Abstract
Antioxidants and diets supplemented with foods high in oxygen radical absorbance capacity (ORAC) reverse age-related decreases in cerebellar β-adrenergic receptor function. We examined whether this effect was related to the antioxidant capacity of the food supplement and whether an antioxidant-rich diet reduced the levels of proinflammatory cytokines in the cerebellum. Aged male Fischer 344 rats were given apple (5 mg dry weight), spirulina (5 mg), or cucumber (5 mg) either in 0.5 ml water by oral gavage or supplied in the rat chow daily for 14 d. Electrophysiologic techniques revealed a significant decrease in β-adrenergic receptor function in aged control rats. Spirulina reversed this effect. Apple (a food with intermediate ORAC) had an intermediate effect on cerebellar β-adrenergic receptor physiology, and cucumber (low ORAC) had no effect, indicating that the reversal of β-adrenergic receptor function decreases might be related to the ORAC dose. The mRNA of the proinflammatory cytokines tumor necrosis factor-α (TNFα) and TNFβ was also examined. RNase protection assays revealed increased levels of these cytokines in the aged cerebellum. Spirulina and apple significantly downregulated this age-related increase in proinflammatory cytokines, whereas cucumber had no effect, suggesting that one mechanism by which these diets work is by modulation of an age-related increase in inflammatory responses. Malondialdehyde (MDA) was measured as a marker of oxidative damage. Apple and spirulina but not cucumber decreased MDA levels in the aged rats. In summary, the improved β-adrenergic receptor function in aged rats induced by diets rich in antioxidants is related to the ORAC dose, and these diets reduce proinflammatory cytokine levels.
Keywords: aging, cerebellum, cytokines, antioxidants, norepinephrine, inflammation
Impaired antioxidant defense mechanisms and increases in reactive oxygen species and reactive nitrogen species are postulated to be causative factors in aging-related functional declines and in neurodegenerative diseases (Harman, 1956; Leibovitz and Siegel, 1980; Ames et al., 1993). Increasing evidence suggests that inflammatory processes are linked to oxidative damage in the CNS. Injection of the antioxidant enzyme superoxide dismutase decreases inflammation in some animal model systems. Antioxidants such as vitamins E, C, and β-carotene enhance some parameters of immune function when added to isolated immune cellsin vitro or when given as supplements to humans or animalsin vivo (Han and Meydani, 2000). Despite extensive evidence of the anti-inflammatory effects of antioxidants, little is known about the underlying molecular mechanisms.
One potential mechanism is the effect of antioxidants on the production of immunoregulatory molecules such as cytokines. Cytokines are induced in response to brain injury and can mediate and inhibit cellular injury and repair. Many clinical studies report increased cytokine expression in the CSF or in postmortem brain tissue of patients that have suffered stroke or brain injury. Several lines of evidence indicate that proinflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and transforming growth factor-β increase with aging (Lynch, 1998; Knoblach et al., 1999).
Several studies have examined antioxidants and their effects in aged animals and humans. Diets enriched in fruits and vegetables that have a high antioxidant capacity as measured by oxygen radical absorbance capacity (ORAC) (Cao et al., 1997) fed to rats for periods as short as 2 weeks to 2 months starting at 18 months of age can reverse the age-related onset of some behavioral and neurochemical deficits (Gould and Bickford, 1997; Joseph et al., 1999; Bickford et al., 2000). Much of the evidence supporting the beneficial role of fruits and vegetables to health comes from epidemiological literature. The traditional common diet of the Mediterranean region, a diet high in fruits and vegetables, is associated with a significant (17%) reduction in overall mortality in the elderly from these regions (Willet et al., 1995). Recent studies with vitamin E indicate that high doses slow the progression of certain aspects of Alzheimer's disease (Sano et al., 1997). The nature of the protective effects of specific nutrients found in fruits and vegetables, such as β-carotene, vitamin C, and vitamin E, however, is unknown. A single factor is not likely to be the only effective agent in these studies. With few exceptions, a single nutrient is not packaged into a single food, and it is possible that combinations of nutrients have greater protective effects than each nutrient alone.
The present study focused on a well characterized model system to examine the effects of dietary alterations in aged rats. The cerebellar noradrenergic system shows age-related changes in neurophysiology, and these changes might underlie age-related deficits in motor learning. The noradrenergic input to cerebellar Purkinje neurons inhibits spontaneous discharge but appears to augment the “signal to noise” ratio for both excitatory and inhibitory neurotransmission, and thus has been characterized previously as a “modulatory” input (Freedman et al., 1976). The modulatory effect of norepinephrine is blunted in aged rats (Bickford, 1993; Gould and Bickford, 1997). In addition, cerebellar norepinephrine is necessary for motor learning. Depletion of cerebellar norepinephrine or blockade of β-adrenergic receptors impairs performance on a runway task where rats must learn to walk on varying patterns of pegs that protrude from the runway walls (Bickford et al., 1992; Heron et al., 1996). Impaired performance in aged rats in this task is correlated with the loss of β-adrenergic receptor function (Bickford et al., 1992; Bickford, 1993).
The present study examined the relationship of the effects of diets designed to improve cerebellar β-adrenergic receptor function to thein vitro antioxidant activity of fruits and vegetables, whether anti-inflammatory mechanisms mediate the effects of a diet high in fruits and vegetables, and whether proinflammatory cytokines are involved in mediating these effects.
