 1998). Millimolar Ca2+ concentrations are required for rigidifying the extracellular domains of cadherins; coordinate binding between these domains stabilizes interactions that promote lateral clustering and homophilic interaction with cadherins from opposing membranes (Shapiro and Colman, 1998; Tamura et al., 1998;Koch et al., 1999). Low-Ca2+ conditions are therefore considered to very effectively prevent strand-dimer formation. Thus, the role of cadherins in rat SC interaction with other SCs or with axons can be studied in vitro by reducing the calcium concentration in the medium from 1.0 to 0.15 or 0.22 mm Ca2+ (on addition of 1% FBS). Under control conditions, SCs extended long, straight processes contacting neighboring cells and forming a network (Fig.4A). At lower calcium ion concentration, some SCs failed to extend processes, whereas others extended shorter, bent processes; most of the SCs failed to form contacts with neighboring cells. Under low calcium conditions, N-cadherin appeared less intense and not concentrated at cell–cell contact areas (Fig. 4B, inset). These results suggest that SC network formation is strongly dependent on functional cadherins.
Fig. 4.
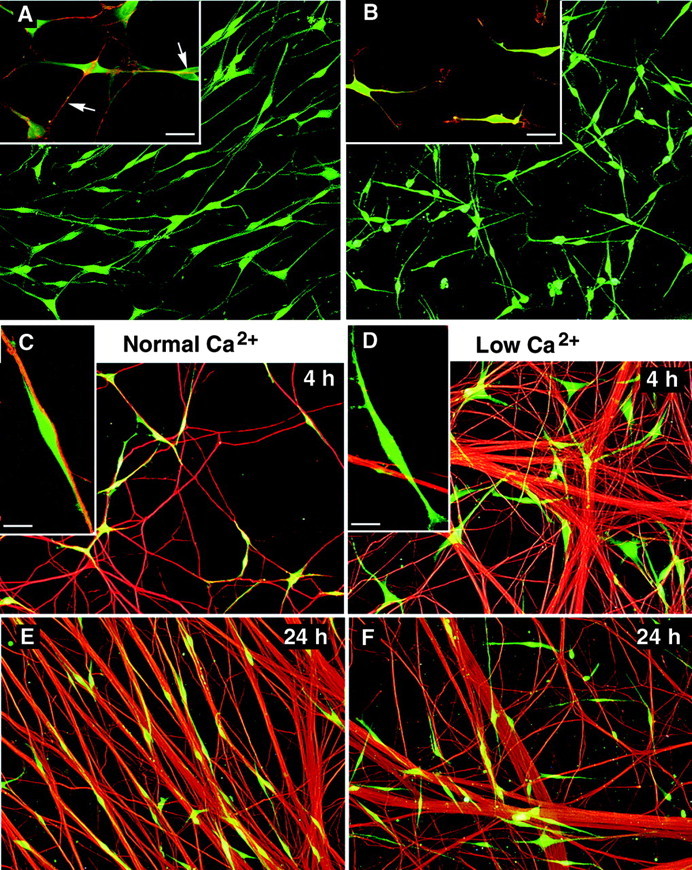
Calcium is required for SC–SC and SC–axon interaction. The contribution of cadherins to SC contact behavior is shown by the effect of lowering calcium in SC–SC cultures (A, B) and SC–DRG cocultures (C–F). A, Vitally labeled SCs (green) cultured for 24 hr in normal calcium (N2 medium, 1% FBS) are lined up in arrays.Inset in A shows SCs in normal calcium with intense N-cadherin-positive cell–cell contacts (red, arrows). B, The SC network is perturbed in low calcium (N2 medium, 1% FBS, 0.22 mm Ca2+), and SCs were not interacting with each other. B, Inset, SC processes failed to contact other SCs, and N-cadherin is shown more evenly distributed on the cell surface (red/yellow). Scale bar, 20 μm.C–F, SCs (Cell Tracker,green) were plated onto axons of DRG neurons (anti-neurofilament M, red) in either normal calcium levels (1.15 mm Ca2+; C,E) or low calcium levels (0.15 mmCa2+; D, F) medium. C, E, SCs are observed in alignment with axons at 4 hr (C) and 24 hr (E) after plating. C,Inset, Confocal image shows an SC adjoining a small axon bundle with its processes aligned. D, F, By comparison, SCs in low calcium medium fail to align with axons at either 4 hr (D) or 24 hr (F) of coculturing. D,Inset, One SC (green) not aligning with axons but crossing the axon bundle (red).
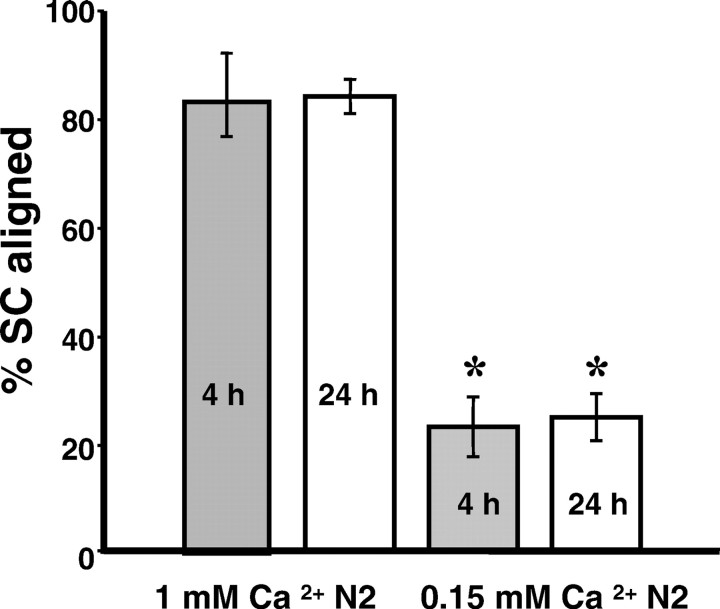
When SCs were added to DRG neurons at normal calcium levels, most of the SCs were seen in alignment to axons by 4 hr (Figs. 4C). Quantifying SCs aligned with axons gave similar results when counting was done on the microscope (90% SC aligned to axons; SD 5;n = 4) or by computer-assisted choice of fields and subsequent analysis of overlaid images (84% SCs aligned; SD 3;n = 4). Comparable percentages of SCs aligned to axons were found at 4 and 24 hr (Fig. 5) and 48 hr (data not shown) of coculturing. In low calcium medium, SCs first settled onto the neuron culture surface (substrate and axons) and then processes grew out not in alignment with axons. Instead, SCs migrated off the axons onto the substrate (in the presence of 1% FBS), and processes extended independently of axons. As a result, cells were located randomly in the culture dish at 4 and 24 hr, without being organized by the underlying neuronal networks (Fig.4D,F). Only a few SCs showed axon alignment under low calcium conditions (microscope: 26% SCs aligned, SD 5, n = 3; overlaid images: 25% SCs aligned, SD 4, n = 4). The lack of sufficient calcium ions prevented proper SC–axon association with the same efficiency from hours to days.
Fig. 5.
Quantification of SC–axon alignment under low calcium conditions. Means of percentages of SCs aligned to axons were plotted at 4 hr (gray) and 24 hr (white) under normal and low calcium conditions. In low calcium the percentages of SCs aligned with axons are decreased to one-fourth of the controls (normal calcium N2: 85% at 4 hr, 84% at 24 hr; low calcium N2: 23% at 4 hr, 25% at 24 hr; for each condition,n = 3). Error bars represent SDs. The differences between control and low calcium conditions were statistically significant at both time points (t test, two tailed; *p ≪ 0.001).
