Abstract
Dopamine effects in the striatum are mediated principally through the D1 and D2 dopamine receptor subtypes, which are segregated to the direct and indirect striatal projection neurons. After degeneration of the nigrostriatal dopamine system, direct pathway neurons display a supersensitive response to D1 dopamine receptor agonists, which is demonstrated by the induction of immediate early genes (IEGs), such as c-fos. Here we show, using analysis of receptor-mediated signal transduction, including protein phosphorylation and induction of IEGs, that D1 dopamine receptor supersensitivity is attributable to a switch to ERK1/2/MAP kinase (extracellular signal-regulated kinase/mitogen-activated protein kinase) in direct pathway neurons. Normally, in the dopamine-intact striatum, activation of ERK1/2/MAP kinase is shown to be restricted to indirect and not direct pathway neurons in response to stimulation of corticostriatal afferents. Moreover, in the dopamine-intact striatum, treatment with full D1 dopamine receptor agonists or stimulation of nigrostriatal dopaminergic afferents, both of which result in the induction of IEGs in direct striatal projection neurons, does not activate ERK1/2/MAP kinase. However, after degeneration of the nigrostriatal dopaminergic pathway, ERK1/2/MAP kinase is activated in direct pathway neurons in response to D1 dopamine receptor agonists either alone or when combined with stimulation of corticostriatal afferents. Inhibitors of MEK (MAP kinase kinase), which is responsible for phosphorylation of ERK1/2/MAP kinase, blocks D1 dopamine receptor agonist activation of ERK1/2/MAP kinase in the dopamine-depleted striatum, as well as the supersensitive induction of IEGs. These results demonstrate that dopamine input to the striatum maintains distinct forms of protein kinase-mediated gene regulation in the direct and indirect striatal projection neurons.
Keywords: dopamine, striatum, Parkinson's disease, gene regulation, signal transduction, MAP kinase, protein kinase
In a current model of the basal ganglia, it is proposed that movement disorders result from imbalanced function of the direct and indirect striatal projection pathways (Albin et al., 1989). Dopamine exerts opposite functional effects on these two striatal output systems as a consequence of the respective segregation of D1 and D2 dopamine receptors to the direct and indirect striatal projection neurons (Gerfen et al., 1990). The loss of dopamine input to the striatum in Parkinson's disease results in bradykinesia and slowed movements caused by the increases and decreases of function in the indirect and direct pathways, respectively (Bergman et al., 1990).l-3,4-Dihydroxyphenylalanine (l-DOPA) and other dopamine agonists used to treat Parkinson's disease restore many of the normal functions of these output systems (Gerfen et al., 1990; Engber et al., 1991); however, long-term treatment invariably leads to the development of uncontrolled movements termed dyskinesias (Bergmann et al., 1987). One effect of dopamine depletion, which is not normalized by dopamine agonist treatment, is the supersensitive response of direct pathway neurons to D1 dopamine receptor agonists demonstrated by the induction of over 30 immediate early genes (IEGs) (Robertson et al., 1990; Gerfen et al., 1995; Steiner and Gerfen, 1996;Berke et al., 1998). This response occurs despite the fact that D1 dopamine receptor levels are actually decreased or unchanged after dopamine depletion (Marshall et al., 1989; Gerfen et al., 1990), which suggests a change in signal transduction mechanisms. Neurotransmitter receptor-mediated induction of IEGs is regulated by protein kinase phosphorylation of transcription factors that bind to promoter response elements (Sheng and Greenberg, 1990; Ghosh and Greenberg, 1995; Karin, 1995; Montminy, 1997; Gutkind, 1998). In the striatum, multiple protein kinase pathways are involved in receptor-mediated gene regulation. D1 dopamine receptor-mediated activation of protein kinase A results in phosphorylation of the transcription factor cAMP response element-binding protein (CREB) (Cole et al., 1994; Konradi et al., 1994), whereas glutamate-receptor mediated mechanisms activate ERK1/2/MAP kinase (extracellular signal-regulated kinase/mitogen-activated protein kinase) (Sgambato et al., 1998a,b) and JN kinase/SAP kinase (c-Jun N-terminal protein kinase/synapse-associated protein kinase) (Schwarzschild et al., 1997). In the present study, we examined the possible role of ERK1/2/MAP kinase in the D1 dopamine receptor-supersensitive response in the dopamine-depleted striatum.
MATERIALS AND METHODS
Animals. Male Sprague Dawley rats (Taconic Farms, Germantown, NY), weighing 250–350 gm, were used. Unilateral lesions of the nigrostriatal dopamine pathway were made with the animals anesthetized with sodium pentobarbital (67 mg/kg, i.p.), and 6-hydroxydopamine (6-OHDA) (4 μg/2 μl) was infused into the right substantia nigra. Animals were allowed to recover from the anesthesia and were put back into their home cages, where they were given access to food and water ad libitum.
Pharmacologic treatments. Three weeks after the 6-OHDA lesions, animals were treated with different pharmacologic agents. In the first experiment, animals were treated with the partial D1 agonist SKF38393 (1–2 mg/kg, i.p.) and were killed 5, 15, or 30 min later by carbon dioxide intoxication. In a second experiment, animals received either the full D1 agonist SKF81297 (1 mg/kg, i.p) alone or a combination of the full D1 agonist SKF81297 (2 mg/kg, i.p.) with the D2 agonist quinpirole (1 mg/kg, i.p.), or the full D1 agonist SKF81297 (2 mg/kg, i.p.) with the D2 agonist quinpirole (1 mg/kg, i.p.) and the muscarinic antagonist scopolamine (5 mg/kg, i.p.) and were killed at 15 or 30 min after drug treatment (all drugs obtained from Sigma, St. Louis, MO).
MEK inhibitors. Two weeks after 6-OHDA lesions, some animals, under sodium pentobarbital anesthesia, had stainless steel guide cannulas (24 gauge) implanted bilaterally, affixed to the skull with screws and dental acrylic, directed at the striatum. One week later, the animals were placed in a Plexiglas bowl, and an infusion cannula was inserted into the guide cannulas, through which one of two MEK (MAP kinase kinase) inhibitors (U0126,100 mm; PD98059, 100 mm; Sigma), (Alessi et al., 1995; Dudley et al., 1995) was infused into the dopamine-depleted striatum at the rate of 1 μl/5 min for 45 min. After 15 min of the intrastriatal infusion, the animals were given an injection of the partial D1 agonist SKF38393 (1 mg/kg, i.p.) and were killed 30 min later.
In another set of animals, the MEK inhibitor (SL327; DuPont, Wilmington, DE), was administered systemically [60 mg/kg, i.p. (Valjent et al., 2000)] to animals with unilateral 6-OHDA nigrostriatal lesions 30 min before treatment with the partial D1 agonist SKF38393 (5 mg/kg, i.p.). Animals were killed either 15 min after SKF38393 treatment and brains were processed for immunohistochemical localization of phosphorylated ERK1/2/MAP kinase and phosphorylated c-jun or 45 min after SKF38393 treatment and brains were processed for in situ hybridization histochemical localization of mRNA encoding c-fos,arc, and c-jun.
Cortical stimulation studies. Two weeks after unilateral 6-OHDA lesions, some animals (n = 20), while under sodium pentobarbital anesthesia, had stimulating electrodes implanted bilaterally into the orofacial area (3 mm anterior, 3.5 mm lateral to bregma, and 1.5 mm below dural surface) of the lateral agranular motor cortex. A ground electrode was placed on the dural surface over the parietal cortex. All of the electrodes and a head holder (to connect a swivel during stimulation) were fixed on the skull with dental acrylic resin. During this surgery, an injection of fluorogold (0.4 μl, 1%, in saline) was placed into the substantia nigra bilaterally. This retrograde tracer was used to label direct projection striatal neurons. Three to seven d after surgery, rats were separated in individual chambers and stayed there at least 3 hr for habituation. Animals were given an injection of saline, the partial D1 agonist SKF38393 (1 mg/kg, i.p.), or the D2 agonist quinpirole (1 mg/kg, i.p.), and then the implanted electrodes were attached to a stimulator (Frederick Haer Co., Bowdoinham, ME) and biphasic pulse trains (100–200 μA, 100 Hz, 160 msec trains repeating once per second). Stimulation was applied for 20 min, and the animals were killed immediately after the stimulation offset. The intensity was 100 μA for most cases but, if necessary to elicit small somatic movements, increased not to exceed 200 μA. The cases that failed to show visible somatic movements were excluded from additional analysis. In no case did animals display evidence of seizure activity from the electrical stimulation.
