Abstract
The different cerebellar phenotypes are generated according to a precise time schedule during embryonic and postnatal development. To assess whether the differentiative potential of cerebellar progenitors is progressively restricted in space and time we examined the fate of embryonic day 12 (E12) or postnatal day 4 (P4) cerebellar cells after heterotopic–heterochronic transplantation into the embryonic rat brain in utero or into organotypic CNS explants in vitro. Donor cells, isolated from transgenic mice overexpressing the enhanced-green fluorescent protein under the control of the β-actin-promoter, engrafted throughout the host brainstem and diencephalon, whereas they rarely incorporated into specific telencephalic structures. In any recipient site, the vast majority of transplanted cells could be recognized as cerebellar phenotypes, and we did not obtain clear evidence that ectopically located cells adopted host-specific identities. Nevertheless, the two donor populations displayed different developmental potentialities. P4 progenitors exclusively generated granule cells and molecular layer interneurons, indicating that they are committed to late-generated cerebellar identities and not responsive to heterotopic–heterochronic environmental cues. In contrast, E12 precursors had the potential to produce all major cerebellar neurons, but the repertoire of adult phenotypes generated by these cells was different in distinct host regions, suggesting that they require instructive environmental information to acquire mature identities. Thus, cerebellar precursors are able to integrate into different foreign brain regions, where they develop mature phenotypes that survive long after transplantation, but they are committed to cerebellar fates at E12. Embryonic progenitors are initially capable, although likely not competent, to generate all cerebellar identities, but their potential is gradually restricted toward late-generated phenotypes.
Keywords: differentiation, in utero neural graft, neural precursor, stem cell, cerebellum, mouse, rat
The relative contribution of cell-autonomous mechanisms and environmental signaling to cell specification during CNS development is still poorly understood. Several lines of evidence indicate that the differentiative potential of neural progenitors is progressively restricted both in space, because of regional specification (Barbe and Levitt, 1991;Alvarado-Mallart 1993; Lumsden et al., 1994; Jessell, 2000; Anderson, 2001; McCarthy et al., 2001), and in time, because of a progressive loss of sensitivity to stage-specific cues (Frantz and McConnell, 1996;Rao, 1999; Desai and McConnell, 2000). On the other hand, progenitor cells heterotopically–heterochronically transplanted into the developing brain integrate in wide areas of the recipient CNS and may acquire site-specific identities (Fishell, 1995; Brüstle et al., 1995; Campbell et al., 1995; Magrassi and Graziadei, 1996; Olsson et al., 1997, 1998). However, under the same experimental conditions grafted precursors may also actively segregate from host cells and develop according to their origin (Magrassi and Graziadei, 1996;Magrassi et al., 1998). Furthermore, the number of successfully integrated cells depends both on the age of the host brain and of donor cells, and different precursor cell populations may preferentially incorporate into defined areas of the recipient CNS. Thus, although transplanted progenitors are able to adopt novel identities, their actual capability to interact with environmental cues in different brain regions at different ontogenetic stages remains to be established.
The rodent cerebellum offers a suitable ground to address these issues. All cerebellar phenotypes originate according to a precise time schedule from two germinative neuroepithelia (Ramón y Cajal, 1911; Miale and Sidman, 1961). During embryonic life, the cerebellar ventricular zone generates deep nuclear neurons, Purkinje cells, Golgi interneurons, and glia. Its proliferative activity ceases after birth, but dividing precursors emigrate into the cerebellar parenchyma, where they generate molecular layer interneurons and glia during postnatal development (Zhang and Goldman, 1996a,b). The other cerebellar neuroepithelium, the external granular layer (EGL), is formed by progenitors from the rhombic lip, which migrate on the cortical surface and proliferate during postnatal development. In rodents, EGL cells are committed to a granule cell fate (Gao and Hatten, 1994; Alder et al., 1996; Wingate, 2001).
Transplantation studies indicate that mid-hindbrain progenitors become regionally specified between embryonic day 10.5 and 13.5 (Olsson et al., 1997) and that postnatal cerebellar precursors are committed toward late-generated phenotypes (Jankovski et al., 1996). However, postnatal cerebellar progenitors acquire site-specific identities when transplanted to the postnatal dentate gyrus (Vicario-Abejón et al., 1995), but they only generate cerebellar phenotypes in the adult subventricular zone (Jankovski and Sotelo, 1996). In most of these studies, grafted cerebellar cells have only been followed for short survival times or examined using limited ranges of molecular markers, so that their actual differentiative potential capability of engraftment and long-term survival into ectopic CNS regions are still unclear. To address these issues, here we examined the fate of embryonic and postnatal cerebellar progenitors after heterotopic–heterochronic transplantation into the embryonic CNSin vivo or in organotypic explants in vitro.
A preliminary report of this work has been published (Carletti et al., 2001).
MATERIALS AND METHODS
Animals and surgical procedures. We transplanted embryonic and postnatal cerebellar progenitors into the telencephalic vesicles of rat embryos in utero or into organotypic explants of mouse brainstem–cerebellum or neocortex. An additional set of in utero experiments was performed using embryonic neocortical precursors. To identify transplanted cells in the recipient tissue, donor cells were isolated from transgenic mice overexpressing the enhanced green fluorescent protein (EGFP) under the control of the β-actin promoter (a generous gift from Dr. M. Okabe, Osaka University; Okabe et al., 1997). In these mice all cells, including all neurons and glia of the CNS, are intensely fluorescent. Wistar rat embryos (Charles River, Calco, Italy) were used as hosts for in utero transplantation, whereas in vitro explants were prepared from CD1 mouse embryos (Charles River). All surgical procedures were performed under deep general anesthesia obtained by intraperitoneal administration of ketamine (100 mg/kg; Ketalar; Bayer, Leverkusen, Germany) supplemented by xylazine (5 mg/kg; Rompun; Bayer) or diazepam (2.5 mg/kg; Roche, Mannheim, Germany). The experimental plan was designed according to the National Institutes of Health guidelines and the Italian law for care and use of experimental animals (DL116/92) and approved by the Italian Ministry of Health.
Preparation of cell suspensions. The preparation of donor cerebellar cells was performed as previously described (Jankovski et al., 1996). To collect embryonic cerebellar progenitors, EGFP mouse embryos were removed by caesarian section from deeply anesthetized timed-pregnant females at embryonic day 12 (E12), rapidly decapitated, and dissected in PBS with 0.6% glucose (PBG) to isolate the cerebellar primordia. Postnatal cerebellar cells were obtained from the cerebellar cortex of transgenic mouse pups at postnatal day (P4). The donor mice were cryoanesthetized in melting ice and rapidly transcardially perfused with 5 ml of PBG to wash out blood cells. The cerebellum was removed from the skull and cut using a tissue chopper into 250-μm-thick parasagittal slices, collected in PBG. From these sections small blocks of cerebellar cortex were dissected by means of fine glass microneedles.
For both donor cell populations, the collected tissue blocks were incubated for 5 min in trypsin (1% in PBG; Sigma, St. Louis, MO) and DNase (0.1%; Sigma) at 37°C, and then mechanically dissociated to a single cell suspension by means of a fire-polished Pasteur pipette. The obtained suspension was centrifuged and resuspended at a final concentration of 1–2 × 104cells/μl. An aliquot of the suspension was immediately examined under the microscope to assess cell viability and EGFP expression. In all instances, virtually all the cells displayed an intense fluorescence. The same procedures were applied to dissect and dissociate neocortical donor cells isolated from the dorsal aspect of the telencephalic vesicle of E12 embryos.
Transplantation in utero. The surgical manipulation of rat embryos in utero was performed according to a previously described approach (Cattaneo et al., 1994). Briefly, timed-pregnant E16 rats were deeply anesthetized, and the uterine horns were exposed. The embryonic CNS was identified under transillumination, and 5 μl of the cell suspension (5–10 × 104 cells) was gently injected in the telencephalic vesicle by means of a glass capillary. The embryos were placed back into the abdomen for spontaneous delivery. Live-born recipient rats (70–80% of the littermate) were killed between P7 and P40 (i.e., 2–6 weeks after transplantation), except for a few animals killed just after birth (P0–P1). On the whole, we analyzed 19 rats receiving postnatal cerebellar grafts, 15 receiving embryonic donor cells, and 11 receiving embryonic neocortical cells.
Transplantation in vitro. Organotypic explants of brainstem–cerebellum were prepared as described by Chédotal et al. (1997). Briefly, the brain region between the tectocerebellar and medullospinal junctions was isolated from E12 CD1 mouse embryos and collected in PBG. The right and left cerebellar plates and the caudal portion of the medulla oblongata were gently opened along the dorsal midline, to place the explant flat in the culture dish. E15 neocortical explants were prepared by dissecting a tissue slab from the dorsal portion of the telencephalon, approximately corresponding to the parietal cortex. The explants were positioned in the culture dishes with their ventricular side up to have direct access to the cerebellar or neocortical germinative neuroepithelia and placed in the incubator for ∼30 min before transplantation. Then, 3–5 μl of the cell suspension (as above) was injected by a glass capillary in several intraparenchymal sites or just deposited on the upper (ventricular) surface of the organotypic culture.
The explants were cultured on the membrane of a 30 mm Millipore culture insert (Millicell; Millipore, Bedford, MA; pore size, 0.4 μm) in 10 cm culture dishes containing 3 ml of a medium composed of 50% basal medium with Earle's salts (Life Technologies, Gaithersburg, MD), 2.5% HBSS (Invitrogen, Gaithersburg, MD), horse serum (25% first week, 15% following weeks; Invitrogen), 22.5% water,l-glutamine (1 mm; Invitrogen), penicillin–streptomycin (200 U/ml; Invitrogen), and glucose (5 mg/ml). The cultures were kept at 37°C in a humidified atmosphere with 5% CO2, and they were fixed after 15–25 d in vitro. On the whole we analyzed 21 cerebellar and 10 neocortical explants grafted with postnatal cerebellar cells and 19 cerebellar and 14 neocortical explants receiving embryonic donor cells.
