Abstract
Basal extracellular glutamate sampled in vivo is present in micromolar concentrations in the extracellular space outside the synaptic cleft, and neither the origin nor the function of this glutamate is known. This report reveals that blockade of glutamate release from the cystine–glutamate antiporter produced a significant decrease (60%) in extrasynaptic glutamate levels in the rat striatum, whereas blockade of voltage-dependent Na+ and Ca2+ channels produced relatively minimal changes (0–30%). This indicates that the primary origin of in vivo extrasynaptic glutamate in the striatum arises from nonvesicular glutamate release by the cystine–glutamate antiporter. By measuring [35S]cystine uptake, it was shown that similar to vesicular release, the activity of the cystine–glutamate antiporter is negatively regulated by group II metabotropic glutamate receptors (mGluR2/3) via a cAMP-dependent protein kinase mechanism. Extracellular glutamate derived from the antiporter was shown to regulate extracellular levels of glutamate and dopamine. Infusion of the mGluR2/3 antagonist (RS)-1-amino-5-phosphonoindan-1-carboxylic acid (APICA) increased extracellular glutamate levels, and previous blockade of the antiporter prevented the APICA-induced rise in extracellular glutamate. This suggests that glutamate released from the antiporter is a source of endogenous tone on mGluR2/3. Blockade of the antiporter also produced an increase in extracellular dopamine that was reversed by infusing the mGluR2/3 agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxlylate, indicating that antiporter-derived glutamate can modulate dopamine transmission via mGluR2/3 heteroreceptors. These results suggest that nonvesicular release from the cystine–glutamate antiporter is the primary source of in vivo extracellular glutamate and that this glutamate can modulate both glutamate and dopamine transmission.
Keywords: microdialysis, glutamate, cystine, striatum, nonvesicular, cystine–glutamate antiporter, system xc
Glutamate is the major excitatory neurotransmitter in the CNS system and is involved in many aspects of brain functioning in normal and diseased states (Greenamyre et al., 1988; Coyle and Puttfarcken, 1993; Moghaddam and Adams, 1998;Tapia et al., 1999; Marino et al., 2001). Despite intensive effort, the cellular mechanisms regulating glutamate neurotransmission have not been fully characterized. Although synaptically released glutamate has been studied in great detail, basal extracellular glutamate measuredin vivo is present in micromolar concentrations in the extracellular space outside the synaptic cleft, and neither the origin nor the function of this pool of glutamate is known (Timmerman and Westerink, 1997). Given the extrasynaptic location of Na+-dependent glutamate transporters, as well as group II and III metabotropic glutamate receptors (mGluR2/3;Alagarsamy et al., 2001; Tamaru et al., 2001), this extracellular pool of glutamate has access to mechanisms for regulating the glutamate transmission.
Although the origin has not been identified, in vivoextrasynaptic glutamate is maintained by nonvesicular release because levels are relatively insensitive to blockade of voltage-dependent Na+ and Ca2+channels (Bradford et al., 1987; Miele et al., 1996; Timmerman and Westerink, 1997). The continuous release of nonvesicular glutamate has also been detected in hippocampal tissue slices using patch-clamp recording (Jabaudon et al., 1999), indicating that it is not an artifact of microdialysis. Akin to the in vivo situation, the source of nonvesicular release in hippocampal tissue slices was not identified, although several mechanisms were excluded, including Ca2+-dependent release by astrocytes, transmembrane diffusion, and leakage from Na+-dependent glutamate transporters or volume-sensitive Cl− channels (Jabaudon et al., 1999).
Another source of nonvesicular glutamate release is from the cystine–glutamate antiporter (Bannai, 1986; Murphy et al., 1990; Warr et al., 1999). The antiporter is a plasma membrane-bound, Na+-independent, anionic amino acid transporter that exchanges extracellular cystine for intracellular glutamate (Bannai, 1986; Danbolt, 2001). It exists as two separate proteins, the light chain xCT that is unique to the cystine–glutamate antiporter and the heavy chain 4F2 that is common to many amino acid transporters (Sato et al., 1999; Bridges et al., 2001). Similar to Na+-dependent glutamate transporters, the antiporter is ubiquitously distributed on cells throughout the body, although in the brain it may be preferentially located on glia (Cho and Bannai, 1990; Murphy et al., 1990; Danbolt, 2001; Pow, 2001).
In the present report it was shown that in vivoextrasynaptic glutamate in the rat striatum is maintained by glutamate released from the cystine–glutamate antiporter. Similarities in the regulation of synaptic vesicular glutamate and extrasynaptic glutamate supplied by antiporter release were revealed by showing that extrasynaptic glutamate derived from the antiporter is regulated by mGluR2/3 and Na+-dependent glutamate transporters. Finally, it was found that glutamate derived from the antiporter provides endogenous in vivo tone on mGluR2/3 that are regulating the extracellular levels of glutamate and dopamine.
MATERIALS AND METHODS
Animals and surgeries. Male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275–300 gm were individually housed in a temperature-controlled colony room with a 12 hr light/dark cycle (lights on at 7:00 A.M.) with food and water available ad libitum. The housing conditions and care of the rats was in accordance with the Animal Welfare Act, and all procedures were approved by the Medical University of South Carolina's Institutional Animal Care and Use Committee. Rats included in the microdialysis studies were anesthetized with a combination of ketamine (100 mg/kg, i.m.) and xylazine (3 mg/kg, i.m.). Using coordinates derived from the Paxinos and Watson atlas (1998) (+1.6 mm anterior and ±2.5 mm mediolateral to bregma and −4.7 mm from the surface of the skull at a 6o angle from vertical), bilateral guide cannulas (20 gauge, 14 mm; Plastics One, Roanoke, VA) were implanted above the ventral striatum to allow the microdialysis probes, which extend beyond the tip of the guide cannulas by 2 mm, to be placed in the ventral striatum. Guide cannulas were secured to the skull using four skull screws (Small Parts, Roanoke, VA) and dental acrylic. After surgery, rats were given at least 5 d to recover before testing.