Electrical stimulation of the nigrostriatal dopamine pathway. In animals under sodium pentobarbital anesthesia, bipolar stimulating electrodes were implanted into the midbrain, with the electrode tip placed at the junction between the dopamine cell groups in the ventral tegmental area and substantia nigra pars compacta. Surgical implantation was similar to that used for implantation of electrodes into the cortex. Three to 7 d after electrode implantation, animals, while awake and freely moving, received electric stimulation (100–200 μA, 100 Hz, 160 msec trains repeating once per second) for 15 min. Animals were killed either immediately or 30 min after offset of the stimulation. Brains from animals killed immediately after the offset of stimulation were processed for immunohistochemical localization of phosphorylated ERK1/2/MAP kinase, whereas brains from animals killed 30 min after offset of the stimulation were processed for immunohistochemical localization of c-fos.
Immunohistochemical and in situ hybridization histochemical methods. All animals were killed by carbon dioxide intoxication, perfused transcardially with a brief rinse of saline (50 ml, 0.9%, 4°C), followed by formaldehyde fixative (4%formaledhyde in 0.5 m sodium phosphate buffer, pH 7.4, plus 0.9% saline), and brains were removed immediately, stored in the fixative solution for an additional 6 hr and then overnight in the fixative solution to which 30% sucrose had been added. After the brains had sunken in the sucrose solution, coronal sections through the striatum were frozen sectioned (30 μm) on a sliding microtome. For sections processed for immunohistochemistry alone, sections were collected into PBS (0.1m, pH 7.4, 0.9% saline). Sections were transferred to solutions (0.05 m phosphate buffer, pH 7.4, 0.9% saline, 2% normal goat serum, and 1% Triton X-100) of primary antisera, including c-fos rabbit antisera (1:4000; Genesys The Woodlands, TX), phosphorylated MAP kinase rabbit antisera (1:500; Cell Signaling Technology, Beverly, MA), phosphorylated c-jun rabbit antisera (1:500; Cell Signaling Technology), and then were incubated overnight, rinsed in PBS, and processed for diaminobenzidine peroxidase staining using the Vectastain avidin–biotin–peroxidase protocol (Vector Laboratories, Burlingame, CA).
Sections processed for combined immunohistochemistry and in situ hybridization histochemistry were immediately mounted onto gelatin-coated glass slides and dried on a slide warmer (30°C). Slide-mounted sections were processed successively through solutions containing the following and air dried: 4%formaldehyde in 0.9% saline (10 min), 0.25% acetic anhydride in triethanolamine (0.1m, pH 8.0; 10 min), 70% alcohol (2 min), 95% alcohol (2 min), 2× 100% alcohol (2 min each), 2× 100% chloroform (10 min each), 2× 100% alcohol (5 min each), and 95% alcohol. Digoxigenin-labeled ribonucleotide probe directed against the mRNA encoding enkephalin (Gerfen et al., 1995) was added to hybridization buffer (50% formamide, 600 mm NaCl, 80 mm Tris Hcl, pH 7.5, 4 mmEDTA, 0.1% sodium pyrophosphate, 0.2% SDS, 2% sodium polyacrylate, 100 mm dithiothreitol, 1 μg of tRNA, 1 μg of total RNA, and 0.4 μg of salmon sperm DNA), was applied to the glass-mounted sections, and was incubated at 55°C overnight. After treatment with RNase A (20 mg/ml) for 30 min, slides were then washed for four times 20 min each at 65°C in 0.2× SSC and rinsed in Tris (0.5 m, pH 7.5) saline (0.9%) at room temperature for 5 min. A solution (0.05 mphosphate buffer, pH 7.4, 0.9% saline, 2% normal goat serum, and 1% Triton X-100) containing a mixture of mouse antisera directed against digoxigenin (1:50; Boehringer Mannheim, Indianapolis, IN) combined with antisera directed against phosphorylated MAP kinase (1:250), phosphorylated c-jun (1:500), or c-fos (1:2000) were applied to the slide-mounted sections (50 μl/section), loosely covered with a coverslip, and incubated for 1–2 d at 4°C. Slide-mounted sections were then rinsed in PBS twice for 15 min each. Solution (0.05 m phosphate buffer, pH 7.4, 0.9% saline, and 2% normal goat serum) containing mixed fluorescent labeled antisera, Alexia 488-labeled rabbit antisera, and Cy3-labeled mouse antisera (Molecular Probes, Eugene, OR) was applied to the sections for 2 hr (50 μl/section; room temperature), rinsed twice in PBS, dried, and examined under appropriate fluorescent illumination.
Western blots. For Western blot analysis of phosphorylated MAP kinase, the striatum remaining from brains processed for immunohistochemistry as described above was dissected frozen, combined with sample buffer (50 mm Tris-HCl, pH 7.0, 2% w/v SDS, and 50 mm DTT) at 100 μl/10 mg tissue, briefly sonicated (5–10 sec), boiled for 10 min, cooled on ice for 5 min, and centrifuged at 14,000 × g for 15 min at room temperature. The supernatant was retrieved, and protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL). Ten to 40 mg of protein was diluted with additional sample buffer and combined 1:1 with sample loading buffer (50 mmTris-HCl, pH 7.0, 2% w/v SDS, 50 mm DTT, 20% glycerol, and 0.2% bromophenol blue). Ten to 20 μl samples were boiled for 5 min, cooled on ice briefly, and then loaded onto precast 10% polyacrylamide Tris-glycine gels with 4% polyacrylamide stacking gels (Bio-Rad, Hercules, CA). Gels were run using the Mini-Protean II (Bio-Rad,) systems and electrophoresed in a 70 mmTris, 192 mm glycine, and 0.1% w/v SDS buffer, pH 8.3. Electrophoresis was performed at 100 V for 5 min, followed by 180 V for 40 min. After SDS-PAGE, gels were bathed briefly in Western running buffer (70 mm Tris, 192 mm glycine, 0.1% w/v SDS, and 20% v/v methanol, pH 8.3), opposed to nylon membranes, and assembled with filter paper into Mini Trans-Blot (Bio-Rad) filled with ice-cold Western running buffer. Blotting was performed at 100 V for 1 hr. After blotting, the membranes were removed and washed in TBS (20 mmTris and 0.9% w/v NaCl) for 5 min and then placed into plastic sealed on three sides. The membranes were blocked for 1 hr shaking in TBST (TBS plus 0.1% v/v Tween 20) plus 5% w/v nonfat dry milk, followed by three washes for 5 min in TBST. TBST plus 5% w/v nonfat dry milk containing phosphorylated MAP kinase antisera (1:500) was added to each membrane and incubated overnight shaking at 4°C. One the following day, the blots were washed three times for 5 min with TBST and then incubated with secondary antibody (goat anti-rabbit IgG-peroxidase; Sigma) at 1:2000 in TBST containing 5% w/v nonfat dry milk for 2 hr shaking at room temperature. The blots were washed three times for 5 min with TBST and processed for chemiluminescence with SuperSignal substrate (Pierce), opposed to autoradiographic film for 5 sec to 10 min, and developed.