Histological procedures. Under deep general anesthesia (as above), the rats were transcardially perfused with 1 l of 4% paraformaldehyde in 0.12 m phosphate buffer, pH 7.2–7.4. The brains were immediately dissected, stored for 2 hr in the same fixative at 4°C, and finally transferred in 0.12m phosphate buffer. The brain was cut in 100-μm-thick vibratome parasagittal slices, whereas the cerebellum was sectioned on the frontal plane. The sections were collected in PBS and immediately examined under the microscope to localize the transplanted cells. Sections containing EGFP-positive cells where incubated with different primary antibodies (in some instances the vibratome slices were further cut in 10-μm-thick sections directly collected on gelatinized slides). The explants were fixed 4% paraformaldehyde in 0.12 m phosphate buffer, pH 7.2–7.4, for 3 hr at room temperature, washed in PBS, and processed for immunocytochemistry.
To detect the expression of cell-specific markers we applied a set of primary antibodies: anti-calbindin (Purkinje cells; Celio, 1990; 1:5000; polyclonal; Swant, Bellinzona, Switzerland), anti-parvalbumin (Purkinje cells and molecular layer interneurons; Celio, 1990; 1:5000; monoclonal; Swant), anti-neurofilament SMI32 (Purkinje cells and deep nuclei neurons; Jankovski et al., 1996; 1:500; monoclonal; Sternberger, Baltimore, MD), anti-α6 subunit of the GABAAreceptor (granule cells; Kato, 1990; Luddens et al., 1990; 1:100; polyclonal; Chemicon, Temecula, CA), anti-glial fibrillary acidic protein (GFAP; astrocytes; 1:1000; polyclonal; Dakopatts, Glostrup, Denmark), anti-NG2 (immature oligodendrocytes; 1:200; polyclonal;Chemicon); anti-MBP (oligodendrocytes; 1:2000; monoclonal; Sternberger). Incubations were made overnight at 4°C in PBS with 1.5% normal serum and 0.25% Triton X-100. To visualize the primary antibodies the sections or the explants were incubated for 1 hr at room temperature with second biotinylated antibodies and then with a solution of streptavidin Texas Red conjugate (1:200; Molecular Probes, Eugene, OR). The reacted material was mounted on microscope slides with PBS-glycerol supplemented with DABCO (2%; Sigma) to avoid fading of fluorescence.
BrdU labeling. To evaluate the proliferative activity of donor cells in the host brain, BrdU (50 μg/g of body weight, dissolved at 5 mg/ml in 0,007N NaOH in normal saline) was injected intraperitoneally to the pregnant mother after transplantation (three injections during 48 hr) or to the live-born recipient rats 24–48 hr (three injections) before the end of the survival time. The brains were fixed and vibratome-sectioned as above. Sections containing transplanted cells were incubated in 2N HCl for 1 hr at room temperature, exposed to anti-BrdU antibodies (1:500; monoclonal; Sigma) overnight at 4°C, and then reacted with biotinylated second antibody and streptavidin Texas Red conjugate as above.
In situ hybridization of RU49 expression. As an additional marker to identify transplanted granule cells, we examined the expression of RU49 mRNA, which is selectively expressed in cerebellar and hippocampal granule cells and olfactory bulb interneurons (Yang et al., 1996). In situ hybridization was performed following previously described protocols (Schaeren-Wiemers and Gerfin-Moser, 1993; Yang et al., 1996). Vibratome slices from two 4% paraformaldehyde fixed brains, which contained EGFP-positive donor cells from P4 cerebella, were selected and cut in 15-μm-thick cryostat sections, collected on superfrost slides, and air-dried at room temperature for ∼30 min. The slides were treated with 10 μg/ml proteinase K, postfixed for 10 min in 4% paraformaldehyde, and permeabilized for 10 min in PBS with 0.1% Triton X-100. Then, the sections were acetylated by 10 min incubation in a solution made of 250 ml of DEPC water with 3.5 ml of triethanolamine (Sigma) and 625 μl of acetic anhydride (Sigma) added dropwise. Prehybridization was performed at room temperature in humid chamber, in 500 μl of the hybridization buffer (50% formamide, 5× SSC, and 2% blocking reagent; Boehringer Mannheim, Mannheim, Germany). The hybridization mixture was prepared by adding 1 μg/ml of a full length RU49 digoxigenated (DIG) riboprobe (a kind gift of Dr. N. Heintz, Rockefeller University, NY) (Yang et al., 1996), first heated for 5 min at 85°C to denature the probe and then chilled on ice. Hybridization was done overnight at 72°C in a hybridization mixture humidified chamber. Stringency washing was performed in 0.2× SSC at 72°C for 60 min, plus 5 additional minutes at room temperature. For the immunological detection of DIG-labeled hybrids, slides were incubated for 1 hr in anti-DIG antibody (Boehringer Mannheim) diluted 1:2000 in a 0.1 mmaleic acid 0.15 NaCl solution, pH 7.5. DIG-labeled hybrids were then visualized by means of the 2-hydroxy-3-naphtoic acid-2-phenylianilide phosphate fluorescent detection set (Roche). Because EGFP fluorescence faded out during the hybridization procedures, we could not obtain double labeling of grafted cells expressing RU49. Thus, to identify the RU49-positive donor cells, the cryostat sections were carefully examined under the microscope before the in situhybridization processing and the position of EGFP-positive cells was determined. In addition, the analysis of this material was restricted to mesodiencephalic regions of the host brain, in which RU49 is not normally expressed. On the basis of these criteria, RU49-labeled cells present in RU49-negative regions of the host brain were identified as donor elements.
Data analysis. The histological preparations were examined by means of a Zeiss Axiophot light microscope. Digital micrographs were taken by means of a Nikon Coolpix 950 digital camera attached to the same microscope. The material was also examined with an Olympus(Hamburg, Germany) Fluoview 300 confocal microscope. Digital images were processed with Adobe Photoshop 6.0 to adjust contrast and to assemble the final plates. Quantitative and morphometric evaluations were made using the Neurolucida software (MicroBrightField Inc., Colchester, VT) connected to an E-800 Nikon microscope via a color CCD camera.
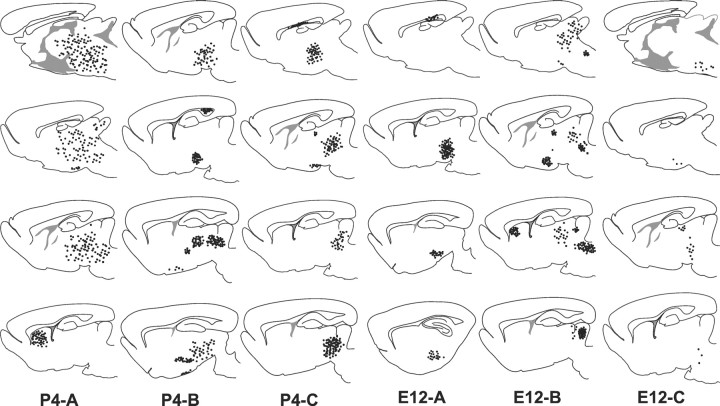
To evaluate the distribution of heterotopically transplanted cells in the host CNS in vivo, we selected three representative cases transplanted with postnatal or embryonic cerebellar cells, killed 3–5 weeks after transplantation, when grafted cells had completed their development. For each case, the outline of several serial sections, encompassing mesencephalon and forebrain, was reproduced using theNeurolucida system, and the position of every EGFP-positive cell were carefully mapped. To allow a better comparison between different cases, the obtained maps were reported on corresponding sections from a stereotaxic atlas of the rat brain (Paxinos and Watson, 1982) (Fig.1). To improve the readability of the drawings, in the final version of the figure each dot represents either a cell cluster or small groups of single scattered cells.
Fig. 1.
Distribution of postnatal (P4-A–C) and embryonic (E12-A–C) cerebellar cells transplanted to the embryonic rat brain in utero. For each case, four serial sections, modified from Paxinos and Watson (1982), are shown (top to bottom = medial to lateral). Each dotrepresents cellular aggregates or groups of single scattered cells.
The mature phenotypes adopted by transplanted cells were investigated by labeling with different cell specific markers (see above). However, although all the selected markers are distinctive for different cerebellar cell populations, some of them are also expressed in extracerebellar regions. Hence, phenotype identification of transplanted cells was based on several concurrent criteria: (1) marker expression; (2) typical morphological features; (3) morphometric parameters. This approach allowed us to unequivocally identify glial cells, Purkinje cells, granule cells, and molecular layer interneurons. The other transplanted neurons, a class of medium-sized or large multipolar neurons, in part labeled by different markers (see Results), included presumptive deep nuclei neurons plus a minority of other types that could not be precisely classified (see Results). All these cells were grouped into a single category of “multipolar neurons.”
