Abstract
Repeated exposure to psychomotor stimulants produces a striking behavioral syndrome involving repetitive, stereotypic behaviors that occur if an additional exposure to the stimulant is experienced. The same stimulant exposure produces specific alterations in gene expression patterns in the striatum. To identify the dopamine receptor subtypes required for the parallel expression of these acquired neural and behavioral responses, we treated rats with different D1-class and D2-class dopamine receptor agonists and compared the responses of drug-naive rats with those of rats given previous intermittent treatment with cocaine. In rats exposed to repeated cocaine treatment, the effects of a subsequent challenge treatment with either a D1-class agonist (SKF 81297) or a D2-class agonist (quinpirole) were not significantly different from those observed in drug-naive animals: the drugs administered singly did not induce robust stereotyped motor behaviors nor produce significantly striosome-predominant expression of early genes in the striatum. In contrast, challenge treatment with the D1-class and D2-class agonists in combination led to marked and correlated increases in stereotypy and striosome-predominant gene expression in the striatum. Thus, immediately after repeated psychomotor stimulant exposure, only the concurrent activation of D1 and D2 receptor subclasses evoked expression of the neural and behavioral phenotypes acquired through repeated cocaine exposure. These findings suggest that D1–D2 dopamine receptor synergisms underlie the coordinate expression of both network-level changes in basal ganglia activation patterns and the repetitive and stereotypic motor response patterns characteristic of psychomotor stimulant sensitization.
Keywords: stereotypy, gene expression, dopamine receptors, basal ganglia, striosomes, striatum
Psychomotor stimulants provoke the appearance of repetitive, stereotyped behaviors that become more pronounced with repeated exposure to the drugs. This augmented responsivity is a core feature of the behavioral sensitization syndrome that results from chronic drug exposure (Robinson and Becker, 1986;Kalivas and Stewart, 1991; Robinson et al., 1998) Studies in the last 30 years suggest that basal ganglia dysfunction is linked to the appearance of such stereotypies. Drug-induced stereotypies in experimental animals are sensitive to lesions of the nigrostriatal projection (Creese and Iversen, 1972; Fibiger et al., 1973), treatments with dopamine receptor antagonists (Iorio et al., 1983; Arnt, 1985;Arnt et al., 1987; Karler et al., 1995; White et al., 1998; Nestler et al., 2001), and mutations affecting dopaminergic transmission (Turski et al., 1984; Richter et al., 1999). In humans, stereotypies are clinical manifestations of a number of psychiatric, neurological, and developmental disorders (Manschreck, 1986; Antony et al., 1988; Ridley, 1994; Rosenberg et al., 1997; Graybiel and Rauch, 2000), and alterations in frontostriatal transmission are associated with conditions featuring compulsive and stereotyped behaviors (Cummings, 1995; Rosenberg et al., 1997; Volkow and Fowler, 2000; Goldstein et al., 2001; Graybiel and Canales, 2001).
How the basal ganglia and their dopamine-containing inputs contribute to the generation of stereotypy is unclear. One key to this problem might be the finding that stereotypy induced by psychomotor stimulants is strongly correlated with the degree of imbalance in the activation of the striosome and matrix compartments of the striatum and their associated basal ganglia output pathways. Cocaine- and amphetamine-induced stereotypy is predicted by the relative predominance of early gene activation of striatal neurons in striosomes, which are thought to modulate the activity of dopamine-containing cell groups in the midbrain (Canales and Graybiel, 2000). On this basis, we reasoned that, if predominant neuronal activation in striosomes were directly linked to motor repetition, such neural adaptation should be readily observed both after acute treatment with stereotypy-inducing drugs and immediately after the sensitization phase of stimulant treatment, without the requisite for an intervening period of drug withdrawal.
A second key finding is that concurrent stimulation of D1-class and D2-class dopamine receptors is required for dopaminergic stimulation to produce robust motor stereotypies and other specific motor behaviors (Clark and White, 1987; Arnt et al., 1988; Canales and Iversen, 1998; Berke and Hyman, 2000; Nestler et al., 2001). Similarly, in early gene assays, only such simultaneous activation of D1-class and D2-class dopamine receptors evokes a striosome-predominant pattern of gene expression in the striatum (LaHoste et al., 1993; Wirtshafter and Asin, 1994). These findings suggest that the coordinate expression of sensitized neural and behavioral responses acquired through repeated exposure to psychomotor stimulants could depend on an interplay between subclasses of dopamine receptors.
To test these hypotheses, we measured, in cocaine-sensitized and drug-naive rats, the stereotypy induced by D1-class, D2-class, or D1–D2 agonist challenges and, in the same animals, measured the patterns of striatal early gene expression induced by these treatments immediately after cocaine sensitization. We found that combined D1–D2 agonist treatment was required to induce such alterations and that a strong positive correlation held between the degree of stereotypy and the degree of striosome-predominant gene expression elicited.
MATERIALS AND METHODS
Drug treatments. Male Sprague Dawley rats weighing 250–350 gm were treated according to procedures approved by the Massachusetts Institute of Technology Committee on Animal Care. Rats were kept under standard conditions of temperature and humidity with a 12 hr light/dark cycle (lights on at 7:00 A.M.), and they were handled daily for 2 d before drug treatments. Throughout, the animals were treated in groups of seven. Injections were given in the home cages. Drug-naive rats (n = 56) received single injections of the D1-class dopamine receptor agonist SKF 81297 in doses of 1 or 3 mg/kg intraperitoneally (dissolved in 0.1% ascorbic acid), combined with the D2-class dopamine receptor agonist quinpirole in doses of 1, 3, or 9 mg/kg intraperitoneally (dissolved in 0.9% saline) or were given injections of one of these agonists alone at a dose level of 3 mg/kg. Control animals received 0.9% saline only. After the injections, the behavior of the rats was observed by at least one observer blind to the treatment type (see below). In a second experiment, rats (n = 49) received repeated cocaine (or, for control, saline) treatments before challenge with SKF 81297 alone, quinpirole alone, or both agonists in combination. The cocaine (cocaine hydrochloride, 25 mg/kg, dissolved in saline) was administered intraperitoneally twice daily (10:00 A.M. and 5:00 P.M.) for 7 consecutive days. On day 8, each rat received a challenge with quinpirole (6 mg/kg, i.p.), SKF 81297 (6 mg/kg, i.p), quinpirole plus SKF 81297 (each at 3 mg/kg, i.p.), or saline. Systematic behavioral observations were made after each 10:00 A.M. treatment with cocaine or saline and after the final challenge with the dopamine receptor agonists or saline. At the end of the final observation period, the rats were deeply anesthetized with sodium pentobarbital (Nembutal; >25 mg/kg) and were perfused transcardially with 4% paraformaldehyde in 0.1 m NaKPO4.
Behavioral analysis. The induction of stereotyped behaviors was assessed during 1 hr after the experimental treatments by following a standardized 10-point rating scale (1, undetectable; 2, very weak; 3, weak; 4, weak-to-moderate; 5, moderate; 6, moderate-to-strong; 7, strong; 8, intense; 9, very intense; 10, extreme) (Canales and Graybiel, 2000) modified from Creese and Iversen (1972).
Stereotypy ratings were computed for each animal and for each observation period by calculating the mean score across four behavioral dimensions ranging between 1 and 10 in severity. The four behavioral dimensions were repetitiveness (degree of switching between different behavioral responses, with the exclusion of feeding and drinking responses), frequency (degree of intensity with which a single motor response was emitted), duration (estimation of the length of time engaging in motor stereotypy), and spatial distribution (degree of spatial confinement of the motor response, with the exclusion of periods of sleep). Scores were based on these four estimates of the motor responses emitted during 1 min periods 20 and 50 min after treatment. The average of these values was recorded as the session score. Monitored responses included movements of the head and limbs, stereotyped sniffing, and directed oral stereotypies. Scoring was done by an observer blind to the treatments given, and extended observation periods and simultaneous observation by two or three observers blind to the treatments were performed periodically. In preliminary experiments, all ratings were made independently by two or three observers, and the scores were found to match closely.