MATERIALS AND METHODS
Animals. Male Fischer 344 rats (National Institute on Aging contract colony; Harlan Sprague Dawley, Indianapolis, IN) (n = 76; 4 and 18 months of age) were used. The rats were housed in pairs, maintained in environmentally controlled chambers on a 12 hr light/dark cycle at 21 ± 1°C, and provided food and water ad libitum.
ORAC assay. The in vitro antioxidant capacity of an aqueous extract of the dietary supplements was measured using an ORAC assay (Cao et al., 1993, 1995). Freeze-dried samples were added to distilled water (2:1), sonicated, and then centrifuged at 10,000 × g for 10 min. The supernatant was used for subsequent analysis with dilutions as needed. The reaction mixture was 1.78 ml of 0.75 mmol/l sodium phosphate buffer (PB), pH 7.0, 0.02 ml of β-phycoerythrin (1.7 × 10−8 mol/l in PB; Sigma-Aldrich, St. Louis, MO), 0.2 ml of 2,2′-azobis (2- amidinopropane) hydrochloride solution (40 mmol/l in PB), and 0.02 ml of sample. The reaction was run at 37°C, and fluorescence was determined at 10 min intervals on a scanning fluorescent spectrophotometer (Acufluor) at excitation and emission wavelengths of 540 and 575 nm, respectively. All samples were compared with a standard reaction mixture of 1 μm Trolox (final concentration) (obtained from Sigma-Aldrich).
Diet preparation and feeding regimens. NIH-31 (TD 00365; Harlan Teklab, Madison, WI) rodent diet was supplemented with 0.33% w/w dry spirulina (Earthrise Co., Petaluma, CA), freeze-dried Spy apples (including skin), or freeze-dried cucumber (Van Drunen Farms, Momence, IL), approximating a 5 mg/d dry weight dose of the supplements. Control diet and the supplements were combined using a Waring (Torrington, CT) Blendor and delivered in powder form to the animals. Animals were allowed to feed ad libitum for 14 d and were then killed; brain areas were dissected and flash frozen in liquid nitrogen. The ORACs, determined as 1 μm Trolox equivalents for the diets, were 0.05 (cucumber), 1.1 (apple), and 320 (spirulina).
For experiment 1, the rats were fed supplements by oral gavage with 5 mg dry weight of either apple (including skin) or spirulina in 0.5 ml of water. Controls received 0.5 ml of water only. In vivoelectrophysiologic measurements under urethane anesthesia were performed before collecting the cerebellum for cytokine measurements. For experiment 2, instead of oral gavage, the apple and spirulina were added to the chow. Cytokine levels were measured. For experiment 3, rats (young and aged) were fed the diets either ad libitumor by oral gavage (0.5 ml of water) and studied electrophysiologically. For experiment 4, rats were fed a chow diet supplemented with cucumber or a control diet and examined using electrophysiologic techniques or used for cytokine measurements.
RNA isolation and RNase protection assay. The animals were decapitated, the brains were removed, the brain regions were dissected, and tissues were frozen in liquid nitrogen and stored at −80°C until total RNA was extracted (Gemma et al., 2000). Total RNA from homogenized tissues was extracted using a Qiagen (Valencia, CA) Rneasy minikit according to the manufacturer's instructions. Total RNA (20 μg) from each sample was hybridized with antisense radiolabeled probes, after which free probe and remaining single-stranded RNA were digested with RNase A/T1. Double-stranded RNase-protected fragments were resolved on 5% denaturing polyacrylamide gels. The probe template used was purchased from PharMingen (San Diego, CA) and included rat-specific sequences for IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, TNFα, TNFβ, and interferon-γ (IFNγ). A positive control transcript was made using a probe specific for the ribosomal protein L32, a housekeeping gene, to calculate the specific activity and obtain a sufficient excess of the L32 probe. The L32 probe was then added to the probe template before the hybridization reaction started. Yeast transfer RNA and rat mRNA were used as negative and positive controls, respectively. Dried gels were placed on a phosphorimager screen for 16–20 hr. The phosphorimaging screen was subsequently scanned with a phosphorimager (Molecular Image System GS-363; Bio-Rad, Hercules, CA). The images were processed using Molecular Analyst software (Bio-Rad). The intensity of a band in the computer-generated image was directly proportional to the amount of radioactivity within the band. The optical density (OD) values obtained from each band were normalized against the OD obtained from the L32 band in that sample by the following equation: [(OD of the sample band/OD of the L32 band) × 100].
Electrophysiology. Rats were anesthetized with urethane (0.75–1.25 gm/kg), intubated, and allowed to breathe spontaneously. Corneal reflex and toe pinch were used to monitor the anesthetic level to establish adequate anesthesia. A heating pad was used to maintain body temperature at 37°C. Animals were placed in a stereotaxic frame, and the skin and muscle over the posterior vermis was removed. The cistern was drained, and the skull and dura over the vermis were removed. The brain was covered with solution of 2% agar in saline. Extracellular recordings were made from Purkinje cells in lobules VI and VII of the cerebellar vermis as identified by anatomic location and the characteristic complex spiking of Purkinje cells (Eccles et al., 1967).
