Abstract
The fly, Ormia ochracea, possess a novel auditory organ, which allows it to detect airborne sounds. The mechanical coupling of its pair of tympanal membranes provides the basis for a unique means of sensing the direction of a sound source. In this study, we characterized the neuroanatomy, frequency tuning, and neurophysiological response properties of the acoustic afferents. Our experiments demonstrate that the fly's nervous system is able to encode and localize the direction of a sound source, although the binaural auditory cues available in the acoustic sound field are miniscule. Almost all of the acoustic afferents recorded in this study responded to short and long sound pulses with a phasic burst of one to four action potentials. A few afferents responded tonically for the duration of the sound stimulus. A prominent class of afferents responds to suprathreshold stimuli with only a single spike discharge, independent of stimulus level, frequency, or duration. We also tested the response of the afferents to speakers separated by 180° along the azimuth of the fly. We found that the afferent responses have a shorter latency because of ipsilateral stimulation. This could be a temporal code of the direction of a sound source. The threshold frequency tuning for the afferents revealed a range of sensitivities to the frequency of the cricket host's calling song frequency. The difference in the number of afferents above threshold on either side of the animal is a population code, which can also be used for sound localization.
Keywords: directional hearing, time coding, population coding, parasitoid, mechanical coupling, arthropod
Sound localization is a fundamental task of the auditory system. The ears of most animals are separated as far apart on their head or body as possible. This anatomical fact permits them to detect differences in a directional sound wave as it sweeps past the near ear and then the far ear. There are two kinds of interaural difference cues. First, the sound level at the near ear may be greater than at the far ear; this is the interaural level difference (ILD), and it is frequency-dependent. If the distance separating the two ears is greater than one-tenth of wavelength of an incident sound, sound is diffracted by the intervening tissues of the head (vertebrates) or body (insects) that casts the far ear in the sound shadow of the animal's body (Michelsen, 1994). Second, the sound wave will arrive at the near ear before the far ear; this is the interaural time difference, or delay, (ITD) depending only on the speed of sound, and so it is frequency-independent. The magnitude of the ITD and ILD will both decrease as an animal's head or body size decreases. However, many small animals, including insects, can hear the same sound frequencies that are heard by much larger animals. They possess auditory adaptations to cope with the mismatch between wavelength and body size to generate interaural cues for sound localization (Michelsen, 1992).
The acoustic parasitoid fly Ormia ochracea finds its host, the field cricket Gryllus rubens, by homing in on the cricket's mating call (Cade, 1976; Walker, 1993). This is remarkable because the interaural distance separating the fly's hearing organs (500 μm) is nearly 15 times shorter than the 7.35 cm wavelength of the host's calling song (4.5 kHz) (Robert et al., 1992).Ormia's small size means it generates an immeasurably small (0 dB) ILD and a miniscule interaural time cue, at most 1.5 μsec, at 90° azimuth. Yet Ormia possesses a hearing organ that is highly sensitive to the direction of G. rubens calling song (Robert and Hoy, 1998; Mason et al., 2001; Muller and Robert, 2001). The key to Ormia's directional sensitivity is the novel mechanical coupling of its tympanal membranes, in the auditory periphery (Miles et al., 1995). In brief, this acoustic coupling mechanism generates significantly greater interaural intensity (5–10 dB) and time differences (>50 μsec) than are available from the impinging sound field, alone. We now turn to the question of how the fly's auditory system processes these small interaural cues. The response properties of single afferents in the fly's auditory afferents indicate that they are acute in the temporal domain. These are the cues that are “handed off” to the second order auditory interneurons. The fly appears to overcome the acoustic disadvantage of its small size relative to the behaviorally salient wavelengths of the signal it must localize by possessing biomechanical innovations in its auditory periphery and by time and intensity coding features in its auditory afferents.
MATERIALS AND METHODS
Animals. The animals used in this study are gravid (larvae-bearing) female flies of the species Ormia ochracea(Diptera: Tachinidae, Ormiini). They were reared from a lab colony descended from field-caught females attracted to sound traps in Gainesville, Florida and at the Gulf Coast Research and Education Center (Bradenton, FL), according to the procedures of Walker (1986)and Walker and Wineriter (1990).
Physiological recordings and staining of single auditory afferents. To prepare the flies for physiological recording, they were immobilized by chilling on ice for 5 min. They were waxed, dorsal side up, to a small round magnet (diameter 1 cm) using a wax mixture (4:1) consisting of Sticky wax (Kerr Manufacturing Company) and beeswax. The fly's dorsum was dissected, and the flight muscles and the gut were removed to reveal the nervous system, the fused thoracic ganglion. The dorsal approach for dissection was preferred because it spares damage to the hearing organs, which are situated ventral and frontal on the fly. For intracellular recording, we used high-resistance, glass pipette microelectrodes, with 70–140 MΩ impedance. The tip of the electrode was filled with 1% Lucifer yellow (catalog #LY-L20964; Sigma, St. Louis, MO) and backfilled with 100 mm LiCl2. Sometimes, we filled the micropipette with other dye-marking solutions such as 100 mm CoCl2 or 4% Neurobiotin (Vector Laboratories, Burlingame, CA), in which case the shanks contained the same solutions. The signals were led to either an A & M Systems Model 1600 or a Dagan (Minneapolis, MN) voltage-clamp amplifier. Whenever we were able to record the physiological response of an acoustically responsive cell, we attempted to inject dye into it. In 8 of 41 single-unit recordings, dye staining confirmed that the identity of the unit as an auditory afferent; the others were incompletely stained or not stained at all. When physiological responses were recorded from a unit but were not stained, the consistency of response among stained and unstained afferents was marked by a very short latency of response (<4 msec) that we took to be a reliable and characteristic identifier. We know that the response latencies of second order interneurons are at least twice as long (Oshinsky, 1998). Our confidence that even unstained auditory units were likely to be afferent cells was based on our knowledge of the anatomy of the auditory system. We confined our recordings to a localized region of the fused thoracic ganglion where the frontal nerve (containing the auditory nerve) enters the thoracic ganglion; this region is distant from the auditory neuropil areas (Stumpner and Lakes-Harlan, 1996; Oshinsky, 1998). This strategy minimizes the possibility that the recordings were made from auditory interneurons, which have most of their projections near the middle each thoracic neuromere (Stumpner and Lakes-Harlan, 1996; Oshinsky, 1998). Our anatomical data show that all afferent fibers are <2 μm in diameter, and many are <0.5 μm. This made long-term intracellular recordings difficult and staining problematic. The identity of stained single afferents was matched against the terminal projections of multiple afferents that were stained, en masse, by backfilling the from the afferent nerve (see below). Confirmation of afferent identity was easy because of the stereotyped course and anatomy of the auditory afferent system.
Auditory stimulus. We calibrated the stimulus field emitted from speakers (high-performance tweeter; ESS Systems AM1) by making measurements in the free field with a Bruel & Kjaer (Norcross, GA) type 4138 condenser microphone connected to a Bruel & Kjaer model 2608 or model 2209 sound level meter. Acoustic stimuli were programmed through a TDT, Inc. (Gainesville, FL) System 2 and fed into a Harmon–Kardon model HK6100 amplifier, and in turn connected to a TDT programmable attenuator. The Bruel & Kjaer microphone was placed at the position of the fly, and the intensity of sound pulses at frequencies between 1 and 25 kHz were calibrated to within 0.5–95 dB sound pressure level (SPL) with reference to a continuous tone. The speakers were placed in the horizontal plane at ±90° azimuth with respect to the anteroposterior axis of the animal. The arrival time of the sound from each speaker to the fly was calibrated to match within 1 μsec using a Gould Instruments (Valley View, OH) model 1604 digital oscilloscope, Bruel & Kjaer 4138 microphone, and a Bruel & Kjaer model 5935 power supply and amplifier.