Compounds.dl-threo-B-benzyloxyaspartate (TBOA) was generously donated by Dr. Keiko Shimamoto of the Suntory Institute for Bioorganic Research (Osaka, Japan) and was dissolved directly into dialysis buffer. Cystine (Sigma, St. Louis, MO) and [35S]cystine (Amersham, Arlington Heights, IL) were dissolved in 0.05 m HCl and diluted using dialysis or Krebs'–Ringer's solution phosphate buffer (KRP) (in mm: 118 NaCl, 25 NaHCO3, 4.7 KCl, 1.3 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 5.0 HEPES, and 10 glucose, pH 7.4) buffer for in vivo or in vitro experiments, respectively. (S)-4-carboxyphenylglycine (CPG), (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), (RS)-1-amino-5-phosphonoindan-1-carboxylic acid (APICA), and (2R,4R)-4-aminopyrrolidine-2,4-dicarboxlylate (APDC; Tocris-Cookson, Ballwin, MO) were dissolved in one equivalent NaOH and diluted in dialysis buffer or KRP. All remaining compounds were purchased from Sigma and dissolved in dialysis buffer for microdialysis experiments or KRP buffer for in vitro uptake experiments, including tetrodotoxin (TTX), the L-type Ca2+ channel blocker diltiazem (DTZ), the P/Q-type Ca2+ channel blocker ω-conotoxin MVIIC (MVIIC), the N-type Ca2+ channel blocker ω-conotoxin GVIA (GVIA), and the cAMP-dependent protein kinase (PKA) inhibitor and activator, Rp-adenosine 3,5-cyclic monophospothioate triethylamine (Rp-cAMPS) and Sp-adenosine 3,5-cyclic monophospothioate triethylamine (Sp-cAMPS), respectively.
In vivo microdialysis. Microdialysis probes were constructed as described by Robinson and Whishaw (1988) except both the inlet and outlet tubing consisted of fused silica. The active region of the dialysis membrane was between 2 and 3 mm in length and ∼0.22 mm in diameter. The recovery for probes with this design ranged from 6 to 11% at 32°C. The night before the experiment, the probes were inserted through the guide cannulas into the ventral striatum. The next day, dialysis buffer (in mm: 5 glucose, 140 NaCl, 1.4 CaCl2, 1.2 MgCl2, and 0.15% PBS, pH 7.4) was pumped through the microdialysis probes at a rate of 2 μl/min. Two hours later, baseline samples were collected. Liquid switches (Uniswitch; Bioanalytical Systems, West Lafayette, IN) were used to minimize pressure fluctuations while changing dialysis buffers with varying concentrations of drug. The standard protocol used for microdialysis experiments involved the collection of five 20 min baseline samples, followed by three additional 20 min samples for each concentration of a given drug or combination of drugs, as indicated in the figures. An exception to the standard protocol is the experiments presented in Figure 6, in which 1.0 μm APICA and 0.5 μm CPG were infused for 2 and 3 hr, respectively. The other exception is the experiment presented in Figure3, in which six 10 min baseline samples were collected. Afterwards, additional 10 min samples were collected during reverse dialysis of CPG (0 or 5.0 μm) for 1 hr, followed by reverse dialysis of the same dose of CPG plus K+(80 mm) for 30 min. This procedure was then repeated 2 hr later with the other dose of CPG. The order of CPG dose (i.e., 0 or 5 μm) was counterbalanced across rats. Because there was not a significant order effect, the data for each CPG dose (i.e., 0 or 5.0 μm) was collapsed across the order of presentation. The concentrations of the compounds were selected from previous microdialysis studies (APDC, APICA, GVIA, CPG, Xi et al., 2002; TTX, Miller and Abercrombie, 1999; DTZ, Hu et al., 1999; GVIA and MVIIC, Okada et al., 1998, Bergquist et al., 1998) or efficacy estimates (TBOA, Shimamoto et al., 1998).
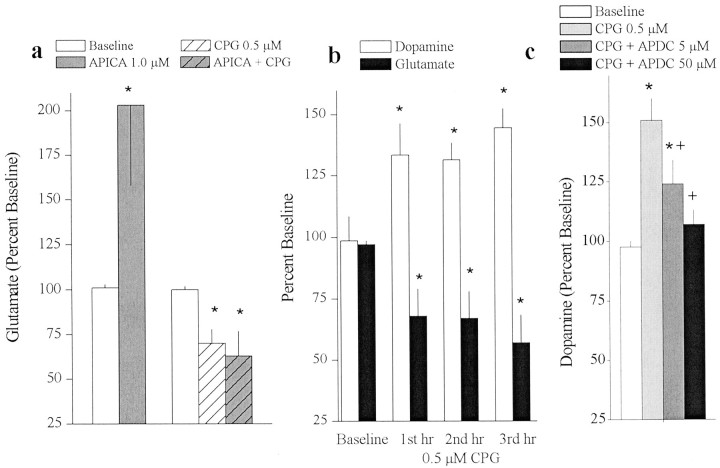
Fig. 6.
Glutamate released from the cystine–glutamate antiporter tonically stimulates group II mGluRs regulating glutamate and dopamine release. a, Extrasynaptic glutamate sampledin vivo from the striatum using microdialysis before and after infusion of the group II mGluR antagonist APICA alone (n = 11) or with CPG (n = 6).b, Extrasynaptic glutamate (n = 6) and dopamine (n = 5) sampled from the striatum before and after infusion of CPG alone for 3 hr. c,Extrasynaptic dopamine sampled from the striatum before and after infusion of CPG with the group II mGluR agonist APDC (n = 4). Data are presented as mean (± SEM) glutamate (percentage of baseline) from samples collected during baseline (100 min) or at each drug concentration (60 min/concentration). A one-way ANOVA indicated a significant effect of APICA alone (F(1,10) = 5.21;p < 0.05) or APICA plus CPG (F(2,10) = 6.652; p< 0.05) on glutamate levels (a), CPG alone on glutamate (F(3,18) = 4.00;p < 0.05) or dopamine levels (F(3,12) = 4.82; p< 0.05) (b), and CPG plus APDC (F(3,9) = 9.21; p< 0.05) (c). *p < 0.05, difference from baseline (Fisher's LSD post hocanalysis). +p < 0.05, difference from CPG alone (Fisher's LSD).
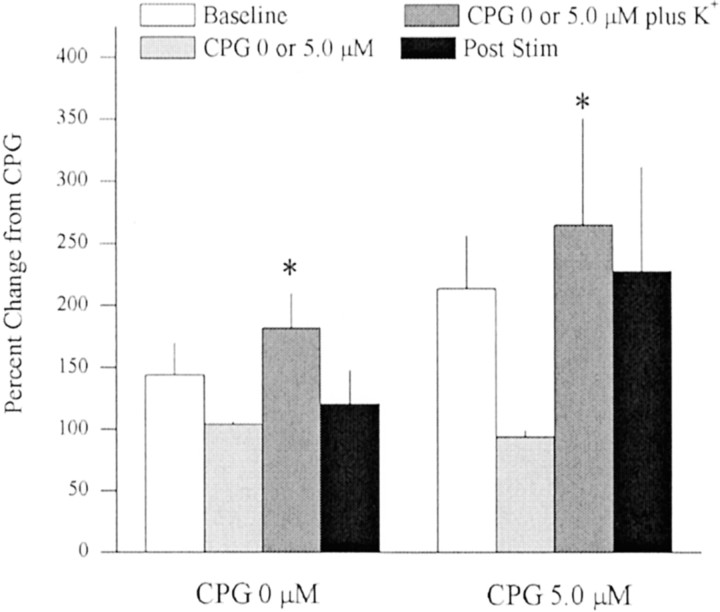
Fig. 3.