RESULTS
D1 dopamine receptor agonists activate ERK/MAP kinase and c-jun in the dopamine-depleted striatum
In rats with a unilateral lesion of the nigrostriatal dopamine system, treatment with the partial D1 dopamine agonist SKF38393, at doses of 1 or 2 mg/kg, results in the induction of the mRNAs encoding IEGs such as c-fos when animals are killed 60 min after agonist treatment (Gerfen et al., 1995; Berke et al., 1998). Activation of ERK1/2/MAP kinase in the striatum after cortical stimulation reportedly occurs within 10–15 min (Sgambato et al., 1998a,b). In a pilot experiment to determine the optimal time point to study phosphorylation of ERK1/2/MAP kinase after agonist treatment and to verify the specificity of the antisera used to detect phosphorylated ERK1/2/MAP kinase (Cell Signaling Technology), Western blot analysis was used. Animals with unilateral lesions of the nigrostriatal dopamine system were killed at 0, 5, 10, 15, 20, and 60 min (n = 3 for each time point) after treatment with the D1 dopamine receptor agonist SKF38393 (2 mg/kg, i.p.). Analysis of Western blots with antibodies directed against the phosphorylated form of ERK1/2/MAP kinase (Cell Signaling Technology) showed labeled bands corresponding to ERK1 and ERK2 (p44 and p42 isoforms of MAP kinase), which were increased in the lesioned but not intact striatum between 15 and 20 min after agonist treatment (data not shown). These qualitative results were confirmed with immunohistochemical analysis of animals killed at the same time points.
Detailed immunohistochemical analysis was performed on brain sections from animals treated with 2 mg/kg SKF38393 and killed 15 min later. In the lesioned striatum, phosphorylated ERK1/2/MAP kinase was evident in numerous neurons throughout the dorsal and ventral striatum (Fig.1). In the dopamine-intact striatum, phosphorylated ERK1/2/MAP kinase immunolabeling was not apparent in most of the dorsal striatum but was present in a few neurons scattered along the medial border of the striatum and in the nucleus accumbens. To determine in which neuron type phosphorylated ERK1/2/MAP kinase was present, double labeling of phosphorylated ERK1/2/MAP kinase immunoreactivity and in situ hybridization histochemistry to detect mRNA encoding enkephalin was used. Enkephalin is a selective marker of indirect projection neurons. In the lesioned striatum, cell counts were conducted on two 500 μm2areas in the dorsal striatum from four animals. In a 500 μm2 area, phosphorylated ERK1/2/MAP kinase was present in an average of 256 neurons, which were enkephalin negative, and in 6 of 291 enkephalin-positive neurons. In a 500 μm2 area in the dopamine intact striatum, phosphorylated ERK1/2/MAP kinase was present in an average of 12 neurons, which were enkephalin negative, and in an average of 6 of 306 neurons, which were enkephalin positive. There are approximately equal numbers of indirect and direct striatal projection neurons, which together constitute >90% of the neuron population of the striatum (Gerfen and Young, 1988). Thus, these results indicate that phosphorylated ERK1/2/MAP kinase was present in the majority of direct-projecting neurons in the lesioned striatum and in a negligible number of neurons in the dopamine-intact striatum.
Fig. 1.

D1 dopamine receptor-mediated phosphorylation of ERK1/2 (p-ERK1/2) in the dopamine-depleted striatum. Unilateral lesion of the nigrostriatal dopamine system is demonstrated by the loss of tyrosine hydroxylase immunoreactivity in the right lesioned striatum (A). After treatment (15 min) with the partial D1 dopamine agonist SKF38393 (2 mg/kg, i.p.), p-ERK1/2 is not evident in the dopamine-intact striatum (B) but is present in numerous neurons in the dopamine-depleted striatum (C). To determine the type of striatal neuron in which p-ERK1/2 is present, sections are processed to display both p-ERK1/2 with a green fluorescent label (D) and enkephalin mRNA with a red fluorescent label (D′). Nearly all p-ERK1/2-immunoreactive neurons (blue arrows) are enkephalin negative. Only a small number of enkephalin-positive neurons display p-ERK1/2 immunoreactivity (yellow arrow), whereas the vast majority are p-ERK1/2 negative (orange arrows). The graph provides quantitative data of the average number of pERK-positive/enkephalin-negative (blue arrows), pERK-positive/enkephalin-positive (yellow), and pERK-negative/enkephalin-positive (red) neurons in a 500 μm2 area from the lateral striatum of four animals. Enkephalin provides a marker of indirect projection neurons, with any given striatal area having an equal number of direct projecting, enkephalin-negative neurons (Gerfen and Young, 1988). Data indicate that, in the dopamine-intact striatum, there are few pERK1/2-immunoreactive neurons, whereas in the dopamine-depleted striatum, D1 agonist-induced p-ERK1/2 occurs selectively in enkephalin-negative, direct striatal projection neurons.
In these same animals, large numbers of neurons in the lesioned striatum also display phosphorylated c-Jun immunoreactivity, whereas few neurons in the intact dorsal striatum are labeled. Cell counts from two 500 μm2 areas in four animals revealed the following numbers: in the dopamine-depleted dorsal striatum, each 500 μm2 area displayed an average of 241 neurons displaying phosphorylated c-Jun immunoreactivity, which were enkephalin mRNA negative, and 9 of 303 enkephalin-positive neurons were labeled, whereas in the dopamine intact striatum, 11 enkephalin-negative neurons were labeled and 8 of 288 enkephalin-positive neurons were labeled. These results are comparable with those for phosphorylated ERK1/2/MAP kinase. Phosphorylation of c-Jun is an indicator of activation of JN kinase/SAP kinase, because c-Jun is a substrate of JN kinase/SAP kinase but not PKA or MAP kinase (Karin, 1995).
In a second experiment, the NMDA antagonist (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate (MK801) (1 mg/kg, i.p.) was given to animals 15 min before D1 dopamine receptor agonist (SKF38393; 1 mg/kg, i.p.). A lower dose of SKF38393 was used to provide a more stringent test of the effect of MK801. When these animals were killed 15 min after the agonist treatment, phosphorylated ERK1/2/MAP kinase was evident in striatal neurons in the dopamine-depleted striatum, similar to animals not receiving MK801 pretreatment (average number of phosphorylated ERK1/2/MAP kinase neurons per 500 μm square area: SKF38393 alone, 285; MK801 pretreated, 265). This is consistent with previous studies, which have shown that MK801 treatment does not affect D1 agonist induction of immediate early genes in the dopamine-depleted striatum (Keefe and Gerfen, 1996).
Effect of inhibitors of MEK on D1-supersensitive responses
Inhibitors of the Ser/Thr MEK, which is responsible for phosphorylating ERK1/2/MAP kinase, have been shown to block cortical stimulation-induced phosphorylation of ERK1/2/MAP kinase and subsequent IEG induction in striatal neurons (Sgambato et al., 1998b). An MEK inhibitor (SL327; DuPont), which may be administered systemically [60 mg/kg, i.p. (Valjent et al., 2000)], was given to animals with unilateral 6-OHDA nigrostriatal lesions 30 min before treatment with the partial D1 agonist SKF38393 (5 mg/kg, i.p.). Animals pretreated with the MEK inhibitor SL327 (n = 6) or with vehicle (n = 6) were killed 15 min after SKF38393 treatment. In the dopamine-intact dorsal striatum, there was no evidence of activation of ERK1/2/MAP kinase. In the dopamine-depleted striatum, D1 dopamine receptor agonist-mediated phosphorylation of ERK1/2 (Fig.2A) and phosphorylation of c-jun (Fig. 2B) is significantly reduced by pretreatment with the MEK inhibitor SL327 (Fig.2C,D). Other animals receiving MEK inhibitor pretreatment or vehicle 30 min before SKF38393 (1 or 2 mg/kg, i.p.) were killed 45 min after the D1 agonist treatment. In these animals, MEK inhibitor pretreatment blocked the induction of mRNA encoding c-fos, arc, and c-jun in the dopamine-depleted striatum compared with the robust induction of mRNAs encoding these IEGs in vehicle-treated animals (Fig.2E–H).
Fig. 2.
Inhibition by MEK inhibitors of D1 dopamine receptor agonist-mediated phosphorylation of ERK1/2 and c-fos IEG induction in the dopamine (DA)-depleted striatum. Animals received either saline control or a systemic treatment with the MEK inhibitor SL327 (60 mg/kg, i.p.) 30 min before treatment with the D1 dopamine receptor agonist SKF38393 (5 mg/kg). Compared with controls, this MEK inhibitor significantly reduces the phosphorylation of ERK1/2 (A,B) and the phosphorylation of c-Jun (C,D) in the dopamine-depleted striatum at 15 min, demonstrated with immunohistochemical labeling. This treatment also blocks the later induction of mRNAs encoding the IEGs c-fos (E, F) and c-jun (G, H) at 45 min after agonist treatment, demonstrated with in situhybridization histochemistry. In a second experiment, either vehicle (I; 1% DMSO in artificial CSF) or the MEK inhibitor PD98059 (J; 100 μm) was infused into the dopamine-depleted striatum of animals before and after systemic treatment with the D1 dopamine receptor agonist SKF38393 (1 mg/kg, i.p.). Animals were killed 45 min after agonist treatment. Contrasted with intrastriatal infusion of vehicle (I), MEK inhibitor blocked D1 dopamine receptor agonist-induced mRNA encoding c-fos(J) around the infusion site.