Neuronal signals were amplified, filtered (−3 dB at 0.3 and 5 kHz), and displayed on a storage oscilloscope. Action potentials were isolated using a window discriminator, and the firing output was displayed using a strip chart recorder. Single units had a signal to noise ratio of at least 2:1. Multibarrel glass micropipettes were used for single-cell recording and local drug application via microiontophoresis. The resistance of the recording electrodes was 1.5–3.3 MΩ. In the multibarrel glass micropipettes, two barrels were filled with 3 m NaCl and the other two barrels were filled with GABA (0.25 m, pH 4.0–4.5) and with the β-adrenergic agonist isoproterenol (ISO; 0.25 m, pH 4.0–4.5), respectively. A constant-current source provided ejection and retention currents for the drug barrels and passed an equal current of opposite polarity through the balance barrel to neutralize the tip potential. Uniform pulses of drug were applied at regular intervals.
Current was adjusted until GABA application produced a 10–30% inhibition of Purkinje cell firing. Four applications of GABA were given with a stable baseline response before ISO was coadministered. The level of ISO was adjusted until either a ≥10% change in GABA-induced Purkinje cell inhibition was observed for four applications of GABA or until the baseline Purkinje cell firing rate was altered. After ISO was turned off, GABA application continued until it could be determined whether the pre-ISO level of GABAergic inhibition returned. Only Purkinje cells in which the post-ISO level of GABAergic inhibition matched the pre-ISO level of GABAergic inhibition were analyzed. Drug-induced responses were quantified by computer. The Datawave (Longmont, CO) system was used to acquire and quantify drug responses.
Determination of malondialdehyde by HPLC with spectrofluorometric detection. Analysis of the lipid peroxidation by product, malondialdehyde (MDA), by HPLC, with spectrofluorometric detection, was used to overcome the limitations of the thiobarbituric acid (TBA) reaction by chromatographically separating the MDA–TBA conjugate from contaminating fluors that arise during the TBA reaction. In a microassay adaptation of the TBA reaction, small aliquots (10–50 μl) of tissue extracts are combined in a 1.5 ml microcentrifuge tube with 250 μl of 0.5 m phosphoric acid, 250 μl of 42 mmol/l TBA in acetic acid, and 50 μl of 500 ppm butylated hydroxytoluene. Samples are then placed into a 100°C heating block for 45 min. A 150 μl postreaction aliquot was neutralized with 1N NaOH in MeOH and immediately injected onto the column for chromatographic analysis. MDA peak identification and quantification were determined by comparison with a standard curve of MDA–TBA adduct generated by acid hydrolysis of working standards of 1,1,3,3-tetraethoxypropane.
Statistical analyses. One-way ANOVA was used to analyze the OD values. Fisher's exact test was used for the electrophysiologic data, because it was nonparametric. A pvalue of <0.05 was considered to be statistically significant.
RESULTS
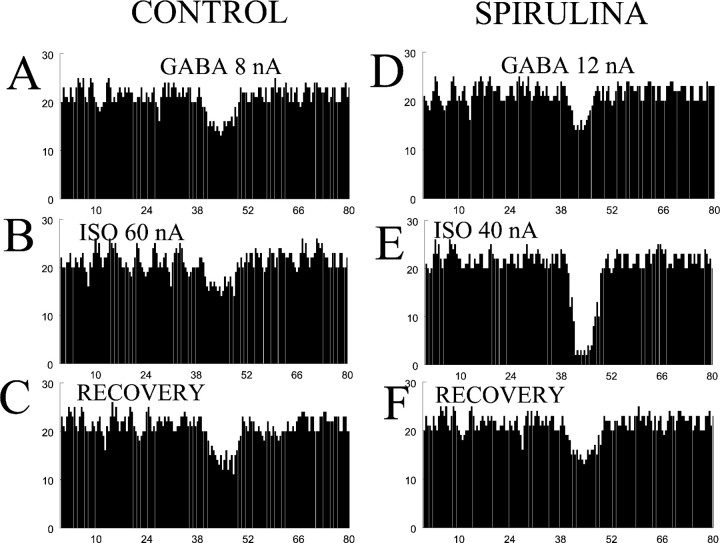
Electrophysiologic recordings were performed to determine whether these diets produced functional changes in the cerebellum, previously observed with other antioxidant-enriched chow-fed diets (blueberry, spinach, strawberry, and vitamin E). There were no significant differences between chow-fed rats or gavage-fed rats (young gavage, 57 ± 3, n = 7; young chow, 59 ± 3,n = 7; old gavage, 19 ± 2, n = 5; old chow, 21 ± 2, n = 6); therefore, these dietary strategies were considered equivalent for the electrophysiologic studies. β-adrenergic receptor function was examined by testing the ability of iontophoretically applied ISO to increase the inhibitory response to iontophoretically applied GABA while recording the spontaneous firing rate of Purkinje neurons using extracellular multibarrel electrodes. There was an age-induced decrease in β-adrenergic function in control aged animals. Figure1A shows a typical response to GABA in an aged rat where a 20% inhibition of spontaneous firing rate is observed. Figure 1B shows that when ISO is applied concurrently with GABA, the increase in GABAergic inhibition typically observed in a Purkinje cell from a young rat does not occur. Conversely, in an aged rat fed spirulina, ISO augments the GABAergic inhibition from a baseline of 18% (Fig.1D) to 91% (Fig. 1E). Figure2 summarizes the results from all rats. In young rats (n = 14), >75% of recorded Purkinje neurons demonstrated an ISO-mediated increase in GABAergic inhibition. In the control 18-month-old rats (n = 11), only 40% of neurons responded to ISO with an increase in GABAergic inhibition (p < 0.05; Fisher's exact test). When rats were fed spirulina (n = 6; 5 mg in 0.5 ml by oral gavage daily), >75% of the Purkinje neurons had an ISO-mediated increase in GABAergic inhibition, a statistically significant reversal of the age-related impairment of β-adrenergic receptor function. Rats fed apple (n = 6; 5 mg in 0.5 ml by oral gavage daily) showed intermediate improvement; they were not statistically different from the aged controls, yet they were also not statistically different from the young rats or the spirulina-treated group. When rats were fed a cucumber diet for 14 d (n = 6; 0.33% freeze-dried powder in NIH-31 diet) there was no improvement in the ISO-mediated GABA inhibition of the cerebellar Purkinje neuron firing rate.