In vivo microdialysis was used to sample extrasynaptic glutamate in the striatum after reverse dialysis of K+ alone (i.e., 0 μm CPG + K+; n = 8) or after reverse dialysis of 5.0 μm CPG followed by 5.0 μmCPG plus K+ (80 mm;n = 8). Mean extracellular glutamate levels (± SEM) in the 0 μm CPG experiment were 1.83 ± 0.36 μm during baseline and 1.52 ± 0.28 μmduring 0 μm CPG. The decrease in extracellular glutamate during the 0 μm CPG treatment was not significantly different from baseline and was essentially caused by a single rat that exhibited stable basal levels but exhibited a drop in glutamate while switching dialysis buffer. In the 5.0 μm CPG experiment, extracellular glutamate levels were 2.01 ± 0.41 μmduring baseline and 1.33 ± 0.24 during 5.0 μm CPG. Because 5.0 μm CPG lowered extracellular glutamate levels, the data presented are normalized to glutamate levels at 0 or 5.0 μm CPG. A two-way ANOVA on glutamate levels across time between rats treated with 0 or 5.0 μm CPG indicated a significant effect of time (F(3,42) = 3.337; p < 0.05). *p < 0.05, difference from respective CPG baseline (0 or 5.0 μm; Fisher's LSD post hoc analysis).
Glutamate and dopamine quantification. Microdialysis samples analyzed for glutamate only were collected into vials containing 10 μl of 0.05 M HCl. Samples analyzed for dopamine were collected into 20 μl of mobile phase (see description below). The concentration of glutamate in dialysis samples was determined using HPLC coupled to fluorescence detection. Precolumn derivitization of glutamate withO-pthalaldehyde was performed using a Gilson (Middleton, WI) 231 XL autosampler. The mobile phase consisted of 13% acetonitrile, 100 mmNa2HPO4, and 0.1 mm EDTA, pH 5.90. Glutamate was separated using a reversed-phase column (3 μm; 100 × 4.2 mm; Bioanalytical Systems) and was detected using a Shimadzu (Columbia, MD) 10RF-A fluorescence detector with an excitation wavelength of 320 nm and an emission wavelength of 400 nm. Dopamine was determined using HPLC coupled to electrochemical detection. The mobile phase consisted of 15% acetonitrile, 10% methanol, 150 mmNaH2PO4, 4.76 mm citric acid, 3 mm SDS, and 50 μm EDTA, pH 5.6. Dopamine was separated using a reversed-phase column (3 μm; 100 × 4.2 mm; Bioanalytical Systems) and was detected using coulometric detection (ESA Inc., Bedford MA). Three electrodes were used, a preinjection port guard cell (+0.25 V) to oxidize the mobile phase, an oxidation analytical electrode (+0.22 V) and a reduction analytical electrode (−0.150). The concentration of glutamate and dopamine in dialysis samples was quantified by comparing peak heights from samples and external standards.
[35S]cystine uptake. Rats were decapitated, and the striatum was rapidly dissected and cut into 350 × 350 μm slices using a McIlwain tissue chopper. The slices were then washed five times for 10 min each at 37°C in oxygenated KRP. The slices were incubated at 37°C in oxygenated KRP buffer containing 1.0 μm[35S]cystine (0.1 μCi) for 15 min. Cystine uptake can also occur via two other mechanisms, XAG and γ-glutamyl transpeptidase (Knickelbein et al., 1997). To isolate cystine uptake to cystine–glutamate exchange, the XAG inhibitor aspartate (1 mm) and the γ-glutamyl transpeptidase inhibitor acivicin (1 mm) were added to the incubation buffer. Incubation was terminated by rapidly washing the tissue three times using ice-cold KRP. Slices were then solubilized using 1% SDS, and the level of radioactivity was determined using a liquid scintillation counter. Radioactivity counts from known concentrations of [35S]cystine were used to determine the concentration of [35S]cystine in tissue slices. Protein content in the slices was measured using the Bradford assay. Cystine uptake in the presence of unlabelled 1 mm cystine was used to identify nonspecific labeling and was subtracted from all data. The concentrations of compounds used in these experiments are similar to those used in previous studies (Rp-cAMP, Bedi et al., 1998; Sp-cAMP, Kaji et al., 1996, Hu et al., 2001; APDC, Mistry et al., 1998, Doi et al., 2002; APICA, Krenz et al., 2000) and/or efficacy estimates in rat (APDC and APICA, Schoepp et al., 1995, 1999). The high dose of the PKA activator Sp-cAMP (1 mm) was chosen to provide a high signal to detect any potential inhibition by APDC (Kaji et al., 1996;Hu et al., 2001).
Histology and statistical analyses. Rats included in the microdialysis studies were given an overdose of pentobarbital, and the brains were fixed by intracardiac infusion of 0.9% saline followed by 1% formalin solution. Brains were then removed and stored in 1% formalin for at least 1 week before sectioning. The tissue was then blocked, and coronal sections (100 μm) were cut and stained with cresyl violet to verify probe placements. All probes were located in the ventral 50% of the striatum, corresponding to the nucleus accumbens and ventral portion of the caudate. The SPSS statistics package was used to perform the statistical analyses. Data were analyzed using one-way ANOVA with drug concentration as a repeated factor. Significant main effects were further analyzed using Fisher's LSD when appropriate. Microdialysis data for each rat are presented as percentage of change from baseline.