The neuronal networks in the images shown in Figure4C–F differ in axon density and fasciculation. We thus counted SC–axon alignment at different neuronal and SC densities. In a sparse control coculture, the average percentage of SCs aligned to axons was 90 ± 5% (SD; n = 3); in low calcium medium 26 ± 5% SCs were aligned (SD; n = 4). In dense SC–DRG cocultures in which five times as many SCs were seeded on a more compact axonal network of three times as many DRG neurons, on average 84 ± 3% (SD; n = 4) of all SCs were aligned in normal medium and 25 ± 4% (SD;n = 4) were aligned under low calcium conditions. This comparison shows that successful SC–axon association or its perturbation by decreased calcium levels was not affected by the differences in density of axons or SCs.
The blockage of SC–axon interaction caused by lowering the Ca2+ concentration was greatest when DRG cultures were kept overnight in low calcium medium. When DRG cultures were not pretreated, SCs were initially seen aligned to axons, and the number of axon-ignoring SCs increased only after 24 hr (data not shown). This finding implies that removal of calcium bound on neuronal cadherins and reversal of dimer formation with glial cadherins was slow. In addition, the blocking of alignment was more pronounced in low calcium conditions when cells were trypsinized in TE compared with TCa2+ digestion (see below). Only 19% of TE-harvested SCs were aligned compared with 35% of TCa2+-harvested SCs when cultured in low calcium with DRG neurons for 4 hr. This suggests that a small percentage of cells from TCa2+ harvest had preserved cadherin dimers with enough calcium bound to be able to interact with axons even under low calcium conditions. SC alignment with axons was not diminished when calcium levels >0.4 mmwere maintained (data not shown). In conclusion, the results show that SCs require at least 0.4 mm calcium ions to complete successful axon association.
Cadherin removal from the SC surface results in failure of SCs to form networks and reduces their ability to align with axons
Is there a difference in SC behavior in the absence or presence of cadherins on their surface? This question was addressed using enzymatic digestion conditions that protect or remove cadherins from the cell surface. Although other cell surface proteins are digested, cadherins are protected from trypsin proteolysis in the presence of at least 1 mm calcium (Volk and Geiger, 1986; Takeichi, 1988). Trypsin treatment in absence of calcium digests cadherins. The kinetics of cadherin replenishment in rat SCs was analyzed in protein samples immediately and 4 and 24 hr after TE or TCa2+ treatment. After a 10 min digestion in the absence of calcium, the full-length 130 kDa band of N-cadherin could no longer be detected (Fig.6A, TE); instead, an ∼66 kDa size N-cadherin fragment was detected. Four hours after TE treatment, half of the 130 kDa N-cadherin band was recovered, and the intact protein was almost fully restored by 24 hr (Fig.6A). In contrast, in the presence of calcium, a 10 min trypsin digestion did not lead to a loss of full-length N-cadherin, because the 130 kDa band was detected in cultured rat SCs with the same intensity at all times (Fig. 6A,TCa2+).
Fig. 6.
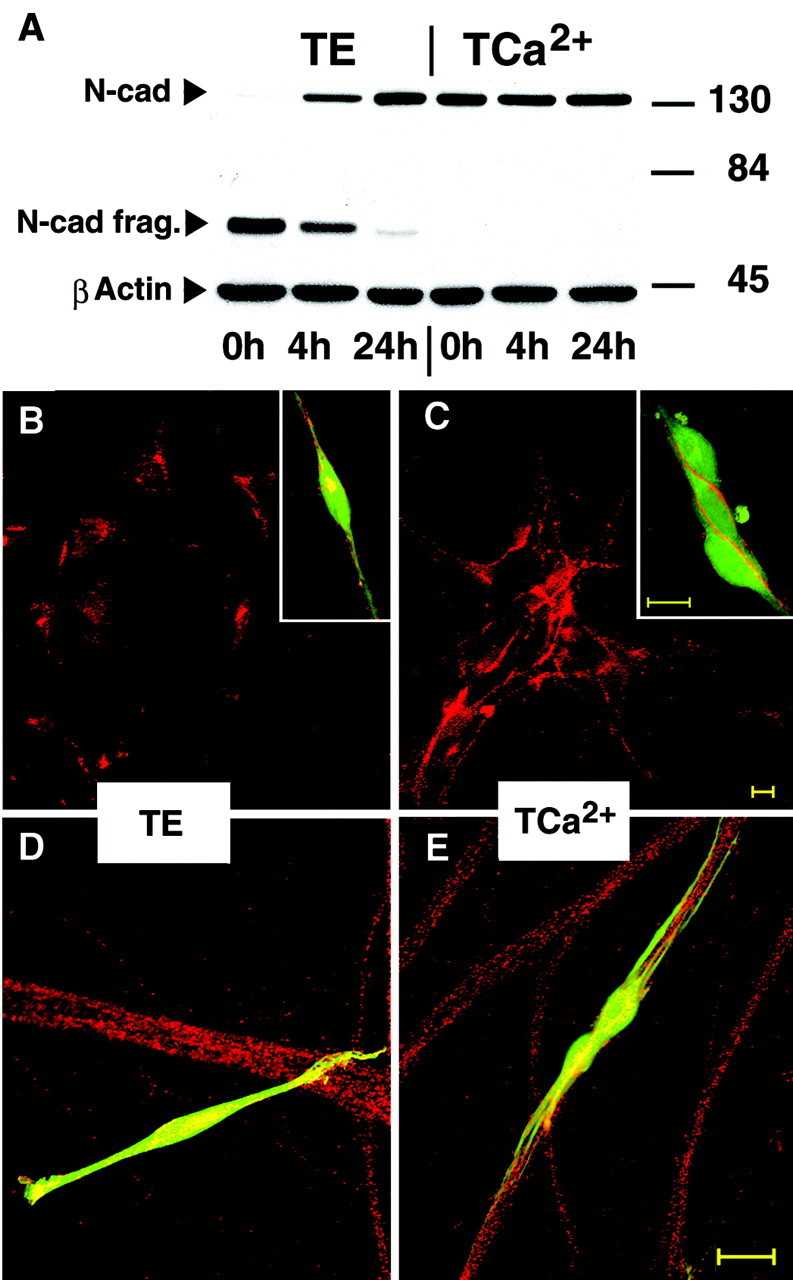
Removal of full-size cadherins from the SC surface prevents SC contact formation and alignment to axons. A, The Western blots show the effect of trypsin digestion on N-cadherin in the presence (TCa2+) and absence (TE) of calcium ions. Cultured rat SCs were treated for 10 min in TE (lanes 1–3) or TCa2+(lanes 4–6), and protein samples were harvested immediately after digestion (lanes 1,4), as well as after 4 hr (lanes 2, 5) and 24 hr (lanes 3,6) of culturing. Samples were probed for N-cadherin and β-actin. The 130 kDa N-cadherin band was almost absent in the TE-treated SCs (lane 1) and then recovered to ∼50% within 4 hr (lane 2) and almost fully within 24 hr (lane 3). TE-treated SCs show an N-cadherin fragment of ∼66 kDa (lane 1–3); this band disappeared within 24 hr. In the TCa2+-treated SCs, the 130 kDa N-cadherin band was detected with unreduced intensity (lanes 4-6), even immediately after trypsin digestion (lane 4), and the 66 kDa fragment was not detectable.B, C, The interaction of SCs treated for 10 min in TE (B) or TCa2+(C) are shown after 4 hr of culturing.B, TE-treated SCs fail to form N-cadherin-positive contacts. B, Inset, A vitally labeled, TE-treated SC exhibits weak and discontinuous surface N-cadherin and an intense aggregate of N-cadherin in the cytoplasm (yellow). C, Cadherin-protected SCs (TCa2+) formed numerous cell–cell N-cadherin-positive contacts. C, Inset, Three SCs (green) show bands of N-cadherin staining (red) at cell surfaces, particularly at cell–cell contact sites. D, E, The interaction of TE- and TCa2+-treated SCs (Cell Tracker, green) with axons (N-cadherin immunofluorescence, red) after 4 hr plating onto DRG neurons is shown. D, The confocal image shows a TE-treated SC not aligning with the axon bundles. E, A pair of cadherin-protected (TCa2+) SCs display all processes in alignment with axons. Scale bar, 20 μm.