Immunohistochemistry. Brains were cut at 24 μm on a sliding microtome, and sections through the caudoputamen were stained by dual-antigen immunohistochemical protocols with antisera raised against either c-Fos (Ab-5; 1:40,000; Oncogene Sciences, Manhasset, NY) or JunB (1:10,000; gift from Dr. R. Bravo) and the C-terminal peptide of the μ-opioid receptor (MOR1) (1:20,000; DiaSorin, Stillwater MN), a marker for striosomes, as described previously (Canales and Graybiel, 2000). Briefly, after incubation with the primary antibody, sections were exposed to a biotinylated secondary antibody and to an avidin–biotin complex conjugated to HRP (ABC Kit; Vector Laboratories, Burlingame, CA). Specific double stains were obtained by exposure to DAB-H2O2 (MOR1) and to DAB-H2O2 with nickel (NiSO4) intensification (c-Fos and JunB).
Data analysis. For each brain, two sections through the rostral caudoputamen were analyzed. A ∼4 mm2 area at the center of the caudoputamen was divided into medial and lateral halves, and the outlines of the MOR1-positive striosomes within these regions were drawn. Fos-positive nuclei were counted with a microscope attached to an image analysis system (Biocom, Les Ulis, France). The counting was done by an observer blind to the experimental treatments. The counting procedure permitted calculations, for each section, of the density of Fos-positive neurons in the μ-opioid receptor-rich striosomes and in the surrounding matrix. For each individual animal, average values for the two sections counted were used to calculate an index of the relative gene expression in striosomes compared with that in the matrix [index of striosome-to-matrix predominance (ISMP)] by taking the ratio between the density values of Fos-positive nuclei in the striosomes and in the matrix of the sampled regions (Canales and Graybiel, 2000). The gene expression data for each treatment group were analyzed statistically by means of ANOVA. Kruskal–Wallis and the Kolmogorov–Smirnov tests were used for analysis of the behavioral observations. Correlations between gene expression values and stereotypy scores were calculated by the method of Spearman (rs values).
RESULTS
Coactivation of D1-class and D2-class dopamine receptors is required to induce strong motor stereotypies in normal and cocaine-pretreated animals
To establish baselines for the behavioral effects of the dopamine receptor agonists, we first measured the stereotypies induced by different single or combined doses of the D1-class and D2-class agonists. Confirming previous results (Arnt et al., 1987, 1988), acute treatment with combinations of the D1-class and D2-class receptor agonists induced oral stereotypies, including nibbling of the cage material, licking, and head-down sniffing (Fig.1A). Treatment with quinpirole alone induced stereotypies of mild intensity, not reaching statistical significance compared with control values (p = 0.063). SKF 81297 treatment alone did not produce such responses but only nondirected behavioral activation. Stereotypy levels appeared to saturate at values of ∼5 on the rating scale after the combined D1–D2 dopamine agonist treatments. Even the lowest doses of the two agonists (1 mg/kg each SKF 81297 and quinpirole), when given in combination, produced high stereotypy ratings, and higher doses of either or both did not produce additional increases in stereotyped behavior. These results suggest that significant levels of dopamine receptor-stimulated stereotypy depend on stimulation of dopamine receptors of both D1 and D2 subclasses and that even large doses of either agonist alone are ineffective in augmenting such stereotypic behavior. The lack of dose dependency of the combined D1–D2 agonist treatments for the expression of stereotypies contrasts with the capacity of apomorphine to evoke such behaviors in a dose-dependent manner (Canales and Graybiel, 2000), which could either be attributable to the differential D1–D2 affinity ratios of the drugs or to the fact that we did not test yet lower doses of the agonists.
Fig. 1.
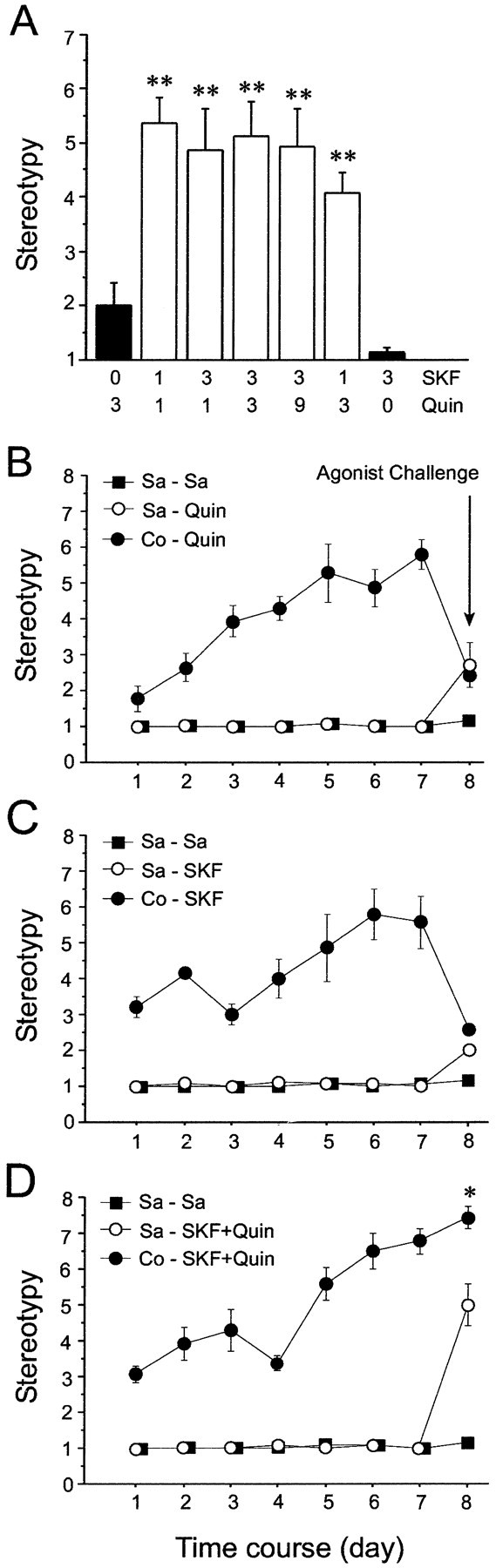
Acute treatment with a combination of dopamine D1-class receptor agonist (SKF 81297) and dopamine D2-class agonist (quinpirole), but not treatments with each class of dopamine agonist given alone, induces motor stereotypy.A, Stereotypy scores after acute administration of different dose combinations of SKF 81297 (0, 1, and 3 mg/kg) and quinpirole (0, 1, 3, and 9 mg/kg; indicated on x-axis). In A, the baseline stereotypy value was 1.0.Black bars show single agonist treatments.White bars show combined D1 and D2agonist treatments. B–D, Induction of stereotypies by acute challenge with 6 mg/kg quinpirole (B), 6 mg/kg SKF 81297 (C), or SKF 81297 plus quinpirole (both 3 mg/kg) (D) after 1 week of either repeated cocaine or repeated saline treatment. Acutely, all combined agonist challenges, but not the single agonist challenges, induced significant levels of stereotypy (Kruskal–Wallis test;H = 36.821; p < 0.001, followed by Kolmogorov–Smirnov tests). Note the progressively increased levels of stereotypy induced by repeated cocaine treatment and the enhanced response to combined D1–D2 agonist challenge in rats exposed to intermittent cocaine treatment (significantly different from controls) (Kruskal–Wallis test;H = 27.528; p < 0.001, followed by Kolmogorov–Smirnov tests). **p < 0.01. Sa , Saline; Co , cocaine;SKF, SKF 81297; Quin, quinpirole.