RESULTS
The origin of extracellular glutamate
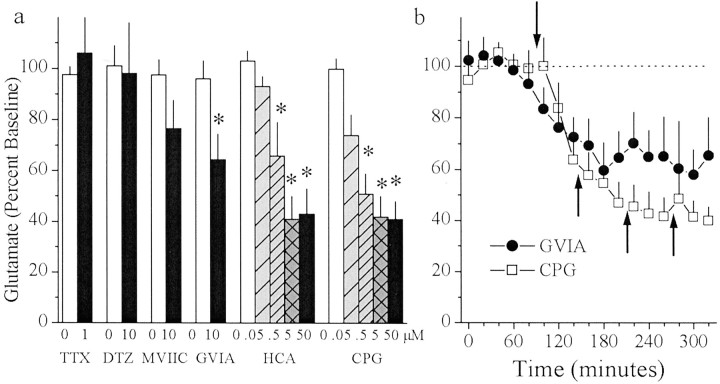
The contribution of vesicular release to extracellular glutamate concentrations in the rat striatum was determined by reverse dialysis of the voltage-dependent Na+ channel blocker TTX, the L-type Ca2+ channel blocker DTZ, the P/Q-type Ca2+ channel blocker MVIIC, or the N-type Ca2+ channel blocker GVIA. Basal concentrations of extrasynaptic glutamate (mean ± SEM) from rats treated with TTX, DTZ, MVIIC, or GVIA were 1.33 ± 0.29, 2.44 ± 0.41, 1.26 ± 0.30, and 3.47 ± 0.67 μm, respectively. Figure1a illustrates that reverse dialysis of TTX, DTZ, and MVIIC failed to significantly decrease extrasynaptic glutamate levels, whereas reverse dialysis of GVIA produced a significant 30% decrease in basal extrasynaptic glutamate levels.
Fig. 1.
In vivo microdialysis was used to sample extrasynaptic glutamate in the striatum before and after reverse dialysis of the Na+-channel blocker TTX (n = 9), the L-type Ca2+ channel blocker diltiazem (n = 7), the P/Q-type Ca2+ channel blocker MVIIC (n = 6), the N-type Ca2+ channel blocker GVIA (n = 8), or the cystine–glutamate antiporter inhibitors HCA (n = 6) and CPG (n = 7). a, Data are presented as mean (± SEM) glutamate (percentage of baseline levels) from samples collected during baseline (100 min) or at each drug concentration (60 min/concentration). b, Data from a are presented as glutamate across 20 min samples for rats receiving GVIA (0 or 10 μm) or CPG (0, 0.05, 0.5, 5.0, and 50 μm). The downward pointing arrow indicates the beginning of the infusion of GVIA or CPG. Upward pointing arrows indicate increases in CPG concentration as described ina. A one-way ANOVA on glutamate levels indicated a significant effect of drug concentration for GVIA (F(1,5) = 6.75; p< 0.05), HCA (F(4,20) = 10.19;p < 0.05), and CPG (F(4,24) = 18.64; p< 0.05). *p < 0.05, compared with baseline levels difference from baseline (Fisher's LSD post hocanalysis).
The contribution of nonvesicular release of glutamate to extracellular levels was determined by inhibiting cystine–glutamate exchange. Inhibition of cystine–glutamate exchange has been previously demonstrated in vitro using homocysteic acid (Bannai and Ishii, 1982; Murphy et al., 1990) and CPG (Ye et al., 1999), which blocks both [35S]cystine uptake and [3H]glutamate release, indicating that it is not transported (Ye et al., 1999). Basal concentrations of extrasynaptic glutamate (mean ± SEM) from rats treated with HCA or CPG were 2.11 ± 0.52 and 4.00 ± 1.03 μm, respectively. In contrast with the relatively modest effect obtained after blockade of vesicular release, reverse dialysis of the cystine–glutamate exchange inhibitors HCA or CPG produced a 60% decrease in basal extrasynaptic glutamate (Fig.1a).
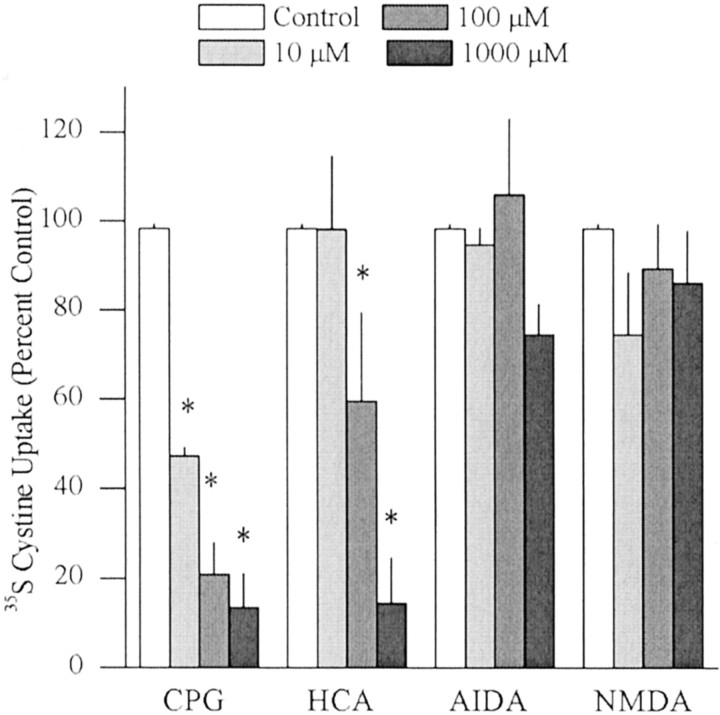
To verify that CPG and HCA directly inhibit cystine–glutamate exchange, the uptake of [35S]cystine in rat striatal tissue slices was measured in the presence and absence of these inhibitors. Figure 2 illustrates that both CPG and HCA decreased in vitro[35S]cystine uptake by >80%. The lack of an effect of the group I mGluR antagonist AIDA or NMDA on the uptake of [35S]cystine in tissue slices demonstrates that blockade of cystine uptake by CPG and HCA was not caused by other known actions of these drugs (Lehmann et al., 1988;Hayashi et al., 1994; Schoepp et al., 1999), including inhibiting group I mGluRs or stimulating NMDA receptors, respectively (Fig. 2).
Fig. 2.
CPG and HCA directly block the cystine–glutamate antiporter. The uptake of [35S]cystine in striatal tissue slices incubated was measured in the presence and absence of CPG, HCA, the group I mGluR antagonist AIDA, or NMDA (N = 4/drug). Data are presented as [35S]cystine uptake in the presence of inhibitors expressed as percentage of change of [35S]cystine uptake in the absence of inhibitors. A one-way ANOVA on [35S]cystine uptake indicated a significant effect of drug concentration for CPG (F(3,9) = 47.23; p < 0.05) and HCA (F(3,9) = 15.74; p< 0.05) . *p < 0.05, difference from [35S]cystine uptake in the absence of inhibitors (Fisher's LSD post hoc analysis).
To examine whether vesicular and nonvesicular release comprises independent pools of glutamate in the striatum, in vivomicrodialysis was used to examine the effect of blocking cystine–glutamate exchange on potassium-induced release of glutamate. Basal concentrations of extrasynaptic glutamate (mean ± SEM) before infusion of 0 or 5.0 μm CPG were 1.83 ± 0.36 and 2.01 ± 0.41 μm, respectively. Figure 3 indicates that reverse dialysis of 80 mmK+ produced a significant increase in extracellular glutamate levels in the striatum relative to the hour before stimulation. Pretreatment with 5.0 μmCPG did not decrease K+-induced release of glutamate. In fact, the 5.0 μm CPG group showed a nonsignificant trend toward potentiating K+-induced increase in extracellular glutamate (0.5 μm CPG infusion ± SEM = 291 ± 95; mean change from 0 μmCPG ± SEM = 194 ± 35).