Neuroanatomy from backfills. The anatomy of the chordotonal auditory sensory organ was obtained by the backfill technique, using fluorescent dextrans (Texas Red D-3328 or FITC D-3306; Molecular Probes, Eugene, OR), horseradish peroxidase (HRP), or cobalt chloride. A small crystal of the stain reagent was applied directly onto the sensory organ exposed by dissection. A small drop of saline (O'Shea and Williams, 1974) was also applied, and the animal or preparation was incubated at 4°C for 24 hr. The thoracic ganglion was removed by dissection and fixed in 4% paraformaldehyde, pH 7.4, prepared from 16% stock solutions (catalog #15710; Electron Microscopy Sciences, Fort Washington, PA). The ganglion was then dehydrated in ethanol, cleared in methyl salicylate, and viewed with a Bio-Rad (Hercules, CA) MRC 600 confocal microscope. The 1 μm optical sections were then digitally projected in the z plane to produce one image (Confocal Assistant, 1994–1996; Todd Clark Brelje). Projections stained by HRP or the cobalt sulfide procedure were viewed and photographed via conventional photomicroscopy procedures. Where cobalt chloride was used as a stain, the cobalt was precipitated by reaction with hydrogen sulfide, and then fixed with Carnoy's solution. The preparation was cleared with methyl salicylate and viewed using conventional light microscopy.
Data acquisition and analysis. Threshold-tuning curves were obtained using custom-designed software (Vreislander et al., 1991) for a Macintosh and a Mac AdiosII analog-to-digital (A/D) board (GW Instruments, Somerville, MA) with an algorithm described in Taylor and Creelman (1967). Briefly, the magnitude and the direction of the intensity steps during the tests for threshold are based on the results for previous trials. This method permitted obtaining threshold-tuning curves in <2 min. This was essential because the small size of the afferent axons made long-term recordings very difficult. The threshold frequency response of the afferent axons was tested using a 10 msec sound pulse with a 1 msec rise–fall ramp. The repetition rate for sound tones used for the tuning curves was 10 Hz. The trapezoidal shape of a stimulus tone closely resembles a cricket's natural sound pulse. Auditory threshold was defined as a response of one or more spikes, at least three times to a set of five stimulus tones. Spikes were detected using a modified World Precision Instruments (Sarasota, FL) window discriminator and a custom spike buffer on the GW board.
Physiological and acoustic data were recorded for off-line analysis on an AR Vetter (Rebersburg, PA) model 420 F tape recorder. Custom software, written on a personal computer, was used for sound presentation using an array processor and A/D equipment from TDT, Inc. All digital signals were filtered to remove aliasing. A sampling rate of 100 kHz allowed for 10 μsec resolution of the spike times. Data analysis was done using IgorPro (WaveMetrics, Lake Oswego, OR) and Mathematica (Wolfram Research). The logistic regression equation in Figure 5 was computed using SAS analysis software.
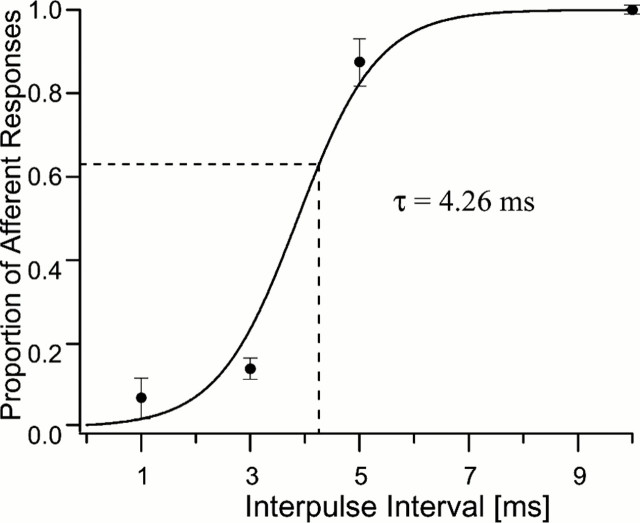
Fig. 5.
The proportion of phasic afferent spikes to sound pulses (10 msec, 5 kHz, 85 dB SPL) presented with varying interpulse intervals. Because the interpulse interval increases the phasic afferent is able to reset before the next sound pulse, so there is a higher proportion of responses. The time constant (τ) for the refractory period of this phasic afferent is defined as the interpulse interval that will allow the afferent to respond to 63% of the sound pulses.
Refractory period measurements. The objective was to quantify the ability of an afferent unit to follow a periodic characteristic of the acoustic stimulus such as the tone pulse envelope itself. The pulse repetition rate was an experimental variable. The stimulus procedure consisted of a series of four different trains of tone pulses. Each train consisted of 20 pulses with either 1, 3, 5, or 10 msec interpulse intervals. Each pulse train was presented at least three times. The proportion of sound pulses that the unit responded to was measured. For example, if the afferent unit responds to 16 of the 20 pulses in a stimulus train, the proportion of afferent responses was 0.80.
RESULTS
Anatomy
The hearing organs (bulba acusticae) of O. ochracea are a pair of white, egg-shaped structures that are suspended within the thoracic cavity and behind the tympanal membranes, to which they are attached. Each sensory organ contains ∼90–100 afferent cells each associated with support cells and scolopales (Robert et al., 1994a; Edgecomb et al., 1995; Robert and Willi, 2000). Each bulba acustica is directly attached to its tympanum by means of a stiff rod-like cuticular apodeme (Robert et al., 1994b). This means that any sound induced vibration of the tympanum would be directly transferred to the sensory organ, with the apodeme acting like a piston. It was possible to make small lesions in the bulba and apply small amounts of marker reagents (such as cobalt chloride, HRP, fluorescent dextran, or neurobiotin), which were taken up by from a few to many afferent cells and transported to their terminals in the central ganglia, thus revealing the branching structure of the afferent projection within the CNS. Cobalt chloride was especially useful because it often resulted in staining scores of afferents and this en masse staining of terminals helped to define the length and breadth of terminal projections, and hence reveal the morphology of the auditory neuropils.
When HRP, neurobiotin, or fluorescent dextrans were applied to the bulba, the stains remained solely within the afferents, and such preparations allowed us to visualize the terminal projections of variable numbers of afferent fibers from many to a few afferents, which permitted us to confirm the identity of single fibers stained from intracellular injections of Lucifer yellow (above). Figure1A is an image of a population of afferent terminals stained en masse using Texas Red dextran and photographed by digital confocal microscopy; the image consists of the summed projection of twenty-three optical sections, each 1-μm-thick. It is consistent with the result of examining >200 stained afferent preparations using various marker dyes. The afferent projections and neurons are shown in Figure 1 and are drawn in the context of a labeled “standard” ganglionic outline, with the aid of camera lucida drawing, as well as from photographs made from confocal microscopy. The thoracic neuromeres and the extent of the auditory neuropils are drawn in dotted lines, but reflect the reliable anatomical compartments seen in our histological preparations. The auditory nerve (frontal nerve–FN) enters the fused thoracic-abdominal ganglion, anteriorly, and forms a compact, well defined nerve tract (Fig. 1A). The auditory tract then turns toward the midline and runs posteriorly through all three thoracic neuromeres, branching within each one and contributing a spray of terminals, thereby defining acoustic neuropils in the prothoracic (T1), mesothoracic (T2), and metathoracic (T3) neuromeres. Taken together, the two bilateral projections of the auditory tract define a ventral ellipse (VE). The auditory tract and its neuropils lie entirely in the ventral region of the fused ganglion and only within the thoracic neuromeres. No afferent projections into the posterior abdominal neuromeres posterior to T3 were ever seen in our preparations.
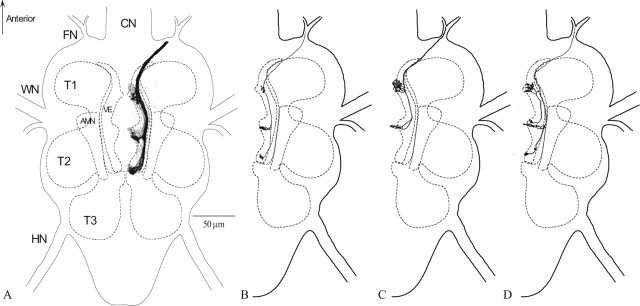
Fig. 1.
