Abstract
Evidence indicates that gonadotropin releasing hormone-1 [GnRH-1, also known as luteinizing hormone releasing hormone (LHRH)] neurons can exhibit synchronized neuroendocrine secretory activity before entrance into the CNS. In this study, we used calcium imaging to evaluate patterns of activity in individual, embryonic, GnRH-1 neurons as well as population dynamics of GnRH-1 neurons in mouse nasal explants maintained for 1 versus 3 weeks. Independent of age, GnRH-1 neurons displayed significant calcium peaks that synchronized at an interval of ∼20 min across multiple GnRH-1 cells within an explant. Acute tetrodotoxin treatment decreased the amplitude of calcium peaks in individual GnRH-1 neurons and the duration but not the frequency of synchronized activity in the population of GnRH-1 neurons. Acute GABAB receptor antagonism increased the frequency of synchronized neuronal activity at both ages, whereas acute GABAA receptor antagonism decreased calcium oscillations in individual GNRH-1 cells as well as synchronization of the calcium pulses within the GnRH-1 population at the 1 week time point to background non-GNRH-1 cell levels. These results indicate that developing GnRH-1 neurons rely heavily on GABAergic signaling to initiate synchronized bouts of activity but thereafter, possess an innate capacity for synchronized activity patterns that are modulated by, but not completely dependent on GABAergic signaling.
Keywords: LHRH, GnRH, GABA, explant culture, development, synchronous pulses, calcium oscillations
Gonadotropin releasing hormone-1 (GnRH-1) peptide [also known as luteinizing hormone releasing hormone (LHRH)] is released into the portal vascular system in a pulsatile pattern in all mammals examined to date (Levine, 1997). This pulsatile GnRH-1 profile is essential for reproduction (Belchetz et al., 1978;Wildt et al., 1980). The source of GnRH-1 is a small number of neurons (∼800 in mouse) (Hoffman and Finch, 1986) diffusely localized within the forebrain, although their axons converge within the median eminence where GnRH-1 is secreted. The signal or signals synchronizing GnRH-1 secretion has eluded discovery for decades. Recent studies using immortalized cell lines (Martinez de la Escalera et al., 1992; Hiruma et al., 1997; Nunez et al., 2000) as well as embryonic, primary GnRH-1 neurons (Terasawa et al., 1999b; Funabashi et al., 2000) suggest that pulsatility is an innate capability of the GnRH-1 neuron.
Explant models of primary GnRH-1 neurons originating within the nasal placode have facilitated investigations of the GnRH-1 neuronal population (Daikoku et al., 1993; Terasawa et al., 1993; Fueshko and Wray, 1994). GnRH-1 neurons in monkey (Terasawa et al., 1999b) and rat (Funabashi et al., 2000) nasal explants exhibit, after 2 weeks in culture, pulsatile GnRH-1 secretion. These data suggest that pulsatility is a characteristic of the GnRH-1 neuron that is independent of cues in the developing CNS. However, from these studies, it is unclear whether pulsatile GnRH-1 secretion develops over time or whether this characteristic is associated with, or shortly after, onset of GnRH-1 gene expression. In addition, other cell types are present in these explants, which could influence GnRH-1 secretion, confounding the autonomous nature of synchronized GnRH-1 secretion.
Our laboratory uses mouse nasal explants to examine the development of GnRH-1 neurons. By 5–7 d in vitro (div), GnRH-1 neurons within these explants have migrated in a unidirectional path away from the tissue mass and are now visible in the periphery of the culture (Fueshko and Wray, 1994). GnRH-1 cells within these explants can be maintained for several weeks (Fueshko and Wray, 1994). Therefore, in these explants studies on individual GnRH-1 neurons as well as on populations of GnRH-1 neurons can be performed chronologically. Asin vivo, GABAergic neurons are present within mouse nasal explants and GABAergic axons parallel the path taken by GnRH-1 neurons as they migrate into the explant periphery (Wray et al., 1996). In addition, GABAergic signaling, via GABAAreceptors, is depolarizing to (Kusano et al., 1995) and stimulates GnRH-1 peptide synthesis and intracellular calcium mobilization in GnRH-1 neurons (Moore and Wray, 2000) in these cultures at 1 week. In light of these findings, it is possible that GABAergic signals influence GnRH-1 pulsatile secretion at early stages of development.
In this investigation we monitored intracellular calcium mobilization as an indicator of GnRH-1 neuronal activity and evaluated patterns of activity in GnRH-1 neurons in 1- and 3-week-old nasal explants. The effect of GABAergic signaling on, and the requirement of sodium conductivity for GnRH-1 neuronal activity was also determined at each age. The experiments reported in this paper demonstrate that primary GnRH-1 neurons, devoid of CNS influences, exhibit calcium oscillations as early as 7 div and that these calcium oscillations synchronized with an interval of ∼20 min across multiple GnRH-1 cells in a single explant. Between 7 and 21 div the mean number of peaks of intracellular calcium in GnRH-1 cells doubled, however synchronization of these events in GnRH-1 cells per explant was similar to that observed at 7 div, e.g., ∼20 min interval. Perturbation of GABAergic systems significantly altered the synchronized pulse interval while the presence of tetrodotoxin, at either age, did not. Inhibition of GABAB signaling decreased the synchronized pulse interval in GnRH-1 cells at both ages. Inhibition of GABAA signaling had no effect on the synchronized pulse interval at 21 div, but totally disrupted the synchronized pulse interval at 7 div, with GnRH-1 cells exhibiting calcium oscillation at background non-GnRH-1 cell levels. These data indicate that although GnRH-1 cells can pulse outside the CNS, GABAergic cues are important for pulse generation and that the response of GnRH-1 cells changes over development.
MATERIALS AND METHODS
Materials. For tissue preparation and culturing,d-glucose, apo-transferrin, putrescine, sodium selenite, bovine insulin, l-ascorbic acid, fluorodeoxyuridine, and thrombin were purchased from Sigma (St. Louis, MO). The supplier of Gey's Balanced Salt Solution, Eagle Basal Medium, Ham's F-12 Nutrient Mixture, l-glutamine, and penicillin–streptomycin–nystatin antibiotic mixture was Invitrogen (Grand Island, NY). Bovine serum albumin and chicken plasma were purchased from Boehringer Mannheim (Indianapolis, IN) and Cocalico Biologicals, Inc. (Reamstown, PA), respectively.
Nasal explant preparations. Nasal regions were cultured as previously described (Fueshko and Wray, 1994). Briefly, embryos were obtained from timed pregnant animals in accordance with National Institutes of Health (NIH) guidelines. Nasal pits of E11.5 staged NIH-Swiss mice were isolated under aseptic conditions and refrigerated for 1 hr in Gey's Balanced Salt Solution enriched with glucose. Nasal explants were adhered onto coverslips by a plasma/thrombin clot. The explants were maintained in a defined serum-free medium (SFM) at 37°C in a culture chamber with a humidified atmosphere with 5% CO2. On culture day 3, a dose of fluorodeoxyuridine (8 × 10−5m) was given for 3 d to inhibit proliferation of dividing olfactory neurons and non-neuronal explant tissue. On culture day 6, and every 2 d afterward, the media were changed to fresh SFM. The explants were used for experiments on culture days 7–8 or 20–28, when GnRH-1 cells have ceased migration.
Experimental groups for calcium imaging consisted of three cultures per group and at least seven GnRH-1 neurons per image field per culture. Calcium activity within GnRH-1 neurons was monitored in explants exposed to SFM alone or SFM containing the sodium channel blocker tetrodotoxin (TTX; 10−6m), the GABAA antagonist picrotoxin (PIC; 10−4m), or the GABAB antagonist saclofen (SAC; 10−8m). A control group was exposed to calcium-depleted SFM that had been adjusted for osmolarity with NaCl.
Calcium imaging. The indicator dye Calcium Green-1 AM (Molecular Probes, Eugene, OR) was chosen based on its fluorescent intensity at low calcium concentrations (very sensitive to changes in calcium), stability of cytoplasmic labeling at 37°C, intermediate rate of photobleaching, and excitation in the visible light range (less phototoxic and does not pose a problem if using UV-absorbing substances during culture treatments) (Thomas et al., 2000).
