Abstract
Glycogen synthase kinase-3 (GSK-3) was generally considered a constitutively active enzyme, only regulated by inhibition. Here we describe that GSK-3 is activated by lysophosphatidic acid (LPA) during neurite retraction in rat cerebellar granule neurons. GSK-3 activation correlates with an increase in GSK-3 tyrosine phosphorylation. In addition, LPA induces a GSK-3-mediated hyperphosphorylation of the microtubule-associated protein tau. Inhibition of GSK-3 by lithium partially blocks neurite retraction, indicating that GSK-3 activation is important but not essential for the neurite retraction progress. GSK-3 activation by LPA in cerebellar granule neurons is neither downstream of Gαi nor downstream of Gαq/phospholipase C, suggesting that it is downstream of Gα12/13. Overexpression of constitutively active Gα12 (Gα12QL) and Gα13(Gα13QL) in Neuro2a cells induces upregulation of GSK-3 activity. Furthermore, overexpression of constitutively active RhoA (RhoAV14) also activates GSK-3 However, the activation of GSK-3 by Gα13 is blocked by coexpression with C3 transferase, whereas C3 does not block GSK-3 activation by Gα12. Thus, we demonstrate that GSK-3 is activated by both Gα12 and Gα13 in neuronal cells. However, GSK-3 activation by Gα13 is Rho-mediated, whereas GSK-3 activation by Gα12 is Rho-independent. The results presented here imply the existence of a previously unknown mechanism of GSK-3 activation by Gα12/13 subunits.
Keywords: Gα12/13, GSK-3 activation, lysophosphatidic acid, neurite retraction, tau hyperphosphorylation, RhoA
Glycogen synthase kinase-3 (GSK-3) is a key regulator in several physiological processes, such as cell cycle, oncogenesis, apoptosis, and development (Ferkey and Kimelman, 2000). GSK-3 was originally identified as one of the serine–threonine protein kinases that phosphorylate and inhibit glycogen synthase (Rylatt et al., 1980), but it has a number of cellular targets (Woodgett et al., 1993). Until recently, it was believed that GSK-3 is a constitutively active enzyme, whose activity is decreased in response to cell stimulation. The best known signaling pathways that, when active, inhibit GSK-3, are the insulin and the Wnt pathways (Welsh and Proud, 1993; Cook et al., 1996). However, activation of kinases such as PKA and integrin-linked kinase also induce GSK-3 inhibition (Delcommenne et al., 1998; Fang et al., 2000).
However, there are some recent reports that indicate that GSK-3 can be activated, in response to some cellular stimuli. GSK-3 activity is increased in some neuronal cell culture models of apoptosis, neurodegeneration, and in an in vivo model of focal ischemia (Bhat et al., 2000). Additionally, different stimuli produce a transient activation of GSK-3, accompanied by an increase of tau phosphorylation in neuroblastoma cells (Hartigan and Johnson, 1999;Lesort et al., 1999). According to this line of evidence, our own recent results indicate that GSK-3 is activated during lysophosphatidic acid (LPA)-induced neurite retraction, in the SH-SY5Y human neuroblastoma cell line. This is accompanied by the hyperphosphorylation of tau protein blocked by the tyrosine kinase inhibitor genistein, suggesting that GSK-3 activation could be downstream of Gα12/13, in the Rho pathway (Sayas et al., 1999).
GSK-3β is highly expressed in central nervous system (Takahashi et al., 1994) and directly phosphorylates several neuronal microtubule-associated proteins (MAPs), involved in microtubule (MT) stabilization (Goold et al., 1999; Sanchez et al., 2000; Sperber et al., 1995). A member of the Wnt family, Wnt-7a, has been recently implicated in the regulation of axonal remodeling by inhibiting GSK-3β (Hall et al., 2000). GSK-3β inhibition by Wnt 7-a induces a decrease in the phosphorylated forms of neuronal MAPs, with a concomitant reorganization of MT (Salinas, 1999). This suggests that the direct cell shape reorganization induced by Wnt signaling could be mediated by GSK-3β inhibition and its effects on MAPs phosphorylation and MT rearrangement.
In the present study, we have tested, first, whether GSK-3 is activated by LPA in primary neurons, and second, where GSK-3 is located in the signaling pathways downstream of LPA binding to its endothelial differentiating gene (EDG) receptor (Contos et al., 2000). Our results indicate that LPA induces neurite retraction of cerebellar granule cells, accompanied by hyperphosphorylation of tau. Both processes are at least partially prevented by lithium, a specific GSK-3 inhibitor. Direct measurement of GSK-3 activity confirms that LPA activates GSK-3 in these neurons. This activation correlates with an increase in GSK-3 tyrosine phosphorylation. Our data show that GSK-3 activation in these neurons is neither downstream of Gi nor downstream of Gq. To investigate further whether the increase of GSK-3 activity is downstream of Gα12/13, we used the LPA-responsive neuroblastoma Neuro2A. We show that both constitutively active Gα12 and Gα13activate GSK-3. The C3 exoenzyme partially inhibits GSK-3 activation by Gα13, indicating that GSK-3 activation by Gα13 is mediated by the small GTPase RhoA. These findings demonstrate a novel mechanism of activation of GSK-3.
MATERIALS AND METHODS
Cell cultures and LPA treatment. Neuro2a mouse neuroblastoma cells were routinely grown in DMEM with 10% FCS containing glutamine (2 mm) and antibiotics (penicillin and streptomycin). To obtain a neuronal-like phenotype, cells were maintained for 24 hr in serum-free Neurobasal medium supplemented with B-27. To initiate neurite retraction, 10 μm lysophosphatidic acid [1-oleyl-9-(cis)-2-lyso-sn-glycero-3 phosphate] (LPA) (Sigma, St. Louis, MO) was added to differentiated cells. Controls without LPA were examined simultaneously.
Cerebellar granule neurons were isolated from postnatal day 7 (P7) rats, as previously described (Levi. et al., 1989), plated onto poly-l-lysine-coated dishes, and grown in serum-free Neurobasal medium, supplemented with B-27 and 12.5 mm KCl, for 24 hr. LPA was added to these neurons at a final concentration of 10 μm. GSK-3 involvement in neurite retraction was tested using lithium, a GSK-3 inhibitor (Klein and Melton, 1996). Because lithium is also an inositol monophosphatase inhibitor, we performed all of our experiments with lithium in the presence of an excess of myo-inositol, to avoid an effect due to inositol depletion. Cells were pretreated with lithium (10 mm) and myo-inositol (5 mm) for 4 hr before LPA addition.
Northern blot analysis of EDG-2 receptor. Total RNA was purified from primary cultures of P7 cerebellar granule neurons and from NIH 3T3. For Northern Blot analysis, RNA (5 μg/lane) was electrophoresed in formaldehyde–agarose gels and blotted onto nylon membranes by a positive-pressure system (Stratagene, La Jolla, CA). The RNA was cross-linked to the nylon filter by UV light (Strata-linker). Hybridization was performed with32P-labeled EDG-2 cDNA, in a solution containing 7.5× SSC, 50% formamide in phosphate buffer (50 mm), pH 7, with tRNA and salmon sperm DNA as carriers. The filters were incubated with the DNA probes in this solution at 42°C. To remove 32P-labeled probe, filters were washed successively with 2× SSC, 0.1% SDS for 30 min and 0.1× SSC, 0.5% SDS for 30 min and then exposed.
Antibodies and Western blot analysis. Antibodies used were anti-α-tyrosinated-tubulin monoclonal antibody (mAb) (Sigma); anti-β-tubulin mAb (Sigma); anti-GSK3β mAb (Transduction Laboratories, Lexington, KY); rabbit antiphospho Y279/216 GSK-3α/β (Biosource International, Camarillo, CA); rabbit anti-Gα13 (Santa Cruz Biotechnology, Santa Cruz, CA); phalloidin-fluorescein (Sigma); PHF-1, which is an antiphospho Ser 396/404 tau mAb (kindly supplied by Dr. P. Davies, Albert Einstein College, Bronx, NY); AD-2, which is an antiphospho Ser396 tau mAb (kind gift of Dr. C. Mourton-Gilles, Institut de Biotechnologie en Inmunoanalyse et Pharmacologie, Centre National de la Recherche Scientifique, Montpellier, France); anti-hemagglutinin (HA) mAb [kind gift of Dr. A. C. Carreras, Centro Nacional de Biotecnología, Consejo Superior de Investigaciones Científicas (CSIC)]; and rabbit anti-Gα12 (a generous gift of Dr. S. Offermans, Pharmakologisches Institut, University of Heidelberg, Heidelberg, Germany).
