Abstract
The dynamics of dopamine receptor signaling efficacy were characterized in developing mice by measuring striatal c-Fos expression after dopaminergic agonist treatment at postnatal day 4 (P4) to P18. Control mice and mutant mice, in which dopamine production is inactivated in dopaminergic neurons by gene targeting, were treated with saline; a synthetic dopamine precursor,l-3,4-dihydroxyphenylalanine (l-DOPA) methyl ester; a direct dopamine D1 receptor agonist,N-allyl-SKF 38393; or a dopamine reuptake inhibitor, cocaine. l-DOPA methyl ester treatment failed to induce striatal c-Fos immunoreactivity in control and mutant mice deficient in dopamine production at P4 and P6 compared with saline treatment. However, at P10 through P18 it induced abundant c-Fos expression in mutants. At these later stages, c-Fos expression remained at basal levels in control mice after l-DOPA methyl ester treatment. Control and mutant mice responded to D1 receptor agonist administration to a similar degree at P4 and P6, but the responses were greatly enhanced in mutants at later stages. Cocaine treatment elicited expression in control mice at P10 through P18 but not at P4 and P6. Mutant mice were largely unresponsive to cocaine treatment. The results suggest that striatal dopamine receptors are capable of transducing extracellular signals at P4 and P6, but dopaminergic neurotransmission begins thereafter. Dopaminoceptive neurons appear to reduce their sensitivity to dopamine as dopaminergic terminals innervate the striatum and functional neurotransmission begins.
Keywords: aromatic l-amino acid decarboxylase, c-Fos, cocaine, D1 receptor, development, dopamine, dopamine-deficient, l-DOPA, mouse, μ-opioid receptor, N-allyl-SKF 38393, postnatal, striatum, tyrosine hydroxylase
The development of the dopaminergic neurotransmission system has been investigated extensively. Proteins involved in catecholamine synthesis, such as tyrosine hydroxylase (TH) and aromatic l-amino acid decarboxylase (AADC), are first expressed in the rodent midbrain at embryonic day 11.5 (E11.5); nerve terminals containing TH and AADC reach the striatum by E19 (Jaeger, 1986; Foster et al., 1988). Other presynaptic dopaminergic markers, including the dopamine transporter, which is required for the reuptake of released dopamine, and the vesicular monoamine transporters (VMATs), which load synaptic vesicles with dopamine, are expressed by postnatal day 1 (P1) (Leroux-Nicollet et al., 1990; Jung and Bennett, 1996). Dopamine can be detected in the striatum at P0; it increases sharply from P1 to P5 (Restani et al., 1990). The concentration of the dopamine metabolites dihydroxyphenylacetic acid and homovanillic acid begins to increase in the striatum at P5 (Restani et al., 1990). Direct measurements of released dopamine by microdialysis indicate that dopamine secretion begins after P5 and increases to adult levels by P10 (Andersen and Gazzara, 1993). D1 and D2 receptors can be detected in the striatum at E18 (Jung and Bennett, 1996). D1 receptors initially exhibit an enriched patchy expression in neurons that comprise the striosomes (Murrin and Zeng, 1989; Liu and Graybiel, 1996). After P6, D1 receptor expression becomes more uniform throughout the striatum (Murrin and Zeng, 1989). D1 receptors are functionally coupled to G-proteins and can elicit changes in intracellular signaling as early as E19 (Jung and Bennett, 1996; Shearman et al., 1997). Although the development of various components of the dopaminergic system has been described previously, the ontogeny of both presynaptic dopaminergic release and postsynaptic propagation of dopaminergic signals has not been thoroughly documented in an in vivo setting that does not rely on responses to electrically evoked dopamine release.
Several studies have demonstrated the inductive effects of D1 receptor agonists, cocaine, or amphetamine on immediate early gene expression during postnatal development in intact animals (Weaver et al., 1992; Arnauld et al., 1995; Shearman et al., 1997; Snyder-Keller and Keller, 1998). In addition, many investigators have shown that selective lesioning of dopaminergic neurons by intracranial delivery of the catecholaminergic neurotoxin 6-hydroxydopamine results in the sensitization of D1 receptors to agonists in terms of immediate early gene expression (Robertson et al., 1989; Paul et al., 1992;LaHoste et al., 1993). One hypothesis is that during early postnatal life the striatum is highly sensitive to dopaminergic agonists before the onset of dopamine release, and that this situation can be mimicked in adults after dopamine depletion. This report describes the onset of functional dopaminergic release and signaling during the postnatal period in an in vivo mouse model of dopamine depletion in which dopaminoceptive neurons display a high sensitivity to dopaminergic agonists (Kim et al., 2000). The striatum of dopamine-deficient (Th−/−;DbhTh/+) mice provides an ideal system for the characterization of the developmental role of dopamine in striatal organization and function, because dopamine production is specifically and stably removed from otherwise intact dopaminergic neurons and can be restored by administration of the dopamine precursorl-3,4-dihydroxyphenylalanine (l-DOPA).
MATERIALS AND METHODS
Mice. A total of 137 control and mutant mice from 26 litters were treated and analyzed in this study. All mice were used in accordance with the guidelines for animal care and experimentation established by the National Institutes of Health and the University of Washington Animal Care Committee.Th−/−;DbhTh/+mice were bred as described previously (Zhou and Palmiter, 1995;Zhou et al., 1995). Null alleles at the Th gene locus, whose product is rate-limiting for catecholamine synthesis, were introduced by gene targeting. TH function was restored in the noradrenergic and adrenergic cells of Th−/− mice by targeting the Th coding region downstream of the transcriptional regulatory elements of the Dbh gene locus. Control andTh−/−;DbhTh/+mice were bred by intercrossingTh+/−;DbhTh/+mice and were on a mixed C57BL/6 × 129/SvEv genetic background. Control mice included animals that wereTh+/+,Th+/−,Dbh+/+,DbhTh/+, and combinations of these genotypes. The day of birth was designated P0. Tail DNA was isolated, and genotypes were determined in retrospect by Southern blot analysis using StuI polymorphisms at both the Th andDbh loci. Blots were hybridized with aKpnI/SacII fragment probe containing part of intron 1 and part of exon 2 of the Th genomic sequence (Zhou and Palmiter, 1995; Zhou et al., 1995).