Nasal explants were exposed to Calcium Green-1 AM for 20 min in a CO2 humidified incubator. The dye was diluted to 2.7 mm concentration in 80% DMSO and 20% pluronic F-127 solution. This solution was diluted 1:200 with SFM to a final Calcium Green concentration of 13.5 μm. Explants were then washed twice with media (10 min each) and loaded into a heated perfusion chamber (Warner Instruments, Hamden CT). Medium was perfused across the cultures at a rate of ∼100 μl/min using a variable speed peristaltic pump (Spectra Hardware, Inc., Westmoreland City, PA). Temperature control was accomplished using a voltage regulator that controlled the temperature of the lower stage of the perfusion chamber. Calcium Green was visualized (Fig.1A,C) using a Nikon microscope equipped with a 20× fluorescence objective and an ICCD camera (Video Scope International, Sterling, VA) linked with a Power Macintosh 7300 series computer equipped with imaging software (IP Lab Spectrum; Signal Analytics Corp., Vienna, VA). Excitation wavelengths were 450–490 nm and emission was monitored at 520–560 nm.
Fig. 1.
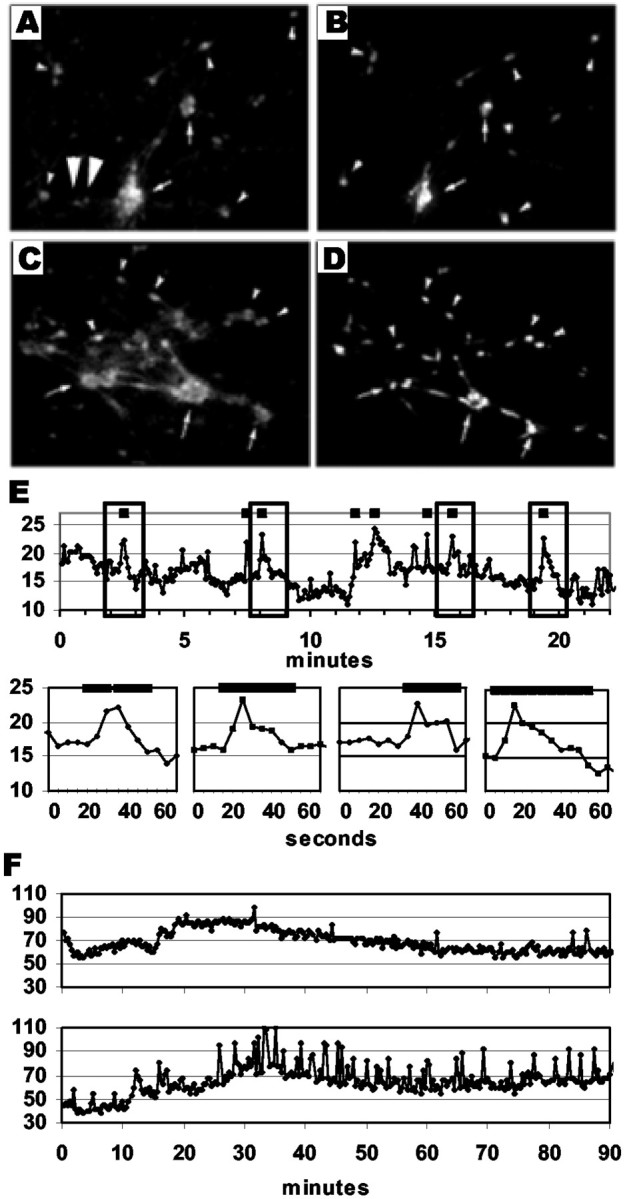
Changes in intracellular calcium can be used to evaluate activity patterns in GnRH-1 neurons. A–D, Digitized images of neurons in nasal explants. Calcium imaging (A, C) and GnRH-1 immunofluorescence (B, D) for explants at 1 week (A, B) and 3 weeks (C, D) in vitro. Neurons in the periphery that label with Calcium Green are predominantly GnRH-1 neurons (compare A, B; C, D).Arrows indicate clusters of GnRH-1 neurons, whereassmall arrowheads indicate single GnRH-1 neurons.Oversized arrowheads (A) indicate non-GnRH-1 cells loaded with Calcium Green. E, Calcium Green trace of SFM-treated culture quantified from digital images obtained at 5 sec intervals. Top graph shows complete recording over 22 min. Hatch marks at the topof the graphs in E demonstrate significant peaks as detected by the PULSAR algorithm. Bottom graphs inE (expanded data from boxed areas intop graph) demonstrate duration of significant peaks.F, Calcium Green traces in two individual cells from the same SFM-treated explant displaying unique activity patterns.
Analysis of calcium data. Intracellular calcium was monitored for 100 min, and images were captured every 20 sec. The time interval of 20 sec was chosen after analysis of data collected at 5 sec intervals for 25 min (Fig. 1E). This analysis revealed that the average duration of intracellular calcium peaks was 28.12 ± 7.04 sec and that the shortest duration observed was interpolated as 23.38 sec. In addition, recordings taken in 2–20 sec intervals revealed that intervals of <10 sec caused quenching of the fluorescent Calcium Green dye over the recording period. Therefore, a 20 sec interval was chosen for the experiments reported here because this interval accurately separated sequential peaks in intracellular calcium while limiting the amount of dye quenching within our preparation. The imaged field routinely contained 12–30 Calcium Green-labeled neurons (Fig. 1). GnRH-1 neurons were identified after Calcium recording, using fluorescent immunocytochemistry (see below). Optical densities (ODs) of the Calcium Green signals were determined using IPLab software. ODs were based on a fixed area within the cell body of the labeled neurons. A background value was subtracted from each OD to yield a corrected OD that was specific to intracellular events. The background values were determined by measuring the OD of a cell-free area adjacent to each monitored neuron. The traces of intracellular calcium for each GnRH-1 neuron were then individually analyzed using the PULSAR algorithm (Merriam and Wachter, 1982) to determine significant changes in OD levels (see Figs. 2-4) equal to peaks in intracellular calcium. Within our cultures, GnRH-1 neurons exhibited variable fluctuations in baseline calcium levels (Fig.1F). PULSAR is frequently used to identify peaks in hormone levels, particularly when there is a naturally fluctuating baseline. Thus, PULSAR could be used on the data directly obtained from our calcium imaging experiments, easily compensating for baseline fluctuations. PULSAR uses an algorithm that determines baseline values in segments of the data and makes evaluations only within these segments. Therefore, significant rises in intracellular calcium can be determined based on the baseline at a preset amount of time before and after the data point. In addition, PULSAR uses different cutoff values for peaks consisting of sequential points. A rise that includes one data point must be higher than one consisting of multiple points to be labeled as a peak. G-values (G1–G5), corresponding to 1–5 sequential data points, are used to evaluate the significance of a rise in the data. A G value of 2.0 means the rise must be >2.0 times the SD of the mean baseline to be considered a significant peak. The peak detection parameters we used for PULSAR, G(1)-G(5), were 2, 1.75, 1.5, 1.25, and 1. Two SDs were chosen to obtain 95% confidence in determining statistically significant peaks. A smoothing window of 5% of the total number of data points was used to determine segmented baseline values. The SD was determined by using the mean of the SDs identified by time series analysis using a window corresponding to 5% of the total number of data points (GB-Stat version 6.5; Dynamic Microsystems, Silver Spring, MD). These parameters were used for every cell monitored, regardless of treatment, to maintain an objective and equal evaluation of the raw data. For each individual GnRH-1 neuron, the number of peaks as well as the amplitude and time of the peaks was determined and used in subsequent analyses.
Fig. 2.
Calcium peaks are rarely observed in non-GnRH-1 cells in 1-week-old (A, B) and 3-week-old (C, D) nasal explants. Calcium traces from individual non-GnRH-1 cells maintained in SFM. Values represent mean optical density, after background correction, of Calcium Green-1 fluorescence within the cell soma.
Fig. 3.