The ability of TE- or TCa2+-treated SCs to form networks and associate with axons in medium containing normal calcium levels was determined. Cultures were fixed, permeabilized, and stained for N-cadherin with an antibody recognizing the cytoplasmic part of N-cadherin to visualize both the cytoplasmic and membrane-associated pool of the molecule. SCs devoid of cadherins did not form intercellular contacts and failed to form a network (Fig.6B, TE). Moreover, TE-treated SCs showed a small amount of punctuate staining at the cell surface but an intense spot of immunoreactivity in the cell body (Fig. 6B,inset). This suggests that TE digestion removes the extracellular domain of cadherins from the cell surface, followed by internalization of the remaining protein fragment toward the perinuclear area (Volberg et al., 1986). Cadherin-protected SCs formed extensive networks (Fig. 6C,TCa2+) and had extensive intercellular membrane contacts shown as strongly N-cadherin positive bands between attached cells (Fig. 6C, inset). The network was formed within 4 hr of culturing, because the cell suspension did not contain groups of clustered cells at the time of seeding. Before plating, most SCs were single and only rarely were cells seen in pairs or triplets (data not shown).
SCs treated with TE for 10 min and seeded on DRG neurons failed to align with axons within the first 4 hr of coculturing, and SC processes were observed crossing N-cadherin-positive axon bundles (Fig.6D) (49–62% of the SCs were axon aligned). In contrast, the majority of cadherin-protected SCs seeded onto DRG neurons attached to axon bundles and formed long processes in alignment with axons within 4 hr of coculturing (86% of the SCs were axon aligned) (Fig. 6E). In addition, cells that failed to align to axons did not display bands of N-cadherin staining at the surface. The time of reappearance of 130 kDa N-cadherin and N-cadherin surface staining corresponded with the time at which SCs formed contacts with other SCs as well as with axons, suggesting that within 24 hr full-size N-cadherin was assembled in SC membranes leading to functional recovery of N-cadherin and enabling the described adhesive behaviors of rat SCs. Replenishment of full-size N-cadherin in SCs had already started at 4 hours (Fig. 6A), and digestion with TE could interfere with the presence of other junction molecules; therefore this protocol was not chosen for more quantitative studies.
N-cadherin is the major cadherin expressed in cultured rat SCs and DRG neurons
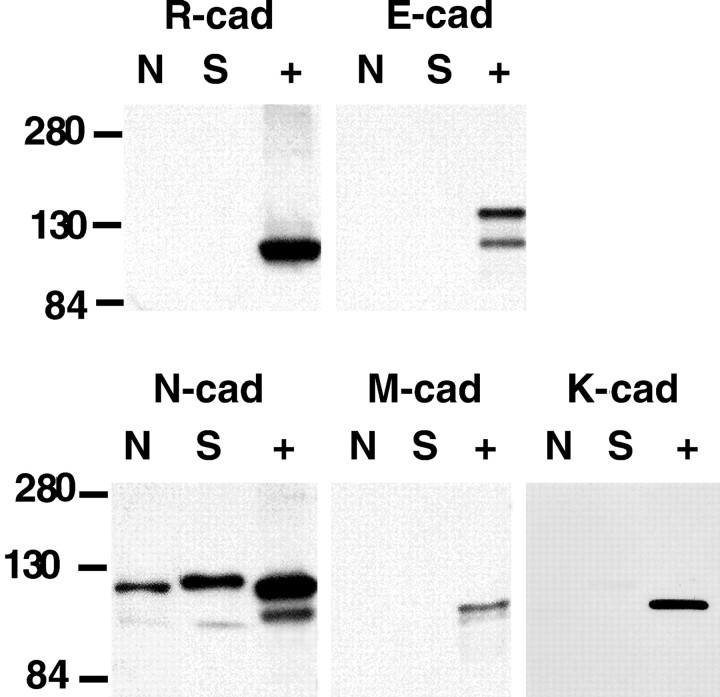
What specific cadherins are expressed in cultures of adult-derived, mitogen-treated SCs and embryonic DRG neurons? The expression of four specific cadherins was assessed using Western blotting. E-cadherin, R-cadherin, and M-cadherin, all class I cadherins, were not detected in cultured rat SCs and DRG neurons (Fig.7). In addition, immunocytochemistry of cultured DRG neurons and SCs did not show specific signals for M- and R-cadherin (data not shown). Specific immunohistochemical staining for E-cadherin was occasionally seen in a small number of DRG neuron cell bodies, but axonal staining was absent (data not shown). E-cadherin signals were not detected in equivalent stages of developing peripheral nerves and DRG roots (I. Wanner and K. Jessen, unpublished observations). Postnatally and in adult rats, E-cadherin is reported in satellite cells and in unmyelinated axons, as well as in myelinating SCs, but exclusively at autotypic junctions of SCs (Shimamura et al., 1992; Uchiyama et al., 1994; Fannon et al., 1995).
Fig. 7.
N-cadherin is the most abundant cadherin expressed in cultured rat SCs and DRG neurons. The Western blots were performed using protein lysates from cultured rat DRG neurons (N) and purified rat SCs, cultured in mitogens (S). They were compared with positive control samples (+) for the various cadherins. These controls were lysates of epidermoid carcinoma cell line A 431 (E-cad), rat brain (N-cad, R-cad), mouse neonate (M-cad), and MDCK cells (K-cad). Positive controls gave strong signals for all tested cadherins. The autoradiogram shows no signals for R-cadherin, E-cadherin, and M-cadherin; however, strong N-cadherin signals were detected in cultured SCs and DRG neurons. Faint bands were detected for K-cadherin in cultured SCs and DRG neurons.
It has been reported that cadherin-6 (K-cadherin), a class II cadherin, is expressed in the developing PNS (Inoue et al., 1997). Thus, a goat polyclonal antibody against K-cadherin was used to determine the presence of K-cadherin in adult-derived cultured rat SCs and E15 DRG neurons. Western blots showed no expression in DRG neurons and a very weak signal in SCs. K-cadherin was present in outgrowing neurons at earlier stages than E15 but not in developing SCs (Wanner and Jessen, unpublished data). Thus, N-cadherin was the most strongly expressed of the tested cadherins in rat SCs and DRG neuronsin vitro. The absence of other cadherins is further evidence that N-cadherin is involved in SC network formation and their association with axons.