In another experiment, the MEK inhibitors PD98059 (Alessi et al., 1995;Dudley et al., 1995) and U0126 (Favata et al., 1998) were infused into the dopamine-depleted striatum for 15 min before systemic treatment of the D1 agonist SKF38393 (1 mg/kg) and for the subsequent 15 min period (100 μm, 6 μl/60 min). In the dopamine-depleted striatum, these MEK inhibitors blocked ERK1/2/MAP kinase phosphorylation at 15 min and blocked the induction of mRNA encoding c-fos at 45 min (Fig.2I,J).
Comparison of D1 dopamine receptor-mediated gene regulation in the dopamine-intact and -depleted striatum
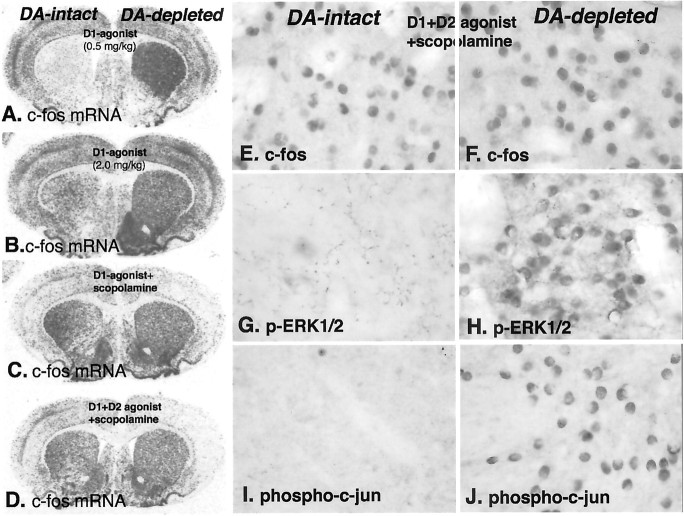
Partial D1 dopamine receptor agonist treatments elicit a robust supersensitive IEG response in the dopamine-depleted striatum. However, IEG induction in the intact striatum requires costimulation of D1 and D2 receptors (LaHoste and Marshall, 1993) or use of a full D1 agonist, such as SKF81297, either alone or in combination with a muscarinic antagonist (Wang and McGinty, 1996). To compare D1 receptor activation of ERK1/2/MAP kinase and JN kinase/SAP kinase responses between the normal and dopamine-depleted striatum, we analyzed treatment paradigms using a full D1 receptor agonist (SKF81297) alone and combined with D2 receptor agonists and with the muscarinic antagonist scopolamine, which produce robust IEG induction in the dopamine-intact striatum. Treatment with a low dose of SKF81297 (0.5 mg/kg) produces induction of mRNA encoding the IEG c-fos that is restricted to the dopamine-depleted striatum (Fig.3A). Induction of c-fos mRNA in the dopamine-intact striatum, which is comparable with that in the dopamine-depleted striatum (Fig.3B–D), is produced by treatments with a high dose of SKF81297 (2.0 mg/kg) or a combination of this D1 dopamine receptor agonist with the muscarinic receptor antagonist scopolamine (5 mg/kg) and the D2 agonist quinpirole (2 mg/kg). Induction of the mRNAs encoding IEGs was demonstrated at a time point 45 min after agonist treatments.
Fig. 3.
Demonstration of distinct mechanisms of D1 dopamine receptor-mediated gene regulation in the dopamine (DA)-intact and -depleted striatum, using the full D1 agonist SKF81297 alone or combined with other drugs.A–D, In situ hybridization histochemical localization of mRNA encoding c-fos 45 min after different drug combinations: A, SKF81297 (0.5 mg/kg); B, SKF81297 (2.0 mg/kg); C, SKF81297 (2.0 mg/kg) combined with the muscarinic receptor antagonist scopolamine (5 mg/kg); or D, SKF81297 (2.0 mg/kg) combined with the D2 dopamine receptor agonist (1 mg/kg) and scopolamine. The low dose of agonist alone (A) demonstrates the supersensitive response by the selective induction of c-fos in the dopamine-depleted striatum. Bilateral induction of c-fos IEG in both the dopamine-intact and -depleted striatum follows treatment with high dose of the full D1 agonist alone (B) or in combination with other drugs (C, D). However, when animals receiving any of these treatments are killed at 15 min, p-ERK1/2-immunoreactive neurons are evident only in the dopamine-depleted striatum and not in the dopamine-intact striatum (data not shown). The treatment combining full D1 agonist with both the D2 agonist and scopolamine produces the most robust c-Fos IEG response in the dopamine-intact striatum at 45 min (E). This treatment also results in persistent p-ERK1/2 (H) and phosphorylated c-Jun (J) in the dopamine-depleted striatum but does not activate p-ERK1/2 (G) or phosphorylated c-Jun (I) in neurons in the dopamine-intact striatum. These results demonstrate that, although D1 dopamine receptor-mediated induction of the IEG c-Fos occurs in both the dopamine-intact and -depleted striatum, activation of ERK1/2 occurs only in the dopamine-depleted striatum.
To examine the activation of ERK1/2/MAP kinase and the induction of c-Fos protein, animals were treated with the same drug treatments, as shown in Figure 3A–D, and killed either 15 or 45 min after treatment. Treatment with SKF81297 alone (0.5 or 2.0 mg/kg) at 15 min resulted in phosphorylated ERK1/2/MAP kinase and c-Jun in the lesioned striatum that was similar to that produced by treatment with the partial agonist SKF38393 (1 or 2 mg/kg) shown in Figure 1. These treatments resulted in negligible phosphorylated ERK1/2/MAP kinase and c-Jun immunolabeling in the dopamine-intact striatum at 15 min. When animals were killed 45 min after these agonist treatments, c-Fos immunoreactivity was present in a comparable number of neurons as labeled with phosphorylated ERK1/2/MAP kinase at 15 min in the dopamine-depleted striatum. In the dopamine-intact striatum of the animals treated with SKF81297 at a dose of 0.5 mg/kg, there was negligible c-Fos immunoreactivity, whereas in animals treated with 2.0 mg/kg, there was robust induction. Similar results were obtained with a combined treatment of SKF81297 and scopolamine. The combination of the full D1 agonist SKF81297 (2.0 mg/kg), the D2 agonist quinpirole (1.0 mg/kg), and scopolamine provides the most robust c-fos mRNA induction in the dopamine-intact striatum. When this combination was used and animals were killed at 15 min, there was negligible c-Fos immunoreactivity in the dopamine-intact or dopamine-lesioned striatum, but both phosphorylated ERK1/2/MAP kinase and c-Jun-immunoreactive neurons were present in large numbers throughout the dopamine-lesioned striatum (data not shown). This treatment resulted in negligible immunolabeling in the dopamine-intact dorsal striatum but in a substantive number of neurons in the nucleus accumbens. When animals treated with this combination were killed at 45 min, c-Fos immunoreactivity was evident throughout the dorsal and ventral striatum in the dopamine-intact and dopamine-lesioned striatum (Fig.3E,F). Moreover, at this time point, both phosphorylated ERK1/2/MAP kinase and c-Jun were present in large numbers of neurons throughout the dopamine-lesioned dorsal striatum but not in the dopamine-intact dorsal striatum (Fig.3G–J).
