Abstract
It is known that leptin, an adipocyte-derived hormone, exerts a stimulatory effect on growth hormone (GH) secretion in various animal species. However, no previous study examined in vivowhether leptin affects the secretion of GH-releasing factor (GRF), somatostatin (SRIH), and some other closely relevant neurohormones in the hypothalamus. Therefore, in this study we investigated the effects of direct leptin infusion into the hypothalamus on the in vivo release of GRF, SRIH, α-melanocyte-stimulating hormone (α-MSH), and neuropeptide Y (NPY) in freely moving adult male rats using the push–pull perfusion. Leptin was infused into the median eminence–arcuate nucleus complex at three different concentrations, i.e., 1.0 (normal feeding level), 3.0, and 10 ng/ml (mild obesity level). In normally fed rats, only 10 ng/ml leptin was able to stimulate GH secretion, whereas in 3 d fasted rats, GH release was dose-dependently stimulated by 1.0 and 3.0 ng/ml leptin, although its 10 ng/ml dose did not produce additional effects. The facilitation of GH secretion occurred as increased pulse amplitudes without significant changes in the pulse frequency. During the leptin infusion, the hypothalamic GRF increased and SRIH decreased in magnitudes that approximately paralleled those of GH changes. Leptin stimulated the release of α-MSH in the fasted but not fed rats. It is likely that the fasting-induced increase in the hypothalamic α-MSH sensitivity to leptin is relevant to ingestive behavior involving leptin. Leptin was without effect on NPY release in either the fed or fasted group. Although it is certain that NPY mediates at least part of the metabolic actions of leptin, NPY is unlikely to be involved in the acute effects of leptin on GH, GRF, and SRIH secretion. These results demonstrate for the first time that leptin can alter the in vivo release of both GRF and SRIH in rat hypothalamus concurrently with the stimulation of GH secretion.
Keywords: leptin, growth hormone, growth hormone-releasing factor, somatostatin, α-melanocyte-stimulating hormone, neuropeptide Y, arcuate nucleus, median eminence, push–pull perfusion
It is well known that alterations in nutritional states markedly influence growth hormone (GH) secretion in both experimental animals and humans. Such disturbance of GH secretion appears to develop as a consequence of altered metabolic conditions, because normal GH secretion can be reinstated after weight reduction in obesity and after refeeding in undernourished conditions (Dieguez and Casanueva, 1995). However, the mechanisms whereby nutritional factors affect GH secretion had mostly been elusive until the recent discovery of leptin, the adipocyte-derived hormone (Zhang et al., 1994). In addition to playing an important role in energy homeostasis, leptin is also known to affect the secretion of various pituitary hormones, including GH (Casanueva and Dieguez, 1999; Ahima et al., 2000).
It has been reported that leptin stimulates the basal and GH-releasing factor (GRF)-induced GH secretion in rats (Carro et al., 1997, 2000;Tannenbaum et al., 1998; Vuagnat et al., 1998). Several studiesin vivo and in vitro suggested that these facilitatory actions of leptin may be mediated by GRF and somatostatin (SRIH), both of which represent principal hypothalamic peptides participating in the neuroendocrine regulation of GH secretion (Quintela et al., 1997; LaPaglia et al., 1998; Carro et al., 1999; Cocchi et al., 1999). On the other hand, it has also been reported that leptin acts directly on the pituitary to modulate GH release in a quite complex manner (Barb et al., 1998; Roh et al., 1998,2001; Shimon et al., 1998; Cocchi et al., 1999; Chen et al., 2001;Korbonits et al., 2001). Neuropeptide Y (NPY) and α-melanocyte-stimulating hormone (α-MSH), the latter of which is a proopiomelanocortin (POMC)-derived peptide, have diverse biological functions in the brain and periphery. In terms of the influence on ingestive behavior, NPY and α-MSH exert orexigenic and anorectic effects, respectively. Abundant data suggest that both peptides serve significant roles in mediating the metabolic and neuroendocrine actions of leptin (Casanueva and Dieguez, 1999; Kalra et al., 1999; Ahima et al., 2000).