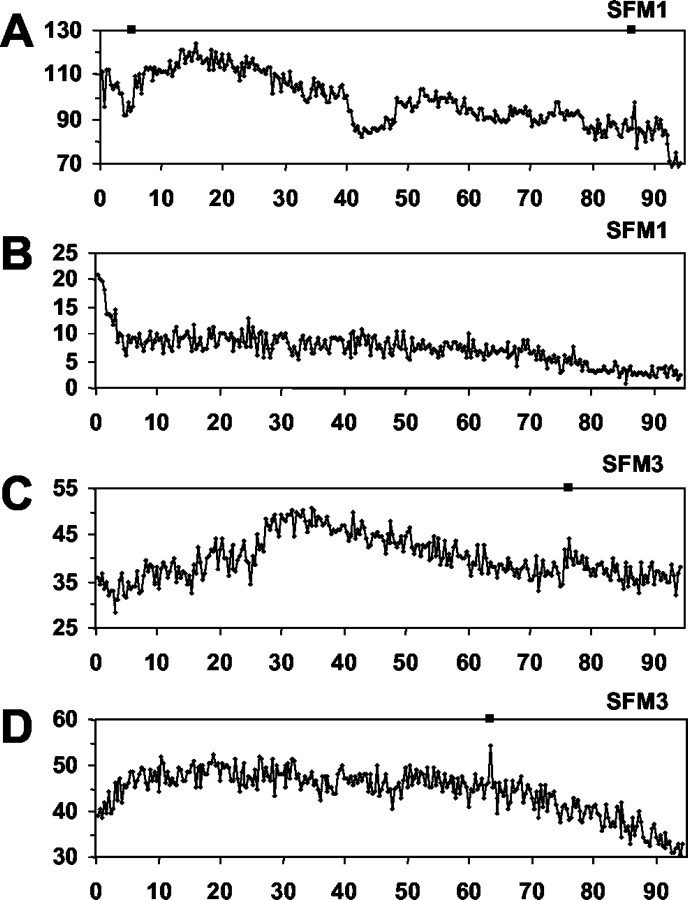
Calcium fluctuations in GnRH-1 neurons in 1-week-old nasal explants. Calcium traces from individual GnRH-1 neurons treated acutely with serum-free media (A, SFM1), tetrodotoxin (B, TTX1), picrotoxin (C, PIC1), or saclofen (D, SAC1). Values represent mean optical density, after background correction, of Calcium Green-1 fluorescence within the cell soma. Asterisks indicate significant peaks in intracellular calcium as determined by PULSAR analysis.
Fig. 4.
Calcium fluctuations in GnRH-1 neurons in 3-week-old nasal explants. Calcium traces from individual GnRH-1 neurons treated acutely with serum-free media (A, SFM3), tetrodotoxin (B, TTX3), picrotoxin (C, PIC3), or saclofen (D, SAC3). Values represent mean optical density, after background correction, of Calcium Green-1 fluorescence within the cell soma. Asterisks indicate significant peaks in intracellular calcium as determined by PULSAR analysis.
For each culture, the time points of the peaks detected in each cell were archived into a single file. These files were analyzed with a script written in the MATLAB program that divided the data into 1 min intervals and converted these data into a series of “0”s and “1”s for each cell. 0s represent 1 min periods where no peaks were detected, and 1s denote that PULSAR detected at least one significant calcium peak during the 1 min interval. Multiplicity of calcium peaks was ignored during that 1 min period. This series of 0s and 1s were summed for each culture and processed using a one-dimensional continuous wavelet transform (WAVELET) using MATLAB scripts. WAVELET analysis provides spectral decomposition of signal data as well as locates the dominant spectral features of the data in time. The 1 min subdivisions normalized the wavelet transform to the 1 min frequency, making our temporal resolution very high. The wavelet transform was plotted in a 40 line contour plot with shading interpolation denoting the relative strength of the pulse activity as a function of time (see Fig. 5). Pulses were defined as periods when multiple GnRH-1 neurons displayed synchronized calcium oscillations that produced activity clusters with values that were at least half the strength of the strongest detectable cluster. A cutoff value of 50% was used because a clear break occurs in the frequency distribution of the amplitudes of the clusters at 45%. For each pulse a duration, from start to end, and an interpulse interval (IPI), from start to start, were calculated. The results of the WAVELET analysis were further supported by Fourier analysis, which uses a Nyquist frequency of half the sampling rate and detected a dominate frequency of 18.7 ± 4.1 min but could not ascribe the temporal location of significant events.
Fig. 5.
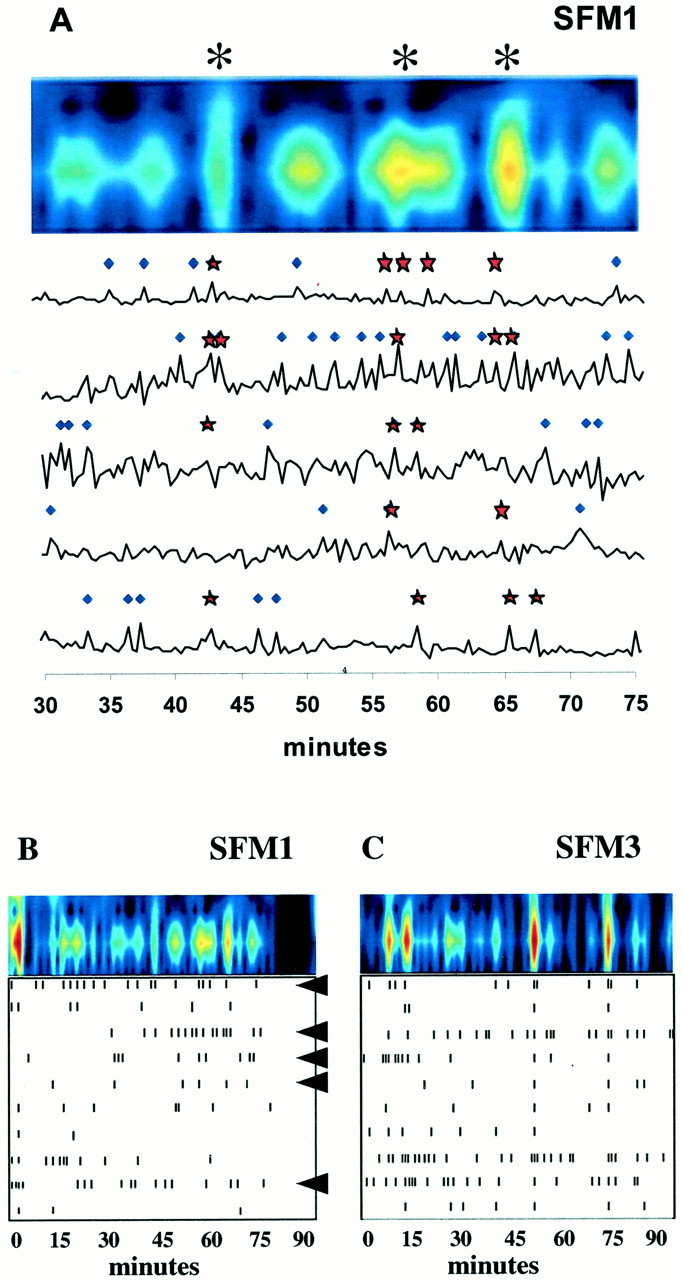
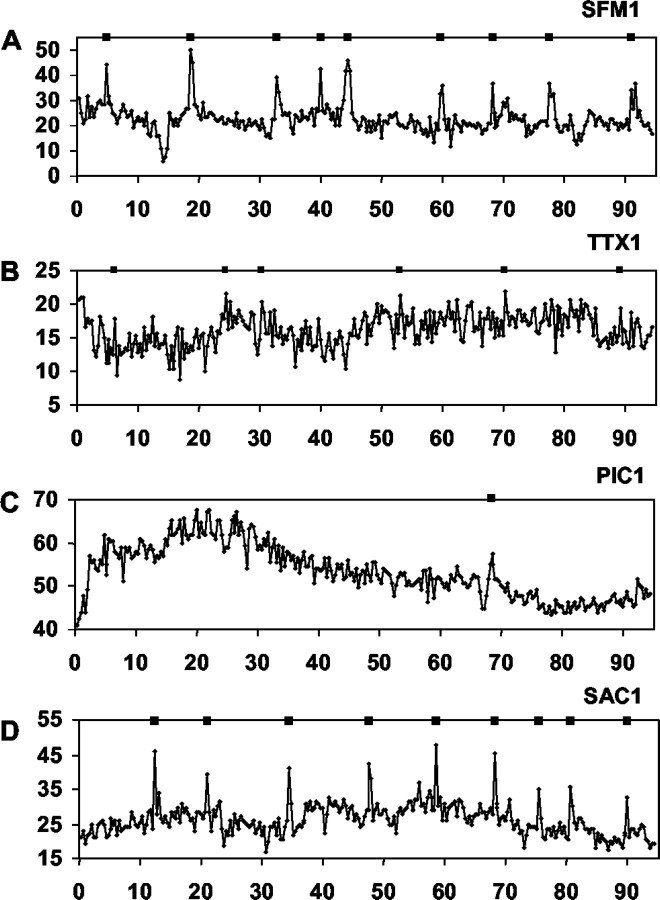
Synchronous neuronal activity is observed in GnRH-1 neuronal populations. The top panel inA demonstrates WAVELET analysis (color contour plot) of a portion of the combined calcium activity of 10 cells (5 of which are shown below) from a single nasal explant preparation treated with SFM alone. Shown below (B, C) are the minute intervals (bottom box, tick marks) in which PULSAR detected calcium peaks and the WAVELET transform (top, color contour plots) for GnRH-1 neurons in a 1-week-old, SFM-treated explant (B, SFM1) and a 3-week-old, SFM-treated explant (C, SFM3). The bottom panels (withtick marks) indicate significant calcium peaks in 10 individual GnRH-1 neurons (rows) in a single explant preparation. The top panels in B andC (with color columns) represent the one-dimensional continuous sum of plotted cell activity (frombottom panel) in each preparation (see Materials and Methods). Asterisks in A indicate significant pulses of synchronized activity in the monitored population of GnRH-1 neurons. Stars on individual activity plots (A, bottom) indicate PULSAR detected peaks that occurred in WAVELET detected synchronized pulses; diamondsindicate PULSAR detected peaks only. Arrowheads inB indicate cells also shown in A. Independent of age, GnRH-1 neuronal populations exhibit synchronized calcium oscillations with an interval of ∼18 min.
