Abstract
Animals can discriminate among many different types of foods. This discrimination process involves multiple sensory systems, but the sense of taste is known to play a central role. We asked how the taste system contributes to the discrimination of different “bitter” taste stimuli in Manduca sexta caterpillars. This insect has approximately eight bilateral pairs of taste cells that respond selectively to bitter taste stimuli. Each bilateral pair of bitter-sensitive taste cells has a different molecular receptive range (MRR); some of these taste cells also contain two signaling pathways with distinctive MRRs and temporal patterns of spiking. To test for discrimination, we habituated the caterpillar's taste-mediated aversive response to one bitter taste stimulus (salicin) and then asked whether this habituation phenomenon generalized to four other bitter taste stimuli (caffeine, aristolochic acid,Grindelia extract, and Canna extract). We inferred that the two compounds were discriminable if the habituation phenomenon failed to generalize (e.g., from salicin to aristolochic acid). We found that M. sexta could discriminate between salicin and those bitter taste stimuli that activate (1) different populations of bitter-sensitive taste cells (Grindeliaextract and Canna extract) or (2) different signaling pathways within the same bitter-sensitive taste cell (aristolochic acid). M. sexta could not discriminate between salicin and a bitter taste stimulus that activates the same signaling pathway within the same bitter-sensitive taste cell (caffeine). We propose that the heterogeneous population of bitter-sensitive taste cells and signaling pathways within this insect facilitates the discrimination of bitter taste stimuli.
Keywords: discrimination, bitter taste, taste cell, habituation-generalization paradigm, insect, Manduca sexta
Many naturally occurring foods taste “bitter” to humans and elicit an aversive response in animals (Garcia and Hankins, 1975; Brower, 1984). Because some of these foods are toxic and yet others are nutritious or medicinally active (Rouseff, 1990; Vitazkova et al., 2001), animals would benefit from an ability to discriminate among them (Glendinning, 1994). In insects, the detection of different bitter taste stimuli is not mediated by a uniform population of broadly tuned bitter-sensitive taste cells. Instead, it is segregated across a heterogeneous population of bitter-sensitive taste cells and signaling pathways that have different molecular receptive ranges (MRRs) (Glendinning and Hills, 1997; van Loon and Schoonhoven, 1999; Glendinning et al., 1999, 2001b). There is evidence that mammals also possess a heterogeneous population of bitter-sensitive taste cells (Caicedo and Roper, 2001), but this result is controversial (Adler et al., 2000). Our goal was to determine whether the presence of bitter-sensitive taste cells and signaling pathways with different MRRs would help insects discriminate among bitter taste stimuli.
We used the herbivorous caterpillar Manduca sexta for three reasons. First, it has only eight bilateral pairs of bitter-sensitive taste cells (bipolar sensory neurons) that are distributed across four different classes of sensillum (see Fig. 1). Second, because the different classes of bitter-sensitive taste cell have different MRRs (see Fig. 2), they do not contribute equally to the aversive response to any given bitter taste stimulus (see Table 1). Third, some of the bitter-sensitive taste cells contain two signaling pathways with different response properties. For example, the bitter-sensitive taste cells in the lateral styloconic and epipharyngeal sensilla each contain one signaling pathway that responds to caffeine and salicin and another that responds to aristolochic acid. The caffeine-activated pathway exhibits a tonic pattern of firing, whereas the aristolochic acid-activated pathway exhibits an accelerating pattern of firing (see Fig. 2B). Furthermore, the maximal firing rate of the aristolochic acid-activated pathway is twice that of the caffeine-activated pathway, at least in the bitter-sensitive taste cell within the lateral styloconic sensillum (see Fig.2B,C).
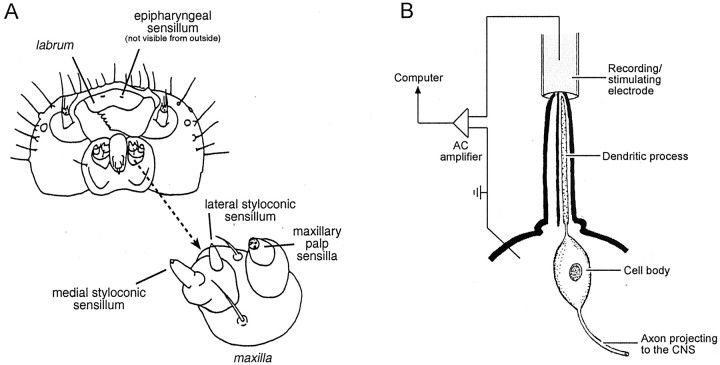
Fig. 1.
A, Diagram of the head of M. sexta, as viewed from below. An enlargement of a maxilla (indicated with an arrow) is provided to show the location of the medial and lateral styloconic sensilla. The epipharyngeal sensilla are located underneath the labrum and thus are not visible in this diagram. There are four taste cells in each of the lateral and medial styloconic sensilla, three taste cells in each of the epipharyngeal sensilla, and three to four taste cells in each of the gustatory sensilla on the maxillary palp. One of the taste cells in each sensillum is bitter sensitive. [This illustration was adapted from Bernays and Chapman (1994), their Fig. 3.4.] B, Diagram of the tip recording method (Hodgson et al., 1955) for recording excitatory responses of taste cells (or neurons) located within a single taste sensillum (for examples, see Fig.2A). During a recording, the tip of a taste sensillum is inserted into the end of a glass recording/stimulating electrode, which is filled with an electrolyte solution (0.1m KCl in deionized water) and the taste stimulus. The stimulus solution diffuses through a pore in the tip of the sensillum and activates a transduction mechanism(s) on the distal end of the dendritic process of the taste cell; the electrode records the ensuing action potentials. For clarity, only one taste cell is indicated. Note that the axonal process of the taste cell projects directly to the CNS.