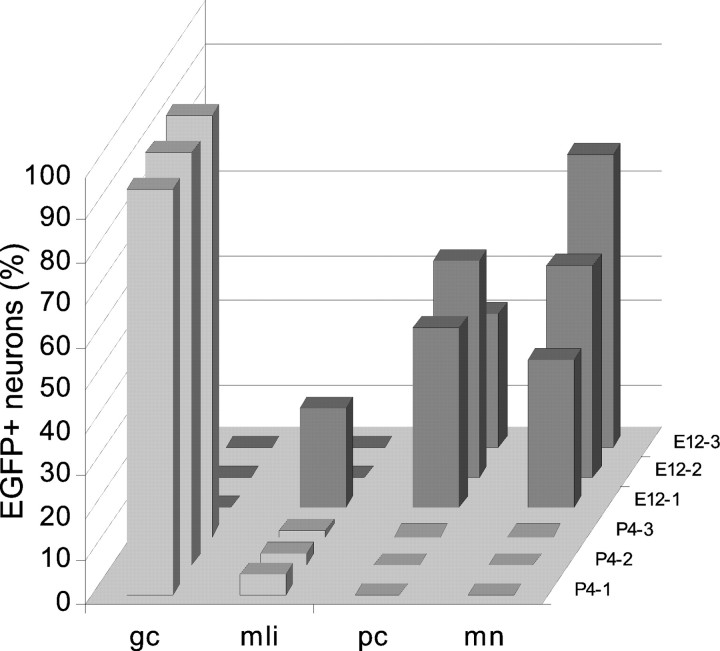
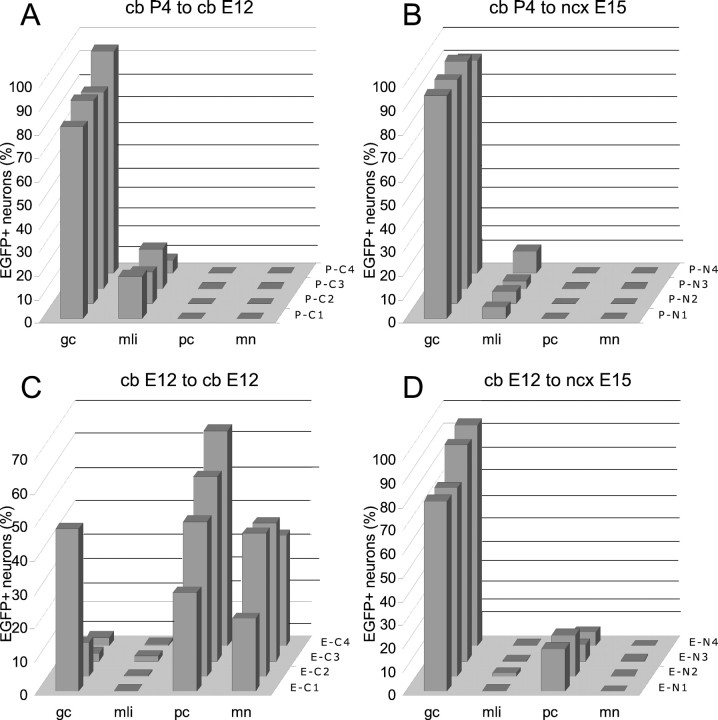
To quantify the phenotypic repertoire generated by ectopically located donor cells we examined parvalbumin immunostained material, where Purkinje cells and molecular layer interneurons could be identified for both morphology and labeling (Celio et al., 1988; Celio, 1990), whereas other cell types were classified according to their structural features. Three cases from each set of animals transplanted in utero with postnatal or embryonic donors (three to five parasagittal sections including mesencephalon and forebrain for each case), and four cerebellar or neocortical explants receiving either donor cell populations were considered for this analysis. The selected animals from in vivo experiments had been killed between P25 and P30, and the explants had been fixed after 15–25 d in vitro, after donor cells had completed their development. All the EGFP-positive cells present in the selected material were counted and classified according to the four categories defined above (i.e., Purkinje cells, granule cells, molecular layer interneurons, multipolar neurons), plus a category of glial cells. In the case of cell clusters, the identification of individual cells was further checked by confocal scanning. The distributions of neuronal phenotypes observed in the different experimental conditions are illustrated in Figures 4 and 8, where each cell type is expressed as percentage of the total number of EGFP-positive neurons.
Fig. 4.
Quantitative analysis of the phenotypic repertoire generated by embryonic (dark gray) and postnatal (light gray) cerebellar cells transplanted to the embryonic rat brain in utero. For each experimental set, three representative cases are illustrated (indicated on thez-axis). The different neuron phenotypes are represented as the percentage on the total number of transplanted nerve cells observed in the relevant brain. Granule cells generated by embryonic progenitors in the lower brainstem are not included in these counts that were performed on sections encompassing mesencephalon and forebrain, where no granule neurons were found. gc, Granule cells; mli, molecular layer interneurons;pc, Purkinje cells; mn, multipolar neurons.
Fig. 8.
A–D, Quantitative analysis of the phenotypic repertoire generated by postnatal (A, B) and embryonic (C, D) cerebellar cells transplanted to cerebellar (A, C) or neocortical (B, D) explants. Each histogram shows the distribution of neuron phenotypes generated by grafted cells in four representative explants from each experimental set (indicated on the z-axis of each histogram). The different cell types are represented as the percentage on the total number of transplanted neurons observed in the relevant explant.gc, Granule cells; mli, molecular layer interneurons; pc, Purkinje cells; mn, multipolar neurons; cb, brainstem-cerebellar explant;ncx, neocortical explant.
To further define the phenotype of the EGFP-positive multipolar neurons, we determined the number of these cells stained by anti-neurofilament SMI32 or anti-parvalbumin antibodies on three vibratome sections from four different cases. The fraction of SMI32-positive neurons was also estimated on a sample of 452 nerve cells from the deep cerebellar nuclei of two adult EGFP mice. In addition, we compared the cell body size of EGFP-positive multipolar neurons in cerebellar explants with that of SMI32-positive host neurons located in the deep nuclei of the same organotypic cultures. To this aim, by means of the Neurolucida system at 40× magnification, we measured the size of the perikaryon of 88 transplanted and 82 host neurons from three different explants grafted with embryonic donor cells. The same approach was used to compare the size of parvalbumin-immunopositive molecular layer interneurons. In this case we sampled 59 transplanted and 47 host cells from three different explants, receiving postnatal donor cells. Statistical comparisons between the obtained values were made by the Student's ttest.
Finally, to estimate the relative numbers of the different types of host cerebellar neurons in the cerebellar explants, we sampled parvalbumin-immunopositive Purkinje cells and molecular layer interneurons and SMI32-positive deep nuclei neurons. The analysis was performed on three hemicerebella from different explants stained by each antibody. The cells were counted on the computer screen in every third microscopic field by moving across the cerebellar region of the explant in a raster manner with the motorized stage of the Neurolucidasystem (magnification was 20×).
RESULTS
To assess the differentiative potential of cerebellar progenitors at different developmental stages, we examined the fate of heterotopically–heterochronically transplanted cells taken from E12 cerebellar anlage or P4 cerebellar cortex. E12 cerebellar cells include proliferating progenitors destined to generate all cerebellar phenotypes, whereas those from the postnatal cortex produce granule cells, molecular layer interneurons, and glia (Miale and Sidman, 1961;Zhang and Goldman, 1996a,b; Altman and Bayer, 1997). Here, we asked whether these progenitors are able to engraft in ectopic positions of the host CNS and acquire local identities, and also whether postnatal cerebellar precursors exposed to the embryonic homotopic environment can be respecified toward cell types generated during fetal life. Donor cells, tagged by EGFP, were transplanted to the E16 rat brain in utero or to mouse explants of E12 brainstem–cerebellum or E15 neocortex.
Fate of postnatal cerebellar progenitors transplanted to the embryonic CNS in utero
Successful transplantation was observed in ∼65% of the treated embryos, in which numerous EGFP-positive cells were dispersed through the host brain or gathered in clusters of variable size and density. The vast majority of transplanted cells were well integrated within extracerebellar regions of the recipient CNS, with very rare elements remaining on the ventricular surface. The distribution of heterotopically located cells was determined by mapping their position on several sections of the recipient brain (Fig. 1). In all the examined animals, transplanted cells were consistently found throughout the host brainstem, mesencephalon, and diencephalon. In addition, EGFP-positive cells were also seen in basal ganglia and corpus callosum, but they were never found in the neocortex, hippocampal formation, or olfactory bulb.
Postnatal cerebellar cells that engrafted in extracerebellar regions of the host CNS generated both neurons and glia. The majority of glial cells were different types of GFAP-positive astrocytes (Fig.2A), well integrated into the recipient glial network. In addition, transplanted cells also produced oligodendrocytes. Immature cells of this category could be identified by anti-NG2 labeling (Fig. 2B), whereas their mature counterparts displayed the typical morphology with several processes aligned to host white matter tracts (Fig. 2C).
Fig. 2.
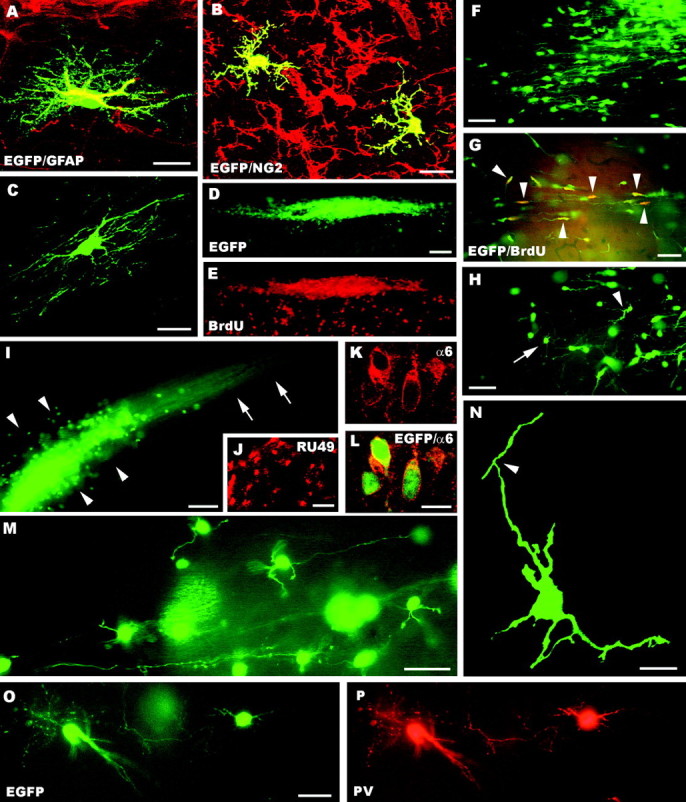
A–P, Fate of postnatal cerebellar cells engrafted in ectopic regions of the embryonic brainin utero. The confocal pictures A–C show glial phenotypes generated by postnatal cerebellar precursors, including GFAP-positive astrocytes (A), NG2-positive immature oligodendrocytes (B, taken from an animal killed at P1), and mature oligodendrocytes properly integrated in host white matter tracts (C). A large aggregate of small, densely packed transplanted cells is shown inD (taken from an animal killed at P1); BrdU labeling of the same microscopic field (E) reveals the intense proliferative activity of these cells (numerous host cells outside the cluster are also labeled). Migrating cells, with typical leading and trailing processes, radiate from such clusters (F) and likely continue to divide, as shown by BrdU incorporation (G, arrowheads point to some double-labeled cells). At the end of their migratory phase (H), the cells show several short processes radiating from the perikaryon (e.g.,arrowhead) and eventually acquire the typical morphology of granule cells (e.g., arrow). Mature granule cells can be found scattered or clustered in aggregates (arrowheads in I) with bundles of parallel fibers (arrows). The identification of these cells is further supported by the expression of RU49 mRNA (J) as well as α6 subunit of the GABAA receptor (K, L). The typical morphology of granule neurons, with small round cell bodies, a few short clawed dendrites and thin varicose axons, is displayed inM. In addition, the confocal image Nshows an immature granule cell bearing the characteristic T-shaped bifurcation (arrowhead) of the parallel fiber.O and P display parvalbumin-immunopositive molecular layer interneurons.GFAP, Glial fibrillary acidic protein; α6, α6 subunit of the GABAA receptor; BrdU, bromodeoxyuridine; PV, parvalbumin; EGFP, enhanced green fluorescent protein. Scale bars: A, C, K, L, N, 10 μm; B, 15 μm; M, O, P, 30 μm; F–H, J, 50 μm; D, E, I, 100 μm.
