Abstract
The mucosal terminals of sensory neurons intrinsic to the wall of the intestine are sensitive to the chemical environment within the lumen. Lumenal stimuli probably release sensory mediators from the mucosal epithelium, which then activate the nerve terminals indirectly. Here, we tested the idea that ATP activates intrinsic sensory nerve terminals in a way consistent with its being a sensory mediator.
We made intracellular recordings from intrinsic sensory neurons located in the myenteric plexus [identified as AH neurons, which are neurons with a long-lasting afterhyperpolarization following the action potential (AP)], located within 1 mm of intact mucosa. Focal electrical stimulation of the mucosa was used to locate and map regions innervated by each neuron. Application of ATP (1–2 mm in the pressure pipette) to these regions elicited trains of APs that originated at the sensory terminals. ATP-γ-S produced a similar response, but α,β-methylene ATP and 2-methylthio-ATP were only weakly active. The P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS) (60 μm in the bath) abolished the APs evoked by ATP and ATP-γ-S but spared similar responses evoked by 5-hydroxytryptamine (5-HT). Another P2 receptor antagonist suramin (100 μm in the bath) did not significantly change the number of APs evoked by ATP. Either ATP or α,β-methylene ATP desensitized the ATP-evoked APs; 50% recovery occurred after ∼5 sec. The number of APs evoked by ATP was reduced, but not abolished, by the selective 5-HT3receptor antagonist granisetron (1 μm in the bath).
ATP was applied to the cell bodies of sensory neurons to investigate whether the cell bodies express the same P2X receptor as the terminals. ATP evoked a fast depolarization associated with a reduction in input resistance and a reversal potential of −11 mV. This depolarization was potentiated by suramin and blocked by PPADS.
We conclude that activation of an atypical excitatory P2X receptor by ATP triggers AP generation in the mucosal processes of the sensory neurons; endogenous 5-HT release may also contribute to activation of the nerve terminals. A similar P2X receptor exists on the cell body of the sensory neuron. Together, these data are consistent with a role for ATP as a sensory mediator in gastrointestinal chemosensory transduction.
Keywords: ATP, electrophysiology, intestine, sensory neuron, sensory transduction, serotonin
The chemical environment of the intestinal lumen determines the behavior of this organ. Intestinal transit is enhanced by the presence of short chain fatty acids within the lumen (Richardson et al., 1991) but slowed by the presence of many other nutrients (Schemann and Ehrlein, 1986; Spiller, 1994). These behaviors are mediated by neurons of the enteric nervous system present in the gut wall. A population of enteric neurons within the myenteric plexus of the guinea pig is sensitive to the chemical environment of the intestinal lumen and may cause changes in intestinal motor behavior (Kunze et al., 1995; Bertrand et al., 1997); similar neurons have been identified in the submucosal plexus (Kirchgessner et al., 1992).
The intrinsic chemosensitive sensory neurons (also referred to as intrinsic primary afferent neurons) are classed electrophysiologically as AH neurons [neurons with a long-lasting afterhyperpolarization (AHP) following the action potential (AP)], have Dogiel type II morphology (for review, see Furness et al., 1998), and provide a dense innervation to surrounding ganglia and the mucosa (Song et al., 1994; Bertrand et al., 1998; Li and Furness, 1998). It has been proposed that the nerve terminals of both the intrinsic afferents and the extrinsic afferents of vagal or spinal origin are activated by the release of sensory mediators from specialized sensory cells in the mucosal epithelium rather than responding directly to lumenal stimuli (for review, see Kirkup et al., 2001). Indeed, there is good evidence that 5-hydroxytryptamine (serotonin, 5-HT) released from enterochromaffin cells can act through 5-HT3receptors to excite the mucosal terminals of the myenteric sensory neurons (Bertrand et al., 2000) or the vagal extrinsic primary afferent neurons (Hillsley and Grundy, 1998) and can act through 5-HT1P receptors to excite the terminals of the submucosal sensory neurons (Pan and Gershon, 2000). However, 5-HT is unlikely to be the only sensory intermediary acting on the mucosal terminals of the sensory neurons, because depletion of 5-HT from the mucosa, or addition of 5-HT receptor antagonists, does not block all of the many reflex actions of the intestine (Gershon, 1991; Sanger, 1996). It is more likely that several substances, acting in concert, encode the changing lumenal environment (Kirkup et al., 2001). One such substance may be ATP.
ATP and P2X receptors are known to be involved in visceral and cutaneous sensory pathways (Wood and Docherty, 1997; Burnstock, 2001). The afferent nerve terminals in these pathways express P2X2 and P2X3 receptors and respond to application of ATP or its analogs (Bland-Ward and Humphrey, 2000). ATP and related purines are also recognized as neurotransmitters (Ralevic and Burnstock, 1998) in the central (Edwards et al., 1992), peripheral (Evans et al., 1992; Silinsky et al., 1992), and enteric (Galligan et al., 2000) nervous systems. This has been most convincingly demonstrated in enteric neurons in which ATP-mediated fast EPSPs have been described (Galligan and Bertrand, 1994) and characterized (LePard and Galligan, 1999) and a role in reflexes defined (Bian et al., 2000; Spencer et al., 2000).
Our preliminary data suggests that the mucosal terminals of myenteric sensory neurons are sensitive to ATP (Bertrand et al., 2000). ATP can also activate the terminals of vagal extrinsic primary afferent neurons (Kirkup et al., 1999), suggesting that ATP could function as a sensory mediator to enteric sensory neurons. In this study, we test the idea that ATP functions as a sensory mediator within the gastrointestinal tract by activating the terminals of intrinsic sensory neurons with cell bodies in the myenteric plexus.
MATERIALS AND METHODS
Tissue preparation. All experiments were performed using guinea pigs of either gender (160–280 gm, Hartley strain, from the University of Melbourne) that were fed a standard laboratory diet until the day of the experiment. Animals were stunned by a blow to the head and killed by severing the carotid arteries and spinal cord; procedures were approved by the University of Melbourne Animal Experimentation Ethics Committee. A 2- to 3-cm-long segment of ileum, 10–20 cm from the ileocaecal junction, was removed and placed in oxygenated (95% O2–5% CO2) physiological saline solution of the following composition (in mm): 117 NaCl, 1.2 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, 4.7 KCl, 25 NaHCO3, and 11 glucose. The physiological saline also contained nicardipine (3 μm) to relax the smooth muscle and scopolamine (1 μm) to minimize neurogenic movements. The segment was cut open along the line of the mesenteric attachment and pinned mucosal side up in a Petri dish lined with a silicone elastomer (Compound 184; Dow Corning, Midland, MI). The mucosa, submucosa, and circular muscle were removed over half the circumference, leaving the myenteric plexus with attached longitudinal muscle exposed (see Fig. 1). The preparation was then transferred to the base of a small recording chamber (volume of 1–2 ml), stretched, and pinned flat with 80 μm pins. The preparation was superfused with warmed (35–36°C) physiological saline at a flow rate of 3 ml/min (for additional details, see Bertrand et al., 1997, 1998,2000).
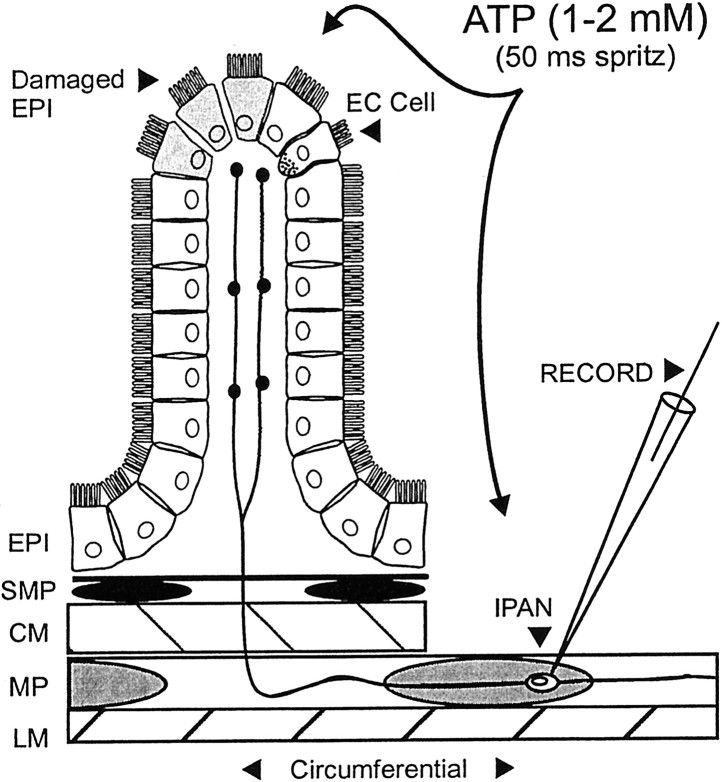
Fig. 1.
