Abstract
Episodic ataxia type 1 (EA1) is an autosomal dominant neurological disorder affecting both central and peripheral nerve function, causing attacks of imbalance and uncontrolled movements. Genetic linkage studies have identified mutations in the gene encoding the voltage-gated delayed rectifier potassium channel Kv1.1 as underlying EA1. The EA1 mutations E325D and V408A, residing near the cytoplasmic ends of S5 and S6, respectively, induce an unstable open state, resulting in an ∼10-fold increase in deactivation rates compared with wild-type (WT) channels. Coexpression of EA1 mutations with human Kvβ1 (hKvβ1) subunits in Xenopusoocytes yielded channels with altered rapid N-type inactivation. Compared with WT channels, inactivation was approximately twofold slower for homomeric E325D or V408A channels and 1.5-fold slower for heteromeric channels composed of two WT and two E325D or V408A subunits. Recovery from inactivation was ∼10-fold faster for homomeric E325D or V408A channels and threefold to fourfold faster for heteromeric WT and E325D or V408A channels compared with WT channels. Currents during successive pulses 3 msec in duration given at a rate of 40 kHz decayed e-fold in approximately four pulses for homomeric E325D or V408A and ∼2.5 pulses for heteromeric channels compared with approximately one pulse for WT channels. These results show that channels containing E325D or V408A subunits, which destabilize the open state, increase the rate of recovery from inactivation. The slower onset and more rapid recovery of hKvβ1-induced inactivation in channels containing these EA1 subunits may affect temporal integration of action potential firing rates.
Keywords: episodic ataxia type 1, inactivation, recovery from inactivation, Kv1.1, Kvβ1, potassium channel
Episodic ataxia type 1 (EA1) is an autosomal dominant neurological disorder affecting central and peripheral nerve function, with symptomatic attacks of uncontrolled movements (Ashizawa et al., 1983). Genetic studies identified mutations in KCNA1, the gene encoding the voltage-gated K+ channel Kv1.1, as underlying EA1, and affected individuals are heterozygous (Browne et al., 1994; Litt et al., 1994). Heterologous expression of EA1 subunits inXenopus oocytes revealed that most EA1 mutations resulted in functional channels with altered biophysical properties (Adelman et al., 1995; Comu et al., 1996; Zerr et al., 1998a;b; Zuberi et al., 1999; Eunson et al., 2000), whereas some channels were nonfunctional because of reduced protein levels or altered intracellular trafficking (Zerr et al., 1998a; Manganas et al., 2001; Rea et al., 2002). Therefore, EA1 mutations may cause the disorder through dominant negative effects or haplotype insufficiency.
Potassium channel diversity results from a large number of α subunit genes (Jan and Jan, 1989, 1990). Heteromeric assembly between different α subunits within the same subfamily (Covarrubias et al., 1991; Li et al., 1992) and interactions with associated regulatory β subunits amplifies K+ channel diversity (Rettig et al., 1994; Shi et al., 1996; Rhodes et al., 1997). Rapid inactivation of Kv1 channels is mediated by an associated Kvβ1 subunit (Rettig et al., 1994), and a second related β subunit, Kvβ2, appears to act as a molecular chaperone, increasing the number of channels in the plasma membrane (Shi et al., 1996; Xu and Li, 1997; Campomanes et al., 2002). Immunohistochemical studies using Kvβ1-specific antibodies indicate that Kvβ1 is extensively colocalized with Kv1.1 and Kv1.4 in cortical interneurons and hippocampal mossy fiber pathways (Rhodes et al., 1997).
Rapid N-type inactivation of Shaker channels occurs through the binding of the N-terminal inactivation domain (ID) to a receptor localized in the inner vestibule of the open channel (Hoshi et al., 1990). For mammalian Kv1 channels, the inactivation domain resides on the N-terminal region of the cytoplasmic Kvβ1 subunit (Rettig et al., 1994). The receptor site in the inner vestibule of the channel is formed from residues within the S6 domain (Zhou et al., 2001), and, in Shaker channels, the ID peptide protects against chemical modification of cysteine residues substituted into S6 (del Camino et al., 2000). One specific residue, V478, is analogous to V408 in Kv1.1, a position that is mutated to an alanine in one of the previously studied EA1 alleles (Adelman et al., 1995). V408A and another EA1 mutation, E325D, which resides at the intracellular boundary with S5, give rise to channels with deactivation rates that are ∼10 times faster than wild type (WT) (Adelman et al., 1995;D'Adamo et al., 1998; Zerr et al., 1998a). The positions of these residues suggest that they may also affect inactivation. To investigate whether EA1 mutations alter channel function solely at the level of the α subunits or also affect inactivation that require ancillary proteins, the effects of human Kvβ1 (hKvβ1) on potassium channel inactivation of V408A or E325D were examined.
MATERIALS AND METHODS
Xenopus care and handling were in accordance with the highest standards of institutional guidelines. Frogs underwent no more than two surgeries, separated by at least 3 weeks. To isolate oocytes, frogs were anesthetized with an aerated solution of 3-aminobenzoic acid ethyl ester. Standard recording solution contained (in mm): 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.4. Two-electrode voltage-clamp recordings were performed at room temperature with a Geneclamp 500 amplifier (Axon Instruments, Foster City, CA) interfaced to a Macintosh Power personal computer (Apple Computers, Cupertino, CA) with an ITC16 data acquisition interface (InstruTech, Port Washington, NY). Linear leak and capacitance currents were corrected with a P/4 leak subtraction procedure. Data collection and analysis were performed using Pulse, PulseFit (Heka Elektronik, Lambrecht/Pfalz, Germany), and IGOR (WaveMetrics, Lake Oswego, OR). Statistical significance was determined by an unpaired Student t test, and p < 0.01 was considered significant.
