Abstract
Although injury-induced afferent discharge declines significantly over time, experimental neuropathic pain persists unchanged for long periods. These observations suggest that processes that initiate experimental neuropathic pain may differ from those that maintain such pain. Here, the role of descending facilitation arising from developing plasticity in the rostral ventromedial medulla (RVM) in the initiation and maintenance of experimental neuropathic pain was explored. Tactile and thermal hypersensitivity were induced in rats by spinal nerve ligation (SNL). RVM lidocaine blocked SNL-induced tactile and thermal hypersensitivity on post-SNL days 6–12 but not on post-SNL day 3. Lesion of RVM cells expressing μ-opioid receptors with dermorphin–saporin did not prevent the onset of SNL-induced tactile and thermal hypersensitivity, but these signs reversed to baseline levels beginning on post-SNL day 4. Similarly, lesions of the dorsolateral funiculus (DLF) did not prevent the onset of SNL-induced tactile and thermal hypersensitivity, but these signs reversed to baseline levels beginning on post-SNL day 4. Lesions of the DLF also blocked the SNL-induced increase in spinal dynorphin content, which has been suggested to promote neuropathic pain. These data distinguish mechanisms that initiate the neuropathic state as independent of descending supraspinal influences and additional mechanism(s) that require supraspinal facilitation to maintain such pain. In addition, the data indicate that these time-dependent descending influences can underlie some of the SNL-induced plasticity at the spinal level. Such time-dependent descending influences driving associated spinal changes, such as the upregulation of dynorphin, are key elements in the maintenance, but not initiation, of neuropathic states.
Keywords: descending facilitation, neuropathic pain, RVM, lidocaine, tactile hypersensitivity, thermal hyperalgesia, dermorphin–saporin
Neuropathic pain may result from increased excitability of injured nerves (Kirk, 1974; Wall and Gutnick, 1974a,b). Persistent spontaneous afferent input also results in sensitization of spinal neurons to promote enhanced pain (Devor, 1991;Woolf, 1991; Woolf and Thompson, 1991). Agents that diminish spontaneous afferent activity are effective in clinical and experimental neuropathic pain (Chaplan et al., 1995; Chapman et al., 1998; Devor and Seltzer, 1999). Spontaneous afferent activity also correlates with expression of neuropathic pain (Han et al., 2000; C.Liu et al., 2000; X. Liu et al., 2000), and the onset of tactile hypersensitivity occurs with the development of afferent discharge (C.Liu et al., 2000). Discharges are most pronounced 1 week after injury but diminish significantly and rapidly over time (Han et al., 2000). Nerve injury elicits a fourfold to sixfold increase in spontaneous ectopic discharge within 24 hr but is largely reduced by postinjury day 5 (C. Liu et al., 2000). Notably, however, once developed, behavioral signs of neuropathic pain remain constant for many weeks (Chaplan et al., 1994; Bian et al., 1999; Malan et al., 2000) despite the diminished rate of afferent discharge. These observations suggest the possibility that although the enhanced discharge associated with nerve injury may be critical in the initiation of neuropathic pain, such increased afferent activity may be insufficient to maintain neuropathic pain in the absence of other mechanisms.
Descending facilitation arising from neuroplastic changes occurring in the rostral ventromedial medulla (RVM) and projecting to the spinal dorsal horn through the dorsolateral funiculus (DLF) has been suggested to be necessary for expression of neuropathic pain (Ossipov et al., 2001). Blocking descending facilitation by lesions of the DLF, RVM microinjection of lidocaine, or cholecystokininB antagonists all block neuropathic behavior (Pertovaara et al., 1996; Kovelowski et al., 2000;Ossipov et al., 2000). Selective lesioning of RVM cells expressing μ-opioid receptors also blocks neuropathic behaviors (Porreca et al., 2001). However, the role of descending facilitation in the processes that initiate or maintain the expression of neuropathic pain is not known.
Peripheral nerve injury is known to elevate spinal dynorphin content, which may promote nociception (Kajander et al., 1990; Bian et al., 1999; Claude et al., 1999; Malan et al., 2000). Spinal dynorphin content is maximal by post-spinal nerve ligation (SNL) day 10 (Malan et al., 2000). Prodynorphin knock-out mice demonstrated tactile and thermal hypersensitivity that fully reversed to preinjury baselines within 8 d after SNL, whereas the wild-type mice sustained pain (Wang et al., 2001). Neuropathic behaviors were reversed by dynorphin antiserum in wild-type mice in late but not early periods after injury, suggesting that dynorphin is required for sustained expression of pain (Wang et al., 2001). The late time course of SNL-induced spinal dynorphin upregulation suggests the novel possibility that some of the nerve injury-induced spinal plasticity may be secondary to neuroplasticity in other parts of the nervous system. One possibility is that spinal dynorphin upregulation may depend on developing neuroplasticity in the RVM. The present experiments explore the hypothesis that descending facilitation from the RVM develops over time and influences the spinal upregulation of dynorphin. These processes may be essential in the maintenance of experimental neuropathic pain.
MATERIALS AND METHODS
Male Sprague Dawley rats (Harlan, Indianapolis, IN), 200–300 gm at time of testing, were maintained in a climate-controlled room on a 12 hr light/dark cycle (lights on at 06:00 A.M.) with food and water available ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals and received approval from the Institutional Animal Care and Use Committee of the University of Arizona.