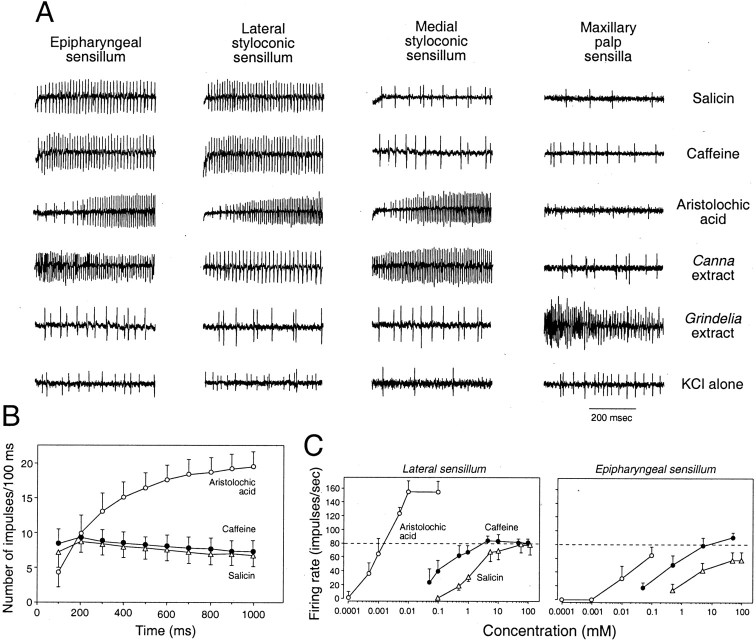
Fig. 2.
Illustration of how the peripheral taste system ofM. sexta mediates the aversive response to the five “bitter” taste stimuli used in this study. A, Excitatory responses of the four different classes of taste sensilla to five bitter taste stimuli (10 mm salicin, 5 mmcaffeine, 0.1 mm aristolochic acid, 0.3×Canna extract, and 1× Grindelia extract; see Materials and Methods for additional details). These neural responses are typical of habituated and nonhabituated caterpillars (Glendinning et al., 1998, 1999, 2001a; J. Glendinning, unpublished data). The onset of stimulation occurred at the beginning of each trace, and each vertical line reflects the occurrence of an action potential. In most of the traces containing a large number of action potentials, there is a single taste cell (the bitter-sensitive taste cell) firing regularly and another taste cell (the salt-sensitive taste cell) firing sporadically. The only exception is the multiunit response of the maxillary palp sensilla to theGrindelia extract, which probably contains action potentials from several bitter-sensitive taste cells. Because all bitter taste stimuli were presented in a 0.1 m KCl solution, we also show representative responses of each sensillum to the electrolyte solution alone for comparison (see bottom row of traces). Note that each bitter taste stimulus selectively activates bitter-sensitive taste cells in a subset of the taste sensilla (e.g., the Grindelia extract stimulated bitter-sensitive taste cells only in the maxillary palp). Furthermore, note that the only taste sensilla that are sufficient to mediate an aversive response to a particular bitter taste stimulus (Table 1) are the ones that contain a bitter-sensitive taste cell that responds vigorously to the same bitter taste stimulus.B, Instantaneous firing rates (total impulses/100 msec; median ± median absolute deviation) of the bitter-sensitive taste cell in the lateral styloconic sensillum to 5 mm caffeine, 10 mm salicin, and 0.1 mm aristolochic acid across 1000 msec of stimulation (Glendinning and Hills, 1997). It is revealed in B (and shown in the traces inA) that caffeine and salicin elicit a relatively tonic pattern of spiking, whereas aristolochic acid elicits an accelerating pattern of spiking. C, Excitatory responses (impulses per second; median ± median absolute deviation) of the bitter-sensitive taste cell in the lateral styloconic and epipharyngeal sensilla to a range of aristolochic acid, caffeine, and salicin concentrations (Glendinning et al., 1999). Note that aristolochic acid elicits a maximal firing rate in the lateral styloconic sensillum that is twice that elicited by caffeine and salicin; no such difference is apparent in the epipharyngeal sensillum.
Table 1.
Location of the different classes of bitter-sensitive taste cells in M. sexta
| Sensillum | Bitter taste stimulus | ||||
|---|---|---|---|---|---|
| Grindelia extract | Canna extract | Aristolochic acid | Caffeine | Salicin | |
| Maxillary palp | X | ||||
| Medial styloconic | X | X | |||
| Lateral styloconic | X | X | X | ||
| Epipharyngeal | X | X | X | X | |
We use an X to indicate the bitter-sensitive taste cells that are sufficient to mediate an aversive response to each of the five bitter taste stimuli (data from de Boer and Hanson, 1987;Peterson et al., 1993; Glendinning et al., 1998, 1999). The excitatory response of each bitter-sensitive taste cell to each of the bitter taste stimuli is provided in Figure 2.
There are two ways to test for taste discrimination in animals. One can determine whether subjects can be trained to respond differentially to two taste stimuli (Spector and Kopka, 2002). Both stimuli are assumed to be discriminable if the training is successful. Alternatively, one can determine whether habituating a subject's aversive response to one taste stimulus generalizes to another taste stimulus. Both taste stimuli are assumed to be discriminable if the habituation phenomenon fails to generalize (Glendinning and Gonzalez, 1995; Wes and Bargmann, 2001). We adopted the latter paradigm becauseM. sexta caterpillars exhibit weak associative learning (Dethier and Yost, 1979). Our approach involved exposing caterpillars to a diet containing an aversive concentration of salicin for 24 hr. This procedure habituates the caterpillar's taste-mediated aversive response to salicin through a central gustatory mechanism (Glendinning et al., 2001a). We predicted that this habituation phenomenon would generalize only to bitter taste stimuli that activate the same taste cells and signaling pathways as salicin.