All EGFP-positive neurons generated by grafting postnatal cerebellar progenitors could be classified in two distinct phenotypes, identified as granule cells and molecular layer interneurons. Both cell types were distributed throughout the different recipient regions, but granule cells always prevailed.
Granule cells were generated through a peculiar developmental sequence. They originated from large clusters made of several hundreds tightly packed small cells (Fig. 2D,E), which were a typical feature of the postnatal cerebellar transplants. Large streams of migratory elements, with characteristic leading and trailing processes, radiated from these clusters into the host parenchyma (Fig.2F). BrdU injections, either before birth or immediately before killing up to several weeks after transplantation, labeled many cells inside the clusters (Fig. 2D,E) or migrating into the host parenchyma (Fig. 2G). In contrast, EGFP-positive cells in such migratory streams were never labeled by any of the applied glial markers, confirming that they belonged to a neuronal lineage.
At the end of the migratory stream the cells displayed transitional morphologies, with ramified processes radiating from the cell body (Fig. 2H), and eventually acquired the structural features of mature granule cells (Fig. 2I,M) with small round perikarya (8–10 μm in diameter), short-clawed dendrites, and thin varicose axons, which sometimes bore the typical T-shaped bifurcation of parallel fibers (Fig. 2N). The identification of these cells was further confirmed by labeling for the α6 subunit of the GABAA receptor (Fig.2K,L), a specific granule cell marker (Kato, 1990;Luddens et al., 1990), and for RU49 mRNA (Fig. 2J), which is selectively expressed in cerebellar and hippocampal granule cells and olfactory bulb interneurons (Yang et al., 1996). Because EGFP fluorescence faded out during in situ hybridization procedures, we could not obtain double labeling for EGFP and RU49. Nevertheless, RU49-positive donor cells could be identified according to the following criteria: (1) in situ hybridization was performed on selected sections in which the position of EGFP-positive cells had been previously assessed, and (2) the analysis was restricted to host regions, in which RU49 is not normally expressed.
On the basis of this sequence of proliferation, migration, and terminal differentiation, we concluded that the clusters of actively dividing cells represent EGL progenitors that generate mature granule neurons after a migratory phase. Granule neurons were either scattered through the recipient parenchyma or clustered as in Figure2I. However, confocal scanning throughout several of such clusters confirmed that, with very rare exceptions, they exclusively contained cells of this type.
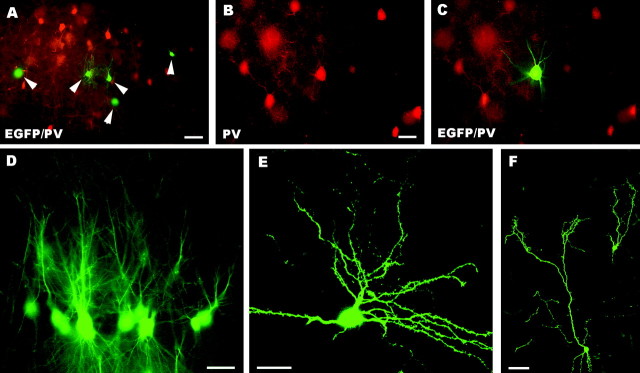
EGFP-positive cells belonging to the second phenotype were small multipolar neurons with slender dendrites and a short axon ending in the vicinity of the parent cell body. These structural characteristics, together with the strong anti-parvalbumin immunolabeling shown by these neurons (Fig. 2O,P), were distinctive features of molecular layer interneurons, i.e., basket and stellate cells. Indeed, even when such cells were close to parvalbumin-positive host neurons they were always clearly recognizable for their size, morphology, and intensity of immunostaining (see Celio, 1990, for an extensive description of parvalbumin distribution in the rat brain).
To quantitatively estimate the phenotypic repertoire generated by grafted cells, we counted all the cells encountered in three to five representative vibratome sections from three different brains (see Fig.4, light gray bars). On the whole, we examined 7057 EGFP-positive cells, including 6858 neurons and 199 glial cells. Among neuronal phenotypes there were 6624 granule cells (96.6%) and 234 molecular layer interneurons (3.4%), uniformly distributed among the different animals. Thus, postnatal cerebellar progenitors are able to engraft in several heterotopic regions of the host CNS, but they exclusively adopt late-generated cerebellar identities.
Fate of embryonic cerebellar progenitors transplanted to the embryonic CNS in utero
The results obtained with P4 cerebellar progenitors indicate that they are committed to cerebellar fates. Hence, we asked whether E12 cerebellar precursors have a wider differentiative potential. Previous observations on the expression of the En-1 gene in heterotopically transplanted mid-hindbrain progenitors indicate that these cells become regionally specified between E10.5 and E13.5 (Olsson et al., 1997). These authors, however, did not follow the fate of donor cells longer than four days post-transplantation and, hence, it is still not clear whether these cells actually developed mature cerebellar phenotypes.
As shown in Figure 1, the distribution of embryonic cerebellar cells in extracerebellar regions of the recipient brain was essentially equivalent to that observed with postnatal donor cells, although the large cell aggregates present in the latter animals were not observed. In addition, in four animals of this set scattered EGFP-positive neurons were also occasionally encountered in the cerebral cortex. In all recipient regions the transplanted cells generated neurons and glia. Among neuronal phenotypes, numerous Purkinje cells could be identified by their morphology and expression of specific markers, such as parvalbumin or calbindin (Fig.3A,B). They displayed highly ramified dendritic trees (Fig. 3A) or, more frequently, less developed arbors surrounding the cell body (Fig. 3B), similar to those of the rare ectopic Purkinje cells present in the intact brain (De Camilli et al., 1984; Rossi and Borsello, 1993). Purkinje axons, recognized for their particularly intense EGFP fluorescence and specific immunostaining, run for long distances through the host parenchyma. Sometimes their terminal branches were apposed to host neurons (Fig. 3C), suggesting heterotypic synaptic contacts. Most frequently, however, they enwrapped the somatodendritic surface of EGFP-positive multipolar nerve cells, identified as presumptive deep nuclear neurons (Fig.3D–F).
Fig. 3.
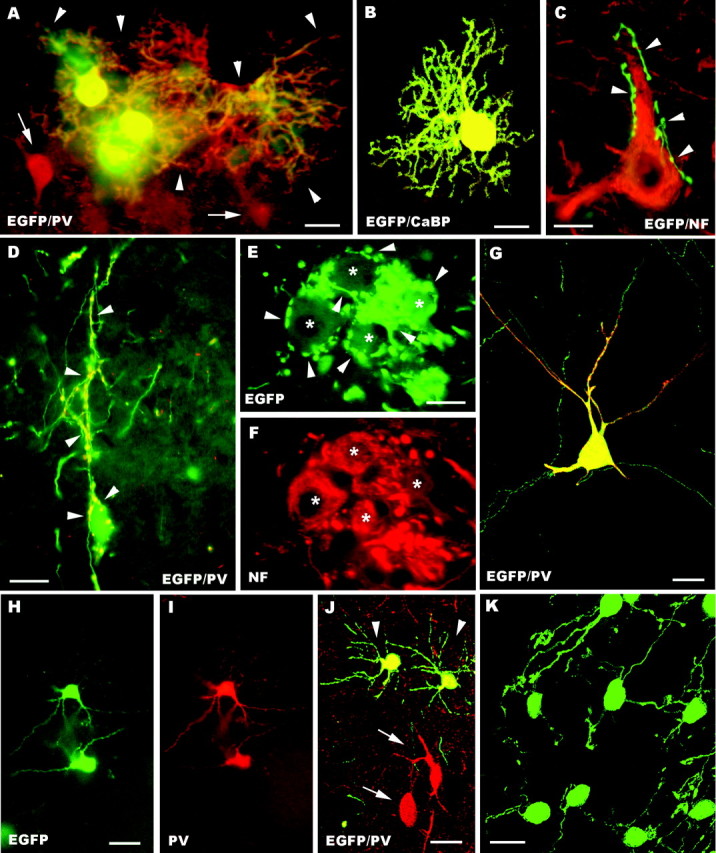
A–K, Fate of embryonic cerebellar cells engrafted in ectopic regions of the embryonic brain in utero. Parvalbumin-immunolabeled Purkinje cells with large dendritic trees (arrowheads) are illustrated inA; arrows point to adjacent parvalbumin-positive host neurons. Another calbindin-immunolabeled transplanted Purkinje cell, bearing less extended dendrites, is shown in the confocal image B. In C a presumptive EGFP-positive Purkinje axon (arrowheads), recognized by the particularly intense fluorescence, terminates on a neurofilament immunostained host neuron. Frequently, Purkinje axons (arrowheads in D point to the parvalbumin-immunolabeled terminal branches) enwrap the somatodendritic surface of EGFP-positive multipolar neurons. E and Fshow a small cluster of these neurons (asterisks), which are labeled by anti-neurofilament antibodies (F) and covered by strongly fluorescent Purkinje axons (arrowheads in E). The morphological features of EGFP-multipolar neurons, bearing slender dendrites with long ramifications, are illustrated in the confocal pictureG; note that this neuron is also double labeled for parvalbumin. H and I show small parvalbumin-immunopositive neurons, classified as molecular layer interneurons. Arrowheads in J point to another two of such neurons settled in the host striatum; note the different size and morphology shown by transplanted and recipient neurons (arrows). A cluster of transplanted granule cells in the host dorsal cochlear nucleus is displayed by the confocal picture K. NF, Neurofilament SMI32; PV, parvalbumin; CaBP, calbindin; EGFP, enhanced green fluorescent protein. Scale bars: K, 10 μm; C, E, F, 15 μm; B, D, 20 μm;A, 25 μm; G–J, 30 μm.