Drug treatments. All drugs were injected intraperitoneally in a volume of 10 μl/gm body weight. Mice were treated with 0.9% saline, l-DOPA methyl ester hydrochloride (10 mg/ml with 2.5 mg/ml ascorbic acid in PBS; Sigma, St. Louis, MO), (±)-N-allyl-SKF 38393 hydrobromide (0.25 mg/ml in 0.9% saline; Sigma/RBI, St. Louis, MO), and (−)-cocaine HCl (2 mg/ml in 0.9% saline; Sigma/RBI). Mice were injected using 1 ml plastic syringes and 30 gauge needles. The injection volumes ranged from 0.02 to 0.14 ml. Mice were weighed before and after treatment to assess the accuracy of the volume delivered.
Immunohistochemistry. Mice were treated with the drugs and then decapitated after 2 hr. Brains were dissected, immersed overnight in 4% paraformaldehyde, cryoprotected in 30% sucrose, and frozen in supercooled isopentane. Free-floating coronal sections (50 μm) were immunostained using rabbit polyclonal antisera directed against c-Fos (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA), μ-opioid receptor (MOR-1, 1:5000; DiaSorin, Stillwater, MN), or AADC (1:1000; Affiniti Research Products, Ltd., Exeter, UK). A rat monoclonal antibody was used to detect dopamine D1 receptors (1:1000; Sigma). For c-Fos, MOR-1, and AADC antibodies, immunoreactivity was revealed using a biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA), streptavidin-conjugated horseradish peroxidase (Zymed Laboratories, San Francisco, CA), and nickel-enhanced, diaminobenzidine chromogen (Sigma). For the dopamine D1 receptor antibody, a biotinylated rabbit anti-rat IgG secondary antibody (Vector Laboratories) that was preadsorbed to remove antibodies that reacted with mouse Igs was used. Sections were mounted on slides, coverslipped, and photographed. c-Fos immunoreactive nuclei were quantitated from three striatal images for each mouse using Scion Image for Windows, release 4.02 (Scion Corp., Frederick, MD). Images were cropped such that only the striatum was visible; immunoreactive nuclei were counted using the computer software. The software excluded pixels with intensities below a preset threshold value, included only groups of contiguous pixels fitting within a user-designated size range, and totaled the number of remaining particles.
Statistical analysis. The low numbers ofTh−/−;DbhTh/+mice obtained as a result of the complex breeding scheme and the small litter sizes caused by lethality in embryos lacking noradrenergic function (Thomas et al., 1995) necessitated the pooling of c-Fos immunohistochemistry results from mice at P4 and P6 and mice at P10, P14, and P18 to achieve sufficient statistical power. ANOVA andpost hoc tests were performed using Statistica for Windows, release 6.0 (StatSoft Inc., Tulsa, OK).
RESULTS
Saline-elicited c-Fos immunoreactivity and MOR-1 expression
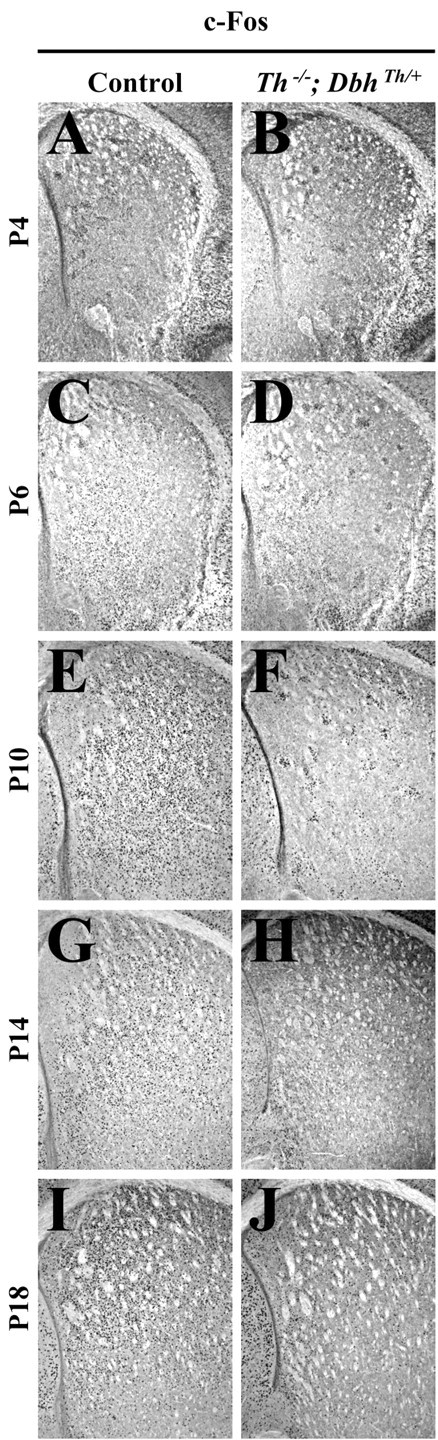
Control and mutant (Th−/−;DbhTh/+) mice were injected with saline at P4, P6, P10, P14, and P18; c-Fos immunoreactivity was assessed in the striatum 2 hr later. c-Fos-positive nuclei from images of coronal sections of the striatum as shown were counted using computer software. Similar basal levels of nuclear immunoreactivity were observed in the striatum of saline-treated control and mutant animals at each stage (see Figs.1A–J, 5A). At P4 and P6, c-Fos immunoreactivity was enriched in clusters of cells resembling striosomes (Fig. 1C–F). The development of striosomes was characterized by examining the striatal expression of MOR-1 (Johnston et al., 1990). MOR-1 immunoreactivity in control andTh−/−;DbhTh/+mice was similar at each stage (Fig. 1K–T). At P4, weak clusters of MOR-1 expression were evident; the number and size of clusters were augmented at P6 and persisted from P10 through P18.