MATERIALS AND METHODS
Subjects and rearing conditions. The M. sexta caterpillars were reared from eggs on a wheat germ-based artificial diet (Bell and Joachim, 1976) and maintained in an environmental chamber with a 16 hr light/8 hr dark cycle at 25°C. All experiments were conducted with caterpillars in the 1st or 2nd day of their fifth larval growth stage (instar). All caterpillars were naive to the taste stimuli before testing. To control for any potential differences among caterpillars from different egg batches, individuals from each batch were interspersed indiscriminately across treatment levels according to a blind procedure. Sample sizes for each experiment are provided in the figure legends.
Habituation protocol. The habituation protocol is described in detail elsewhere (Glendinning et al., 2001a). In brief, the “habituated” caterpillars received a block of their rearing diet containing a 6% concentration (fresh mass) of salicin (157 mm/kg diet) as their only source of food and water for 24 hr. This procedure completely habituates the aversive response of the caterpillars to salicin concentrations as high as 12% fresh mass (Glendinning et al., 2001a). The nonhabituated caterpillars received a salicin-free block of the same diet containing 6% alphacel (an indigestible form of cellulose; ICN Biomedicals) as their only source of food and water for 24 hr.
Substrates for presenting taste stimuli. We used two substrates for presenting the taste stimuli. In some tests, the taste stimuli were presented in the caterpillar's rearing diet. Specific concentrations of each compound were produced by heating the diet to ∼60°C, adding the appropriate quantity of compound, stirring vigorously for 3 min, and then pouring the diet into a test cake mold (1.5 cm in diameter and 0.4 cm deep). Once the diet cooled, it assumed a firm and rubbery consistency. Consumption was quantified by weighing the caterpillar before and after the taste test (to the nearest 0.1 mg) on an analytical balance; any increase in mass reflected the amount of diet eaten. The control diets were treated identically, except that no taste stimulus was added to them.
In the other taste tests, chemical stimuli were presented in a glass-fiber disk. Four hundred microliters of taste stimulus (dissolved in deionized water) were pipetted onto a glass-fiber disk (Whatman GF/A, 4.25 cm diameter; Whatman International, Maidstone, UK) and then offered to a caterpillar immediately afterward (to minimize evaporative water loss). Consumption was quantified by recording the number of bites taken over the 2 min taste test, using a software-based event recorder (Glendinning et al., 2000b).
Brief-access taste test. After a 30 min period of food deprivation, the caterpillar was weighed and then placed on the test platform (i.e., an inverted Petri dish) so that its head contacted the test diet. Care was taken to ensure that the caterpillar's legs grasped the test diet. The 2 min taste test began once the caterpillar took its first bite. After the test ended, the caterpillar was weighed a second time and then returned for 30 min to the diet it received during the habituation period so that it could overcome any hunger that might have developed over the course of the test. Next, it was food deprived for 30 min and then subjected to another brief-access taste test. The caterpillar was offered the control diet during the first test and a bitter diet during the second test.
To control for individual differences in biting rate and motivational state during the brief-access taste tests, the following response variable was calculated separately for each caterpillar: percentage of control response = (amount of bitter taste stimulus eaten/amount of control taste stimulus eaten) × 100. This parameter yielded a value of 100% when the caterpillar ingested equal amounts of the bitter and control taste stimulus, indicating that the two stimuli were not treated differently. Values approaching 0 indicated that the bitter taste stimulus was substantially less palatable than the control taste stimulus.
Same/different taste test. After a 30 min period of food deprivation, the caterpillar was placed on a test platform with the control disk (see below). The test platform was an inverted Petri dish with a piece of cork (1 cm diameter, 0.4 cm tall) attached to the center; the glass-fiber disk was pinned to the cork. Care was taken to ensure that the caterpillar's legs and prolegs grasped the control disk. Once the caterpillar began to bite the control disk (but before it had taken >30 bites), it was transferred to the experimental disk (see below). To minimize disturbance of the caterpillar during the transfer, the experimental disk was positioned next to its head, and the animal was permitted to walk onto the disk on its own. Once the caterpillar grasped the experimental disk with its prolegs, the disk was pinned to a second test platform. The 2 min taste test began once the caterpillar took its first bite from the experimental disk. We recorded the timing of all bites.
We assumed that the transfer from control to experimental disk did not disturb the caterpillar if it took ≥20 bites from the experimental disk during the taste test. However, if the caterpillar took <20 bites on the experimental disk, then we could not be certain whether the lack of biting was produced by (1) the aversive taste stimulus in the experimental disk or (2) the transfer procedure. To distinguish between these two possibilities, we transferred the caterpillar back to the control disk immediately after the 2 min test. If it initiated vigorous biting on the control disk (i.e., took >20 bites in <30 sec), then we assumed that the aversive taste stimulus inhibited biting. If the caterpillar failed to initiate feeding on the control disk, then we assumed that the transfer procedure itself inhibited biting. We used data only from those caterpillars that either took ≥20 bites from the experimental disk during the feeding test or reinitiated vigorous biting on the control disk after the 2 min feeding test.
After the first same/different test, the caterpillar was permitted to feed ad libitum on its rearing diet for 30 min. Then, it was food deprived for 30 min and run through a second same/different taste test with an experimental disk containing a different bitter taste stimulus. Because each caterpillar was tested with two bitter taste stimuli, the presentation order of each taste stimulus was randomized separately for each caterpillar.