Human Kv1.1 and Kvβ1 cDNA was cloned into the vector pS− (Promega, Madison, WI). Site-directed mutagenesis, nucleotide sequencing, and in vitro mRNA synthesis were performed as described previously (Adelman et al., 1995).
RESULTS
Inactivation by hKvβ1
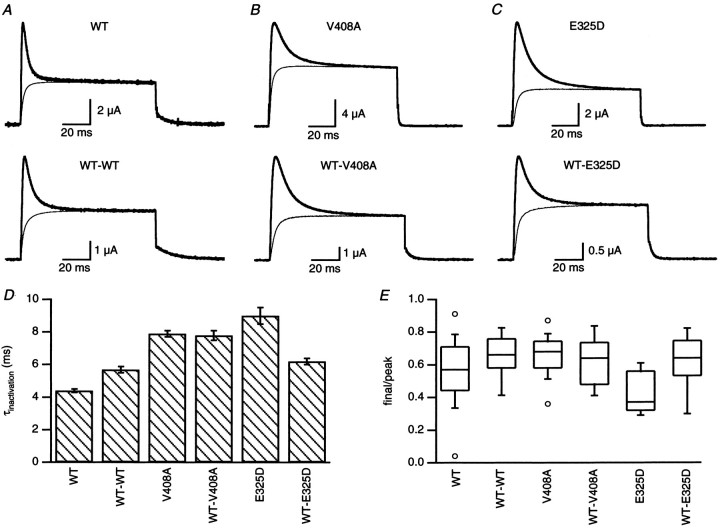
Episodic ataxia is a heterozygous disorder; affected individuals possess one WT and one mutant allele. Therefore, hKvβ1-mediated inactivation was examined for heteromeric Kv1.1 channels containing a fixed stoichiometry of two WT and two EA1 subunits using concatenated dimers of WT with either V408A (WT-V408A) or E325D (WT-E325D), as well as homomeric Kv1.1 channels containing only WT, V408A, or E325D subunits. Figure 1 shows that coexpression with hKvβ1 endowed each of the channel types with rapid inactivation during depolarization to 40 mV (Fig. 1A–C). The time course of inactivation was described with a double exponential and showed that the fast component accounted for >90% of the inactivation. The mean value for the time constant of the fast component of inactivation of homomeric V408A (8.1 ± 0.2 msec;n = 61) or E325D (9.0 ± 0.5 msec;n = 15) coexpressed with hKvβ1 was significantly increased compared with WT (4.4 ± 0.1 msec; n = 139) (Fig. 1D). Constraining the C and N termini of two WT subunits by expressing the WT-WT dimer decreased the rate of inactivation induced by hKvβ1 (5.7 ± 0.2 msec;n = 47) compared with homomeric WT channels. Expression of WT-V408A or WT-E325D dimers with hKvβ1 yielded inactivation rates (7.8 ± 0.3 msec, n = 17; and 6.4 ± 0.2 msec, n = 24, respectively) that were slower than WT-WT channels. These results show that V408A and E325D reduce the inactivation rates of heteromeric channels formed from WT and EA1 subunits that coassemble with hKvβ1.
Fig. 1.
Inactivation of WT, V408A, and E325D Kv1.1 currents induced by coexpression with hKvβ1. A, Currents recorded from oocytes expressing homomeric WT channels (top traces) or dimers of WT-WT (bottom traces). Superimposed noninactivating current recorded in oocytes without hKvβ1 were scaled to the steady-state inactivated current obtained with hKvβ1. For the voltage protocol, from a holding potential of −80 mV, the membrane potential was stepped to 40 mV for 100 msec and back to −50 mV. B, Currents recorded from oocytes expressing homomeric V408A or dimers of WT-V408A.C, Current recorded from oocytes expressing homomeric E325D or dimers of WT-E325D. D, Bar graph of the time constant of inactivation of the dominant fast component (see Results). Data are displayed as mean ± SEM (Table 2). E, Box plot of the distribution of the ratio of final to peak current for indicated constructs expressed with hKvβ1.
The amount of inactivation was variable between batches of oocytes, and a box plot of the ratio of the final current to the peak current revealed minimal differences between homomeric or heteromeric channels (Fig. 1E). The peak of the inactivating current measured in oocytes coexpressing homomeric WT or V408A channels with hKvβ1 was always smaller than the non-inactivating current measured on the same day in oocytes injected with the same amount of WT or V408A RNA without hKvβ1. The ratio of the maximum outward current of homomeric WT expressed with and without hKvβ1 was 0.47 ± 0.08 (n = 15 d). The ratio for V408A was 0.56 ± 0.15 (n = 6 d, 44 oocytes). In contrast, coexpression of hKvβ1 with E325D always yielded larger currents than E325D expressed without hKVβ1, with a ratio of 2.8 ± 1.15 (n = 3 d, 16 oocytes). These results show that Kvβ1 has a much larger affect on expression of E325D compared with WT or V408A. Consistent with this finding is the result that E325D does not express well by itself, yielding current amplitudes that were ∼30-fold less than homomeric WT compared with V048A, which were 0.5 of WT (Zerr et al., 1998a). This suggests that E325D by itself does not traffic well to the membrane and that Kvβ1, in which the C-terminal domain is ∼80% homologous with Kvβ2 (Rettig et al., 1994), may stabilize E325D channels and enhance surface expression.