Cell extracts were prepared as follows: cells were washed with 1× PBS and then resuspended in a buffer containing 20 mm HEPES, pH 7.4, 100 mm NaCl, 100 mm NaF, 1 mmsodium ortho-vanadate, 5 mm EDTA, 1% Triton X-100, and protease inhibitors (Complete; Roche Products, Hertfordshire, UK). The soluble fraction was obtained by centrifugation at 14,000 × g for 10 min at 4°C. Proteins (25–50 μg) were separated in SDS-PAGE gels and electrotransferred to a nitrocellulose filter. Filters were blocked with 5% nonfat milk in PBS, 0.1% Tween 20 (PBS-T), and then incubated with primary antibodies overnight at 4°C. Filters were rinsed and then incubated with the corresponding peroxidase-conjugated secondary antibody (Promega, Madison, WI), for 1 hr at room temperature. Immunoreactivity was visualized by the use of an enhanced chemiluminescence detection system (Amersham Biosciences, Arlington Heights, IL).
Densitometric analysis was performed on different Western blot lanes (3–5 replicates), and the data was processed with an imaging densitometer (GS-710 model; Bio-Rad, Hercules, CA). Data were analyzed with the Quantity One software (Bio-Rad). The densitometric values were normalized with respect to the values obtained for a control antibody to correct for any variations in the amounts of total loaded protein.
Quantitative analysis of GSK-3 activity. GSK-3 assays were performed essentially as previously described (Cross, 2001). Cells were collected with a cell scraper and homogenized in a buffer of 20 mm HEPES, pH 7.4, containing 100 mm NaCl, 100 mm NaF, 1 mm sodium ortho-vanadate, 5 mm EDTA, 100 nm okadaic acid, 1% Triton X-100, and protease inhibitors (Complete; Roche). The soluble fraction was obtained after centrifugation at 14,000 ×g for 15 min at 4°C.
Samples of 7 μg of protein were incubated in a buffer containing 25 mm Tris, pH 7.5, 1 mm DTT, 10 mmMgCl2, with the specific substrate peptide 2B-SP (Welsh et al., 1997) at a final concentration of 0.75 mg/ml, in the presence of γ[32P]ATP. After 1 hr, the reaction was stopped, and the activity was quantified by spotting aliquots on P81 phosphocellulose paper. The difference between the kinase activity in the presence or absence of 20 mm LiCl was considered a measure of GSK-3 protein kinase activity. The average range of incorporated counts per minute in a standard assay was 10 × 103 to 50 × 103 cpm. The activity values were normalized with respect to the expression levels of GSK-3 and Gα12QL-HA or Gα13QL-HA in each case, to correct for any variations in the amounts of total proteins.
Indirect immunofluorescence. After treatments, cell cultures were fixed with PBS containing paraformaldehyde (4%) for 30 min. After several washes with PBS, cells were preincubated in PBS–0.1% Triton X-100–1% FCS, for 30 min. After a brief wash with PBS, they were incubated overnight at 4°C with primary antibodies diluted in PBS–0.1% Triton X–100–1% FCS. After incubation with the primary antibody, cultures were extensively washed and then incubated for 45 min with the appropriate secondary antibody, conjugated either with fluorescein or Texas Red (The Jackson Laboratory, Bar Harbor, ME). After washing, they were immediately mounted with Fluoromount and examined under a Zeiss microscope coupled to a CCD camera, that directly captured digital micrographs. Photographs were analyzed, and neurite length was measured using the Spot software (Diagnostic Instruments, Sterling Heights, MI).
Plasmids and transfections. Plasmids used were: expression vectors for the Gα12Q229 (QL) and Gα13Q226L (QL) (ATCC reference numbers 63450 and 63451; supplied from Dr. H. Bourne, University of California San Francisco, San Francisco, CA). Both cDNAs were tagged with an HA epitope. The RhoV14-HA expression vector was kindly provided by Dr. A. Hall (Medical Research Council, University College, London, UK); the one for Clostridium botulinum C3 exoenzyme cDNA was a gift from Dr. A. C. Carreras (CNB, CSIC, Madrid, Spain), and the expression vector for green fluorescent protein (GFP) pEGFP was obtained from Clontech (Cambridge, UK).
Neuro2A cells were transiently transfected using the method of transferrin receptor endocytosis as described (DuoFect 80, Quantum; Appligene, Heidelberg, Germany). This transfection method yielded >50% transfection efficiency under optimal conditions. In the cotransfection experiments, total amounts of DNAs were kept constant (10 μg), and 7 μg of Gα12/13/Rho/C3 or plasmid without cDNA insert were mixed with 3 μg of pEGFP. Twenty-four hours after transfection, cells were processed differently, depending of the kind of experiment to be done. A proportion of the cells were fixed for immunofluorescence analysis, and the remainders were lysed for use in Western blot analysis or for in vitroGSK-3 kinase assays.
RESULTS
LPA induces neurite retraction in cerebellar granule cells
Our previous results indicated that GSK-3 is activated during LPA-induced neurite retraction in the SH-SY5Y human neuroblastoma cell line (Sayas et al., 1999). In this study, we have tested whether GSK-3 activation by LPA is a more general process, not only occurring in a particular neuroblastoma cell line, but also in primary neurons. To determine this, we selected a cerebellar granule cell culture, as a well described and homogenous primary neuron culture, which can be maintained in a serum-free medium (LPA is a component of serum).
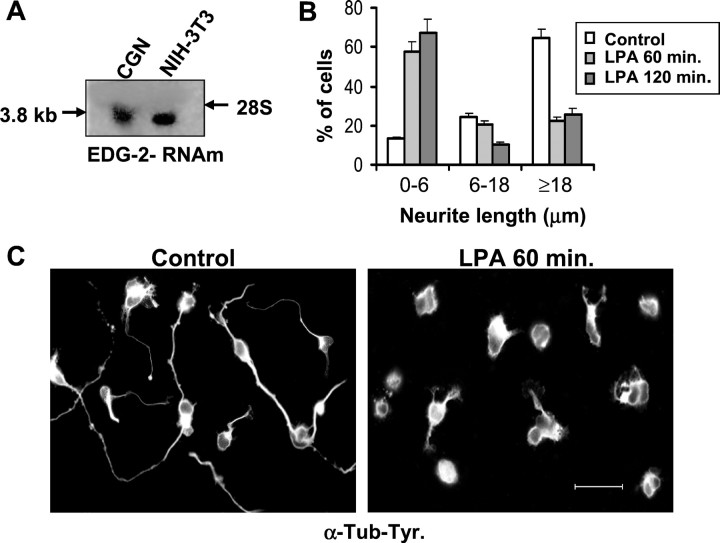
Before examining the response of cerebellar granule neurons to LPA, we analyzed by Northern blot, the expression of EDG-2, a specific LPA receptor, in these cells. These neurons expressed a 3.8 kb mRNA that corresponds to EDG-2 (Fig.1A).
Fig. 1.
LPA induces neurite retraction in cerebellar granule neurons. A, Northern blot analysis of EDG-2. Total RNA from NIH-3T3 (positive control) and cerebellar granule neurons (CGN) were hybridized with a32P-labeled cDNA probe of EDG-2. A 3.8 kb mRNA is detected in both cell types. B, Neurons were cultured for 24 hr and then treated with 10 μm LPA for 60 and 120 min. Neurite lengths were measured in control and LPA-exposed cells. Error bars represent the SD of the mean values (>400 cells per data point).C, Immunofluorescence staining of control and LPA-treated neurons with an anti-α-tyrosinated tubulin antibody. Scale bar, 20 μm.
Subsequently, we examined whether cerebellar granule cells responded to LPA. Neurons were incubated in the presence of 10 μm LPA, and cell morphology was analyzed at different times after lipid addition (60 and 120 min). Before the LPA treatment, almost 85% of the cells bore neurites. LPA addition induced a time-dependent neurite retraction. After 60 min, 55–65% of cells were rounded or had very short neurites, whereas 120 min after LPA treatment, ∼70% of cells showed no cytoplasmic extensions (Fig. 1B).
We also analyzed the tubulin network during the neurite retraction process, showing that 60 min after LPA addition, tubulin was no longer present in the axon-like extension but had accumulated in the cell body (Fig. 1B,C), clearly correlating with the morphological retraction (Fig. 1C).
These results show that cerebellar granule cells express EDG-2, a specific LPA receptor, and respond to LPA in a time-dependent manner, reorganizing their microtubular network.