Regulation of the cystine–glutamate antiporter by mGluR2/3 and PKA
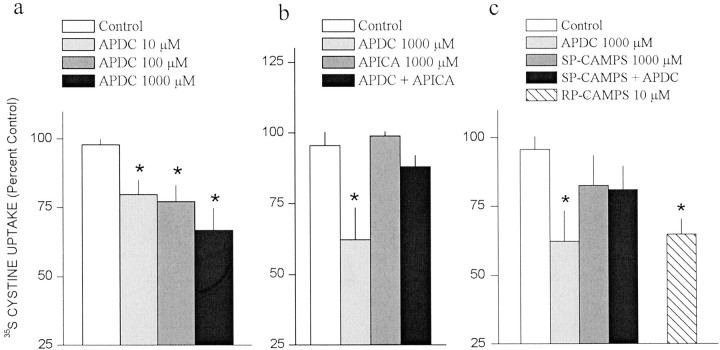
Because group II mGluRs have been shown to inhibit vesicular release of glutamate (Conn and Pin, 1997; Anwyl, 1999), cystine uptake in striatal tissue slices was used to determine whether mGluR2/3 also regulates cystine–glutamate antiporters. Figure4a illustrates that the mGluR2/3 agonist APDC produced a dose-dependent decrease in [35S]cystine uptake in striatal tissue slices that was reversed by coadministration of the mGluR2/3 antagonist APICA (Fig. 4b). The role of PKA in the inhibition of cystine–glutamate exchange by APDC was examined because mGluR2/3 is Gi-coupled and inhibits PKA activity (Cartmell and Schoepp, 2000), as well as the fact that the sequence of human Xct contains two consensus PKA phosphorylation sites (revealed in a search for common motifs using GCG; Biomedical Computing Resources, Medical University South Carolina). Figure 4c illustrates that the PKA inhibitor Rp-cAMPS produced a significant decrease in [35S]cystine uptake, revealing a role for PKA phosphorylation in regulating cystine–glutamate exchange. Although activation of PKA by Sp-cAMPS did not affect [35S]cystine uptake, in the presence of Sp-cAMPS, APDC failed to significantly decrease [35S]cystine uptake (Fig.4c).
Fig. 4.
The cystine–glutamate antiporter is regulated by group II mGluR autoreceptors via a PKA-dependent mechanism. The uptake of [35S]cystine in striatal tissue slices was measured in the presence and absence of the group II agonist APDC, the group II antagonist APICA, the PKA activator Sp-cAMPS, or the PKA inhibitor Rp-cAMPS (n = 4–10/group). Data are presented as [35S]cystine uptake in the presence of inhibitors expressed as percentage of change of [35S]cystine uptake in the absence of inhibitors. A one-way ANOVA on [35S]cystine uptake indicated a significant effect of drug concentration for APDC alone (F(3,27) = 5.34; p< 0.05), APDC plus APICA (F(3,9) = 5.23; p < 0.05), and Rp-cAMPS (F(1,3) = 10.47; p< 0.05). *p < 0.05, difference from [35S]cystine uptake in the absence of inhibitors (Fisher's LSD post hoc analysis).
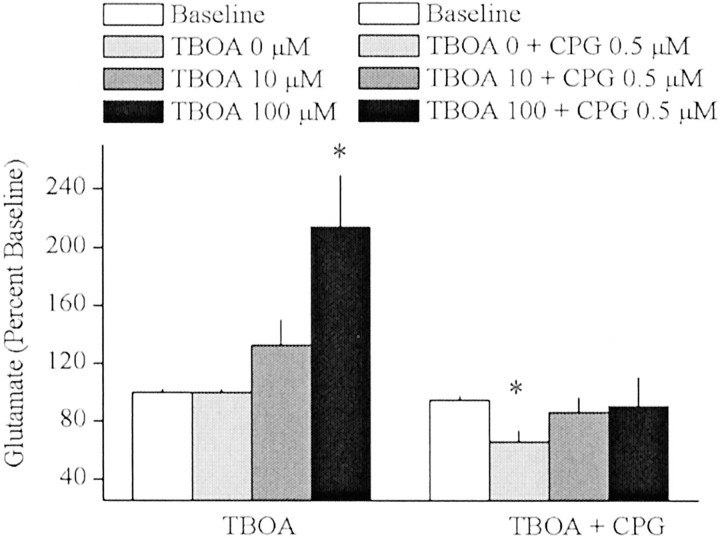
In vivo microdialysis was used to determine whether Na+-dependent glutamate transporters regulate the extracellular glutamate that is derived from cystine–glutamate exchange. Basal concentrations of extrasynaptic glutamate (mean ± SEM) from rats treated with TBOA or TBOA plus CPG were 3.87 ± 0.11 and 1.67 ± 0.20 μm, respectively. Figure5 illustrates that reverse dialysis of the broad-spectrum inhibitor of Na+-dependent glutamate transport TBOA (Shimamoto et al., 1998) produced a significant increase in extrasynaptic glutamate in the striatum. The TBOA-induced rise in extracellular glutamate was prevented by previous infusion of the cystine–glutamate exchange inhibitor CPG.
Fig. 5.
Na+-dependent glutamate transporters shape the effect of glutamate released from the cystine–glutamate antiporter. In vivo microdialysis was used to sample extrasynaptic glutamate in the striatum before and after infusion of the broad spectrum Na+-dependent glutamate transport inhibitor TBOA alone (n = 6) or with the cystine–glutamate exchange inhibitor CPG (n = 6). A one-way ANOVA on glutamate levels indicated a significant effect of drug concentration for only TBOA alone (F(3,30) = 9.45;p < 0.05). *p < 0.05, difference from baseline (Fisher's LSD post hocanalysis).