Repeated administration of cocaine produced a progressive enhancement of stereotyped behavior over time, in accord with previous findings (Jung et al., 1999; Canales and Graybiel, 2000; Vanderschuren and Kalivas, 2000; Haile et al., 2001), with scores reaching values of ∼6 on the stereotypy scale by day 7 (Fig. 1B–D). To test whether subsequent expression of this sensitized response required coactivation of D1-class and D2-class dopamine receptors, we administered D1 and D2 agonist challenges at dose levels within the ranges established in the drug-naive animals (3 mg/kg for each in the combined agonist treatments; for matching single agonist treatments, 6 mg/kg). Challenge with either SKF 81297 alone or quinpirole alone failed to induce intense stereotyped behaviors (Fig.1B,C). In contrast, challenge with these D1-class and D2-class agonists combined elicited a significantly elevated motor response relative to that observed in saline-pretreated controls (Fig.1D). The profile of behaviors induced by the drug combination included vigorous oral responses, such as persistent licking and nibbling. These results suggest that expression of the changes in motor behavior acquired as a result of repeated cocaine treatment require activation of both D1-class and D2-class dopamine receptors.
Coactivation of D1-class and D2-class dopamine receptors is required to induce differentially enhanced early gene activation in striosomes
The requirement for coactivation of D1-class and D2-class dopamine receptors in producing stereotypy in the drug-naive and cocaine-treated animals raised the possibility that D1–D2synergism would also be essential to produce compartmental shifts in striatal early gene expression. We examined this possibility by performing immunohistochemistry to detect c-Fos and JunB together with the striosomal marker MOR1 in the same tissue sections. The results were clear-cut: in both groups, combined D1–D2 agonist challenge was required for expression of major shifts in the compartmental distribution of the early gene protein within the caudoputamen.
Figures 2 and3 illustrate these findings for c-Fos. In the drug-naive rats, the D2 agonist quinpirole slightly depressed the levels of Fos expression in the caudoputamen relative to control values (Figs. 2, 3A), whereas the D1 agonist SKF 81297 produced a widespread expression of Fos, with only a twofold predominance in striosomes (Figs. 2, 3B) and slightly greater expression medially than laterally within the caudoputamen. A dramatic shift in the pattern of Fos distribution occurred in the caudoputamen after the combined D1–D2 agonist treatments. Fos expression was significantly increased in striosomes both medially and laterally (Figs. 2, 3C,D), but, in the matrix, the levels of Fos expression were mainly unaffected. The result was an elevation of ISMP values by several-fold (Fig.4). The scatter in the data (Fig. 4) suggests that the D1–D2combinations that included the high dose of SKF 81297 (3 mg/kg) activated the matrix more than the lower (1 mg/kg) doses without proportionally elevating striosomal activation, suggesting that high levels of D1-class receptor stimulation did not further elevate the ISMP ratios beyond those obtained with low-dose D1–D2 combinations. Within the dose combinations we tested, any combination of the D1 and D2 agonists, but not treatment with either class of agonist given alone, producing a pronounced pattern of relative striosomal expression of Fos–Jun proteins.
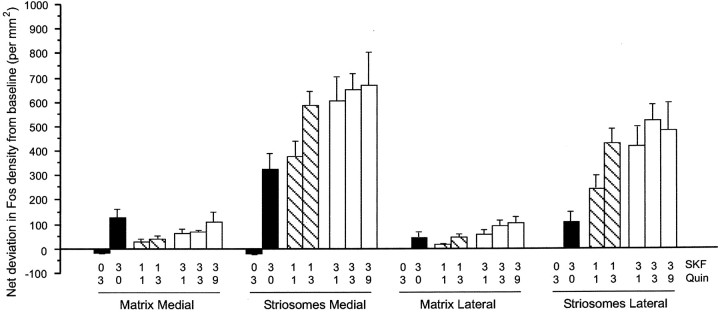
Fig. 2.
Acute dopamine D1-class and D2-class receptor agonist treatments induce varying levels of early-gene expression in striosomes and matrix of the striatum. Values indicate net deviations from baseline levels of Fos expression in striosome and matrix compartments compared with values in saline-treated animals. Baseline values were as follows: medial matrix, 30.50 ± 9.51; medial striosomes, 39.60 ± 16.42; lateral matrix, 2.78 ± 1.32; and lateral striosomes, 3.81 ± 1.6. Of the single agonist challenges (black bars), only SKF 81297 induced significant levels of Fos expression in the striatum. Activation in the striosomes was much greater than activation in the matrix when increasing doses of quinpirole were combined with 1 mg/kg (hatched bars) or 3 mg/kg (white bars) SKF 81297. ANOVA indicated a significant high-order interaction of agonist × sector × compartment (F(6,42) = 3.113; p< 0.013). Quin, Quinpirole; SKF, SKF 81297.
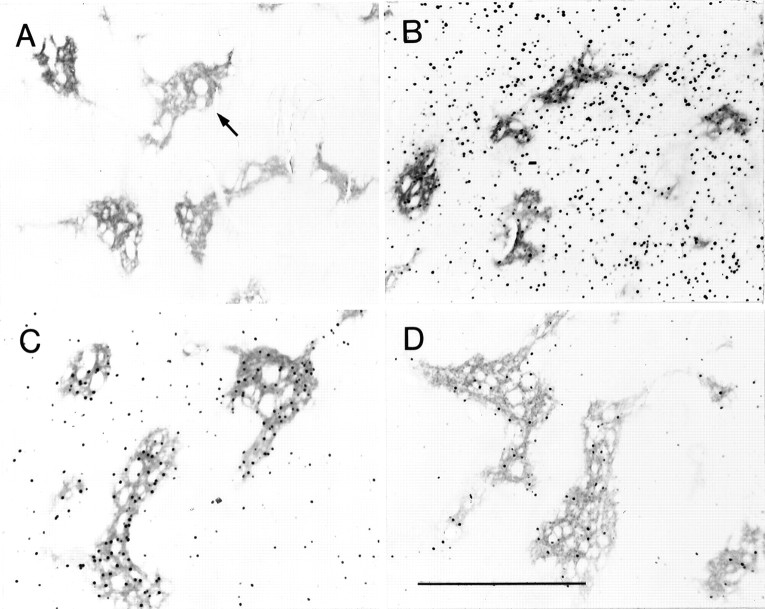
Fig. 3.
Dopamine D1-class and D2-class receptor agonists have differential effects on early-gene expression in the matrix and striosomes of the caudoputamen. Transverse coronal sections through the caudoputamen illustrating distributions of Fos-immunoreactive neurons in rats given acute challenges with the D2 agonist quinpirole (3 mg/kg) (A), the D1 agonist SKF 81297 (3 mg/kg) (B), and the D1–D2 agonist combinations of SKF 81297 plus quinpirole (3 mg/kg each) (C) or SKF 81297 plus quinpirole (1 mg/kg each) (D). Note that, in the animals given combined agonist challenge (C,D), clusters of Fos-positive nuclei are concentrated in striosomes, marked by strong μ-opioid receptor immunoreactivity.Arrow in A points to a μ-opioid receptor-positive striosome. Scale bar, 0.5 mm.