N-cadherin function-blocking peptides and antibodies impede SC network formation and reduce the number of SCs associating with axons
N-cadherin function-blocking agents and their potency
N-cadherin function-blocking peptides and antibodies were used to further test the role of N-cadherin in SC adhesion and interaction with axons. The effect of CHAVC peptide, containing the presumptive N-cadherin interaction region (HAV at 0.5–0.75 mg/ml), was tested in parallel with CHGVC, a nonactive control peptide (HGV, same concentrations). In addition, two different N-cadherin blocking antibodies were used: IgGs of a rabbit antibody and of a guinea pig antibody, both against the first extracellular domain of N-cadherin, were used in SC–SC adhesion assays. Potencies of purified antibodies were validated determining their effect on the aggregation of NC+L-cells (provided by D. Colman). Cells were cultured overnight in the presence of the blocking antibodies (L7, gp1260) and their controls (L4 IgG, guinea pig preimmune serum), respectively (Shan et al., 2000). After 24 hr, Cell Tracker-labeled L-cells were fixed and immunostained for N-cadherin. NC+L-cells form cobblestone-like networks displaying strong N-cadherin-positive bands between neighboring cells (Fig. 8A) (N2) that do not develop under low calcium conditions (Fig. 8B) (low Ca2+). This pattern made up of numerous N-cadherin-positive intercellular junctions was also present in control antibody-treated cultures (guinea pig preimmune serum, rabbit IgG, L4 IgG) (Fig. 8C). Analyzed at similar intermediate densities in the presence of N-cadherin-blocking antibodies, markedly fewer N-cadherin-positive cell–cell contact zones were observed (example L7 IgG) (Fig. 8D). The L-cell aggregation assay confirmed the potency of N-cadherin blocking antibodies in perturbing N-cadherin-mediated cell adhesion.
Fig. 8.
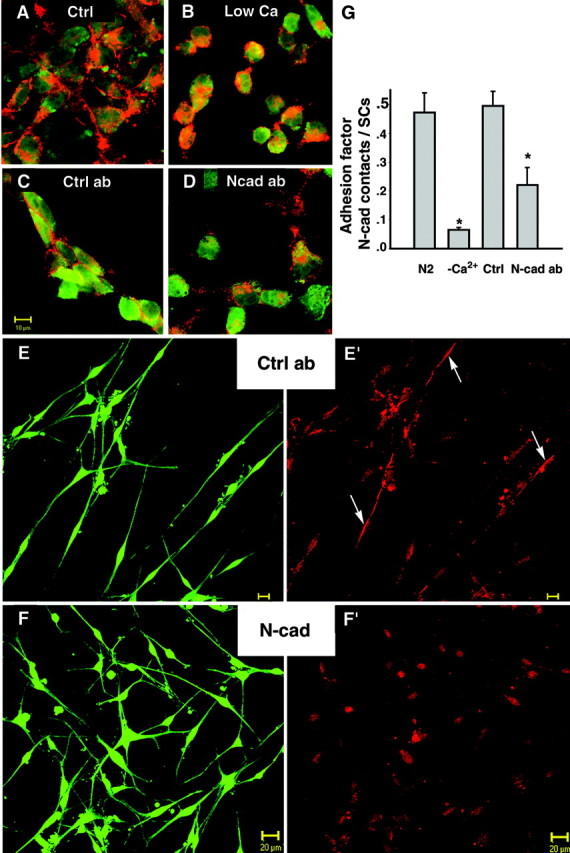
N-cadherin-blocking antibodies perturb SC network formation. A–D, L7 IgG perturbs adhesion of NC+L cells using a 24 hr aggregation assay. A, Control (ctrl) culture of Cell Tracker-labeled NC+L cells (green), kept in N2 medium plus 1% FBS, show “cobblestone”-like arrangement and N-cadherin-positive bands between cells (red).B, Cultures in low calcium (low Ca2+) exhibit spherically shaped, single L-cells and only rare N-cadherin-positive cell–cell contacts.C, In the presence of control antibody (ctrl ab; L4 IgG 1:50 in N2), cell clusters with N-cadherin-positive junctions are shown similar to those of controls (A). D, Cultures treated with N-cadherin-binding antibody (N-cad ab; L7 IgG, 1:50 in N2) display single cells and less frequent N-cadherin-positive cell–cell contacts. E, F, Vitally stained SCs (green) cultured for 24 hr in the presence of control antibody (ctrl ab; rabbit IgG 1:50 in N2) form arrays of cells (E) and display N-cadherin-positive bands (E′, red,arrows). SCs treated with N-cadherin-binding antibodies (N-cad ab; L7 IgG 1:50 in N2 medium) fail to form arrays (F), and N-cadherin-containing contacts between SCs are rarely seen (F′). Scale bar, 20 μm.G, Plotted are the averages of the adhesion factor obtained from four SC cultures under each condition. The adhesion factor was determined by dividing the number of N-cadherin-positive cell–cell contacts by the number of SCs found in groups (contacting neighboring SCs). Control cultures were SCs in normal calcium medium (N2) as well as SCs in low calcium medium (−Ca2+). Treated cultures were incubated with N-cadherin-binding antibodies (N-cad ab; L7 IgG) and nonbinding control antibody (ctrl; L4 IgG). The adhesion factor is significantly lower under low calcium conditions and in L7 IgG-treated SC cultures (p < 0.01; Tukey–Kramer multiple comparisons).
SC network formation under N-cadherin blocking conditions
SCs were cultured at medium density in the presence of N-cadherin function-blocking antibodies (see Materials and Methods). The result of blocking N-cadherin was essentially the same using either the L7 antibody (Fig. 8F,F′) (N-cad ab) or the gp1260-blocking antibody (Table 1) when compared with their respective controls (rabbit IgG) (Fig.8E,E′, ctrl ab) [Table1, preimmune serum (gp preim.)]. Under low calcium conditions, more cells were single (Table 1). Among cells that were contacting neighboring cells (SCs in groups), fewer N-cadherin-positive contacts were seen under blocking conditions (Fig. 8F′) compared with controls (Fig. 8E′). Control cultures had an average “adhesion factor” (see Materials and Methods) of ∼0.5 (n = 6); i.e., one N-cadherin-positive band was seen between every pair of contacting SCs. In low calcium conditions the adhesion factor was reduced to approximately one-seventh of the control value, meaning that on average only one N-cadherin-positive junction remained among 14 neighboring cells (Fig. 8G, Table1). In the presence of N-cadherin blocking antibodies, this value declined significantly (in L7-treated cultures the adhesion factor was half of that of L4 or rabbit IgG-treated cultures; in gp1260-treated cultures it was 0.7 times that of preimmune serum-treated cultures;p < 0.01) (Table 1). This experiment demonstrates that low calcium and N-cadherin-blocking antibodies perturbed the formation of N-cadherin bands between SCs and SC network formation.
SC–axon interaction in the presence of N-cadherin blocking agents
The effect of the peptides on SC–axon interaction was analyzed at 4 hr of coculturing in three independent experiments. In the presence of the control peptide, SCs were associated with axons and had bipolar processes in alignment with axons (Fig.9A, HGV), whereas many SCs in cocultures treated with the blocking peptide displayed multiple processes that crossed axons, and their cell bodies were not associated with axons (Fig. 9B,HAV). As shown in Figure 9B, their cell bodies were not attached to axons, and their process tips were not contacting axons. However, some axon-aligned SCs and SCs with short or no processes were observed in HAV-treated cultures as well; therefore the number of SCs aligning to axons was compared with controls without peptides as well as low calcium treatments. The data (Fig.9E, Table 2) show significantly fewer SCs aligned to axons in the presence of HAV peptides compared with HGV peptides (p < 0.01;n = 3) or normal medium (p < 0.001; n = 3).