Illustration of the experimental arrangement and the relation of the epithelium and the AH–sensory nerve terminals. A side view of the preparation used in the present study. From thebottom: LM, the longitudinal muscle;MP, myenteric plexus; CM, circular muscle; SMP, submucosal plexus; EPI, epithelium. Note that the circular muscle, submucosal plexus, and epithelium have been dissected away from the right half of the preparation to allow an intracellular recording electrode (RECORD) to impale myenteric AH neurons [sensory neurons and intrinsic primary afferent neurons (IPAN, at the open circle)]. When the cell body of the AH neuron is close enough to the intact mucosa (<1 mm), there is a good chance that one or more of its projections is intact and innervates the mucosa. ATP and other agonists were applied to the mucosa and to the cell body of AH neurons via short-duration pressure ejection. The serotonin-containing enterochromaffin cells (EC Cell) are evenly spaced among the enterocytes. They represent ∼1% of the total population of endothelial cells. The mucosal epithelium is likely to be damaged when maintained in vitro (Damaged EPI).
Electrophysiology. Myenteric ganglia were visualized at 200 to 300× magnification using an inverted microscope (IMT-2; OlympusOptical, Tokyo, Japan) with Nomarski differential interference contrast optics. Neurons or muscle were impaled with glass microelectrodes (120–200 MΩ tip resistance, 1 mm outer diameter, 0.5 mm inner diameter; Clark Electromedical Instruments, Kent, UK) containing 1m KCl (or in previous experiments, 2% biocytin in 1 m KCl) using a mechanical micromanipulator (MP-1; Narishige, Tokyo, Japan). Voltage recordings were made in bridge mode using an Axoprobe or AxoClamp2A, digitized at 2–20 kHz (Digidata 1200A), recorded on a personal computer using Axoscope 8.0 (all fromAxon Instruments, Foster City, CA), and then analyzed with Origin 6.0 (Microcal Software, Northampton, MA). Measurements of the electrophysiological properties of AH neurons commenced 10 min or more after impalement (for classification, see Bornstein et al., 1994). The mucosa was stimulated with a bipolar electrode (114 μm stainless steel insulated with Teflon; MedWire, Mt. Vernon, VT) to generate maps of the region innervated by single AH neurons that assisted in finding regions sensitive to chemical application (Bertrand et al., 1998). Stimulus pulses from 0.5 to 2.0 mA (typically 1.0 mA) and a 0.5 msec duration (Master-8 stimulator, ISO-Flex stimulus isolation unit; both from A.M.P.I., Jerusalem, Israel) were used. All other protocols were detailed previously (Bertrand et al., 1997, 1998).
Solutions containing agonist or antagonist. Agonists were made up in a HEPES (10 mm)-buffered saline (120 mm NaCl, pH 7.2) and used on the day of the experiment or frozen in aliquots for later use. Application of small volumes of saline to the mucosa does not cause firing in myenteric AH neurons (Kunze et al., 1995; Bertrand et al., 1997). Agonists were applied to the mucosa by pressure ejection (10 psi, 10–100 msec duration; Picospritzer II; General Valve, Fairfield, NJ) from one of two micropipettes mounted on matched micromanipulators and positioned ∼0.5 mm above the intact mucosa and 2–5 mm from the impaled neuron; micropipettes were withdrawn from the superfusing solution between trials to guard against leakage of agonist onto the mucosa. The location of agonist application was visually confirmed under 40× magnification. Agonist was applied to the cell body with the same micropipettes used for application to the mucosa. Pressure application protocols were described previously (Bertrand and Galligan, 1992).
Antagonists were made up and stored in distilled water at a concentration of 10 mm. Tetrodotoxin (TTX) was an exception; it was initially dissolved in a small amount of acetic acid and stored at 1 mm. Antagonists were added to physiological saline solution at a 1000-fold dilution and stored in one or more 50 ml reservoirs that were connected in-line with the superfusion system. Visual observations of pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS), which is bright orange in solution, suggested that substantial concentrations of antagonists reached the bath within 2 min. The practical time limit that antagonists could be superfused was 20–40 min; on reaching the bath, antagonists were allowed to equilibrate with the tissue for 8 min, leaving a window of 10–30 min, during which measurements could be taken. Initial measurements, taken after ∼5 min equilibration, helped to define the onset of antagonist action but were not included in the averaged data.
Quantification of agonist-evoked responses. The number of APs and/or proximal process potentials (PPPs) (axonal APs that failed to excite somatic APs) elicited by agonist application to the mucosa were counted and are collectively referred to as APs; the results of two to three such applications were then averaged. The latency to the first AP (latency), the number of APs, the duration over which AP discharge took place (duration), and the average (f) and instantaneous (fINT) firing frequency during AP discharge were measured and used to determine agonist rank-order potency (see Fig. 2) and the extent to which antagonists were effective in blocking the actions of the agonists (see Figs. 3-5). In some neurons, slow depolarizations, similar to the slow EPSPs evoked in AH neurons by focal electrical stimulation, occurred 1–2 sec after mucosal application of agonist. These slow EPSP-like events were quantified by measuring the peak amplitude, time-to-peak, and the membrane potential before stimulation. The extent to which the excitability or the input resistance of the cell was altered during the slow depolarization was determined by passing 500 msec positive and negative current pulses through the recording electrode. The effects of agonists applied to the cell body of AH neurons were characterized in the same way as were the slow depolarizations, with the exception that changes in input resistance were assessed using 50 msec current pulses.
Fig. 2.
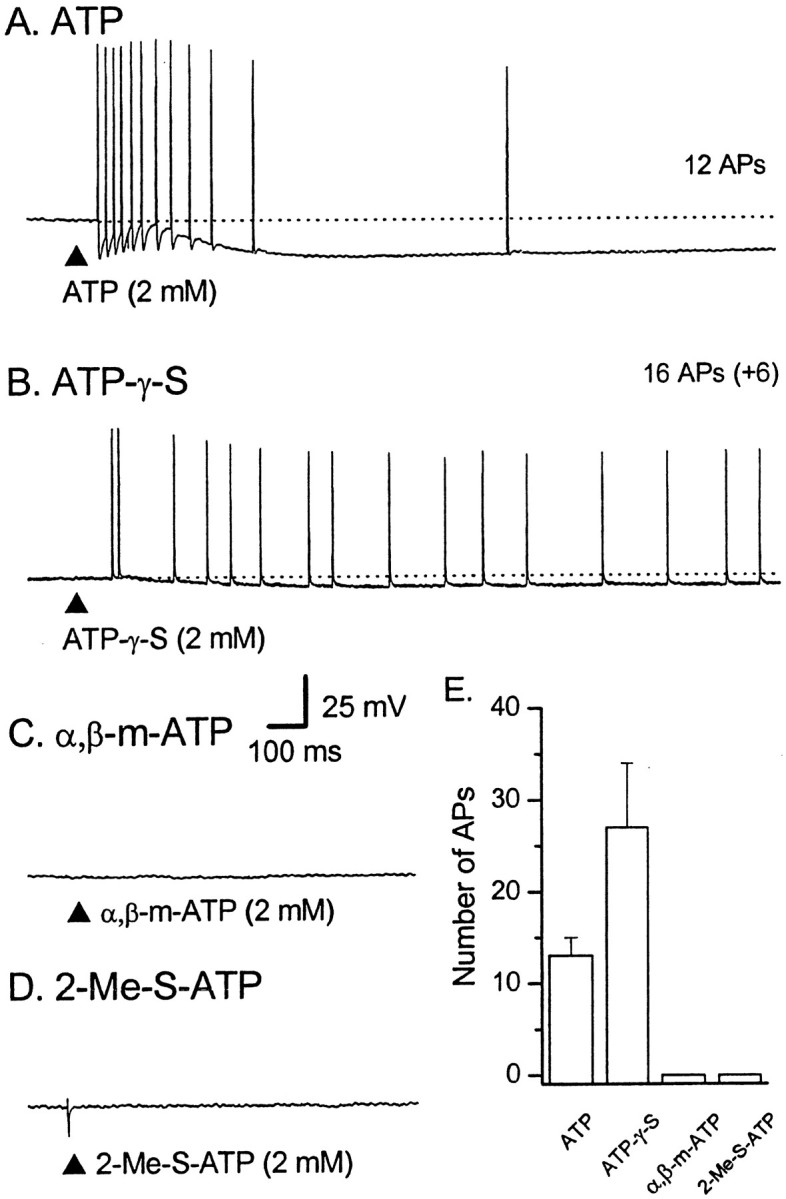
Responses in AH–sensory neurons to mucosal application of ATP, ATP-γ-S, α,β-me-ATP or 2-Me-S-ATP. Representative voltage traces from four different AH neurons during application of ATP or ATP analogs to the mucosa; dotted lines indicate RMP. Calibration in Capplies to all traces. The total number of APs is shown to the right of each trace.A, Brief application (100 msec; at the filled triangle) of ATP (2 mm) elicited a train of APs that showed a slowing in frequency during the 1.1 sec duration of the discharge; the average instantaneous frequency (ƒINT) was 29 Hz [resting membrane potential (RMP) of −67 mV]. B, ATP-γ-S (2 mm) elicited a 3.0-sec-long train of 22 APs (16 APs shown) that fired at a slow, relatively constant frequency (fINT of 12 Hz; RMP of −82 mV).C, α,β-methylene ATP (2 mm) failed to elicited any APs in the majority of cases (RMP of −68 mV).D, 2-Me-S-ATP (2 mm) also failed to elicit any APs under control conditions (n = 3).E, Histogram showing the average number of APs evoked by agonists for two to three repetitions per cell. ATP,n = 18; ATP-γ-S, n = 7; α,β-methylene ATP, n = 7; 2-Me-S-ATP,n = 3.