LPA activates GSK-3 in cerebellar granule neurons
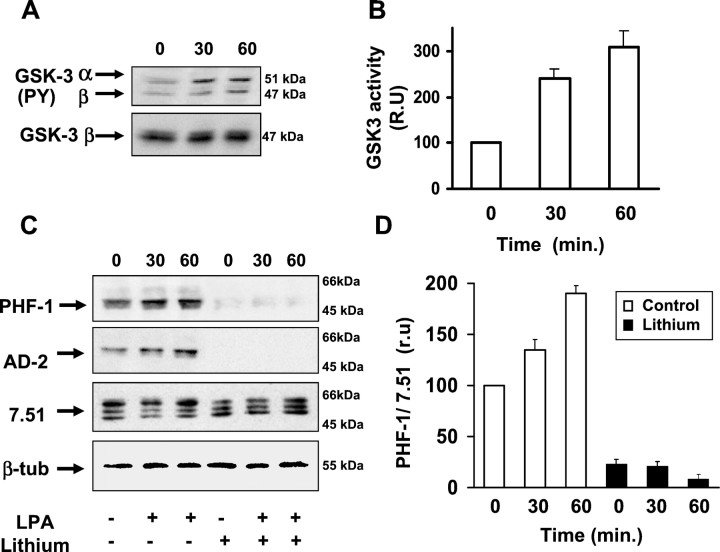
Phosphorylation of GSK-3 in a tyrosine residue (Y216 in GSK-3β, and Y279 in GSK-3α), is necessary for its activity. Recently, an increase in the level of tyrosine phosphorylation has been correlated to GSK-3 activation by different stimuli (Lesort et al., 1999; Hartigan and Johnson, 1999; Bhat et al., 2000). Therefore, we examined whether LPA addition induced an increase in GSK-3 tyrosine phosphorylation in cerebellar granule neurons. For this purpose, we performed Western blot analysis of lysates of control and LPA-treated cells. The antibody used recognizes both GSK-3 α and β isoforms, phosphorylated in tyrosine. LPA addition induced a time-dependent increase on the level of tyrosine-phosphorylated GSK-3 (α and β), whereas no differences were detected in the total amount of the GSK-3β protein (Fig.2A). In addition, the GSK3 pool phosphorylated in serine did not change along the LPA treatment (data not shown). These results suggest that GSK-3 is activated by LPA in these neurons.
Fig. 2.
LPA activates GSK-3 in cerebellar granule neurons.A, Western blot of soluble cell extracts obtained 30 and 60 min after LPA treatment. 0 represents cell extracts from control cells. Identical samples were incubated with antiphospho Y279/216 GSK-3 α/β (top blot) and with anti-GSK-3β (bottom blot) antibodies.B, Quantitative analysis of the GSK-3 kinase activity in control cells (0) and cells exposed to LPA (30 and 60 min). Each time point was normalized with respect to total GSK-3β protein present in each cell extract. Data are expressed as the mean of four different experiments. Data from control cells were considered 100 relative units (r.u.). Error bars represents SDs of the mean values. C,Western blots of phospho tau (PHF-1 and AD-2 antibodies), total tau (7–51 antibody), and β-tubulin. LPA induces an increase in tau phosphorylation (PHF-1 and AD-2), with no changes in the protein amount (7.51 and β-tubulin). Pretreatment of the cells with 10 mm LiCl, 4 hr before LPA addition, blocks LPA-induced tau hyperphosphorylation. D, The phosphorylation degree of tau protein in the PHF-1 epitope was determined for each culture by densitometry, normalized with respect to the values corresponding to total tau (7.51), and graphically represented. Diagram shows the mean normalized densitometry values and the corresponding SDs (n = 5).
To test this hypothesis directly, GSK-3 activity was measured in extracts of control and LPA-treated cells, by using a specific peptide as a GSK-3 substrate. Results from four different experiments showed that the GSK-3-specific activity increased to three times that of control levels, 60 min after LPA addition (Fig.2B).
We then investigated whether GSK-3 activation modifies the phosphorylation level of the MAP tau, during neurite retraction. To test whether tau is hyperphosphorylated after LPA treatment, we analyzed the phosphorylation level of tau protein by Western blot in lysates of control and LPA-treated neurons. Antibodies that recognized specifically phosphorylated (PHF-1 and AD-2) and nonphosphorylated tau epitopes (7.51), were used. The LPA treatment results in a site-specific phosphorylation of tau, as confirmed by the time-dependent increase of PHF-1 and AD-2 immunoreactivity (Fig.2C). No changes were detected in the total amount of tau or β-tubulin proteins with the treatment (Fig. 2C). Results from five independent experiments showed a twofold increase of PHF-1 immunoreactivity 60 min after LPA addition, when densitometric data were related to the total amount of tau protein (Fig.2D).
The phosphorylation of tau in PHF-1 or AD-2 epitopes has been attributed to three main kinases (GSK-3, MAP kinases, and stress kinases) (Paudel et al.,. 1993). To confirm that GSK-3 is the kinase responsible for tau phosphorylation by LPA in cerebellar granule cells, we used lithium, an inhibitor of this kinase (Klein and Melton, 1996;Stambolic et al., 1996). Treatment of these neurons with LiCl (10 mm) 4 hr before LPA addition almost completely prevented the reaction with PHF-1 and AD-2 antibodies (Fig. 2C,D). As seen in Figure 2, C and D, lithium also totally inhibited the hyperphosphorylation of tau induced by LPA, without changing the total amount of tau protein (Fig. 2C).
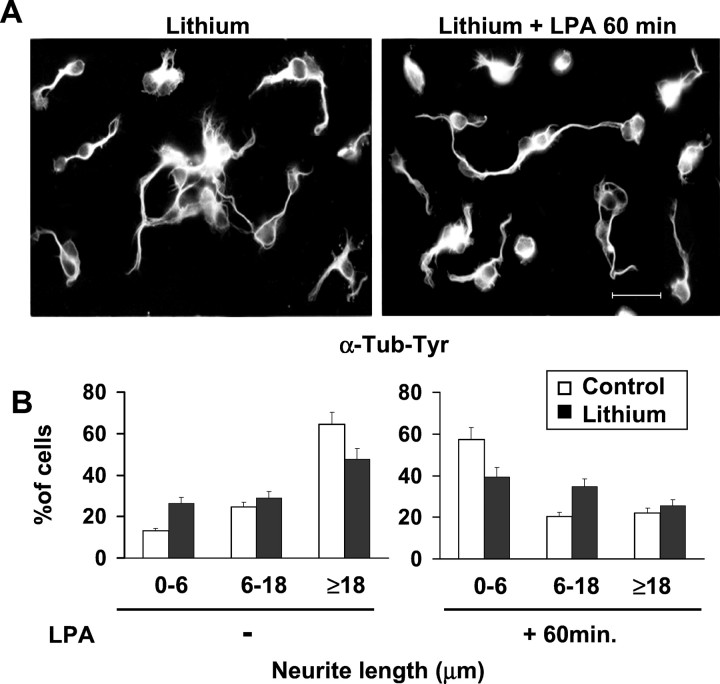
To determine the importance of GSK-3 activation for the progress of the LPA-induced neurite retraction, we investigated whether its inhibition with lithium could prevent the process. We compared the morphology of cerebellar granule cells treated only with lithium and cells pretreated with lithium and subsequently treated with LPA for 1 hr. Lithium induced neurite shortening and thickening, accompanied by microtubule unbundling, as previously described (Lucas et al., 1998) (Fig.3A,B). However, inhibition of GSK-3 by lithium partially prevented the neurite retraction process initiated by LPA (Fig. 3A,B).
Fig. 3.
GSK-3 inhibition partially prevents LPA-induced neurite retraction. A, Immunostaining of cerebellar granule neurons with an antibody against α-tyrosinated tubulin antibody. Neurons were treated with 10 mm lithium for 4 hr (left picture) or pretreated with lithium and then treated with 10 μm LPA for 60 min (right picture). Scale bar, 20 μm. B, Bar graphs represent the measurement of neurite length of control neurons and neurons treated with lithium, exposed or not to 10 μm LPA for 60 min.
Next we examined which of the general signal transduction pathways triggered by LPA, GSK-3 activation was located. LPA receptors can differentially couple to three distinct G-proteins: Gi, which produces Ras-GTP accumulation and MAPK activation; Gαq, which causes a rise in intracellular phosphoinositides and calcium levels, mediated by phospholipase C (PLC) signaling; and Gα12/13, which induces morphological changes through the activation of the small GTPase RhoA. We thus analyzed the effect of pharmacological inhibitors of the different pathways mentioned above on LPA-induced GSK-3 activation. Neither pretreatment of neurons with pertussis toxin (PTX), which ADP-ribosylates and inhibits Gαi, nor with the PLC inhibitor U-73122, blocked GSK-3 tyrosine phosphorylation and LPA-induced activation (data not shown). These results indicate that LPA-induced GSK-3 activation is neither downstream of Gi nor downstream of Gq. Furthermore, our results indicate that pretreatment of cerebellar neurons with the tyrosine kinase inhibitor genistein blocks tyrosine phosphorylation of GSK-3 α and β and significantly reduces kinase activation induced by LPA (data not shown). According to this, it has been postulated that one or more tyrosine kinases, which can be inhibited by genistein, exist in the Gα12/13 pathway, upstream or downstream of RhoA (Kranenburg et al., 1999). These data suggest that GSK-3 activation could be downstream of Gα12/13 in cerebellar granule cells, in agreement with our previous results that suggested that GSK-3 activation by LPA was downstream of Gα12/13 in the SH-SY5Y human neuroblastoma cell line (Sayas et al., 1999).
le;&.2qThese results demonstrate that GSK-3 is activated during LPA-induced neurite retraction in cerebellar granule cells. GSK-3 activation correlates with an increase in its tyrosine phosphorylation level and induces hyperphosphorylation of tau protein in certain epitopes (PHF-1 and AD-2). Activation of GSK-3 is important but not essential for the course of the neurite retraction process in these neurons. LPA-induced GSK-3 activation in neurons is neither downstream of Gi nor downstream of Gq, suggesting that it could be downstream of Gα12/13.