Fig. 1.
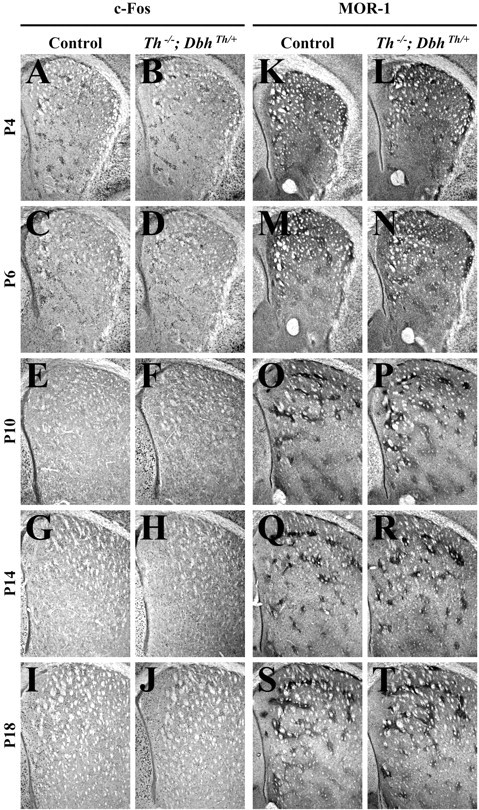
Striatal c-Fos and MOR-1 immunoreactivity 2 hr after treatment with saline. A, C, E, G, I, K, M, O, Q, S, Representative control coronal sections showing the striatum (top, dorsal; right, lateral). B, D, F, H, J, L, N, P, R, T, RepresentativeTh−/−;DbhTh/+sections. A, B, c-Fos immunoreactivity in P4 striatum.C, D, c-Fos immunoreactivity in P6 striatum. E, F, c-Fos immunoreactivity in P10 striatum. G, H, c-Fos immunoreactivity in P14 striatum. I, J, c-Fos immunoreactivity in P18 striatum. K, L, MOR-1 immunoreactivity in P4 striatum. M, N, MOR-1 immunoreactivity in P6 striatum. O, P, MOR-1 immunoreactivity in P10 striatum. Q, R, MOR-1 immunoreactivity in P14 striatum. S, T, MOR-1 immunoreactivity in P18 striatum.
Fig. 5.
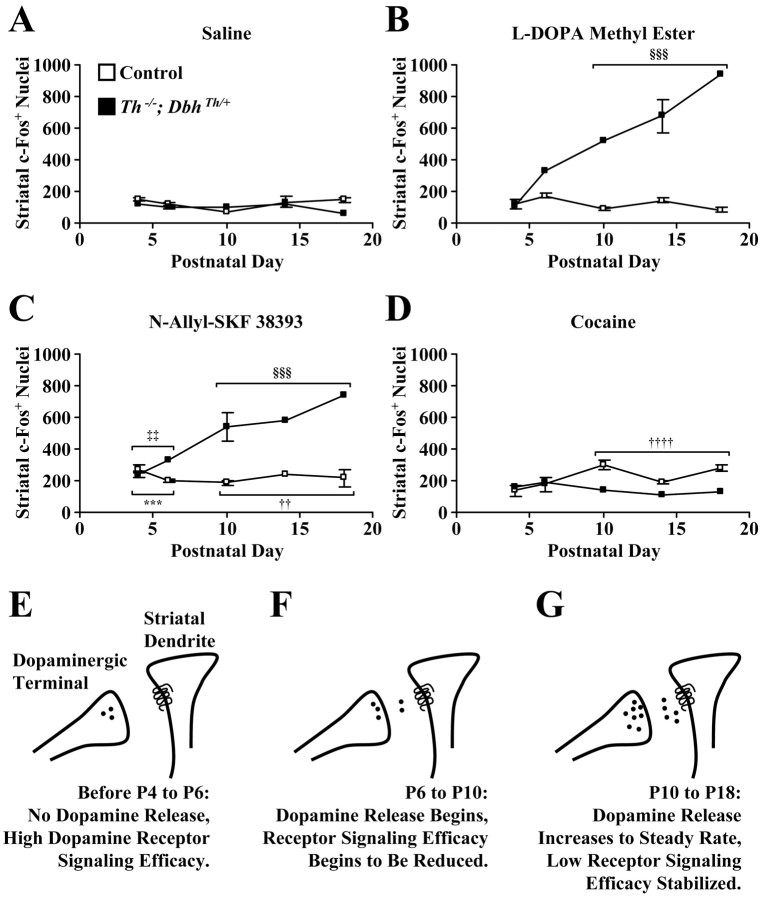
Quantitation of striatal c-Fos-positive nuclei 2 hr after treatments and a homeostatic model of dopamine receptor signaling efficacy during postnatal development. Values represent c-Fos-positive nuclei quantitated from images of entire striatal sections and are reported as means ± SEM at various postnatal stages. A, Values after saline treatment.B, Values after l-DOPA methyl ester (100 mg/kg) treatment. C, Values afterN-allyl-SKF 38393 (2.5 mg/kg) treatment.D, Values after cocaine (20 mg/kg) treatment. When mice at P4 and P6 and mice at P10, P14, and P18 were grouped together, three-way ANOVA revealed a significant interaction of phenotype, age, and drug (F = 20.99; df = 3, 121;p < 0.0001). Investigation of the causes of the complex interaction by two-way ANOVA revealed a significant interaction of age and drug for control mice (F = 9.24; df = 3, 92; p < 0.0001) and mutant mice (F = 7.25; df = 3, 29; p< 0.0001). ***p < 0.001, greater than saline response of pooled P4 and P6 control mice by one-tailed Dunnett's test. ††p < 0.01, ††††p< 0.0001, greater than saline response of pooled P10, P14, and P18 control mice by one-tailed Dunnett's test. ‡‡p< 0.01, greater than saline response of pooled P4 and P6Th−/−;DbhTh/+mice by one-tailed Dunnett's test. §§§p< 0.001, greater than saline response of pooled P10, P14, and P18Th−/−;DbhTh/+mice by one-tailed Dunnett's test. E, Before P4 and P6, midbrain dopaminergic terminals migrate into the striatum and produce dopamine, and some striatal neurons already express dopamine receptors. Dopamine receptor signaling efficacy is high. F, Between P6 and P10, presynaptic dopaminergic release and the propagation of signals in postsynaptic neurons begin. Dopamine signaling efficacy is reduced. G, From P10 to P18 and during adulthood, dopaminergic neurotransmission continues at a steady rate. Dopamine receptor signaling efficacy stabilizes at a low plateau.White squares, Control mice; black squares,Th−/−;DbhTh/+mice.