Glutamate released by the cystine–glutamate antiporter modulates mGluR2/3 and the levels of extracellular glutamate and dopamine
Group II mGluRs are located perisynaptically on axon terminals and are therefore directly accessible by extrasynaptic glutamate released via the cystine–glutamate antiporter (Tamaru et al., 2001). Using mGluR2/3 antagonists it was previously shown that endogenous tone exists on these receptors in vivo (Baskys and Malenka, 1991;Cochilla and Alford, 1998; Hu et al., 1999; Xi et al., 2002). In vivo microdialysis was used to determine whether the cystine–glutamate antiporter is the source of glutamate that provides tonic in vivo stimulation to mGluR2/3. Figure6a illustrates that infusion of the mGluR2/3 antagonist APICA into the striatum significantly increased extrasynaptic glutamate levels. Co-infusion of the cystine–glutamate exchange inhibitor CPG prevented the APICA-induced rise in extrasynaptic glutamate (Fig. 6a). In parallel with the reduction in glutamate, blockade of cystine–glutamate exchange produced a significant increase in extrasynaptic dopamine levels (Fig.6b). The CPG-induced increase in dopamine was reversed after co-infusion of the group II agonist APDC (Fig. 6c). Basal concentrations of extrasynaptic glutamate (mean ± SEM) from rats treated with APICA alone, CPG alone, or APICA plus CPG were 3.36 ± 0.70, 3.70 ± 0.74, and 4.25 ± 0.87 μm, respectively. Basal concentrations of extrasynaptic dopamine from rats treated with CPG alone or CPG plus APDC were 1.73 ± 0.26 and 1.85 ± 0.42 nm, respectively.
DISCUSSION
The major finding in the present report is that in vivoextracellular glutamate in the rat striatum is maintained primarily by nonvesicular glutamate release from the cystine–glutamate antiporter. The importance of this finding is evident, in part, from the observation that nonvesicular glutamate release from the antiporter regulates extracellular concentrations of both glutamate and dopamine by providing endogenous tone to mGluR2/3. This finding poses the possibility that nonvesicular release of glutamate shapes synaptic activity.
The origin of extrasynaptic glutamate
Extracellular glutamate in vivo is present in micromolar concentrations outside the synapse and is primarily maintained by nonvesicular release (Herrera-Marschitz et al., 1996;Timmerman and Westerink, 1997). The present data reveals that blockade of nonvesicular glutamate release from cystine–glutamate antiporters by reverse dialysis of HCA or CPG produces a 60% decrease in extrasynaptic glutamate measured in vivo. Furthermore, CPG and HCA directly inhibited [35S]cystine uptake in striatal tissue slices, whereas NMDA and the group I mGluR antagonist AIDA were ineffective. The latter finding indicates that the effects of CPG and HCA on the cystine–glutamate antiporter are independent of previously described actions at group I mGluRs or NMDA receptors, respectively (Lehmann et al., 1988; Hayashi et al., 1994;Schoepp et al., 1999). This is consistent with previous research showing that blockade of group I mGluR or stimulation of NMDA receptors does not decrease extracellular glutamate levels (Yamamoto et al., 1999; Hashimoto et al., 2000; Swanson et al., 2001). Thus, these data are the first to reveal that nonvesicular glutamate release from cystine–glutamate antiporters is the primary source of extracellular glutamate in the striatum. A recent finding that continuous release of nonvesicular glutamate detected in hippocampal tissue slices regulates synaptic transmission (Jabaudon et al., 1999) poses the possibility that glial cells regulate synaptic activity throughout the brain not only by clearing extrasynaptic glutamate, but by releasing it as well.
Vesicular glutamate has also been shown to contribute to basal glutamate levels in the striatum. For instance, blockade of vesicular glutamate release by has been demonstrated to deplete basal glutamate levels in the striatum as much as 50% (Newcomb and Palma, 1994;Herrera-Marschitz et al., 1996) (but see, Westerink et al., 1989; You et al., 1994). The present data obtained similar results insofar as blockade of vesicular release after reverse dialysis of the N-type Ca2+ channel blocker GVIA produced a statistically significant decrease in extracellular glutamate levels (30%). As opposed to N-type Ca2+channels, blockade of voltage-dependent Na+ channels failed to decrease extracellular glutamate levels; a finding demonstrated previously (Westerink et al., 1987). This indicates that although a vesicular component contributes to basal glutamate levels, it is not in response to action potentials. Interestingly, recent evidence indicates that N-type Ca2+ channels, as well as vesicular scaffolding proteins, are present in glial cells (Parpura et al., 1995;Jeftinija et al., 1997; Verkhratsky et al., 1998). Thus, the detected vesicular component of basal glutamate levels may arise, in part, by vesicular release of glutamate from glia, which has been demonstratedin vitro (Parpura et al., 1994; Bezzi et al., 1998; Araque et al., 2000; Haydon, 2001; Pasti et al., 2001). Alternatively, it could be caused by spontaneous vesicular release of glutamate in neurons (Pare et al., 1998). Regardless of the source of vesicular glutamate, the present data demonstrate that although basal glutamate levels in the striatum are derived by vesicular and nonvesicular glutamate release, the major contributor is from nonvesicular cystine–glutamate exchange. Furthermore, the vesicular and nonvesicular components of basal glutamate levels are distinct pools of glutamate because blockade of cystine–glutamate exchange did not alter potassium-induced glutamate release, which has been shown repeatedly to be of vesicular origin (Paulsen and Fonnum, 1989; Forray et al., 1999;Ueda et al., 2000).
Regulation of the cystine–glutamate antiporter
In parallel with the regulation of synaptic vesicular glutamate release, extrasynaptic nonvesicular glutamate release from cystine–glutamate antiporters was found to be regulated by mGluR2/3 receptors and Na+-dependent glutamate transport. Vesicular glutamate release from neurons is negatively regulated by mGluR2/3 autoreceptors (Baskys and Malenka, 1991; Cochilla and Alford, 1998; Hu et al., 1999; Xi et al., 2002). Similarly, the mGluR2/3 agonist APDC decreased the rate of cystine–glutamate exchange, evident as a decrease in [35S]cystine uptake into striatal tissue slices and a reduction in the extracellular level of in vivoglutamate. Also, mGluR2/3 is GI-coupled to inhibit adenylyl cyclase and the subsequent activation of PKA (Conn and Pin, 1997; Schoepp et al., 1999). Thus, decreased vesicular glutamate release from neurons by stimulating mGluR2/3 has been shown to arise in part from reducing PKA phosphorylation of N-type calcium channels (Lin et al., 2000; Alagarsamy et al., 2001). Similarly, the APDC-induced decrease in [35S]cystine uptake in the present study was mimicked by the PKA inhibitor Rp-cAMPS and reversed by the PKA activator Sp-cAMPS. Finally, Na+-dependent glutamate transporters shape the effects of vesicular glutamate on synaptic activity by clearing extracellular glutamate from the synaptic space (Danbolt, 2001). In parallel, blockade of Na+-dependent glutamate transporters in the present study produced a significant increase in extracellular glutamate levels in the striatum that was prevented by blockade of the cystine–glutamate antiporter, indicating that Na+-dependent glutamate transporters clear nonvesicular glutamate released by the antiporter. Thus, just as synaptic vesicular glutamate release is regulated by mGluR2/3 and by Na+-dependent transporters, extrasynaptic nonvesicular glutamate released from the antiporter is also regulated by these cellular mechanisms. The primary location of the antiporter may be on glia (Pow, 2001) posing the existence of parallel mGluR2/3-mediated mechanisms for regulating two pools of glutamate, one arising from neurons maintained by vesicular release and one arising from glia maintained by nonvesicular release.