The combination treatment is illustrated because it provides the most stringent test of the absence of activation of ERK1/2/MAP kinase and c-Jun in the dopamine intact striatum and because it is possible to compare the effect in both the dopamine-intact and dopamine-lesioned striatum in the same animals as a result of the persistent activation of ERK1/2/MMAP kinase and c-Jun at this longer time point. These results demonstrate that, in the dopamine-intact dorsal striatum, pharmacologic treatment paradigms that produce robust D1 dopamine receptor-mediated induction of IEGs is not accompanied by activation of ERK1/2/MAP kinase. Interestingly, the regulation of D1 dopamine receptor-mediated activation of ERK1/2/MAP kinase appears to be regulated differentially in the dorsal and ventral striatum in the dopamine-intact striatum.
Comparison of induction of c-Fos and activation of ERK1/2/MAP kinase after stimulation of the nigrostriatal dopamine pathway
To further examine the absence of activation of ERK1/2/MAP kinase in direct pathway neurons in the dopamine-intact striatum, we stimulated the nigrostriatal pathway and compared the activation of ERK1/2/MAP kinase and the induction of the IEG c-Fos in the striatum and nucleus accumbens. Animals with implanted electrodes placed in the rostral medial substantia nigra pars compacta received electrical stimulation (15 min duration) and were killed either immediately (15 min after stimulation onset) or 45 min after stimulation onset. Sections through the striatum were processed for immunohistochemical localization of the IEG c-Fos and the phosphorylated form of ERK1/2/MAP kinase. In animals killed 45 min after stimulation onset (n = 10), c-Fos-immunoreactive nuclei were observed throughout the dorsal striatum and nucleus accumbens (Fig. 4A–D). In these animals, immunoreactive phosphorylated ERK1/2/MAP kinase was present at very low levels in neurons only in the nucleus accumbens. In animals killed 15 min after stimulation onset (n = 10), phosphorylated ERK1/2/MAP kinase was present in medium-sized neurons in the nucleus accumbens but was absent in such neurons in the dorsal striatum (Fig. 4E–H). In the dorsal striatum, a few large neurons displayed immunoreactive phosphorylated ERK1/2/MAP kinase. In these animals, c-Fos immunoreactivity was not present in neurons in any part of the striatum. The absence of c-Fos induction at this short time point presumably reflects that such induction requires a longer time for activation of the protein kinases and phosphorylation of transcription factors required for the induction of c-Fos.
Fig. 4.
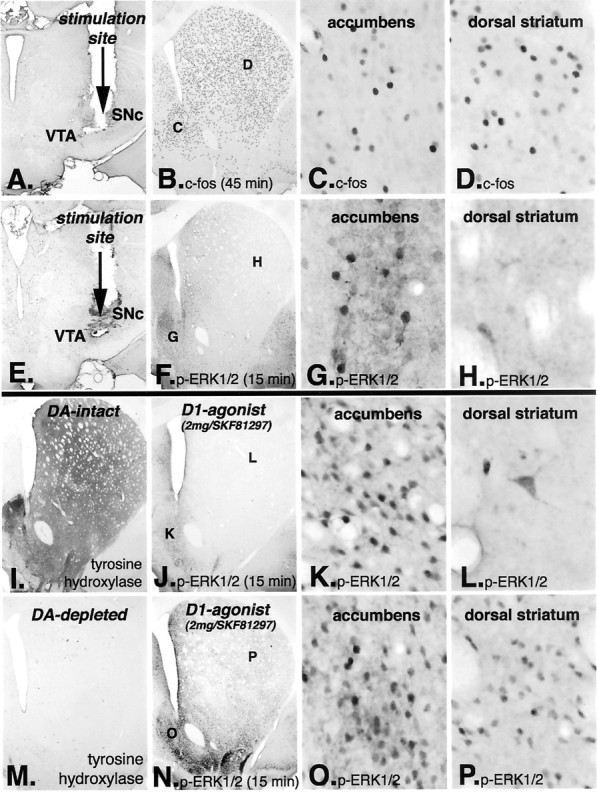
Electrical stimulation of the nigrostriatal pathway results in the induction of the IEG c-fosthroughout the striatum and nucleus accumbens, but activation of ERK1/2 occurs only in the nucleus accumbens. Electrodes were placed in the junction between dopamine (DA) neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) and stimulated (A,E). In animals killed 45 min after stimulation onset (A–D), c-Fos is induced throughout the dorsal striatum and nucleus accumbens (B). Higher-power photomicrographs reveal c-Fos-immunoreactive nuclei in the nucleus accumbens (C) and in the dorsal striatum (D). In animals killed 15 min after stimulation onset (E–H), the time point that is optimal for detecting phosphorylated ERK1/2, immunoreactive neurons are observed only in the nucleus accumbens (F). Higher-power photomicrographs reveal numerous immunoreactive neurons in the nucleus accumbens (G), whereas in the dorsal striatum, only scattered large immunoreactive neurons are observed (H) and not medium-sized projection neurons. In the dopamine-intact striatum (I; indicated by tyrosine hydroxylase immunoreactivity), animals killed 15 min after treatment with the full D1 dopamine receptor agonist SKF81297 (2.0 mg/kg) display phosphorylated ERK1/2 immunoreactivity only in the nucleus accumbens (J). Higher-power photomicrographs reveal numerous immunoreactive projection neurons in the nucleus accumbens (K), whereas in the dorsal striatum, only scattered large immunoreactive neurons are observed (L) and not medium-sized projection neurons. This pattern of p-ERK1/2 matches that observed after stimulation of the nigrostriatal dopamine pathway (E–H). On the other hand, in the dopamine-depleted striatum (M; indicated by the absence of tyrosine hydroxylase-immunoreactive fibers), treatment with the full D1 dopamine receptor agonist SKF81297 (2.0 mg/kg) displays phosphorylated ERK1/2 immunoreactivity throughout the nucleus accumbens and dorsal striatum (N). Higher-power photomicrographs reveal numerous immunoreactive medium-sized projection neurons in the nucleus accumbens (O) and in the dorsal striatum (P).
The pattern of neurons displaying phosphorylated ERK1/2/MAP kinase after electrical stimulation of the nigrostriatal pathway matched the response in the dopamine-intact striatum after treatment with the full D1 dopamine receptor agonist SKF81297 (2 mg/kg) (Fig.4I–L). Medium-sized immunoreactive neurons were present in the nucleus accumbens, but only scattered, large neurons were immunoreactive in the dorsal striatum (Fig. 4L). This pattern contrasts markedly with that in the dopamine-depleted striatum, in which numerous medium-sized neurons displayed immunoreactive phosphorylated ERK1/2/MAP kinase in both the nucleus accumbens and dorsal striatum (Fig. 4M–P).
These results demonstrate that, in the dopamine intact striatum, stimulation of the nigrostriatal dopamine pathway or treatment with a full D1 dopamine receptor agonist results in the induction of the IEG c-Fos throughout the striatum and nucleus accumbens. However, such treatments result in activation of ERK1/2/MAP kinase only in the nucleus accumbens and not in the dorsal striatum. This is in marked contrast to the dopamine-depleted striatum, in which activation of ERK1/2/MAP kinase occurs in response to D1 dopamine receptor agonist in the dorsal striatum.
D2 dopamine receptor-mediated activation of ERK1/2/MAP kinase
Previous studies reported that D2 agonist treatment results in ERK1/2/MAP kinase activation in the dopamine-depleted striatum (Cai et al., 2000). In animals with unilateral lesions of the nigrostriatal dopamine system, the D2 agonist quinpirole (1 mg/kg, i.p.) results in increased ERK1/2/MAP kinase phosphorylation in the lesioned but not intact striatum at 15 min (Fig.5A). However, such labeling in the lesioned striatum appears mainly within the neuropil and in only scattered neurons (Fig. 5B), primarily localized in the dorsolateral striatum. The sparse numbers of phosphorylated ERK1/2/MAP kinase-immunoreactive neurons and their scattered distribution suggest that these are striatal interneurons. To further examine possible D2 mechanisms mediating MAP kinase activation, animals were treated with the D2 dopamine receptor antagonist eticlopride (1 mg/kg, i.p.). After such treatment (15 min), phosphorylated ERK1/2/MAP kinase is apparent in striatal neurons in the dopamine-intact but not dopamine-lesioned striatum (Fig. 5C,E). Furthermore, phosphorylated ERK1/2/MAP kinase is localized exclusively to indirect striatal projection neurons, which express enkephalin and the D2 dopamine receptor. Pretreatment of animals with the NMDA receptor antagonist MK801 (1 mg/kg, i.p.) blocked eticlopride activation of ERK1/2/MAP kinase.