Surgical procedures. Rats were prepared for bilateral RVM drug administration by placing anesthetized (100 mg/kg ketamine/xylazine, i.p.) animals in a stereotaxic headholder. For intracranial bilateral drug administrations, the skull was exposed and two 26 ga guide cannula separated by 1.2 mm (Plastics One Inc., Roanoke, VA) were directed toward the lateral portions of the RVM (anteroposterior, −11.0 mm from bregma; lateral, ±0.6 mm from midline; dorsoventral, −8.5 mm from the cranium) (Paxinos and Watson, 1986) and cemented in place. Drug administrations into the RVM were performed by slowly expelling 0.5 μl of drug solution or saline through a 33 ga injection cannula inserted through the guide cannula and protruding an additional 1 mm into fresh brain tissue to prevent backflow of drug into the guide cannula. At the termination of the experiments, pontamine blue was injected into the site of the RVM injections and cannula placement was verified histologically. Data from animals with incorrectly placed cannula were discarded. Dermorphin, saporin, dermorphin–saporin (Advanced Targeting Systems, San Diego, CA), or vehicle were administered as a single dose of 3 pmol into the RVM (1.5 pmol in 0.5 μl each side). Lidocaine was given in a dose of 4% w/v in 0.5 μl.
SNL. Tight ligation of the L5/L6 spinal nerve was performed according to the method of Kim and Chung (1992). The rats were maintained under anesthesia with halothane vaporized in 95% O2 and 5% CO2. After surgical preparation of the rats and exposure of the dorsal vertebral column from L4 to S2, the exposed L5 and L6 spinal nerves were tightly ligated with 4-0 silk suture. The incision was closed, and the animals were allowed to recover. Rats that exhibited motor deficiency or a lack of subsequent increased sensitivity to innocuous mechanical stimulation were excluded from additional testing. Sham control rats underwent the same operation and handling as the experimental animals, but without SNL.
Spinal DLF lesions. Spinal lesions at T8 were performed in halothane-anesthetized rats as described previously (Kovelowski et al., 1999; Ossipov et al., 2000). The spinal cord was exposed by laminectomy and the DLF was crushed with fine forceps. Sham spinal surgery was performed by exposing the vertebrae and performing the laminectomy, but without cutting any neuronal tissue. Hemostasis was confirmed and the wound over the exposed spinal cord was packed with gelfoam and closed. All lesions were verified histologically at the termination of the experiment by fixing the spinal sections obtained from the lesion site in paraffin. Sections (40 μm thick) were mounted and stained with Luxor Fast Blue myelin stain to visualize intact and disrupted white matter. Behavioral results and dynorphin content obtained only from animals that had appropriately placed DLF lesions were included in analysis.
Thermal hyperalgesia. The method of Hargreaves et al. (1988)was used to assess paw-withdrawal latency to a thermal nociceptive stimulus. Rats were allowed to acclimate within Plexiglas enclosures on a clear glass plate maintained at 30°C. A radiant heat source (i.e., high-intensity projector lamp) was activated with a timer and focused onto the plantar surface of the hindpaw. Paw-withdrawal latency was determined by a motion detector that halted both lamp and timer when the paw was withdrawn. A maximal cutoff of 40 sec was used to prevent tissue damage. Significant changes from baseline control values were detected by ANOVA followed by the post hoc least significance test. Significance was set at p ≤ 0.05.
Tactile hypersensitivity. The paw-withdrawal thresholds of the hindpaws of the rats were determined in response to probing with eight calibrated von Frey filaments (Stoelting, Wood Dale, IL) in logarithmically spaced increments ranging from 0.41 to 15 gm (4–150 mN). Each filament was applied perpendicularly to the plantar surface of the ligated paw of rats kept in suspended wire-mesh cages. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (“up and down” method), analyzed using a Dixon nonparametric test (Chaplan et al., 1994) and expressed as the mean withdrawal threshold. Significant changes from baseline control values were detected by ANOVA followed by the post hoc least significance test. Significance was set atp ≤ 0.05.
Dynorphin enzyme immunoassay. Spinal dynorphin content was assayed as described previously (Malan et al., 2000). The dorsal quadrant of lumbar spinal cord from the ipsilateral side of sham-operated or ligated rats was placed in 1 macetic acid, disrupted with a Polytron homogenizer (Kinematica Kriens, Lucerne, Switzerland), and boiled at 95°C for 20 min. The samples were centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was analyzed for protein content and lyophilized. A commercial enzyme immunoassay system using anti-dynorphin A(1–17) antiserum (Peninsula Laboratories, Belmont, CA) was used to determine the content of dynorphin in the spinal cord extracts against a standard curve of dynorphin A(1–17). The reaction product is quantified by absorbance at 450 nm. Standard curves were constructed, and the dynorphin content was determined with Prism (GraphPad Software, San Diego, CA). Pairwise comparisons between treatments were detected using Student's t test. Significance was determined atp ≤ 0.05.