In addition to Purkinje cells, the most frequent EGFP-positive nerve cells were a population of medium-sized or large multipolar neurons, characterized by a fainter fluorescence (Fig. 3D–G). These neurons displayed triangular or spindle-shaped cell bodies and long ramified dendrites (Fig. 3D,G), which were frequently covered by the terminal branches of Purkinje axons (Fig.3D–F). Many of these neurons could be stained by anti-neurofilament antibodies (55%, of 72 sampled neurons) (Fig.3E,F). The SMI32 antibodies stain deep nuclei neurons (Jankovski et al., 1996), which nonetheless encompass different subtypes (De Zeeuw and Berrebi, 1995), including 31% SMI32-negative cells (our personal observation, see Materials and Methods). In addition, in 4 of 15 animals belonging to this experimental set, we found some multipolar neurons stained by anti-parvalbumin antibodies (Fig. 3G) (43 neurons of 323 multipolar neurons sampled from these four animals). Deep nuclei neurons are not normally labeled by these antibodies, but they become immunopositive in particular conditions, such as after colchicine application (Celio, 1990).
Thus, although all these features indicate that many EGFP-positive multipolar nerve cells were deep nuclei neurons, part of these transplanted cells could not be definitely classified. Nevertheless, EGFP-positive neurons with these characteristics were present in many different regions of the host CNS, and they did not display clear site-specific features. For instance, most of the parvalbumin-immunopositive neurons were not in the vicinity of recipient neurons stained by the same antibody. Thus, on the basis of these considerations, as well as of the results of the in vitro experiments (see below), we concluded that these transplanted cells comprise different populations of deep nuclei neurons plus a minority of other cell types, likely including other cerebellar phenotypes such as Golgi and Lugaro cells, and, possibly, cells that had acquired extracerebellar features.
The embryonic cerebellar cells also produced parvalbumin-immunopositive molecular layer interneurons (Fig. 3H–J), although this phenotype was not present in all the examined cases (Fig.4). As for postnatal transplants, these cells could be readily identified for their intense immunolabeling and morphology even when they were intermingled with parvalbumin-immunolabeled host neurons (Fig. 3J). In contrast, EGL progenitors or mature granule cells were never observed in extracerebellar regions of the host CNS, except for the dorsal region of the lower brainstem and, most notably, the dorsal cochlear nucleus (Fig. 3K), a structure bearing strict neuroanatomical (Mugnaini et al., 1980; Mugnaini, 1985) and ontogenetic (Altman and Das, 1966; Taber Pierce, 1967) relationships with the cerebellum. Hence, among 1928 cells sampled from three brains (Fig. 4,dark gray bars), embryonic cerebellar cells generated 1283 glia and 645 neurons, including 268 Purkinje cells (41.6%), 269 multipolar neurons (41.7%), and 108 molecular layer interneurons (16,7%). Altogether, these observations indicate that most E12 cerebellar cells are already specified toward cerebellar identities, but they are endowed with a broader developmental potential than their postnatal counterparts.
Fate of embryonic neocortical progenitors transplanted to the embryonic CNS in utero
Both embryonic and postnatal cerebellar progenitors showed a preferential incorporation into defined regions of the recipient forebrain (Fig. 1), suggestive of site-specific interactions between donor cells and local cues. Alternatively, however, it is possible that the distribution of transplanted cells was influenced by mechanical constraints linked to the injection procedure. To address this issue, we performed control experiments in which E12 neocortical progenitors were transplanted to the embryonic CNS in utero.
As shown in Figure 5A–F, neocortical progenitors showed a wide distribution throughout the host forebrain, including many regions in which cerebellar donor cells did not engraft, such as neocortex (Fig. 5A–C), hippocampus (Fig. 5D), and olfactory bulb (Fig. 5F). In addition, most of the grafted neocortical cells displayed distinct structural features according to their integration site (Fig.5B–F), suggesting that their morphological maturation was influenced by local cues. Thus, the region-specific distribution of cerebellar progenitors within the host forebrain cannot be attributed to particular conditions related to the transplantation procedure.
Fig. 5.
A–F, Fate of embryonic neocortical precursors grafted to the embryonic CNS in utero. Micrograph A shows EGFP-positive embryonic neocortical cells (some are pointed by arrowheads) engrafted in the host neocortex. Some of such neurons (B, C) show the morphology of stellate cells and can be immunolabeled by anti-parvalbumin antibodies. Many of these grafted cells show site-specific morphological features, such as presumptive pyramidal neurons in hippocampal CA1 (D), medium-sized spiny neurons in the striatum (E), and olfactory bulb interneurons (F). PV, Parvalbumin, EGFP, enhanced green fluorescent protein. Scale bars: B–E, 20 μm; A,F, 50 μm.
Fate of postnatal and embryonic cerebellar progenitors homotopically engrafted in the embryonic cerebellum
Despite the high frequency of heterotopically incorporated elements, EGFP-positive cells were found in the cerebellum only in five brains receiving postnatal progenitors and in four grafted with embryonic donor cells. In postnatal cerebellar transplants, aggregates of proliferating EGL progenitors were present in the region of the deep nuclei (Fig. 6A). Mature granule neurons were correctly positioned in the internal granular layer of several cortical lobules (Fig. 6B). These granule cells displayed characteristic clawed dendrites (Fig.6E) and were immunolabeled by antibodies against the α6 subunit of the GABAA receptor (Fig.6F,G). In addition, in some instances we also observed typical ascending axons and parallel fibers oriented along the longitudinal axis of the folium (Fig. 6C,D). Postnatal donor cells also generated some GFAP-positive astrocytes (Fig.6H,I).
Fig. 6.
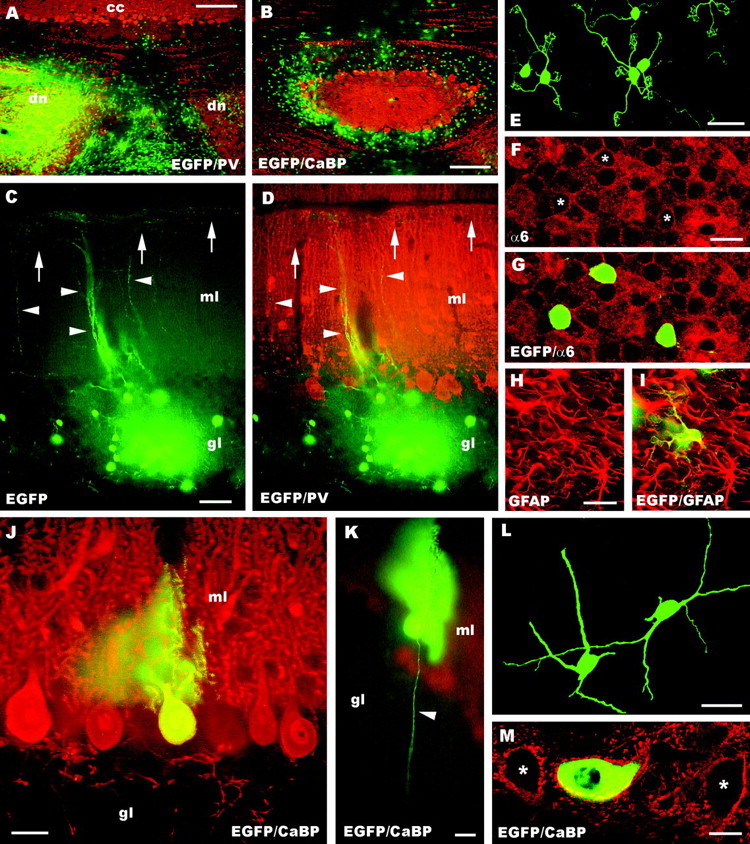
A–M, Fate of postnatal (A–I) and embryonic (J–M) cerebellar cells homotopically engrafted in the embryonic cerebellumin utero. Micrograph A, taken from a rat transplanted with postnatal donor cells, shows a survey of the host cerebellum, stained with anti-parvalbumin antibodies (dn, deep cerebellar nuclei; cc,cerebellar cortex): note the large aggregates of EGFP-positive cells in the region of the deep nuclei. Numerous EGFP-positive cells are also located in the granular layer (B), where they develop into mature granule cells. Such neurons display typical ascending axons (arrowheads in C, D) and parallel fibers (arrows) running along the longitudinal axis of the folium. In addition, they bear characteristic short clawed dendrites (E) and express the α6 subunit of the GABAA receptor (F–G) as their host counterparts (asterisks in F indicate the position of the EGFP-positive neurons). Finally, postnatal cerebellar progenitors also develop into some GFAP-positive astrocytes (H, I). Embryonic progenitors produce granule cells (data not shown) and Purkinje cells (J, K) correctly positioned in the Purkinje cell layer, with monoplanar dendritic trees and thin axons (arrowhead in K) running across the granular layer. In addition, they develop deep nuclei neurons, properly positioned in the central gray matter (L, M). Such transplanted cells acquire the typical multipolar shape (L) and are innervated by Purkinje axons: the confocal picture M shows an EGFP-positive neuron surrounded by calbindin immunopositive Purkinje axon boutons (asterisks point to two adjacent host neurons that are similarly innervated). CaBP, Calbindin;PV, parvalbumin; α6, α6 subunit of the GABAA receptor; EGFP, enhanced green fluorescent protein; ml, molecular layer;gl granular layer. Scale bars: E–G, M, 10 μm; K, 20 μm; J, 25 μm;H, I, 30 μm; L, 40 μm; C, D, 50 μm; B, 150 μm; A, 250 μm.
Embryonic cerebellar cells that settled in the host cerebellum comprised rare Purkinje cells or deep nuclei neurons, usually <10 cells per animal, except for one cerebellum that contained several dozens of Purkinje cells. Purkinje cells, scattered over different host lobules, were correctly positioned in their cortical layer and bore characteristic monoplanar dendritic trees and thin axons traversing the granular layer (Fig. 6J,K). Nuclear neurons were located within the central gray matter, displayed the typical multipolar morphology (Fig. 6L), and were surrounded by numerous parvalbumin- or calbindin-immunolabeled Purkinje cell synaptic boutons (Fig. 6M). Finally, two of these cerebella also contained mature granule cells (data not shown), localized in the recipient granular layer or ectopically positioned in the region of the deep nuclei. These neurons displayed the same morphological features and anti-α6 immunostaining described above for transplantation of postnatal donor cells. Thus, despite the low efficiency of cerebellar incorporation, both embryonic and postnatal donor cells were able to become properly integrated in the host environment, where they generated phenotypes characteristic of their donor age.