Fig. 5.
Effect of D2 dopamine receptor agonist and antagonist treatment on the phosphorylation of ERK1/2/MAP kinase in the dopamine (DA)-intact and -depleted striatum. In animals treated with the D2 dopamine receptor agonist quinpirole (2 mg/kg), phosphorylation of ERK1/2 is produced in the DA-depleted striatum (A) in a small number of scattered large neurons (B), which are likely striatal interneurons. On the other hand, in animals treated with the D2 dopamine receptor antagonist eticlopride (2 mg/kg), phosphorylation of ERK1/2 occurs exclusively in the DA-intact striatum (C) in numerous medium-sized neurons (D). Double-labeling studies demonstrate that these neurons are indirect striatal projection neurons (data not shown).
Corticostriatal activation ofERK1/2/MAP kinase
The cerebral cortex provides excitatory, glutamatergic synaptic inputs to striatal neurons, which, when stimulated, activate ERK1/2/MAP kinase in the striatum (Sgambato et al., 1998b). In this study, we further examined interactions between corticostriatal stimulation-mediated activation of ERK1/2/MAP kinase and dopamine D1 and D2 receptor agonists in the dopamine-intact and -depleted striatum. In a first experiment (Fig. 6), we determined that bilateral stimulation of the primary motor cortex in awake behaving rats, with or without unilateral lesions of the nigrostriatal dopamine system, results in phosphorylated ERK1/2/MAP kinase in the lateral striatal region, which receives inputs from the stimulated area. Cell counts were performed in animals with unilateral dopamine lesions. An area of 150 μm2 in the lateral striatal area that receives inputs from the stimulated cortical area from two sections in four animals was quantified in the dopamine-intact and -depleted striatum. In the dopamine-intact striatum, phosphorylated ERK1/2/MAP kinase immunoreactivity was present in an average of in 70% (64 of 91) of enkephalin-positive neurons and in an average of seven neurons that were enkephalin negative. In the dopamine-depleted striatum, phosphorylated ERK1/2/MAP kinase immunoreactivity was present in an average of 29% (29 of 97) of enkephalin-positive neurons and in an average of 10 neurons that were enkephalin negative. Thus, cortical stimulation–activation of ERK1/2/MAP kinase occurred selectively in indirect striatal projection neurons, in both the dopamine-intact and -depleted striatum.
Fig. 6.

Effect of corticostriatal stimulation on the activation of ERK1/2 in direct and indirect striatal neurons compared between the dopamine-intact and -depleted striatum. In animals with unilateral lesions of the nigrostriatal pathway (dopamine-depleted side on the right), electrodes were implanted bilaterally into the orofacial region of the dorsal agranular insular motor cortex (A, AGl). These animals received cortical stimulation for 30 min, at which point they were killed. This stimulation results in phosphorylation of ERK1/2 bilaterally in the lateral region of the striatum (B), which receives inputs from the stimulated cortical area. Phosphorylated MAP kinase (p-ERK1/2) immunoreactivity is present in neurons in the lateral region of the dopamine-intact (C) and dopamine-depleted (D) striatum. Indirect striatal projection neurons are demonstrated by in situhybridization histochemical localization of enkephalin mRNA (C′, D′, red fluorescence). Determination of which striatal neurons display p-ERK1/2 is demonstrated by merging p-ERK1/2 and enkephalin images (C", D"). The graph insetshows the average counts of neurons displaying p-ERK1/2 in the dopamine-intact and -depleted striatum. In the dopamine-intact striatum, phosphorylated-ERK1/2/MAP kinase immunoreactivity was present in an average of 70% (64 of 91) of enkephalin-positive neurons (yellow) and in an average of seven neurons that were enkephalin negative (green). In the dopamine-depleted striatum, phosphorylated-ERK1/2/MAP kinase immunoreactivity was present in an average of 29% (29 of 97) of enkephalin-positive neurons (yellow) and in an average of 10 neurons that were enkephalin negative (green). These results demonstrate that, in the dopamine-intact and -depleted striatum, corticostriatal stimulation results in activation of ERK1/2 selectively in indirect (enkephalin-positive) striatal projection neurons.
In a second experiment (Fig. 7), animals with unilateral lesions of the nigrostriatal dopamine pathway were given a low dose of the partial D1 dopamine receptor agonist SKF38393 (0.5 mg/kg), and the primary motor cortex was stimulated bilaterally in awake, freely moving rats for 20 min. Again, cortical stimulation resulted in phosphorylation of ERK1/2/MAP kinase bilaterally in the lateral striatal region. The average number and percentage of direct or indirect projection neurons labeled were counted from two sections from four animals. In the dopamine-intact striatum, phosphorylated ERK1/2/MAP kinase was evident principally in indirect/D2 striatal neurons [in a 150 μm2 area, an average of 59 of 87 (68%) of indirect striatal pathway neurons were labeled, whereas only an average of 8 of 98 (8%) of direct striatal pathway neurons were labeled]. Conversely, in the dopamine-depleted striatum, phosphorylated ERK1/2/MAP kinase was localized in both direct striatal projection neurons [in a 150 μm2 area, an average of 65 of 93 (70%) of direct pathway neurons] and indirect striatal projection neurons [22 of 119 (18%) of indirect pathway neurons]. In the dopamine-depleted striatum, neurons in which phosphorylated ERK/MAP kinase was present were localized to the lateral region of the striatum, which receives inputs from the stimulated cortical area. The absence of labeling in the medial striatum suggests that the low dose D1 agonist used was not solely responsible for activation of ERK/1/2/MAP kinase, because a higher-dose D1 agonist treatment results in activation throughout the striatum.
Fig. 7.

Effect of corticostriatal stimulation combined with a low dose of the D1 dopamine receptor agonist SKF38393 (0.5 mg/kg) on the activation of ERK1/2/MAP kinase in direct and indirect striatal neurons compared between the dopamine-intact and -depleted striatum. In animals with unilateral lesions of the nigrostriatal pathway (dopamine-depleted side on the left), electrodes were implanted bilaterally into the orofacial region of the dorsal agranular insular motor cortex (A,AGl). These animals were treated with a low dose of the D1 agonist SKF38393 (0.5 mg/kg) and received cortical stimulation for 30 min, at which point they were killed. This stimulation results in phosphorylation of ERK1/2/MAP kinase bilaterally in the lateral region of the striatum (B), which receives inputs from the stimulated cortical area. Phosphorylated ERK1/2/MAP kinase (p-ERK1/2) immunoreactivity is present in neurons in the lateral region of the dopamine-intact (C) and dopamine-depleted (D) striatum. Direct striatal projection neurons are demonstrated by the retrograde transport of fluorogold (C′, D′, blue fluorescence), which had been injected into the substantia nigra. Indirect striatal projection neurons are demonstrated byin situ hybridization histochemical localization of enkephalin mRNA (C′, D′, red fluorescence). Determination of which striatal neurons display p-ERK1/2 is demonstrated by merging images displaying p-ERK1/2 and direct and indirect pathway neurons (C", D"). Thegraph inset displays quantitative data of the percentage of p-ERK1/2-positive neurons in the dopamine-intact and -depleted striatum. In the dopamine-intact striatum, 8% of the direct pathway striatal neurons display p-ERK1/2 labeling, whereas 68% of the indirect pathway neurons in lateral striatal region display p-ERK1/2 labeling. In the dopamine-depleted striatum, 70% of the direct pathway neurons display p-ERK1/2, whereas 28% of the indirect pathway neurons display p-ERK1/2 labeling. These results demonstrate that, in the dopamine-depleted striatum, there is a switch in the mechanism responsible for activation of ERK1/2 in direct pathway striatal neurons in response to stimulation of corticostriatal afferents.
In a third experiment, animals with unilateral lesions were treated with the D2 agonist quinpirole (1 mg/kg), and the primary motor cortex was stimulated bilaterally for 20 min. In these animals, phosphorylated ERK1/2/MAP kinase was absent in both direct and indirect pathway neurons in both the dopamine-intact and -lesioned striatum (data not shown).