LPA induces cell rounding and GSK-3 activation in Neuro2-A cells
To confirm whether GSK-3 activation by LPA was downstream of Gα12/13, we selected a murine neuroblastoma cell line, Neuro2a cells, which can be transfected highly efficiently.
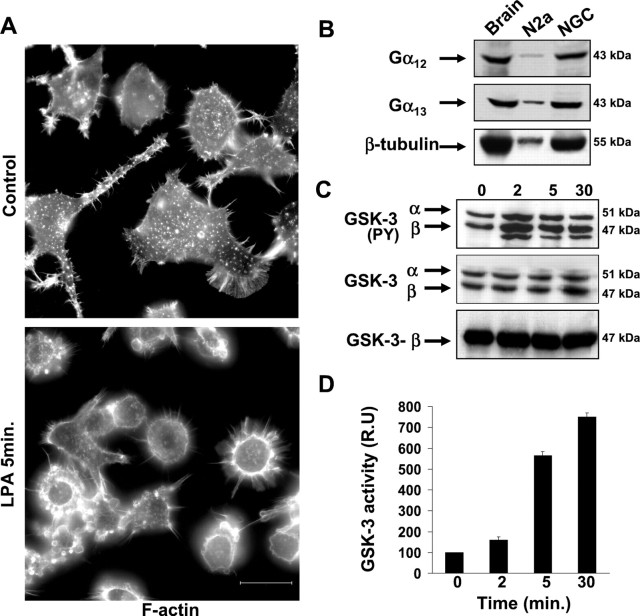
Initially, we tested whether differentiated Neuro2a cells responded to LPA. Under the differentiation conditions used, 10% of the cells were rounded, whereas 55% presented a flat morphology, and 35% bore short neurites (data not shown). Five minutes after LPA addition, 98% of cells were rounded with a cortical ring of actin cytoskeleton and exhibited obvious membrane blebbing (Fig.4A). Thus, Neuro2A cells undergo rapid cell rounding in response to LPA treatment.
Fig. 4.
LPA induces cell rounding and GSK-3 activation in Neuro2a cells. A, Differentiated Neuro2a cells were treated with 10 μm LPA for 5 min. Cells were then fixed and immunostained with phalloidin-fluorescein to visualize F-actin. Scale bar, 20 μm. B, Western blot analysis of Gα12 and Gα13 expression in Neuro2A and cerebellar granule cells. Identical samples were incubated with an anti-β-tubulin antibody as a loading control. A soluble lysate of mouse brain was used as a positive control. C, Analysis of GSK-3 tyrosine phosphorylation. Western blots were performed on soluble lysates of control cells (0), and cells treated with LPA for 2, 5, and 30 min. An anti-phospho Y279/216 GSK-3α/β antibody was used. Western blots were reproved with anti-GSK3α/β and anti-GSK3β-specific antibody to confirm that equivalent amounts of protein were loaded. D, Bar graph represents the GSK-3 kinase activity in control cells (0) and LPA-treated cells (2, 5, and 30 min). Control cell activity was considered 100 r.u. GSK-3 activity, normalized with respect to total GSK-3 protein present in each cell extract, was expressed as the mean of three different experiments. Error bars represent SD of the mean values.
We then tested whether Neuro2a cells expressed Gα12 and/or Gα13, the α subunits of the heterotrimeric G-proteins, which are hypothesized as being upstream of GSK-3 activation, in the LPA activated signaling pathways. As confirmed by Western blot analysis, Neuro2a cells expressed both Gα12 and Gα13. Cerebellar granule neurons also expressed both α subunits (Fig. 4B).
Because in primary neurons, GSK-3 activation by LPA correlated with an increase in tyrosine phosphorylation of GSK-3, we investigated whether this also occurred in Neuro2a treated with this lipid. For that purpose, we performed Western blot analysis, again using an antibody that recognizes α and β GSK-3 isoforms, phosphorylated in tyrosine residues. The level of GSK-3 (α and β) phosphorylated in tyrosine residues increased 2 min after LPA addition. A third immunoreactive band with lower molecular weight, appeared at similar times. The nature of this band is currently under study. Both the increase in GSK-3 tyrosine phosphorylation and the novel band were still present 30 min after LPA treatment. No changes in amounts of GSK-3 were detected during the lipid treatment (Fig. 4C). This result suggests that the increase in GSK-3-tyrosine phosphorylation levels could be attributable to the activation of the enzyme, instead of an increase in the amount of the protein.
To confirm this hypothesis, GSK-3 activity was measured in extracts of control and LPA-treated cells, using a specific peptide as a kinase substrate. LPA addition induced a time-dependent increase in GSK-3 activity, which reaches 7.5 times that of control levels, 30 min after the treatment. Therefore, in Neuro2A cells, LPA induces a time-dependent activation of GSK-3 that correlates with an increase in GSK-3 tyrosine phosphorylation.
Constitutively activated Gα12 and Gα13activate GSK-3
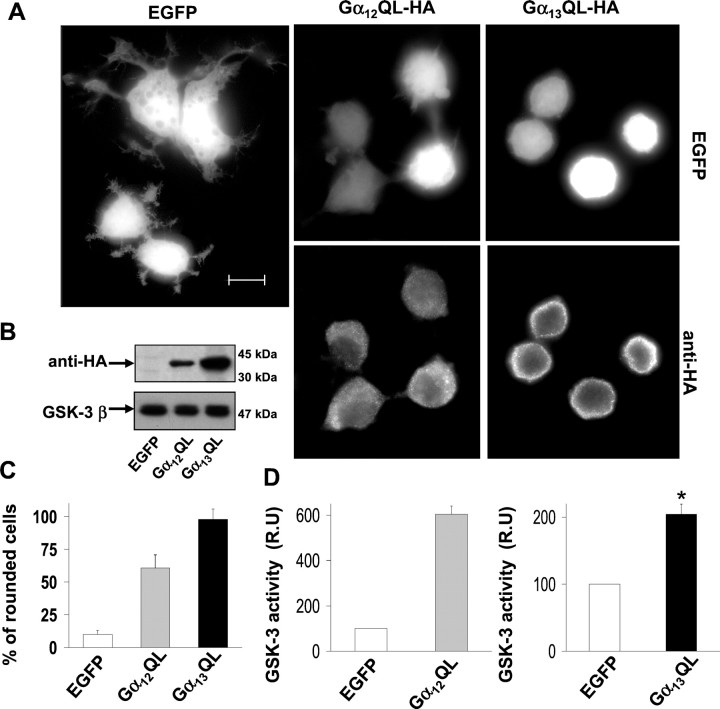
To determine whether the α subunits of the G12 family could mimic the effects of LPA, we transiently transfected expression plasmids for the activated forms of Gα12 (Gα12QL) and Gα13 (Gα13QL) in Neuro2a cells. Coexpression of pEGFP and the mutant forms of Gα12/13 were performed in all of these transfection experiments. Approximately 90% of the cells transfected with the control plasmid (pEGFP) had flat or process-bearing shape like that seen in untransfected cells (Fig.5A). Overexpression of constitutively activated Gα12 or Gα13 induced a loss of neurites and produced a rounded morphology in 60 (Gα12--QL) to 95% (Gα13-QL) of the transfected cells (Fig.5A,C). The expression of these proteins was confirmed using and anti-HA antibody, because the expression plasmids used were tagged with an HA epitope (Fig. 5A,B). Both activated Gα12 and Gα13 showed a punctuate pattern of expression in the transfected cells, Gα13 being localized preferentially on the plasma membrane, whereas Gα12 had a more diffuse localization, probably circumscribed to membranes of cytoplasmic organelles (Fig. 5A). Although the same amount of cDNA from both plasmids was used in every transfection experiment, the expression level of Gα13 protein was always 2.5–3-times that obtained for Gα12 (Fig.5B), probably because of differences in the purity of the two plasmids.