RESULTS
RVM lidocaine
Behavioral signs of tactile hypersensitivity and thermal hyperalgesia were clearly evident within 3 d after SNL (Fig.1). The preligation baseline paw-withdrawal threshold to probing with von Frey filaments was 14.25 ± 0.30 gm and the paw-withdrawal latency to noxious radiant heat was 21.0 ± 0.19 sec (Fig. 2). After SNL, the paw-withdrawal threshold was significantly (p ≤ 0.05) reduced to 3.67 ± 0.44 gm and the paw-withdrawal latency was significantly (p≤ 0.05) reduced to 14.5 ± 0.20 sec (Fig. 2). In contrast, sham surgery had no significant effect on behavioral signs of neuropathic pain; the postsurgical paw-withdrawal tactile threshold and thermal latency were 14.5 ± 0.37 gm and 20.5 ± 0.38 sec, respectively (Fig. 2). The bilateral microinjection of lidocaine (4% w/v; 0.5 μl) or saline into the RVM on day 3 after SNL did not elicit any changes in paw-withdrawal thresholds to probing with von Frey filaments (Fig. 1A) or to noxious radiant heat over the 60 min observation period (Fig. 1B). The paw-withdrawal threshold to von Frey filaments was 4.13 ± 0.77 gm 10 min after lidocaine (Fig. 1A), and the paw-withdrawal latency to noxious heat was 14.3 ± 0.45 sec 10 min after lidocaine (Fig. 1B). However, behavioral manifestations of neuropathic pain were reversed when lidocaine was microinjected into the RVM on the sixth day after SNL (Fig.1C,D). The maximal effect of lidocaine was observed 10 min after microinjection into the RVM, significantly (p ≤ 0.05) raising paw-withdrawal thresholds to light tactile stimuli to 11.7 ± 1.0 gm (Fig. 1C) and mean paw-withdrawal latencies to radiant heat to 19.4 ± 1.46 sec (Fig. 1D). The blockade of tactile hypersensitivity and thermal hyperalgesia by RVM lidocaine rapidly returned to baseline values within 30 min of the injection. Similarly, lidocaine microinjected into the RVM also reversed signs of neuropathic pain on the ninth and 12th day after SNL (Fig. 2). The paw-withdrawal thresholds to probing with von Frey filaments were significantly (p ≤ 0.05) elevated to 12.1 ± 0.77 and 12.4 ± 0.76 gm, respectively, on those days (Fig.2A). Similarly, the paw-withdrawal latencies to radiant heat were significantly (p ≤ 0.05) elevated to 19.8 ± 1.63 and 20.0 ± 0.73 sec on the same days (Fig. 2B). Furthermore, the effects of lidocaine against behavioral signs of neuropathic pain were maximal at 10 min after microinjection and returned to baseline values by 30 min (data not shown). Microinjection of lidocaine into the RVM did not alter responses to either tactile or thermal stimuli in the sham-operated rats over the entire course of the study. Furthermore, the microinjection of saline into the RVM of either sham-operated or SNL rats did not produce any changes in either tactile or thermal responses over the time course of this study (Fig. 2).
Fig. 1.
Lidocaine (4% w/v) or saline was microinjected bilaterally into the RVM of sham-operated male Sprague Dawley rats and of rats with L5/L6 SNL 3 and 6 d after nerve injury. Baseline responses to tactile and thermal stimuli were determined in sham-operated and SNL rats before injections (BL). Tactile hypersensitivity (A, C) and thermal hyperalgesia (B, D), indicated by significant decreases in the response thresholds, were measured at 10 min intervals for 60 min after each lidocaine or saline microinjection. Lidocaine did not reverse tactile hypersensitivity and thermal hyperalgesia on post-SNL day 3 (A, B) but was effective on day 6 (C, D). Behavioral responses were not altered by lidocaine in sham-operated rats or by saline in either group. *p ≤ 0.05 compared with pre-SNL values.
Fig. 2.
Lidocaine (4% w/v) or saline was microinjected bilaterally into the RVM of sham-operated male Sprague Dawley rats and of rats with L5/L6 SNL 3, 6, 9, and 12 d after nerve injury. Baseline responses to tactile and thermal stimuli were determined before surgery (pre-SNL) and on day 3 after surgery before injections (BL). Tactile hypersensitivity (A) and thermal hyperalgesia (B), indicated by significant decreases in the response thresholds, were measured 10 min after each lidocaine or saline microinjection. Lidocaine did not reverse tactile hypersensitivity and thermal hyperalgesia on post-SNL day 3 but was effective thereafter. Behavioral responses were not altered by lidocaine in sham-operated rats or by saline in either group. *p ≤ 0.05 compared with pre-SNL values;+p ≤ 0.05 compared with SNL baseline values.
Dermorphin–saporin microinjection
Rats received a single bilateral injection of saline (0.5 μl), dermorphin (3 pmol), saporin (3 pmol), or the dermorphin–saporin conjugate (3 pmol) into the RVM. Our previous investigations revealed that this protocol of dermorphin–saporin treatment elicited a selective loss of RVM neurons expressing the μ-opioid receptor at postinjection day 28 (Porreca et al., 2001). These microinjections did not produce any changes in the baseline hindpaw responses to probing with von Frey filaments or to noxious radiant heat when evaluated after 28 d (Fig. 3). On the 28th day after the RVM microinjections, each of the pretreated groups was divided into two groups, one receiving L5/L6 SNL and the other receiving sham surgery. The behavioral responses to light tactile and noxious heat stimuli were measured on a daily basis. None of the groups of rats with sham surgery demonstrated any significant decreases in behavioral responses to either tactile or thermal stimuli over the entire 14 d observation period (Fig. 3). All groups of rats with SNL demonstrated tactile hypersensitivity and thermal hyperalgesia evident by the second day after SNL. Paw-withdrawal thresholds to light tactile stimuli ranged between 14.4 ± 0.6 and 15 ± 0 gm before SNL and were significantly (p ≤ 0.05) reduced to between 3.7 ± 0.84 and 4.9 ± 1.85 gm (Fig.3A). Similarly, the paw-withdrawal latencies to noxious heat ranged from 19.9 ± 0.56 to 20.3 ± 0.23 sec before SNL and were significantly (p ≤ 0.05) reduced to between 14.0 ± 0.38 and 15.8 ± 0.46 sec by the second day after SNL (Fig. 3B). Tactile and thermal hypersensitivity remained evident throughout the 14 d observation period in the rats with SNL that were pretreated with saline, dermorphin, or saporin microinjected into the RVM (Fig. 3). In contrast, the rats that were pretreated with dermorphin–saporin demonstrated a time-related reversal of heightened sensitivity to tactile and thermal stimuli that was seen beginning at the fifth day after SNL; ultimately these thresholds were not significantly different from pre-SNL baseline values (Fig. 3).