Anatomy of the acoustic afferents of the right bulba acustica in Ormiaochracea.A, This is a dorsal view of az-projection of 28, 1-μm-thick confocal optical sections. Texas Red dextran dye was applied to the bulba acustica, and >50 afferents were stained. Stained afferents project to three acoustic neuropil regions in the ventral region of the three neuromeres in the fused thoracic ganglion of the fly. B, A single auditory afferent stained with fluorescent dextrans. There is a dense projection into the T2 auditory neuropil area from this afferent. The T1 and T3 neuropil regions receive minimal projections from this afferent. C, A single auditory afferent stained with fluorescent Texas Red dextrans. There are dense projections into the T1 and T2 auditory neuropil area from this afferent. The T3 neuropil region does not receive any projections from this afferent.D, A single auditory afferent stained by Lucifer yellow injection. A single branch projects into the T1 auditory area from the primary neurite. This branch then continues projecting to the T2 auditory neuromere. There is a separate projection from the primary neurite into the T2 auditory area and a minimal projection to the T3 auditory area. AMN, Accessory mesothoracic neuromere;CN, cervical connective; FN, frontal nerve; HN, haltere nerve; T1, prothoracic neuromere; T2, mesothoracic neuromere;T3, metathoracic neuromere; VE, ventral ellipse; WN, wing nerve.
Figure 1B–D is an anatomical depiction of individually stained afferent fibers. Figure 1, B andC, resulted from retrograde staining made by applying dye to the bulba, but Figure 1D is a Lucifer yellow preparation from an apparent intracellular penetration. Although most of the single fiber stains seen in our preparations contributed projections to all three neuromeres, a few stained only two: T1 and T2 (Fig. 1C). The extent of efflorescence for any given terminal fiber within a particular thoracic neuromere appears to be variable, as also seen from Figure 1C. Such differences may have functional significance, but we realize that incomplete staining could also produce the appearance of differential efflorescence. However, examination of many afferent fiber terminals gives the impression that such differences are real.
Frequency tuning of the auditory afferents
Despite their small diameters, we were able to record from the axons of single auditory afferent fibers in the auditory tract with glass micropipette electrodes. Their small axons (maximum diameters of 2 μm) made it difficult to maintain the penetration for long enough periods to get complete tuning information for every unit. We present tuning curves from a sample of 28 afferents from 26 flies where reasonable amounts of data were obtained. In the two flies where we obtained two tuning curves, there was no significant difference in best frequency or threshold. A population tuning curve from the entire sample of 28 fibers was plotted in Figure2A. The collective best frequencies (BFs) fall within a range from 4 to 9 kHz. In this sample, the BFs were also displayed categorically as a frequency histogram in Figure 2B, and it reinforces the impression gained from the population tuning curve. In Figure 2C, we compared the physiological tuning of our afferent sample with the mechanical tuning of the tympanal membrane, as measured by laser vibrometry (Miles et al., 1995; Robert et al., 1996). It should not be surprising that the neural tuning curve closely follows the mechanical tuning curve in frequency and dynamic range. The tympanal movements are directly reflected in the movements of the auditory organ; they are, after all, mechanically coupled (Miles et al., 1995).
Fig. 2.
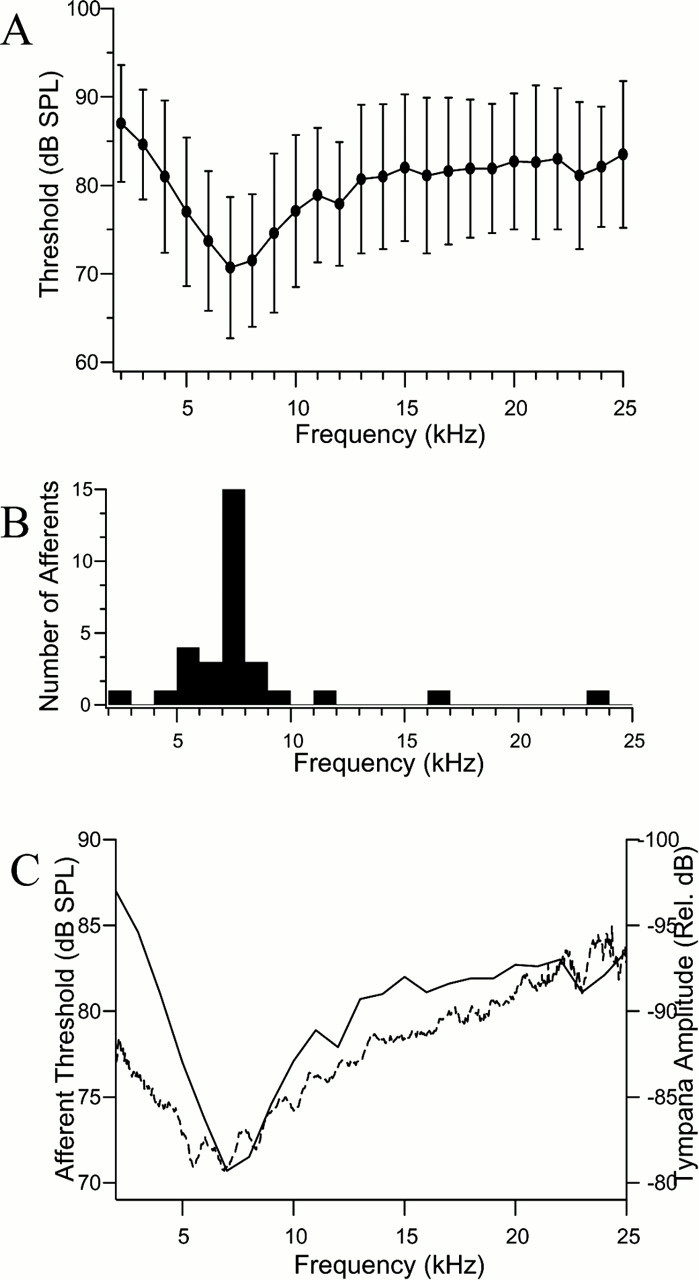
Summary of the frequency threshold response in the auditory afferents of Ormia ochracea. A,Average ± SDs for 28 afferents (type 1, type 2, and type 3) obtained with 10 msec pulses repeated at 1 Hz. B,Histogram of the number of afferents with best frequencies at a particular frequency. These data were compiled from the same tuning curves that are averaged in A. C,Comparison of the average tuning of the afferents in this study (solid line, left ordinate) with the membrane displacement measured with laser vibrometry measured in relative decibels (dotted line, right ordinate). The membrane response amplitude was measured with laser vibrometry, using a broad band white noise stimulus between 1 and 25 kHz (Robert et al., 1996).
The tuning curves of individual afferent fibers are often sharper than the average curve of Figure 1A. The average Q3dB, the ratio of the BF over the bandwidth at 3 dB above the BF threshold, of the combined grand average tuning curve is 2.6. This is sharper than the predicted Q3dB from the mechanical response of the tympana of 1.9 (Fig. 2C). Figure3A shows three examples, each from different animals, of threshold tuning curves for afferents with BFs <10 kHz. Although none of these afferents have a BF of 4.5 kHz, which is the frequency with the most power in the host's calling song, the tuning at BF is sufficiently broad to be relatively sensitive to 4.5 kHz. Figure 3B demonstrates the tuning curves for three single afferent fibers with BFs >10 kHz. In general, the tuning curves for afferents tuned to high frequencies (BFs >10 kHz; mean Q3dB, 6.4) are sharper than for those tuned to low frequencies (BFs <10 kHz; mean Q3dB, 2.1).
Fig. 3.
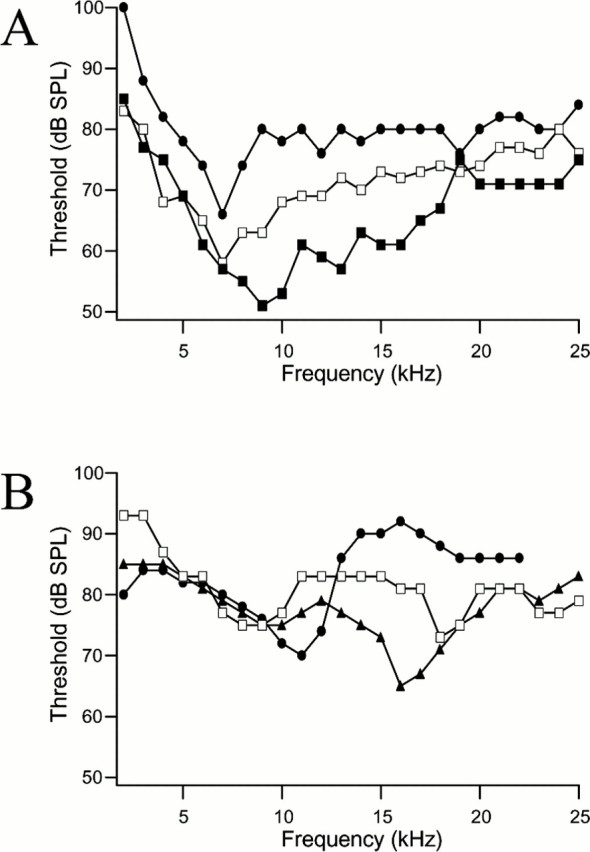
A, Examples of three tuning curves for afferents with best frequencies <10 kHz. B,Examples of three tuning curves for afferents with best frequencies >10 kHz.