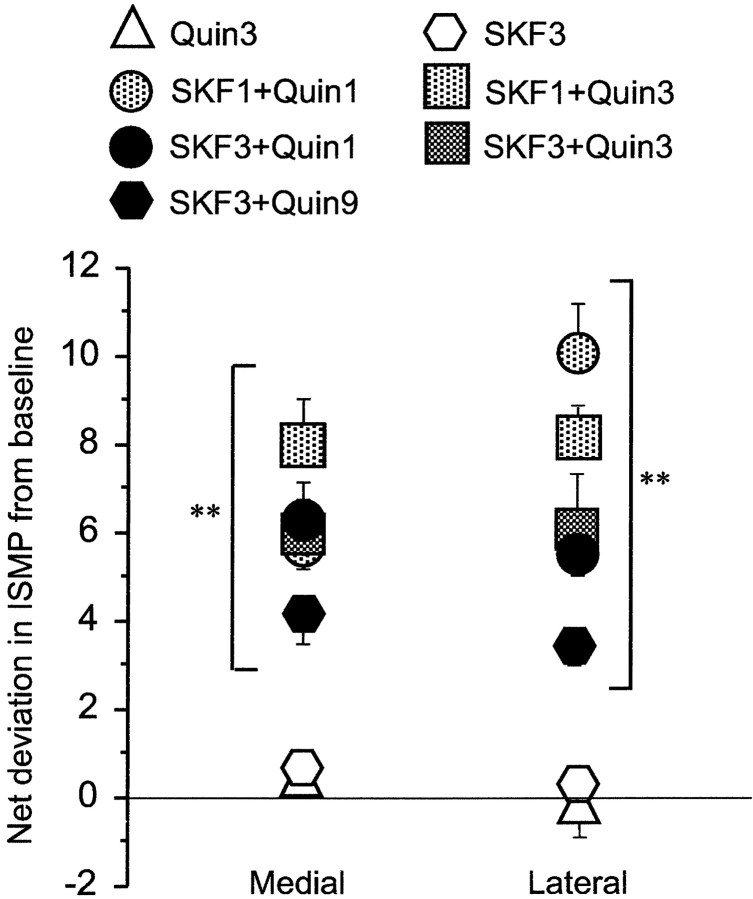
Fig. 4.
Indices of striosome-to-matrix predominance of early-gene activation (ISMP values) are increased by acute combinations of D1-class (SKF 81297) and D2-class (quinpirole) dopamine receptor agonist treatments. Values indicate deviations from baseline ISMP values. These were 1.30 ± 0.21 and 1.69 ± 0.98 for the medial and lateral sectors of the caudoputamen, respectively. Dosage (in milligrams per kilogram) is indicated with corresponding symbols. Single D1 or D2 agonist treatments did not elevate ISMP values significantly, whereas all combined D1-class and D2-class agonist challenges, regardless of dose levels, produced significant increases in ISMP values. ANOVA showed a significant effect of the interaction of agonist × sector (F(6,42) = 3.360; p< 0.009), and only the combined treatments differed significantly from baseline (Newman–Keuls). **p < 0.01.Quin, Quinpirole; SKF, SKF 81297.
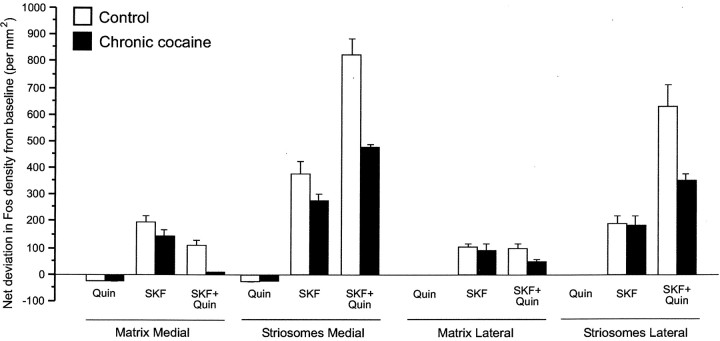
In the rats treated repeatedly with cocaine and then given a final challenge with either the D1 or the D2 agonist or the D1–D2 agonist combination, there was less striatal Fos expression than in the saline-pretreated control animals, reflecting a general downregulation of excitability. Only the combined D1–D2agonist treatment, however, produced significant alterations in the balance of Fos expression between striosomes and matrix (Fig.5). With single D1-class or D2-class agonist challenge after the intermittent cocaine treatment, the compartmental patterns of Fos expression (Figs.6,7A,A′,B,B′) and ISMP values (Fig. 5) were not different from those of rats repeatedly treated with saline. In contrast, when SKF 81297 and quinpirole were combined for the final agonist challenge after the cocaine exposure, there was a marked reduction of Fos expression in the matrix of the medial caudoputamen relative to control levels, and this reduction was much greater than that in striosomes (Figs. 6,7C,C′). ISMP values therefore were significantly increased in the medial striatum (Fig. 5). Thus, combined activation of D1-class and D2-class receptors was necessary for the expression of the cocaine-induced changes in Fos–Jun protein distributions in the striatum. Qualitatively similar results were found for JunB (Fig.7D,D′).
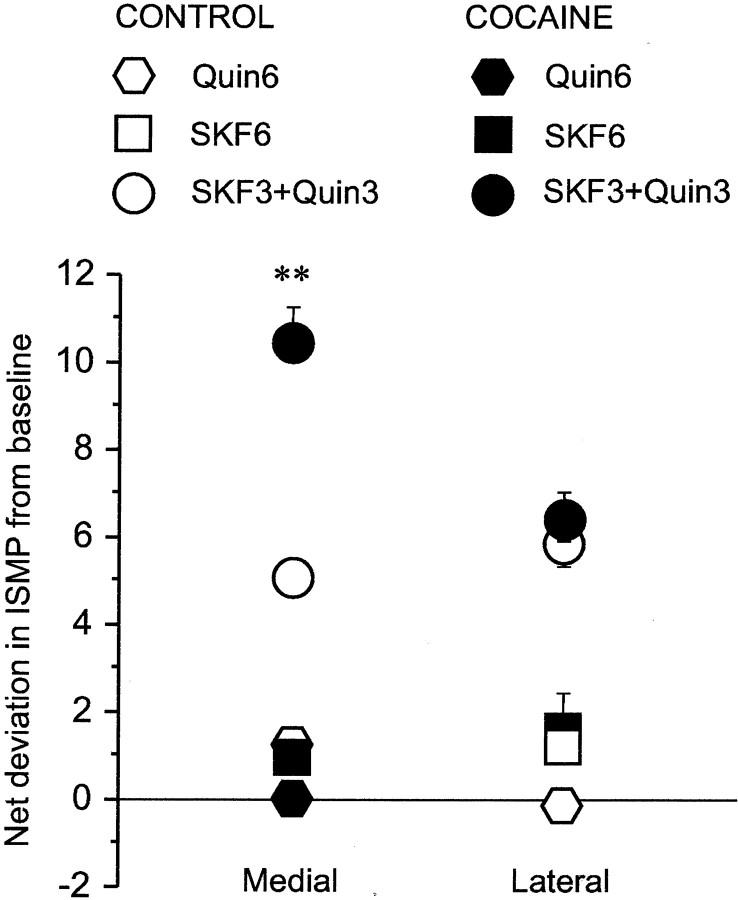
Fig. 5.
Repeated exposure to cocaine enhances striosome-predominant Fos expression in the medial caudoputamen in response to combined D1-class and D2-class dopamine receptor agonist challenge. The ISMP values shown indicate deviations from baseline ISMP values, which were 1.10 ± 0.16 and 0.66 ± 0.33 for the medial and lateral caudoputamen, respectively. Dosage is indicated with correspondingsymbols. Combined, but not single, D1-class or D2-class agonist challenges elevated ISMP values significantly in control and cocaine-sensitized animals. In the latter group, the effect was potentiated in the medial sector of the caudoputamen. ANOVA indicated a significant drug × agonist × sector interaction (F(2,36) = 11.674; p < 0.001). ISMP values differed significantly in response to D1–D2 challenge in saline-pretreated controls compared with cocaine-pretreated animals (Newman–Keuls). **p < 0.01. Quin, Quinpirole; SKF, SKF 81297.
Fig. 6.
Challenges with combined D1-class and D2-class dopamine receptor agonists after repeated cocaine treatment show differential downregulation of Fos expression in the striatal matrix. Graphs show levels of Fos expression in striatal compartments after combined D1-class and D2-class dopamine receptor agonist challenges in rats that were saline pretreated (white bars) or cocaine pretreated (black bars). Values represent net deviations from baseline values, which were as follows: medial matrix, 29.32 ± 7.06; medial striosomes, 35.93 ± 13.12; lateral matrix, 4.60 ± 2.39; and lateral striosomes, 6.40 ± 3.73. Repeated exposure to cocaine (25 mg/kg, 7 d, twice per day) markedly downregulated Fos expression induced by all dopamine receptor agonist treatments. Note that, after cocaine plus combined D1–D2 challenge (3 mg/kg each), matrix Fos activation is almost nil in the caudoputamen medially, whereas net deviation from baseline in the striosomes is close to 500 cells/mm2. ANOVA showed a significant interaction of drug × agonist × compartment (F(2,36) = 15.060;p < 0.001) and a nonsignificant interaction of drug × agonist × sector × compartment (F(2,36) = 0.485; p< 0.620).