For the sake of clarity, peaks are defined as significant rises in calcium levels (calcium oscillations) in individual neurons as determined with PULSAR analysis. Pulses are defined as periods when two or more GnRH-1 neurons are displaying simultaneous peaks. The characteristics of GnRH-1 pulses were determined by WAVELET analysis. It is important to note that pulses can be made up of a variable number of peaks based on the strength of calcium signaling in the population of neurons.
Statistical comparisons of data were calculated with the statistical software GB-STAT version 6.5 (Dynamic Microsystems, Inc., Silver Spring, MD). ANOVA followed by Fisher's LSD post hocanalysis was used to compare groups. P values < 0.05 were considered statistically significant. All data are expressed as mean ± SEM.
Immunocytochemistry. For identification of GnRH-1 neurons after calcium imaging, cultures were fixed with 4% formaldehyde in PBS for 1 hr. After fixation, samples were washed with PBS, incubated for 1 hr in 10% NGS and 0.3% triton X-100, washed several times in PBS, and incubated in GnRH-1 antibody (1:3000; SW1) (Wray et al., 1988) overnight at 4°C. The next day, cultures were washed several times in PBS and incubated in a directly conjugated secondary antibody: goat anti-rabbit conjugated Cy3 (1:1000; Jackson ImmunoResearch, West Grove, PA).
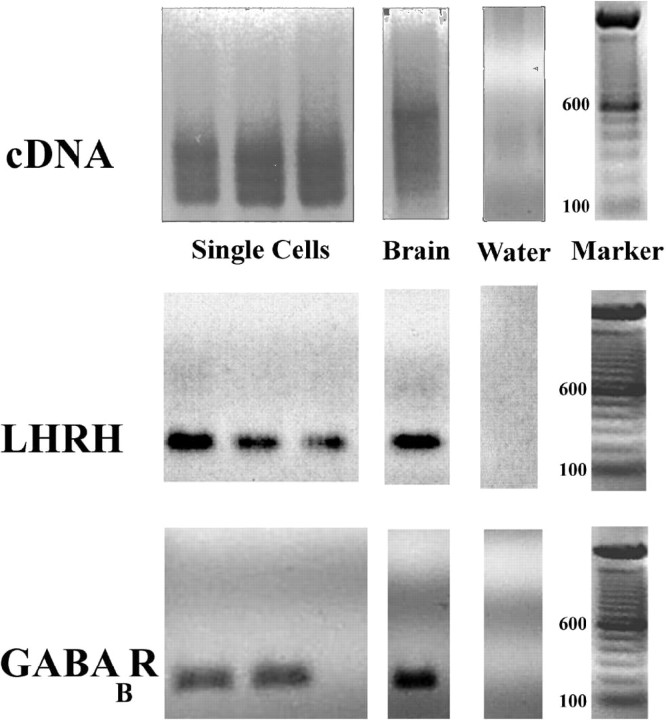
Generation of cDNA libraries for examination of GABAB receptors. At 7 div and 28 div, explants were observed under a Nikon inverted microscope, GnRH-1-like neurons were identified using their bipolar morphology, and single GnRH-1-like cells were isolated with a micromanipulator fitted with a pulled microcapillary pipette (Kramer et al., 2000; Kramer and Wray, 2000). Construction of cDNA libraries was based on published protocols (Dulac and Axel, 1995; Kramer et al., 2000). Briefly, 1 μg of brain RNA or a single cell was placed into a reaction mixture containing 4 μl of lysis buffer [for 100 μl of mix on ice, 20 μl of Moloney murine leukemia virus (MMLV) buffer 5× [Bethesda Research Labs (BRL), Branchburg, NJ], 76 μl of H2O, 0.5 μl of NP40 (Amersham Biosciences, Piscataway, NJ), 1 μl of PrimeRNase inhibitor (3′5′ Inc., Boulder, CO), 1 μl of RNAguard (Amersham), 2 μl of a freshly made 1:24 dilution of the stock primer mix [10 μl of each 100 mm dNTP (BRL), 10 μl of 50 OD/ml pd(T) 19–24, and 30 μl of H2O]]. The mixture was incubated (65°C, 1 min; ice, 1 min; room temperature, 2 min). A 1:1 (volume, 0.5 μl) mix of avian myeloblastosis virus and MMLV–reverse transcriptases (BRL) were added (37°C, 15 min; 65C, 10 min) followed by stock tailing buffer (4.5 μl; 100 μl 5× BRL TdT buffer, 3.75 μl of 100 mmdATP, 146.25 μl of H2O; 37C for 15 min; 65°C for 10 min). Samples were kept on ice until PCR. PCR mix containing 10 μl of 10× PCR buffer II (PerkinElmer Life Sciences, Foster City, CA), 10 μl of 2 mm MgCl2(PerkinElmer Life Sciences), 0.5 μl of 20 mg/ml BSA (Boehringer Mannheim, Indianapolis, IN), 1 μl of each 100 mm dNTP (BRL), 1 μl of 5% Triton X-100 (Sigma), 5 μg of AL1 primer (ATT GGA TCC AGG CCG CTC TGG ACA AAA TAT GAA TTC (T) 24), 2 μl of AmpliTaq (PerkinElmer Life Sciences), and 57.5 μl of H2O was mixed on ice. PCR mix (90 μl) was added to each PCR thin wall tube (Stratagene, La Jolla, CA) containing 10 μl of the template, and then placed in a Perkin-Elmer Life Sciences DNA Thermal Cycler for 25 cycles (94°C, 1 min; 42°C, 2 min; 72°C, 6 min with 10 sec extension time at each cycle). After the first 25 cycles, 1 μl of AmpliTaq was added to each tube, and 25 more cycles were performed with the same program, but without the 10 sec extension at each cycle. Phenol–chloroform extraction was performed, samples were then ethanol-precipitated and run on a 1.5% agarose gel (see Fig. 7a)
Fig. 7.
GnRH-1 neurons express GABABreceptors. Representative gel documentation of PCR products from single-cell RT-PCR performed on individual cells extracted from nasal explants. (1) cDNA produced from poly(A) targeted, AL1 primer, PCR amplification; (2) products produced by PCR amplification of above cDNA using primers specific for GnRH-1 (LHRH), or (3) primers specific for GABAB receptor.
PCR analysis. Primers were designed for GnRH-1 (5′-ACTGGTCCTATGGGTTGCGCCCTG-3′), (5′-CGGGGCCAGTGGACAGTACATTCG-3′), β 3 tubulin (5′-GAGGACAGAGCCAAGTGGAC-3′), (5′-CAG-GGCCAAGACAAGCAG-3′), and GABAB receptors (5′-CGCCTTTGCCTCTCTGGCC-3′), (5′-GGCG-CAGTTCAGAGACACGC-3′) and (5′-GGTTGATCACTCGAGGTGAATGG-3′), (5′-GCGGATCTGCTGCCGAGACTGC-3′). For each reaction 24.8 μl of nuclease-free water, 4.0 μl of 10× PCR buffer (PerkinElmer Life Sciences), 3.2 μl of 25 mm MgCl2 (PerkinElmer Life Sciences), 2.5 μl of dNTP mix (10 μl of each dNTP, 360 μl water), and 0.5 μl AmpliTaq Gold (PerkinElmer Life Sciences) were mixed. Two microliters of each primer were added to the mixture so that the primer final concentration was between 100 and 500 nmol. One μl of template cDNA was added to the mix. Total brain cDNA was used as a positive control. Samples were run on RoboCycler (Stratagene): 10 min 94°C pre-run, 1 min 94°C, 1 min 55°C or 65°C (depending on the primers), 2 min 72°C for 40 cycles, and 10 min 72°C post run. Amplified samples were run on a 1.5% agarose gel (Fig. 7). Specific bands were observed in all control total brain lanes, whereas no bands were seen in water lanes.