Fig. 1.
Peri-event time histograms showing the response to iontophoretically applied GABA onto single cerebellar Purkinje neurons from an aged rat on the control diet (left) or an aged rat fed a spirulina-enriched diet for 2 weeks (right). The top panels (A, D) show baseline responses to a 5 sec application of GABA. The middle panels (B, E) show responses to GABA during concurrent application of ISO. The bottom panels(C, F) represent recovery after termination of the ISO. Histograms represent averages of four drug responses. Thex-axis represents time in seconds and they-axis represents action potentials per 0.5 sec time bin.
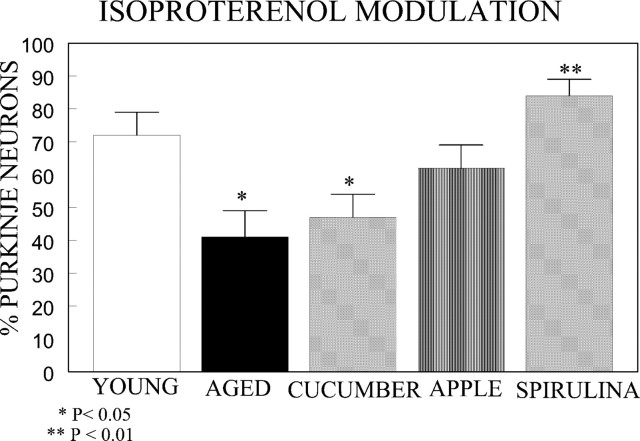
Fig. 2.
The ability of ISO to augment GABAergic inhibition of cerebellar Purkinje neurons was examined in young rats (n = 7 rats, 42 neurons for gavage;n = 7 rats, 57 neurons for control diet) and aged rats fed apple (n = 6 rats, 38 neurons, fed via oral gavage), spirulina (n = 6 rats, 38 neurons, fed via oral gavage), vehicle (n = 5 rats, 40 neurons, fed via gavage; n = 6 rats, 33 neurons, normal diet), or cucumber (n = 6 rats, 35 neurons, fed via diet supplement) for 2 weeks. There were no differences between young or aged controls on the diet versus gavage treatment; therefore the samples were pooled. The ability of ISO to augment GABAergic inhibition of Purkinje cell spontaneous firing was the dependent measure. The spirulina diet significantly reversed the age-induced decrease in the β-adrenergic receptor response (p < 0.05; Fisher’s exact test). The apple diet had an intermediate effect (not significantly different from either control or spirulina), and the cucumber diet did not produce any change.
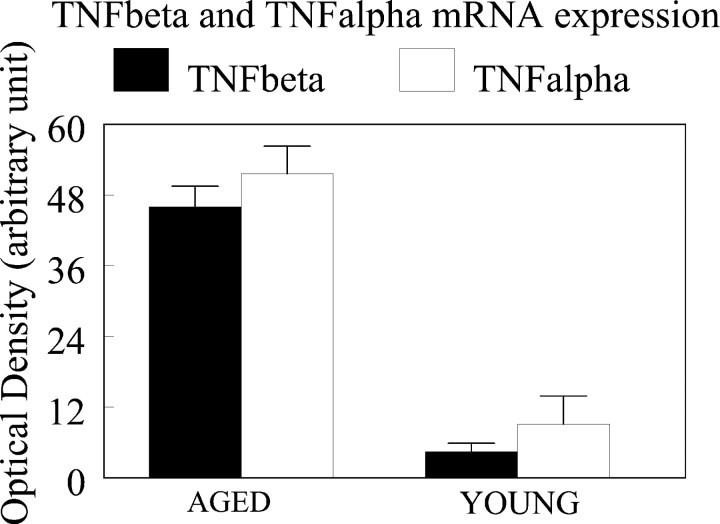
To evaluate neuroinflammatory processes that occur during normal aging, we used the RNase protection assay (RPA) to determine the mRNA expression of proinflammatory cytokines. A representative example of a gel taken from the phosphorimaging system is depicted in Figure3. In this particular RPA, total cerebellar RNA from 20-month-old and 4-month-old rats was assayed. In the young rats, there is very low expression of mRNA for proinflammatory cytokines. In contrast, bands corresponding to TNFβ, IL-6, IL-10, TNFα, and IFNγ are observed in the aged rat samples. Because TNFα and TNFβ were the major bands observed in the aged rats, they were analyzed quantitatively. The expression of TNFα and TNFβ mRNA was age-related (Fig. 4). The cerebellum from 20-month-old rats (n = 6) showed significantly higher expression of mRNA for both cytokines when compared with those obtained from 4-month-old animals [n = 6; TNFα, F = 49.3 (one-way ANOVA), p < 0.001; TNFβ, F = 152,p < 0.001].