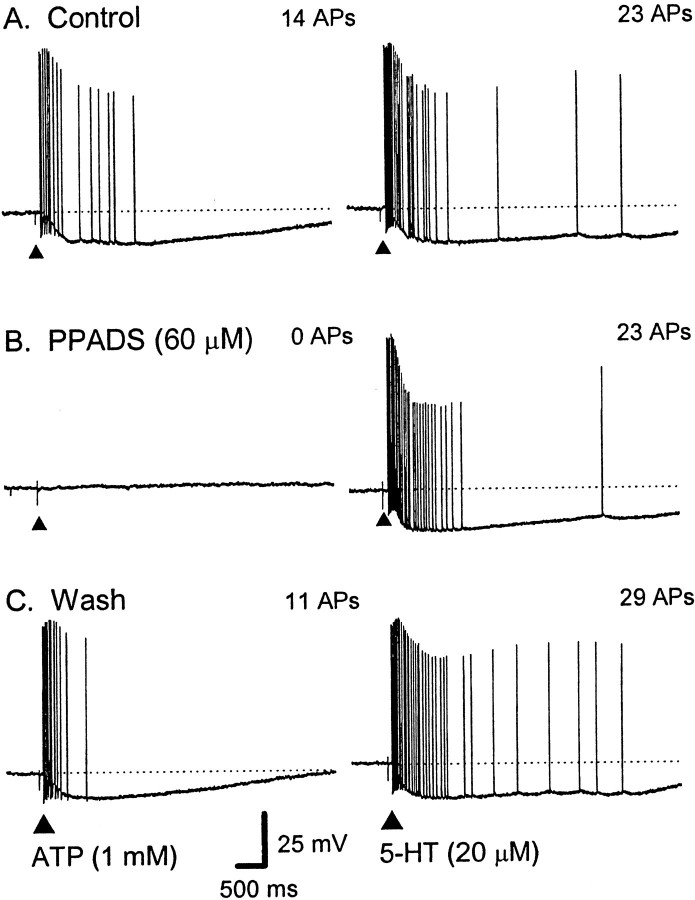
Fig. 3.
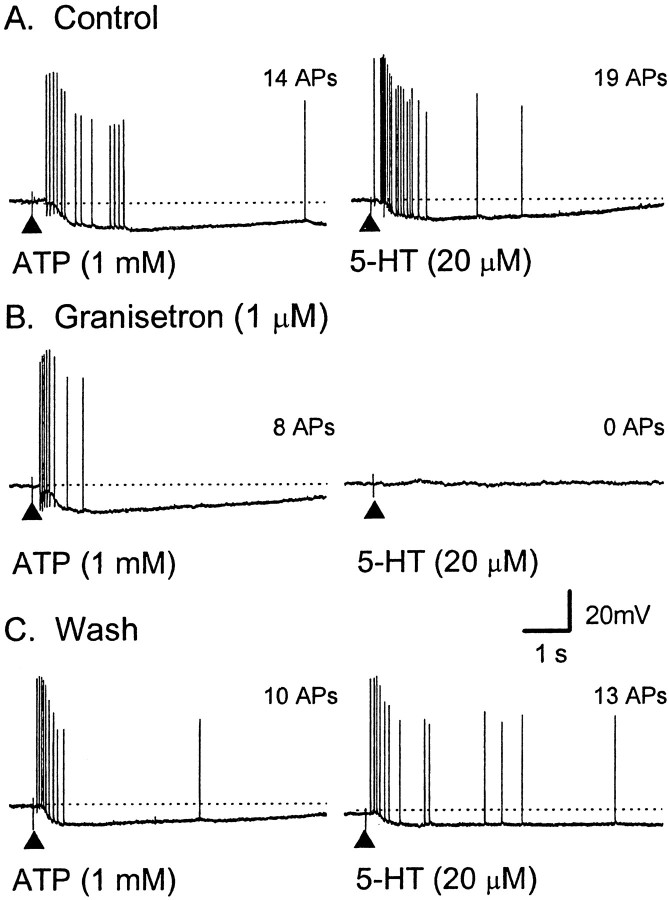
Effect of the P2 receptor antagonist PPADS on the train of APs evoked by ATP and 5-HT. Representative voltage traces from a single AH neuron; dotted lines indicate RMP. Calibration in C applies to all traces.Left, A, ATP (2 mm, 100 msec; applied at the filled triangle) elicited a 1.8 sec duration train of 14 APs at an average instantaneous frequency (fINT) of 13 Hz.B, The response was blocked by PPADS (60 μm). C, Twenty-five minutes after washout of PPADS, APs have reappeared, and the response is shorter in duration and of a higher frequency; fINT of 26 Hz (RMP, control, −63 mV; PPADS, −46 mV; recovery, −59 mV).Right, A, 5-HT (20 μm, 100 msec; applied at the filled triangle) elicited a train of 23 APs; fINT of 24 Hz. B, The response was not affected during superfusion with PPADS (23 APs;fINT of 22 Hz) and remained stable during washout of PPADS (C, 29 APs;fINT of 22 Hz) (RMP, control, −64 mV; PPADS, −48 mV; wash, −56 mV).
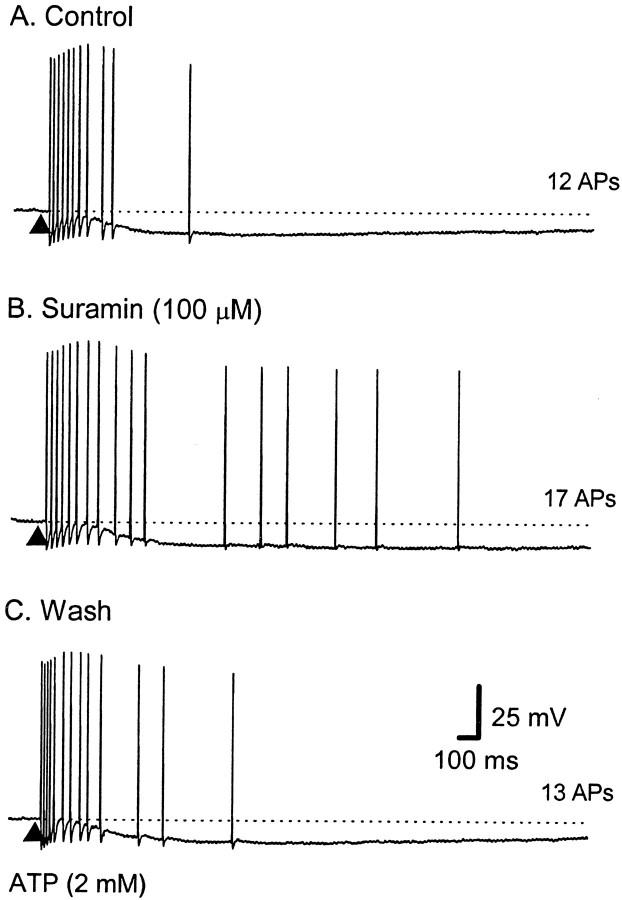
Fig. 4.