Fig. 3.
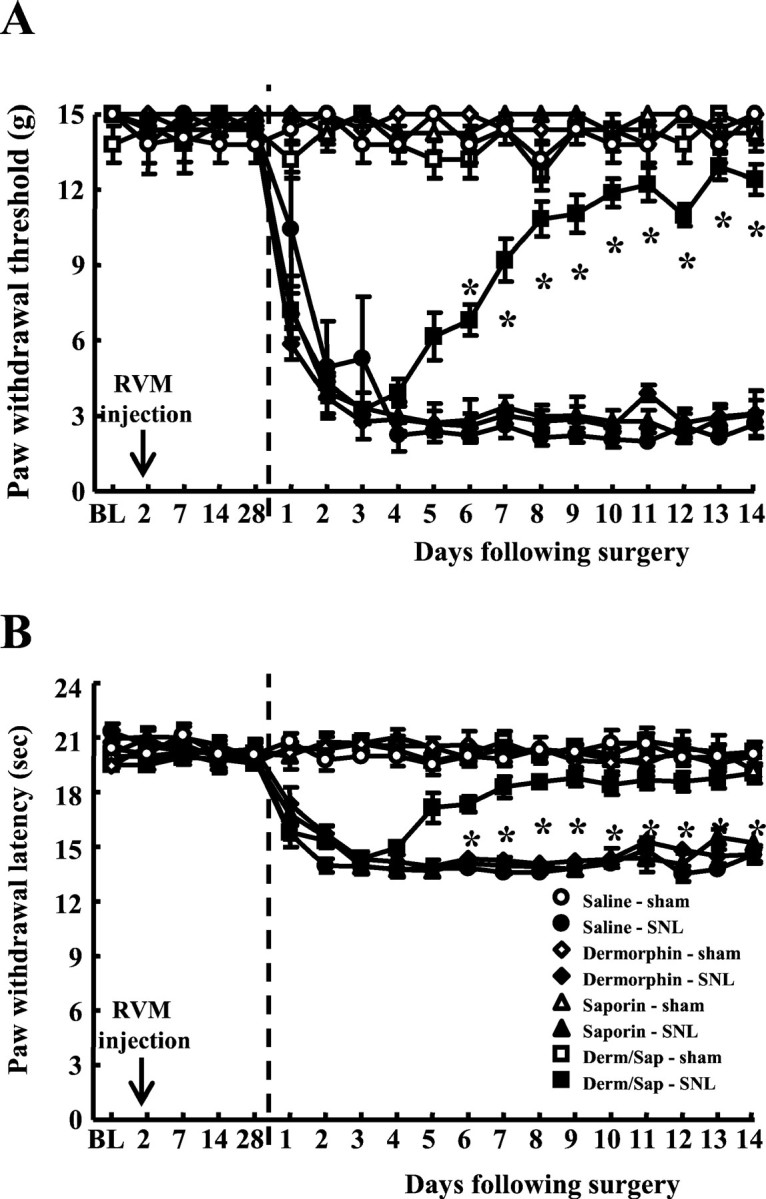
Male Sprague Dawley rats received bilateral microinjections of saline or of saporin, dermorphin, or the dermorphin–saporin conjugate (Derm/Sap) (1.5 pmol on each side of the RVM). After 28 d, the rats were subjected to either L5/L6 SNL or sham surgery.Vertical dashed lines represent time of surgery. Paw-withdrawal thresholds to light tactile stimuli (A) and to noxious radiant heat (B) were determined before microinjections (BL), weekly after the microinjections, and daily for 14 d after SNL or sham surgery. Tactile hypersensitivity (A) and thermal hyperalgesia (B) were evident in all groups with SNL during the initial 4 d of testing, as indicated by the significant decreases in response thresholds. However, the rats pretreated with the dermorphin–saporin conjugate demonstrated clear reversal of SNL-induced threshold changes commencing at postsurgery day 5. *p ≤ 0.05 compared with premicroinjection values.