Fate of postnatal cerebellar progenitors transplanted to organotypic explants of the embryonic CNS
In line with a previous report (Jankovski et al., 1996), the results of in vivo transplantation experiments indicate that postnatal cerebellar progenitors cannot be respecified toward earlier generated cerebellar phenotypes. However, because we could not transplant into recipient embryos younger than E15–E16, we asked whether the fate of such postnatal progenitors could be changed if they were placed in a more immature cerebellar environment, at the time when Purkinje cells and deep nuclei neurons are generated. To address this issue, we grafted postnatal cerebellar progenitors into E12 embryonic explants of mouse brainstem and cerebellum. In addition, to further test their ability to integrate and differentiate in an ectopic position where they fail to penetrate in vivo, we made similar transplantation experiments into E15 neocortical explants.
The cerebellar region of the brainstem–cerebellar explants (Chédotal et al., 1997; Tashiro et al., 2001) comprised two distinct hemicerebella, with a peripherally located cortex surrounding the deep nuclei. Despite the rather high degree of organization achieved by these organotypic cultures, they remained underdeveloped compared with the cerebellum in vivo. For instance, from three different explants we sampled on the average 35 ± 5 (mean ± SD) neurofilament immunostained deep nuclei neurons, 339 ± 49 Purkinje cells, and 207 ± 25 molecular layer interneurons. The ratio of deep nuclei neurons to Purkinje cells to molecular layer interneurons in the adult cerebellum is 1:15:300 (Ito, 1984), whereas in the explants it was 1:9.7:5.9. The number of granule cells was not estimated, but they were also far less abundant than in the normal cerebellum, because the explants were placed in vitro before the migration of granule cell precursors from the rhombic lip to the cerebellar surface. Thus, although the cultured cerebella contained all major phenotypes, both the total number of neurons and the relative amount of different cell populations were strongly altered, late generated types being particularly affected. The E15 cortical explants were not thoroughly characterized, but they contained all major cortical phenotypes, including large pyramidal neurons and stellate cells.
Numerous EGFP-positive cells engrafted in the brainstem–cerebellar explants and generated neurons and glia. Neuronal types comprised granule cells and molecular layer interneurons. Granule cells showed a similar behavior to that observed in vivo, with large clusters of densely packed precursors, numerous migrating cells radiating into the host tissue (Fig.7A), and mature elements with typical morphology (Fig. 7B). Molecular layer interneurons were readily identified by their morphology and parvalbumin immunolabeling (Fig. 7C). In addition, their perikaryal size [97.5 ± 19 μm2; mean ± SD; minimum (min), 62 μm2; maximum (max), 156 μm2; n = 59] was equivalent to that of their host counterparts (101.7 ± 19.8 μm2; min, 64 μm2; max, 160 μm2; n = 47). In four different explants maintained in vitro for 15–25 d we counted 6426 EGFP-positive cells (4776 neurons and 1650 glia). Neuronal phenotypes comprised 4307 granule cells (90%) and 469 molecular layer interneurons (10%). As shown in Figure8A, the distribution of neuronal phenotypes was essentially similar in all the cultures, with no clear differences between cerebellar and extracerebellar regions.
Fig. 7.
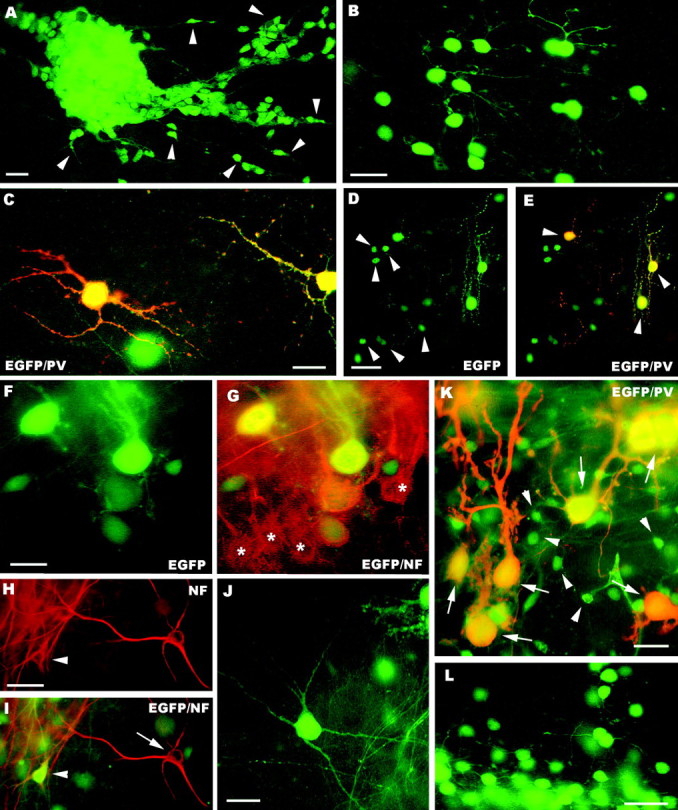
A–H, Fate of postnatal (A–E) and embryonic (F–L) cerebellar cells transplanted to cerebellar (A–C, F–J) or neocortical explants (D, E, K, L). In the cerebellar explants, postnatal cerebellar progenitors form dense aggregates (A) with numerous migrating elements (some are pointed byarrowheads). Mature granule cells, bearing typical morphological features, are shown in B, whereas parvalbumin-immunopositive molecular layer interneurons are displayed in C. D and E illustrate the neuronal phenotypes generated by postnatal cerebellar cells grafted to neocortical explants, granule cells (arrowheads in D) and molecular layer interneurons (arrowheadsin E). The latter are also labeled by anti-parvalbumin antibodies (E). The micrographs Fand G show EGFP-positive Purkinje cells derived from embryonic precursors intermingled with their host counterparts (asterisks in G) in the cerebellar cortex of the explant. Examples of EGFP-positive multipolar neurons in cerebellar explants are illustrated in H–J. Some of these neurons (arrowhead in H andI) are localized in the deep nuclei of the explant, and can be stained by anti-neurofilament antibodies (arrow in I points to an immunolabeled host neuron). In neocortical explants embryonic cerebellar progenitors also generate parvalbumin-immunopositive Purkinje cells (arrows in K) with variably shaped dendritic trees and granule cells (K, L, some are pointed by arrowheads in K).PV, Parvalbumin; NF, neurofilament;EGFP, enhanced green fluorescent protein. Scale bars:B, C, 20 μm; A, F, G, K, 25 μm;L, 30 μm; D, E, H–J, 50 μm.
Similar results were obtained when the postnatal cerebellar progenitors were grafted to neocortical explants, although a lower amount of cells survived in this condition. Among 1167 EGFP-positive cells from four different cultures there were 972 neurons and 195 glial cells (Fig.7D,E), including 912 granule and (93.8%) and 60 parvalbumin-immunopositive molecular layer interneurons (6.2%) (Fig.8B). Hence, the results of these in vitroexperiments confirmed those obtained in vivo by showing that postnatal cerebellar progenitors are strictly committed to generate postnatal cerebellar phenotypes.
Fate of embryonic cerebellar progenitors transplanted to organotypic explants of the embryonic CNS
Embryonic cerebellar progenitors also engrafted in the brainstem–cerebellar explants and produced different mature phenotypes. Numerous Purkinje cells were dispersed in the extracerebellar regions of the explant or well integrated in the cerebellar cortex, and they developed mature features in parallel with their host counterparts (Fig. 7F,G). Similar to the in vivo experiments, these explants also contained a population of medium-sized or large EGFP-positive multipolar neurons (Fig.7H–J). These cells, frequently labeled by anti-neurofilament antibodies, were present all over the explant, including the deep nuclei (Fig. 7H,I), where they were mixed to host neurons and innervated by Purkinje axons (data not shown). Morphometric evaluation of these neurons revealed a mean cell body size of 322.6 μm2 (±141.7; mean ± SD; min, 110 μm2; max, 836 μm2; n = 88). This value was clearly higher than that of molecular layer interneurons (see above; Student's t test;p < 0.0001), but also significantly lower than that of host neurofilament-immunopositive deep nuclei nerve cells (mean perikaryal size, 385 ± 117.7 μm2; min, 191 μm2; max, 836 μm2; n = 82; Student'st test; p = 0.002). Comparison of the frequency distributions of cell body sizes indicated that the EGFP-positive cells include the large neurofilament-positive neurons plus a subset of medium sized nerve cells that are not stained by SMI-32 antibodies. These data corroborate the conclusion drawn fromin vivo experiments that this category of transplanted cells encompasses deep nuclei neurons and a minority of other cell types that could not be definitely identified.
In addition to these phenotypes, EGFP-positive cells also generated parvalbumin-immunopositive molecular layer interneurons and granule cells (data not shown). These types, however, represented a minority of the transplanted cells. Indeed, among 6980 cells from four explants we counted 4515 neurons (64.7%) and 2465 glial cells (35.3%). Neuronal phenotypes included 1997 Purkinje cells (44.2%), 1395 multipolar neurons (31%), 1095 granule cells (24.2%), and 28 molecular layer interneurons (0.6%). As shown in Figure 8C, granule cells were not uniformly distributed in the four examined explants, being rare in several cases. Thus, although E12 cerebellar cells grafted to homotopic–homochronic explants are able to produce all cerebellar phenotypes, they preferentially adopt earlier generated identities.
In a parallel set of experiments we tested whether E12 cerebellar progenitors can integrate in explants of neocortex, a region where they rarely penetrate in vivo. Transplanted cells were viable also in these explants and acquired different neuronal and glial phenotypes. The vast majority of EGFP-positive nerve cells were Purkinje cells, with variably shaped dendritic trees (Fig.7K) and granule cells displaying typical morphological features (Fig. 7K,L). In contrast, other phenotypes, such as multipolar neurons or molecular layer interneurons, were extremely rare. Cell counts (Fig. 8D) yielded 5996 neurons and 2461 glial cells, which included 5261 granule cells (87.7%), 683 Purkinje cells (11.3%), 35 molecular layer interneurons (0.6%), and 17 multipolar neurons (0.3%). On the whole, the results of in vitro experiments further confirm the conclusion that embryonic cerebellar cells are committed to cerebellar fates, but, contrary to their postnatal counterparts, they have the potential to generate all major cerebellar types. Nevertheless, the ratio of mature phenotypes that will be actually generated is influenced by the environment in which the transplanted cells incorporate.