N-type inactivation of the ShakerK+ channel requires only one of four N-terminal IDs, and the rate of inactivation of channels containing four IDs is 3.5 times the rate of inactivation of channels containing one ID (MacKinnon et al., 1993). Kv1 channels in vivo are likely comprised of four α and four β subunits (Shamotienko et al., 1997). The results presented above do not distinguish between an altered interaction between the inactivation domain of Kvβ1 and a reduced number of coassembled Kvβ1 subunits; it is possible that E325D and V408A slow the onset of inactivation as a result of a reduced number of Kvβ1 subunits incorporated with the channel. Figure1E shows that the extent of inactivation of WT channels as inferred from the ratio of the final current to the peak current varied from 0.04 to 0.91. It is possible that oocytes showing a greater extent of inactivation contain a greater number of channels with more than one Kvβ1 subunit (MacKinnon et al., 1993). By regrouping the data according to the extent of inactivation, the mean time constant of inactivation of WT for ratios ≤0.2 or ≥0.8 was 4.2 ± 0.2 (n = 6) and 4.5 ± 0.2 (n = 11) msec, respectively (p = 0.3). Thus, the rate of inactivation of WT channels did not vary with the extent of inactivation, suggesting that Kv1.1 and Kvβ1 are coassembled in a fixed stoichiometry. Similar results were seen for V408A and E325D when data were grouped according to inactivation ratios that were ≤0.5 or >0.5. The time constant of inactivation for V408A was 7.5 ± 0.5 (n = 6) and 8.1 ± 0.2 (n = 55), respectively (p = 0.3) and for E325D was 9.3 ± 0.7 (n = 10) and 8.3 ± 0.2 (n = 5), respectively (p= 0.2). These data show no correlation between the extent of inactivation and the rate of inactivation for WT, V408A, or E325D channels.
Voltage dependence of availability of EA1 channels coexpressed with hKvβ1
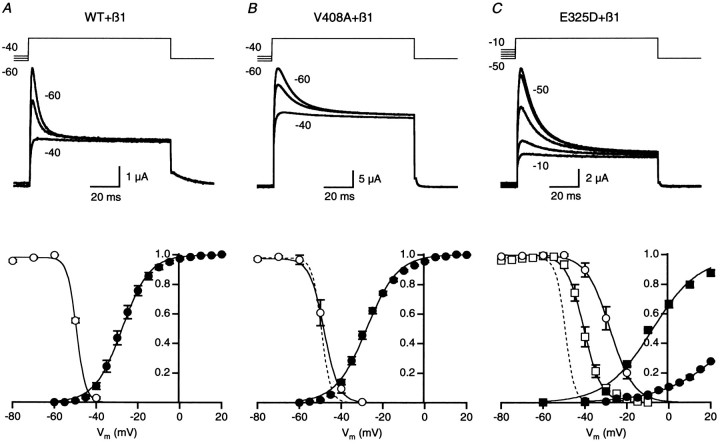
In a neuron, the membrane potential will affect the number of Kv1 channels available to open during an action potential, as well as the recovery rate, or repriming, of the channels that inactivated during the action potential. Therefore, the effect of holding potential on channel availability was measured for homomeric and heteromeric channels coexpressed with hKvβ1 (Fig.2). Availability relationships were determined from currents evoked by a family of test pulses to 40 mV preceded by a 1 sec prepulse to potentials ranging from −80 to −20 mV. Representative current traces for homomeric WT, V408A, and E325D channels coexpressed with hKvβ1 are shown in Figure2A–C. Depolarization of the prepulse potential decreased the amount of current available to inactivate with minimal effect on the steady-state current at the end of the pulse. The small reduction in steady current for V408A and E325D reflects enhanced rates of C-type inactivation reported previously for these mutations (Adelman et al., 1995). The relative amount of inactivation, determined from the peak outward current measured 5–7 msec after depolarization to 40 mV, was plotted as a function of the prepulse potential. Data for each oocyte was normalized by the maximum current at the plateau of the relationship. Availability relationships were averaged for several oocytes and fit with a Boltzmann function (Fig. 2, bottom row). For comparison, the voltage dependence of activation, determined in separate experiments without hKvβ1 coexpression (Adelman et al., 1995; Zerr et al., 1998a), was plotted on the same graph. Comparison of the two relationships revealed that WT Kv1.1 channels became preinactivated near the foot of the activation curve. The voltage for half-maximal inactivation,V1/2, determined from the averaged availability relationship of WT Kv1.1 coexpressed with hKvβ1, was −49.5 mV, with a steepness factor of 2.7 mV. Average values determined from several oocytes are listed in Table1.
Fig. 2.
Voltage dependence of availability for WT, V408A, and E325D channels coexpressed with hKvβ1. A, Current traces, recorded from oocytes expressing WT and hKvβ1 subunits, evoked by a depolarizing pulse to 40 mV from a 1 sec prepulse potential of −60, −50, and −40 mV. The voltage template is shown in thetop traces. The peak current measured relative to steady-state inactivation was normalized by the maximum value and plotted versus the prepulse potential; mean ± SEM data are displayed as open circles in the bottom graph (n = 26). A Boltzmann relationship was fit to the data, yielding a V1/2 of −49.5 mV and slope factor of 2.7 mV. The voltage dependence of activation was determined in separate experiments without hKvβ1 subunit coexpression. Tail currents were measured at −50 mV after a test pulse to potentials between −60 and 40 mV, and the peak outward tail current was plotted versus test pulse potential. The data for each oocyte was normalized to the maximum current averaged over several pulses at the plateau of the voltage relationship. The data from several oocytes were averaged (filled circles;n = 7) and fit with a Boltzmann relationship, yielding a V1/2 of −27.6 mV and a slope factor of 6.5 mV. B, Current traces recorded from oocytes expressing V408A and hKvβ1 subunits. Dashed line represents WT availability curve from A.C, Current traces recorded from oocytes expressing homomeric E325D (open and filled circlesfor inactivation and activation, respectively) or heteromeric WT-E325D (open and filled squares for inactivation and activation, respectively) and hKvβ1 subunits. Dashed line represents WT availability curve fromA.
Table 1.