Cystine–glutamate exchange provides endogenous tone on mGluR2/3 regulating extracellular glutamate and dopamine
Although glial and neuronal glutamate release systems are parallel, the cellular localization of cystine–glutamate antiporters on glial cells neighboring neurons (Pow, 2001) and the extrasynaptic location of mGluR2/3 on axon terminals (Alagarsamy et al., 2001) pose the possibility that these systems influence one another. In support, earlier studies have shown that blockade of mGluR2/3 produces an increase in extracellular levels of glutamate and dopamine, implying the existence of endogenous stimulation of these receptors that is capable of modulating synaptic activity (Hu et al., 1999; Xi et al., 2002). The present data demonstrate that cystine–glutamate antiporters are a source of glutamate supplying endogenous stimulation to mGluR2/3. In support and consistent with recent reports showing that nonvesicular glutamate release in vitro regulates glutamatergic synaptic transmission (Jabaudon et al., 1999; Warr et al., 1999), blockade of the antiporter prevented the rise in extracellular glutamate associated with mGluR2/3 blockade. Blockade of the antiporter also increased extracellular dopamine levels, which are well characterized to be of vesicular, synaptic origin (Timmerman and Westerink, 1997). Moreover, the increase in dopamine was reversed by stimulating mGluR2/3, indicating that glutamate derived from cystine–glutamate exchange is providing tone to presynaptic mGluR2/3 heteroreceptors. The relevance of glutamate sampled using microdialysis has been questioned because it was derived from nonvesicular release (Timmerman and Westerink, 1997). Thus, an important implication of the present data set is to provide anin vivo demonstration that nonvesicular neurotransmitter release can contribute to synaptic activity.
These data reveal the primary source of basal extrasynaptic glutamate in the striatum is the cystine–glutamate antiporter, with a lesser contribution by vesicular glutamate. Moreover, nonvesicular glutamate released from this antiporter, which may be primarily in glial cells (Pow, 2001), was shown to provide in vivo tone to mGluR2/3 that regulate both glutamate and dopamine transmission. These data also indicate that parallels exist in the cellular regulation of nonsynaptic glutamate from glia and synaptic glutamate from neurons because both are controlled by mGluR2/3 and Na+-dependent transporters. Recently, the traditional view of the synapse has been challenged by in vitro studies suggesting that vesicular glutamate release from glial cells contributes to synaptic activity (Parpura et al., 1994;Bezzi et al., 1998; Araque et al., 2000; Haydon, 2001; Pasti et al., 2001). The present data complement the notion that our current view of neurotransmission is incomplete by providing in vivoevidence that nonvesicular glutamate release, via cystine–glutamate exchange, also contributes to synaptic transmission.
Footnotes
This work was supported in part by United States Public Health Service Grants MH-40817, DA-03960, DA-06074, and DA007288.
Correspondence should be addressed to Dr. David A. Baker, Department of Physiology and Neuroscience, Medical University of South Carolina, 173 Ashley Avenue, BSB Suite 403, Charleston, SC 29425. E-mail:bakerda@musc.edu.
C. J. Swanson's present address: Neuroscience Division, Eli Lilly and Company, Indianapolis, IN 46285.
REFERENCES
- 1.Alagarsamy S, Sorensen SD, Conn PJ. Coordinate regulation of metabotropic glutamate receptors. Curr Opin Neurobiol. 2001;11:357–362. doi: 10.1016/s0959-4388(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 2.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 3.Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. Neuroscience. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- 5.Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol. 1982;112:265–272. doi: 10.1002/jcp.1041120216. [DOI] [PubMed] [Google Scholar]
- 6.Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol (Lond) 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedi SS, Salim A, Chen S, Glanzman DL. Long-term effects of axotomy on excitability and growth of isolated Aplysia sensory neurons in cell culture: potential role of cAMP. J Neurophysiol. 1998;79:1371–1383. doi: 10.1152/jn.1998.79.3.1371. [DOI] [PubMed] [Google Scholar]
- 8.Bergquist F, Jonason J, Pileblad E, Nissbrandt H. Effects of local administration of L-, N-, and P/Q-type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: a microdialysis study in rat. J Neurochem. 1998;70:1532–1540. doi: 10.1046/j.1471-4159.1998.70041532.x. [DOI] [PubMed] [Google Scholar]
- 9.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 10.Bradford HF, Young AM, Crowder JM. Continuous glutamate leakage from brain cells is balanced by compensatory high-affinity reuptake transport. Neurosci Lett. 1987;81:296–302. doi: 10.1016/0304-3940(87)90399-5. [DOI] [PubMed] [Google Scholar]
- 11.Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function, and regulation of human cystine–glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:47–54. [PubMed] [Google Scholar]
- 12.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 13.Cho Y, Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J Neurochem. 1990;55:2091–2097. doi: 10.1111/j.1471-4159.1990.tb05800.x. [DOI] [PubMed] [Google Scholar]
- 14.Cochilla A, Alford S. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 15.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 16.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 17.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 18.Doi A, Ishibashi H, Jinno S, Kosaka T, Akaike N. Presynaptic inhibition of GABAergic miniature currents by metabotropic glutamate receptor in the rat CNS. Neuroscience. 2002;109:299–311. doi: 10.1016/s0306-4522(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 19.Forray MI, Bustos G, Gysling K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1999;55:311–320. doi: 10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Greenamyre JT, Maragos WF, Albin RL, Penney JB, Young AB. Glutamate transmission and toxicity in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:421–430. doi: 10.1016/0278-5846(88)90102-9. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto A, Kanda J, Oka T. Effects of N-methyl-d-aspartate, kainate or veratridine on extracellular concentrations of free d-serine and l-glutamate in rat striatum: an in vivo microdialysis study. Brain Res Bull. 2000;53:347–351. doi: 10.1016/s0361-9230(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y, Sekiyama N, Nakanishi S, Jane DE, Sunter DC, Birse EF, Udvarhelyi PM, Watkins JC. Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. J Neurosci. 1994;14:3370–3377. doi: 10.1523/JNEUROSCI.14-05-03370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 24.Herrera-Marschitz M, Goiny M, Meana JJ, Silveira R, Godukhin OV, Chen Y, Espinoza S, Pettersson E, Loidl CF, Lubec G, Andersson K, Nylander I, Terenius L, Ungerstedt U. On the origin of extracellular glutamate levels monitored in the basal ganglia of the rat by in vivo microdialysis. J Neurochem. 1996;66:1726–1735. doi: 10.1046/j.1471-4159.1996.66041726.x. [DOI] [PubMed] [Google Scholar]