Experiment 1: does the habituation phenomenon generalize to other bitter taste stimuli? To address this question, we used both of the taste-testing protocols described above. We used the brief-access taste test to determine whether the habituation phenomenon would generalize to four other bitter taste stimuli. To this end, we tested each habituated and nonhabituated caterpillar twice, once with a control substrate and a second time with a bitter substrate. The bitter substrates contained salicin (157 mm/kg diet, fresh mass; Sigma-Aldrich), caffeine (7.7 mm/kg diet; Sigma-Aldrich), aristolochic acid sodium salt (0.76 mm/kg diet; Sigma-Aldrich), Grindeliaextract [1.2% fresh mass; see Glendinning et al. (1998) for the extraction procedure], or Canna extract [a 0.3× concentration; see Peterson et al. (1993) for details]. All bitter taste stimuli, except for the Canna extract, were presented in the test diet. The Canna extract was presented in a glass-fiber disk; the control disk for this test was treated with water alone. We selected the indicated concentrations of each bitter taste stimulus because they are isoaversive (i.e., the lowest concentrations of each taste stimulus that elicit a robust aversive response inM. sexta) and because their aversive behavioral effects are mediated exclusively by gustatory input (de Boer and Hanson, 1987;Glendinning et al., 1998, 1999).
We ran two different types of same/different taste tests. First, we asked whether habituated and nonhabituated caterpillars would exhibit an aversive response after being transferred from the control disk containing 5 mmmyo-inositol (a palatable taste stimulus) (Glendinning et al. 2000b) to an experimental disk containing 5 mm caffeine or 0.1 mmaristolochic acid. Second, we asked whether habituated caterpillars would exhibit an aversive response after being transferred from the control disk containing 5 mm caffeine to an experimental disk containing 5 mm caffeine or 0.1 mm aristolochic acid.
Experiment 2: can caterpillars discriminate sensory input from different signaling pathways within the same taste cells? This experiment asked whether habituated caterpillars, lacking their medial sensilla, would still exhibit an aversive response to salicin, caffeine, or aristolochic acid. We wanted to determine whether sensory input from the medial sensilla was necessary to elicit an aversive response to aristolochic acid in habituated caterpillars. The medial styloconic sensillum contains a bitter-sensitive taste cell that exhibits a vigorous excitatory response to aristolochic acid but not to salicin or caffeine. We reasoned that the central projection sites for the bitter-sensitive taste cell in this sensillum may not become habituated by 24 hr of dietary exposure to salicin and thus may play a key role in mediating the aversive response to aristolochic acid in habituated caterpillars.
We used standard procedures (Glendinning et al., 1999) for surgically ablating the medial sensilla from all caterpillars on the first day of the fifth instar (Fig. 1). Then, we exposed the ablated caterpillars to the control or salicin diet for 24 hr. Finally, we ran these nonhabituated and habituated caterpillars through two consecutive brief-access taste tests, the first with the control diet and the second with a bitter diet containing salicin (157 mm/kg diet; fresh mass), caffeine (7.7 mm/kg diet), or aristolochic acid (0.76 mm/kg diet) (Fig.2).
Data analysis. Because the behavioral data were not distributed normally, we used nonparametric statistical procedures throughout. When comparing the “percentage of control response” to 100%, we used a one-sample Wilcoxon matched-pairs signed-rank test. When comparing “number of bites” taken from the two experimental disks, we used a two-sample Wilcoxon matched-pairs signed-rank test. The α level was 0.05. We uses the median as a measure of central tendency and the median absolute deviation (i.e., the median absolute difference of all values from the sample median) as a measure of variation.
RESULTS
Experiment 1: does the habituation phenomenon generalize to other bitter taste stimuli?
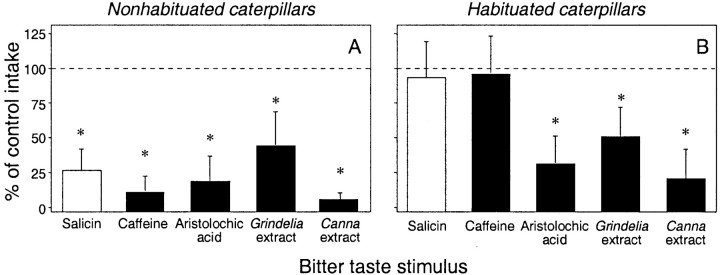
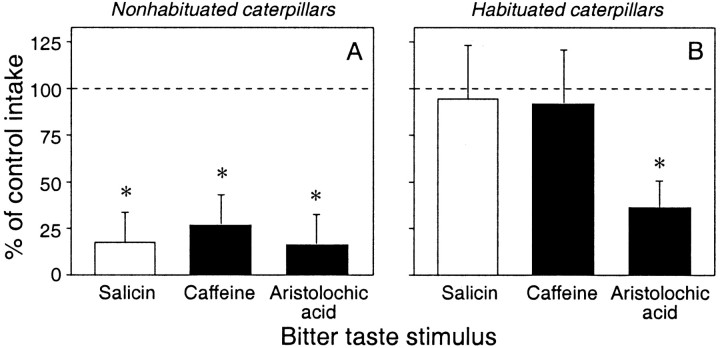
In the brief-access taste tests, the nonhabituated caterpillars ate significantly less of the bitter diets than of the corresponding control diets, yielding a percentage of control response that was significantly below 100% in all cases (p < 0.05) (Fig.3A). The habituated caterpillars, on the other hand, exhibited a more complex pattern of response (Fig. 3B). The percentage of control response for salicin and caffeine was statistically indistinguishable from 100% (p > 0.05), whereas that for aristolochic acid,Canna extract, and Grindelia extract was significantly below 100% (p < 0.05). These data show that the habituation phenomenon generalized to caffeine but not to aristolochic acid, Canna extract, orGrindelia extract.