Effect of the P2 receptor antagonist suramin on the train of APs evoked by ATP. Representative voltage traces from a single AH neuron; the dotted lines indicate RMP. Calibration in C applies to all traces. The total number of APs is shown to the right of eachtrace. A, ATP (2 mm, 100 msec; applied at the filled triangle) elicited a train of 12 APs at an average instantaneous frequency (fINT) of 30 Hz for a duration of 0.8 sec. B, The response was not reduced by suramin (100 μm) (17 APs; ƒINT of 16 Hz; duration of 2.1 sec). C, Thirty minutes after washout, the response is shorter and of a higher frequency than in suramin (13 APs;fINT of 30 Hz; duration of 1.0 sec) (RMP, control, −69 mV; suramin, −70 mV; recovery, −65 mV).
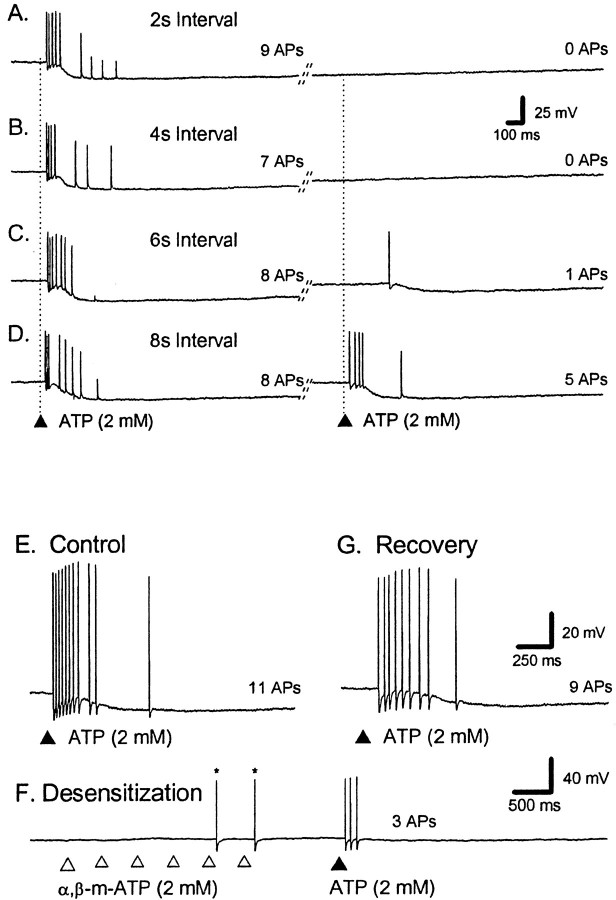
Fig. 5.
ATP can desensitize the train of APs evoked by ATP. A–D, Representative voltage traces from a single AH neuron; the dotted lines indicate RMP. Calibration in B applies to all traces. ATP was applied twice to the mucosa at the vertical lines with a 2, 4, 6, or 8 sec interval (A–D, respectively); the total number of APs is shown to theright of each trace. The diagonal lines indicate a break in the trace of a variable length of time. In control (A–D), ATP evokes a burst of APs consisting of between seven and nine APs. Subsequent APs evoked by the second application of ATP are depressed when the interval is shorter than 8 sec. Note that the long AHP after AP generation does not prevent incoming APs from the distal processes from evoking PPPs.E–G, Calibration in G also applies to E. The total number of ATP-evoked APs is shown to the right of each trace.E, In control, ATP evoked a burst of APs consisting of between seven and nine APs (3 repetitions). F, α,β-me-ATP was then applied six times in rapid succession to the same region of mucosa, and ATP was applied immediately after.G, Two minutes later, the ATP response was fully recovered.
Chemicals. TTX was purchased from Alomone Labs (Jerusalem, Israel);granisetron was the kind gift of SmithKline Beecham (Middlessex, UK). All other chemicals were purchased from Sigma (St. Louis, MO).
Statistics. Unless otherwise noted, all numbers are given as mean ± SEM. The range, where relevant, is given in parentheses. Student's t test was used to compare data for significant differences, with an α of 0.05 taken as the cutoff for significance. All t tests were one-tailed and paired unless noted; Bonferroni's correction for multiple comparisons was used when appropriate.
RESULTS
Electrophysiology
Intracellular recordings were made from 45 myenteric neurons with AH type electrophysiological characteristics. This type of neuron has been characterized previously as a sensory neuron (Furness et al., 1998) with chemosensitive projections to the mucosa (Kunze et al., 1995; Bertrand et al., 1997; Bertrand et al., 2000). Each AH neuron, whose shape was determined in the present study, had Dogiel type II morphology and electrophysiological characteristics that were consistent with previous studies. All of these neurons responded to a single electrical stimulus (0.5 msec pulse duration) applied to the mucosa with one or more APs; these APs could still be elicited when the soma was hyperpolarized by intracellular current injection, indicating that these AH neurons had an intact projection to the mucosa. Recordings were also made from S neurons (interneurons and motor neurons) and circular muscle cells to examine the effects of activation of AH neurons on downstream circuits. All neurons or muscle cells studied were within 1 mm of the intact mucosa in the circumferential direction (Fig. 1).
Effect of ATP applied to the mucosa
Twenty-six of 35 AH neurons responded to mucosal application of ATP (1–2 mm in a HEPES-buffered saline, pH 7.2), with a train of APs arising from a flat baseline (Fig.2A). ATP was ineffective when applied to regions from which electrical stimulation did not evoke APs. The APs were often converted into PPPs by direct application of hyperpolarizing current passed through the recording electrode or by an AHP (see Fig. 5A–D), suggesting that the APs are generated distal to the soma and normally invade the cell body. The latency to the first AP evoked by ATP (1–2 mm) was 0.7 ± 0.3 sec, and the bursts consisted of 15 ± 3 APs, which occurred within 2.1 ± 0.4 sec of the start of the burst (n = 18). The average frequency during the bursts was 10 ± 2 Hz, with instantaneous frequencies up to 30 Hz at the start of the bursts. ATP applied to the same area of mucosa typically evoked 10 or more bursts of APs. Seven of the 26 AH neurons also responded to ATP with a slow EPSP-like event. An additional two AH neurons responded to ATP with a slow EPSP-like event but failed to respond with a burst of APs (data not shown).
Comparison of ATP with 5-HT
5-HT has been shown previously to activate the mucosal terminals of a subset of myenteric sensory neurons (Bertrand et al., 2000). To test whether ATP and 5-HT activate the same populations, 14 of the AH neurons that responded to ATP (above) were also tested with 5-HT (20 μm) applied to the same regions of mucosa. Ten of the 14 AH neurons (71%) responded to 5-HT with a short latency burst of APs. In these 10 AH neurons, near-maximal responses to ATP and 5-HT were compared directly. ATP evoked 12 ± 2 APs (latency, 0.34 ± 0.11 sec; duration, 6.5 ± 1 sec), whereas 5-HT evoked more APs (18 ± 3; p < 0.05; n = 10) with a similar latency (0.31 ± 0.12 sec) and a similar duration (6.9 ± 2.2 sec).