Fig. 9.
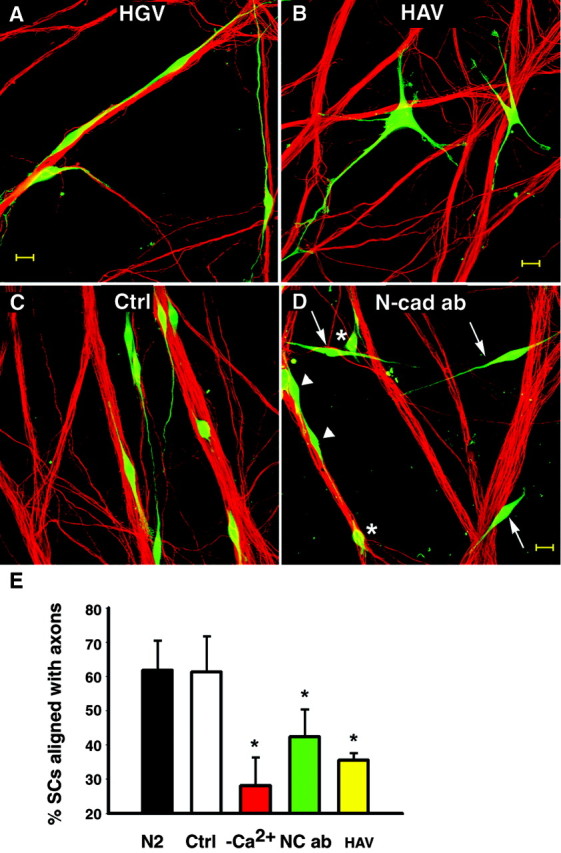
N-cadherin function-blocking agents decrease the number of SCs aligning to axons. A, B, Cell Tracker-labeled SCs (green) were cocultured with DRG neurons in the presence of 0.5 mg/ml of the cyclic pentapeptides HGV (0.5 mg/ml) or HAV (0.5 mg/ml). After 4 hr, cultures were fixed and axons were stained for neurofilament M (red). A, Spindle-shaped SCs are seen with their extensions aligned to axons in cultures treated with the control peptide (HGV). B, In the presence of blocking peptide HAV, SCs display multiple processes that failed to align with axons. Scale bar, 20 μm. C,D, SCs (Cell Tracker, green) were cocultured with DRG neurons for 24 hr in defined medium (ctrl; N2) or the presence of N-cadherin function-blocking guinea pig antibody (Ncad ab; gp1260 IgG, 1:100). C, In control cultures (N2), most SCs show processes well aligned to axon bundles. Both N-cadherin-blocking antibodies (L7 IgG and gp1260) were used to treat SC–DRG cocultures.D, A coculture treated with N-cadherin-blocking antibody (here gp1260 IgG) shows SCs that failed to associate with axons (arrows) as well as SCs with short or no processes (∗) and some SCs with axon-aligned processes (arrowheads). Scale bar, 20 μm. E, Plotted are average percentages of SCs aligned with axons. Error bars represent SDs from three cultures of each condition. Significantly fewer SCs align to axons in HAV as well as N-cadherin-blocking antibody (here data from L7 IgG)-treated cocultures compared with untreated (N2) and controls treated (average of HGV, rabbit IgG, and L4 IgG combined;p < 0.01, Tukey–Kramer multiple comparisons). There was no significant difference between HAV and low Ca2+ (Tukey–Kramer, multiple comparisons).
Table 2.
SC–axon alignment was decreased using N-cadherin blocking agents
| Coculture time | Controls | Blocking agent | Low calcium | ||
|---|---|---|---|---|---|
| 4 hr | 59 ± 4 (N2) | HAV | |||
| 49 ± 1 (HGV) | 0.5 mg/ml | 34 ± 3 | 18 ± 4 | ||
| 4 hr | 71 ± 3 (N2) | HAV | |||
| 58 ± 5 (HGV) | 0.75 mg/ml | 37 ± 3 | 35 ± 2 | ||
| % SC aligned with axons | 65 ± 6 (N2) | Average peptides | |||
| 54 ± 5 (HGV) | 36 ± 3 | 27 ± 9 | |||
| 24 hr | 71 ± 3 (N2) | L7 IgG | |||
| 74 ± 1 (rb IgG) | 1:50 | 51 ± 5 | 35 ± 2 | ||
| 24 hr | 66 ± 3 (N2) | L7 IgG | |||
| 64 ± 4 (L4 IgG) | 1:50 | 41 ± 2 | 32 ± 8 | ||
| 69 ± 3 (N2) | Average antibodies | ||||
| 69 ± 5 (proper) | 46 ± 5 | 34 ± 2 | |||
SCs aligned with axons were counted in cultures (n = 3 for each condition) treated with N-cadherin-blocking peptides (HAV) and antibodies (L7 IgG) and their controls (HGV, L4 IgG) at the concentration given, together with untreated cultures (N2) and cultures under low calcium conditions (low Ca2+). Shown are means with SDs. The percentage of SCs that were not aligned with axons varied among experiments depending on SC harvesting conditions and the presence of serum in the cocultures. Therefore, the percentage of axon-aligned SCs in controls (N2) of each experiment was used to determine the relative blockage of treated cultures.
In four independent experiments, SC process growth in alignment with axons was analyzed in the presence of N-cadherin-blocking antibodies. Alignment was compared with controls (Fig. 9C) after treating cocultures for 24 hr with L7 or gp1260 antibodies, respectively. The percentage of SCs aligned with axons was significantly lower in the presence of N-cadherin-blocking antibodies, similar to results with peptides, compared with their controls, respectively (Fig. 9E, Table 2). A large population of N-cadherin-blocked SCs showed no processes or noticeably shorter extensions than in control cultures, suggesting that SC process outgrowth was impeded. However, even under blocking conditions, some SC processes were aligned to axons. Peptides and blocking antibodies compete with cellular N-cadherin in binding and thus could be expected to show less complete blocking. This interpretation is supported by the observation that blocking agents failed to prevent aggregation of NC+L-cells at high cell density (data not shown), implying that N-cadherin–N-cadherin affinity is stronger than the affinity of peptides or antibodies with cell surface N-cadherin.
DISCUSSION
Structural analysis at subcellular resolution combined with molecular information about involved adhesion molecules are needed to improve our knowledge of the mechanisms underlying changing modes of SC–axon association. SCs attach immediately to axons when cocultured with DRG neurons and rapidly extend processes in association with axons. The preceding results document a role for N-cadherin in directing the extension of SC processes along axons. N-cadherin was detected in axons of DRG neurons derived from E15 rats and cultured rat SCs. Intense focal accumulations of N-cadherin in process tips of migrating SCs and at axon contact sites were observed. Removing N-cadherin from the cell surface by trypsination in the absence of calcium, or blocking N-cadherin binding by lowering the extracellular calcium concentration as well as using specific perturbing agents, prevented the axon-aligned outgrowth of SC processes. The presented studies also demonstrate that N-cadherin was required for SC–SC contact behavior and the ability of SCs to form networks. Low calcium conditions, N-cadherin-blocking agents, and conditions in which SC surface N-cadherin was absent (trypsination in TE or culture in over-confluent conditions) caused the loss of cell–cell contacts and lack of the cellular swirling pattern that SCs form in vitro. These results constitute evidence that N-cadherin plays an essential role in mediating mammalian SC–SC adhesion and early events in the association of SCs with axons.