l-DOPA methyl ester-elicited c-Fos immunoreactivity and AADC expression
l-DOPA methyl ester (100 mg/kg) was administered to control andTh−/−;DbhTh/+mice; striatal c-Fos immunoreactivity was assessed 2 hr after treatment. This dose restores striatal dopamine levels in adultTh−/−;DbhTh/+mice to 24% of the levels of control mice (Szczypka et al., 1999).l-DOPA methyl ester treatment did not induce striatal c-Fos expression at P4 and P6 in either control orTh−/−;DbhTh/+mice (see Figs. 2A–D,5B). However, a regional analysis, in which dorsomedial, dorsolateral, ventromedial, and ventrolateral striatal quadrants were examined separately, showed that l-DOPA methyl ester treatment significantly elevated c-Fos expression in the ventromedial striatum ofTh−/−;DbhTh/+mice at P4 and P6 (data not shown). In control mice,l-DOPA methyl ester administration did not significantly enhance c-Fos expression above that observed with saline treatment at P10, P14, and P18 (Fig. 2E,G,I). However, inTh−/−;DbhTh/+mice, l-DOPA methyl ester administration induced c-Fos expression 433, 442, and 1363% above that observed with saline treatment at P10, P14, and P18, respectively (Fig.2F,H,J).
Fig. 2.
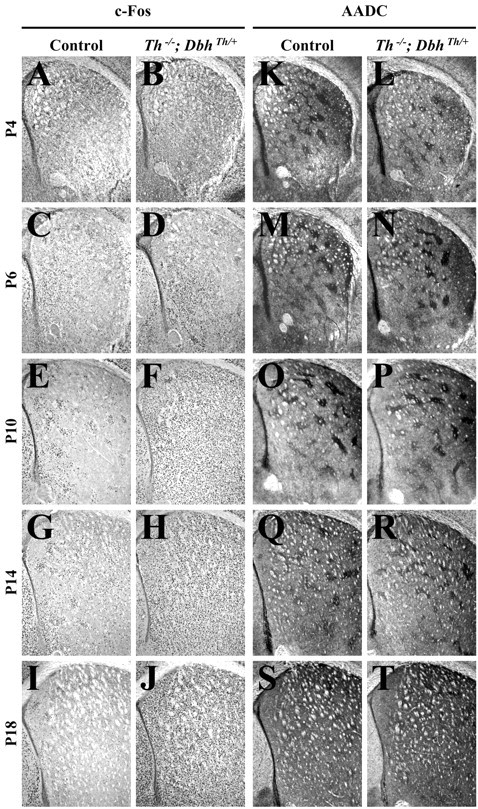
Striatal c-Fos and AADC immunoreactivity 2 hr after treatment with l-DOPA methyl ester (100 mg/kg).A, C, E, G, I, K, M, O, Q, S, Representative control coronal sections showing the striatum (top, dorsal;right, lateral). B, D, F, H, J, L, N, P, R, T, RepresentativeTh−/−;DbhTh/+sections. A, B, c-Fos immunoreactivity in P4 striatum.C, D, c-Fos immunoreactivity in P6 striatum. E, F, c-Fos immunoreactivity in P10 striatum. G, H, c-Fos immunoreactivity in P14 striatum. I, J, c-Fos immunoreactivity in P18 striatum. K, L, AADC immunoreactivity in P4 striatum. M, N, AADC immunoreactivity in P6 striatum. O, P, AADC immunoreactivity in P10 striatum. Q, R, AADC immunoreactivity in P14 striatum. S, T, AADC immunoreactivity in P18 striatum.
AADC expression was examined in striatal sections as a marker of presynaptic dopaminergic terminals. AADC immunoreactivity in control andTh−/−;DbhTh/+mice was similar at each stage (Fig. 2K–T). At P4, weak clusters of AADC expression were evident in the lateral striatum. The number and size of clusters was augmented from P6 to P10; AADC expression increased outside of clustered terminals from P14 to P18.