RESULTS
Bipolar cells in the periphery of the nasal explants, associated with outgrowing fibers, readily loaded with the calcium indicator. As predicted from their morphology (Fueshko and Wray, 1994), the majority of these cells were GnRH-1-positive neurons (Fig. 1). Initial analysis by PULSAR of the calcium traces from 1-week-old cultures exposed to SFM alone revealed that 76% of the Calcium Green-loaded GnRH-1 neurons displayed very prominent calcium oscillations. After 3 weeks in culture, the percentage of GnRH-1 neurons displaying calcium oscillations (63%) was not significantly different than the percentage observed in GnRH-1 cells at 1 week. Calcium traces of individual GnRH-1 cells revealed a wide variety of patterns (Fig. 1F). Control cultures treated with calcium-depleted SFM lacked visible calcium oscillations (data not shown).
Non GnRH-1 cells
In the imaged fields, occasional non-GnRH-1 calcium-loaded cells were present (Fig. 1A, large arrowheads). Few calcium peaks were detected in these cells (Fig. 2). Non-GnRH-1 calcium-loaded cells from at least two different cultures (≥7 cells per culture) from each time point and treatment paradigm were evaluated with the PULSAR algorithm for peaks in intracellular calcium. In SFM, individual non-GnRH-1 cells displayed 1.25 ± 1.5 and 0.75 ± 1.04 peaks/100 min at 1 and 3 weeks, respectively. In all other treatments, at both ages, individual non-GnRH-1 calcium-loaded cells showed similar peak calcium values of ∼1 peak/100 min. TTX: 1 week = 0.2 ± 0.45 peaks/100 min and 3 weeks = 1.0 ± 1.41 peaks/100 min; PIC: 1 week = 0 peaks/100 min and 3 weeks = 0.5 ± 0.55 peaks/100 min; SAC: 1 week = 1.0 ± 1.41 peaks/100 min and 3 weeks = 1.0 ± 0.82 peaks/100 min. WAVELET analysis of non-GnRH-1 cells was not performed because these cells exhibited no synchronous clusters of activity unless depolarized with KCl (data not shown).
GnRH-1 neurons at 1 week in culture
SFM
PULSAR analysis revealed numerous calcium peaks in individual SFM-treated GnRH-1 neurons (Table 1, Fig. 3A). Twenty-six cells were analyzed, and the mean number of peaks detected was 7.5 ± 0.96/100 min (∼4.5/hr). WAVELET analysis of PULSAR-identified peaks per culture revealed that the calcium oscillations synchronized with an interval of 17.9 ± 1.99 min (see Fig. 5A) and that the duration of these events was 4.9 ± 0.63 min. An IPI of 17.9 ± 1.99 min is comparable to data reported for the pulsatile profile of GnRH-1 secretion in the immortalized mouse GnRH-1 neuronal cell line GT1–7 (Martinez de la Escalera et al., 1992).
Table 1.
Summary of results from PULSAR and WAVELET analysis for all treatments
| PULSAR | WAVELET | ||||
|---|---|---|---|---|---|
| Number of cells with peaks (%) | Number of peaks/100 min | Amplitude (OD) | Interpulse interval (min) | Pulse duration (min) | |
| 1 week | |||||
| SFM 1 | 26 /26 (100) | 7.5 ± 0.961-b | 19.09 ± 3.611-b | 17.88 ± 2.00 | 4.91 ± 0.63 |
| TTX 1 | 22 /22 (100) | 4.7 ± 0.771-b | 6.75 ± 0.521-a,1-b | 18.67 ± 2.68 | 2.61 ± 0.631-a,1-b |
| PIC 1 | 9 /23 (40) | 1.0 ± 0.361-a,1-b | 10.21 ± 0.571-a | 42.11 ± 8.601-a,1-b | 3.72 ± 0.97 |
| SAC 1 | 20 /24 (83) | 5.5 ± 1.271-b | 21.43 ± 1.611-b | 11.65 ± 1.241-a,1-b | 3.02 ± 0.401-a |
| 3 weeks | |||||
| SFM 3 | 34 /34 (100) | 14.7 ± 1.651-a | 12.07 ± 0.741-a | 18.45 ± 1.29 | 4.33 ± 0.50 |
| TTX 3 | 18 /21 (84) | 4.3 ± 0.611-b | 10.07 ± 0.701-a | 20.27 ± 2.88 | 2.44 ± 0.231-a,1-b |
| PIC 3 | 33 /39 (84) | 6.1 ± 0.901-b | 9.53 ± 0.371-a | 23.17 ± 3.16 | 4.98 ± 0.99 |
| SAC 3 | 33 /35 (94) | 6.8 ± 1.501-b | 9.70 ± 0.451-a | 13.03 ± 1.871-b | 4.18 ± 0.56 |
Different from SFM 1.
Different from SFM 3.
P < 0.05.
Propagation of membrane depolarization
TTX visibly attenuated the calcium oscillations in GnRH-1 neurons (Fig. 3B, note change in y-axis scale). However, because the SD of the ODs of the TTX-treated cells was small, PULSAR analysis identified calcium peaks in GnRH-1 neurons exposed to TTX. Twenty-two GnRH-1 neurons were analyzed, and the mean number of peaks was 4.7 ± 0.77/100 min, or 2.82 peaks/hr. The amplitude of the calcium peaks was significantly reduced as compared with GnRH-1 neurons in SFM-treated explants (19.09 ± 3.61 vs 6.75 ± 0.52 OD units). These calcium oscillations synchronized with an interval of 18.67 ± 2.68 min, an IPI similar to that observed in the SFM-treated cultures (17.9 ± 1.99 min). Although the IPI was similar to that of GnRH-1 cells in SFM, the duration was significantly decreased (2.61 ± 0.63 min vs 4.9 ± 0.63 min). Taken together, these data indicate that a pulsatile pattern of intracellular calcium activity was maintained in GnRH-1 neurons despite blockade of membrane propagation of electrochemical depolarization. However, the amplitude of calcium peaks and duration of the calcium pulses were diminished without the availability of sodium conductivity.
GABAergic signaling
The influence of GABAergic neurons on GnRH-1 neuronal activity was examined by application of the GABAA and GABAB antagonists picrotoxin (PIC) and saclofen (SAC). PIC (Fig. 3C) abolished calcium peaks to a greater extent than that observed after application of TTX. PULSAR analysis detected only 0.6 calcium peaks per cell per hour (23 GnRH-1 neurons were analyzed and the mean number of peaks detected was 1 ± 0.36/100 min). WAVELET analysis found few calcium oscillations to be synchronized. However, in the cultures that did exhibit synchronized calcium pulses, the IPI increased to 42.07 ± 8.63 min (see Fig.6A), although the duration (3.72 ± 0.97 min) of these pulses was not significantly altered from GnRH-1 cells in SFM. These data indicate that GABA influences the number and amplitude of calcium oscillations within individual GnRH-1 neurons, reducing calcium peaks to background non-GnRH-1 cell values, and that GABAA signaling is important for the timing of synchronous activity in the population of GnRH-1 neurons.
Fig. 6.
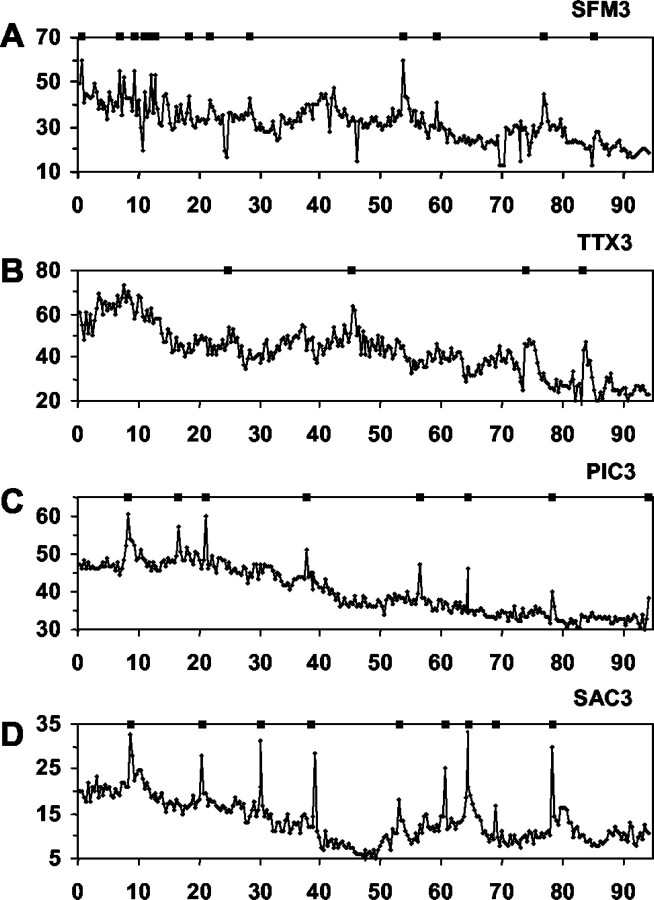
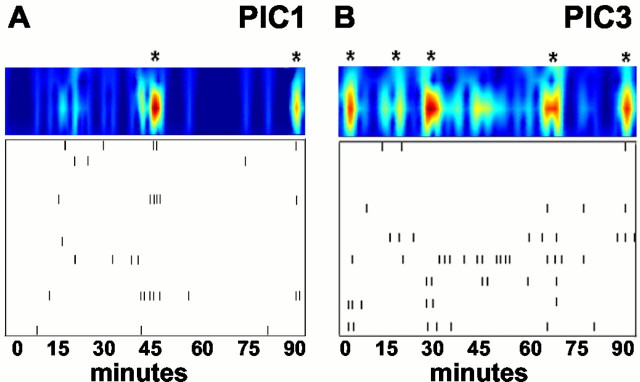
Picrotoxin treatment alters GnRH-1 neuronal activity. Shown are the minute intervals (box, tick marks) in which PULSAR detected calcium peaks and the WAVELET transform (top, shaded contour plots) for a 1-week-old, picrotoxin-treated explant (A, PIC1) and a 3-week-old, picrotoxin-treated explant (B, PIC3). The bottom panels (with tick marks) indicate significant calcium peaks in nine individual GnRH-1 neurons (rows) in a single explant preparation. Note that some cells exhibited no detectable peaks (empty rows). The top panels (with color columns) represent the one-dimensional continuous sum of plotted cell activity (frombottom panel) in each preparation (see Materials and Methods). Asterisks indicate significant pulses of synchronized activity in the monitored population of GnRH-1 neurons. Disruption of GABAA receptors decreased calcium peaks in individual GnRH-1 cells at both ages but only altered (lengthened) the interpulse interval in cells from 1-week-old explants.