Our results show that N-cadherin is present during different stages of SC–axon interaction. In the following we discuss how N-cadherin could mediate these interactions.
First, what could be the role of N-cadherin in SC process growth along axons? The behavior of axon-aligned SC process growth is similar to neuronal growth cone movement (high-resolution time-lapse imaging; data not shown) (Polinsky et al., 2000). Evidence suggests that neurite outgrowth stimulated by N-cadherin requires the presence of functional fibroblast growth factor (FGF) receptor in neurons (Williams et al., 1994). An interaction between N-cadherin and FGF receptor via the HAV motif has been reported recently and is suggested to signal neurite outgrowth of cerebellar neurons on N-cadherin-transfected 3T3 cells (Williams et al., 2001). FGF receptor is present in SCs (Dong et al., 1997; Grothe et al., 2001), suggesting that a similar interaction of N-cadherin with FGF receptor may promote axon-aligned SC process growth.
Second, when SCs acquired bipolar morphology with processes aligned to axons, N-cadherin was accumulated at the SC–axon interface. At this stage, N-cadherin establishes and maintains axolemma–SC membrane juxtaposition, therefore contributing to contact-dependent signaling between SCs and axons as suggested by Yap et al. (1997). Thus, in addition to signaling process outgrowth and providing adhesive contact (see below), N-cadherin could instate localized ligand-receptor interaction at the SC–axon interface.
Third, N-cadherin might be involved in morphological changes of SCs and thus should be linked to the actin cytoskeleton. Influences of focal adhesion proteins on SC morphology have been reported independently for SC–SC as well as SC–axon interactions. Focal adhesion assembly in SCs and F-actin rearrangements induced by lysophosphatidic acid have been reported recently to upregulate N-cadherin and alter SC morphology and adhesion (Weiner et al., 2001). Actin cytoskeleton changes at focal adhesion sites coincide with morphological changes of SCs interacting with axons (Fernandez-Valle et al., 1997, 1998; Chen et al., 2000). When staining for N-cadherin and ezrin, a member of the ERM family, N-cadherin was enriched in SC process tips where its signals were colocalized with ezrin (I. Wanner and C. Fernandez-Valle, unpublished observations).
Immunohistochemical signals for β-catenin and plakoglobin were observed in cultured rat SCs (data not shown). Cadherin-based contacts appear in specialized junctions called zonula adherentia and focal adhesions (Yap et al., 1997). The intense N-cadherin-positive bands demonstrated here likely represent cadherin–catenin–actin complexes typical for adherens junctions, particularly because numerous adherens junctions were indeed found in electron microscopic observations at juxtaposed SC–SC and SC–axon membranes (our unpublished data). These additional observations make it very likely that N-cadherin is functionally linked to the actin cytoskeleton.
Fourth, as part of focal adhesion sites or adherens junctions, could N-cadherin mediate dynamic shape changes in SCs interacting with axons? Early SC–axon interaction shows dynamic morphological changes accompanying SC movement along axons while maintaining contact with the axolemma (Martin and Webster, 1973; Billings-Gagliardi et al., 1974;Billings-Gagliardi, 1977; Webster, 1993). Therein, transient contacts were suggested to underlie SC movement along axons. Thus we asked the following: could N-cadherin-based junctions be of temporary nature? Indeed, the existence of transient adherens junctions between SCs and axons has been shown previously (Tetzlaff, 1982; Sims et al., 1988;Dezawa and Nagano, 1993, 1996). How is contact of adherens junctions regulated? In other words, is the connection of the cadherin-based protein complex to the actin cytoskeleton subjected to cell signaling? It has been shown recently in other systems that cadherin-based adhesive contacts are indeed dynamic, regulated multiprotein complexes with rearrangements regulated by various factors, including the Rho family of GTPases and phosphotyrosine phosphatases (Balsamo et al., 1998; Brady-Kalnay et al., 1998; Braga et al., 1999) and receptor tyrosine kinases (Takeda et al., 1995). These points thus lead to the conclusion that transient N-cadherin-based adhesive contacts between SCs and axons could indeed be part of SC shape changes during early interaction with axons.
Other adhesion molecules are candidates to participate in SC–axon interaction. L1 binding antibodies were reported to interfere with SC engulfment and myelination of axons reflecting later stages of SC–axon interaction (Seilheimer et al., 1989; Wood et al., 1990). However, initial SC–axon association was not investigated, and axon-induced proliferation of SCs was not affected in these studies. Although nerve development and myelination occur normally in L1 (−/−) mice (Dahme et al., 1997; Haney et al., 1999), incomplete ensheathment of nonmyelinating sensory axons was observed in sciatic nerve transplants into L1 knock-out mice (Haney et al., 1999). In studies now underway using the same alignment assay described in the present study, L1 binding antibodies blocked SC–axon alignment as effectively as N-cadherin-blocking antibodies or low calcium did, but there was no additional blocking seen when both antibodies were used together (our unpublished observations).
What is the relevance for N-cadherin mediated SC–SC interaction and the formation of bands and networks by SCs in vivo? N-cadherin-based adherens junctions between SCs stabilize the formation of bands. Such SC–SC contacts could reflect the behavior of SC precursors during peripheral nerve development when strong adhesion among them is common (Jessen et al., 1994). Indeed, short-term cultured SC precursors from developing peripheral nerves of E14 rats do show N-cadherin signals (Wanner and Jessen, unpublished data).
A first step in identifying factors that may regulate N-cadherin expression in adult rat SCs is the finding of increased N-cadherin protein amounts in the presence of mitogens (serum factors, forskolin, pituitary extract, and heregulin) shown here. Furthermore, although SCs acutely isolated from newborn sciatic nerves did not express N-cadherin, the protein was detected when these SCs were treated with heregulin β1 in defined medium (Wanner and Jessen, unpublished data). Because DRG neurons are reported to be a source of neuregulins (Bermingham-McDonogh et al., 1997; Meyer et al., 1997), these findings could reflect the regulation of N-cadherin in SCs contacting axons. The presented finding is the first report indicating a regulated N-cadherin expression by mitogens, including neuregulin, in SCs.
In SC–DRG cocultures, the intensity of N-cadherin immunostaining appeared decreased a few days after SCs contacted axons. This decrease could be caused in part by changes in N-cadherin distribution from focally clustered to evenly spread along the SC–axon surfaces. However, the reduction could also indicate an axon-induced downregulation of N-cadherin expression after completion of alignment and process growth, suggesting that N-cadherin might not be needed at later stages of SC–axon interaction. Interestingly, during development of peripheral nerves, N-cadherin was present in neuronal and glial structures at E14–E15; however, specific staining substantially declined when analyzed at E18 (Wanner and Jessen, unpublished observations). A comparable time-regulated expression of N-cadherin during chicken PNS development has been reported (Akitaya and Bronner-Fraser, 1992). In adult sciatic nerves in rats (as shown) and in chicken (Shibuya et al., 1995), only low levels of N-cadherin were present. The subcellular localization of N-cadherin at SC–SC and SC–axon junctions demonstrated here and the blocking of their formation in vitro advocate a role for N-cadherin in early SC interaction with axons relevant within a narrow developmental window during early stages of nerve formation. N-cadherin is also present in olfactory ensheathing glia (Lakatos et al., 2000) at axon–olfactory ensheathing glia interface areas (Wanner, unpublished observations), in SC precursors (Wanner and Jessen, unpublished observations), and in oligodendrocytes where it is suggested to be involved in interaction of oligodendrocytes with axons (Schnädelbach et al., 2001). Taken together, these data speak generally for a role of N-cadherin in initial stages of axon–glia interaction in PNS and CNS glia.