Fig. 7.
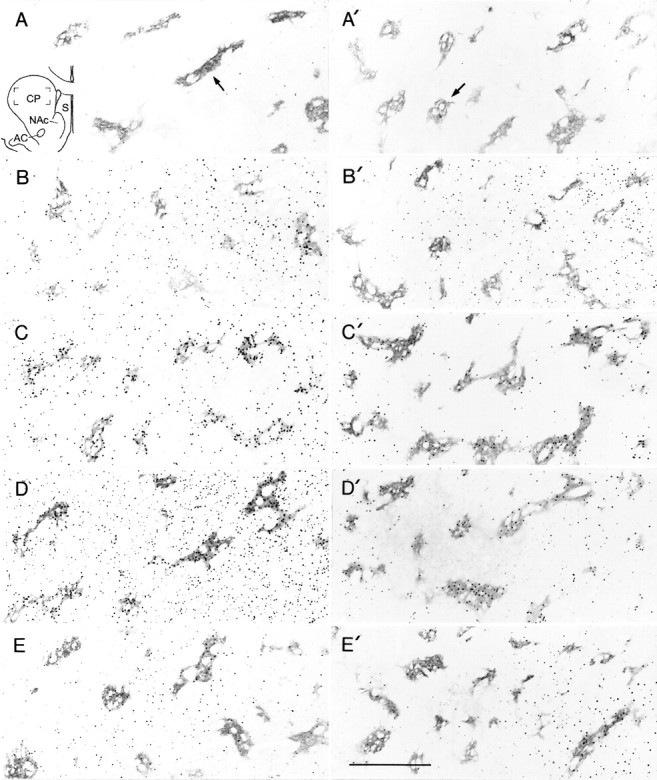
Immunohistochemical localization of c-Fos and JunB demonstrates synergistic effects of D1-class and D2-class receptors in striatum. Sections through the caudoputamen illustrating Fos (A, A′,B, B′, C,C′) or JunB (D, D′,E, E′) immunoreactivity in the striosomal and matrix compartments of the caudoputamen. Examples of striosomes are shown at arrows in A andA′. A, A′, A final challenge with the D2-class agonist quinpirole (6 mg/kg) did not elicit Fos expression in rats previously exposed to saline (A) or to cocaine (25 mg/kg, 7 d, twice per day) (A′). B, B′, A final challenge with the D1 agonist SKF 81297 (6 mg/kg) produced prominent, homogenous levels of Fos expression in saline-pretreated rats (B) and reduced levels in both compartments after repeated intermittent cocaine exposure (25 mg/kg, 7 d, twice per day) (B′). C,C′, D, D′, Combined D1–D2 agonist challenge with coadministered SKF 81297 and quinpirole (3 mg/kg each) produced a detectably striosome-predominant pattern of Fos expression (C) and JunB (D) in the saline-pretreated animals, and this pattern became much accentuated in animals pretreated with cocaine, especially medially (Fos,C′; JunB, D′). E,E′, A final challenge with cocaine (25 mg/kg) in cocaine-pretreated animals (E′) also produced a pattern of striosome predominance compared with control animals (E), but the striosomal pattern was more lateral in the caudoputamen (JunB illustrated). Drawing in Ashows the region of the caudoputamen included in the photomicrographs.CP, Caudoputamen; NAc, nucleus accumbens;S, septum; AC, anterior commisure. Scale bar, 0.5 mm.
Enhanced activation of striosomes parallels enhanced behavioral stereotypy
To test whether the changes in stereotypy values and density values for Fos expression in the caudoputamen were correlated, we performed nonparametric versions of regression analysis. Figures8 and 9show scatter plots with all data points from both experiments with corresponding Spearman correlation values and significance levels. In our acute model (Fig. 8), early gene activation in the matrix medially was poorly correlated with the emergence of stereotypies, despite the fact that all acute challenges in which D1 and D2 agonists were coadministered increased Fos levels in this striatal compartment. Lateral activation in the matrix compartment did show a significant correlation. Early gene expression in striosomes correlated better with motor stereotypy than matrix activation, and both medial and lateral Spearman correlation values for striosomes were significant. Overall activation in the caudoputamen, regardless of compartmental effects, showed correlation values of 0.24 and 0.48 for medial and lateral sectors, respectively (scatter plots not shown). The best neural estimate of motor stereotypy was given by the gene expression values in striosomes relative to expression values in the matrix, as defined by ISMP values. These exceeded the predictive power of the striosomal activation alone by 10% on average.
Fig. 8.
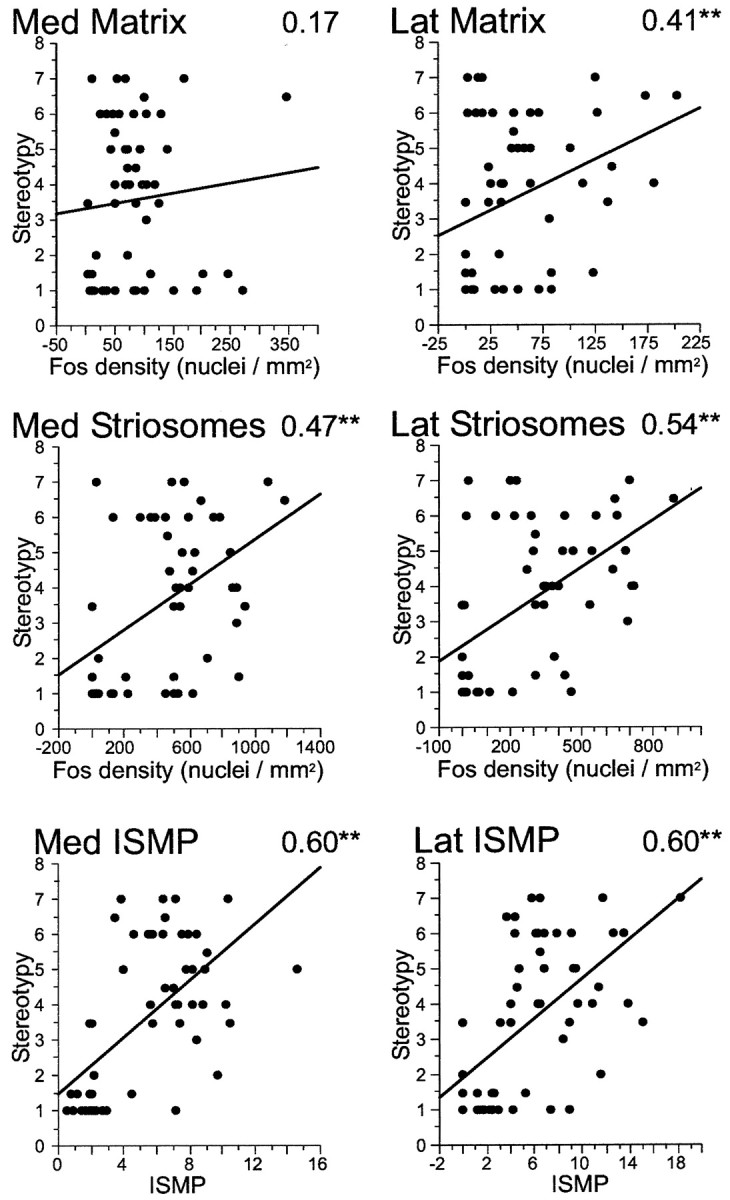
After acute treatments with combined D1-class and D2-class dopamine receptor agonists, the best predictor of motor stereotypy is the relative predominance of Fos expression in the striosomal compartment (ISMP value). Scatter plots representing individual data points for all animals included in the agonist–dose experiment and regression lines relating measures of Fos activation in the caudoputamen (abscissa) and stereotypy values (ordinate). The number at the top right corner of each plot is the Spearman correlation value (**p < 0.01). Med Matrix, Medial matrix; Lat Matrix, lateral matrix; Med Striosomes, medial striosomes; Lat Striosomes, lateral striosomes; Med ISMP, medial ISMP; Lat ISMP, lateral ISMP.