GnRH-1 neurons exposed to SAC appeared very active (Fig.3D). PULSAR analysis determined that these neurons had 3.3 calcium peaks per hour (24 GnRH-1 cells were analyzed, and the mean number of calcium peaks was 5.5 ± 1.27/100 min) with an average amplitude of 21.43 ± 1.61 OD units. These values were not significantly different than for GnRH-1 cells maintained in SFM alone. However, WAVELET analyses revealed a significant decrease in the IPI (11.2 ± 1.24 min) and duration (3.0 ± 0.4 min) of synchronized calcium pulses in SAC-treated cultures as compared with untreated controls. These findings suggest that GABA is acting through GABAB receptors to alter the frequency of synchronized calcium events within the GnRH-1 neuron population.
GnRH-1 neurons at 3 weeks in culture
Basal (SFM)
To determine the properties of GnRH-1 neurons that change as a function of development in vitro, calcium dynamics were also monitored in GnRH-1 neurons in 3-week-old cultures (Fig.4A). GnRH-1 neurons in older cultures exhibited 8.82 calcium peaks per hour (34 GnRH-1 cells were analyzed, and the mean number of calcium peaks was 14.7 ± 1.65/100 min) with an amplitude of 12.07 ± 0.74 OD units. WAVELET analyses (Fig. 5B) revealed a pulsatile pattern of synchronized calcium oscillations with an IPI of 18.45 ± 1.29 min and pulse duration of 4.3 ± 0.5 min.
Propagation of membrane depolarization (TTX)
Exposure of GnRH-1 neurons in 3-week-old cultures to TTX (Fig.4B) resulted in significantly fewer calcium peaks (2.58/hr, 21 GnRH-1 cells were analyzed, and the mean number of calcium peaks was 4.3 ± 0.61/100 min). The amplitude of the peaks was not different than those in age-matched SFM-treated GnRH-1 neurons. The synchronized pulses in these cultures maintained a similar IPI (20.27 ± 2.88 min) as that observed in the 3-week-old SFM-treated group. However, the duration of the synchronized pulses was significantly less (2.44 ± 0.23 min) than that of GnRH-1 cells in SFM-treated cultures.
GABAergic signaling (PIC and SAC)
Treatment of GnRH-1 neurons in 3-week-old cultures with PIC (Fig.4C) caused a significant decrease in the number of calcium peaks (3.66/hr; 39 GnRH-1 neurons were analyzed, and the mean number of peaks = 6.1 ± 0.9/100 min) as compared with those observed in SFM-treated, 3-week-old GnRH-1 neurons. Peak amplitude remained similar. WAVELET analysis revealed that PIC had no effect on the IPI or duration of the synchronized calcium pulses in 3-week-old cultures (Fig. 6B). These data suggest that GABA signaling through GABAAreceptors is important for nonsynchronous activity of individual GnRH-1 neurons but is no longer influencing the synchronous activity of the GnRH-1 neuronal population.
Treatment of GnRH-1 neurons in 3-week-old cultures with SAC also caused a significant decrease in the number of detected calcium peaks (4.08/hr; 35 GnRH-1 neurons were analyzed, and the mean number of peaks = 6.8 ± 1.5/100 min), with no effect on the amplitude of the calcium oscillations (Fig. 4D). Unlike PIC treatment, SAC treatment significantly decreased the IPI (13.0 ± 1.9 min) of the synchronized calcium oscillations, whereas the duration of these pulses was unaltered. These findings strengthen the earlier results suggesting that GABA is acting through GABAB receptors to influence the timing of synchronized calcium events within the GnRH-1 neuron population.
Comparison of activity of GnRH-1 neurons at 1 versus 3 weeks
SFM
GnRH-1 neurons in older cultures maintained in SFM exhibited a doubling in mean number of peaks as detected by PULSAR (1 week = 7.5 ± 0.96/100 min; 3 weeks = 14.7 ± 1.65/100 min). However, the amplitude of these calcium peaks was significantly less than those observed in 1-week-old cultures (1 week = 19.09 ± 3.61; 3 week = 12.1 ± 0.74). WAVELET analyses revealed no changes in the IPI or pulse duration between GnRH-1 neurons maintained in SFM for 1 week versus 3 weeks. However, in the 3-week-old cultures, the pulses revealed by WAVELET analysis were comprised of cells more active during the 1 min intervals. At both ages, peaks were detected in all GnRH-1 cells imaged (29/29 at 1 week and 34/34 at 3 weeks).
TTX
TTX decreased the mean number of peaks in GnRH-1 neurons to similar levels at both ages (1 week = 4.7 ± 0.77/100 min; 3 weeks = 4.3 ± 0.61/100 min). However, the effect of TTX was much greater at 3 weeks (decrease of 71%) than at 1 week (decrease of 37%). Also, unlike their 1 week counterparts, the amplitude of the peaks at 3 weeks was not significantly different than in age-matched SFM treated GnRH-1 neurons. In the presence of TTX, peaks were observed in all GnRH-1 cells at 1 week (23/23) and 84% of the cells at 3 weeks (16/19).
PIC and SAC
PIC decreased the mean number of calcium peaks in GnRH-1 neurons at both ages. However, the change that occurred was greater at 1 week (decrease of 86%) as compared with 3 weeks (59%). Of all the perturbations examined, PIC treatment at 1 week resulted in the fewest GnRH-1 cells that exhibited any peaks (18/45, i.e., 40%). Eighty-four percent still showed peaks in the presence of PIC at 3 weeks (32/39). SAC also decreased the mean number of peaks in GnRH-1 neurons but only at 3 weeks (decrease of 54%). SAC treatment did not change the number of cells that exhibited peaks [83% at 1 week (20 of 24) and 94% at 3 weeks (33 of 35)]. PIC and SAC were the only perturbations that influenced the IPI of the synchronized calcium events, although in different directions. PIC increased the IPI but only at 1 week (57% longer than that observed in SFM), whereas SAC reduced the IPI at both ages (35% at 1 week and 30% at 3 weeks).
Presence of GABAB receptors
Previous electrophysiological studies revealed the presence of functional GABAA receptors in explant GnRH-1 neurons (Kusano et al., 1995), however functional GABAB receptors were not identified. In this investigation, single GnRH-1 cells were examined for the presence of GABAB receptor transcripts (Fig.7). At 7 and 28 div, 8 of 10 and 7 of 10 GnRH-1 cells expressed GABAB receptor transcript.
DISCUSSION
This study evaluated activity patterns of primary GnRH-1 neurons within mouse nasal explants. We report here that GnRH-1 neurons maintained in serum-free conditions exhibit synchronized pulses of intracellular calcium as early as 7 div. The intervals between the pulses were ∼18 min. This study also demonstrates that in situ GABAergic tone influences GnRH-1 neuronal activity. At 1 week in culture, GABAA signaling was essential for individual as well as synchronous activity of GnRH-1 neurons. However, after 3 weeks in vitro, individual GnRH-1 neuronal activity was only attenuated by blockage of GABAAsignaling, and synchronous activity within the GnRH-1 neuronal population was now GABAA independent.