N-allyl-SKF 38393-elicited c-Fos immunoreactivity and dopamine D1 receptor expression
The D1-like receptor agonistN-allyl-SKF 38393 (2.5 mg/kg) was administered to control andTh−/−;DbhTh/+mice; striatal c-Fos immunoreactivity was assessed 2 hr after treatment. A preliminary experiment indicated that this dose elicited a submaximal c-Fos response in control mice. At a 12.5 mg/kg dose, both control and mutant mice responded equivalently at P18 (data not shown). A submaximal dose (2.5 mg/kg) was used to reveal potential differences between control and mutant mice. The D1 receptor agonist induced striatal c-Fos expression in both control andTh−/−;DbhTh/+mice at each stage (see Figs.3A–J, 5C). In control mice, D1 receptor agonist administration induced c-Fos expression 87, 64, 155, 75, and 49% above that observed with saline treatment at P4, P6, P10, P14, and P18, respectively (Fig.3A,C,E,G,I). InTh−/−;DbhTh/+mice, D1 receptor agonist administration induced expression 108, 236, 458, 368, and 1055% above that observed with saline treatment at P4, P6, P10, P14, and P18, respectively (Fig.3B,D,F,H,J). Although regional analysis revealed significant induction of immunoreactivity in the ventromedial and ventrolateral striatum for control andTh−/−;DbhTh/+mice at both P4 and P6 and P14, P10, and P18, significant induction in the dorsal striatum was observed only in the dorsolateral striatum of control mice at P4 and P6 andTh−/−;DbhTh/+mice at P10, P14, and P18 (data not shown).
Fig. 3.
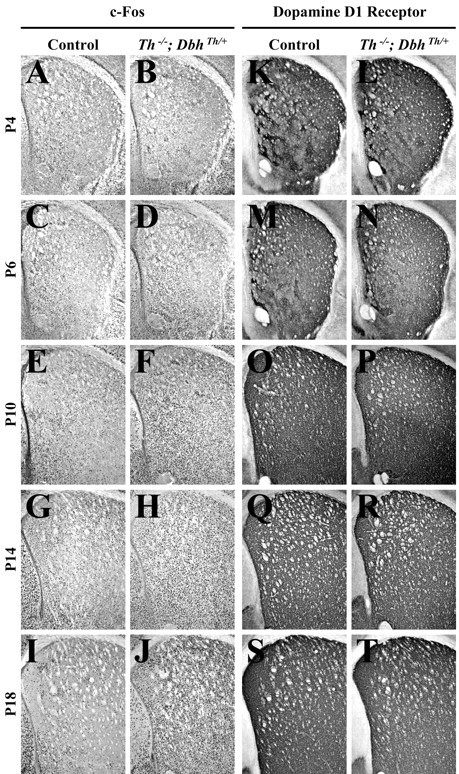
Striatal c-Fos and dopamine D1receptor immunoreactivity 2 hr after treatment withN-allyl-SKF 38393 (2.5 mg/kg). A, C, E, G, I, K, M, O, Q, S, Representative control coronal sections showing the striatum (top, dorsal; right, lateral).B, D, F, H, J, L, N, P, R, T, RepresentativeTh−/−;DbhTh/+sections. A, B, c-Fos immunoreactivity in P4 striatum.C, D, c-Fos immunoreactivity in P6 striatum. E, F, c-Fos immunoreactivity in P10 striatum. G, H, c-Fos immunoreactivity in P14 striatum. I, J, c-Fos immunoreactivity in P18 striatum. K, L, D1receptor immunoreactivity in P4 striatum. M, N, D1 receptor immunoreactivity in P6 striatum. O, P, D1 receptor immunoreactivity in P10 striatum.Q, R, D1 receptor immunoreactivity in P14 striatum. S, T, D1 receptor immunoreactivity in P18 striatum.
D1 receptor expression was examined in striatal sections as a marker of postsynaptic dopaminoceptive neurons. D1 receptor immunoreactivity in control andTh−/−;DbhTh/+mice was similar at each stage (Fig. 3K–T). At P4, weak clusters of D1 receptor expression were evident in the lateral striatum. The number and size of clusters were augmented from P6 to P10; D1 receptor expression increased outside of clustered terminals from P14 to P18.
Cocaine-elicited c-Fos immunoreactivity
Control andTh−/−;DbhTh/+mice were also treated with cocaine (20 mg/kg), a selective monoaminergic reuptake inhibitor; c-Fos immunoreactivity was assessed in the striatum 2 hr later. This dose was chosen because treatment with higher doses caused mice at P4 and P6 to die. In control mice, cocaine administration did not enhance c-Fos expression at P4 and P6, but it induced responses 304, 43, and 94% above those observed with saline treatment at P10, P14, and P18, respectively (Figs. 4A,C,E,G,5D). Cocaine significantly elevated c-Fos expression in the dorsolateral, ventromedial, and ventrolateral striatum of control mice at P10, P14, and P18 (data not shown). InTh−/−;DbhTh/+mice, cocaine administration induced some striatal c-Fos expression. However, the responses of mutants to cocaine were less than those of control mice at P10, P14, and P18 (Fig.4F,H,J). Cocaine can have nonselective effects at monoamine transporters other than the dopamine transporter, such as the serotonin and norepinephrine transporters (Kuhar et al., 1991;Rocha et al., 1998), so a component of the immediate early gene response to cocaine can be independent of dopamine secretion.
Fig. 4.
Striatal c-Fos immunoreactivity 2 hr after treatment with cocaine (20 mg/kg). A, C, E, G, I, Representative control coronal sections showing the striatum (top, dorsal; right, lateral). B, D, F, H, J, RepresentativeTh−/−;DbhTh/+sections. A, B, c-Fos immunoreactivity in P4 striatum.C, D, c-Fos immunoreactivity in P6 striatum. E, F, c-Fos immunoreactivity in P10 striatum. G, H, c-Fos immunoreactivity in P14 striatum. I, J, c-Fos immunoreactivity in P18 striatum.