Fig. 9.
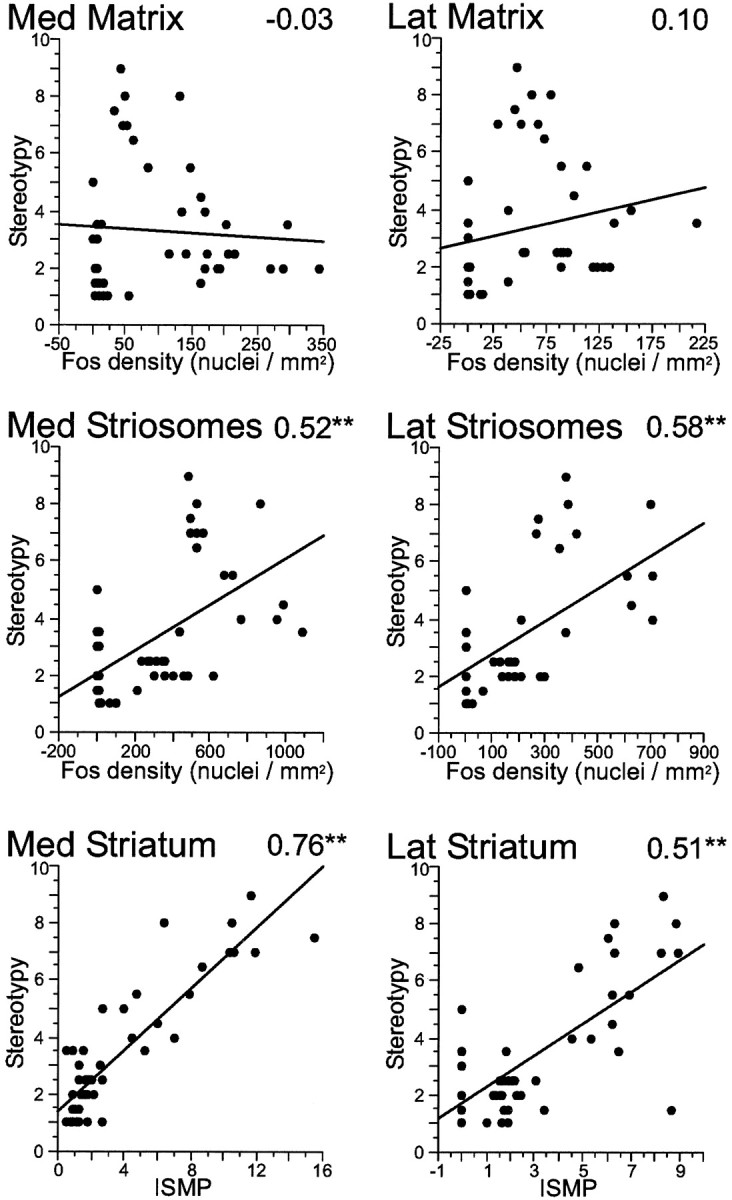
After repeated exposure to cocaine or saline, a strong correlation is found between stereotypy and the relative predominance of Fos expression in the striosomes (ISMP) induced by combined D1–D2 dopamine receptor agonist challenge. Scatter plots show individual data points for all animals included in the cocaine sensitization experiment and corresponding regression lines. Top four plots show data for Fos expression in medial (Med) and lateral (Lat) matrix and striosomes relative to stereotypy values, and two bottom plots show ISMP values for medial and lateral caudoputamen relative to stereotypy scores. Thenumber at the top rightcorner of each plot represents the Spearman correlation value. **p < 0.01.
In the cocaine-pretreated animals (Fig. 9), decreases in Fos expression in the matrix occurred after combined D1–D2 agonist challenge relative to values in saline-pretreated animals, but, again, the density values for Fos expression in this compartment were not correlated with changes in behavior. Activation values for the striosomes were also decreased in rats given the combined D1–D2 challenge after exposure to cocaine, and the correlations between striosomal gene expression and behavior clearly exceeded those for the matrix compartment, reaching in both instances statistical significance. Overall levels of gene expression in the caudoputamen were not correlated with stereotypy (Spearman values were 0.06 and 0.30 for medial and lateral sectors, respectively; scatter plots not shown). Combined D1–D2 receptor agonist challenge after repeated cocaine treatment brought down levels of activation in the matrix to nearly baseline levels medially but only reduced activation levels in striosomes twofold. This differential downregulation produced significantly elevated ISMP values in the medial sector of the caudoputamen, and these ISMP values were better predictors of motor stereotypy than any other index of neural activation, including activation in the individual compartments. The Spearman rank correlation value for ISMP in medial caudoputamen was 0.76. This value (Fig. 9) was higher than that found for any of the analyses performed for the acutely treated animals (Fig. 8). In the chronic cocaine experiments, when calculations were performed for individual subgroups, similar correlations were observed between stereotypy and ISMP values in both the sensitized and naive subgroups of animals (data not shown). This analysis suggests that the relationship between relative striosome predominance and stereotypy does not depend on one particular preceding condition of drug exposure but, rather, bears on a general aspect of the regulation of the motor behavior.
DISCUSSION
The alterations in behavior and neural function induced by repeated exposure to psychomotor stimulants represent a striking example of prolonged, environmentally modulated neurobehavioral plasticity. These alterations can remain covert until an additional challenge with the drug occurs or until a stimulus contingently paired with the drug is presented. Our findings demonstrate that compartment-based neural alterations in the striatum are already evident during the sensitization regimen and that concurrent stimulation of D1-class and D2-class dopamine receptors is a necessary condition for uncovering them. Our findings further demonstrate that this neural plasticity is strongly correlated with the behavioral patterns induced by the D1–D2 challenges. Our observations suggest a link between the expression of repetitive behavior evoked by conjoint stimulation of D1-class and D2-class dopamine receptors and the expression of patterned alterations in relative activation of striosome-based and matrix-based basal ganglia circuits.
Requirement for synergisms between D1-class and D2-class dopamine receptors in evoking the expression of sensitized stereotypy
Many of the acute behavioral effects that are dependent on the activation of D2-class dopamine receptors, including stereotypy and hyperlocomotion, are known to require simultaneous activation of D1-class receptors. In the acute agonist treatment model that we used, combinations of D1-class and D2-class dopamine receptor agonist treatments were required to induce stereotypy, in accord with previous findings (Arnt et al., 1987; Clark and White, 1987; Walters et al., 1987;LaHoste et al., 1993; Waddington et al., 1995). Remarkably, after repeated cocaine treatments, the enhanced motor stereotypy that could be evoked by combined D1–D2 agonist challenge did not transfer to single-receptor class agonist challenge. Thus, despite the fact that sensitization had developed in the cocaine-pretreated animals, it remained covert even when animals were exposed to very high levels of either D1-class or D2-class dopamine receptor agonists alone.
Our observations are based on coordinated neural and behavioral measurements made directly after acute challenge or directly after postsensitization challenge. It is not known whether this requirement for combined D1–D2 dopamine receptor activation, evident at the end of the sensitizing period, holds also after extended periods of withdrawal. Enhanced stereotypy in response to D2 receptor agonist challenge has been reported in one study in which rats were exposed to repeated cocaine treatment, followed by an intervening period of withdrawal (Ujike et al., 1990), which is thought to be critical for the development of some neural adaptations in dopamine and opioid neurotransmission. Our results suggest that whatever sensitization of D1-class and D2-class receptor function occurred as a result of the drug exposure given to the animals, agonist challenge of either one of these receptor systems was insufficient to evoke the expression of the latent, behavioral adaptations that took place during the sensitization phase.