Physiological response properties of single afferent fibers
We made our recordings from single axons in the auditory tract near its origin, where the frontal nerve joins the central ganglion. This location made it much more likely that the electrodes would encounter afferent fibers than auditory interneurons (Fig.1A) (Oshinsky, 1998). The search tone used to elicit auditory responses was a 10-msec-long pulse (rise–fall ramp time of 1 msec) at one of two frequencies, either 5 or 7 kHz. Also, we could choose to increase the pulse duration up to 100 msec to characterize the tonic response of a unit. The 41 afferents in our data set fell into one of three physiological response categories, type 1 (predominantly), type 2, and type 3.
Type 1 afferents
Type 1 afferents, which comprised 23 of the 41 afferents sampled, were the most common kind. Their response to auditory tone stimuli was distinctive and unusual in that one and only one action potential was elicited by any given suprathreshold tone pulse. Regardless of the stimulus intensity or duration, and whether in response to a single tone or to tones in trains, type 1 afferents discharged only one spike per tone (Fig. 4A,B). Spontaneous activity in type 1 afferents was very rarely observed (Fig.4C); in particular, no bursts of spikes, such as occur in injury discharges, were recorded.
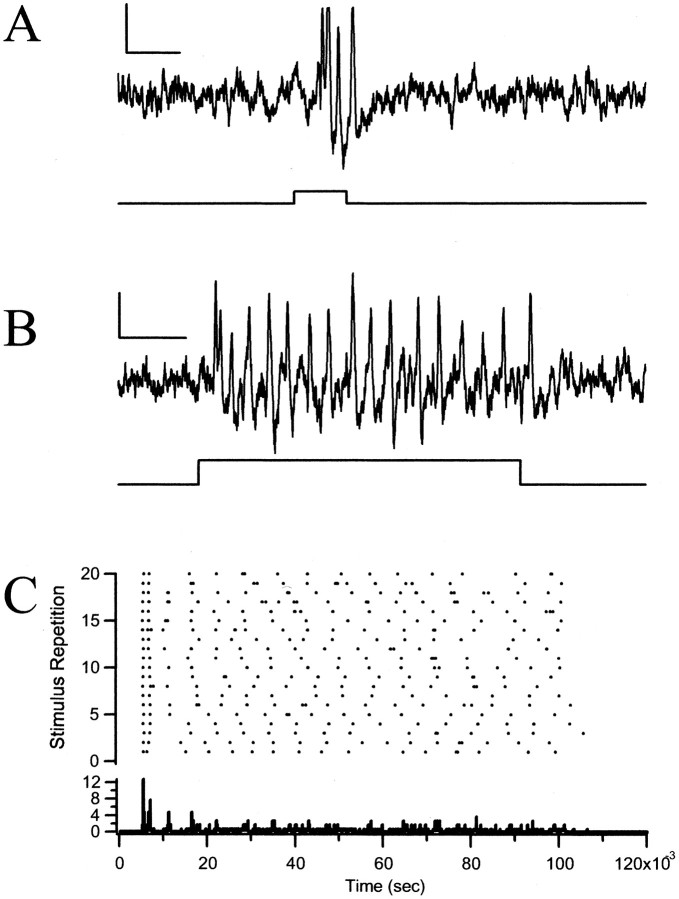
Fig. 4.
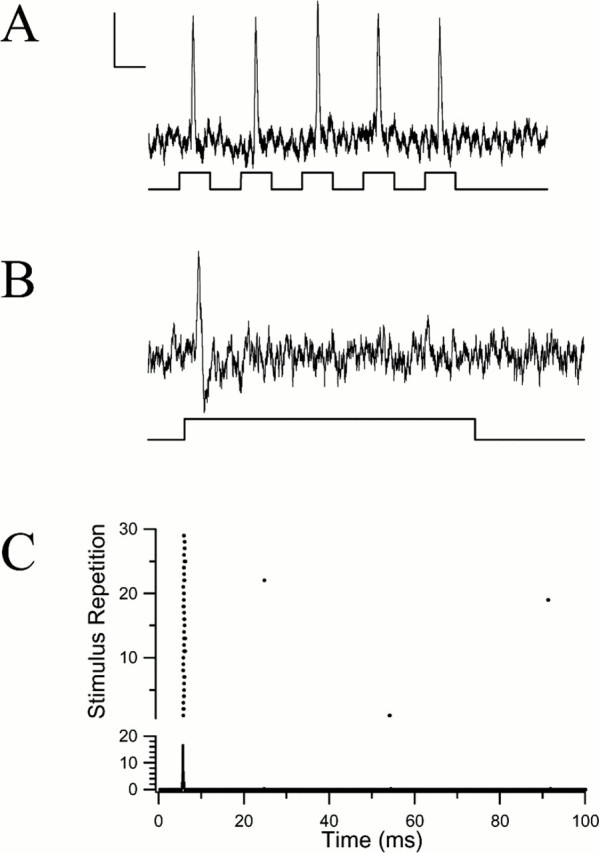
Physiological recordings of a type 1 phasic acoustic afferent. A, Response of a type 1 afferent to synthetic cricket song (50 pps, 5 kHz, 50% duty cycle, 85 dB SPL). Calibration: 2 mV, 10 msec. B, Phasic response of a type 1 afferent to a 100 msec sound pulse at its BF, 7 kHz at 80 dB SPL. Calibration: same as in A.C, Raster and PST histogram of a type 1 afferent in response to the repetition of the 100 msec stimulus at 1 Hz. Very little variation can be seen in the latency of the spike at this resolution (bin width, 20 μsec).
A second notable response characteristic of type 1 cells is their strict time-locking to the stimulus onset. At any given suprathreshold intensity level, type 1 response latencies were reproducible within a very narrow time window (Fig. 4C). For example, in Figure 4C, the occurrence of the spike was time-locked to the onset of the stimulus so precisely that its SD, or jitter, was 0.080 msec. The latencies of the spike responses of seven different type 1 afferents, each to a set of 30 successive tones revealed an average latency of 2.84 msec, but an average jitter of just 76 μsec (0.076 msec) (Table 1). It appears that the parasitoid fly's auditory afferents are very sensitive to the pulse repetition rate of the host's calling song, as evidenced from the tight time-locking (low jitter) to the onset of each pulse.
Table 1.
Spike latency and jitter for type 1 afferents
| Animal | Frequency (kHz) | Stimulus intensity (dB SPL) | Afferent threshold (dB SPL) | Latency (msec) | Jitter (msec) |
|---|---|---|---|---|---|
| 2–61 | 5 | 85 | 65 | 2.71 | 0.073 |
| 2–77 | 5 | 85 | 63 | 2.29 | 0.080 |
| 2–93 | 5 | 85 | 80 | 3.05 | 0.121 |
| 2–93b | 7 | 85 | 71 | 2.65 | 0.081 |
| 2–109a | 7 | 85 | 78 | 3.19 | 0.052 |
| 2–109c | 7 | 85 | 75 | 2.97 | 0.075 |
| 2–119b | 7 | 75 | 75 | 3.02 | 0.012 |
Type 1 afferents appear to be good candidates for the function of time coding. We measured the refractory period for type 1 afferents because the precision of the discharge of a neuron is sensitive to its refractory period (Berry and Meister, 1998). For example, longer refractory periods allow sensory neurons to respond to trains of repetitive stimuli with a consistent latency. To determine the refractory period or time constant of type 1 afferents, we measured the minimum interval of time between two stimulus pulses that allowed the afferent neuron to respond to both pulses. We defined the refractory period as the length of the interpulse interval required to elicit an afferent discharge in at least 63% of the stimulus presentations, which conforms to conventional definitions of refractory period (Rieke et al., 1997). The stimulus pulse was defined as a 5 kHz stimulus tone, 10 msec in duration, and presented at a level of 85 dB SPL; the interpulse interval was varied at 1, 3, 5, and 10 msec. Trains of 20 pulses were presented at 1 Hz. Data were collected from 10 presentations of such pulse trains and so data from a total of 200 stimulus presentations for each interpulse interval were collected from three different animals. Figure 5displays the data and a logistical equation describing the best fit for the data. The graph shows that the proportion of afferent responses (single spikes) to the train of tone pulses is highly sensitive to the interpulse interval. The best fit follows equation:f(x) = (e 1.36x − 5.26)/(1 +e 1.36x − 5.26) (p << 0.01). The value of the time constant, 4.26 msec, was extrapolated from the sigmoidal curve in Figure 5. We interpret these data to mean that the phasic afferents require a “quiet” period (no acoustic stimulation) of >4 msec between sound pulses to respond to >63% of a train of pulses. Thus, although a type 1 afferent discharges only a single action potential during the first 4 msec of the 10 msec sound pulse and is inactive for the remaining 6 msec while the sound stimulus is still present, it still requires an additional 4.26 msec of silence during the interpulse interval before it can be predicted with confidence to discharge again if stimulated by a following sound pulse. These temporal characteristics “fit” nicely the task of detecting the mate calling song of its cricket host, Gryllus rubens (in Florida, Walker, 2000) orTeleogryllus oceanicus (in Hawaii, Zuk et al., 1993) which have a ∼10 or ∼41 msec interpulse interval in their calling songs, respectively.