Fig. 3.
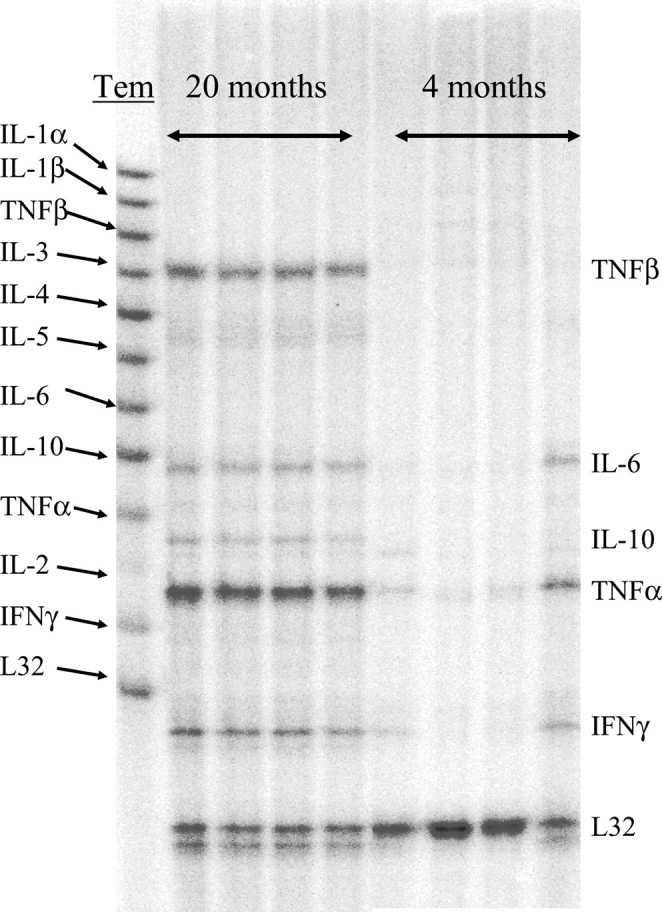
Representative image of a 5% denaturing polyacrylamide gel obtained by a phosphorimager on which double-stranded RNase-protected fragments were resolved. Total mRNA was extracted from the cerebellum of rats aged 4 and 20 months. The template consisted of radiolabeled antisense RNA probes for IL-1α (protected probe size, 403 nt), IL-1β (361 nt), TNFβ (322 nt), IL-3 (286 nt), IL-4 (256 nt), IL-5 (226 nt), IL-6 (202 nt), IL-10 (181 nt), TNFα (160 nt), IL-2 (142 nt), and IFNγ (129 nt).Tem, Template.
Fig. 4.
Age-related mRNA expression of TNFα and TNFβ in cerebellar tissue of male Fischer 344 rats. TNFα and TNFβ mRNA expression was significantly increased in cerebellar tissues dissected from aged rats (20 months; n = 6) compared with young rats (4 months; n = 6) (one-way ANOVA: TNFα, F = 49, p < 0.0001; TNFβ, F = 152, p < 0.0001). An internal standard control gene, ribosomal protein L32, was included in the template, and the OD of each band for cytokine mRNA was normalized relative to the OD of the L32 band by the following expression: (OD of the cytokine mRNA band/OD of the L32) × 100.
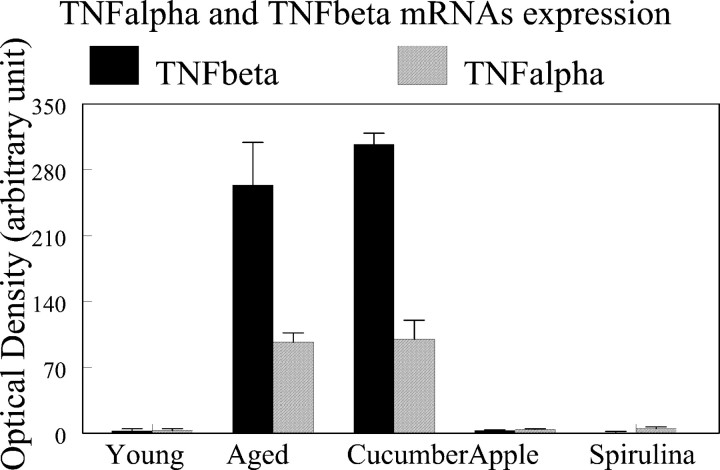
We subsequently examined whether the expression of proinflammatory cytokines was modulated by diets enriched with foods high in antioxidant activity using spirulina and apple diets. Rats were also fed a cucumber-enriched diet, a food with low ORAC. Cytokine measurements were identical between diet-fed rats or gavage-fed rats; however, there was less variability, so the cytokine measurement data from chow-fed rats are presented. The cerebella obtained from the aged animals fed the spirulina-supplemented diet (n = 8) and apple (n = 7) showed decreased expression of TNFα or TNFβ mRNA [spirulina: TNFα, F = 13 (one-way ANOVA), p < 0.0004; TNFβ, F = 22.6,p < 0.0006; apple: TNFα, F = 11.5,p < 0.007; TNFβ, F = 19,p < 0.001] (Fig. 5). In contrast, in animals fed cucumber (n = 6), the expression of mRNA was not different from that in animals maintained on the control diet.