In view of past investigations (Terasawa et al., 1999a; Funabashi et al., 2000; Moore and Wray, 2000), it is expected that the rises in intracellular calcium correlate with pulses of GnRH-1 secretion.Terasawa et al. (1999a), using primate embryonic nasal explants 2–7.5 weeks of age, reported synchronous and pulsatile activity among GnRH-1 neurons. These GnRH-1 cells showed an IPI that correlated with GnRH-1 secretion in vitro (Terasawa et al., 1999b) and mimicked the 50 min pulsatile pattern observed in vivo. Our analysis revealed that mouse GnRH-1 neurons had a pulsatile behavior with an interval of ∼18 min. Unfortunately, little data are available on GnRH-1 secretion in mouse. However, our results are comparable with the 20 min IPI observed in the immortalized GT1 mouse GnRH-1 cell line (Martinez de la Escalera et al., 1992) and similar to the 28 min interval reported for LH in castrated male mice (Kokoris et al., 1998).
The PULSAR software used to analyze calcium-imaging traces provides an objective method for monitoring intracellular calcium within individual neurons. PULSAR determines where significant peaks occur and provides descriptive data such as peak amplitude. PULSAR analysis is performed on individual cells, and hence evaluation of population dynamics is not possible per se. PULSAR does provide a method for normalizing individual calcium traces into time series data that can be used by WAVELET to determine population activity. WAVELET analysis provides a time-frequency representation of the data. By combining values for every recorded GnRH-1 neuron per culture, a temporal representation of the calcium dynamics was obtained. This then allows one to detect synchronized events within the population.
Terasawa et al. (1999a) used Fura-2 as the calcium indicator dye and observed dramatic spikes in calcium levels in GnRH-1 neurons in 2- to 7.5-week-old primate cultures. Our analysis used Calcium Green, a visible light indicator that is less phototoxic to tissues (Thomas et al., 2000). Using this dye we detected significant calcium changes, although our spikes are clearly less dramatic then those reported in primate. Certainly, the difference in magnitude of calcium changes could be attributable to the indicator dye used and/or the fact that the primate experiments (Terasawa et al., 1999a) were performed in the presence of serum, whereas ours were done in serum-free conditions. However, GnRH-1 cells in primate explants 10–12 div had little oscillatory activity (Terasawa et al., 1999a), whereas GnRH-1 neurons in mouse nasal explants exhibited calcium oscillations by 7 div. Thus, the differences in magnitude of calcium peaks and onset of oscillatory behavior may have a physiological basis and be caused by a species difference or related to chronological age of the explants.
Mouse GnRH-1 cells maintained for 1 week exhibited ∼4.5 calcium peaks per hour. This number approximately doubled by 3 weeks to 8.8 peaks per hour. Independent of number of peaks, GnRH-1 neurons at these two ages exhibited calcium oscillations that synchronized with an interval of ∼18 min. Because the number of calcium peaks increased in GnRH-1 neurons at 3 weeks, but the IPI remained unchanged, the number of peaks per 1 min intervals clearly increased in older GnRH-1 neurons. The increase in GnRH-1 neuronal activity may reflect intrinsic developmental changes in GnRH-1 neurons and/or extrinsic changes such as an increase in stimulatory or decrease in inhibitory signaling. Independent of the mechanism or mechanisms, the increase in activity of GnRH-1 neurons documented here correlates with the developmental increase in GnRH-1 peptide observed in explants as well as in vivo (Moore and Wray, 2000).
To examine the mechanisms underlying GnRH-1 neuronal activity, TTX-sensitive Na+ currents were blocked, and intracellular calcium levels were monitored. At both ages, TTX significantly decreased the duration of synchronized calcium pulses, but only significantly reduced the number of calcium peaks at 3 weeks. Because these changes did not correlate with the decrease in synchronized pulse duration, the TTX-induced change in the latter is probably caused by a generalized decrease in spontaneous activity in the GnRH-1 neuronal population. This would appear as a “shortening” of the synchronized pulse duration. At both ages, TTX treatment did not change the IPI. Acute TTX treatments in earlier studies (Kusano et al., 1995) inhibited presynaptic input and reduced GnRH-1 electrical activity. Therefore, the inability of TTX to abolish calcium peaks in individual GnRH-1 cells coupled with the observation that TTX did not alter the pattern of synchronous calcium pulses in the GnRH-1 population, suggests a “presynaptic” signal is not necessary for the “GnRH-1 pulse generator.” However, the increase in TTX-sensitive peaks at 3 weeks implies that stimulatory afferents that can alter GnRH-1 neuronal activity develop in vitro.
GABAergic transmission is an important signal during neuronal development. GABAergic signaling has been shown to modulate process outgrowth (Joo et al., 1987; Mattson and Kater, 1989; Behar et al., 1996), synaptogenesis (Redburn, 1992; Ramakers et al., 1994), and GABAA receptor expression (Frieder and Grimm, 1985; Hablitz et al., 1989; Montpied et al., 1989; Liu et al., 1997;Poulter et al., 1997). In nasal explants, muscimol, a GABAA agonist, causes depolarization of GnRH-1 neurons, which leads to a significant rise in intracellular calcium as well as increased synthesis of GnRH-1 peptide (Kusano et al., 1995;Moore and Wray, 2000). To determine whether GABAergic signals were extrinsic mechanisms (stimulatory afferents) that were altering GnRH-1 neuronal activity during in vitro development, pharmacological agents were used to selectively disrupt signaling through GABAA and GABABreceptors.
GABAA-elicited calcium rises result from chloride efflux (Chen et al., 1996) and subsequent activation of voltage-dependent calcium channels. In 1-week-old explants, native GABAergic signaling through GABAA receptors was found to profoundly modulate number and amplitude of calcium peaks in GnRH-1 neurons as well as synchronized calcium oscillations in the GnRH-1 neuronal population. At 1 week, blockage of GABAA receptors virtually eliminated GnRH-1 activity, reducing calcium peaks to background non-GnRH-1 cell levels. In contrast, GABAA signaling in 3-week-old explants did not influence interval or duration of synchronized calcium oscillations, although the number of calcium peaks in GnRH-1 neurons decreased in the presence of the antagonist. These results suggest that depolarizing GABAA signaling is important for the ontogeny of pulsatile GnRH-1 neuronal activity and synchronization, but not required for maintenance of this pattern as GnRH-1 neurons mature.
GABAergic signaling though GABAB receptors is also functional during development and primarily results in an inhibition of GABAA signaling (Obrietan and van den Pol, 1998). Signaling through GABAB receptors appears to be inhibitory to GnRH-1 neurons because in the absence of GABAB signal transduction, synchronous activity in the GnRH-1 neuronal population at both ages occurred more frequently. These data are consistent with activation of GABAB receptors influencing the timing of synchronous activity by increasing the interval between synchronized pulses of activity. GABAB receptors are metabotropic receptors that are coupled to calcium and potassium channels via G-proteins and second messenger signaling that inhibits stimulus-evoked cAMP accumulation (Chen et al., 1996). Thus, GABAB receptor signaling may act to counterbalance GABAA receptor activation and allow GnRH-1 neurons to maintain pulsatile activity, i.e., in the absence of GABAB receptor signaling, GnRH-1 neurons could become hyper-reactive to the depolarizing influence of GABAA receptor activation. Evidence for this type of compensation is observed in fetal hypothalamic neurons where application of GABAB antagonists results in a rise in basal levels of intracellular calcium in active neurons (Obrietan and van den Pol, 1998).
Similarly, the inhibitory action of PIC on synchronous activity of the GnRH-1 neuronal population may also be the result of unabated signaling through GABAB receptors. PIC nearly abolished intracellular calcium peaks in GnRH-1 neurons in 1-week-old explants and reduced the number of peaks in GnRH-1 neurons in 3-week-old explants. Without the depolarizing GABAA signal, the GABAB response would predominate and cause an inhibitory response to the GABAergic tone in the explants. This signaling could inhibit the intrinsic, non-TTX-sensitive, pulsatile activity of the GnRH-1 neuronal population, especially if some of the GABAergic influences were via trophic influences (Behar et al., 1994). The “age-related” effect of PIC on the synchronous activity of the GnRH-1 neuronal population may also reflect a developmental transition of GABAergic signaling from excitatory to inhibitory (Obrietan and van den Pol, 1995; Lee et al., 2000; Auger et al., 2001). The stimulatory effect of GABA on GnRH-1 neurons at early developmental stages may be essential for differentiation into mature GnRH-1 neurons possessing a pulsatile pattern of activity. Once the maturity of the GnRH-1 system is established, the transition of GABA signaling to inhibitory may allow for appropriate postnatal modulation of GnRH-1 pulsatile secretion. At partial odds with this idea is the effect of GABAergic signaling (with regard to number of calcium peaks) on GnRH-1 neurons within 3-week-old explants treated with PIC. In these cultures, blockade of GABAergic signaling decreased overall activity of GnRH-1 neurons, albeit to a lesser extent than that observed in 1-week-old explants. This suggests that stimulatory GABAergic signaling was still present. However, it is clear that multiple cell types are present in mouse nasal explants (Wray et al., 1996), and therefore GnRH-1 neurons may respond to other native signals in this preparation which themselves are effected by GABAergic signals. Alternatively, full maturity of the GnRH-1 system may require factors within the CNS.