Requirement for D1–D2 dopamine receptor synergisms in evoking the expression of striosome-predominant early gene distributions
Based on our previous finding that stereotypy induced by psychomotor stimulants is highly correlated with striosome-predominant early-gene expression evoked by the same treatments, we tested whether these neural changes, like the behavioral changes, developed during drug sensitization and required concurrent activation of D1-class and D2-class dopamine receptors. They did. After repeated cocaine treatment, the early-gene responses induced by single D1-class or D2-class agonist challenge were downregulated relative to those in the striatum of drug-naive animals, but there was no difference in the relative response of neurons in striosomes and matrix. In contrast, in animals challenged with D1–D2agonist combinations, there was a significantly greater downregulation in the matrix than in striosomes, especially medially, leading to greater relative activation in the striosomal compartment. The degree of stereotypy induced by the combined D1–D2 agonist challenge showed poor correlation with the matrix downregulation or with estimates of overall striatal gene expression but strong correlation with striosomal activation and even higher correlation with the relative striosomal predominance of gene expression in the medial striatum.
A similar dependency on combined activation of D1-class and D2-class dopamine receptors held for the full range of acute single agonist treatments that we used to treat drug-naive rats, extending previous observations on the regulation of early response genes by dopamine receptor agonists (LaHoste et al., 1993; Wirtshafter and Asin, 1994;Wirtshafter et al., 1997). We did not observe dose dependency for this effect, but lower doses of D1 and D2 agonists than those used in our experiments might show dose dependency just as apomorphine, a mixed D1–D2 agonist, increases both stereotypy and striosomal predominance in a dose-dependent manner (Canales and Graybiel, 2000).
Correlations between stereotypy and striosome-predominant gene activation in the striatum
The degree of stereotypy induced by direct dopamine receptor agonists in our experiments was correlated with a relative enhancement of striosomal early-gene activation not only in cocaine-sensitized animals but also in drug-naive animals. In naive animals, neuronal activation in the matrix and the striosomes increased in parallel with stereotypy, whereas activation in both compartments was reduced after repeated cocaine exposure, a treatment that conversely produced higher levels of stereotypy. However, levels of activity in the striosomes alone showed significant correlations with the stereotypy ratings, which is in accord with previous findings (Canales and Graybiel, 2000). The results we report here suggest that these behavioral and neural responses are regulated either in parallel or in common by a mechanism involving concomitant activation of D1-class and D2-class dopamine receptors. Such synergistic D1–D2 effects on activation of striosomes and matrix may give clues to the specific mechanisms underlying the behavioral expression of sensitized stereotypies. For example, the cholinergic and nitric oxide synthase-containing interneurons of the striatum have been suggested to act in the induction of the striosome-predominant pattern of gene expression in response to drug challenge (Graybiel et al., 2000;Svenningsson et al., 2000; Saka et al., 2002), as has upregulation of dynorphin in striatal neurons and enhanced corticostriatal transmission (Moratalla et al., 1996; Canales and Graybiel, 2000).
In rats exposed to repeated cocaine treatment, combined D1–D2 agonist challenge, but not single agonist treatments, reproduced the main phenotypes of cocaine sensitization: increased stereotypy after challenge and increased striosome-to-matrix neuronal activation. Some important differences between D1–D2agonist challenge and cocaine challenge were also noted. In cocaine-sensitized animals, a final challenge with cocaine produces an enhanced pattern of striosomal activation in the lateral caudoputamen (Canales and Graybiel, 2000), whereas with combined D1–D2 agonist challenge, the striosomal enhancement was mainly medial. Activity in striosomes was decreased by combined D1–D2 challenge, but increased by cocaine challenge, after a sensitizing regimen of cocaine exposure. The combined D1–D2 treatment elicited effects reminiscent of amphetamine challenge after repeated amphetamine exposure, reducing intrinsic striosomal activation yet increasing relative striosomal activation both medially and laterally in the caudoputamen attributable to even greater reduction in the matrix. Acute apomorphine treatment, which directly stimulates D1 and D2 receptors, also enhances relative striosomal activity medially (Canales and Graybiel, 2000). Such topographic differences might relate to the fact that the motor responses emitted in response to different psychomotor stimulants and D1–D2 agonists have different specific patterns, despite sharing common characteristics of repetitiveness and stereotypy. The medial pattern of striosome predominance is also found in rodent models of parkinsonism, in which rats with 6-hydroxydopamine-induced lesions of the nigrostriatal pathway are chronically exposed to levodopa (Cenci et al., 1999) or are given apomorphine (Saka et al., 1999) or combined D1–D2 dopamine receptor agonist treatment (Paul et al., 1992). Jonathan M. Brotchie (University of Manchester, Manchester, UK) (personal communication) has reported such striosome-enhanced patterns of preproenkephalin in the brains of dyskinetic Parkinson's disease patients, and a similar enhancement of striosomal prodynorphin mRNA was noted in a case report of a cocaine addict (Hurd and Herkenham, 1993).
Our findings suggest a bridge between the effects of direct dopamine receptor agonists and the effects of drugs of abuse. A key factor for both is combined activation of D1-class and D2-class dopamine receptors. Such conjoint dopamine receptor stimulation is thought to lead to activation of the direct pathway and inhibition of the indirect pathway leading out of the striatum (Gerfen and Wilson, 1996) and thus to activation of the motor thalamus and frontal neocortex through basal ganglia–thalamocortical loop pathways. Repeated overactivation of these striatocortical loops may result in effects akin to “kindling” of the frontal cortex that could favor the emergence of repetitive behaviors in the absence of external cues for adaptive movement. A close relationship between stereotypy and striosome-predominant neuronal activity could, in particular, reflect a persistent differential recruitment of striosome-based cortico-basal ganglia circuits involving medial and orbital frontal cortex (Gerfen, 1984; Donoghue and Herkenham, 1986; Ragsdale and Graybiel, 1990; Eblen and Graybiel, 1995; Volkow and Fowler, 2000; Goldstein et al., 2001). Such persistent pathway selection could trigger the compulsion to repeat the same few behaviors at the expense of the initiation of adaptive, switching responses requiring threshold activation of matrix-based basal ganglia circuits (Peterson et al., 1998; Graybiel and Rauch, 2000; Leckman et al., 2001).
Another possibility is that the presumably inhibitory striatonigral projection could have a homeostatic function to decrease dopaminergic stimulation of the striatum and, in turn, to decrease increased repetitions of elicited actions. Such an adaptive control mechanism would not, however, be expected to be completely effective if the source of dopaminergic stimulation is from exogenous sources rather than from intrinsic activity of the part of the substantia nigra pars compacta interconnected with the striosomal system. The ineffectiveness of such a striosome-based homeostatic mechanism could contribute to the development of stereotypy in behavioral sensitization, as studied here, as well as levodopa-induced dyskinesias, drug-induced compulsions, Tourette syndrome, and obsessive-compulsive spectrum disorders.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS25529 and NS38372, National Institute on Drug Abuse Grant DA08037, the National Parkinson's Foundation, the Stanley Foundation, the Swiss National Science Foundation, and the Science and Technology Ministry of Spain. We thank Patricia Harlan and Henry F. Hall for their expert help with the experiments, Dr. Paul DiZio for his help with data analysis, and Dr. Esen Saka Topcuoglu for her insightful comments on this manuscript.
Correspondence should be addressed to Dr. Ann M. Graybiel, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, E25-618, 45 Carleton Street, Cambridge, MA 02139. E-mail:graybiel@mit.edu.