Fig. 5.
The age-related increase in TNFα and TNFβ mRNA expression in rat cerebellar tissue is prevented by diets rich in antioxidants. The TNFα and TNFβ mRNA expression was significantly decreased in cerebellar tissue obtained from aged rats (18 months) fed for 2 weeks with either spirulina (n = 6) (0.33% w/w dry; spirulina vs aged controls; p < 0.005 andp < 0.001 for TNFα and TNFβ, respectively) or apple (n = 6) (5 mg/d dry weight; apple vs aged controls; p < 0.005 and p < 0.001 for TNFα and TNFβ, respectively) compared with aged rats fed on control diet. The cucumber diet did not produce any significant change in TNFα and TNFβ.
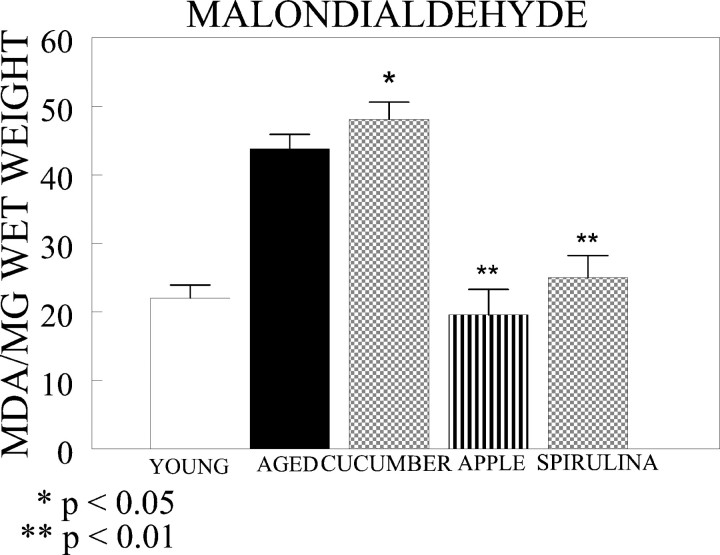
To evaluate the effects of the apple, spirulina, and cucumber diets on an index of oxidative stress, we measured the levels of MDA in the cerebellum. MDA levels were significantly higher in the aged control rats (Fig. 6). The levels of MDA were significantly lower in aged rats fed apple- and spirulina-enriched diets compared with aged controls. The cucumber diet did not decrease MDA levels.
Fig. 6.
MDA values are increased in control aged rats (n = 6) compared with young rats (n = 6), and apple (n = 6) and spirulina (n = 6) diets bring those values back toward those observed in young rats (p < 0.01; one-way ANOVA). Lipid peroxidation by MDA was measured by a TBA reaction where the MDA–TBA adduct end-product was chromatographically separated. A single MDA–TBA peak is detected by spectrophotometry with absorbance at 532 nm and emission at 553 nm. Values are expressed in picomoles of MDA per milligram wet weight of tissue.
DISCUSSION
Aged Fischer 344 rats fed either spirulina- or apple-enriched diets for 14 d have improved β-adrenergic receptor function, downregulation of proinflammatory cytokines, and decreased MDA in the cerebellum. There was no improvement, however, in rats fed a diet supplemented with equivalent amounts of cucumber, a food with a low ORAC.
It is widely thought that diet-derived antioxidants have a role in the prevention of human disease. Diets rich in fruits, grains, and vegetables are protective against several human diseases, especially cardiovascular disease and some types of cancer (Halliwell and Gutteridge, 1999). We have demonstrated previously that vitamin E and/or extracts of strawberries, blueberries, and spinach fed to rats for 2 weeks to 2 months starting at 18 months of age can reverse the age-related onset of some behavioral and neurochemical deficits (Gould et al., 1998; Joseph et al., 1999; Bickford et al., 2000). It is unlikely, however, that a single factor is the effective agent; there are many constituents in food that might exert additional antioxidant effects or protect by completely different mechanisms. It is important to take into account that some compounds that have limited direct antioxidant activity might exert antioxidant action in vivoby upregulating endogenous antioxidant factors.
In the present study, mRNA expression of TNFα and TNFβ in the cerebellum of aged rats was dramatically enhanced when compared with the cerebellum of young rats, suggesting that inflammatory mechanisms are involved in normal aging processes and that central mechanisms for the production of proinflammatory cytokines are age dependent. The increase in mRNA expression of TNFα and TNFβ in the cerebellum of old rats completely reversed when aged animals were fed apple- or spirulina-supplemented diets for 2 weeks, but not when they were fed cucumber, suggesting that at least some of the beneficial effects of these enriched diets on physiologic parameters, such as β-adrenergic receptor function, might be exerted through the downregulation of inflammatory mediators.