Fig. 3.
The habituation phenomenon generalizes to caffeine but not to three other bitter taste stimuli in the brief-access taste test. We show the percentage of control response in nonhabituated (A) and habituated (B) caterpillars during two sequential tests. The caterpillars received the control taste stimulus in the first test and one of the following bitter taste stimuli in the second test: salicin (157 mm/kg diet; fresh mass), caffeine (7.7 mm/kg diet), aristolochic acid (0.76 mm/kg diet), Grindelia extract [1.2% fresh mass; seeGlendinning et al. (1998) for details], and Cannaextract [400 μl of 0.3× stock solution/glass-fiber disk; seePeterson et al. (1993) for details]. We calculated the percentage of control response by dividing total intake from a bitter diet by total intake from the corresponding control diet (and then multiplying this number by 100). To determine whether a bitter diet elicited an aversive response (i.e., elicited a median response that was significantly <100%), we used a one-sample Wilcoxon matched-pairs signed-rank test (* p ≤ 0.05). A dashed line was placed at 100% for comparison. Each bar indicates the median ± median absolute deviation (n = 20–25 caterpillars per median).
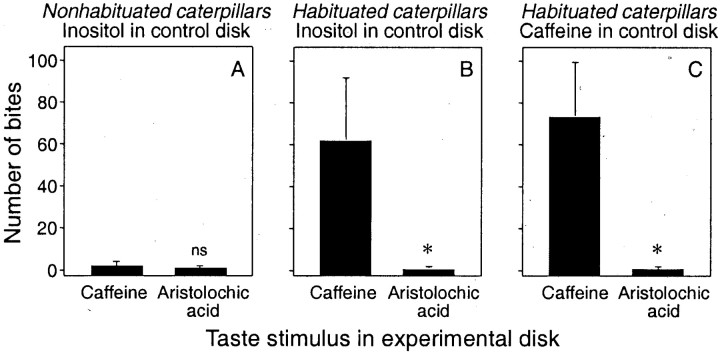
We conducted two types of same/different taste tests. In the first, we treated the control disk with 5 mmmyo-inositol. This taste stimulus elicited vigorous biting in the nonhabituated and habituated caterpillars. However, when the nonhabituated caterpillars were transferred from the control disk to the experimental disk treated with 0.1 mmaristolochic acid or 5 mm caffeine, they ceased biting immediately (Fig.4A). There was no significant difference in the number of bites taken from the aristolochic acid-treated (median = 1.0) or caffeine-treated (median = 1.5; p > 0.05) disks. The habituated caterpillars, on the other hand, behaved quite differently after being transferred to the experimental disks. They continued biting vigorously on the caffeine-treated disk but ceased biting precipitously on the aristolochic acid-treated disk (Fig. 4B). In fact, the habituated caterpillars took significantly fewer bites from the aristolochic acid disk (median = 1.0; p < 0.05) than from the caffeine disk (median = 62.0).
Fig. 4.
The habituation phenomenon generalizes to caffeine but not to aristolochic acid in the same/different test. Once each caterpillar initiated vigorous biting on the control disk, it was transferred to the experimental disk. We show the median (± median absolute deviation) number of bites taken by each caterpillar from the experimental disk across the 2 min trial. Each panelcontains results from two tests that were conducted sequentially on the same caterpillar. In A, nonhabituated caterpillars were offered a control disk treated with 5 mmmyo-inositol and experimental disks treated with 0.1 mm aristolochic acid or 5 mm caffeine. InB, habituated caterpillars were offered a control disk treated with 5 mmmyo-inositol and an experimental disk treated with 0.1 mm aristolochic acid or 5 mm caffeine. In C, habituated caterpillars were offered a control disk treated with 5 mm caffeine and an experimental disk treated with 0.1 mm aristolochic acid or 5 mm caffeine. We compared total number of bites taken from the caffeine- and aristolochic acid-treated disks, separately for each panel, using the Wilcoxon matched-pairs signed-rank test (NS, p > 0.05; *p ≤ 0.05). n = 14–16 caterpillars perpanel.
In the second same/different taste test, we used only habituated caterpillars and treated the control disk with 5 mmcaffeine. All of the caterpillars initiated rapid biting on the control disk. They continued to bite rapidly after being transferred to the experimental disk containing 5 mm caffeine but ceased biting after being transferred to the experimental disk containing 0.1 mm aristolochic acid. The habituated caterpillars took significantly fewer bites from the aristolochic acid disk (median = 1.0; p < 0.05) than from the caffeine disk (median = 73.0).
Taken together, the results from the same/different test corroborate those from the brief-access taste test, confirming that the habituation phenomenon generalizes to caffeine but not to aristolochic acid.
Experiment 2: can caterpillars discriminate sensory input from different signaling pathways within the same taste cells?
This experiment sought to determine whether ablating the medial sensilla altered the behavioral response of habituated and nonhabituated caterpillars to salicin, caffeine, or aristolochic acid. The nonhabituated (ablated) caterpillars, as expected, exhibited an aversive response to the caffeine, salicin, and aristolochic acid diets (Fig.5A). In contrast, the habituated (ablated) caterpillars exhibited an aversive response that was exclusive to the aristolohchic acid diet (Fig. 5B). Consumption from the salicin and caffeine diets was nearly identical to that from the control diet. These results show that habituation phenomenon generalized to caffeine but not aristolochic acid, regardless of whether the medial styloconic sensilla were present or absent.
Fig. 5.