- 25.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- 26.Hu SJ, Song XJ, Greenquist KW, Zhang JM, LaMotte RH. Protein kinase A modulates spontaneous activity in chronically compressed dorsal root ganglion neurons in the rat. Pain. 2001;94:39–46. doi: 10.1016/S0304-3959(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 27.Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeftinija SD, Jeftinija KV, Stefanovic G. Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res. 1997;750:41–47. doi: 10.1016/s0006-8993(96)00610-5. [DOI] [PubMed] [Google Scholar]
- 29.Kaji H, Sugimoto T, Kanatani M, Nasu M, Chihara K. Estrogen blocks parathyroid hormone (PTH)-stimulated osteoclast-like cell formation by selectively affecting PTH-responsive cyclic adenosine monophosphate pathway. Endocrinology. 1996;137:2217–2224. doi: 10.1210/endo.137.6.8641168. [DOI] [PubMed] [Google Scholar]
- 30.Knickelbein RG, Seres T, Lam G, Johnston RB, Warshaw JB., Jr Characterization of multiple cysteine and cystine transporters in rat alveolar type II cells. Am J Physiol. 1997;273:L1147–1155. doi: 10.1152/ajplung.1997.273.6.L1147. [DOI] [PubMed] [Google Scholar]
- 31.Krenz WD, Nguyen D, Perez-Acevedo NL, Selverston AI. Group I, II, and III mGluR compounds affect rhythm generation in the gastric circuit of the crustacean stomatogastric ganglion. J Neurophysiol. 2000;83:1188–1201. doi: 10.1152/jn.2000.83.3.1188. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann J, Tsai C, Wood PL. Homocysteic acid as a putative excitatory amino acid neurotransmitter: I. Postsynaptic characteristics at N-methyl-d-aspartate-type receptors on striatal cholinergic interneurons. J Neurochem. 1988;51:1765–1770. doi: 10.1111/j.1471-4159.1988.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, Wang SJ, Luo MZ, Gean PW. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J Neurosci. 2000;20:9017–9024. doi: 10.1523/JNEUROSCI.20-24-09017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21:7001–7012. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miele M, Boutelle MG, Fillenz M. The source of physiologically stimulated glutamate efflux from the striatum of conscious rats. J Physiol (Lond) 1996;497:745–751. doi: 10.1113/jphysiol.1996.sp021805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller DW, Abercrombie ED. Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous l-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem. 1999;72:1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
- 37.Mistry R, Golding N, Challiss RA. Regulation of phosphoinositide turnover in neonatal rat cerebral cortex by group I- and II- selective metabotropic glutamate receptor agonists. Br J Pharmacol. 1998;123:581–589. doi: 10.1038/sj.bjp.0701626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 39.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- 40.Newcomb R, Palma A. Effects of diverse omega-conopeptides on the in vivo release of glutamic and gamma-aminobutyric acids. Brain Res. 1994;638:95–102. doi: 10.1016/0006-8993(94)90637-8. [DOI] [PubMed] [Google Scholar]
- 41.Okada M, Wada K, Kiryu K, Kawata Y, Mizuno K, Kondo T, Tasaki H, Kaneko S. Effects of Ca2+ channel antagonists on striatal dopamine and DOPA release, studied by in vivo microdialysis. Br J Pharmacol. 1998;123:805–814. doi: 10.1038/sj.bjp.0701675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pare D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- 43.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 44.Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG. alpha-Latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett. 1995;360:266–270. doi: 10.1016/0014-5793(95)00121-o. [DOI] [PubMed] [Google Scholar]
- 45.Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulsen RE, Fonnum F. Role of glial cells for the basal and Ca2+- dependent K+ release of the transmitter amino acids investigated by microdialysis. J Neurochem. 1989;52:1823–1829. doi: 10.1111/j.1471-4159.1989.tb07263.x. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 4. Academic; New York: 1998. [Google Scholar]
- 48.Pow DV. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia. 2001;34:27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- 49.Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in the striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- 50.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine–glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 51.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 52.Schoepp DD, Johnson BG, Salhoff CR, Valli MJ, Desai MA, Burnett JP, Mayne NG, Monn JA. Selective inhibition of forskolin-stimulated cyclic AMP formation in rat hippocampus by a novel mGluR agonist, 2R, 4R-4-aminopyrrolidine-2, 4-dicarboxylate. Neuropharmacology. 1995;34:843–850. doi: 10.1016/0028-3908(95)00061-a. [DOI] [PubMed] [Google Scholar]
- 53.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. dl-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 54.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 56.Tapia R, Medina-Ceja L, Pena F. On the relationship between extracellular glutamate, hyperexcitation and neurodegeneration, in vivo. Neurochem Int. 1999;34:23–31. doi: 10.1016/s0197-0186(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 57.Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 58.Ueda Y, Doi T, Tokumaru J, Mitsuyama Y, Willmore LJ. Kindling phenomena induced by the repeated short-term high potassium stimuli in the ventral hippocampus of rats: on-line monitoring of extracellular glutamate overflow. Exp Brain Res. 2000;135:199–203. doi: 10.1007/s002210000509. [DOI] [PubMed] [Google Scholar]
- 59.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 60.Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol (Lond) 1999;514(3):783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westerink BH, Damsma G, Rollema H, De Vries JB, Horn AS. Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 1987;41:1763–1776. doi: 10.1016/0024-3205(87)90695-3. [DOI] [PubMed] [Google Scholar]
- 62.Westerink BH, Damsma G, de Vries JB. Effect of ouabain applied by intrastriatal microdialysis on the in vivo release of dopamine, acetylcholine, and amino acids in the brain of conscious rats. J Neurochem. 1989;52:705–712. doi: 10.1111/j.1471-4159.1989.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 63.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto Y, Kakigi T, Maeda K. Intra-striatal phencyclidine inhibits N-methyl-d-aspartic acid-stimulated increase in glutamate levels of freely moving rats. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:161–174. doi: 10.1016/s0278-5846(98)00085-2. [DOI] [PubMed] [Google Scholar]
- 65.Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.You ZB, Herrera-Marschitz M, Nylander I, Goiny M, O'Connor WT, Ungerstedt U, Terenius L. The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis–II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience. 1994;63:427–434. doi: 10.1016/0306-4522(94)90540-1. [DOI] [PubMed] [Google Scholar]