DISCUSSION
Two significant findings emerge from the present results (Fig. 8). First, direct and indirect striatal projection neurons, which both receive corticostriatal glutamatergic and nigrostriatal dopaminergic inputs (Hersch et al., 1995), display distinct receptor-mediated activation of the protein kinase pathways that are responsible for IEG induction. Specifically, in the dopamine-intact striatum, the ERK1/2/MAP kinase signaling pathway is normally used by indirect but not direct striatal projection neurons. Second, after lesions of nigrostriatal dopamine input, there is a switch in the regulation of D1 dopamine receptor-mediated signal transduction pathways such that ERK1/2/MAP kinase and JN kinase/SAP kinase signaling pathways are activated in direct striatal projection neurons. The switch to ERK1/2/MAP kinase signaling in direct pathway neurons in the dopamine-depleted striatum appears to be responsible for the D1 dopamine receptor-supersensitive response.
Fig. 8.
Diagram depicting the direct and indirect pathway neurons in the striatum. Both neuron types receive excitatory inputs from the cerebral cortex and dopaminergic inputs from the substantia nigra pars compacta, whereas D1 and D2 dopamine receptors are segregated to the direct and indirect pathway neurons, respectively.A, In the dopamine-intact striatum, stimulation of corticostriatal inputs results in activation of ERK1/2/MAP kinase (pERK) and subsequent IEG induction that is restricted to indirect pathway neurons. B, Also, in the dopamine-intact striatum, both stimulation of the nigrostriatal dopamine pathway or treatment with a full D1 dopamine receptor agonist results in the induction of IEGs in direct pathway neurons, without activation of pERK. C, After lesions of the nigrostriatal dopamine input to the striatum, without cortical stimulation, low doses of partial or full D1 dopamine receptor agonist result in a supersensitive induction of IEGs in direct pathway neurons that results from activation of pERK. D, After lesions of the nigrostriatal dopamine input to the striatum, cortical stimulation, coupled with a very low dose of partial D1 agonist treatment, results in activation of perk and subsequent IEG induction in both direct and indirect striatal pathway neurons. Thus, activation of ERK1/2/MAP kinase is normally restricted to indirect striatal projection neurons; however, after dopamine denervation of the striatum, direct pathway neurons display a supersensitive response to D1 dopamine receptor agonist treatment that is dependent on the aberrant activation of ERK1/2/MAP kinase.
Restriction of activation of ERK1/2/MAP kinase to indirect striatal projection neurons
Both direct and indirect striatal pathway neurons receive corticostriatal afferent synapses (Hersch et al., 1995), and both display excitatory postsynaptic responses to cortical afferent stimulation (Kawaguchi et al., 1990). However, the present findings demonstrate that, in the dopamine-intact striatum, corticostriatal stimulation activates ERK1/2/MAP kinase principally in indirect striatal neurons and not in direct striatal projection neurons. This is consistent with reports that corticostriatal stimulation results in IEG induction selectively in indirect striatal projection neurons (Berretta et al., 1997; Parthasarathy and Graybiel, 1997). ERK1/2/MAP kinase appears to be responsible for induction of IEGs in response to corticostriatal stimulation because their induction is blocked by inhibitors of the MEK that is responsible for phosphorylation of ERK1/2/MAP kinase (Sgambato et al., 1998b).
Additional evidence that indirect striatal neurons use ERK1/2/MAP kinase signaling is provided by the finding that D2 dopamine receptor antagonist treatment results in phosphorylation of ERK1/2/MAP kinase in indirect striatal neurons. Previous studies have reported that ERK1/2/MAP kinase is induced in the dopamine-depleted striatum by D2 agonist treatment (Cai et al., 2000). However, in the present study, it is shown that D2 dopamine receptor agonist treatment results in phosphorylation of ERK1/2/MAP kinase only in a small percentage of striatal interneurons and not in either direct or indirect striatal projection neurons. In fact, the present results demonstrate that activation of ERK1/2/MAP kinase in indirect striatal projection neurons by corticostriatal stimulation is inhibited by treatment with a D2 dopamine receptor agonist. These results suggest that dopamine, acting through the D2 dopamine receptors either presynaptically or postsynaptically, functions to inhibit activation of ERK1/2/MAP kinase in indirect striatal neurons in response to stimulation of corticostriatal afferents.
Striatal dopamine depletion alters D1-mediated signal transduction in direct projection neurons
In the dopamine-intact striatum, ERK1/2/MAP kinase is not normally activated in direct pathway neurons by either glutamatergic or dopaminergic receptor stimulation. Two experiments in the present study demonstrate that, in the dopamine-intact striatum, stimulation of the D1 dopamine receptor does not activate ERK1/2/MAP kinase in direct striatal projection neurons. In one experiment, pharmacologic treatments with a full D1 dopamine receptor agonist, which results in c-fos induction, does not activate ERK1/2/MAP kinase in the dopamine-intact dorsal striatum. One might question whether the absence of D1-mediated activation of ERK1/2/MAP kinase reflects simply a low-level response in the dopamine-intact striatum. However, combined drug treatments that enhance the IEG response in the dopamine-intact striatum to levels that are comparable with the dopamine-depleted striatum also fail to activate ERK1/2/MAP kinase. In a second experiment, electrical stimulation of the nigrostriatal dopamine pathway resulted in the induction of the IEG c-Fos throughout the dorsal striatum and nucleus accumbens. However, such stimulation failed to activate ERK1/2/MAP kinase in the dopamine-intact dorsal striatum. These results indicate that, although stimulation of D1 dopamine receptors with an agonist or endogenous dopamine results in the induction of IEGs, neither activates ERK1/2/MAP kinase in direct pathway neurons in the dorsal dopamine-intact striatum.
Interestingly, both D1 dopamine receptor agonist treatment and stimulation of the nigrostriatal dopamine pathway activate ERK1/2/MAP kinase direct striatal pathway neurons in the nucleus accumbens. This indicates that direct pathway neurons in the nucleus accumbens appear to normally use ERK1/2/MAP kinase in the dopamine-intact striatum, whereas dorsal direct striatal pathway neurons do not. In stark contrast to the dopamine-intact striatum, in the dopamine-depleted striatum, D1 dopamine receptor agonist treatment results in activation of ERK1/2/MAP kinase in direct pathway neurons in the dorsal striatum. This indicates that D1 dopamine receptor-mediated activation of this protein kinase is regulated by distinct mechanisms in the dopamine-intact dorsal and ventral striatum.
A recent study has demonstrated that cocaine treatment results in activation of ERK1/2/MAP kinase in the dopamine-intact striatum (Valjent et al., 2000). However, cocaine-induced activation of ERK1/2/MAP kinase is dependent on NMDA glutamate receptor activation (Valjent et al., 2000). This contrasts with D1-mediated activation of ERK1/2/MAP kinase in the dopamine-depleted striatum, which is shown here to be independent of NMDA receptor activation, as is the induction of IEGs such as c-fos (Keefe and Gerfen, 1996). These results indicate that the cellular mechanisms by which D1 receptors are linked to ERK1/2/MAP kinase are present in direct striatal pathway neurons in the dopamine-intact striatum. However, it appears that, after dopamine depletion in the striatum, there is a switch in the mechanism by which ERK1/2/MAP kinase activation is mediated by the D1 dopamine receptor.