Behavioral observations and body weight data
There were few visible effects on the motor function of mice treated with various dopaminergic compounds from P4 to P10.l-DOPA methyl ester and N-allyl-SKF 38393 induced locomotion, rearing, and stereotyped grooming movements at P14 and P18; the effects were more pronounced in mutant mice. Cocaine depressed activity at P14 and P18. The body weights of all of the control and mutant mice used in this study were similar at P4 and P6 (means ± SEM; P4 control, 3.39 ± 0.07 gm, n= 22; P4 mutant, 3.21 ± 0.18 gm, n = 9; P6 control, 4.70 ± 0.15 gm, n = 20; P6 mutant, 4.32 ± 0.31 gm, n = 9). However, the body weights of control and mutant mice began to diverge at P10 (P10 control, 8.19 ± 0.13 gm, n = 20; P10 mutant, 6.04 ± 0.22 gm, n = 8). At P14, control mice weighed 9.17 ± 0.35 gm (n = 18), and mutant mice weighed 5.41 ± 0.38 gm (n = 7). By P18, control mice (10.18 ± 0.40 gm, n = 20) weighed almost twice as much as mutant mice (5.49 ± 0.55 gm, n = 4). Two-way ANOVA revealed a significant interaction of phenotype and age in terms of body weight (F = 17.59; df = 4, 127;p < 0.0001). Mean body weights between control and mutant mice were significantly different at P10, P14, and P18 (p < 0.01, p < 0.0001,p < 0.0001, respectively, by Scheffé's test).
DISCUSSION
The results suggest that dopamine is not required for the structural organization of the dopaminergic neurotransmission machinery, but that dopamine is necessary for normal maturation of striatal dopaminoceptive neurons. The role of dopamine in striatal development was explored by measuring c-Fos expression after treatment with dopaminergic agonists. This approach was useful, because activation of postsynaptic D1 receptors, which are coupled to Gs- and Golf-proteins, leads to stimulation of adenylyl cyclase (Hervé et al., 1993). Immediate early gene induction reflects acute elevations of cAMP-dependent signaling within striatal neurons (Konradi et al., 1996; Liu and Graybiel, 1996; Andersson et al., 2001), although it has been shown that c-fostranscriptional activation requires convergent cAMP- and calcium-dependent signals (Robertson et al., 1995; Konradi et al., 1996). Thus, striatal c-Fos induction can be used as an indicator of D1 receptor signal transduction in vivo.
At P4 and P6, both control andTh−/−;DbhTh/+mice displayed increased c-Fos expression in response to the direct D1 receptor agonist, indicating that functional D1 receptor signaling can occur at these early stages. However, l-DOPA methyl ester failed to induce c-Fos expression at P4 and P6. l-DOPA methyl ester is presumably taken up into neurons, converted into dopamine in AADC-expressing cells, and loaded into synaptic vesicles by VMATs. The expression of c-Fos after acute l-DOPA methyl ester treatment ofTh−/−;DbhTh/+mice likely results from the activity-dependent release of dopamine from presynaptic neurons, the activation of postsynaptic D1 receptors, and the induction of cAMP-dependent gene transcription. The lack of c-Fos induction afterl-DOPA methyl ester treatment inTh−/−;DbhTh/+mice, even in the presence of functional D1receptors, suggests that dopaminergic neurotransmission has not reached significant levels at P4 and P6. However, a regional analysis of c-Fos expression showed that l-DOPA methyl ester treatment significantly elevated immunoreactivity in the ventromedial striatum of mutants at P4 and P6. In the ventromedial striatum, it may thus appear that dopamine release begins at or before P6 (Figs.1D, 2D). But cocaine failed to induce striatal c-Fos expression at P4 and P6 in control mice. Furthermore, significant cocaine responses were not observed in control mice at P4 and P6 when striatal quadrants were analyzed separately. The ability of cocaine to induce striatal c-Fos expression in control mice requires functional dopamine synthesis, release, activation of postsynaptic receptors, and reuptake through presynaptic transporters (Drago et al., 1996; Rocha et al., 1998), which are all hallmark features of dopaminergic neurotransmission. The absence of a cocaine-elicited response, although a D1 receptor agonist induced expression, suggests that dopaminergic neurotransmission does not reach significant levels until after P4 and P6 in the striatum (Fig. 5E).
At P10, P14, and P18, cocaine treatment elicited an immediate early gene response in the control striatum, indicating that dopamine release has begun by these stages (Fig. 5F). This idea is supported by the observation that l-DOPA methyl ester also evoked abundant c-Fos expression in mutant mice at P10, P14, and P18. Thus, although the presynaptic machinery required for dopamine production, vesicular loading, and release and postsynaptic systems needed for dopaminergic signaling are present at P1 (Jaeger, 1986;Foster et al., 1988; Leroux-Nicollet et al., 1990; Restani et al., 1990; Andersen and Gazzara, 1993; Jung and Bennett, 1996), striatal dopaminergic neurotransmission apparently does not reach significant levels until after P6. This delay suggests that some factor not considered here limits the onset of functional synaptic transmission. This limiting factor could be electrical activity driven by afferents received by midbrain dopaminergic neurons. It is also possible that the development of other inputs to the striatum, such as cortical glutamatergic neurons, may be limiting, because glutamate receptor activation is believed to act in concert with dopamine signaling to induce c-Fos expression (Konradi et al., 1996). The responsiveness of D1 receptors in control mice declined from P10 through P18, as evidenced by the stable level of c-Fos induction after D1 receptor agonist administration even as the absolute number of D1 receptor-expressing striatal cells increased. The D1 agonist, which acts directly on postsynaptic neurons, elicited much more immunoreactivity in mutants compared with control mice at P10 through P18, indicating that striatal D1 receptor signaling efficacy is reduced in control mice as a consequence of the onset of functional dopaminergic neurotransmission after P6 (Fig.5G).