DLF lesions
Rats received either lesions of the DLF or sham surgery at T8. Each group was further subdivided and received either sham surgery or L5/L6 SNL after an additional 7 d. Neither sham DLF nor DLF lesions caused any changes in behavioral responses to tactile or thermal stimuli. Paw-withdrawal thresholds to light touch were 15 ± 0 gm before and after spinal surgery, and the paw-withdrawal latencies to noxious radiant heat were 20.2 ± 0.17 sec before spinal surgery and 20.2 ± 0.23 sec after sham DLF and 20.1 ± 0.25 sec after DLF lesions (Fig. 4). Sham ligation did not produce any significant decreases in behavioral responses to either light tactile stimuli or noxious radiant heat over the entire 14 d observation period (Fig. 4). Both groups of rats with SNL demonstrated tactile hypersensitivity and thermal hyperalgesia by the second day after SNL. Paw-withdrawal thresholds of the sham-operated and DLF-lesioned rats to light tactile stimuli were significantly (p ≤ 0.05) reduced to 3.94 ± 0.57 and 2.76 ± 0.48 gm, respectively (Fig. 4A). Similarly, the paw-withdrawal latencies of the sham-operated and DLF-lesioned rats to noxious heat were significantly (p ≤ 0.05) reduced to 14.6 ± 0.27 and 13.8 ± 0.38 sec, respectively, on the second day after SNL (Fig.4B). The behavioral responses of the rats with SNL and sham DLF surgery remained constant throughout the 14 d observation period. In contrast, the heightened sensory responses of the rats with SNL and DLF lesions began a gradual return to pre-SNL baseline values within 4–5 d after SNL (Fig. 4). The paw-withdrawal threshold to light tactile stimuli was significantly (p ≤ 0.05) increased to 11.7 ± 1.66 gm, and the paw-withdrawal latency to radiant heat was significantly (p ≤ 0.05) increased to 18.9 ± 1.42 sec by the seventh day after SNL (Fig. 4).
Fig. 4.

Male Sprague Dawley rats received bilateral surgical lesions of the DLF or sham DLF surgery at T8. After 7 d, the rats were subjected to either L5/L6 SNL or sham surgery. Paw-withdrawal thresholds to light tactile stimuli (A) and to noxious radiant heat (B) were determined before spinal surgery before SNL (BNL) and daily for 14 d after SNL or sham surgery. Tactile hypersensitivity (A) and thermal hyperalgesia (B) were evident in all groups with SNL during the initial 4 d of testing, as indicated by the significant decreases in behavioral responses. However, the rats that received both L5/L6 SNL and lesions of the DLF demonstrated a clear reversal of SNL-induced threshold changes commencing at postsurgery days 4–5. *p ≤ 0.05 compared with premicroinjection values. N, Naive.
Spinal dynorphin content
Spinal cords were extruded and assayed for dynorphin content in the dorsal quadrant ipsilateral to SNL or sham ligation on the 10th day after surgery. This time point was chosen because our previous investigations demonstrated that dynorphin levels were maximally increased at this time point (Malan et al., 2000). Rats with sham DLF lesions and L5/L6 SNL demonstrated a significant (p ≤ 0.05) elevation in spinal dynorphin content 10 d after SNL. The spinal dynorphin content of rats with sham DLF lesion and with SNL was 991 ± 63 pg dynorphin/mg protein, whereas that of rats with sham DLF lesion and sham SNL was 698 ± 53 pg dynorphin/mg protein (Fig.5). In contrast, lesions of the DLF prevented the elevation in spinal dynorphin content. The spinal dynorphin content in the rats with SNL and DLF lesions was 656 ± 22 pg dynorphin/mg protein, which was not significantly different (p > 0.05) from that of the control group (Fig.5). Rats with DLF lesions and sham SNL surgery also had a spinal dynorphin level (667 ± 93 pg dynorphin/mg protein) that was similar to that seen for the rats with sham DLF and sham SNL surgery.
Fig. 5.
The spinal cords of sham-operated rats (sham SNL) and rats with L5/L6 SNL that had received either lesions of the DLF or sham spinal surgery (sham DLF) were removed on day 10 after surgery. The dorsal half of the lumbar cord was isolated and assayed for dynorphin content with enzyme immunoassay. The rats with SNL that had also received sham DLF surgery showed a significant (p ≤ 0.05; Student'st test) increase in spinal dynorphin content when compared with the sham SNL/sham DLF group. In contrast, the spinal dynorphin content of the rats with SNL or sham SNL that also received lesions of the DLF was not significantly different (p > 0.05; Student's ttest) than that of the SNL/sham DLF group.
DISCUSSION
The results of the present experiments provide supporting evidence for the hypothesis that mechanisms that initiate neuropathic pain differ from those that maintain such pain. In addition, the data support the hypothesis that some of the nerve injury-induced plasticity occurring at the spinal level may be secondary to developing plasticity in other regions of the neuroaxis. Although the initiation of neuropathic pain is likely to be mediated by increased afferent drive occurring shortly after the injury, such enhanced activity is insufficient to maintain the neuropathic state in the absence of time-related development of descending facilitation arising in the RVM and an attendant elevation in spinal dynorphin content. The need for descending modulatory influences and enhancement of spinal dynorphin does not exclude the possibility of other mechanisms that may also be important in maintaining the neuropathic state.
Considerable evidence supports the importance of afferent drive as a mechanism of neuropathic pain (for review, see Devor et al., 1992;Dickenson et al., 2001). Behavioral signs of neuropathic pain in nerve-injured mice were blocked by spinal (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate (MK-801) at postinjury day 3, although they were insensitive to dynorphin antiserum at this time, suggesting the importance of excitatory transmission possibly arising in part from increased afferent input (Wang et al., 2001). Tactile and thermal hypersensitivity in nerve-injured rats was significantly attenuated by the application of lidocaine directly at the injury site (Ossipov et al., 1995; Kovelowski et al., 2000; Malan et al., 2000) and local lidocaine application has been used to successfully treat postherpetic neuralgia (Rowbotham et al., 1995, 1996). These observations suggest that enhanced afferent discharge is an important component of the neuropathic state at both the initial stage and at subsequent stages after injury. Experimental observations also confirm, however, that although maintained above preinjury baselines, spontaneous ectopic activity diminishes quite rapidly within the first week after injury (Han et al., 2000; C. Liu et al., 2000; X. Liu et al., 2000). Despite the diminishing afferent input over time, the behavioral hypersensitivity remains unchanged for many weeks once it is established, suggesting that other mechanisms may be also necessary to maintain the neuropathic state.