Type 1 afferents exhibit an intensity-level dependent latency shift, commonly observed in other sensory systems, in which latency of response and stimulus intensity level are inversely related. InOrmia, however, this common sensory property has potential implications for processing sound direction, as will be discussed below.
Type 2 afferents
In our recording sample, type 2 afferents were encountered less frequently than type 1. Sixteen of the 41 cells examined in detail were of this type. Their response properties are depicted in Figure6. Like type 1 afferents, they are also phasic in their response but differ by generating from two to four action potentials per stimulus tone, at a discharge rate on the order of 300 Hz, depending on stimulus level, as opposed to the single spikes from type 1 afferents. Figure 6A shows the response of a type 2 afferent to a stimulus consisting of five repetitions of a tone pulse: 4 kHz carrier frequency, 10 msec pulse duration, delivered at a 50% duty cycle and at a level of 85 dB SPL. Figure6B shows the response to a 5 kHz, 100 msec duration tone pulse delivered at 85 dB SPL. Clearly, increasing the stimulus duration does not necessarily elicit more spikes. Figure 6Cis a raster display (above) and its peristimulus time (PST) histogram (below) showing 35 repetitions of a 100 msec, 5 kHz duration tone stimulus. It shows that the response intervals and their jitter in type 2 afferents, as in type 1, are time-coded with precision. For the first responding spike, the average latency and jitter for this afferent is 2.90 ± 0.17 msec. The first four spikes show time locking, and the precision of the latency lock is greater for the first two spikes than for the third or fourth. A few spikes occur sporadically after the initial burst of four spikes; this may reflect a low level of spontaneous activity. Type 2 afferents also exhibit an inverse relationship between response latency and stimulus intensity level.
Fig. 6.
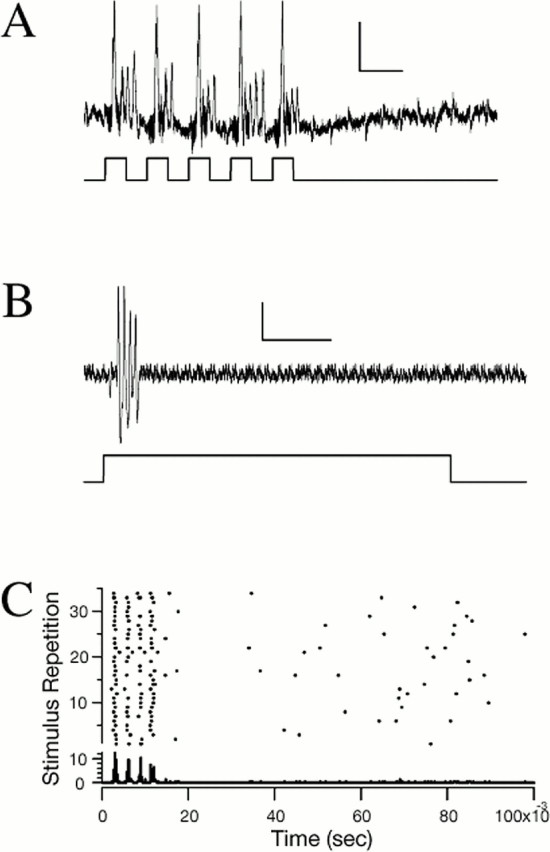
Physiological recordings of a type 2 phasic acoustic afferent. A, Response to synthetic cricket song (50 pps, 4 kHz, 50% duty cycle, 95 dB SPL). Calibration: 2 mV, 10 msec. B, Phasic response to a 100 msec sound pulse at the BF of the cell, 5 kHz at 85 dB SPL. C, Raster and PST histogram of a type 2 afferent in response to the repetition of the 100 msec stimulus at 1 Hz. Four distinct clusters of spikes are seen in the response to this 100 msec sound pulse (bin width, 20 μsec).
Type 3 afferents
Type 3 afferents were rarely encountered in this study, accounting for only 2 of the 41 cells sampled. These cells discharge spikes throughout the duration of the stimulus tone, hence the term for this kind of cell, “tonic”. Figure7A shows a typical type 3 response to a brief tone (5 kHz carrier, 10 msec duration, at 75 dB SPL); the four spikes elicited would not distinguish this cell from a type 2 cell. However, when the same stimulus was increased in duration to 100 msec, the afferent discharged a train of spikes that persisted for the full length of the tone (Fig. 7B). Type 3 cells have a low level of spontaneous activity, similar to the other two afferent types. The spontaneous rate of this cell in the absence of acoustic stimulation was less than one spike per second. Figure 7C is another raster plot and PST histogram of a type 3 cell. Type 3 cells show time locking to the stimulus onset only for the first two action potentials, after which the latency lock is no longer apparent. The latency and jitter for the first spike is 3.79 ± 0.082 msec.
Fig. 7.
Physiological recordings of a type 3 tonic acoustic afferent. A, Response to a single 10 msec cricket-like sound pulse (1 msec rise–fall, 5 kHz, 85 dB SPL). Calibration: 2 mV, 10 msec. B, Tonic response for the duration of the 100 msec at its BF, 7 kHz at 85 dB SPL. Calibration: 2 mV, 10 msec. C, Raster and PST histogram of a type 3 afferent in response to the repetition of the 100 msec stimulus at 1 Hz. The latency for the first two spikes is relatively invariant compared with the timing of the subsequent spikes during the rest of the stimulus (bin width, 20 μsec).
Directional sensitivity
Latency coding
In all three classes of auditory afferents, the response latency is dependent on the stimulus intensity level. As the stimulus level is systematically increased, the latency of the first spike (and only spike in type 1 afferents) decreases monotonically to some demonstrable minimum value. Figure8A illustrates the response of a typical type 1 afferent cell to a series of five 5 kHz tone pulses, each of which is increased stepwise in intensity level, in 5 dB steps. Reading left to right, the first spike is elicited to the most intense stimulus (95 dB) i.e., it occurs with the shortest delay. The succeeding spikes appear in order of stimulus intensity. The least intense stimulus, 75 dB, elicited the right-most spike with the longest delay. This elementary intensity/latency relationship was observed for all three types of afferent cells.
Fig. 8.