Boltzmann parameters for voltage dependence of availability and activation
| Construct | Inactivation | Activation | ||
|---|---|---|---|---|
| V1/2 (mV) | k(mV) | V1/2 (mV) | k (mV) | |
| WT | −49.5 ± 0.3 | 2.6 ± 0.1 (26) | −27.6 ± 1.2 | 6.2 ± 0.3 (7) |
| V408A | −45.8 ± 1.0 | 3.2 ± 0.1 (14) | −27.9 ± 0.7 | 7.2 ± 0.2 (9) |
| E325D | −28.2 ± 1.8 | 5.3 ± 0.1 (5) | 35.8 ± 0.4 | 16.4 ± 0.3 (5) |
| WT-WT | −49.1 ± 1.0 | 2.7 ± 0.2 (19) | −27.0 ± 1.2 | 5.8 ± 0.2 (6) |
| WT-V408A | −49.4 ± 2.2 | 3.5 ± 0.2 (4) | −26.7 ± 2.5 | 6.6 ± 0.3 (6) |
| WT-E325D | −42.1 ± 1.2 | 3.3 ± 0.2 (9) | −12.4 ± 0.7 | 9.1 ± 0.7 (5) |
V1/2 and k represent voltage for half-maximal effect and steepness factor determined from Boltzmann fits; mean ± SEM. Numbers in parentheses indicate number of oocytes.
Previous studies have shown that the voltage dependence of activation for homomeric V408A and heteromeric WT-V408A channels are not significantly different from WT channels (Table 1). Figure2B shows that the voltage dependence of availability was not markedly different for V408A channels coexpressed with hKvβ1 compared with WT channels coexpressed with hKvβ1 (dashed line vs open symbols). Similarly, the voltage dependence of availability for heteromeric WT-V408A channels coexpressed with hKvβ1 was not different from homomeric V408A coexpressed with hKvβ1 (Table 1).
In contrast, the voltage dependence of activation of homomeric E325D and heteromeric WT-E325D channels was considerably right-shifted, by 63.4 and 14.6 mV, respectively (Fig. 2C; Table 1), and the steepness of activation was reduced. In concert with the shift in the voltage dependence of activation, the availability relationships for homomeric E325D and heteromeric WT-E325D channels coexpressed with hKvβ1 were right-shifted by 21.3 and 7.4 mV, respectively (Fig.2C; Table 1). Similarly, the steepness factor was increased from 2.6 to 5.3 and 3.3 mV, respectively.
Inactivation of Kv1 channels conferred by Kvβ1 subunits may influence membrane excitability by affecting the number of channel available to open at the resting membrane potential. For WT Kv1.1 and V408A subunits coexpressed with hKvβ1, depolarizing the resting membrane potential from −80 to −50 mV decreased the fraction of channels available to open from 1 to ∼½ (Fig.2A,B). In contrast, E325D, which shifted the voltage dependence of availability to more positive potentials by ∼20 and 9 mV for homomeric E325D and heteromeric WT and E325D channels, the fraction of channels available to open at a resting membrane potential of −50 mV was 0.98 and 0.9, respectively (Fig.2C). Thus, for heteromeric complexes of WT or V408A with hKvβ1, the number of Kv1 channels available to open will be influenced by the resting membrane potential over the physiological voltage range of −60 to −50 mV and may thus affect membrane excitability. In contrast, availability of heteromeric WT and E325D channels when complexed with hKvβ1 subunits will not be very sensitive to the resting potential.
Recovery from inactivation
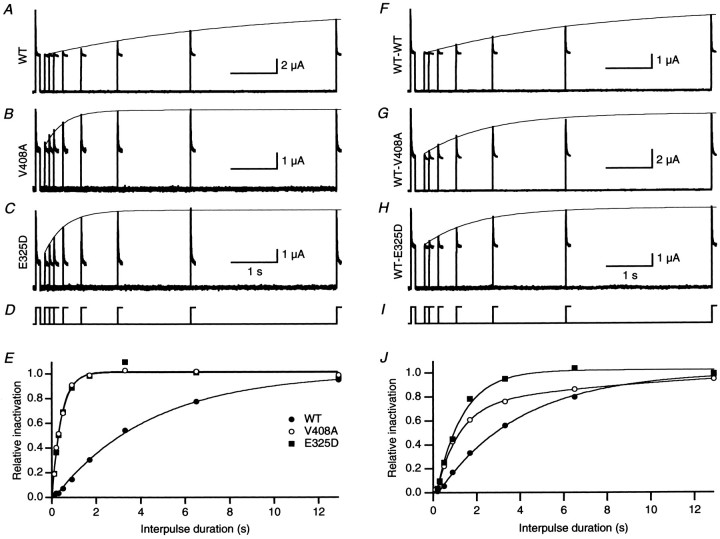
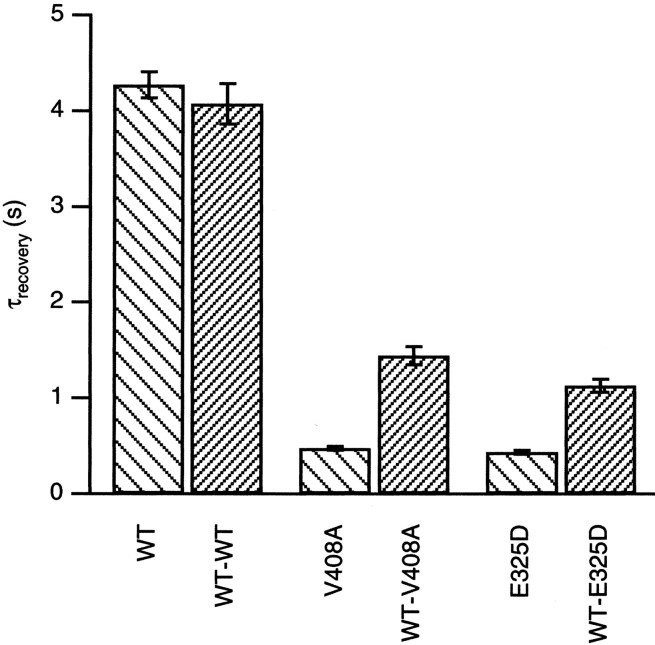
The deactivation rates of the EA1 mutations E325D and V408A were increased ∼10-fold compared with WT channels (Adelman et al., 1995;Zerr et al., 1998a). The faster deactivation is likely to affect the kinetics of repriming after inactivation. Recovery from inactivation induced by hKvβ1 was determined using a double-pulse protocol (Fig.3). From a holding potential of −80 mV, inactivation was induced by depolarization to 40 mV for 100 msec and repolarized back to the holding potential. To assess the recovery from inactivation, a second pulse to 40 mV was applied after a delay of 