Previous work has shown that the behavioral expression of neuropathic pain is dependent on descending facilitatory systems that arise in the RVM. Such facilitation may be time dependent, resulting from plasticity in the RVM and may act to further enhance the now diminished afferent input from injured (Devor and Seltzer, 1999) or adjacent (Tal and Bennett, 1994; Yoon et al., 1996; Wu et al., 2001) fibers. Evidence supports a role for pontine–medullary sites in the manifestation of experimental neuropathic pain (Pertovaara et al., 1996, 2001;Kovelowski et al., 2000). Manipulations that disrupt communication between the brain and spinal cord have been shown to block the expression of tactile and thermal hypersensitivity. Spinal transection and hemisection eliminate nerve injury-induced tactile hypersensitivity, indicating the critical contribution of a supraspinal component in the expression of neuropathic pain (Bian et al., 1998;Kauppila et al., 1998; Sun et al., 2001). Similarly, enhanced responses of wide dynamic-range neurons to tactile stimuli induced by mustard oil are blocked by transection of the spinal cord (Mansikka and Pertovaara, 1997; Pertovaara, 1998).
The RVM has been well characterized in regard to spinopetal modulatory control of nociception mediating both inhibition and facilitation of nociception (Fields, 1992; Zhuo and Gebhart, 1992, 1997). Persistent input from injured or adjacent fibers to supraspinal sites (Sun et al., 2001) may ultimately elicit neuroplastic changes within the RVM that might elicit a time-related activation of descending facilitation. One possibility for such descending facilitation is the class of RVM neurons identified as “ON” cells, because they accelerate firing immediately before a nociceptive reflex occurs (Fields et al., 1983;Fields and Heinricher, 1985; Fields, 1992; Heinricher et al., 1992;Heinricher and Roychowdhury, 1997). Enhanced nociceptive sensitivity has been noted when ON cell activity is increased (Heinricher et al., 1989; Bederson et al., 1990; Kim et al., 1990). Consistent with this, RVM lidocaine blocks both SNL-induced enhanced activity of spinal dorsal horn units and neuropathic behavior, suggesting the presence of a facilitatory influence from this region (Pertovaara et al., 1996,2001; Mansikka and Pertovaara, 1997; Pertovaara, 1998; Kovelowski et al., 2000; Porreca et al., 2002). The possible time dependency of neuropathic pain on such descending facilitation has not been explored previously. The present studies reveal that descending influences are not apparent for the first 3 d after injury but are clearly present by day 6, when RVM lidocaine blocks both SNL-induced tactile and thermal hypersensitivity. Critically, RVM lidocaine was inactive at postinjury day 3, suggesting that at this time, tonic activity of cells in this region is unlikely.
Evidence supports the possibility that RVM cells that mediate descending facilitation may express μ-opioid receptors (Fields et al., 1983; Fields and Heinricher, 1989; Pan et al., 1990; Heinricher et al., 1994). It has been shown previously that dermorphin–saporin produced a partial lesion of μ-opioid receptor-expressing neurons in the RVM and prevented as well as reversed the behavioral manifestation of neuropathic pain when evaluated at postinjury day 7 (Porreca et al., 2001). Similarly, selective ablation of the DLF, which includes the spinopetal projections from the RVM, also prevented and reversed experimental neuropathic pain behavior when evaluated at postinjury day 7 (Ossipov et al., 2000). Together, these observations provide strong evidence that descending facilitation from the RVM is a critical factor in the expression of pain. The present studies show that such descending facilitation does not play a role in the early phase of the postinjury state but seems to be critical to the maintenance of the neuropathic condition. Lesions of the DLF or of μ-opioid receptor-expressing cells in the RVM show a reversal of SNL-induced behavior that is apparent by approximately postinjury day 5 and a return to preinjury baselines by approximately day 8. The time course of the reversal of both tactile and thermal hypersensitivity after lesion of the DLF or of RVM cells with dermorphin–saporin is remarkably similar, suggesting that RVM plasticity over this time period and later is crucial to the neuropathic state. These data are also consistent with the observed reversible blockade of nerve injury-induced pain by RVM lidocaine.
The time course over which descending facilitation develops is also consistent with the time course of nerve injury-induced upregulation of spinal dynorphin content, which may provide insights into spinal mechanisms by which facilitation may occur. The relatively late peak in expression of spinal dynorphin after nerve injury (Malan et al., 2000;Wang et al., 2001) suggests the possibility that upregulation depends on the time-related development of descending modulatory influences and may ultimately function to maintain the neuropathic state. This possibility is supported by the data, because manipulations that blocked the maintained state of neuropathic pain also blocked the SNL-induced elevation of spinal dynorphin content. For example, disruption of the spinopetal tracts from the RVM through the DLF prevented SNL-induced upregulation levels of spinal dynorphin. Because neither DLF lesion or dorsal rhizotomy blocks basal expression of spinal dynorphin, it is highly likely that upregulation of dynorphin results from local interneurons (Cho and Basbaum, 1988). Significantly, lidocaine in the RVM also did not block neuropathic pain behaviors at postinjury day 3, a time at which spinal dynorphin is not significantly elevated, suggesting the presence of a transitional period for descending influence (Malan et al., 2000; Wang et al., 2001). Dynorphin antiserum was shown to abolish tactile and thermal hypersensitivity at postinjury day 14, but not at day 2, whereas MK-801 was effective at both time points (Wang et al., 2001). Finally, mice with deletions of the prodynorphin gene displayed the behavioral signs of neuropathic pain only up to postinjury day 5, with complete reversal by day 8, whereas wild-type littermates maintained pain for the entire 14 d observation period (Wang et al., 2001). These data are all consistent with the view that upregulation of spinal dynorphin is a mechanism that maintains the neuropathic state, perhaps through a nonopioid action, to enhance release of excitatory neurotransmitters such as glutamate or excitatory peptides from primary afferents (Faden, 1992; Skilling et al., 1992; Arcaya et al., 1999; Claude et al., 1999; Vanderah et al., 2001).