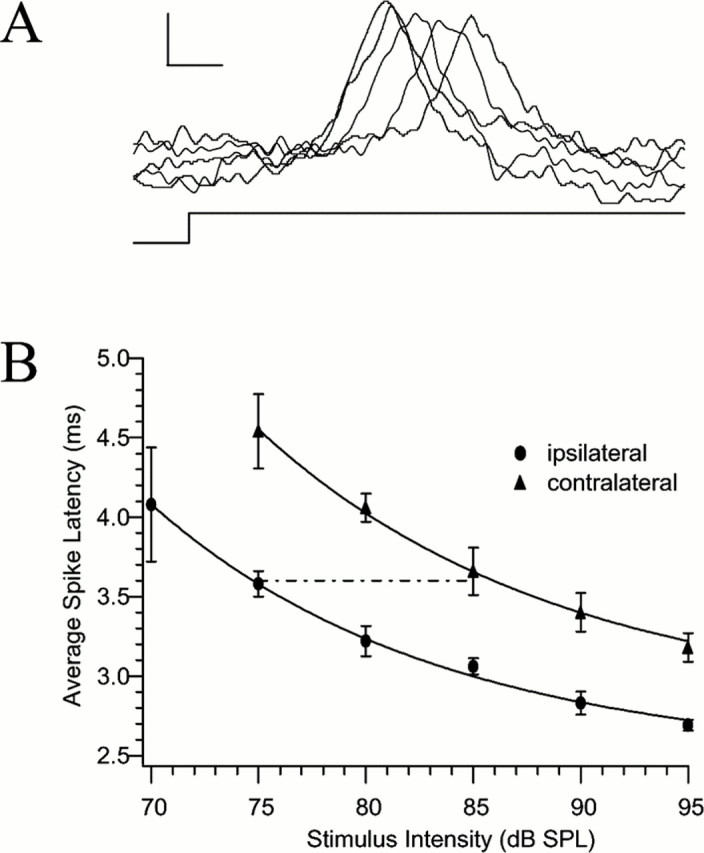
Direction-dependent physiology of the type 1 phasic afferents. A, There is a latency shift of the spikes elicited by stimuli of different intensities. In this experiment, sound was presented from 95–75 dB SPL in −5 dB steps. Calibration: 1 mV, 1 msec. B, There is a latency difference in the spikes elicited by sound on either side of the animal. This is caused by a decrease in the amplitude of the movement of the tympanal membrane contralateral to a sound stimulus. Thedashed reference line shows that a 10 dB difference in intensity would produce a latency shift consistent with the difference in the latency caused by ipsilateral versus contralateral stimulation.
The relationship between latency coding and directional sensitivity emerges only when we compare the response latencies between sensory organs (either at the level of spikes from single units or compound action potential spikes from the whole auditory nerve) to a directional sound source (Fig. 8B). Let us say we record from an afferent cell from the auditory organ on the animal's right side in response to cricket-like sounds played back from a loudspeaker on the animal's left side, meaning that the sound source is coming from the “far” (or contralateral) speaker relative to the ear that provides the input to the afferent. In this configuration, we would generate the upper latency–intensity curve in Figure 8B. Then, change the experimental configuration simply by playing back the same set of stimuli from a loudspeaker placed on the animal's right side, such that the sound source comes from the “near” (or ipsilateral) speaker relative to the ear (both speakers placed symmetrically with respect to 0° azimuth). This change would generate the lower latency–intensity curve in Fig. 8B. For either ipsilateral or contralateral stimulation, the data points represent the mean spike latencies for five repetitions of the stimulus at each intensity level. The variation in the response latency, or spike jitter, is plotted as SD of the mean latency. Although the two latency–intensity curves parallel each other, they are also well separated from each other (p << 0.01, two-way ANOVA). This is a graphical depiction of the directionally dependent difference in the two latency–intensity curves in each independent auditory channel. The lack of any overlap between the mean spike latencies at any intensity means the source direction could be coded by response latency between left and right channels and could serve as a significant cue for interneurons, on which the afferent channels converge. Furthermore, the jitter is negligible compared with the value of the spike latencies themselves. Thus, when the sound source is shifted from the ipsilateral to the contralateral side (from 90° right to 90° left, for example), the response latencies of the afferents are increased from 0.5 to 1 msec, depending on intensity (Fig. 8B).
The two curves can be used to determine “trading relations” between latency and intensity levels in the two auditory organs. Thus, for example, the response delay of a given afferent neuron to an ipsilateral sound source with a sound level of 75 dB is approximately equivalent to the response delay to a contralateral sound source with a sound level of 85 dB (Fig. 8B). Conversely, a 10 dB decrease in stimulus intensity level resulting from shifting the sound source from one side to the other produces nearly equivalent time delays. The curves generated from our neural response data again find close parallels with earlier findings from investigations of the mechanical properties of the tympanal membranes. Robert et al. (1996)reported that when the sound source is switched from one side to the other the amplitude of vibrations in the contralateral tympanal membrane is decreased by 10–12 dB relative to those of the ipsilateral tympanum, to a 5 kHz tone. It seems likely, therefore, that the directionally sensitive response latencies recorded in the afferents reported here have their basis in the mechanical resonance properties of the peripheral auditory apparatus (Miles et al., 1995).
Population coding: range fractionation and the emergence of directionality
We compiled the thresholds at the afferents' best frequencies of all 28 of the 5 kHz-tuned afferents and found that they are distributed over a wide range, from 50 to 93 dB SPL (Fig.9). The 43 dB dynamic range provides a physiological mechanism for sound intensity discrimination and subsequent sound localization by the animal, as has been suggested earlier by Römer et al. (1998). In the Discussion, we will propose a hypothesis of the population coding for sound localization based on intensity differences.
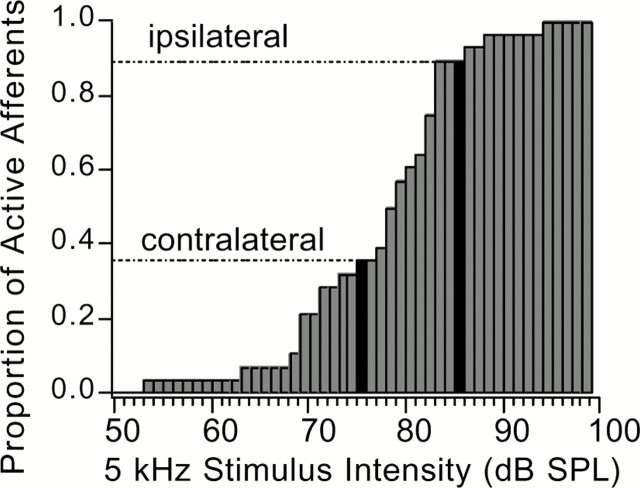
Fig. 9.
Range fractionation of the all the afferent types. The cumulative histogram of the proportion of afferents above threshold in response to a 5 kHz stimulus at various intensities. The proportion of afferents above threshold on the contralateral side of the animal will be less because of the amplitude decrease in the movement of the contralateral tympanal membrane caused by the mechanical coupling of the tympana. The lines show the relative proportion of the afferents that would be above threshold in response to an 85 dB SPL ipsilateral stimulus. The contralateral side of the animal will be subject to a 10 dB attenuation of the stimulus, therefore 55% fewer afferents will be above threshold on that side.
Figure 9 is a cumulative histogram of afferent thresholds. The histogram depicts the proportion of afferents that would be above threshold at any given intensity, for a 5 kHz tone pulse. The horizontal lines provide a marker to compare the proportion of afferents that would be above threshold to a 75 dB SPL (n = 10 of 28) stimulus and the proportion that would respond to stimulus at 85 dB stimulus (n = 25 of 28). This example can be interpreted to mean that a directional sound source that impinges on the nearest ear would elicit spikes from 89% of the afferents in the ipsilateral auditory channel. However, in the contralateral channel of the far ear, just 36% of the afferents are above threshold. This marked difference in the interaural neural input supports a plausible neural mechanism for directional sensitivity in the auditory system. The 10 dB difference in stimulus intensity highlighted in Figure 8B approximates the difference in the vibration amplitudes of the ipsilateral and contralateral tympanal membranes when the sound is on one side of the fly's body (Robert et al., 1996). This again emphasizes the parallel in the biomechanical response in the auditory periphery and the neural response in the auditory system.
DISCUSSION
Temporal response characteristics of the auditory afferents
The prevalence of type 1 neurons is the most striking feature in the physiological response properties of Ormia's auditory afferents. Afferents of this type discharged only a single spike when stimulated by a threshold or suprathreshold 5 kHz tone pulse, no matter how high the intensity level or how long its duration. Type 1 phasic neurons also exhibited very precise latency locking with the stimulus onset; i.e., the jitter in the time delay was exceedingly small, on the order of 75–80 μsec. Neurons with similar properties have been reported in vertebrate sensory systems that feature high acuity in the temporal domain and appear to part of time-coding systems (Carr, 1993). Even type 2 phasic cells showed precise time locking for the first one or two spikes. Type 3 tonic cells also exhibited reliable time locking for the first spike or two but variability increased greatly in succeeding spikes. Thus, the response properties of nearly all ofOrmia's auditory afferents seem well suited to code the temporal onset of pulsatile acoustic stimuli that occur in cricket songs.