In control mice, clustered c-Fos expression at P10 and P14 was observed after l-DOPA methyl ester treatment, whereas in mutants, widespread striatal nuclear immunoreactivity was evident. Clustered c-Fos expression may represent enhanced cAMP/calcium signaling within striosomal neurons, which receive preferential presynaptic dopaminergic innervation (Roffler-Tarlov and Graybiel, 1987) and in which D1 receptors are enriched in their expression during perinatal life (Murrin and Zeng, 1989; Liu and Graybiel, 1996). One possibility is that this difference in the pattern of c-Fos expression between control and mutant mice is attributable to a requirement for dopamine in the formation and organization of striosomes. However, striosomes appeared to develop comparably in control and mutant mice, as assessed by examination of MOR-1 expression. Another possibility is that presynaptic terminals from midbrain afferents are disorganized in the striatum ofTh−/−;DbhTh/+mice. But dopaminergic differentiation and axonal guidance into the striatum also proceeded normally in control and mutant mice, with clustered AADC immunoreactivity appearing at early stages, followed by increased AADC expression in regions around clusters at later stages. A third possibility is that enriched D1 receptor localization in striosomes is disrupted in the mutant striatum, but D1 receptor expression appeared similar in both groups with enriched striosomal expression at P4 and P6, followed by more uniform distribution at later stages. A final possibility is that the responsiveness of striatal D1 receptors is dampened as functional dopaminergic neurotransmission begins. In the absence of dopaminergic input in the striatum ofTh−/−;DbhTh/+mice, the sensitivity of D1 receptors remained high throughout the striatum, and dopamine release induced widespread c-Fos expression after l-DOPA methyl ester treatment. D1 receptor abundance appeared similar in the control andTh−/−;DbhTh/+striatum, which is consistent with previous results showing that receptor levels in adult control and mutant mice are similar (Kim et al., 2000). The results are consistent with enhanced c-Fos responses observed in D1 receptor agonist- and cocaine-treated rats during early postnatal life and the subsequent decline of sensitivity to these drugs during adolescence (Weaver et al., 1992; Arnauld et al., 1995; Shearman et al., 1997; Snyder-Keller and Keller, 1998).
cAMP signaling contributes to striatal immediate early gene induction, but calcium-dependent signaling resulting from NMDA receptor activation is also important (Konradi et al., 1996). Previous electrophysiological studies have characterized the development of excitatory cortical inputs to striatal medium spiny neurons. The number of glutamatergic synapses increases until P18, but spontaneous electrical activity in the striatum is rare before P14 because of low cortical activity (Tepper et al., 1998). c-Fos expression elicited by dopaminergic agonists before P14 occurs against a stable background of low glutamatergic signaling. After P14, cortical excitatory input may play a role in shaping the responsiveness of dopamine receptors in terms of immediate early gene induction. The probability of glutamate release at striatal synapses is relatively high until P19, when it is reduced in a dopamine D2 receptor-dependent manner (Tang et al., 2001). Thus, dopamine secretion contributes to the development of glutamatergic synapses after P19. This change in glutamatergic function may in turn alter the responsiveness of dopamine receptors in terms of c-Fos expression.
The absence of behavioral responses to dopaminergic agonists at P4, P6, and P10 suggests that dopamine plays a minimal role in modulating motor activity at these stages. At P10, mutant mice had lower body weights than control mice. By P14, the severe hypoactive phenotype ofTh−/−;DbhTh/+mice becomes apparent (Zhou and Palmiter, 1995). Previous reports have shown that dopaminergic agonists can induce suckling behavior in young rodents (Fon et al., 1997), which is consistent with a role for dopamine in the ingestive behavior of pups or behaviors related to eliciting maternal care.
The hypothesis that synaptic formation and maintenance are dependent on activity-related neurotransmitter release has been addressed in several experimental systems (Shatz, 1996; Constantine-Paton and Cline, 1998;Sanes and Lichtman, 1999; Verhage et al., 2000). The results presented here suggest that dopaminergic neurotransmission is not absolutely required for the targeting of presynaptic neurons to their sites of innervation. Instead, the role of dopamine in developing synapses appears to be related to dampening the response of striatal postsynaptic receptors to the neurotransmitter itself such that a slowly shifting homeostatic relationship between the quantity of dopamine released and the efficacy of dopamine receptor signaling is established (Fig. 5E–G). Developing neuronal circuits are thought to achieve balanced synaptic transmission through the excessive elaboration of presynaptic contacts and the subsequent pruning of synapses to reduce neuronal information flow to appropriate levels (Purves and Lichtman, 1980). This in vivo study suggests that developing dopaminergic synapses are tuned by the reduction of the sensitivity of receptors in striatal neurons. It would be interesting to determine whether this type of modulation of postsynaptic sensitivity occurs at other developing synapses; if this were not the case, it would be interesting to understand what makes dopaminergic synapses peculiar.
Footnotes
This work was supported by a graduate research fellowship from the National Science Foundation (D.S.K.). We thank V. Denenberg for help with statistical analysis.
Correspondence should be addressed to Richard D. Palmiter, Department of Biochemistry, Howard Hughes Medical Institute, University of Washington, Health Sciences Building, Room J661E, 1959 Northeast Pacific Street, Seattle, WA 98195-7370. E-mail:palmiter@u.washington.edu.