Effects of purine analogs applied to the mucosa
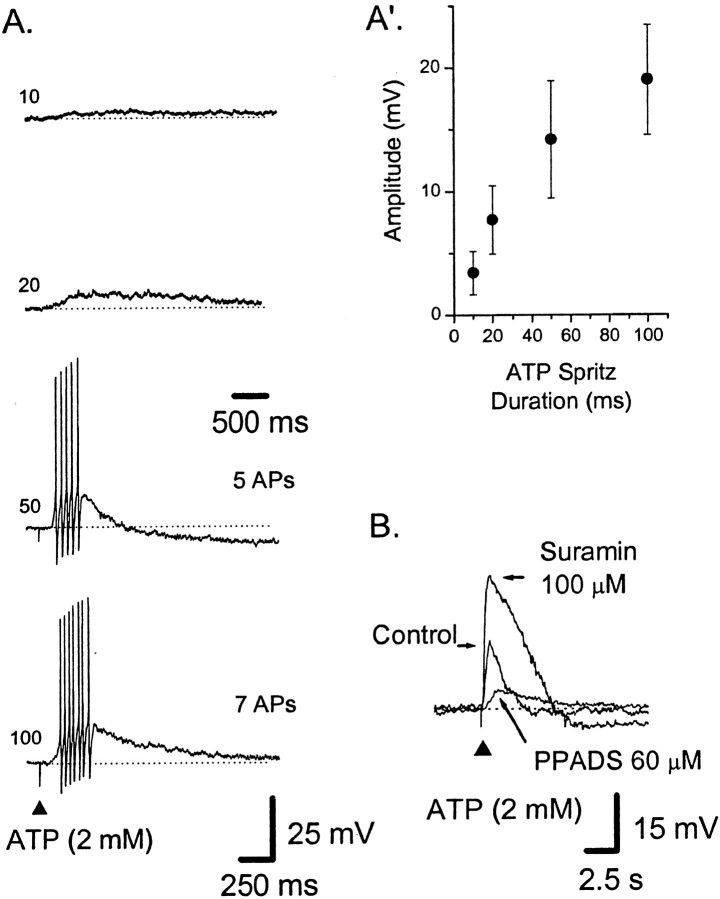
Different P2X receptors have different affinities for ATP and ATP analogs (Khakh et al., 2001). To characterize the receptor underlying the responses to ATP, three ATP analogs were applied to the mucosa with ATP as a direct comparison; these data were then used to construct an agonist rank-order potency relationship (see below). ATP-γ-S, when applied at the same concentrations as ATP, produced similar short-latency trains of APs (Fig. 2B) and/or slow EPSP-like depolarizations (data not shown). ATP-γ-S (1–2 mm) evoked 27 ± 7 APs (latency, 0.7 ± 0.5 sec; duration, 4.3 ± 1.8 sec; n = 7); the average frequency during the bursts was 11 ± 2 Hz, with instantaneous frequencies of up to 20 Hz at the start of the bursts. Compared with ATP, ATP-γ-S-evoked trains of APs were longer in duration and of a lower frequency.
α,β-Methylene ATP (α,β-me-ATP) (Fig. 2C) (see Fig.6B) and 2-methylthio-ATP (2-Me-S-ATP) (Fig.2D) were only weakly active when applied to the mucosa. α,β-methylene ATP (1–2 mm) elicited either a short train of APs in 2 of 11 AH neurons (two and four APs; this proportion was significantly less than the proportion of neurons responding to ATP; p < 0.01; χ2 test; df = 1) or a slow EPSP-like event in another 2 of the 11 AH neurons. When α,β-me-ATP (1–2 mm) was repeatedly applied (see below), it elicited a few APs in an additional two of three AH neurons (see Fig.6B). Similarly, 2-Me-S-ATP (2 mm) failed to elicit any short-latency bursts of APs in an additional three AH neurons (Fig. 2D), each of which responded to ATP; in one of these neurons, the presence of suramin (100 μm) seemed to reveal a small burst of APs in response to 2-Me-S-ATP.
Fig. 6.
Effect of granisetron on trains of APs evoked by ATP and 5-HT. Representative voltage traces from a single AH neuron; the dotted lines indicate RMP. Calibration inC applies to all traces. The total number of APs is shown to the right of eachtrace. Left, A, ATP (1 mm, 100 msec; applied at the filled triangle) elicited a train of 10 APs at an average instantaneous frequency (fINT) of 12 Hz for a duration of 2.1 sec. B, Granisetron (1 μm) caused a significant reduction in the number of APs (8 APs; fINT of 13 Hz; duration of 0.6 sec; RMP of −77 mV). C, The train of APs recovered after washout of granisetron (9 APs;fINT of 11 Hz; duration of 0.8 sec).Right, A, 5-HT (20 μm, 100 msec; applied at the filled triangle) elicited a train (15 APs; fINT of 11 Hz; duration of 3.9 sec). B, Granisetron (1 μm) fully blocked this response. C, Twenty-five minutes after washout of granisetron, the number of APs had increased (8 APs;fINT of 10 Hz; duration of 3.1 sec). Note that the long AHP after AP generation in C does not prevent incoming APs from the distal processes from evoking PPPs.
Based on the average number of APs evoked per agonist application (each at ∼1 mm), the agonist rank-order potency is as follows: ATP-γ-S = ATP > α,β-me-ATP = 2-Me-S-ATP, in which “greater than” denotes an ∼10-fold greater relationship (Fig.2E).
Effect of the P2 receptor antagonists PPADS and suramin
To further characterize the receptor underlying the responses to ATP, two nonselective P2 receptor antagonists were used: PPADS and suramin (Khakh et al., 2001). Superfusion of PPADS (60 μm), but not suramin (100 μm), into the bath caused a progressive decrease in the number of ATP-evoked APs from the mucosa in AH neurons (see below). After 10 min, PPADS abolished the train of APs and any slow EPSP-like responses but left trains of APs evoked by 5-HT (20 μm; applied to the same region of mucosa) unaffected (Fig. 3). Lower concentrations of PPADS (10 or 30 μm), sufficient to block fast synaptic transmission in myenteric ganglia (Galligan and Bertrand, 1994; Bian et al., 2000) did not depress ATP-evoked APs during a 30 min incubation (data not shown) (n = 3).
Before addition of PPADS (60 μm), mucosal application of ATP (1 or 2 mm) evoked 13 ± 2 APs (latency, 0.6 ± 0.2 sec; duration, 1.4 ± 0.4 sec; n = 6). In the presence of PPADS (60 μm), one AH neuron responded with a single AP, whereas the remaining AH neurons failed to respond (p < 0.05; n = 6) (Fig.3B). Partial recovery was observed in three AH neurons after 20 min wash (7 ± 1 APs; n = 3)
The effects of PPADS on responses to ATP-γ-S, applied to the mucosa, were also tested for three of the six AH neurons. ATP-γ-S (1 mm) evoked 14 ± 1.5 APs (latency, AP of 1.2 ± 1.2 sec; duration, 1.8 ± 1.2 sec; n = 3), whereas, in the presence of PPADS (60 μm), one AH neuron responded with a burst of 10 APs, and the remaining two AH neurons failed to respond (p < 0.05;n = 3).
In contrast, PPADS (60 μm) had no effect on the bursts of APs evoked by mucosally applied 5-HT in one AH neuron (whose ATP response was blocked) or in two additional AH neurons whose responses to ATP were not tested (Fig. 3). 5-HT evoked 17 ± 6 APs (latency, 0.2 ± 0.1 sec; duration, 2.1 ± 1.4 sec; n = 3), whereas, in the presence of PPADS (60 μm), it evoked 19 ± 7 APs (latency, 0.2 ± 0.1 sec; duration, 1.8 ± 0.7 sec; p > 0.05; n = 3).
When suramin (100 μm) was superfused into the bath, it was accompanied on three of six occasions by bursts of spontaneous APs that persisted when the soma was hyperpolarized and, thus, apparently originated in axonal processes rather than the soma. This activity subsided after ∼5 min.
Before the addition of suramin, ATP (1 mm) evoked 10 ± 1 APs (latency, 0.7 ± 0.3 sec; duration, 1.4 ± 0.7 sec;n = 6), whereas, in the presence of suramin (100 μm), it evoked 11 ± 2 APs (latency, 0.6 ± 0.3 sec; duration, 1.6 ± 1.0 sec; p> 0.05; two-tailed t test; n = 6) (Fig.4). Overall, there was no significant effect of suramin; however, in two of the six AH neurons, suramin caused an increase in the number of APs in a burst (Fig.4B), whereas in the other four AH neurons, there was little change from control.