The observation that these effects are rapid is consistent with previous literature indicating that these antioxidants, such as the free radical scavenging spin-trap agent, phenyl-butyl-tert-nitrone and vitamin E, can have beneficial effects in this short period of time (Carney et al., 1991; Gould et al., 1998). Thus, the action of these foods might be to downregulate ongoing oxidative stress and/or inflammatory processes, allowing for the recovery of function of cellular homeostasis. There is a rapid reversal of oxidative damage to protein and membranes (Carney et al., 1991), which might underlie the functional recovery observed. The in vitro ORACs of spirulina, apple, and cucumber are different. Spirulina has an ORAC activity that is 300-fold higher than that measured in apples. Apple, spirulina, and cucumber were given to the animals in the same milligram quantities so that the relative ORAC present in each diet was different. The ORAC determination was performed on the aqueous extract; therefore it could be an underestimate of the total ORAC for the sample, because some of the phytochemicals would not be extracted into an aqueous extract. Nonetheless, the reversal of the electrophysiologic alterations in β-adrenergic receptor function is consistent with an ORAC dose relationship, because the apple data were intermediate between the results observed with spirulina and cucumber. The spirulina produced results similar to those reported previously with blueberry and spinach, foods with ORAC equivalents of ∼3 (Joseph et al., 1999). In previous studies, an ∼60 times higher amount of the food was given, so the studies are not directly comparable, yet this also indicates a dose relationship of the antioxidant amounts on the observed changes in β-adrenergic receptor function. The ORAC dose relationship is less clear when changes in proinflammatory cytokines are examined. In aged rats fed either apple or spirulina, the overproduction of TNF was completely reversed, whereas in aged rats fed cucumber, which is low in ORAC, TNF levels were not affected. The in vitro ORAC of apples is lower than that of spirulina, but both apple and spirulina completely reversed the increase in the mRNA for TNFα and TNFβ, suggesting that the phytochemicals in apple were sufficient to produce this downregulation of cytokines. Spirulina had no other detectable effects, suggesting that the effect was maximal and that there was a floor so that no additional change could be observed. A similar profile was observed for the MDA levels in the cerebellum. The apple diet, however, had an intermediate effect on the electrophysiologic measures of β-adrenergic receptor function. One explanation for this divergence is that the antioxidant and/or anti-inflammatory dose requirement for observing a difference in the β-adrenergic receptor signal transduction cascade is different from what is needed to observe a change in proinflammatory cytokines and MDA. Alternately, ORAC is a crude in vitro measure, and there are multiple components in both apple and spirulina that result in the observed effects on cytokines, MDA, and the electrophysiologic measures. It is possible that the differences observed between apple and spirulina relate to these different phytochemical profiles. In addition, these different phytochemicals might have varying abilities to cross the blood–brain barrier and thus produce effects in the CNS. Additional research is required to examine subcomponents of these foods and dose responses to these components to examine this question in more detail.
Spirulina is a microalga, rich in protein and other essential nutrients. Spirulina contains phycobiliprotein (phycocyanin and allophycocyanin), phenolic acid, tocopherols, and β-carotene. Although all of these components exhibit high antioxidant properties, the antioxidant activity of spirulina, when evaluated against oxidation of methyl linoleate in a hydrophobic system, is primarily related to the biliprotein phycocyanin component (Hirata et al., 2000). The contribution of phycocyanin to the antioxidant effects of spirulina has been investigated in other systems. An increase in the antioxidant activity of different fractions of spirulina, obtained during the phycocyanin purification process, is related to an increase in phycocyanin content (Pinero Estrada et al., 2001). Other investigators report that when administered orally to mice at 100 or 200 mg/kg, phycoerythrin inhibits glucose oxidase-induced inflammation of the paw in a dose-dependent manner (Romay et al., 1998). However, apple contains phenolic, flavonoid, and flavonoid-sugar compounds, and quercetin is major dietary flavonoid. There are more phenolics in the skin of apples than in the flesh, and quercetin glycosides are found only in the skins. The antioxidant effects of apple extracts with skin and apple extracts without skin have been tested on the proliferation of several kinds of cancer cell lines. The whole-apple extract either with skin or without skin inhibits the growth of colon and cancer cellsin vitro in a dose-dependent manner, suggesting that the combination of phytochemicals including phenolic acid and flavonoids present in the skin and flesh are responsible for the antioxidant effects of apple (Eberhardt et al., 2000). These observations suggest that not only one component but perhaps a combination of nutrients in foods have greater protective effects than each nutrient alone.
There is a possible link between the β-adrenergic receptor and proinflammatory cytokines. Several studies report anti-inflammatory properties of β-adrenergic receptors on immune cells under stress conditions (Haskoó et al., 1998; Zhang et al., 1999). In addition, a large body of evidence suggests that β-adrenergic receptor agonists decrease TNF release through a mechanism mediated by cAMP (Zhang et al., 1999). Because there is a downregulation of β-adrenergic receptor function in Purkinje neurons of the aged cerebellum, which translates at least as far as induction of cAMP (Gould and Bickford, 1997), it is possible that a blunted β-adrenergic response of immune cells is related to the observed increase in proinflammatory cytokines. Additional studies are needed to determine whether this mechanism acts in the cerebellum of aged rats.