DISCUSSION
To assess the differentiative potential of cerebellar precursors at different developmental stages, we examined the fate of embryonic and postnatal progenitors after heterotopic–heterochronic transplantation to the embryonic CNS. Our results show that: (1) cerebellar cells of both ages engraft and produce mature neurons and glia that survive long after transplantation in wide areas of the host brain, but they show a characteristic region-specific distribution; (2) most ectopically located cells adopt cerebellar identities; (3) embryonic progenitors are able to generate all cerebellar neuron phenotypes, whereas postnatal ones are restricted to granule cells and molecular layer interneurons. Altogether, these observations indicate that cerebellar progenitors are regionally specified to local fates already at E12, and their potential is gradually restricted toward late-generated identities as development advances.
Distribution of transplanted cerebellar cells into the host CNS
Both embryonic and postnatal cerebellar cells transplantedin utero integrate in the host brainstem, diencephalon, and in some telencephalic structures, but they are seldom found in the cerebral cortex. Such a distribution pattern is consistent with another report concerning E14 donor cells (Olsson et al., 1998). However, despite their poor engraftment into the recipient neocortex in vivo, cerebellar cells survive and differentiate into neocortical explants. Although this might simply reflect different conditions between in vivo and in vitroexperiments, it is likely that preferential integration rather than survival capability limits in vivo colonization of the telencephalon by grafted cerebellar precursors.
Different types of neural progenitors transplanted into the developing brain incorporate in ectopic positions, but each donor population preferentially engrafts into specific recipient regions (Brüstle et al., 1995; Campbell et al., 1995; Fishell, 1995; Magrassi et al., 1996; Lim et al., 1997; Na et al., 1998; Olsson et al., 1998; Yang et al., 2000). Previous reports indicate that transplanted mid-hindbrain precursors rarely settle in rostral CNS regions (Campbell et al., 1995;Olsson et al., 1997). We also show differential distributions for cerebellar and neocortical donors, suggesting that region-specific engraftment is not caused by mechanical constraints, but may be an active phenomenon. Integration preferences of transplanted progenitors may be determined by their adhesive properties (Götz et al., 1996) and can be modified by removing surface recognition molecules (Olsson et al., 1998). Alternatively, cells initially transplanted into a certain region may sense external cues and emigrate into more favorable ones. Finally, the distribution of grafted cells might be also conditioned by their capability of surviving in different host sites. In any case, the ability of cerebellar cells to engraft preferentially into specific ectopic regions is defined since early embryonic development and maintained thereafter.
EGFP-positive cells incorporate in the cerebellum only in some cases. It is likely that very few proliferating donor cells engraft into the E16 cerebellar anlage, because of its small dimensions and distant position from the transplantation site in the forebrain ventricles. Indeed, cerebellar localization of transplanted cells has been rarely reported (Lim et al., 1997; Yang et al., 2000), and transplanted cells are frequently in the deep nuclei (Yang et al., 2000; this study), suggesting that they enter the cerebellar primordium through the ventricular neuroepithelium.
Mature phenotypes of transplanted cerebellar cells
Another goal of our work was to determine whether heterotopically transplanted cerebellar cells adopted site-specific phenotypes. Respecification of grafted precursors toward local identities has been shown for several progenitor cell populations (Brüstle et al., 1995; Campbell et al., 1995; Magrassi and Graziadei, 1996; Lim et al., 1997). This phenomenon, however, is influenced by different factors such as the age of donor cells (Olsson et al., 1998; Desai and McConnell, 2000), their ability to aggregate after grafting (Magrassi and Graziadei, 1996; Magrassi et al., 1998), the neurogenic activity in the recipient region (Brüstle et al., 1995; Campbell et al., 1995), and the position of the integration site along the neuraxis (Na et al., 1998). Consequently, in many instances grafted cells retain donor-specific features (Magrassi and Graziadei, 1996; Magrassi et al., 1998; Na et al., 1998; Yang et al., 2000).
The intraventricular injection of single cell suspensions and the wide dispersion of transplanted cells through the host parenchyma indicate that, in our experiments, proliferating cerebellar progenitors were effectively exposed to the host environment. Nevertheless, most transplanted cells generated cerebellar neuron phenotypes, which could be readily identified by morphological criteria and cell-specific markers. Only a minority of the EGFP-positive multipolar neurons derived from E12 progenitors escaped a reliable classification. These cells lacked clear distinctive characteristics, but they were consistently observed in all recipient regions, including homotopic cerebellar explants, and they did not display definite site-specific features. Furthermore, although some of the markers used in this study also stained defined populations of extracerebellar neurons, we never observed any grafted cerebellar cells displaying unusual host-specific expression of such markers (e.g., calbindin- or parvalbumin-immunopositive granule cells). Thus, although we could not identify every single transplanted cell, and we cannot exclude that a lean minority of them actually changed their fate or expressed host-specific markers, our results indicate that respecification of cerebellar progenitors toward heterotypic phenotypes was a rare event, limited to embryonic progenitors.
Previous observations limited to 4 d after transplantation also indicate that mid-hindbrain progenitors become regionally specified between E10.5 and E13.5 (Olsson et al., 1997). Nevertheless, it has been reported that postnatal cerebellar progenitors adopt site-specific phenotypes in the postnatal dentate gyrus (Vicario-Abejón et al., 1995), although they fail to change their fate in the adult subventricular zone (Jankovski and Sotelo, 1996) or hippocampus (Bahn et al., 1999). We did not find cerebellar cells in the hippocampus, and we cannot exclude that they may acquire local identities when directly implanted in this region. Nonetheless, our observations indicate that, despite their ability to integrate in many ectopic positions, most, if not all, cerebellar progenitors are committed toward cerebellar identities, at least by E12.
The differentiative potential of embryonic and postnatal cerebellar cells
Despite their specification toward cerebellar fates, embryonic and postnatal progenitors show different developmental potentialities. Embryonic precursors transplanted to the early postnatal cerebellum produce a variety of mature phenotypes, whereas EGL progenitors are restricted to a granule cell fate (Gao and Hatten, 1994), although their potential can be enlarged by immortalization (Snyder et al., 1992; Gao and Hatten, 1994; Snyder, 1994). In addition, postnatal cerebellar cells inserted into embryonic cerebellar grafts exclusively generate granule cells and molecular layer interneurons, suggesting that they are not responsive to embryonic cues (Jankovski et al., 1996). The present observations further extend this conclusion by showing that, in any heterotopic and heterochronic conditions, postnatal progenitors only produce late-generated identities, whereas embryonic precursors are able to generate all major cerebellar neuron types. Altogether, our results suggest that cerebellar progenitors are committed to local fates soon after the formation of the cerebellar primordium, and they are progressively restricted to late phenotypes as development advances.
In the different transplants, postnatal precursors always produced granule cells and molecular layer interneurons. In contrast, the phenotypic repertoire generated by the embryonic donors was not constant in all conditions. Both in heterotopic positions in vivo and in the cerebellar explants they preferentially adopted earlier-generated identities, whereas granule cells and molecular layer interneurons were less frequent. Nevertheless, the latter phenotypes were abundant in other conditions, most notably granule cells in the cerebellum in vivo or in neocortical explants, showing that embryonic cell suspensions did contain progenitors able to produce these types. As shown for rhombic lip cells (Alder et al., 1996), it is likely that E12 cerebellar precursors are committed to cerebellar fates, but they are not competent to produce all phenotypes and require additional instructive information to adopt late-generated identities. Alternatively, however, it is possible that embryonic donor cells stopped proliferating soon after transplantation and, hence, just failed to generate appropriate numbers of late phenotypes. Indeed, host neurons in the cerebellar explants also showed a similar prevalence of early over late-generated identities. On the other hand, the peculiar phenotypic repertoire generated by embryonic cerebellar cells in some conditions, e.g., neocortical explants, where they mostly produced Purkinje and granule cells, indicates that different brain regions may provide peculiar patterns of environmental signals, which favor the development of specific cell types. For instance, the engraftment of granule cells might be conditioned by the local availability of Sonic Hedgehog (Wechsler-Reya and Scott, 1999; Solecki et al., 2000). Hence, although embryonic cerebellar precursors have the potential to produce all cerebellar identities, the actual repertoire of mature phenotypes that will be generated is conditioned by local cues.
It is unclear whether such cues select distinct subsets of precursors already oriented toward specific identities or they provide instructive information to multipotent progenitors. Some studies indicate that individual cerebellar lineages get separated during early embryonic development (Alder et al., 1996; Baader et al., 1999). On the other hand, the observation that multiple cerebellar phenotypes may be clonally related (Mathis et al., 1997) suggests that precisely timed sequences of environmental signals induce the differentiation of specific phenotypes and progressively restrict progenitors' potential, as proposed for the generation of layer-specific types in the cerebral cortex (Desai and McConnell, 2000). Specific experiments are now required to elucidate this point.
Footnotes
This work was supported by grants from Ministero dell'Universitàe della Ricerca Scientifica e Tecnologica, Ministero della SanitàProgetto Alzheimer (300RFA00/01–05) (F.R., L.M.), and Consiglio Nazionale delle Ricerche (Progetto Strategico basi biologiche delle malattie degenerative del sistema nervoso centrale). We are indebted to Dr. Masaru Okabe for the gift of EGFP mice and to Dr. Nathaniel Heintz for providing us with the RU49 mRNA probe. We also thank Mrs. Luisella Milano, Pina Dellisanti, and Graziella Milano for precious technical assistance.
Correspondence should be addressed to Ferdinando Rossi, Rita Levi Montalcini Centre for Brain Repair, Department of Neuroscience, University of Turin, Corso Raffaello 30, I-10125 Turin, Italy. E-mail:ferdinando.rossi@unito.it.