Fig. 5.
Constitutively activated Gα12 and Gα13 activate GSK-3 in Neuro2a cells. A,Overexpression of constitutively active Gα12 and Gα13 in Neuro2a cells induce cell rounding. Gα12QL-HA and Gα13QL-HA were coexpressed along with EGFP. Gα12QL-HA and Gα13QL-HA were detected using an anti-HA antibody. Control cells expressed only EGFP. Scale bar, 10 μm. B, Western blots of transfected cells using anti-HA (top blot) and anti-GSK-3β (bottom blot) antibodies.C, Percentage of rounded cells overexpressing EGFP (control) or EGFP along with Gα12QL-HA or Gα13QL-HA. Diagram shows the mean values obtained from three different experiments (>300 cells per data point and experiment). Error bars represent the SD of the mean values.D, Quantification of GSK-3 kinase activity in control cells (EGFP) and cells overexpressing Gα12QL-HA (left diagram) or Gα13QL-HA (right diagram) (along with EGFP). Data are expressed in r.u., and we considered 100 r.u. as the specific GSK-3 activity of control cells. GSK-3 activity was normalized with respect to total GSK-3β protein present in each cell extract. n = 3 experiments, and error bars represent SDs of the mean values. *p < 0.001 is statistically significant.
We next examined whether the overexpression of constitutively active Gα12 and Gα13 could activate GSK-3. GSK-3 activity was measured in extracts of control cells (transfected only with pEGFP) and of Gα12- or Gα13-overexpressing cells, using a specific peptide as a GSK-3 substrate. Both active Gα12and Gα13 increased GSK-3 activity to a similar level. However, when GSK-3 activity was normalized with respect to the expression of each protein, Gα13QL induced a twofold (Fig. 5D, right diagram) increase of GSK-3 activity, whereas Gα12QL produced a sixfold increase of GSK-3 activity over that of controls (Fig.5D, left diagram). This GSK-3 activity increase was not attributable to changes in GSK-3 protein levels (Fig.5B).
Taken together these results indicate that constitutively active α subunits of the G12/13 family not only induce cell rounding of Neuro2a cells, but also activate GSK-3.
Constitutively activated RhoA activates GSK-3
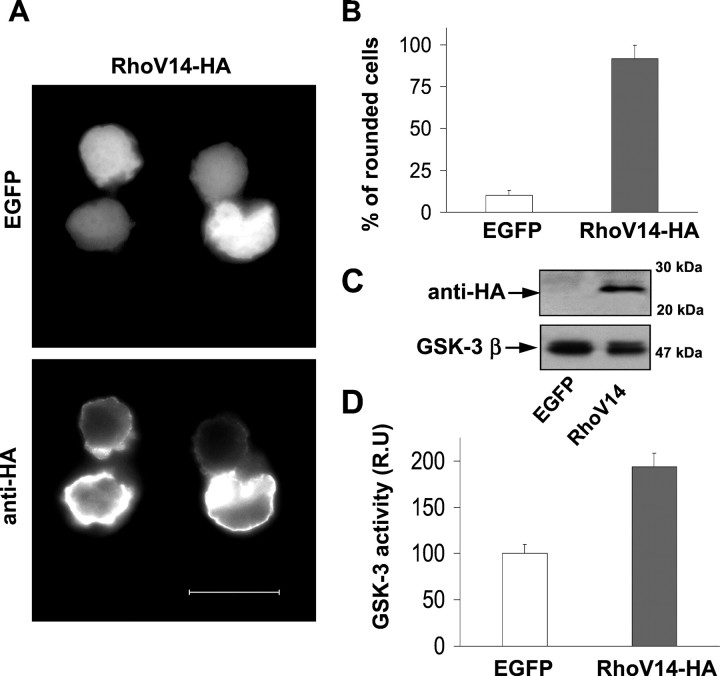
It has been reported that GTPase-deficient mutants of Gα12 and Gα13 induce cell rounding through a Rho-dependent mechanism, in the N1E-115 murine neuroblastoma cell line (Kranenburg et al., 1999) and in PC-12 cells (Katoh et al., 1998). To examine the effect of RhoA on Neuro2a morphology, we transfected constitutively activated RhoA (RhoAV14). Overexpression of RhoAV14 induced cell rounding in 90% of the transfected cells (Fig.6A,B). Activated RhoA was preferentially localized at the plasma membrane of the transfected cells (Fig. 6A).
Fig. 6.
Constitutively activated RhoA activates GSK-3.A, Overexpression of constitutively active RhoA in Neuro2a cells induces cell rounding. RhoAV14-HA was coexpressed along with EGFP, and its expression was detected with an anti-HA antibody. Scale bar, 20 μm. B, Percentage of rounded cells overexpressing EGFP (control) or RhoAV14-HA along with EGFP. Diagram shows the mean values obtained from three different experiments (>300 cells per data point and experiment). Error bars represent the SD of the mean values. C, Western blot of transfected cells performed with anti-HA and anti-GSK-3β antibodies. D,GSK-3 kinase activity was measured and quantified as previously, in control cells (EGFP) and cells overexpressing RhoAV14-HA.
We then addressed the possibility that Rho itself could activate GSK-3. To test this hypothesis, we measured GSK.-3 activity in extracts of control cells (transfected with pEGFP) and of RhoAV14-overexpressing cells, confirming that constitutively active RhoA induces a twofold increase of GSK-3 activity above that of the control. No changes in the GSK-3 level were found in RhoA-overexpressing cells (Fig.6C). These data indicate that RhoA, which is a specific mediator of Neuro2a cell rounding, activates the kinase GSK-3.
Activation of GSK-3 by Gα13 is mediated by RhoA
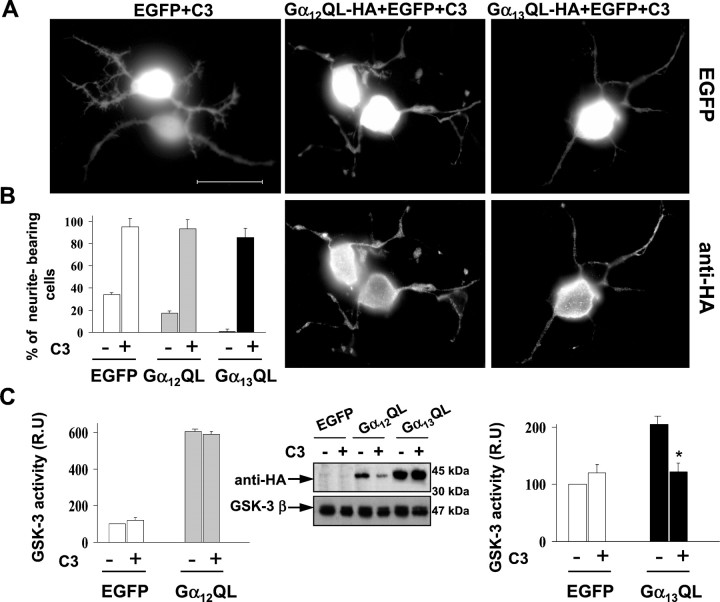
To test further whether Gα12 and Gα13 stimulated GSK-3 activity through a Rho-dependent mechanism, we coexpressed C3 exoenzyme along with Gα12QL and Gα13QL plasmids in Neuro2a cells. The C3 exoenzyme of Clostridium botulinum ADP-ribosylates and inhibits Rho. Coexpression of C3 blocked the ability of constitutively activated Gα12 and Gα13 to induce cell rounding (Fig. 7A). Only a small percentage of Gα12 or Gα13 overexpressing cells bore neurites (17 and 0.1%, respectively), whereas the number of neurite-bearing cells dramatically increased when Gα12 or Gα13 were coexpressed with C3 (95 and 89%, respectively) (Fig. 7B). Coexpression of C3 significantly reduced the expression level of Gα12 without affecting Gα13 level (Fig. 7C,center). Measurements of GSK-3 activity in extracts of control cells and cells cotransfected with Gα12or Gα13 and C3 indicated that C3 significantly reduced GSK-3 activation induced by Gα13 (Fig.7C, right diagram) but not that induced by Gα12 (Fig. 7C , left diagram). These data indicate that, although both Gα12 and Gα13 induce Rho-mediated cell rounding, these Gα subunits activate GSK-3 through different mechanisms. GSK-3 activation by Gα13is mediated at least in part by RhoA, whereas the kinase activation by Gα12 does not involve RhoA function.
Fig. 7.