0.1–13 sec after the first pulse. Figure 3 shows traces from a representative oocyte for each channel type. A single exponential described the recovery from inactivation applied to the envelope of peak currents evoked by the second pulse (line connecting peaks of second pulse). The relative recovery from inactivation plotted as a function of interpulse duration shows that rate of recovery from inactivation was markedly increased for homomeric E325D and V408A compared with WT (Fig. 3E). Coexpression of WT-EA1 dimers with hKvβ1 yielded a recovery time course that was intermediate between homomeric WT and EA1 channels (Fig. 3F–J). Average data from several oocytes were plotted on a bar graph (Fig.4). The recovery from inactivation of homomeric WT or WT-WT channels was similar, 4.27 ± 0.13 and 4.28 ± 0.21 sec, respectively (Table2). Homomeric V408A and E325D had recovery time constants that were ∼10 times faster than homomeric WT channels, with time constants of 0.48 ± 0.02 and 0.43 ± 0.02 sec, respectively. The faster rate of recovery attributable to the EA1 subunits was also endowed on heteromeric channels composed of two WT and two V408A or E325D subunits, with time constants of 1.44 ± 0.10 and 1.18 ± 0.07 sec, respectively.
Fig. 3.
EA1 mutations V408A and E325D accelerate recovery from inactivation induced by hKvβ1. A–C, Recovery from inactivation was determined using a double-pulse protocol to 40 mV separated by an interpulse interval of 0.1–13 sec at a holding potential of −80 mV (D). Superimposed current traces recorded for interpulse intervals from 0.1 to 13 sec for homomeric WT, V408A, and E325D. A single exponential (continuous line) determined in E was overlaid with the envelope of peaks of the second pulse. E, The relative amount of inactivation normalized by the first pulse plotted as a function of interpulse duration for homomeric WT, V408A, and E325D fromA–C. The data were fit with a single-exponential function, yielding a time constant of recovery of 4.1, 0.4, and 0.4 sec for WT (filled circles), V408A (open circles), and E325D (filled squares), respectively. F–I, Recovery from inactivation of heteromeric channels formed by WT-WT, WT-V408A, and WT-E325D coexpressed with hKvβ1. J, Plot of relative recovery from inactivation versus interpulse duration for WT-WT (filled circles), WT-V408A (open circles), and WT-E325D (filled squares). The time constants of recovery determined from single exponentials were 3.3, 1.1, and 1.2 sec, respectively.
Fig. 4.
Time constant of recovery from hKvβ1-induced inactivation of K+ channels formed from homomeric WT, V408A, and E325D and WT-WT, WT-V408A, and WT-V408A dimers. Data are presented as mean ± SEM values.
Table 2.
Time constants of inactivation and recovery from inactivation of WT, homomeric EA1, and heteromeric WT-EA1 channels
| Construct | τinactivation at 40 mV (msec) | τrecovery at −80 mV (sec) |
|---|---|---|
| WT | 4.4 ± 0.1 (139) | 4.27 ± 0.13 (102) |
| V408A | 8.1 ± 0.2 (61) | 0.48 ± 0.02 (42) |
| E325D | 9.0 ± 0.5 (15) | 0.43 ± 0.02 (15) |
| WT-WT | 5.7 ± 0.2 (47) | 4.28 ± 0.21 (31) |
| WT-V408A | 7.8 ± 0.3 (17) | 1.44 ± 0.10 (13) |
| WT-E325D | 6.2 ± 0.2 (27) | 1.18 ± 0.07 (17) |
Data are presented as mean ± SEM. Numbers in parentheses indicate number of oocytes.
These results show that channels containing two EA1 subunits of V408A or E325D have faster rates of recovery than channels containing only WT subunits; the number of mutant EA1 subunits defines the rate of recovery from hKvβ1-induced N-type inactivation. Heteromeric Kv1 channels containing one or more E325D or V408A subunits will thus be available sooner to contribute to repolarization after an action potential.