Whereas we assert that type 1 afferents should be expected in a sensory system such as Ormia's, in which temporal acuity is of the essence, we are aware of the possibility that the type 1 hallmark, a single-spike response, could be an injury artifact. Injury might arise because the diameters of afferent axons are in the range of 1–2 μsec, and intracellular penetration might disrupt the membrane at the point of penetration. However, we believe that the type 1 response is real for the several reasons. First, locally rupturing the axonal membrane would be expected to cause a sudden and massive inward sodium current as well as an outward potassium current that would result in a burst of spikes, well known to intracellular electrophysiologists as the injury discharge. We rarely recorded such discharges during either intracellular or extracellular recording. Second, the consistency of the auditory response from unit to unit, among the 23 type 1 afferents sampled implies a certain predictability that would be expected of a stable (real) physiological characteristic. Third, not all of the 23 afferents recorded were from intracellular penetrations, as judged from the failure to inject dyes into the cell; such recordings were likely to have been extracellular. Yet the response characteristics of the unstained preparations were the same as in stained ones. Fourth, the low level of spontaneous activity is consistent with the high “signal value” in occurrences of spikes. For these reasons we believe that the type 1 auditory response is real and not artifactual.
In those cases where intracellular recordings were held for more than a few minutes, the response to suprathreshold 5 kHz tones was very stereotyped and predictable; the thresholds were stable throughout the recordings, and the response latencies and jitter were stable. It is worth comparing the response of type 1 with the more conventional type 2 phasic and type 3 tonic units. The same level of stability and predictability of response was seen in type 1 and 2 afferents. The level of spontaneous activity was low in type 1 and 2, as would be expected for phasic afferents, and by comparison, somewhat higher, in type 3, as would be expected for tonic afferents. The overwhelming proportion of afferents from which stable recordings could be made were of type 1 or 2, but type 3 were very rare. The argument that type 1 afferents are actually “injured” type 2 afferents is not supported for reasons given above and by the fact that both 1 and 2 demonstrate the same level of stability in their response characteristics from experiment to experiment, within type as well as between types. As for the rarity of type 3, possibly there are simply fewer of them but we can also speculate it reflects a “size principle” at work. By this hypothesis, the largest diameter axons (1–2 μm) are the most phasic, hence types 1 and 2 would be relatively oversampled by intracellular recording, whereas the tonic type 3 axons are the smallest in diameter (< 1 μm) and least likely to be penetrated by microelectrodes.
Frequency tuning of auditory afferents
The power spectrum of the calling songs of the two host species of field crickets have peaks, ∼4.5 kHz (in Gryllus rubens) and ∼5 kHz (in Teleogryllus oceanicus). The tuning characteristic of Ormia's afferents range from 4 to 8 kHz; the average best frequency of all afferents in our sample is ∼7 kHz. We know that the mechanical tuning of the auditory periphery dominates the neural tuning caused by the nature of mechanical resonance of the tympana, which is broadly tuned from 4 to 8 kHz (Miles et al., 1995;Robert and Hoy, 1998). Thus, Ormia's mechanical and neural tuning is broad enough to include the songs of its known cricket hosts. Finally, we emphasize that our physiological data are based on microelectrode recordings, which may bias our sampling toward cells with larger axon diameters, i.e., in the range of 1–2 μm. It is possible that the smallest diameter axons (≤1 μm) are tuned to ∼5 kHz, but we were unable to verify this experimentally because the recordings were near the limits of our recording techniques.
Coding schemes for directional sensitivity in the auditory system
Interaural cues and directionality
From previous and present studies of Ormia's auditory behavior, its auditory periphery, and sensory organs, the processing of temporal cues is of the essence (Miles et al., 1995; Robert and Hoy, 1998; Müller and Robert, 2001; Mason et al., 2001). In the fly's sound field, the arriving sound wave of the cricket's call does not generate any interaural intensity cues. The large mismatch (>130:1) between the 68 mm wavelength of the 5 kHz carrier frequency in the call and the 0.5 mm interaural separation between the fly's tympanal membranes makes diffraction extremely unlikely to occur, and indeed it cannot be measured (Miles et al., 1995; Robert and Hoy, 1998). Hence, no interaural intensity difference caused by a sound shadow, created by the fly's body, can be generated. This leaves the fly with only one measurable interaural cue in the sound field, the interaural time difference. Even this cue is miniscule because the sound wave sweeps past the 500 μm that separate right and left tympanal membranes swiftly. The maximum value of the interaural time difference that occurs when the sound source is at 90° azimuth is only 1.5 μsec. Moreover, as the sound source approaches the main body axis, the interaural time difference decreases (Miles et al., 1995;Robert and Hoy, 1998), from a few hundred microseconds down to tens of nanoseconds, and to zero when the sound source is head-on. If the afferent response exhibited variability (“jitter”) in response latency on the order of hundreds of microseconds, sound localization would be extremely difficult given the temporal constraints in fly hearing. As described above, type 1 afferents seem well suited for the task; they encode stimulus onset with a predictable latency and very little jitter (75–80 μsec) (Thorpe, 1990). Nonetheless, Ormia is faced with a notable lack of sizable interaural sound cues.
Ormia's “solution” to its impoverishment of interaural cues is novel. Miles et al. (1995) and Robert et al. (1998) found that the resonance properties of the fly's mechanically coupled tympanal membranes converts the interaural time difference into an amplified mechanical ITD (MITD). Thus, at 90° azimuth, the 1.5 μsec ITD in the sound field is lengthened mechanically to 55 μsec MITD. In addition, the resonance mechanism in the fly's ears generates a mechanical interaural level difference (MILD) of up to 10 dB, de novo, from none in the sound field. These MITDs and MILDs are passed on to the auditory afferents. Finally, the mechanoelectric sensory transduction in Ormia's auditory organs further amplify the 55 μsec interaural time difference to ∼350 μsec for a 5 kHz sound source, at 90° azimuth and 90 dB SPL, based on recording from the whole auditory nerve (Robert et al., 1996). The sensory interaural time difference reported here (Fig. 8b, e.g.) is somewhat greater, possibly because of differences in recording technique, stimulus level, and placement of the electrodes. In any case, as a result of mechanical and mechanoneural processing of interaural cues by the fly's auditory periphery and sensory system, interaural level and time cues are generated that are of the same order of magnitude as seen in other insect and vertebrate auditory systems. These cues are passed on to the auditory interneurons, for further processing.
Population coding and directionality
In addition to taking advantage of time coding properties of single units in the afferent system, directional sensitivity of the auditory system may benefit from the range fractionation of the entire population of auditory afferents, divided along the dimension of threshold intensity. Thus, at any given intensity level (at or above threshold) for a 5 kHz tone, the proportion of afferent fibers activated by that stimulus will depend on whether the origin of the sound source is ipsilateral or contralateral to the auditory organ.
In Figure 9, we depict the thresholds of 28 afferents (all tuned to ∼5 kHz) and showed that they are spread over a wide range of thresholds, from 50 to 93 dB SPL. Such a wide dynamic range can be fractionated as a function of sound intensity level in each auditory channel, and the proportion of activated afferents in each channel can be compared centrally. This could serve a basis for sound localization, as pointed out by Römer et al. (1998) in his investigation of auditory afferents in bush crickets. The proportion of the 28 afferents that reaches spike threshold is plotted against the intensity level of the stimulus that “recruits” them. As shown in Figure 9, only a quarter, 10 of 28 afferents, had reached threshold at 75 dB; but fully 25 of 28 afferents had been recruited by 95 dB. A noteworthy feature of the display is the gradual rate of recruitment from 65 dB to 90 dB, which we believe is relevant for directional sensitivity because intensity dependent-recruitment of afferent activity is a function of location of the sound source.
The dashed lines in Figure 9 illustrate how stimulus intensity is encoded in the activity of different numbers of afferents (population coding). The proportion of ipsilateral afferents that responded to a stimulus intensity of 85 dB is ∼90%, but the pool of afferents recruited on the contralateral side is only ∼38%. Simply switching the sound source from one side to the other would turn on or off the neural activity for half of the afferents in the sample pool. This appears to be a highly efficient coding scheme for interaural intensity level differences. The 10 dB interaural level difference in this example is not unreasonable for O. ochracea. Robert et al. (1998) measured 10 dB interaural differences in the mechanical response of the tympanal membranes in laser vibrometry experiments.