In conclusion, the beneficial effects of diets rich in fruits and vegetables are related, at least in part, to the ORAC of the compounds present in the diet. In addition, the nutrients influence the appearance of proinflammatory cytokines in the cerebellum of aged rats, inducing an anti-inflammatory response in the aged rat cerebellum. This raises the possibility that some of the effects of diets enriched with foods with high antioxidant activity are related to an interaction with the proinflammatory cytokines.
Footnotes
This work was supported by the Veterans Affairs Medical Research Service and United States Public Health Service Grants AG04418 and AG00728.
Correspondence should be addressed to Dr. Paula C. Bickford, Center for Aging and Brain Repair, Department of Neurosurgery, MDC-78, University of South Florida, College of Medicine, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612. E-mail: pbickfor@hsc.usf.edu.
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickford P. Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Res. 1993;620:133–138. doi: 10.1016/0006-8993(93)90279-v. [DOI] [PubMed] [Google Scholar]
- 3.Bickford P, Heron C, Young DA, Gerhardt GA, de la Garza R. Impaired acquisition of novel locomotor tasks in aged and norepinephrine-depleted F344 rats. Neurobiol Aging. 1992;13:475–481. doi: 10.1016/0197-4580(92)90075-9. [DOI] [PubMed] [Google Scholar]
- 4.Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 5.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 6.Cao G, Verdon CP, Wu AHB, Wang H, Prior RL. Automated oxygen radical absorbance capacity assay using COBAS FARA II. Clin Chem. 1995;41:1738–1744. [PubMed] [Google Scholar]
- 7.Cao G, Sofic E, Prior RL. Antioxidant capacity of tea and common vegetables. J Agric Food Chem. 1997;44:3426–3431. [Google Scholar]
- 8.Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-α-phenylnitrone. Proc Natl Acad Sci USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- 10.Eccles JC, Ito M, Szentogothai J. The cerebellum as a neuronal machine. Springer; New York: 1967. [Google Scholar]
- 11.Freedman R, Hoffer BJ, Puro D, Woodward DJ. Noradrenaline modulation of the responses of the cerebellar Purkinje cell to afferent synaptic activity. Br J Pharmacol. 1976;57:603–605. doi: 10.1111/j.1476-5381.1976.tb10391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gemma C, Smith EM, Hughes TKJ, Opp MR. Human immunodeficiency virus glycoprotein 160 induces cytokine mRNA expression in the rat central nervous system. Cell Mol Neurobiol. 2000;20:419–431. doi: 10.1023/A:1007053129686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould TJ, Bickford P. Aged-related deficits in the cerebellar β adrenergic signal transduction cascade in Fischer 344 rats. J Pharmacol Exp Ther. 1997;281:965–971. [PubMed] [Google Scholar]
- 14.Gould TJ, Chadman K, Bickford PC. Antioxidant protection of cerebellar β-adrenergic receptor function in aged F344 rats. Neurosci Lett. 1998;250:165–168. doi: 10.1016/s0304-3940(98)00477-7. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford UP; Oxford: 1999. [Google Scholar]
- 16.Han SN, Meydani SN. Antioxidant, cytokines, and influenza infection in aged mice and elderly humans. J Infect Dis. 2000;182:S74–S80. doi: 10.1086/315915. [DOI] [PubMed] [Google Scholar]
- 17.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:289–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Haskoó G, Németh ZH, Szabó C, Zsilla G, Salzman A, Vizi ES. Isoproterenol inhibits IL-10, TNF-α, and nitric oxide production in RAW 264.7 macrophages. Brain Res Bull. 1998;45:183–187. doi: 10.1016/s0361-9230(97)00337-7. [DOI] [PubMed] [Google Scholar]
- 19.Heron C, Gould TJ, Bickford P. Acquisition of a runway motor learning task is impaired by a β-adrenergic antagonist in F344 rats. Behav Brain Res. 1996;78:235–241. doi: 10.1016/0166-4328(95)00252-9. [DOI] [PubMed] [Google Scholar]
- 20.Hirata T, Tanaka M, Ooike M, Tsunomura T, Sakaguchi M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J Appl Phycol. 2000;12:435–439. [Google Scholar]
- 21.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford P. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-α after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- 23.Leibovitz BE, Siegel BV. Aspects of free radical reactions in biological systems: aging. J Gerontol. 1980;35:45–56. doi: 10.1093/geronj/35.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1β? Prog Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 25.Pinero Estrada JE, Bermejo Bescos P, Villar del Fresno AM. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco. 2001;56:497–500. doi: 10.1016/s0014-827x(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 26.Romay C, Armesto J, Gonzales R, Ledon N, Garcia I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 27.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, α-tocopherol, or both as treatment for Alzheimer‘s disease. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 28.Willet CW, Sacks F, Trichopoulou A, Drescher G, Ferro-Luizz A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Kim YK, Govindarajan A, Baba A, Binnie M, Ranieri VM, Liu M, Slutsky AS. Effect of adrenoreceptors on endotoxin-induced cytokines and lipid peroxidation in lung explants. Am J Respir Crit Care Med. 1999;160:1703–1710. doi: 10.1164/ajrccm.160.5.9903068. [DOI] [PubMed] [Google Scholar]