Activation of GSK-3 by Gα13 is mediated by RhoA. A, Coexpression of C3 along with constitutively active Gα12 or Gα13 blocks cell rounding induced by Gα12 and Gα13 in Neuro2a cells. Cells coexpress EGFP along with C3 and Gα12QL-HA or Gα13QL-HA. These cells were immunostained using an anti-HA antibody. Control cells coexpress EGFP and C3. Scale bar, 20 μm. B, Diagram showing the percentage of neurite-bearing cells overexpressing EGFP, Gα12QL-HA, or Gα13QL-HA, along with C3 transferase or not. Mean values obtained from three different experiments are represented (>300 cells per data point and experiment). Error bars represent the SD of the mean values.C, Western blot of transfected cells performed with anti-HA and anti-GSK3β antibodies (center). Quantification of GSK-3 kinase activity in lysates of transfected cells, as previously described, shows that GSK-3 activation induced by Gα13QL-HA is inhibited by C3 (right diagram), whereas GSK-3 activation by Gα12QL-HA is not (left diagram). *p < 0.001 is statistically significant.
DISCUSSION
GSK-3 is activated by LPA during neurite retraction in primary neuronal cells
Initially, we investigated whether cerebellar granule cells from 7-d-old rat pups express an LPA receptor and respond to LPA. LPA is an intercellular lipid mediator that induces diverse biological responses in many types of cells and tissues (Moolenaar, 1999). LPA signals through its binding to specific G-protein-coupled receptors. Our data indicate that these neurons express at least EDG-2, which is the most widely expressed LPA receptor in brain during development (Hecht et al., 1996). We do not rule out the possibility that these neurons express other LPA receptors (EDG-4 and/or EDG-7) that are also expressed in mouse brain at postnatal day 7 (Contos and Chun, 2001).
LPA induces morphological changes in neuronal cells: neurite retraction and cell rounding in neuroblastoma and PC-12 cells (Jalink et al., 1993; Tigyi et al., 1996), growth cone collapse in primary chick neurons (Saito, 1997), and cell rounding, membrane retraction, and cellular and nuclear migration in cortical neuroblasts (Fukushima et al., 2000). We show here that cerebellar granule neurons undergo a time-dependent neurite retraction in response to LPA.
Our previous results indicate that GSK-3 is activated during LPA-induced neurite retraction in the SH-SY5Y human neuroblastoma cell line (Sayas et al.,. 1999). In this study, we demonstrate that LPA induces a time-dependent activation of GSK-3 in cerebellar granule cells that correlates to the neurite retraction process. We also show here that GSK-3 activity is increased during LPA-induced cell rounding in the mouse neuroblastoma Neuro2a. Therefore, the present study confirms that GSK-3 activation by LPA is not a peculiarity of the cell line used as it occurs in neuronal cells from different species, suggesting that it could be a widespread physiological process in neuronal cells
Until recently, it was believed that GSK-3 could be regulated only by inhibition, but some recent reports have indicated that GSK-3 is activated in neuronal cells in response to diverse extracellular signals such as apoptotic stimuli, ischemia, a transient rise in intracellular calcium, and insulin (Hartigan and Johnson, 1999; Lesort et al., 1999; Bhat et al., 2000). This activation is accompanied by increased GSK-3 tyrosine phosphorylation. Accordingly, our results indicate that GSK-3 activation by LPA in cerebellar granule neurons and in Neuro2a cells correlates with an increase in tyrosine phosphorylation of both GSK-3 isoforms, α and β. In addition, pretreatment of cerebellar neurons with the tyrosine kinase inhibitor genistein blocks tyrosine phosphorylation of GSK-3α/β and significantly reduces kinase activation (data not shown).
A new tyrosine kinase has recently been cloned in Dictyostelium discoideum, ZAK-1, that directly phosphorylates and activates GSK-3 (Kim et al., 1999). In addition, it has been suggested that the tyrosine kinases Fyn and Pyk2 phosphorylate GSK-3 (Lesort et al., 1999;Hartigan et al., 2001). Therefore, the tyrosine kinase, genistein-sensitive, responsible for LPA-induced GSK-3 activation could be either a putative mammalian ZAK-1 homologous or a member of Fyn or Pyk families. However, the identification of this tyrosine kinase requires further investigation. Taken together, these results indicate that: (1) GSK-3 is activated by LPA in neuronal cells, and (2) an unidentified tyrosine kinase, which can be inhibited by genistein, may be involved in GSK-3 activation by LPA in cerebellar granule neurons.
GSK-3 activation contributes to the reorganization of the microtubular network during LPA-induced neurite retraction
We tested how GSK-3 activation by LPA could be contributing to neurite retraction. Among GSK-3 targets are the main neuronal MAPs (Sperber et al., 1995; Lucas et al., 1998; Sanchez et al., 2000). Tau, a prominently axonal MAP, promotes microtubule assembly and stabilizes the structure of MTs (Mandelkow et al., 1995). Abnormally hyperphosphorylated tau loses its binding to MTs, causing their disruption (Sontag et al., 1996).
Our previous results indicated that tau is hyperphosphorylated by GSK-3 during LPA-induced neurite retraction in SH-SY5Y cells (Sayas et al., 1999). In this study, we show that tau is hyperphosphorylated in two analyzed epitopes (PHF-1 and AD-2) during LPA-induced neurite retraction in cerebellar granule neurons. This increase in tau phosphorylation is blocked by the GSK-3 inhibitor lithium, confirming that GSK-3 is the serine–threonine kinase responsible for this hyperphosphorylation. Furthermore, pretreatment of neurons with lithium partially blocks neurite retraction induced by LPA. Considering that the lithium treatments contain an excess of myo-inositol (5 mm) to avoid the hypothetical inositol depletion, because of inhibition of myo-inositol monophosphatase This result indicates that GSK-3 activation contributes to the execution of the neurite retraction process, possibly by tau and other MAP phosphorylation, thus facilitating MT reorganization. The fact that GSK-3 inhibition does not completely block neurite retraction implies that GSK-3 activation is important but not essential for the process, in which the participation of other factors is needed.
Physiological and pathophysiological implications of GSK-3 activation by LPA in neuronal cells
LPA induces proliferation as well as changes in cell morphology in ventricular zone cortical neuroblasts during neurogenesis (Hecht et al., 1996). MTs play a key role in the mitotic spindle formation during mitosis and in the maintenance of cell morphology. Because MAPs are phosphorylated by GSK-3, GSK-3 activation could promote MT reorganization, contributing to LPA-induced mitosis progression and cell rounding in ventricular zone neuroblasts. In addition, GSK-3 phosphorylates several transcription factors, which regulation could be involved in mitosis progression of neuroblasts. Thus, GSK-3 activation by LPA could be involved in mitosis of neuroblasts through its phosphorylation of different targets such as MAPs and transcription factors. Hyperphosphorylation of MAPs by GSK-3 could also contribute to MT rearrangement during LPA-induced neurite retraction of differentiating neurons.
It has been reported that postmitotic hippocampal neurons undergo apoptosis and necrosis in response to LPA, by an unknown mechanism (Steiner et al., 2000). On the other hand, GSK-3 activation has also been related to apoptosis in neuronal cells (Crowder and Freeman, 2000). Therefore, activation of GSK-3 by LPA could be, at least in part, mediating the apoptotic response induced by LPA in mature neurons.
Neurite retraction is a significant process not only during development (neurogenesis and neuritogenesis), but also in some pathological circumstances such as neurodegeneration. In some neurodegenerative diseases, such as Alzheimer's disease (AD), the affected neurons bear dystrophic neurites (Onorato et al., 1989). One of the hallmarks of Alzheimer's disease is the accumulation of paired helical filaments (PHFs) in the neurofibrillary tangles (Wischik et al., 1985). Hyperphosphorylated tau is the main constituent of PHFs (Goedert, 1993), and GSK-3 is one of the kinases responsible for tau hyperphosphorylation in Alzheimer's PHFs (Hanger et al., 1992). Thus, GSK-3 activation might be one of the causes of PHF formation and, in part, of neurodegeneration. In this sense, our group has recently reported that conditional transgenic mice overexpressing GSK-3β show hyperphosphorylation of tau in hippocampal neurons, resulting in pretangle-like somatodendritic localization of tau, and neuronal stress and death (Lucas et al., 2001). As we have shown that GSK-3 is activated by LPA in neuronal cells, LPA might be one of the factors that play an important role in accumulation of highly phosphorylated tau and PHF formation in AD brains. Thus, it would be of interest to determine whether LPA levels are elevated in AD brains when compared with age-matched control brains. Moreover, during impairment of the blood–brain barrier, LPA could leak into the CNS, elevating its levels in injured brain. Brain injury, which constitutes one of the major risk factors of AD, is frequently accompanied by blood–brain barrier impairment. Accordingly, LPA receptors might be overexpressed in AD neurons, leading to the upregulation of LPA neuronal responses, such as GSK-3 activation, during AD.