Despite these accumulating data implicating the roles of GRF, SRIH, NPY, and α-MSH in mediating the biological functions of leptin, no previous study demonstrated that leptin actually regulates the release of these peptides in the hypothalamus in vivo. To address this important issue, in the present study we examined the effects of direct leptin infusion into the median eminence–arcuate nucleus (ME–ARC) complex on the release of GRF, SRIH, NPY, and α-MSH at this site, and also of plasma GH, using the push–pull perfusion (PPP) technique as in our previous studies (Watanobe and Takebe, 1993a,b,1994). We also compared the hormonal effects of leptin infusion between fed and fasted rats. This attempt was made on the basis of previous reports that the neuroendocrine GH axis showed differential responses to exogenous leptin in fed versus food-restricted animals (Carro et al., 1997; Barb et al., 1998; Tannenbaum et al., 1998; Vuagnat et al., 1998; Henry et al., 1999, 2001; Lado-Abeal et al., 2000;Nagatani et al., 2000; Morrison et al., 2001).
MATERIALS AND METHODS
Animals and PPP protocol. All of the following procedures were approved by the Ethical Committee for Animal Experimentation of the International University of Health and Welfare. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Male rats (240–260) of the Wistar strain were used. They were housed in an air-conditioned room with controlled lighting (light on from 8 A.M. to 8 P.M.) and were given ad libitum access to laboratory chow and tap water. Two weeks before PPP, a guide cannula with a removable inner stylet was stereotaxically implanted in the ME–ARC complex under anesthesia with sodium pentobarbital (40 mg/kg body weight, i.p.). Stereotaxic coordinates for the cannula placement were taken from the atlas of Pellegrino et al. (1979), and they were 0.0 mm anterior to and 0.5 mm lateral to the bregma, and 9.8 mm ventral from the dura. The PPP cannulas used were the same as described in our previous studies (Watanobe and Takebe, 1993a,b, 1994). The device was fixed onto the skull with anchor screws and dental cement. Seven to 10 d after the PPP cannula placement, the body weight of every animal returned to the presurgical level. The animals were divided into two subsets. One subset was allowed to feed ad libitum (fed group), and the other subset was deprived of food for 3 d (fasted group) until the day of PPP. Two days before PPP, all animals were implanted with a jugular vein catheter filled with heparin solution under light ether anesthesia.
At ∼7 A.M. on the day of PPP, an extension of the jugular vein catheter was installed for frequent blood sampling, and the inner stylet within the guide cannula was replaced with the inner cannula perfusion assembly. Thereafter, artificial CSF (aCSF) with the same composition as in our previous reports (Watanobe and Takebe, 1993a,b,1994) was infused through the push cannula and collected from the pull cannula at a flow rate of 15 μl/min. The dead space of the pull system (from the tip of the guide cannula to the distal end of the pull tubing) was adjusted to 225 μl (corresponding to a 15-min period of perfusion). Until the experiment was over, not only the fasted but also the fed groups were deprived of food, although they were given ad libitum access to tap water. After a 2-hr equilibration period, blood samples (150 μl) to measure GH were collected from the freely moving animals every 15 min between 9 A.M. and 7 P.M. Only at 9 A.M., an additional 50 μl of blood was drawn to also measure leptin. The blood was collected in tubes containing EDTA-2Na (2.5 mg/ml blood) and immediately centrifuged, and the plasma was stored at –70°C until assayed for GH and leptin. The red blood cells were resuspended in 0.9% NaCl and returned to the animal after removal of the next blood sample. Perfusion fractions (450 μl) were collected every 30 min over a total period of 630 min (9 A.M. to 7:30 P.M.). The reason for collecting a perfusate also between 7 and 7:30 P.M. is the existence of the above-mentioned dead space within the pull system. Both the fed and the fasted groups were perfused with 1.0, 3.0, or 10 ng/ml recombinant rat leptin (R & D Systems, Minneapolis, MN) from 2 to 7:30 P.M. The rat leptin was dissolved in the aCSF immediately before use. Control groups were perfused with the pure aCSF from 9 A.M. to 7:30 P.M. The actual time of day during which leptin was infused was between 1:45 and 7:15 P.M., because the dead space of the push system (from the tip of the push cannula to the distal end of the push tubing) was adjusted to 225 μl (corresponding to a 15 min period of perfusion). The perfusates were immediately frozen on dry ice, lyophilized, and stored at –70°C until assayed for GRF, SRIH, α-MSH, and NPY. Within 30 min after completion of the experiment, the animals were killed by decapitation, and their brains were removed and stored at –70°C for histological examination.