Stimulation-dependent recruitment of inactivation of EA1 subunits during rapid pacing
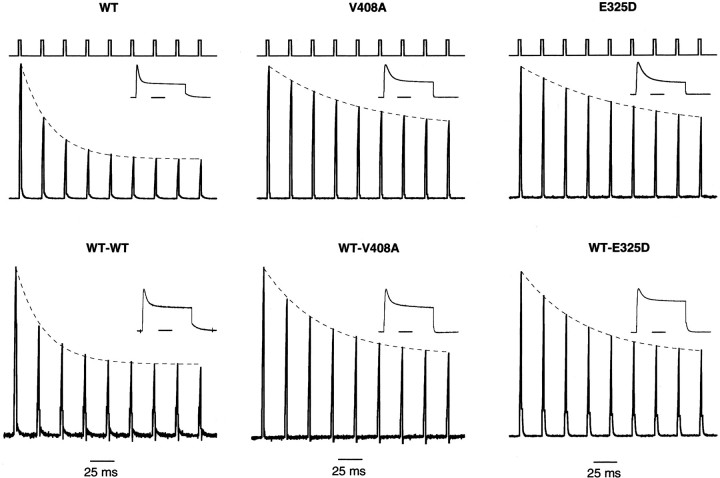
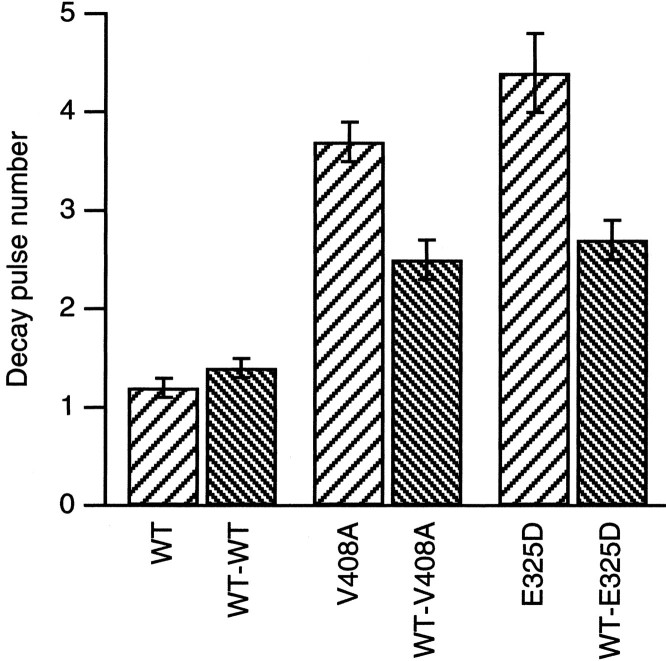
The slower onset of inactivation and faster recovery from hKvβ1-induced inactivation seen for V408A and E325D channels may affect the recruitment of inactivation of these channels during a train of action potentials. To mimic a burst of action potentials, a train of 3 msec pulses to 40 mV at 40 kHz from a holding potential of −80 mV was applied to oocytes coexpressing hKvβ1 with WT or the EA1 subunits (Fig. 5). For homomeric WT channels, the peak outward current during the 3 msec pulse decreased to a steady-state level over four to five pulses. However, for channels containing two or four V408A or E325D subunits, the peak current decreased to a steady state over seven to eight pulses. A single exponential described the current reduction applied to the peak current as a function of the pulse number (Fig. 5, dashed line). Average data were plotted in Figure 6. WT channels decayed e-fold in 1.2 ± 0.1 (n = 23) pulses. In contrast, homomeric V408A or E325D decayed e-fold in 3.7 ± 0.2 (n = 21) and 4.4 ± 0.4 (n = 6) pulses, respectively. Currents recorded from oocytes expressing WT-V408A or WT-E325D and hKvβ1 decayed e-fold in 2.5 ± 0.2 (n= 7) and 2.7 ± 0.2 (n = 2) pulses, respectively. The results indicate that the EA1 mutations V408A and E325D will affect the repriming of K+ channels during a train of action potentials. Increasing the number of EA1 subunits in a Kv1 channel results in faster repriming and slower inactivation rates.
Fig. 5.
Stimulation-dependent recruitment of inactivation induced by hKvβ1. Currents were evoked by a train of depolarizing pulse to 40 mV for 3 msec given at a frequency of 40 kHz. The voltage template is shown in the top trace of eachcolumn. Dashed lines represent a single exponential fitted to the envelope of peak currents. Theinsets show currents recorded from a test pulse to 40 mV for 100 msec. The holding potential was −80 mV.
Fig. 6.
V408A and E325D reduce stimulation-dependent recruitment of hKvβ1-induced inactivation. Bar graph of the mean decay pulse number determined from plots of peak current during a 40 kHz train of 3 msec depolarizing pulses to 40 mV versus pulse number. Data are presented as mean ± SEM.
DISCUSSION
In central neurons, K+ channels contribute to the resting membrane potential and repolarization of the action potential (Hille, 2001). Genetic linkage studies have identified multiple independent missense mutations and one nonsense mutation that results in a truncation in the Kv1.1 coding sequence, which underlie the inherited neurological disorder EA1. Native Kv channels are either heteromeric assemblies between different α subunits within the same subfamily (Covarrubias et al., 1991; Li et al., 1992), and some are associated with a regulatory Kvβ subunit, resulting in remarkable molecular diversity (Rettig et al., 1994; Shi et al., 1996; Rhodes et al., 1997). The results presented here indicate that the EA1 missense mutations E325D and V408A will not only affect the biophysical properties of channels formed from heteromeric Kv1.1 α subunits but will also affect the properties conferred when the mutant channels are associated with Kvβ1 subunits.
E325D and V408A are conservative substitutions that retain the nature of the residue, hydrophobic or negatively charged, but reduce the side chain length by two or one methyl groups, respectively. For both mutations, the shorter side chain results in an ∼10-fold slowing of the deactivation rate for homomeric channels and, for E325D, a 60 mV positive shift in voltage dependence (Adelman et al., 1995; Zerr et al., 1998a). Compared with WT channels, the rapid N-type inactivation conferred by interactions with hKvβ1 is slower in homomeric V408A or E325D and heteromeric WT-V408A or WT-E325D channels. The rate of recovery from inactivation is ∼10-fold faster for homomeric V408A and E325D and approximately fourfold faster for heteromeric channels compared with WT channels. For V408A, the more rapid recovery from N-type inactivation suggests that the shorter side chain of alanine decreases the stability of the inactivated state. Studies ofShaker channels show that V478, the homologous site to V408A in Kv1.1, contributes to the receptor for the inactivation particle. In a channel with the V478C substitution, application of the inactivatingShaker “ball peptide” protected the cysteine from chemical modification. In addition, the analogous site in Kv1.4 resides in a domain that has also been strongly implicated as the binding site for the N-terminal inactivation particle of Kvβ1 (Zhou et al., 2001). Therefore, the slowed onset and faster recovery from inactivation exhibited by V408A channels might reflect a change in the affinity of the receptor for the inactivation particle.