In conclusion, GSK-3 activation by LPA may have different and important roles during nervous system development and in the course of some neurodegenerative diseases, such as AD.
GSK-3 is activated by Gα12 and Gα13: a new mechanism for GSK-3 activation
We investigated in which of the signaling pathways triggered by LPA GSK-3 is located. LPA receptors can differentially couple to three distinct G-proteins: Gi; Gαq, and Gα12/13(Moolenaar, 1999). Our data indicate that pharmacological inhibition of the signaling pathways downstream of Gi and Gαq does not block tyrosine phosphorylation and activation of GSK-3 by LPA in cerebellar granule neurons, whereas genistein treatments do (data not shown). These results indicate that GSK-3 activation is neither downstream of Gi, nor downstream of Gαq in these neurons, and suggest that GSK-3 activation is downstream Gα12 or Gα13.
Here we demonstrate that overexpression of constitutively active Gα12 or Gα13 induces GSK-3 activation, which correlates with cell rounding. Gα12 is a more potent GSK-3 activator than Gα13. This could be caused by the use of different signaling pathways by Gα12 and Gα13 to converge on GSK-3 activation. This possibility has been demonstrated in several studies (Wadsworth et al., 1997; Katoh et al., 1998; Gohla et al., 1999)
Constitutively active Gα12 and Gα13 induce stress fiber formation in fibroblasts (Gohla et al., 1999) and cell rounding in neuroblastoma cell lines (Kranenburg et al., 1999) through activation of the small GTPase RhoA. In this study, we show that overexpression of constitutively active RhoA (RhoAV14) induces upregulation of GSK-3 activity accompanied by cell rounding in Neuro2a cells. Furthermore, coexpression of the bacterial toxin C3, which ADP-ribosylates and inhibits Rho, along with each of the Gα subunits, completely blocks cell rounding induced by Gα12 and Gα13, whereas it only inhibits GSK-3 activation promoted by Gα13. These results indicate that GSK-3 activation promoted by Gα13 is Rho-mediated, whereas the Gα12 promoted seems to be Rho-independent. Additionally, these data indicate that cell rounding and GSK-3 activation induced by Gα12are mediated by different signaling pathways. By contrast, Gα13 causes cell rounding and upregulation of GSK-3 activity by use of the same signaling mechanisms. This confirms our postulated hypothesis concerning the existence of different mechanisms of GSK-3 activation by Gα12 and Gα13.
As mentioned above, upregulation of GSK-3 activity has been correlated with a rise in GSK-3 tyrosine phosphorylation (Hughes et al., 1993). Furthermore, in this study we show that LPA induces an increase in GSK-3 α and β tyrosine phosphorylation in cerebellar neurons and mouse neuroblastoma cells, which is apparently not downstream of Gi or Gq. This suggests that activation of a tyrosine kinase by Gα12 or Gα13 could mediate GSK-3 activation. In addition, a number of evidence suggests that tyrosine kinases may be mediating Rho activation by Gα12 and/or Gα13. Among the proposed tyrosine kinases involved in this process are some of the Bruton's tyrosine kinase family (Tec and Bmx) (Mao et al., 1998), Pyk family (Pyk2 and FAK) (Needham and Rozengurt, 1998; Shi et al., 2000), and the EGF-receptor tyrosine kinase (Gohla et al., 1998). Thus, GSK-3 activation could be mediated by a tyrosine kinase activated by Gα12 and/or Gα13 in the Rho pathway. However, in Neuro2a cells, the neuroblastoma used in this study, none of the tyrosine kinase inhibitors used (tyrphostin A25 and genistein) blocked cell rounding induced by constitutively active Gα12 and Gα13 (data not shown). This result indicates that if a tyrosine kinase is present in the Rho signaling pathway downstream from Gα12and/or Gα13, in Neuro2a cells, it cannot be inhibited by tyrphostin A25 or genistein.
On the other hand, GSK-3 can be inhibited by phosphorylation in a serine residue. This phosphorylation is achieved by different serine–threonine kinases depending of the cellular stimulus: PKB in the insulin-PI3-K pathway (Cross et al., 1995), PKC in the Wnt pathway (Cook et al., 1996), integrin-linked kinase downstream of integrin binding or PI3-K pathway (Delcommenne et al., 1998), or PKA, when the signal is extracellular cAMP (Fang et al., 2000). Thus, downregulation of any of these inhibitory pathways, and/or upregulation of the activity of a phosphatase, which dephosphorylates the serine residue, are other possible mechanisms for GSK-3 activation by Gα12 and Gα13 that cannot be ruled out.
We demonstrate here that GSK-3 can be activated by Gα12 and Gα13. Both proteins are ubiquitously expressed. Gα12/13are not only coupled to LPA receptors, but also to a number of other seven transmembrane domain receptors including: sphingosine 1-phosphate, thrombin, thromboxane A2, endothelin, angiotensin II, bradykinin B2, vasopressin V1A, neurokinin-1, and serotonin 5-HT2C (Djellas et al., 1999; Gohla et al., 1999; Windh et al., 1999) As with LPA, sphingosine 1-phosphate and thrombin induce neurite retraction and cell rounding in neuronal cells (Suidan et al., 1992; Postma et al., 1996). Thus, different signals that promote neurite retraction could converge on GSK-3 activation in these cells. Further research is needed to establish whether GSK-3 can be activated by any of the aforementioned molecules, in neuronal and/or non-neuronal cells, and what the biological implications of GSK-3 activation are.
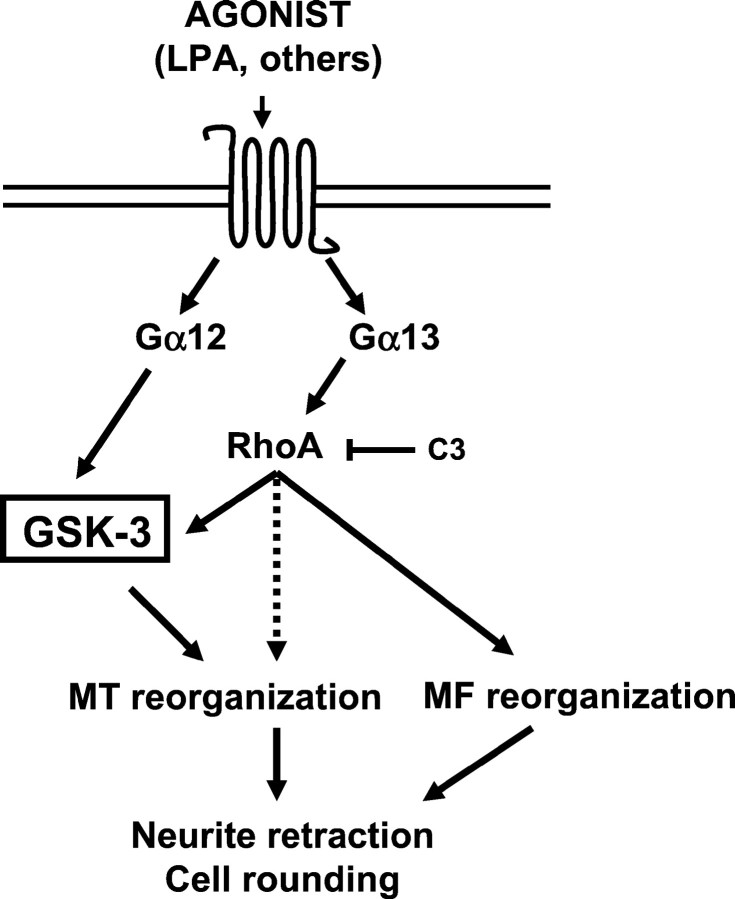
In summary, the data presented here show that GSK-3 is activated by extracellular LPA in primary cerebellar granule neurons. LPA-induced GSK-3 activation correlates with tau hyperphosphorylation and neurite retraction. It is not downstream of Gi or Gαq pathways, indicating that it could be downstream of Gα12 or Gα13. We show that constitutively active Gα12 or Gα13 induce an increase in GSK-3 activity in Neuro2a cells. GSK-3 activation by Gα13 is RhoA-mediated, whereas its activation by Gα12 is Rho-independent (Fig.8). These results taken together suggest a physiological role of GSK-3 activation during neurite retraction, which is an important process during development and neurodegeneration. Additionally, our results point to the existence of a hitherto undescribed mechanism of GSK-3 activation by Gα12 and Gα13proteins.
Fig. 8.
Schematic model of GSK-3 activation by Gα12 and Gα13. LPA activates GSK-3 in neuronal cells, and this activation contributes to neurite retraction. The stimulation of seven transmembrane domain receptors that couple to Gα12/13 may potentially activate GSK-3. Although Gα12 may activate RhoA, GSK-3 activation by Gα12 does not involve RhoA activity. However, Gα13 activates GSK-3 through a Rho-dependent mechanism.