REFERENCES
- 1.Andersen SL, Gazzara RA. The ontogeny of apomorphine-induced alterations of neostriatal dopamine release: effects on spontaneous release. J Neurochem. 1993;61:2247–2255. doi: 10.1111/j.1471-4159.1993.tb07466.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersson M, Konradi C, Cenci MA. cAMP response element-binding protein is required for dopamine-dependent gene expression in the intact but not the dopamine-denervated striatum. J Neurosci. 2001;21:9930–9943. doi: 10.1523/JNEUROSCI.21-24-09930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnauld E, Arsaut J, Tafani JA, Demotes-Mainard J. Dopaminergic control of gene transcription during striatal ontogeny: c-fos induction by D1 receptor activation in the developing striosomes. Brain Res Mol Brain Res. 1995;30:223–232. doi: 10.1016/0169-328x(95)00011-g. [DOI] [PubMed] [Google Scholar]
- 4.Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 5.Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 6.Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 7.Foster GA, Schultzberg M, Hökfelt T, Goldstein M, Hemmings HC, Jr, Ouimet CC, Walaas SI, Greengard P. Ontogeny of the dopamine and cyclic adenosine-3′:5′-monophosphate-regulated phosphoprotein (DARPP-32) in the pre- and postnatal mouse central nervous system. Int J Dev Neurosci. 1988;6:367–386. doi: 10.1016/0736-5748(88)90020-2. [DOI] [PubMed] [Google Scholar]
- 8.Hervé D, Rogard M, Lévi-Strauss M. Molecular analysis of the multiple Golf α subunit mRNAs in the rat brain. Brain Res Mol Brain Res. 1995;32:125–134. doi: 10.1016/0169-328x(95)00070-9. [DOI] [PubMed] [Google Scholar]
- 9.Jaeger CB. Aromatic l-amino acid decarboxylase in the rat brain: immunocytochemical localization during prenatal development. Neuroscience. 1986;18:121–150. doi: 10.1016/0306-4522(86)90183-1. [DOI] [PubMed] [Google Scholar]
- 10.Johnston JG, Gerfen CR, Haber SN, van der Kooy D. Mechanisms of striatal pattern formation: conservation of mammalian compartmentalization. Brain Res Dev Brain Res. 1990;57:93–102. doi: 10.1016/0165-3806(90)90189-6. [DOI] [PubMed] [Google Scholar]
- 11.Jung AB, Bennett JP., Jr Development of striatal dopaminergic function. I. Pre- and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Brain Res Dev Brain Res. 1996;94:109–120. doi: 10.1016/0165-3806(96)00033-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 15.LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leroux-Nicollet I, Darchen F, Scherman D, Costentin J. Postnatal development of the monoamine vesicular transporter in mesencephalic and telencephalic regions of the rat brain: a quantitative autoradiographic study with [3H]dihydrotetrabenazine. Neurosci Lett. 1990;117:1–7. doi: 10.1016/0304-3940(90)90110-u. [DOI] [PubMed] [Google Scholar]
- 17.Liu FC, Graybiel AM. Spatiotemporal dynamics of CREB phosphorylation: transient versus sustained phosphorylation in the developing striatum. Neuron. 1996;17:1133–1144. doi: 10.1016/s0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 18.Murrin LC, Zeng WY. Dopamine D1 receptor development in the rat striatum: early localization in striosomes. Brain Res. 1989;480:170–177. doi: 10.1016/0006-8993(89)91579-5. [DOI] [PubMed] [Google Scholar]
- 19.Paul ML, Graybiel AM, David JC, Robertson HA. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- 21.Restani P, Corsini E, Galimberti R, Galli CL. Postnatal ontogenesis of dopaminergic and serotoninergic systems in rat caudate nucleus. Pharmacol Res. 1990;22:343–350. doi: 10.1016/1043-6618(90)90732-s. [DOI] [PubMed] [Google Scholar]
- 22.Robertson HA, Peterson MR, Murphy K, Robertson GS. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res. 1989;503:346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- 23.Robertson LM, Kerppola TK, Vendrell M, Luk D, Smeyne RJ, Bocchiaro C, Morgan JI, Curran T. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron. 1995;14:241–252. doi: 10.1016/0896-6273(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 24.Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 25.Roffler-Tarlov S, Graybiel AM. The postnatal development of the dopamine-containing innervation of dorsal and ventral striatum: effects of the weaver gene. J Neurosci. 1987;7:2364–2372. [PMC free article] [PubMed] [Google Scholar]
- 26.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 27.Shatz CJ. Emergence of order in visual system development. Proc Natl Acad Sci USA. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shearman LP, Zeitzer J, Weaver DR. Widespread expression of functional D1-dopamine receptors in fetal rat brain. Brain Res Dev Brain Res. 1997;102:105–115. doi: 10.1016/s0165-3806(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 29.Snyder-Keller A, Keller RW., Jr Stimulant-mediated c-fos induction in striatum as a function of age, sex, and prenatal cocaine exposure. Brain Res. 1998;794:88–95. doi: 10.1016/s0006-8993(98)00226-1. [DOI] [PubMed] [Google Scholar]
- 30.Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, Palmiter RD. Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci USA. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang K, Low MJ, Grandy DK, Lovinger DM. Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci USA. 2001;98:1255–1260. doi: 10.1073/pnas.031374698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tepper JM, Sharpe NA, Koós TZ, Trent F. Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci. 1998;20:125–145. doi: 10.1159/000017308. [DOI] [PubMed] [Google Scholar]
- 33.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 34.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Südhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 35.Weaver DR, Rivkees SA, Reppert SM. D1-dopamine receptors activate c-fos expression in the fetal suprachiasmatic nuclei. Proc Natl Acad Sci USA. 1992;89:9201–9204. doi: 10.1073/pnas.89.19.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]