In summary, this investigation has shown that primary GnRH-1 neurons in embryonic nasal explants display synchronized and pulsatile calcium oscillations with an interval of ∼18 min. The mechanisms underlying this pattern of synchronicity involves, in an age-dependent manner, both TTX-sensitive sodium currents and GABAergic signals. Our results indicate that the embryonic GnRH-1 neuronal population relies heavily on GABAergic signals for initiation of synchronized pulsatile activity but thereafter possesses an innate capacity for this behavior that is only modified by signals from neighboring cells.
Footnotes
We thank Andree Reuss and Neda Sharifi for their technical skills in performing the single-cell RT-PCR analysis, as well as Dr. Phillip Kramer for his guidance in the use of this technique.
Correspondence should be addressed to Susan Wray, Cellular and Developmental Neurobiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Building 36, Room 5A-21, Bethesda, MD 20895-4156. E-mail: swray@codon.nih.gov.
J. P. Moore's present address: University of Louisville School of Medicine, Division of Endocrinology and Metabolism, Louisville, KY 40202.
REFERENCES
- 1.Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci USA. 2001;98:8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behar TN, Schaffner AE, Colton CA, Somogyi R, Olah Z, Lehel C, Barker JL. GABA-induced chemokinesis and NGF-induced chemotaxis of embryonic spinal cord neurons. J Neurosci. 1994;14:29–38. doi: 10.1523/JNEUROSCI.14-01-00029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belchetz PP, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol (Lond) 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daikoku S, Koide I, Chikamori-Aoyama M, Shimomura Y. Migration of LHRH neurons derived from the olfactory placode in rats. Arch Histol Cytol. 1993;56:353–370. doi: 10.1679/aohc.56.353. [DOI] [PubMed] [Google Scholar]
- 7.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 8.Frieder B, Grimm VE. Some long-lasting neurochemical effects of prenatal or early postnatal exposure to diazepam. J Neurochem. 1985;45:37–42. doi: 10.1111/j.1471-4159.1985.tb05471.x. [DOI] [PubMed] [Google Scholar]
- 9.Fueshko SM, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166:331–348. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- 10.Funabashi T, Daikoku S, Shinohara K, Kimura F. Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology. 2000;71:138–144. doi: 10.1159/000054529. [DOI] [PubMed] [Google Scholar]
- 11.Hablitz JJ, Tehrani MH, Barnes EM. Chronic exposure of developing cortical neurons to GABA down-regulates GABA/benzodiazepine receptors and GABA-gated chloride currents. Brain Res. 1989;501:332–338. doi: 10.1016/0006-8993(89)90650-1. [DOI] [PubMed] [Google Scholar]
- 12.Hiruma H, Uemura T, Kimura F. Neuronal synchronization and ionic mechanisms for propagation of excitation in the functional network of immortalized GT1–7 neurons: optical imaging with a voltage-sensitive dye. J Neuroendocrinol. 1997;9:835–840. doi: 10.1046/j.1365-2826.1997.00645.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7:45–48. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Joo F, Siklos L, Dames W, Wolff JR. Fine-structural changes of synapses in the superior cervical ganglion of adult rats after long-term administration of GABA. A morphometric analysis. Cell Tissue Res. 1987;249:267–275. doi: 10.1007/BF00215509. [DOI] [PubMed] [Google Scholar]
- 15.Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology. 1998;48:45–52. doi: 10.1159/000124988. [DOI] [PubMed] [Google Scholar]
- 16.Kramer PK, Wray S. Novel gene expressed in nasal regions influences outgrowth of olfactory axons and migration of luteinizing hormone releasing hormone (LHRH) neurons. Genes Dev. 2000;14:1824–1834. [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer PR, Krishnamurthy R, Mitchell PJ, Wray S. Transcription factor activator protein-2 is required for continued luteinizing hormone-releasing hormone expression in the forebrain of developing mice. Endocrinology. 2000;141:1823–1838. doi: 10.1210/endo.141.5.7452. [DOI] [PubMed] [Google Scholar]
- 18.Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA. 1995;92:3918–3922. doi: 10.1073/pnas.92.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 20.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- 21.Liu QY, Schaffner AE, Chang YH, Vaszil K, Barker JL. Astrocytes regulate amino acid receptor current densities in embryonic rat hippocampal neurons. J Neurobiol. 1997;33:848–864. [PubMed] [Google Scholar]
- 22.Martinez de la Escalera G, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1–1 GnRH neuronal cell line. Proc Natl Acad Sci USA. 1992;89:1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattson MP, Kater SB. Excitatory and inhibitory neurotransmitters in the generation and degeneration of hippocampal neuroarchitecture. Brain Res. 1989;478:337–348. doi: 10.1016/0006-8993(89)91514-x. [DOI] [PubMed] [Google Scholar]
- 24.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 25.Montpied P, Ginns EI, Martin BM, Stetler D, O'Carroll AM, Lolait SJ, Mahan LC, Paul SM. Multiple GABAA receptor alpha subunit mRNAs revealed by developmental and regional expression in rat, chicken and human brain. FEBS Lett. 1989;258:94–98. doi: 10.1016/0014-5793(89)81623-0. [DOI] [PubMed] [Google Scholar]
- 26.Moore JP, Jr, Wray S. Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology. 2000;141:4486–4495. doi: 10.1210/endo.141.12.7814. [DOI] [PubMed] [Google Scholar]
- 27.Nunez L, Villalobos C, Boockfor FR, Frawley LS. The relationship between pulsatile secretion and calcium dynamics in single, living gonadotropin-releasing hormone neurons. Endocrinology. 2000;141:2012–2017. doi: 10.1210/endo.141.6.7491. [DOI] [PubMed] [Google Scholar]
- 28.Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obrietan K, van den Pol AN. GABAB receptor-mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons. J Neurophysiol. 1998;79:1360–1370. doi: 10.1152/jn.1998.79.3.1360. [DOI] [PubMed] [Google Scholar]
- 30.Poulter MO, Ohannesian L, Larmet Y, Feltz P. Evidence that GABAA receptor subunit mRNA expression during development is regulated by GABAA receptor stimulation. J Neurochem. 1997;68:631–639. doi: 10.1046/j.1471-4159.1997.68020631.x. [DOI] [PubMed] [Google Scholar]
- 31.Ramakers GJ, van Galen H, Feenstra MG, Corner MA, Boer GJ. Activity-dependent plasticity of inhibitory and excitatory amino acid transmitter systems in cultured rat cerebral cortex. Int J Dev Neurosci. 1994;12:611–621. doi: 10.1016/0736-5748(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 32.Redburn DA. Development of GABAergic neurons in the mammalian retina. Prog Brain Res. 1992;90:133–147. doi: 10.1016/s0079-6123(08)63612-2. [DOI] [PubMed] [Google Scholar]
- 33.Terasawa E, Quanbeck C, Schulz C, Burich LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133:2379–2390. doi: 10.1210/endo.133.5.8404690. [DOI] [PubMed] [Google Scholar]
- 34.Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999a;19:5898–5909. doi: 10.1523/JNEUROSCI.19-14-05898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terasawa E, Keen K, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999b;140:1432–1441. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- 36.Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- 37.Wildt L, Marshall G, Knobil E. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207:1371–1373. doi: 10.1126/science.6766566. [DOI] [PubMed] [Google Scholar]
- 38.Wray S, Gähwiler B, Gainer H. Slice explant cultures of postnatal LHRH neurons in the presence and absence of brainstem and anterior pituitary co-cultures. Peptides. 1988;9:1151–1175. doi: 10.1016/0196-9781(88)90103-9. [DOI] [PubMed] [Google Scholar]
- 39.Wray S, Fueshko SM, Kusano K, Gainer H. GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol. 1996;180:631–645. doi: 10.1006/dbio.1996.0334. [DOI] [PubMed] [Google Scholar]