Clearly, the auditory afferents of Ormia ochracea exhibit directionally dependent differences in their activity within each auditory channel as well as between channels. Thus, ipsilateral sound pulses elicit spikes at shorter response latencies than those elicited in the contralateral afferent channel. Furthermore, a larger number of afferents will be activated on the sound-ipsilateral side because of the disparate vibration amplitudes of the mechanically coupled tympanal membranes. The phasic discharge properties, low jitter, and the dynamic range of afferent thresholds are essential features of physiology of the afferents that allow them to code the direction of sound. Whether a latency code or a population code is used by the central auditory interneurons to “compute” source direction cannot be decided by the data we present here and will be unresolved until recordings are made in the CNS. What we present are the response properties of afferent units, individually and collectively, in response to directional sounds. It is clear that single units are acutely sensitive in the temporal features of acoustic stimuli, but single unit responses are probably not sufficient to account for the fly's directional acuity. Alternatively, differences in the population coding of ipsilateral and contralateral by different numbers of afferents in each channel offer a feasible basis for Ormia's directional acuity.
It is clear that Ormia's ability to localize its cricket hosts by hearing and homing in on the hosts' calling songs are enabled by remarkable sensory adaptations, especially in the parasitoid fly's auditory periphery, but as well by the response properties of its auditory afferents. Further investigation of the auditory processing in this inconspicuous fly is clearly warranted by recent reports of its behavioral capabilities in sound localization (Mason et al., 2001;Müller and Robert, 2001).
Footnotes
We acknowledge the technical help of Marie Read and helpful discussions with Drs. Andrew Mason, Tim Forrest, and Hamilton Farris.
Correspondence should be addressed to Dr. Ronald R. Hoy, Department of Neurobiology and Behavior, Cornell University, Seeley G. Mudd Hall, Ithaca, NY 14853. E-mail: rrh3@cornell.edu.
REFERENCES
- 1.Berry MJ, II, Meister M. Refractoriness and neural precision. J Neurosci. 1998;18:2200–2211. doi: 10.1523/JNEUROSCI.18-06-02200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cade WH. Acoustically orienting parasitoid: fly phonotaxis to cricket song. Science. 1976;190:1312–1313. [Google Scholar]
- 3.Carr CE. Processing of temporal information in the brain. Annu Rev Neurosci. 1993;16:223–243. doi: 10.1146/annurev.ne.16.030193.001255. [DOI] [PubMed] [Google Scholar]
- 4.Edgecomb RS, Robert D, Read MP, Hoy RR. The tympanal hearing organ of a fly: phylogenetic analysis of its morphological origins. Cell Tissue Res. 1995;282:251–268. doi: 10.1007/BF00319116. [DOI] [PubMed] [Google Scholar]
- 5.Mason AC, Oshinsky ML, Hoy RR. Hyperacute directional hearing in a microscale auditory system. Nature. 2001;410:686–690. doi: 10.1038/35070564. [DOI] [PubMed] [Google Scholar]
- 6.Michelsen A. Hearing and sound communication in small animals: evolutionary adaptations to the laws of physics. In: Webster DB, Fay RR, Popper A, editors. The evolutionary biology of hearing. Springer; Berlin: 1992. pp. 61–77. [Google Scholar]
- 7.Michelsen A. Directional hearing in crickets and other small animals. In: Schildberger K, Elsner N, editors. Neural basis of behavioural adaptations. Fischer; New York: 1994. pp. 195–207. [Google Scholar]
- 8.Miles RN, Robert D, Hoy RR. Mechanically coupled ears for directional hearing in the parasitoid fly Ormia ochracea (Diptera: Tachinidae). J Acoust Soc Am. 1995;98:3059–3070. doi: 10.1121/1.413830. [DOI] [PubMed] [Google Scholar]
- 9.Müller P, Robert D. A shot in the dark: the silent quest of a free-flying phonotactic fly. J Exp Biol. 2001;204:1039–1052. doi: 10.1242/jeb.204.6.1039. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea M, Williams JLD. The anatomy and output connection of a locust visual interneurons: the lobular giant movement detector (LGMD) neuron. J Comp Physiol [A] 1974;91:257–266. [Google Scholar]
- 11.Oshinsky ML. Physiological correlates of sound localization in an acoustic parasitoid fly (Ormia ochracea). Thesis. Cornell University; 1998. [Google Scholar]
- 12.Rieke F, Warland D, Stevenick R, Bialek W. Spikes: exploring the neural code. MIT; Cambridge, MA: 1997. [Google Scholar]
- 13.Robert D, Hoy RR. The evolutionary innovation of tympanal hearing in Diptera. In: Hoy RR, Popper AN, Fay RR, editors. Comparative hearing: Insects. Springer; New York: 1998. pp. 197–227. [Google Scholar]
- 14.Robert D, Willi U. The histological architecture of the auditory organs in the parasitoid fly Ormia ochracea. Cell Tissue Res. 2000;301:447–457. doi: 10.1007/s004410000257. [DOI] [PubMed] [Google Scholar]
- 15.Robert D, Amoroso J, Hoy RR. The evolutionary convergence of hearing in a parasitoid fly and its cricket host. Science. 1992;258:1135–1137. doi: 10.1126/science.1439820. [DOI] [PubMed] [Google Scholar]
- 16.Robert D, Miles RN, Hoy RR. A novel mechanism for directional hearing in a parasitoid fly. J Acoust Soc Am. 1994a;96:3296. doi: 10.1121/1.413830. [DOI] [PubMed] [Google Scholar]
- 17.Robert D, Read MP, Hoy RR. The tympanal hearing organ of the parasitoid fly Ormia ochracea (Diptera, Tachinidae, Ormiini). Cell Tissue Res. 1994b;271:63–78. doi: 10.1007/BF00305376. [DOI] [PubMed] [Google Scholar]
- 18.Robert D, Miles RN, Hoy RR. Directional hearing by mechanical coupling in a parasitoid fly, Ormia ochracea. J Comp Physiol [A] 1996;179:29–44. doi: 10.1007/BF00193432. [DOI] [PubMed] [Google Scholar]
- 19.Römer H, Spickermann M, Bailey W. Sensory basis for sound intensity discrimination in the bushcricket Requena verticalis (Tettigoniidae, Orthoptera). J Comp Physiol. 1998;182:595–607. [Google Scholar]
- 20.Stumpner A, Lakes-Harlan R. Auditory interneurons in a hearing fly (Therobia leonidei, Ormiini, Tachinidae, Diptera). J Comp Physiol [A] 1996;178:227–233. [Google Scholar]
- 21.Taylor MN, Creelman CD. PEST: efficiency estimates on probability functions. J Acoust Soc Am. 1967;41:782–787. [Google Scholar]
- 22.Thorpe SJ. Spike arrival times: a highly efficient coding scheme for neural networks. In: Eckmiler R, Hartmann G, Hauske G, editors. Parallel processing in neural systems and computers. Elsevier; Amsterdam: 1990. pp. 91–94. [Google Scholar]
- 23.Vreislander JD, Skovira JF, Capranica RR. Modular expandable microcomputer workstation for synthesizing complex auditory stimuli and for recording and analyzing neurophysiological responses. Soc Neurosci Abstr. 1991;17:1213. [Google Scholar]
- 24.Walker TJ. Monitoring the flights of field crickets and a tachinid fly (Euphasiopterix ochracea) in north Florida. Fla Entomol. 1986;69:678–685. [Google Scholar]
- 25.Walker TJ. Phonotaxis in female Ormia ochracea (Diptera: Tachinidae), a parasitoid of field crickets. J Insect Behav. 1993;6:389–410. [Google Scholar]
- 26.Walker TJ. Pulse rates in the songs of trilling field crickets (Orthoptera: Gryllidae: Gryllus). Ann Entomol Soc Am. 2000;93:565–572. [Google Scholar]
- 27.Walker TJ, Wineriter SA. Rearing phonotactic parasitoid flies (Diptera: Tachinidae, Orminii, Ormia spp.). Entomophaga. 1990;35:621–632. [Google Scholar]
- 28.Zuk M, Simmons LW, Cupp L. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav Ecol Sociobiol. 1993;33:339–343. [Google Scholar]