Desensitization of the ATP-evoked train of APs
Many P2X receptors rapidly desensitize, and this has been used to distinguish between different subtypes of receptor (Ralevic and Burnstock, 1998). The receptors responsible for the burst of APs evoked by ATP were tested for desensitization. The ATP-evoked burst of APs was suppressed (Fig.5A–D,F) when either ATP or α,β-methylene ATP was applied to the mucosa a few seconds before a test stimulus; in the case of α,β-methylene ATP, repeated applications were required. When ATP was applied 1 sec after a prepulse of ATP, the second application usually failed to evoke any APs; however, these data were hard to quantify because the durations of the control bursts were variable. When the interval was extended to 2 sec, the number of APs evoked by the second application of ATP was reduced from 8 ± 1 to 1 ± 1 APs (p < 0.05; n = 5). ATP-evoked APs recovered from desensitization with ATP, with a half-time to recovery of ∼5 sec. When ATP was applied 1 sec after six prepulses of α,β-methylene ATP, the ATP-evoked burst of APs was reduced from 17 ± 5 to 2 ± 1 APs (p < 0.05;n = 3).
Effect of granisetron, a 5-HT3 receptor antagonist
Trains of APs evoked by ATP or 5-HT had similar latencies, comparable numbers of APs, and occurred in a similar population of AH neurons (see above). We tested the idea that the response to ATP is attributable to the release of endogenous 5-HT from mucosal enterochromaffin cells (Fig. 6). In control, ATP (1 mm; n = 4) or ATP-γ-S (1 mm; n = 2) evoked 18 ± 4 APs (latency, 0.3 ± 0.1 sec; duration, 3.1 ± 0.6 sec;n = 6), whereas, in the presence of granisetron (1 μm), they evoked 10 ± 3 APs (latency, 0.7 ± 0.3 sec; duration, 1.3 ± 0.3 sec). This decrease in the number of APs, from 18 to 10 (45%), was found to be significant (p < 0.05; n = 6). In four of the same neurons, 5-HT evoked 19 ± 8 APs (latency, 0.2 ± 0.1 sec; duration, 5.0 ± 3.5 sec; n = 4); these bursts were completely blocked in the presence of granisetron. These data suggest that the actions of endogenously released 5-HT are also blocked by granisetron and, thus, that the granisetron-resistant response to ATP is not mediated by 5-HT.
Effects on enteric microcircuitry
S neurons (interneurons and motor neurons) located within the same ganglia as were the AH neurons were also studied. In 9 of 12 S neurons of unknown functional class, ATP or ATP-γ-S (1 mm), applied to a region of mucosa directly circumferential to the impaled neuron, caused a sustained burst of fast EPSPs that consisted of 25 or more distinct peaks (Fig. 7A). When hexamethonium (300 μm) was added to the bath, most of these fast EPSPs were blocked (n = 3) (data not shown), indicating that ATP is acting at upstream neurons rather than at the cell body of the S neuron. Five of these 12 S neurons were also tested with 5-HT (20 μm) applied to the same region of mucosa, and all responded with a similar burst of fast EPSPs (data not shown). In 5 of 12 S neurons, ATP applied to the mucosa evoked a slow EPSP-like depolarization with (n = 2) or without (n = 3) a burst of fast EPSPs (Fig. 7A).
Fig. 7.
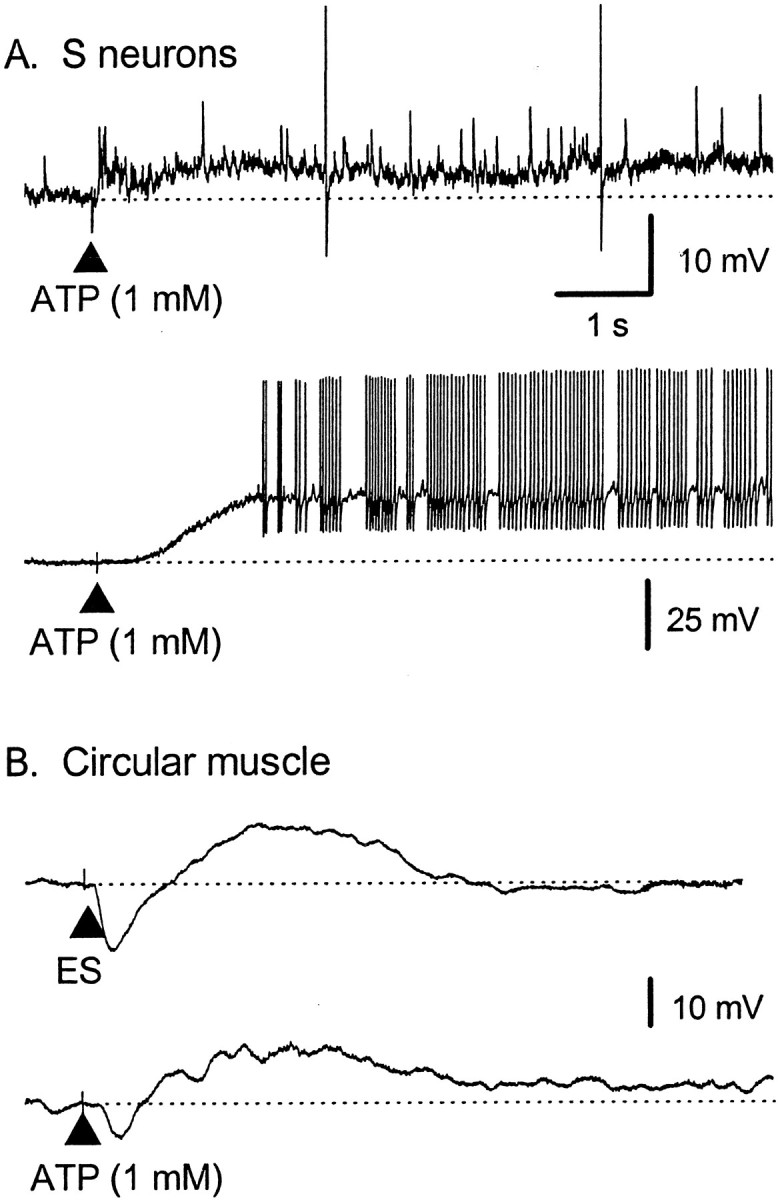
Effect of ATP applied to the mucosa on myenteric S neurons and circular muscle. ATP (1 mm) was applied to mucosa directly circumferential from the impalement. Calibrations inA applies to all traces.A, Representative voltage traces from two S neurons; thedotted lines indicate RMP. Top, ATP evoked a burst of fast EPSPs, two of which reach threshold for AP generation. Bottom, ATP evoked a slow EPSP-like depolarization that evoked many APs. B, Voltage traces from a single circular muscle cell. Top, Electrical stimulation of the mucosa, directly circumferential to the recording site, evoked a biphasic inhibitory junction potential (RMP of −48 mV).Bottom, Application of ATP to the same site on the mucosa evoked a biphasic inhibitory event, similar to that evoked electrically (RMP of −55 mV). ES, Electrical stimulation.
When circular smooth muscle cells were impaled, ATP (1 mm) applied to a region of mucosa directly circumferential to the impaled cell evoked a hyperpolarization, followed by a depolarization (n = 8) or a prolonged hyperpolarization (n = 2) in cells from 10 separate preparations (Fig.7B). The hyperpolarizations were similar in amplitude to inhibitory junction potentials evoked by electrical stimulation of the mucosa and were blocked by addition of TTX (1 μm; n = 3) (data not shown), suggesting that ATP did not act directly on the smooth muscle. ATP-γ-S (1 mm) evoked similar hyperpolarizations in the circular muscle cells in an additional three preparations. No cholinergic excitatory junction potentials were recorded in these experiments because the muscarinic receptor antagonist scopolamine (1 μm) was present in the bathing solution.
Effect of ATP applied to the neuronal soma
To investigate whether AH neurons express the same P2X receptor on the cell body as on the terminal, ATP (1–2 mm) was applied to the cell bodies of 16 AH neurons, including four that had responded to mucosal application of ATP. Twelve of the AH neurons (including three that had responded to mucosal application) responded to ATP with a large somatic depolarization (12 ± 3 mV at −65 mV) that had a short latency (∼100 msec) and initiated somatic APs (Fig.8A); in 4 of the 12 AH neurons, only the first application of ATP evoked a depolarization. When ATP was applied under direct visual control to either ganglionic or interganglionic regions, it caused movements of the underlying longitudinal muscle but did not affect AH neurons.