The habituation phenomenon generalizes to caffeine but not to aristolochic acid in caterpillars lacking their medial styloconic sensilla. We show the percentage of control response by nonhabituated (A) and nonhabituated (B) caterpillars during two sequential brief-access taste tests. The insects received the control diet in the first test and one of the following bitter diets in the second test: salicin (157 mm/kg diet; fresh mass), caffeine (7.7 mm/kg diet), or aristolochic acid (0.76 mm/kg diet). We calculated the percentage of control response by dividing total intake from a bitter diet by total intake from the corresponding control diet (and then multiplying this number by 100). To determine whether a bitter diet elicited an aversive response (i.e., elicited a median response that was significantly <100%), we used a one-sample Wilcoxon matched-pairs signed-rank test (*p ≤ 0.05). A dashed line was placed at 100% for comparison. Each bar indicates the median ± median absolute deviation (n = 20–24 caterpillars per median).
DISCUSSION
We found that the 24 hr of dietary exposure to salicin completely habituated the aversive response of M. sexta to salicin. This habituation phenomenon generalized to caffeine but not to aristolochic acid, Canna extract, or Grindeliaextract. Because the aversive response to all of these bitter taste stimuli is mediated exclusively by sensory input from the bitter-sensitive taste cells (de Boer and Hanson, 1987; Glendinning et al., 1998, 1999), the habituation phenomenon must have been limited to the gustatory neuraxis. Furthermore, because the habituation phenomenon is mediated centrally (Glendinning et al., 2001a), the observed pattern of generalization cannot be explained by a reduction in peripheral responsiveness to the bitter taste stimuli (Glendinning et al., 2001b). It follows, therefore, that exposure to the salicin diet must have selectively habituated the CNS of M. sexta to the pattern of gustatory input elicited by salicin and caffeine.
Why did the habituation phenomenon generalize to some but not all bitter taste stimuli?
Salicin and caffeine stimulate a common signaling pathway within the same bitter-sensitive taste cells (Glendinning and Hills, 1997;Glendinning et al., 1999), and as a result, elicit excitatory responses that are virtually identical in terms of maximal firing rate and temporal pattern of firing (Fig. 2). We propose that the habituation phenomenon generalized from salicin to caffeine because the central gustatory system could not discriminate the pattern of activity elicited by these two taste stimuli.
In contrast, because salicin, Grindelia extract, andCanna extract are all sensed by different populations of bitter-sensitive taste cells (Fig. 2A, Table1), they would all produce a distinct spatial pattern of activation within the primary projection site of the bitter-sensitive taste cells, the subesophageal ganglion (SOG). If we assume that the habituation phenomenon was restricted to loci in the SOG that receive input from taste cells responsive to salicin, then we would not expect it to generalize to loci in the SOG that receive input from taste cells responsive to Grindelia or Cannaextract. Although knowledge of the central projection sites within the insect SOG is fragmentary (Mitchell et al., 1999), there is evidence that axons from individual taste cells in Drosophila melanogaster (adults) project to spatially restricted regions of the SOG (Shanbhag and Singh, 1992; Dunipace et al., 2001).
Our most surprising finding was that the salicin habituation phenomenon did not generalize to aristolochic acid, although salicin and aristolochic acid stimulate the same bitter-sensitive taste cells (Glendinning and Hills, 1997; Glendinning et al., 1999). We can propose two (non-mutually exclusive) explanations for this finding. First, the CNS of M. sexta may have habituated selectively to the low rate of firing rate or the tonic temporal pattern of firing generated by the salicin-activated signaling pathway, (Fig. 2). Second, because salicin stimulates the bitter-sensitive taste cell within the medial styloconic sensilla only weakly (Fig. 2A, Table 1), it is unlikely that 24 hr of dietary exposure to salicin would have habituated the loci in the SOG that receive input from this taste cell. If so, then sensory input from the medial sensilla alone could have mediated the aversive response to aristolochic acid. We were able to reject this second explanation, however, by showing that habituated caterpillars, lacking their medial sensilla, still exhibited an aversive response to aristolochic acid (Fig. 5). This result indicates that although sensory input from the medial sensilla may have contributed to the aversive response to aristolochic acid in intact (habituated) caterpillars, it was not necessary.
Several investigators have suggested that the colocalization of more than one receptor type or signaling pathway within the same chemosensory cell should reduce the potential for discrimination (Bernhardt et al., 1996; Adler et al., 2000; Dunipace et al., 2001). Our findings (and those from the nematode, C. elegans; see below) contradict this speculation. We show that the CNS of M. sexta can discriminate readily between gustatory input from two signaling pathways that are colocalized within the same taste cells. Further work is needed to determine the mechanistic basis of this discrimination. The most likely mechanism would involve a circuit in the CNS that discriminates between the firing rate and temporal patterns of spiking generated by the two signaling pathways (Fig. 2). Previous studies in insects and vertebrates indicate that firing rate (Scott and Giza, 1987; van Loon and Schoonhoven, 1999) or temporal pattern of firing (Katz et al., 2001; Varkevisser et al., 2001; Vickers et al., 2001) could serve as a basis for chemosensory discrimination.
Like M. sexta, the nematode C. elegans expresses multiple signaling pathways within individual chemosensory neurons and readily discriminates among chemical stimuli that activate these colocalized signaling pathways. However, unlike M. sexta, the nematode expresses these signaling pathways asymmetrically across each homologous pair of chemosensory neurons, resulting in neurons with different molecular receptive ranges (Pierce-Shimomura et al., 2001;Wes and Bargmann, 2001). The nematode appears to use this functional asymmetry to help differentiate chemical stimuli (Pierce-Shimomura et al., 2001; Wes and Bargmann, 2001). It is unlikely that M. sexta uses an analogous discrimination mechanism because each bilateral pair of bitter-sensitive taste cells displays symmetrical response properties. For instance, the left and right bitter-sensitive taste cells within the lateral styloconic (or epipharyngeal) sensilla both respond vigorously to caffeine, salicin, and aristolochic acid (J. Glendinning, unpublished data).