During the process of Wallerian degeneration, denervated SCs proliferate and show increased expression of various cell adhesion molecules (Daniloff et al., 1986; Martini, 1994). The presence of adhesion molecules is considered to be important for reestablishing SC–axon relationships required for regeneration and nerve repair after injury. Living SCs are indispensable for optimal axonal regrowth in peripheral nerves, and they support the regeneration of CNS axons (Kromer and Cornbrooks, 1985, 1987). It has been shown in various transplantation and culture models that an intimate contact of axolemma to SC surface is required for these beneficial effects (Ard et al., 1987; Kleitman et al., 1988; Hopkins and Bunge, 1991). These findings suggest that the expression of N-cadherin is an important property of SCs that are to be used as transplants for treatment of demyelination or nervous tissue injury. The enhanced expression of N-cadherin in SCs used for transplantation strategies may potentially improve cell–cell interactions and thus their ability for nervous system repair.
Footnotes
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant RO1-NS09923 and the Miami Project to Cure Paralysis. We thank W. Shan, G. Huntley, and D. Colman (Mt. Sinai, NY) for N-cadherin-transfected L-cells and N-cadherin-blocking guinea pig antibody (gp1260). We also thank Adherex Technologies Inc. (Ottawa, Quebec, Canada) for providing N-cadherin function-perturbing cyclic pentapeptides. A special thanks goes to O. Blaschuk at Division of Urology, Department of Surgery, McGill University (Montreal, Quebec) for providing N-cadherin antiserum L7 and L4. Finally, we thank A. Gomez, Y. Presman, and L. White for excellent technical assistance.
Correspondence should be addressed to Dr. Ina Wanner, The Miami Project to Cure Paralysis, University of Miami School of Medicine, Lois Pope Life Center, 1095 NW 14th Terrace, Miami, FL 33136. E-mail:iwanner@miamiproject.med.miami.edu.
REFERENCES
- 1.Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JS, Blaschuk OW, Haselton FR. An N-cadherin-like protein contributes to solute barrier maintenance in cultured endothelium. J Cell Physiol. 1993;156:610–618. doi: 10.1002/jcp.1041560321. [DOI] [PubMed] [Google Scholar]
- 3.Ard MD, Bunge RP, Bunge MB. Comparison of the Schwann cell surface and Schwann cell extracellular matrix as promoters of neurite growth. J Neurocytol. 1987;16:539–555. doi: 10.1007/BF01668507. [DOI] [PubMed] [Google Scholar]
- 4.Balsamo J, Arregui C, Leung T, Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermingham-McDonogh O, Xu YT, Marchionni MA, Scherer SS. Neuregulin expression in PNS neurons: isoforms and regulation by target interactions. Mol Cell Neurosci. 1997;10:184–195. doi: 10.1006/mcne.1997.0654. [DOI] [PubMed] [Google Scholar]
- 6.Billings-Gagliardi S. Mode of locomotion of Schwann cells migrating in vivo. Am J Anat. 1977;150:73–87. doi: 10.1002/aja.1001500106. [DOI] [PubMed] [Google Scholar]
- 7.Billings-Gagliardi S, Webster HF, O'Connell MF. In vivo and electron microscopic observations on Schwann cells in developing tadpole nerve fibers. Am J Anat. 1974;141:375–391. doi: 10.1002/aja.1001410308. [DOI] [PubMed] [Google Scholar]
- 8.Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol. 1988;107:353–361. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 11.Bottenstein J, Hayashi I, Hutchings S, Masui H, Mather J, McClure DB, Ohasa S, Rizzino A, Sato G, Serrero G, Wolfe R, Wu R. The growth of cells in serum-free hormone-supplemented media. Methods Enzymol. 1979;58:94–109. doi: 10.1016/s0076-6879(79)58127-0. [DOI] [PubMed] [Google Scholar]
- 12.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 13.Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPmu with multiple cadherins in vivo. J Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LM, Bailey D, Fernandez-Valle C. Association of β1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20:3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- 17.Daniloff JK, Levi G, Grumet M, Rieger F, Edelman GM. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J Cell Biol. 1986;103:929–945. doi: 10.1083/jcb.103.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dezawa M, Nagano T. Contacts between regenerating axons and the Schwann cells of sciatic nerve segments grafted to the optic nerve of adult rats. J Neurocytol. 1993;22:1103–1112. doi: 10.1007/BF01235752. [DOI] [PubMed] [Google Scholar]
- 19.Dezawa M, Nagano T. Immunohistochemical localization of cell adhesion molecules and cell-cell contact proteins during regeneration of the rat optic nerve induced by sciatic nerve autotransplantation. Anat Rec. 1996;246:114–126. doi: 10.1002/(SICI)1097-0185(199609)246:1<114::AID-AR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Dong Z, Dean C, Walters JE, Mirsky R, Jessen KR. Response of Schwann cells to mitogens in vitro is determined by pre-exposure to serum, time in vitro, and developmental age. Glia. 1997;20:219–230. [PubMed] [Google Scholar]
- 21.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 22.Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL, Jr, Colman DR. Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol. 1995;129:189–202. doi: 10.1083/jcb.129.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene expression associated with Schwann cell myelination. J Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Valle C, Wood PM, Bunge MB. Localization of focal adhesion kinase in differentiating Schwann cell/neuron cultures. Microsc Res Tech. 1998;41:416–430. doi: 10.1002/(SICI)1097-0029(19980601)41:5<416::AID-JEMT8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Grothe C, Meisinger C, Claus P. In vivo expression and localization of the fibroblast growth factor system in the intact and lesioned rat peripheral nerve and spinal ganglia. J Comp Neurol. 2001;434:342–357. doi: 10.1002/cne.1181. [DOI] [PubMed] [Google Scholar]
- 26.Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, Trapp BD. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol. 1999;146:1173–1184. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopkins JM, Bunge RP. Regeneration of axons from adult rat retinal ganglion cells on cultured Schwann cells is not dependent on basal lamina. Glia. 1991;4:46–55. doi: 10.1002/glia.440040106. [DOI] [PubMed] [Google Scholar]
- 28.Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- 29.Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 30.Kleitman N, Wood P, Johnson MI, Bunge RP. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J Neurosci. 1988;8:653–663. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleitman N, Wood PW, Bunge R. Tissue culture methods for the study of myelination. In: Banker G, Goslin K, editors. Culturing nerve cells, Ed 2. MIT; Boston: 1998. pp. 545–594. [Google Scholar]
- 32.Koch AW, Bozic D, Pertz O, Engel J. Homophilic adhesion by cadherins. Curr Opin Struct Biol. 1999;9:275–281. doi: 10.1016/S0959-440X(99)80038-4. [DOI] [PubMed] [Google Scholar]