Fig. 8.
Effect of ATP applied to the cell body of myenteric AH–sensory neurons. Representative voltage traces from two AH neurons (A, B); the dotted lines indicate RMP. A, ATP was applied to mucosa with an increasing duration in milliseconds (numbers to the left of the trace). Thetop calibration bar applies to the top two traces; the bottom calibration applies to alltraces. A′, Summary data from three AH neurons in which ATP evoked depolarizations that did not generate APs.B, ATP was applied to the cell body under control conditions and during superfusion with PPADS (60 μm), which blocked, and suramin (100 μm), which potentiated, the depolarization.
The somatic depolarization was associated with a decrease in input resistance and was increased in amplitude by increasing the duration of ATP ejection (10 msec, 3 ± 2 mV; 20 msec, 8 ± 3 mV; 50 msec, 14 ± 5 mV; 100 msec, 19 ± 4 mV; n = 5) (Fig. 8B). Hyperpolarizing the cell up to −90 mV also resulted in an increase in the amplitude of the somatic depolarization; the reversal potential for the depolarization was estimated to be −11 ± 13 mV from data taken at holding potentials between −20 and −80 mV (n = 4). TTX (1 μm) did not alter the size or duration of the depolarization in three AH neurons in which the somatic depolarization did not initiate APs. PPADS (60 μm), superfused into the bath, blocked the response to ATP (2 mm, 50 msec) (control, 15 mV; PPADS, 2 mV; wash, 17 mV; n = 4), whereas suramin (100 μm) doubled the amplitude of the depolarization (control, 7 mV; suramin, 15 mV; wash, 6 mV; n = 8). Both PPADS and suramin blocked the ATP-evoked movements of the longitudinal muscle (see above).
In 5 of these 12 AH neurons, the somatic depolarization was followed by a hyperpolarization. This hyperpolarization was associated with a decrease in the membrane resistance, and its amplitude was increased during depolarization of the cell (Fig. 8B, end oftrace); this response was not dependent on AP generation and was similar to that reported by Katayama and Morita (1989).
DISCUSSION
In this study, we found that local application of ATP to the mucosal epithelium of the intestine generates APs in the mucosal processes of intrinsic sensory neurons innervating that region. These APs then propagate back to the cell bodies of the sensory neurons in the myenteric plexus. This effect is mimicked by a P2X receptor agonist and blocked by a P2 receptor antagonist. An additional finding is that the same P2X receptor appears to be expressed on both the terminals and the cell bodies of the sensory neurons.
ATP activates the mucosal terminals of enteric sensory neurons
The following observations support our conclusion that ATP activates the sensory neurons by an action on the mucosal nerve terminals. Application of ATP to the cell body and to the mucosa generate distinctly different responses: ATP acting at the cell body produces a large depolarization capable of evoking somatic APs, whereas mucosal application causes APs arising from a flat baseline. Somatic APs evoked by mucosal application of ATP are converted to PPPs by somatic hyperpolarization, whereas those evoked by somatic application of ATP are blocked by such a hyperpolarization. This indicates APs attributable to mucosal application were generated at a site distant from the soma.
ATP evoked APs from the mucosa only when applied to relatively small regions. When electrical stimuli were applied to these same regions, an antidromically conducted AP with a 3–7 msec latency was always found; this suggests a site of initiation several millimeters from the soma, consistent with the position of the stimulating electrode. These regions of mucosa were well correlated with areas from which 5-HT or electrical stimulation evoked trains of APs (Bertrand et al., 1998). This suggests that, when ATP is applied to the mucosa, it interacts with specialized regions of mucosa that contain the terminals of sensory neurons.
We did not find any evidence that 5-HT stimulated the release of ATP, although we did find evidence that ATP causes the release of endogenous 5-HT. The selective 5-HT3 receptor antagonist granisetron consistently reduced the number of APs in a train evoked by ATP in a reversible manner, whereas the responses to 5-HT were blocked. These data, combined with results from our previous study (Bertrand et al., 2000), suggest that the actions of endogenously released 5-HT would also be blocked by granisetron and, thus, that the granisetron-resistant response to ATP is not mediated by 5-HT. Thus, 5-HT, released by ATP, activates the same sensory nerve terminals as ATP itself, suggesting that 5-HT3 receptors and P2X receptors are colocalized on the same sensory nerve terminals.
Similar receptors are on the cell bodies of enteric sensory neurons
P2X receptors mediate fast synaptic transmission to descending interneurons and inhibitory motor neurons of the enteric nervous system (Johnson et al., 1999; LePard and Galligan, 1999; Bian et al., 2000). This is, however, the first study to show P2X-like depolarizations in identified sensory neurons. In the present study, we applied high concentrations of ATP to the cell bodies of AH–sensory neurons and found depolarizations associated with a reduction in membrane resistance. Of these neurons, three of four AH neurons responded to mucosal application of ATP with a train of APs; thus, they appeared to express the same receptor at the nerve terminal at the cell body. In almost half of the AH neurons tested, the depolarization was followed by a longer-lasting hyperpolarization associated with a decrease in membrane resistance and was increased in amplitude during depolarization of the cell. This hyperpolarization was similar to that seen previously by Katayama and Morita (1989) who did not, however, find a fast P2X-like depolarization of AH neurons as was demonstrated here.
ATP acts at a novel P2X receptor subtype
Several lines of evidence suggest that ATP activates the mucosal processes of AH neurons through a P2X receptor rather than a P2Y receptor or a P1 adenosine receptor. First, agonists active at P2X receptors ATP and ATP-γ-S reliably elicited responses, whereas the P2Y-selective agonist 2-methylthio-ATP did not. Interestingly, α,β-methylene ATP, which is also a P2X agonist, was only weakly active. Second, PPADS, a P2 receptor antagonist, blocked both ATP- and ATP-γ-S-elicited responses. Another P2 receptor antagonist, suramin, was not effective but appeared to potentiate some ATP-evoked responses; suramin is known to block the ectonucleotidases that cause degradation of ATP to adenosine (Ralevic and Burnstock, 1998). Third, either ATP or α,β-methylene ATP could be used to desensitize the ATP-evoked response. Finally, only relatively high concentrations of ATP were effective, and the response had a short latency, suggesting a low-affinity, ligand-gated receptor such as the P2X receptor.
Barajas-Lopez et al. (1996), working with cultured myenteric neurons of unknown functional class (although they were likely to be AH-sensory neurons), demonstrated a fast P2X-mediated current with several similarities in pharmacology to the receptor described here. For example, α,β-methylene ATP was only a weak agonist at both receptors, and both were insensitive to blockade by suramin.
Together, these data suggest that ATP acts on intrinsic sensory neurons through a P2X receptor with a pharmacology not seen for the homomeric receptors studied thus far (Burnstock and Wood, 1996). Lack of a correlation with known P2X receptor types is not unusual for native receptors, which are likely to be assembled from several different subunit types (Lewis et al., 1995; Torres et al., 1999; Patel et al., 2001). Recent evidence suggests that guinea pig pelvic neurons express a P2X receptor with characteristics not seen in rat pelvic neurons but similar to that reported here (Zhong et al., 2001). Our findings extend this observation to the enteric nervous system of the guinea pig and suggest that guinea pigs may express a P2X receptor subtype with characteristics not seen in rat or human receptors identified to date. We conclude that myenteric AH neurons express a novel subtype of P2X receptor, the study of which should be facilitated by its presence on both the soma and terminals.
ATP may act as a sensory mediator
In this study, we present two converging lines of evidence that ATP acts as a sensory mediator in gastrointestinal sensory transduction: demonstration that the intrinsic sensory nerve terminals express functional excitatory P2X receptors and that ATP can excite local reflex pathways mediating inhibition in the circular muscle. The data also suggest that enterochromaffin cells in the guinea pig ileum possess excitatory P2X receptors that mediate an increase in 5-HT release (Racké et al., 1996) and that a large proportion of the sensory neurons activated by ATP are also activated by 5-HT. Thus, ATP may act either directly or indirectly to excite the mucosal terminals of intrinsic sensory neurons.