However, equivalent changes in inactivation were also observed for E325D, a position located at the intracellular boundary of S5 and removed from the hydrophobic S6 inactivation receptor domain (Zhou et al., 2001). The S4–S5 linker could serve as a predocking site for the Kvβ1 inactivation domain before inserting into the hydrophobic region of the inner vestibule (Zhou et al., 2001), and mutations in this region may affect docking of the Kvβ1 inactivation domain. It is also possible that reducing the side chain at E325 by one methyl group affects the overall packing of the S5–S6 structure, giving rise to a wider internal vestibule that destabilizes the interaction with Kvβ1. Alternatively, it has been shown that the inactivation particle binds to its receptor domain when the channel is in the open state and that the inactivation particle is expelled when the open-inactivated channel transitions into the closed state (Zagotta et al., 1990). Both E325D and V408A show accelerated macroscopic deactivation rates and reduced single-channel mean open times (Liu et al., 1998), reflecting a destabilized open state such that the channels flicker in and out of the open state more often than WT channels. In these mutations, the destabilized open state could reduce the availability of the channel for binding of the inactivation particle, thus decreasing the rate of inactivation. Indeed, single-channel analysis showed an approximately twofold decrease in the mean open time of homomeric V408A or E325D channels (Liu et al., 1998), consistent with the twofold slowing in the rate of inactivation induced by hKvβ1. The ∼10-fold increase in the macroscopic deactivation rates of V408A and E325D may induce a more rapid expulsion of the inactivation particle, consistent with the ∼10-fold increase in the rate of recovery. Therefore, the slowed onset and faster recovery from inactivation seen for both E325D and V408A may reflect a similarly destabilized open state and not only an altered affinity of the receptor site for the inactivation domain.
The Kv1.1 gene is widely expressed in the brain, with specific subcellular distributions (Tsaur et al., 1992; Wang et al., 1994). In cerebellar GABAergic basket cells that synapse onto the soma of Purkinje cells, Kv1.1 subunits are clearly localized to juxtaparanodal regions and presynaptic terminals but do not appear to colocalize with Kvβ1 subunits, consistent with the lack of a fast inactivating K+ current in basket cell presynaptic terminals (Rhodes et al., 1997; Southan and Robertson, 1998).). Kv1.1 is not apparently expressed in the postsynaptic Purkinje cell. Dendrotoxin (DTX) affects basket cell transmitter release, increasing the frequency and amplitude of spontaneous IPSCs in the Purkinje cells (Southan and Robertson, 1998, 2000). This is attributable at least in part to DTX-sensitive KV1.1-containing channels because Kv1.1 null mice have a twofold higher spontaneous frequency of IPSCs than WT mice (Zhang et al., 1999). These results suggest that the EA1 mutations also affect GABAergic signaling in the cerebellum by their direct action Kv1.1 channels even without coassembled Kvβ1, consistent with preliminary results (data not shown).
Kvβ1 does colocalize with Kv1.1 and Kv1.4 in hippocampal mossy fibers that have a fast-inactivating K+ current in the presynaptic boutons mossy fiber boutons (MFBs) (Rhodes et al., 1997; Geiger and Jonas, 2000). Synaptic strength is, in part, determined by the shape of the presynaptic action potential, and mossy fibers show an activity-dependent spike broadening that is K+ channel dependent and associated with a slow recovery from K+ channel inactivation (Geiger and Jonas, 2000). The functional properties of the native K+ channel recorded in MFB patches are similar to those of cloned Kv1.1 or Kv1.4 coexpressed with Kvβ1 (Heinemann et al., 1996). The results presented here show that the EA1 mutations V408A and E325D in Kv1.1 accelerate the recovery from inactivation induced by Kvβ1, giving rise to a slowing of activity-dependent recruitment of inactivation. In mossy fibers, these EA1 mutations would be expected to reduce presynaptic activity-dependent spike broadening and reduce the activity-dependent increase in synaptic strength. Indeed, in Kvβ1-deficient mice, activity-dependent spike broadening was reduced and learning was impaired (Giese et al., 1998). These results may reflect a similar process, attributable to the EA mutations, underlying the cognitive dysfunction reported by V408A and E325D EA1 patients (Gancher and Nutt, 1986).
The mutations in Kv1.1 that underlie EA1 have been examined by heterologous expression of the α subunits. In most cases, the studies revealed biophysical differences with wild-type channels, but some EA1 subunits do not form functional homomeric channels. Coassembly of EA1 mutations with wild-type subunits, more accurately reflecting thein vivo condition, reduces the effects of the mutant subunits. Indeed, the effects of V408A subunits in a population of heteromeric channels are surprisingly subtle, yet the V408A mutation results in the symptoms of EA1. The results presented here show that the effects of EA1 subunits are also imposed on processes involving associated proteins, such as Kvβ1. Therefore, the effects of EA1 mutations will manifest at multiple levels of channel function, with distinct consequences for signaling in multiple neural networks.
Footnotes
This work was supported by National Institutes of Health grants (J.M. and J.P.A.) and a National Ataxia Foundation grant (J.M.).
Correspondence should be addressed to Dr. James Maylie, Department of Obstetrics and Gynecology, Oregon Health Sciences University, L-458, 3181 Southwest Sam Jackson Park Road, Portland, OR 97201. E-mail:mayliej@ohsu.edu.