REFERENCES
- 1.Antony MM, Downie F, Swinson RP. Diagnostic issues and epidemiology in obsessive-compulsive disorder. In: Swinson RP, Antony MM, Rachman S, Richter MA, editors. Obsessive-compulsive disorder: theory, research and treatment. Guildford; Oxford: 1988. [Google Scholar]
- 2.Arnt J. Antistereotypic effects of dopamine D-1 and D-2 antagonists after intrastriatal injection in rats. Pharmacological and regional specificity. Naunyn Schmiedebergs Arch Pharmacol. 1985;330:97–104. doi: 10.1007/BF00499901. [DOI] [PubMed] [Google Scholar]
- 3.Arnt J, Hyttel J, Perregaard J. Dopamine D-1 receptor agonists combined with the selective D-2 agonist quinpirole facilitate the expression of oral stereotyped behaviour in rats. Eur J Pharmacol. 1987;133:137–145. doi: 10.1016/0014-2999(87)90144-0. [DOI] [PubMed] [Google Scholar]
- 4.Arnt J, Bogeso KP, Hyttel J, Meier E. Relative dopamine D1 and D2 receptor affinity and efficacy determine whether dopamine agonists induce hyperactivity or oral stereotypy in rats. Pharmacol Toxicol. 1988;62:121–130. doi: 10.1111/j.1600-0773.1988.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 5.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 6.Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 7.Canales JJ, Iversen SD. Behavioural topography in the striatum: differential effects of quinpirole and D-amphetamine microinjections. Eur J Pharmacol. 1998;362:111–119. doi: 10.1016/s0014-2999(98)00752-3. [DOI] [PubMed] [Google Scholar]
- 8.Cenci MA, Tranberg A, Andersson M, Hilbertson A. Changes in the regional and compartmental distribution of FosB- and JunB-like immunoreactivity induced in the dopamine-denervated rat striatum by acute or chronic l-dopa treatment. Neuroscience. 1999;94:515–527. doi: 10.1016/s0306-4522(99)00294-8. [DOI] [PubMed] [Google Scholar]
- 9.Clark D, White FJ. Rev: D1 dopamine receptor—the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1:347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- 10.Creese I, Iversen SD. Amphetamine response in rat after dopamine neurone destruction. Nat New Biol. 1972;238:247–248. doi: 10.1038/newbio238247a0. [DOI] [PubMed] [Google Scholar]
- 11.Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann NY Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- 12.Donoghue JP, Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 1986;365:397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- 13.Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fibiger HC, Fibiger HP, Zis AP. Attenuation of amphetamine-induced motor stimulation and stereotypy by 6-hydroxydopamine in the rat. Br J Pharmacol. 1973;47:683–692. doi: 10.1111/j.1476-5381.1973.tb08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen CR, Wilson CJ. The basal ganglia. In: Swanson LW, Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy. Elsevier; Amsterdam: 1996. pp. 371–468. [Google Scholar]
- 17.Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. NeuroReport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graybiel AM, Canales JJ. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. In: Cohen D, Goetz C, Jankovic J, editors. Tourette syndrome. Williams and Wilkins; Philadelphia: 2001. pp. 123–131. [PubMed] [Google Scholar]
- 19.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 20.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 21.Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–190. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- 22.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 23.Iorio LC, Barnett A, Leitz FM, Houser VP, Korduba CA. SCH23390 a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- 24.Jung BJ, Dawson R, Jr, Sealey SA, Peris J. Endogenous GABA release is reduced in the striatum of cocaine-sensitized rats. Synapse. 1999;34:103–110. doi: 10.1002/(SICI)1098-2396(199911)34:2<103::AID-SYN3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Kalivas PW, Stewart J. Dopamine transmission in drug- and stress-induced behavioral sensitization. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 26.Karler R, Calder LD, Thai LH, Bedingfield JB. The dopaminergic, glutamatergic, GABAergic bases for the action of amphetamine and cocaine. Brain Res. 1995;671:100–104. doi: 10.1016/0006-8993(94)01334-e. [DOI] [PubMed] [Google Scholar]
- 27.LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leckman JF, Cohen DJ, Goetz CG, Jankovic J. Tourette syndrome: pieces of the puzzle. Adv Neurol. 2001;85:369–390. [PubMed] [Google Scholar]
- 29.Manschreck TC. Motor abnormalities in schizophrenia. In: Nasrallah HA, Weinberger DR, editors. The neurobiology of schizophrenia. Elsevier; Amsterdam: 1986. [Google Scholar]
- 30.Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ, Hyman SE, Malenka RC. Molecular basis of neuropharmacology: a foundation for clinical neuroscience. McGraw-Hill; New York: 2001. [Google Scholar]
- 32.Paul ML, Graybiel AM, David J-C, Robertson HA. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 34.Ragsdale CW, Jr, Graybiel AM. A simple ordering of neocortical areas established by the compartmental organization of their striatal projections. Proc Natl Acad Sci USA. 1990;87:6196–6199. doi: 10.1073/pnas.87.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter A, Ebert U, Nobrega JN, Vallbacka JJ, Fedrowitz M, Loscher W. Immunohistochemical and neurochemical studies on nigral and striatal functions in the circling (ci) rat, a genetic animal model with spontaneous rotational behavior. Neuroscience. 1999;89:461–471. doi: 10.1016/s0306-4522(98)00321-2. [DOI] [PubMed] [Google Scholar]
- 36.Ridley RM. The psychology of perseverative and stereotyped behaviour. Prog Neurobiol. 1994;44:221–231. doi: 10.1016/0301-0082(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 38.Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg DR, Dick EL, O'Hearn KM, Sweeney JA. Response-inhibition deficits in obsessive-compulsive disorder: an indicator of dysfunction in frontostriatal circuits. J Psychiatry Neurosci. 1997;22:29–38. [PMC free article] [PubMed] [Google Scholar]
- 40.Saka E, Elibol B, Erdem S, Dalkara T. Compartmental changes in expression of c-Fos and FosB proteins in intact and dopamine-depleted striatum after chronic apomorphine treatment. Brain Res. 1999;825:104–114. doi: 10.1016/s0006-8993(99)01231-7. [DOI] [PubMed] [Google Scholar]
- 41.Saka E, Iadarola M, Fitzgerald DJ, Graybiel AM. Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc Natl Acad Sci USA. 2002;99:9004–9009. doi: 10.1073/pnas.132212499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svenningsson P, Fredholm BB, Bloch B, Le Moine C. Co-stimulation of D(1)/D(5) and D(2) dopamine receptors leads to an increase in c-fos messenger RNA in cholinergic interneurons and a redistribution of c-fos messenger RNA in striatal projection neurons. Neuroscience. 2000;98:749–757. doi: 10.1016/s0306-4522(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 43.Turski L, Schwarz M, Sontag KH. Stereotypy, locomotor and cataleptic effects produced by drugs influencing dopaminergic systems in a mutant strain of Wistar rats: a genuine model of basal ganglia dysfunction? Behav Brain Res. 1984;12:29–37. doi: 10.1016/0166-4328(84)90200-6. [DOI] [PubMed] [Google Scholar]
- 44.Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology. 1990;102:459–464. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- 45.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 47.Waddington JL, Daly SA, Downes RP, Deveney AM, McCauley PG, O'Boyle KM. Behavioural pharmacology of “D-1-like” dopamine receptors: further subtyping, new pharmacological probes and interactions with “D-2-like” receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:811–831. doi: 10.1016/0278-5846(95)00130-n. [DOI] [PubMed] [Google Scholar]
- 48.Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987;236:719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- 49.White IM, Doubles L, Rebec GV. Cocaine-induced activation of striatal neurons during focused stereotypy in rats. Brain Res. 1998;810:146–152. doi: 10.1016/s0006-8993(98)00905-6. [DOI] [PubMed] [Google Scholar]
- 50.Wirtshafter D, Asin KE. Interactive effects of stimulation of D1 and D2 dopamine receptors on fos-like immunoreactivity in the normosensitive rat striatum. Brain Res Bull. 1994;35:85–91. doi: 10.1016/0361-9230(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 51.Wirtshafter D, Schardt G, Asin K. Compartmentally specific effects of quinpirole on the striatal Fos expression induced by stimulation of D1-dopamine receptors in intact rats. Brain Res. 1997;771:272–277. doi: 10.1016/s0006-8993(97)00795-6. [DOI] [PubMed] [Google Scholar]