Mucosal ATP also excites local interneurons and motor neurons and, because these neurons do not project to the mucosa, this is presumably secondary to the activation of intrinsic sensory neurons. The monosynaptic and polysynaptic reflexes that are initiated control the excitability of the neighboring circular muscle. ATP-evoked reflexes appear to be similar to those that would be initiated by physiological, or pathophysiological, activation of the sensory nerve terminals by an abrupt change in the chemical environment of the lumen, as when contents are first pushed into a new region, by distortion of the villi or by the arrival of an inflammatory agent. This then raises the question as to what may release ATP and from where.
In preliminary experiments, we found that blockade of P2X receptors in the lumen with PPADS (60 μm) had little effect on the threshold for initiation of peristaltic reflexes by increased luminal pressure (P. P. Bertrand, unpublished observations). This suggests that stretching the intestinal wall does not release sufficient ATP to excite the mucosal P2X receptors, which contrasts with the bladder (Vlaskovska et al., 2001). This stimulus is unlikely to excite enteroendocrine cells in the mucosa, however, so the possibility that these cells mediate sensory transduction via ATP remains open. Interestingly, it has been suggested that the serotonin storing enterochromaffin cells do not contain ATP (Tamir and Gershon, 1990), although other types of enteroendocrine cells may. An alternative source of ATP would be the mast cells that are prominent in the intestinal mucosa. Activation of these cells markedly enhances intestinal motility and secretion (Cooke, 1994), making them a prime candidate for release of pathophysiological sensory mediators. Thus, it may be that ATP is a mediator of chemosensory information or nociception in the intestine, as has been more generally suggested (Burnstock, 2001).
Conclusions
We found that the mucosal processes of intrinsic sensory neurons in the guinea pig small intestine generate APs in response to ATP acting via excitatory P2X receptors. ATP released within the mucosa may, thus, initiate or enhance reflexes in the guinea pig small intestine. ATP may be a sensory mediator in the guinea pig ileum and in other gastrointestinal tissues.
Footnotes
This work was supported by National Health and Medical Research Council (Australia) Grant 114103 and Fellowship 007703 (to P.P.B.). We thank Prof. John Furness and Prof. Marcello Costa for helpful comments.
Correspondence should be addressed to Dr. Paul P. Bertrand, Department of Physiology, University of Melbourne, Parkville, Victoria 3010, Australia. E-mail: p.bertrand@unimelb.edu.au.
REFERENCES
- 1.Barajas-Lopez C, Huizinga JD, Collins SM, Gerzanich V, Espinosa-Luna R, Peres AL. P2X-purinoceptors of myenteric neurones from the guinea-pig ileum and their unusual pharmacological properties. Br J Pharmacol. 1996;119:1541–1548. doi: 10.1111/j.1476-5381.1996.tb16070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertrand PP, Galligan JJ. Alfaxalone, pentobarbital and diazepam potentiate γ-aminobutyric acid-induced depolarizations in single myenteric neurons of guinea pig intestine. J Pharmacol Exp Ther. 1992;262:677–682. [PubMed] [Google Scholar]
- 3.Bertrand PP, Kunze WAA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;273:G422–G435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand PP, Kunze WA, Bornstein JC, Furness JB. Electrical mapping of the projections of intrinsic primary afferent neurons to the mucosa of the guinea-pig small intestine. Neurogastroenterol Motil. 1998;10:533–541. doi: 10.1046/j.1365-2982.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand PP, Kunze WAA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 6.Bian X-C, Bertrand PP, Bornstein JC. P2X receptors mediate synaptic transmission to inhibitory motor neurons in descending reflexes in the guinea-pig ileum. J Physiol (Lond) 2000;528:551–560. doi: 10.1111/j.1469-7793.2000.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland-Ward PA, Humphrey PP. P2X receptors mediate ATP-induced primary nociceptive neurone activation. J Auton Nerv Syst. 2000;81:146–151. doi: 10.1016/s0165-1838(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein JC, Furness JB, Kunze WAA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J Auton Nerv Syst. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 11.Cooke HJ. Neuroimmune signaling in regulation of intestinal ion transport. Am J Physiol. 1994;266:G167–G178. doi: 10.1152/ajpgi.1994.266.2.G167. [DOI] [PubMed] [Google Scholar]
- 12.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 13.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 14.Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 15.Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galligan JJ, LePard, Schneider, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 17.Gershon MD. Enteric nervous system: neural connections, neurotransmitters, and the function of 5-hydroxytryptamine. In: Kamm MA, Lennard-Jones JE, editors. Gastrointestinal transit: pathophysiology and pharmacology. Wrightson Biomedical Publishing; Petersfield, UK: 1991. pp. 21–32. [Google Scholar]
- 18.Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol (Lond) 1998;509:717–727. doi: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson PJ, Shum OR, Thornton PD, Bornstein JC. Evidence that inhibitory motor neurons of the guinea-pig small intestine exhibit fast excitatory synaptic potentials mediated via P2X receptors. Neurosci Lett. 1999;266:169–172. doi: 10.1016/s0304-3940(99)00275-x. [DOI] [PubMed] [Google Scholar]
- 20.Katayama Y, Morita K. Adenosine 5′-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. J Physiol (Lond) 1989;408:373–390. doi: 10.1113/jphysiol.1989.sp017464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 22.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol (Lond) 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- 25.Kunze WA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- 26.LePard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am J Physiol. 1999;276:G529–G538. doi: 10.1152/ajpgi.1999.276.2.G529. [DOI] [PubMed] [Google Scholar]
- 27.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 28.Li ZS, Furness JB. Immunohistochemical localization of cholinergic markers in putative intrinsic primary afferent neurons of the guinea-pig small intestine. Cell Tissue Res. 1998;294:35–43. doi: 10.1007/s004410051154. [DOI] [PubMed] [Google Scholar]
- 29.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MK, Khakh BS, Henderson G. Properties of native P2X receptors in rat trigeminal mesencephalic nucleus neurones: lack of correlation with known, heterologously expressed P2X receptors. Neuropharmacology. 2001;40:96–105. doi: 10.1016/s0028-3908(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 31.Racké K, Reimann A, Schwörer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1996;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 32.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 33.Richardson A, Delbridge AT, Brown NJ, Rumsey RD, Read NW. Short chain fatty acids in the terminal ileum accelerate stomach to caecum transit time in the rat. Gut. 1991;32:266–269. doi: 10.1136/gut.32.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanger GJ. 5-hydroxytryptamine and functional bowel disorders. Neurogastroenterol Motil. 1996;8:319–331. doi: 10.1111/j.1365-2982.1996.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 35.Schemann M, Ehrlein HJ. Postprandial patterns of canine jejunal motility and transit of luminal content. Gastroenterology. 1986;90:991–1000. doi: 10.1016/0016-5085(86)90878-4. [DOI] [PubMed] [Google Scholar]
- 36.Silinsky EM, Gerzanich V, Vanner SM. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992;106:762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song Z-M, Brookes SJH, Costa M. All calbindin-immunoreactive myenteric neurons project to the mucosa of the guinea-pig small intestine. Neurosci Lett. 1994;180:219–222. doi: 10.1016/0304-3940(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 38.Spencer NJ, Walsh M, Smith TK. Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol (Lond) 2000;522:321–331. doi: 10.1111/j.1469-7793.2000.t01-1-00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiller RC. Intestinal absorptive function. Gut. 1994;35:S5–S9. doi: 10.1136/gut.35.1_suppl.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamir H, Gershon MD. Serotonin-storing secretory vesicles. Ann NY Acad Sci. 1990;600:53–66. doi: 10.1111/j.1749-6632.1990.tb16872.x. [DOI] [PubMed] [Google Scholar]
- 41.Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- 42.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood JN, Docherty R. Chemical activators of sensory neurons. Annu Rev Physiol. 1997;59:457–482. doi: 10.1146/annurev.physiol.59.1.457. [DOI] [PubMed] [Google Scholar]
- 44.Zhong Y, Dunn PM, Burnstock G. Multiple P2X receptors on guinea-pig pelvic ganglion neurons exhibit novel pharmacological properties. Br J Pharmacol. 2001;132:221–233. doi: 10.1038/sj.bjp.0703778. [DOI] [PMC free article] [PubMed] [Google Scholar]