D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch to activation of ERK1/2/MAP kinase
In the dopamine-depleted striatum, D1 dopamine receptor agonist treatment results in a supersensitive response in direct striatal projection neurons, as indicated by the robust induction of >30 IEGs (Berke et al., 1998). This response is supersensitive because IEG induction occurs in the dopamine-depleted striatum when doses of a partial D1 dopamine receptor agonist, such as SKF38393 (1 mg/kg), are used that produce little if any IEG induction in the dopamine-intact striatum. We show in the present study that the supersensitive IEG induction in the dopamine-depleted striatum is preceded by phosphorylation of ERK1/2/MAP kinase and c-Jun. A number of protein kinase signaling pathways converge on transcriptional regulation of c-fos, whose promoter contains SRE (serum response element), TRE/AP-1 (thyroid response element/activator protein-1), and CRE response elements (Sheng and Greenberg, 1990; Ghosh and Greenberg, 1995; Karin, 1995; Montminy, 1997; Gutkind, 1998). ERK1/2/MAP kinase phosphorylates transcription factors that bind to the SRE site, JN kinase phosphorylates c-Jun, which binds to the TRE/AP-1 site, and both MAP kinase and PKA phosphorylates CREB, which binds to the CRE site. Thus, the D1 dopamine receptor-supersensitive response in the dopamine-depleted striatum could involve activation of protein kinase A, ERK1/2/MAP kinase, or JN kinase. In the present study, we showed that treating animals with an inhibitor of MAP kinase kinase (MEK), which is responsible for the phosphorylation of ERK1/2/MAP kinases, before treatment with a D1 dopamine receptor agonist, blocks the phosphorylation of ERK1/2/MAP kinases in direct projecting neurons in the dopamine-depleted striatum. Additionally, such treatment blocks the subsequent induction of other IEGs, including c-fos,arc, and c-jun. Although ERK1/2/MAP kinase does not directly phosphorylate c-Jun, inhibition of MEK and activation of MAP kinase inhibits c-Jun phosphorylation. At this time, we have no explanation for the mechanism responsible. These results suggest that the ERK1/2/MAP kinase signaling pathway is critical for the D1 dopamine receptor-supersensitive response in the dopamine-depleted striatum.
Functional consequences of D1 dopamine receptor supersensitivity
Among the receptor coupled protein kinase signaling systems, the ERK1/2/MAP kinase pathway is emerging as critical to activity-dependent enhancement of synaptic neurotransmission underlying learning and memory (Kornhauser and Greenberg, 1997; Silva et al., 1998; Impey et al., 1999). The present results demonstrate that the protein kinase signaling pathways, including protein kinase A, ERK1/2/MAP kinase, and JN kinase, are normally differentially regulated in the direct and indirect striatal projection neurons. Moreover, dopamine may function to inhibit activation of the ERK1/2/MAP kinase signaling pathway. In indirect striatal neurons, in which ERK1/2/MAP kinase is activated in response to stimulation of corticostriatal afferent input, dopamine, acting through D2 dopamine receptors, appears to inhibit such activation. In the dopamine-intact striatum, the direct striatal pathway neurons do not appear to normally use the ERK1/2/MAP kinase signaling pathway. Whether dopamine, acting on D1 dopamine receptors, is normally responsible for inhibiting activation of ERK1/2/MAP kinase remains to be determined. What is clear from the present study is that, in the dopamine-depleted striatum, D1 dopamine receptor activation results in the aberrant activation of the ERK1/2/MAP kinase signaling pathway neurons in direct pathway neurons.
A current model of basal ganglia function suggests that normal movement depends on a balance in the activity of the direct and indirect striatal projection pathways (Albin et al., 1989). Although this model is generally considered in terms of the physiologic activity in these pathways, the current results suggest an alternative view. We propose that the normal function of the basal ganglia in affecting movement behavior depends on the normal regulation of the ERK1/2/MAP kinase signaling, by which it is normally restricted to the indirect striatal projection pathway. ERK1/2/MAP kinase activation appears to be an evolutionarily conserved mechanism underlying learning and memory (Brambilla et al., 1997; Atkins et al., 1998; Blum et al., 1999). A reasonable speculation follows that the learning and memory of habitual movements that is attributed to the basal ganglia (Graybiel et al., 1994; Knowlton et al., 1996) involves activation of ERK1/2/MAP kinase in the indirect striatal projection pathway. Depletion of dopamine in the striatum results in the aberrant activation of ERK1/2/MAP kinase by dopamine agonist treatments that activate the D1 dopamine receptor in direct striatal pathway neurons. In the treatment of Parkinson's disease, l-DOPA is effective at reversing bradykinesia in the short term, but long-term treatment invariably leads to the development of uncontrolled dyskinetic movements (Bergmann et al., 1987). We propose that the development of dyskinesias result from the repeated aberrant activation of ERK1/2/MAP kinase in direct striatal pathway neurons in response to l-DOPA activation of the D1 dopamine receptor. The present results suggest that inhibitors of MEK, which blocks the aberrant supersensitive response of direct striatal pathway neurons to D1 dopamine receptor agonists, may provide a novel therapeutic adjunct to the use of l-DOPA in the treatment of Parkinson's disease.
Footnotes
We acknowledge the excellent technical assistance of Ron Harbaugh, Alex Cummins, and Bob Gelhard.
Correspondence should be addressed to Charles R. Gerfen, Building 36, Room 2D-30, 36 Convent Drive, Bethesda, MD 20892-4075. E-mail:gerfen@codon.nih.gov.
REFERENCES
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 4.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann KJ, Mendoza MR, Yahr MD. Parkinson's disease and long-term levodopa therapy. Adv Neurol. 1987;45:463–467. [PubMed] [Google Scholar]
- 6.Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, Grant SGN, Chapman, Hans-Peter Lipp PF, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 10.Cai G, Zhen X, Uryu K, Friedman E. Activation of extracellular signal-regulated protein kinases is associated with a sensitized locomotor response to D(2) dopamine receptor stimulation in unilateral 6-hydroxydopamine-lesioned rats. J Neurosci. 2000;20:1849–1857. doi: 10.1523/JNEUROSCI.20-05-01849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole DG, Kobierski LA, Konradi C, Hyman SE. 6-Hydroxydopamine lesions of rat substantia nigra up-regulate dopamine-induced phosphorylation of the cAMP-response element-binding protein in striatal neurons. Proc Natl Acad Sci USA. 1994;91:9631–9635. doi: 10.1073/pnas.91.20.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engber TM, Susel Z, Kuo S, Gerfen CR, Chase TN. Levodopa replacement therapy alters enzyme activities in striatum and neuropeptide content in striatal output regions of 6-hydroxydopamine lesioned rats. Brain Res. 1991;552:113–118. doi: 10.1016/0006-8993(91)90667-k. [DOI] [PubMed] [Google Scholar]
- 14.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 15.Gerfen CR, Young WS. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 17.Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1 and D2 dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 19.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 20.Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 21.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 23.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keefe KA, Gerfen CR. D1 dopamine receptor-mediated induction of zif268 and c-fos in the dopamine-depleted striatum: differential regulation and independence from NMDA receptors. J Comp Neurol. 1996;367:165–176. doi: 10.1002/(SICI)1096-9861(19960401)367:2<165::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 27.Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornhauser JM, Greenberg ME. A kinase to remember: dual roles for MAP kinase in long-term memory. Neuron. 1997;18:839–842. doi: 10.1016/s0896-6273(00)80322-0. [DOI] [PubMed] [Google Scholar]
- 29.LaHoste GJ, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JF, Navarrete R, Joyce JN. Decreased striatal D1 binding density following mesotelencephalic 6-hydroxydopamine injections: an autoradiographic analysis. Brain Res. 1989;493:247–257. doi: 10.1016/0006-8993(89)91160-8. [DOI] [PubMed] [Google Scholar]
- 31.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 32.Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson GS, Vincent SR, Fibiger HC. Striatonigral projection neurons contain D1 dopamine receptor-activated c-fos. Brain Res. 1990;523:288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- 34.Schwarzschild MA, Cole RL, Hyman SE. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J Neurosci. 1997;17:3455–3466. doi: 10.1523/JNEUROSCI.17-10-03455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sgambato V, Vanhoutte P, Pages C, Rogard M, Hipskind R, Besson MJ, Caboche J. In vivo expression and regulation of Elk-1, a target of the extracellular-regulated kinase signaling pathway, in the adult rat brain. J Neurosci. 1998a;18:214–226. doi: 10.1523/JNEUROSCI.18-01-00214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998b;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 38.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 39.Steiner H, Gerfen CR. Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contributions of pre- and postsynaptic mechanisms in dorsal and ventral striatum demonstrated by altered immediate-early gene induction. J Comp Neurol. 1996;376:530–541. doi: 10.1002/(SICI)1096-9861(19961223)376:4<530::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Valjent E, Corvol JC, Pages C, Besson MJ, Makdonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;28:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D1 dopamine receptor agonist SKF-82958 in the intact rat striatum. Neuroscience. 1996;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]