REFERENCES
- 1.Adelman JP, Bond CT, Pessia M, Maylie J. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron. 1995;15:1449–1454. doi: 10.1016/0896-6273(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 2.Ashizawa T, Butler IJ, Harati Y, Roongta SM. A dominantly inherited syndrome with continuous motor neuron discharges. Ann Neurol. 1983;13:285–290. doi: 10.1002/ana.410130310. [DOI] [PubMed] [Google Scholar]
- 3.Browne DL, Gancher ST, Nutt JG, Brunt ERP, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 4.Campomanes CR, Carroll KI, Manganas LN, Hershberger ME, Gong B, Antonucci DE, Rhodes KJ, Trimmer JS. Kvβ subunit oxidoreductase activity and Kv1 potassium channel trafficking. J Biol Chem. 2002;277:8298–8305. doi: 10.1074/jbc.M110276200. [DOI] [PubMed] [Google Scholar]
- 5.Comu S, Giuliani M, Narayanan V. Episodic ataxia and myokymia syndrome: a new mutation of potassium channel gene Kv1. 1. Ann Neurol. 1996;40:684–687. doi: 10.1002/ana.410400422. [DOI] [PubMed] [Google Scholar]
- 6.Covarrubias M, Wei A, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- 7.D'Adamo CM, Liu Z, Adelman JP, Maylie J, Pessia M. Episodic ataxia type-1 mutations in the hKv1.1 cytoplasmic pore region alter the gating properties of the channel. EMBO. 1998;17:1200–1207. doi: 10.1093/emboj/17.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Camino D, Holmgren M, Liu Y, Yellen G. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 2000;403:321–325. doi: 10.1038/35002099. [DOI] [PubMed] [Google Scholar]
- 9.Eunson LH, Rea R, Zuberi SM, Youroukos S, Panayiotopoulos CP, Liguori R, Avoni P, McWilliam RC, Stephenson JB, Hanna MG, Kullmann DM, Spauschus A. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48:647–656. [PubMed] [Google Scholar]
- 10.Gancher S, Nutt J. Autosomal dominant episodic ataxia: a heterogeneous syndrome. Mov Disord. 1986;1:239–253. doi: 10.1002/mds.870010404. [DOI] [PubMed] [Google Scholar]
- 11.Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 12.Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvβ1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. [PMC free article] [PubMed] [Google Scholar]
- 13.Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. J Physiol (Lond) 1996;493:625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hille B. Ionic channels of excitable membranes, Ed 3. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 15.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 16.Jan LY, Jan YN. Voltage-sensitive ion channels. Cell. 1989;56:13–25. doi: 10.1016/0092-8674(89)90979-3. [DOI] [PubMed] [Google Scholar]
- 17.Jan LY, Jan YN. How might the diversity of potassium channels by generated? Trends Neurosci. 1990;13:415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Jan YN, Jan LY. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992;257:1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- 19.Litt M, Kramer P, Browne D, Gancher S, Brunt ER, Root D, Phromchotikul T, Dubay CJ, Nutt J. A gene for episodic ataxia/myokymia maps to chromosome 12p13. Am J Hum Genet. 1994;55:702–709. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ZP, Adelman JP, Maylie J. Single channel and gating currents of episodic ataxia mutations in the human Kv1.1 potassium channel. Biophys J. 1998;74:A216. doi: 10.1016/s0014-5793(98)00814-x. [DOI] [PubMed] [Google Scholar]
- 21.MacKinnon R, Aldrich RW, Lee AW. Functional stoichiometry of Shaker potassium channel inactivation. Science. 1993;262:757–759. doi: 10.1126/science.7694359. [DOI] [PubMed] [Google Scholar]
- 22.Manganas LN, Wang Q, Scannevin RH, Antonucci DE, Rhodes KJ, Trimmer JS. Identification of a trafficking determinant localized to the Kv1 potassium channel pore. Proc Natl Acad Sci USA. 2001;98:14055–14059. doi: 10.1073/pnas.241403898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rea R, Spauschus A, Eunson LH, Hanna MG, Kullmann DM. Variable K+ channel subunit dysfunction in inherited mutations of KCNA1. J Physiol (Lond) 2002;538:5–23. doi: 10.1113/jphysiol.2001.013242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channel altered by presence of β-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvβ1 and Kvβ2 β-subunits with Kv1 α-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamotienko OG, Parcej DN, Dolly JO. Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry. 1997;36:8195–8201. doi: 10.1021/bi970237g. [DOI] [PubMed] [Google Scholar]
- 27.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. β subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 28.Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsaur ML, Sheng M, Lowenstein DH, Jan YN, Jan LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8:1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Li M. Kvβ2 inhibits the Kvβ1-mediated inactivation of K+ channels in transfected mammalian cells. J Biol Chem. 1997;272:11728–11735. doi: 10.1074/jbc.272.18.11728. [DOI] [PubMed] [Google Scholar]
- 33.Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- 34.Zerr P, Adelman JP, Maylie J. Episodic ataxia mutations in Kv1.1 alter potassium channel function by dominant negative effects and haploinsufficiency. J Neurosci. 1998a;18:2842–2848. doi: 10.1523/JNEUROSCI.18-08-02842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerr P, Adelman JP, Maylie J. Characterization of three episodic ataxia mutations in the human Kv1.1 potassium channel. FEBS Lett. 1998b;431:461–464. doi: 10.1016/s0014-5793(98)00814-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang CL, Messing A, Chiu SY. Specific alteration of spontaneous GABAergic inhibition in cerebellar Purkinje cells in mice lacking the potassium channel Kv1.1. J Neurosci. 1999;19:2852–2864. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Morais-Cabral JH, Mann S, MacKinnon R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 2001;411:657–661. doi: 10.1038/35079500. [DOI] [PubMed] [Google Scholar]
- 38.Zuberi SM, Eunson LH, Spauschus A, De Silva R, Tolmie J, Wood NW, McWilliam RC, Stephenson JP, Kullmann DM, Hanna MG. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain. 1999;122:817–825. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]