- 33.Kromer LF, Cornbrooks CJ. Transplants of Schwann cell cultures promote axonal regeneration in the adult mammalian brain. Proc Natl Acad Sci USA. 1985;82:6330–6334. doi: 10.1073/pnas.82.18.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kromer LF, Cornbrooks CJ. Identification of trophic factors and transplanted cellular environments that promote CNS axonal regeneration. Ann NY Acad Sci. 1987;495:207–224. doi: 10.1111/j.1749-6632.1987.tb23676.x. [DOI] [PubMed] [Google Scholar]
- 35.Lakatos A, Franklin RJ, Barnett SC. Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia. 2000;32:214–225. doi: 10.1002/1098-1136(200012)32:3<214::aid-glia20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V. Nerve growth cone migration onto Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev Biol. 1990;138:430–442. doi: 10.1016/0012-1606(90)90209-2. [DOI] [PubMed] [Google Scholar]
- 37.Letourneau PC, Roche FK, Shattuck TA, Lemmon V, Takeichi M. Interactions of Schwann cells with neurites and with other Schwann cells involve the calcium-dependent adhesion molecule, N-cadherin. J Neurobiol. 1991;22:707–720. doi: 10.1002/neu.480220706. [DOI] [PubMed] [Google Scholar]
- 38.Martin JR, Webster HD. Mitotic Schwann cells in developing nerve: their changes in shape, fine structure, and axon relationships. Dev Biol. 1973;32:417–431. doi: 10.1016/0012-1606(73)90251-0. [DOI] [PubMed] [Google Scholar]
- 39.Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol. 1994;23:1–28. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- 40.Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 41.Peters A, Muir AR. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. J Exp Physiol. 1959;44:117–130. doi: 10.1113/expphysiol.1959.sp001366. [DOI] [PubMed] [Google Scholar]
- 42.Polinsky M, Balazovich K, Tosney KW. Identification of an invariant response: stable contact with Schwann cells induces veil extension in sensory growth cones. J Neurosci. 2000;20:1044–1055. doi: 10.1523/JNEUROSCI.20-03-01044.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prestige MC, Wilson MA. Growth of a limb spinal nerve: an ultrastructural study. J Comp Neurol. 1980;194:235–287. doi: 10.1002/cne.901940112. [DOI] [PubMed] [Google Scholar]
- 44.Salzer JL, Williams AK, Glaser L, Bunge RP. Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J Cell Biol. 1980a;84:753–766. doi: 10.1083/jcb.84.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salzer JL, Bunge RP, Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980b;84:767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnädelbach O, Blaschuk OW, Symonds M, Gour BJ, Doherty P, Fawcett JW. N-cadherin influences migration of oligodendrocytes on astrocyte monolayers. Mol Cell Neurosci. 2000;15:288–302. doi: 10.1006/mcne.1999.0819. [DOI] [PubMed] [Google Scholar]
- 47.Schnädelbach O, Ozen I, Blaschuk OW, Meyer RL, Fawcett JW. N-cadherin is involved in axon-oligodendrocyte contact and myelination. Mol Cell Neurosci. 2001;17:1084–1093. doi: 10.1006/mcne.2001.0961. [DOI] [PubMed] [Google Scholar]
- 48.Seilheimer B, Schachner M. Studies of adhesion molecules mediating interactions between cells of peripheral nervous system indicate a major role for L1 in mediating sensory neuron growth on Schwann cells in culture. J Cell Biol. 1988;107:341–351. doi: 10.1083/jcb.107.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seilheimer B, Persohn E, Schachner M. Antibodies to the L1 adhesion molecule inhibit Schwann cell ensheathment of neurons in vitro. J Cell Biol. 1989;109:3095–3103. doi: 10.1083/jcb.109.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shan WS, Tanaka H, Phillips GR, Arndt K, Yoshida M, Colman DR, Shapiro L. Functional cis-heterodimers of N- and R-cadherins. J Cell Biol. 2000;148:579–590. doi: 10.1083/jcb.148.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shapiro L, Colman DR. Structural biology of cadherins in the nervous system. Curr Opin Neurobiol. 1998;8:593–599. doi: 10.1016/s0959-4388(98)80086-x. [DOI] [PubMed] [Google Scholar]
- 52.Shibuya Y, Mizoguchi A, Takeichi M, Shimada K, Ide C. Localization of N-cadherin in the normal and regenerating nerve fibers of the chicken peripheral nervous system. Neuroscience. 1995;67:253–261. doi: 10.1016/0306-4522(95)00015-b. [DOI] [PubMed] [Google Scholar]
- 53.Shimamura K, Takahashi T, Takeichi M. E-cadherin expression in a particular subset of sensory neurons. Dev Biol. 1992;152:242–254. doi: 10.1016/0012-1606(92)90132-z. [DOI] [PubMed] [Google Scholar]
- 54.Sims TJ, Gilmore SA, Waxman SG. Temporary adhesions between axons and myelin-forming processes. Brain Res. 1988;468:223–232. doi: 10.1016/0165-3806(88)90134-4. [DOI] [PubMed] [Google Scholar]
- 55.Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and beta catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 57.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 58.Tetzlaff W. Tight junction contact events and temporary gap junctions in the sciatic nerve fibres of the chicken during Wallerian degeneration and subsequent regeneration. J Neurocytol. 1982;11:839–858. doi: 10.1007/BF01153522. [DOI] [PubMed] [Google Scholar]
- 59.Uchiyama N, Hasegawa M, Yamashima T, Yamashita J, Shimamura K, Takeichi M. Immunoelectron microscopic localization of E-cadherin in dorsal root ganglia, dorsal root and dorsal horn of postnatal mice. J Neurocytol. 1994;23:460–468. doi: 10.1007/BF01184070. [DOI] [PubMed] [Google Scholar]
- 60.Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J Cell Biol. 1986;102:1832–1842. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volk T, Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol. 1986;103:1441–1450. doi: 10.1083/jcb.103.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webster HD. Development of peripheral nerve fibers. In: Dyck PJ, editor. Peripheral neuropathy, Ed 3. W. B. Saunders; Philadelphia: 1993. pp. 243–266. [Google Scholar]
- 63.Webster HD, Martin R, O'Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973;32:401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- 64.Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 2001;21:7069–7078. doi: 10.1523/JNEUROSCI.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilby MJ, Muir EM, Fok-Seang J, Gour BJ, Blaschuk OW, Fawcett JW. N-Cadherin inhibits Schwann cell migration on astrocytes. Mol Cell Neurosci. 1999;14:66–84. doi: 10.1006/mcne.1999.0766. [DOI] [PubMed] [Google Scholar]
- 66.Williams E, Williams G, Gour BJ, Blaschuk OW, Doherty P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J Biol Chem. 2000;275:4007–4012. doi: 10.1074/jbc.275.6.4007. [DOI] [PubMed] [Google Scholar]
- 67.Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 68.Williams EJ, Williams G, Howell FV, Skaper SD, Walsh FS, Doherty P. Identification of an N-cadherin motif that can interact with the fibroblast growth factor receptor and is required for axonal growth. J Biol Chem. 2001;276:43879–43886. doi: 10.1074/jbc.M105876200. [DOI] [PubMed] [Google Scholar]
- 69.Wood PM, Schachner M, Bunge RP. Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J Neurosci. 1990;10:3635–3645. doi: 10.1523/JNEUROSCI.10-11-03635.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 71.Ziskind-Conheim L. Physiological and morphological changes in developing peripheral nerves of rat embryos. Brain Res Dev Brain Res. 1988;42:15–28. doi: 10.1016/0165-3806(88)90198-8. [DOI] [PubMed] [Google Scholar]