Are there different subqualities of bitterness?
We found that the CNS of M. sexta can use the segregated input from different bitter-sensitive taste cells and signaling pathways to discriminate among bitter taste stimuli. This discrimination could be mediated by one of the following cognitive mechanisms. First, all of the bitter taste stimuli may elicit the same taste quality (e.g., bitterness), but nevertheless be discriminable, because the habituation phenomenon makes the central gustatory system selectively unresponsive to sensory input elicited by salicin and caffeine. Second, if the discriminable bitter taste stimuli elicit different taste qualities (e.g., different subqualities of bitterness), then the habituation phenomenon could make the gustatory system selectively unresponsive to the taste quality evoked by salicin and caffeine. To evaluate these two cognitive mechanisms, one could attempt to train nonhabituated caterpillars to respond differentially to isoaversive concentrations of salicin, aristolochic acid,Grindelia extract, and Canna extract (Spector and Kopka, 2002). Successful training would indicate that the bitter taste stimuli each produce distinct taste qualities.
The question of whether mammals perceive different subqualities of bitterness is unresolved. On the one hand, electrophysiological studies indicate that the peripheral taste system of mammals segregates the detection of different bitter taste stimuli into distinct populations of bitter-sensitive taste cells (Caicedo and Roper, 2001) and signaling pathways (for review, see Glendinning et al., 2000a) and that this segregation is preserved, albeit weakly, as information flows up the primary afferent fibers (Dahl et al., 1997; Danilova et al., 1999) and into the insular cortex (Scott et al., 1999). Furthermore, short-term (McBurney et al., 1972) and long-term (Zellner et al., 1985) exposure to one bitter taste stimulus appears to reduce the aversiveness of some but not all bitter taste stimuli in humans and rats. On the other hand, the fact that individual taste cells in rodents each express a large repertoire of bitter taste receptors has led some to infer a limited potential for bitter taste discrimination (Adler et al., 2000). In support of this molecular work, psychophysical studies with rats and humans indicate that different bitter taste stimuli produce indiscriminable gustatory sensations (Lindsey and Breslin, 2001; Keast and Breslin, 2002; Spector and Kopka, 2002). Clearly, more studies are needed to resolve these contradictory findings.
CONCLUSION
Taste discrimination mechanisms help animals distinguish among foods that vary in nutritive value and potential toxicity. The ability to discriminate among bitter-tasting foods would be particularly useful for herbivorous animals because many plant tissues taste bitter, but only a fraction of these bitter plant tissues are actually toxic (Rouseff, 1990; Glendinning, 1994). Our results show that the herbivorous M. sexta can readily discriminate among bitter taste stimuli and that its heterogeneous population of bitter-sensitive taste cells and signaling pathways facilitates this discrimination process. Given that the gustatory system of insects also segregates the detection of nutrients into different taste cells (Glendinning et al., 2000a) and signaling pathways (Dahanukar et al., 2002), it is possible that the taste discrimination mechanisms proposed herein for bitter taste stimuli may also be used to differentiate different types of carbohydrates and amino acids.
Footnotes
This project was supported in part by research Grant 5 R29 DC 02416 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (J.I.G.). We thank Alan Spector and several anonymous reviewers for valuable editorial comments.
Correspondence should be addressed to John I. Glendinning, Department of Biological Science, Barnard College, Columbia University, 3009 Broadway, New York, NY 10027. E-mail:jglendinning@barnard.edu.
REFERENCES
- 1.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zucker CS. A novel family of mammalian bitter taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 2.Bell RA, Joachim FA. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 1976;69:365–373. [Google Scholar]
- 3.Bernays EA, Chapman RF. Host-plant selection by phytophagous insects. Chapman and Hall; New York: 1994. [Google Scholar]
- 4.Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol (Lond) 1996;490:325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brower LP. Chemical defense in butterflies. In: Vane-Wright RI, Ackery PR, editors. The biology of butterflies. Academic; London: 1984. pp. 109–134. [Google Scholar]
- 6.Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2002;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 8.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 9.Danilova V, Roberts T, Hellekant G. Responses of single taste fibers and whole chorda tympani and glossopharyngeal nerve in the domestic pig, Sus scrofa. Chem Senses. 1999;24:301–316. doi: 10.1093/chemse/24.3.301. [DOI] [PubMed] [Google Scholar]
- 10.de Boer G, Hanson FE. Differentiation of roles of chemosensory organs in food discrimination among host and non-host plants by larvae of the tobacco hornworm, Manduca sexta. Physiol Entomol. 1987;12:387–398. [Google Scholar]
- 11.Dethier VG, Yost MT. Oligophagy and absence of food-aversion learning in tobacco hormworms, Manduca sexta. Physiol Entomol. 1979;4:125–130. [Google Scholar]