Footnotes
This research was supported by grants from Spanish Comisión Interministerial de Ciencia y Tecnología and an institutional grant from Ramon Areces Foundation. We thank Drs. A. Hall and A. C. Carreras for generously providing us with RhoV14-HA and C3-transferase cDNAs, respectively, and Drs. P. Davies, C. Mourton-Gilles, A. C. Carrera, and S. Offermans for kind gifts of antibodies. We also thank Dr. J. Díaz-Nido for helpful comments.
Correspondence should be addressed to Francisco Wandosell, Centro de Biología Molecular “Severo Ochoa”, CSIC-Universidad Autónoma de Madrid, Cantoblanco-Madrid 28049, Spain. E-mail:FWANDOSELL@cbm.uam.es.
REFERENCES
- 1.Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci USA. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Contos JJ, Chun J. The mouse lp(A3)/Edg7 lysophosphatidic acid receptor gene: genomic structure, chromosomal localization, and expression pattern. Gene. 2001;267:243–253. doi: 10.1016/s0378-1119(01)00410-3. [DOI] [PubMed] [Google Scholar]
- 3.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 4.Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a PKC. EMBO J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 5.Cross D. Assays for glycogen synthase kinase-3 (GSK-3). Methods Mol Biol. 2001;124:147–159. doi: 10.1385/1-59259-059-4:147. [DOI] [PubMed] [Google Scholar]
- 6.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 7.Crowder RJ, Freeman RS. Glycogen synthase kinase-3 beta activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J Biol Chem. 2000;275:34266–34271. doi: 10.1074/jbc.M006160200. [DOI] [PubMed] [Google Scholar]
- 8.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djellas Y, Manganello JM, Antonakis K, Le Breton GC. Identification of Gα13 as one of the G-proteins that couple to human platelet thromboxane A2 receptors. J Biol Chem. 1999;274:14325–14330. doi: 10.1074/jbc.274.20.14325. [DOI] [PubMed] [Google Scholar]
- 10.Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferkey DM, Kimelman D. GSK-3: new thoughts on an old enzyme. Dev Biol. 2000;225:471–479. doi: 10.1006/dbio.2000.9816. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima N, Weiner JA, Chun J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev Biol. 2000;228:6–18. doi: 10.1006/dbio.2000.9930. [DOI] [PubMed] [Google Scholar]
- 13.Goedert M. tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993;16:460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 14.Gohla A, Harhammer R, Schultz G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J Biol Chem. 1998;273:4653–4659. doi: 10.1074/jbc.273.8.4653. [DOI] [PubMed] [Google Scholar]
- 15.Gohla A, Offermanns S, Wilkie TM, Schultz G. Differential involvement of Gα12 and Gα13 in receptor-mediated stress fiber formation. J Biol Chem. 1999;274:17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- 16.Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J Cell Sci. 1999;112:3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- 17.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 18.Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 19.Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site- selective increases in tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem. 1999;274:21395–21401. doi: 10.1074/jbc.274.30.21395. [DOI] [PubMed] [Google Scholar]
- 20.Hartigan JA, Xiong WC, Johnson GV. Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem Biophys Res Commun. 2001;284:485–489. doi: 10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- 21.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- 24.Katoh H, Aoki J, Yamaguchi Y, Kitano Y, Ichikawa A, Negishi M. Constitutively active Gα12, Gα13, and Gαq induce Rho-dependent neurite retraction through different signaling pathways. J Biol Chem. 1998;273:28700–28707. doi: 10.1074/jbc.273.44.28700. [DOI] [PubMed] [Google Scholar]
- 25.Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 26.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Gα12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10:1851–1857. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesort M, Jope RS, Johnson G V. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- 29.Levi G, Aloisi F, Ciotti MT, Thangnipon W. Preparation of 98% pure cerebellar granule cell cultures. In: Shahar A, de Vellis J, Vernadakis A, Harber B, editors. A dissection and tissue culture manual of the nervous system. Liss; New York: 1989. pp. 211–214. [Google Scholar]
- 30.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 31.Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. SAAS Bull Biochem Biotechnol. 1995;16:355–362. doi: 10.1016/0197-4580(95)00025-a. [DOI] [PubMed] [Google Scholar]
- 33.Mao J, Xie W, Yuan H, Simon MI, Mano H, Wu D. Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by Gα12/13. EMBO J. 1998;17:5638–5646. doi: 10.1093/emboj/17.19.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 35.Needham LK, Rozengurt E. Gα12 and Gα13 stimulate Rho-dependent tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130 Crk-associated substrate. J Biol Chem. 1998;273:14626–14632. doi: 10.1074/jbc.273.23.14626. [DOI] [PubMed] [Google Scholar]
- 36.Onorato M, Mulvihill P, Connolly J, Galloway P, Whitehouse P, Perry G. Alteration of neuritic cytoarchitecture in Alzheimer's disease. Prog Clin Biol Res. 1989;317:781–789. [PubMed] [Google Scholar]
- 37.Paudel HK, Lew J, Ali Z, Wang JH. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J Biol Chem. 1993;268:23512–23518. [PubMed] [Google Scholar]
- 38.Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- 39.Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980;107:529–537. [PubMed] [Google Scholar]
- 40.Saito S. Effects of lysophosphatidic acid on primary cultured chick neurons. Neurosci Lett. 1997;229:73–76. doi: 10.1016/s0304-3940(97)00397-2. [DOI] [PubMed] [Google Scholar]
- 41.Salinas PC. Wnt factors in axonal remodelling and synaptogenesis. Biochem Soc Symp. 1999;65:101–109. [PubMed] [Google Scholar]
- 42.Sanchez C, Perez M, Avila J. GSK3beta-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur J Cell Biol. 2000;79:252–260. doi: 10.1078/s0171-9335(04)70028-x. [DOI] [PubMed] [Google Scholar]
- 43.Sayas CL, Moreno-Flores MT, Avila J, Wandosell F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer's disease-like tau phosphorylation. J Biol Chem. 1999;274:37046–37052. doi: 10.1074/jbc.274.52.37046. [DOI] [PubMed] [Google Scholar]
- 44.Shi CS, Sinnarajah S, Cho H, Kozasa T, Kehrl JH. G13α-mediated PYK2 activation. PYK2 is a mediator of G13α-induced serum response element-dependent transcription. J Biol Chem. 2000;275:24470–24476. doi: 10.1074/jbc.M908449199. [DOI] [PubMed] [Google Scholar]
- 45.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 46.Sperber BR, Leight S, Goedert M, Lee VM. Glycogen synthase kinase-3 beta phosphorylates tau protein at multiple sites in intact cells. Neurosci Lett. 1995;197:149–153. doi: 10.1016/0304-3940(95)11902-9. [DOI] [PubMed] [Google Scholar]
- 47.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 48.Steiner MR, Holtsberg FW, Keller JN, Mattson MP, Steiner SM. Lysophosphatidic acid induction of neuronal apoptosis and necrosis. Ann NY Acad Sci. 2000;905:132–141. doi: 10.1111/j.1749-6632.2000.tb06545.x. [DOI] [PubMed] [Google Scholar]
- 49.Suidan HS, Stone SR, Hemmings BA, Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992;8:363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M, Tomizawa K, Kato R, Sato K, Uchida T, Fujita SC, Imahori K. Localization and developmental changes of tau protein kinase I/glycogen synthase kinase-3 beta in rat brain. J Neurochem. 1994;63:245–255. doi: 10.1046/j.1471-4159.1994.63010245.x. [DOI] [PubMed] [Google Scholar]
- 51.Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- 52.Wadsworth SJ, Gebauer G, van Rossum GD, Dhanasekaran N. Ras-dependent signaling by the GTPase-deficient mutant of Gα12. J Biol Chem. 1997;272:28829–28832. doi: 10.1074/jbc.272.46.28829. [DOI] [PubMed] [Google Scholar]
- 53.Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh GI, Patel JC, Proud CG. Peptide substrates suitable for assaying glycogen synthase kinase-3 in crude cell extracts. Anal Biochem. 1997;244:16–21. doi: 10.1006/abio.1996.9838. [DOI] [PubMed] [Google Scholar]
- 55.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 56.Wischik CM, Crowther RA, Stewart M, Roth M. Subunit structure of paired helical filaments in Alzheimer's disease. J Cell Biol. 1985;100:1905–1912. doi: 10.1083/jcb.100.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodgett JR, Plyte SE, Pulverer BJ, Mitchell JA, Hughes K. Roles of glycogen synthase kinase-3 in signal transduction. Biochem Soc Trans. 1993;21:905–907. doi: 10.1042/bst0210905. [DOI] [PubMed] [Google Scholar]