Our data provide evidence for the presence of time-related descending facilitatory influences arising in the RVM that are critical to the maintenance but not the initiation of experimental neuropathic pain. In addition, the data show the importance of descending influences in eliciting plasticity at the spinal level. It is not known whether other changes observed in the spinal dorsal horn after nerve injury similarly depend on descending influences. Together, these and possibly other events appear to be established by the initial processes of peripheral nerve injury to maintain the expression of abnormal pain. Patients experiencing neuropathic pain are likely to require intervention at time points substantially long after the precipitating injury has occurred, suggesting that the understanding of the processes that maintain neuropathic pain will be critically important in the development of rational approaches for therapeutic interventions.
Footnotes
Correspondence should be addressed to Dr. Frank Porreca, Department of Pharmacology, College of Medicine, University of Arizona Health Sciences Center, Tucson, AZ 85724. E-mail: frankp@u.arizona.edu.
REFERENCES
- 1.Arcaya JL, Cano G, Gomez G, Maixner W, Suarez-Roca H. Dynorphin A increases substance P release from trigeminal primary afferent C-fibers. Eur J Pharmacol. 1999;366:27–34. doi: 10.1016/s0014-2999(98)00897-8. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 3.Bian D, Ossipov MH, Zhong C, Malan TP, Jr, Porreca F. Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal transection in rats with nerve injury. Neurosci Lett. 1998;241:79–82. doi: 10.1016/s0304-3940(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 4.Bian D, Ossipov MH, Ibrahim M, Raffa RB, Tallarida RJ, Malan TP, Jr, Lai J, Porreca F. Loss of antiallodynic and antinociceptive spinal/supraspinal morphine synergy in nerve-injured rats: restoration by MK-801 or dynorphin antiserum. Brain Res. 1999;831:55–63. doi: 10.1016/s0006-8993(99)01393-1. [DOI] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Shafer SL, Yaksh TL. Prolonged alleviation of tactile allodynia by intravenous lidocaine in neuropathic rats. Anesthesiology. 1995;83:775–785. doi: 10.1097/00000542-199510000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Chapman V, Suzuki R, Chamarette HL, Rygh LJ, Dickenson AH. Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain. 1998;75:261–272. doi: 10.1016/s0304-3959(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Basbaum AI. Increased staining of immunoreactive dynorphin cell bodies in the deafferented spinal cord of the rat. Neurosci Lett. 1988;84:125–130. doi: 10.1016/0304-3940(88)90395-3. [DOI] [PubMed] [Google Scholar]
- 9.Claude P, Gracia N, Wagner L, Hargreaves KM. Effect of dynorphin on ICGRP release from capsaicin-sensitive fibers. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Abstracts of the Ninth World Congress on Pain. International Association for the Study of Pain; Vienna: 1999. p. 262. [Google Scholar]
- 10.Devor M. Neuropathic pain and injured nerve: peripheral mechanisms. Br Med Bull. 1991;47:619–630. doi: 10.1093/oxfordjournals.bmb.a072496. [DOI] [PubMed] [Google Scholar]
- 11.Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; London: 1999. pp. 129–164. [Google Scholar]
- 12.Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–268. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- 13.Dickenson AH, Matthews EA, Suzuki R. Central nervous system mechanisms of pain in peripheral neuropathy. In: Hansson PT, Fields HL, Hill RG, Marchettini P, editors. Neuropathic pain: pathophysiology and treatment. International Association for the Study of Pain; Seattle: 2001. pp. 85–106. [Google Scholar]
- 14.Faden AI. Dynorphin increases extracellular levels of excitatory amino acids in the brain through a non-opioid mechanism. J Neurosci. 1992;12:425–429. doi: 10.1523/JNEUROSCI.12-02-00425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields HL. Is there a facilitating component to central pain modulation? APS J. 1992;1:71–78. [Google Scholar]
- 16.Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- 17.Fields HL, Heinricher MM. Brainstem modulation of nociceptor-driven withdrawal reflexes. Ann NY Acad Sci. 1989;563:34–44. doi: 10.1111/j.1749-6632.1989.tb42188.x. [DOI] [PubMed] [Google Scholar]
- 18.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han HC, Lee DH, Chung JM. Characteristics of ectopic discharges in a rat neuropathic pain model. Pain. 2000;84:253–261. doi: 10.1016/s0304-3959(99)00219-5. [DOI] [PubMed] [Google Scholar]
- 20.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new, sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 21.Heinricher MM, Roychowdhury SM. Reflex-related activation of putative pain facilitating neurons in rostral ventromedial medulla requires excitatory amino acid transmission. Neuroscience. 1997;78:1159–1165. doi: 10.1016/s0306-4522(96)00683-5. [DOI] [PubMed] [Google Scholar]
- 22.Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- 23.Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 24.Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 25.Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11:719–728. doi: 10.1016/0196-9781(90)90187-a. [DOI] [PubMed] [Google Scholar]