- 12.Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 13.Garcia J, Hankins WG. The evolution of bitter and the acquisition of toxiphobia. In: Denton DA, Coghlan JP, editors. Olfaction and taste. V. Proceedings of the 5th International Symposium in Melbourne, Australia. Academic; New York: 1975. pp. 39–45. [Google Scholar]
- 14.Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56:1217–1227. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 15.Glendinning JI, Hills TT. Electrophysiological evidence for two transduction pathways within a bitter-sensitive taste receptor. J Neurophysiol. 1997;78:734–745. doi: 10.1152/jn.1997.78.2.734. [DOI] [PubMed] [Google Scholar]
- 16.Glendinning JI, Gonzalez NA. Gustatory habituation to deterrent compounds in a grasshopper: concentration and compound specificity. Anim Behav. 1995;50:915–927. [Google Scholar]
- 17.Glendinning JI, Valcic S, Timmermann BN. Maxillary palps can mediate taste rejection of plant allelochemicals by caterpillars. J Comp Physiol [A] 1998;183:35–44. doi: 10.1007/s003590050232. [DOI] [PubMed] [Google Scholar]
- 18.Glendinning JI, Tarre M, Asaoka K. Contribution of different bitter-sensitive taste cells to feeding inhibition in a caterpillar (Manduca sexta). Behav Neurosci. 1999;113:840–854. [PubMed] [Google Scholar]
- 19.Glendinning JI, Chaudhari N, Kinnamon SC. Taste transduction and molecular biology. In: Finger T, Silver WL, Restrepo D, editors. The neurobiology of taste and smell, Ed 2. Wiley; New York: 2000a. pp. 315–351. [Google Scholar]
- 20.Glendinning JI, Nelson N, Bernays EA. How do inositol and glucose modulate feeding in Manduca sexta caterpillars? J Exp Biol. 2000b;203:1299–1315. doi: 10.1242/jeb.203.8.1299. [DOI] [PubMed] [Google Scholar]
- 21.Glendinning JI, Domdom S, Long E. Selective adaptation to noxious foods by an insect. J Exp Biol. 2001a;204:3355–3367. doi: 10.1242/jeb.204.19.3355. [DOI] [PubMed] [Google Scholar]
- 22.Glendinning JI, Brown H, Capoor M, Davis A, Gbedemah A, Long E. A peripheral mechanism for behavioral adaptation to specific bitter taste stimuli in an insect. J Neurosci. 2001b;21:3688–3696. doi: 10.1523/JNEUROSCI.21-10-03688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson ES, Lettvin JY, Roeder KD. Physiology of a primary chemoreceptor unit. Science. 1955;122:417–418. doi: 10.1126/science.122.3166.417-a. [DOI] [PubMed] [Google Scholar]
- 24.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;27:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keast RS, Breslin PA. Cross-adaptation and bitterness inhibition of l-tryptophan, l-phenylalanine and urea: further support for shared peripheral physiology. Chem Senses. 2002;27:123–131. doi: 10.1093/chemse/27.2.123. [DOI] [PubMed] [Google Scholar]
- 26.Lindsey AT, Breslin PA. Taste matching among five bitter compounds: continuing studies. Chem Senses. 2001;26:1037. [Google Scholar]
- 27.McBurney DH, Smith DV, Shick TR. Gustatory cross adaptation: sourness and bitterness. Percept Psychophys. 1972;11:228–232. [Google Scholar]
- 28.Mitchell BK, Itagaki H, Rivet M-P. Peripheral and central structures involved in insect gustation. Microsc Res Tech. 1999;47:401–415. doi: 10.1002/(SICI)1097-0029(19991215)47:6<401::AID-JEMT4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Peterson SC, Hanson FE, Warthen JD., Jr Deterrence coding by a larval Manduca chemosensory neurone mediating rejection of a non-host plant, Canna generalis L. Physiol Entomol. 1993;18:285–295. [Google Scholar]
- 30.Pierce-Shimomura JT, Faumont S, Gaston MR, Pearson BJ, Lockery SR. The homeobox gene lim-6 is required for distinct chemosensory representation in C. elegans. Nature. 2001;410:694–698. doi: 10.1038/35070575. [DOI] [PubMed] [Google Scholar]
- 31.Rouseff RL. Bitterness in foods and beverages. Elsevier; New York: 1990. [Google Scholar]
- 32.Scott TR, Giza BK. A measure of taste intensity discrimination in the rat through conditioned taste aversions. Physiol Behav. 1987;41:315–320. doi: 10.1016/0031-9384(87)90394-5. [DOI] [PubMed] [Google Scholar]
- 33.Scott TR, Giza BK, Yan J. Gustatory neural coding in the cortex of the alert cynomolgus macaque: the quality of bitterness. J Neurophysiol. 1999;81:60–71. doi: 10.1152/jn.1999.81.1.60. [DOI] [PubMed] [Google Scholar]
- 34.Shanbhag SR, Singh RN. Functional implications of the projections of neurons from individual labellar sensillum of Drosophila melanogaster as revealed by neuronal-marker horseradish peroxidase. Cell Tissue Res. 1992;267:273–282. [Google Scholar]
- 35.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J Neurosci. 2002;22:1937–1941. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Loon JJA, Schoonhoven LM. Specialist deterrent chemoreceptors enable Pieris caterpillars to discriminate between chemically different deterrents. Entomol Exp Appl. 1999;91:29–35. [Google Scholar]
- 37.Varkevisser B, Peterson D, Ogura T, Kinnamon SC. Neural networks distinguish between taste qualities based on receptor cell population responses. Chem Senses. 2001;26:499–505. doi: 10.1093/chemse/26.5.499. [DOI] [PubMed] [Google Scholar]
- 38.Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odour-plume dynamics influence the brain's olfactory code. Nature. 2001;410:466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- 39.Vitazkova SK, Long E, Paul A, Glendinning JI. Mice suppress malaria infection by sampling a “bitter” chemotherapy agent. Anim Behav. 2001;61:887–894. [Google Scholar]
- 40.Wes PD, Bargmann CI. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature. 2001;410:698–701. doi: 10.1038/35070581. [DOI] [PubMed] [Google Scholar]
- 41.Zellner DA, Berridge KC, Grill HJ, Ternes JW. Rats learn to like the taste of morphine. Behav Neurosci. 1985;99:290–300. doi: 10.1037//0735-7044.99.2.290. [DOI] [PubMed] [Google Scholar]