REFERENCES
- 1.Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure and functions. CRC; New York: 1997. [Google Scholar]
- 3.Altman J, Das G. Autoradiographic and histological studies of postnatal histogenesis. II. A longitudinal investigation of kinetics, migration and transformation of cells incorporating thymidine in infant rat with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–390. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 4.Alvarado-Mallart RM. Fate and potentialities of the avian mesencephalic/metencephalic neuroepithelium. J Neurobiol. 1993;24:1341–1355. doi: 10.1002/neu.480241007. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:19–35. doi: 10.1016/s0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 6.Baader SL, Bergmann M, Merz K, Fox PA, Gerdes J, Oberdick J, Schilling K. The differentiation of cerebellar interneurons is independent of their mitotic history. Neuroscience. 1999;90:1243–1254. doi: 10.1016/s0306-4522(98)00563-6. [DOI] [PubMed] [Google Scholar]
- 7.Bahn S, Wisden W, Dunnett SB, Svendsen C. The intrinsic specification of γ aminobutyric acid type A receptor α6 subunit gene expression in cerebellar granule cells. Eur J Neurosci. 1999;11:2194–2198. doi: 10.1046/j.1460-9568.1999.00662.x. [DOI] [PubMed] [Google Scholar]
- 8.Barbe MF, Levitt P. The early commitment of fetal neurons in the limbic cortex. J Neurosci. 1991;11:519–533. doi: 10.1523/JNEUROSCI.11-02-00519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brüstle O, Maskos U, McKay RDG. Host-guided migration allows targeted introduction of neurons into the embryonic brain. Neuron. 1995;15:1275–1285. doi: 10.1016/0896-6273(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K, Olsson M, Björklund A. Regional incorporation and site-specific differentiation of striatal precursors transplanted to the embryonic forebrain ventricle. Neuron. 1995;15:1259–1273. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 11.Carletti B, Rossi F, Magrassi L. Specification of cerebellar progenitors after heterochronic/heterotopic transplantation. Soc Neurosci Abstr. 2001;27:234.6. doi: 10.1523/JNEUROSCI.22-16-07132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo E, Magrassi L, Butti G, Santi L, Giavazzi A, Pezzotta S. A short term analysis of the behaviour of conditionally immortalized neuronal progenitors and primary neuroepithelial cells implanted into the fetal rat brain. Brain Res Dev Brain Res. 1994;83:197–208. doi: 10.1016/0165-3806(94)00137-5. [DOI] [PubMed] [Google Scholar]
- 13.Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 14.Celio MR, Baier W, Schärer L, De Viragh PA, Gerday CH. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988;9:81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 15.Chédotal A, Bloch-Gallego E, Sotelo C. The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development. 1997;124:861–870. doi: 10.1242/dev.124.4.861. [DOI] [PubMed] [Google Scholar]
- 16.De Camilli P, Miller PE, Levitt P, Walter U, Greengard P. Anatomy of cerebellar Purkinje cells in the rat determined by a specific immunohistochemical marker. Neuroscience. 1984;11:761–817. doi: 10.1016/0306-4522(84)90193-3. [DOI] [PubMed] [Google Scholar]
- 17.Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 18.De Zeeuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 19.Fishell G. Striatal precursors adopt cortical identities in response to local cues. Development. 1995;121:803–812. doi: 10.1242/dev.121.3.803. [DOI] [PubMed] [Google Scholar]
- 20.Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 21.Gao W-Q, Hatten ME. Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development. 1994;120:1059–1070. doi: 10.1242/dev.120.5.1059. [DOI] [PubMed] [Google Scholar]
- 22.Götz M, Wizenmann A, Reinhardt S, Lumsden A, Price J. Selective adhesion of cells from different telencephalic regions. Neuron. 1996;16:551–564. doi: 10.1016/s0896-6273(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 23.Ito M. The cerebellum and neural control. Raven; New York: 1984. [Google Scholar]
- 24.Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Jankovski A, Rossi F, Sotelo C. Neuronal precursors in the postnatal mouse cerebellum are fully committed cells: evidence from heterochronic transplantation. Eur J Neurosci. 1996;8:2308–2320. doi: 10.1111/j.1460-9568.1996.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 26.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 27.Kato KJ. Novel GABAA receptor α subunit is expressed only in cerebellar granule cells. J Mol Biol. 1990;214:619–624. doi: 10.1016/0022-2836(90)90276-r. [DOI] [PubMed] [Google Scholar]
- 28.Lim DA, Fishell GJ, Alvarez-Buylla A. Postnatal mouse subventricular zone neuronal precursors can migrate and differentiate within multiple levels of the developing neuraxis. Proc Natl Acad Sci USA. 1997;94:14832–14836. doi: 10.1073/pnas.94.26.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luddens H, Pritchett DB, Kohler M, Killisch I, Keinamen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for a behavioral alcohol antagonists. Nature 346 1990. 648:651. [DOI] [PubMed] [Google Scholar]
- 30.Lumsden A, Clarke JDW, Keynes R, Fraser S. Early phenotypic choices by neuronal precursors, revealed by clonal analysis of the embryonic chick hindbrain. Development. 1994;120:1581–1589. doi: 10.1242/dev.120.6.1581. [DOI] [PubMed] [Google Scholar]
- 31.Magrassi L, Graziadei PPC. Lineage specification of olfactory neural precursor cells depends on continuous cell interactions. Brain Res Dev Brain Res. 1996;96:11–27. doi: 10.1016/0165-3806(96)00068-5. [DOI] [PubMed] [Google Scholar]
- 32.Magrassi L, Pezzotta S, Butti G, Conti L, Govoni S, Cattaneo E. Scattered primary and conditionally immortalized neuroepithelial cells transplanted into the embryonic rat brain differentiate into neurons and glia. Neurosci Res Commun. 1996;18:175–183. [Google Scholar]
- 33.Magrassi L, Ehrlich ME, Butti G, Pezzotta S, Govoni S, Cattaneo E. Basal ganglia precursors found in aggregates following embryonic transplantation adopt a striatal phenotype in heterotopic locations. Development. 1998;125:2847–2855. doi: 10.1242/dev.125.15.2847. [DOI] [PubMed] [Google Scholar]
- 34.Mathis L, Bonnerot C, Puelles L, Nicolas JF. Retrospective analysis of the cerebellum using genetic laacZ/lacZ mouse mosaics. Development. 1997;124:4089–4104. doi: 10.1242/dev.124.20.4089. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy M, Turnbull DH, Walsh CA, Fishell G. Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J Neurosci. 2001;21:6772–6781. doi: 10.1523/JNEUROSCI.21-17-06772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- 37.Mugnaini E. GABA neurons in the superficial layers of the rat dorsal cochlear nucleus: light and electron microscopy immunocytochemistry. J Comp Neurol. 1985;235:61–81. doi: 10.1002/cne.902350106. [DOI] [PubMed] [Google Scholar]
- 38.Mugnaini E, Warr BW, Osen KK. Distribution and light microscopic observations of granule cells in the cochlear nuclei of the cat, rat, and mouse. J Comp Neurol. 1980;191:581–606. doi: 10.1002/cne.901910406. [DOI] [PubMed] [Google Scholar]
- 39.Na E, McCarthy M, Neyt C, Lai E, Fishell G. Telencephalic progenitors maintain anteroposterior identities cell autonomously. Curr Biol. 1998;8:987–990. doi: 10.1016/s0960-9822(98)70403-8. [DOI] [PubMed] [Google Scholar]
- 40.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 41.Olsson M, Campbell K, Turnbull DH. Specification of mouse telencephalic and mid-hindbrain progenitors following heterotopic ultrasound-guided embryonic transplantation. Neuron. 1997;19:761–772. doi: 10.1016/s0896-6273(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 42.Olsson M, Bjerregaard K, Winkler C, Gates M, Björklund A, Campbell K. Incorporation of mouse neural progenitors transplanted in the rat embryonic forebrain is developmentally regulated and dependent on regional adhesive properties. Eur J Neurosci. 1998;10:71–85. doi: 10.1046/j.1460-9568.1998.00015.x. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1982. [DOI] [PubMed] [Google Scholar]
- 44.Ramón y Cajal S. Histologie du Système Nerveux de l'Homme et des Vertébrés. Maloine; Paris: 1911. [Google Scholar]
- 45.Rao M. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Rossi F, Borsello T. Ectopic Purkinje cells in the adult rat: olivary innervation and different capabilities of migration and development after grafting. J Comp Neurol. 1993;377:70–82. doi: 10.1002/cne.903370105. [DOI] [PubMed] [Google Scholar]
- 47.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labeled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 48.Solecki DJ, Liu X, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of granule neuron precursors by maintaining proliferation. Neuron. 2000;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 49.Snyder EY. Grafting immortalised neurons to CNS. Curr Opin Neurobiol. 1994;4:742–751. doi: 10.1016/0959-4388(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 50.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartweig EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 51.Taber Pierce E. Histogenesis of the dorsal and ventral cochlear nuclei in the mouse. An autoradiographic study. J Comp Neurol. 1967;131:27–54. doi: 10.1002/cne.901310104. [DOI] [PubMed] [Google Scholar]
- 52.Tashiro Y, Miyahara M, Shirasako R, Okabe M, Heizmann CW, Murakami F. Local nonpermissive and oriented permissive cues guide vestibular axons to the cerebellum. Development. 2001;128:973–981. doi: 10.1242/dev.128.6.973. [DOI] [PubMed] [Google Scholar]
- 53.Vicario-Abejón C, Cunningham MG, Mc Kay RDG. Cerebellar precursors transplanted to the neonate dentate gyrus express features characteristic of hippocampal neurons. J Neurosci. 1995;15:6351–6363. doi: 10.1523/JNEUROSCI.15-10-06351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 55.Wingate RJT. The rhombic lip and early cerebellar development. Curr Opin Neurobiol. 2001;11:82–88. doi: 10.1016/s0959-4388(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Mujtaba T, Venkatraman G, Wu YY, Rao MS, Luskin MB. Region-specific differentiation of neural tube-derived neuronal restricted progenitor cells after heterotopic transplantation. Proc Natl Acad Sci USA. 2000;97:13366–13371. doi: 10.1073/pnas.97.24.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang XW, Zhong R, Heintz N. Granule cell specification in the developing mouse brain as defined by expression of the zinc-finger transcription factor RU49. Development. 1996;122:555–566. doi: 10.1242/dev.122.2.555. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Goldman JE. Generation of cerebellar interneurons by dividing progenitors in the white matter. Neuron. 1996a;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Goldman JE. Developmental fates and migratory pathways of dividing progenitors in the postnatal rat cerebellum. J Comp Neurol. 1996b;370:536–550. doi: 10.1002/(SICI)1096-9861(19960708)370:4<536::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]