- 26.Kauppila T, Kontinen VK, Pertovaara A. Influence of spinalization on spinal withdrawal reflex responses varies depending on the submodality of the test stimulus and the experimental pathophysiological condition in the rat. Brain Res. 1998;797:234–242. doi: 10.1016/s0006-8993(98)00379-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim DH, Fields HL, Barbaro NM. Morphine analgesia and acute physical dependence: rapid onset of two opposing, dose-related processes. Brain Res. 1990;516:37–40. doi: 10.1016/0006-8993(90)90894-h. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 29.Kirk EJ. Impulses in dorsal spinal nerve rootlets in cats and rabbits arising from dorsal root ganglia isolated from the periphery. J Comp Neurol. 1974;155:165–175. doi: 10.1002/cne.901550203. [DOI] [PubMed] [Google Scholar]
- 30.Kovelowski CJ, Ossipov MH, Hruby VJ, Porreca F. Lesions of the dorsolateral funiculus block supraspinal opioid δ receptor mediated antinociception in the rat. Pain. 1999;83:115–122. doi: 10.1016/s0304-3959(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 31.Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000;84:309–318. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- 34.Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 35.Mansikka H, Pertovaara A. Supraspinal influence on hindlimb withdrawal thresholds and mustard oil-induced secondary allodynia in rats. Brain Res Bull. 1997;42:359–365. doi: 10.1016/s0361-9230(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 36.Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995;199:87–90. doi: 10.1016/0304-3940(95)12022-v. [DOI] [PubMed] [Google Scholar]
- 37.Ossipov MH, Hong Sun T, Malan P, Jr, Lai J, Porreca F. Mediation of spinal nerve injury induced tactile allodynia by descending facilitatory pathways in the dorsolateral funiculus in rats. Neurosci Lett. 2000;290:129–132. doi: 10.1016/s0304-3940(00)01338-0. [DOI] [PubMed] [Google Scholar]
- 38.Ossipov MH, Lai J, Malan TP, Jr, Vanderah TW, Porreca F. Tonic descending facilitation as a mechanism of neuropathic pain. In: Hansson PT, Fields HL, Hill RG, Marchettini P, editors. Neuropathic pain: pathophysiology and treatment. International Association for the Study of Pain; Seattle: 2001. pp. 107–124. [Google Scholar]
- 39.Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol (Lond) 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 41.Pertovaara A. A neuronal correlate of secondary hyperalgesia in the rat spinal dorsal horn is submodality selective and facilitated by supraspinal influence. Exp Neurol. 1998;149:193–202. doi: 10.1006/exnr.1997.6688. [DOI] [PubMed] [Google Scholar]
- 42.Pertovaara A, Wei H, Hamalainen MM. Lidocaine in the rostroventromedial medulla and the periaqueductal gray attenuates allodynia in neuropathic rats. Neurosci Lett. 1996;218:127–130. doi: 10.1016/s0304-3940(96)13136-0. [DOI] [PubMed] [Google Scholar]
- 43.Pertovaara A, Keski-Vakkuri U, Kalmari J, Wei H, Panula P. Response properties of neurons in the rostroventromedial medulla of neuropathic rats: attempted modulation of responses by [1DMe]NPYF, a neuropeptide FF analogue. Neuroscience. 2001;105:457–468. doi: 10.1016/s0306-4522(01)00187-7. [DOI] [PubMed] [Google Scholar]
- 44.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the μ-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 46.Rowbotham MC, Davies PS, Fields HL. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol. 1995;37:246–253. doi: 10.1002/ana.410370216. [DOI] [PubMed] [Google Scholar]
- 47.Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65:39–44. doi: 10.1016/0304-3959(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 48.Skilling SR, Sun X, Kurtz HJ, Larson AA. Selective potentiation of NMDA-induced activity and release of excitatory amino acids by dynorphin: possible roles in paralysis and neurotoxicity. Brain Res. 1992;575:272–278. doi: 10.1016/0006-8993(92)90090-v. [DOI] [PubMed] [Google Scholar]
- 49.Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- 50.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 51.Vanderah TW, Gardell LR, Suenaga NM, Zhong CM, Malan TP, Jr, Lai J, Porreca F. Enhanced evoked cgrp after spinal nerve injury is mediated by spinal dynorphin. Soc Neurosci Abstr. 2001;27:1894. [Google Scholar]
- 52.Wall PD, Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature. 1974a;248:740–743. doi: 10.1038/248740a0. [DOI] [PubMed] [Google Scholar]
- 53.Wall PD, Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974b;43:580–593. doi: 10.1016/0014-4886(74)90197-6. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolf CJ. Generation of acute pain: central mechanisms. Br Med Bull. 1991;47:523–533. doi: 10.1093/oxfordjournals.bmb.a072490. [DOI] [PubMed] [Google Scholar]
- 56.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation: implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 57. Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci 21 2001. RC140:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon YW, Na HS, Chung JM. Contributions of injured and intact afferents to neuropathic pain in an experimental rat model. Pain. 1996;64:27–36. doi: 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 59.Zhuo M, Gebhart GF. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars α in the rat. J Neurophysiol. 1992;67:1599–1614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- 60.Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]