Hormone assays. The lyophilized perfusates were reconstituted with 450 μl of an assay buffer (0.1% bovine serum albumin, 100 mm PBS, 0.1% sodium azide, and 0.1% Triton X-100, pH 7.4) and subjected to radioimmunoassays (RIAs) for GRF, SRIH, α-MSH, and NPY. A 100 μl aliquot was applied to each assay. All these neuropeptides were measured using specific RIA kits purchased from Peninsula Laboratories, Inc. (San Carlos, CA). The sensitivities of these assays (expressed per tube) were 1.0 pg for both GRF and SRIH, 0.5 pg for α-MSH, and 10 pg for NPY. These four peptides were also measured in reconstituted lyophilizates from blank perfusates (five samples per rat) containing 450 μl of the pure aCSF, and their mean values were subtracted from the levels in all the actual perfusates from every animal. NPY was detectable in all actual perfusates from every animal, but the other three neuropeptides were sometimes undetectable (in fewer than three samples in one rat). By convention, such samples that contained undetectable levels of the peptides were allotted the sensitivity thresholds of the respective assays for calculation. GRF, SRIH, α-MSH, and NPY did not cross-react with each other or with their respective related compounds. Plasma leptin concentrations were measured by a rat leptin ELISA kit (Morinaga Institute of Biological Sciences, Yokohama, Japan), and its sensitivity was 0.2 ng/ml. GH levels were determined by RIA using reagents kindly donated by Dr. A. F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases). Rat GH-RP-2 was used as the standard, and the sensitivity of the GH assay was 1.0 ng/ml. For all the hypothalamic and plasma hormones, samples from individual rats were analyzed within the same assay. In these six hormone assays, both intra-assay and interassay coefficients of variation were <10%.
Histology. Histological examination of the PPP cannula placement was done in the same manner as we have reported previously (Watanobe and Takebe, 1993a). Only animals that had the tip of the push cannula within the ME–ARC region were allowed to contribute to the data given in Results.
Statistical analyses. To determine whether observed temporal fluctuations in plasma GH and perfusate peptide levels constituted endogenous pulses, results were analyzed by the cluster analysis method (Veldhuis and Johnson, 1986). A t statistic of 2.0 was selected to maintain a maximal false-positive rate of ≤2.5%, by using cluster sizes of one or two in the nadir and peak. Results were expressed as the mean ± SEM. For the purpose of detecting significant alterations within groups, data of individual experimental groups were analyzed by two-way ANOVA with repeated measures. One-way ANOVA was used to compare data among different groups. When significantF values were obtained, a Bonferroni multiple comparisons test was performed. Differences were considered significant atp < 0.05.
RESULTS
The plasma leptin concentrations in the fed and fasted groups were 1.32 ± 0.05 (n = 39) and 0.28 ± 0.01 ng/ml (n = 34), respectively. These results were consistent with our previous data in male rats determined under the two different nutritional conditions (Watanobe and Suda, 1999; Watanobe et al., 2000). In this study, we perfused the ME–ARC region with three different concentrations of leptin (1.0, 3.0, and 10 ng/ml). The lowest concentration was chosen as the one that mimics the circulating leptin levels in normally fed male rats (Watanobe and Suda, 1999; Watanobe et al., 2000). The highest concentration is comparable with that found in mild obesity. Circulating leptin concentrations in normally fed adult Otsuka–Long–Evans–Tokushima fatty rats, a genetically obese rat strain exhibiting non-insulin-dependent diabetes mellitus and mild obesity, were reported to be 8.6 ± 0.9 and 9.7 ± 1.8 ng/ml in males and females, respectively (Shimizu et al., 1998; Watanobe et al., 2001). The middle concentration (3.0 ng/ml) of leptin infused was set between this mild obesity level and that in normally fed male rats.
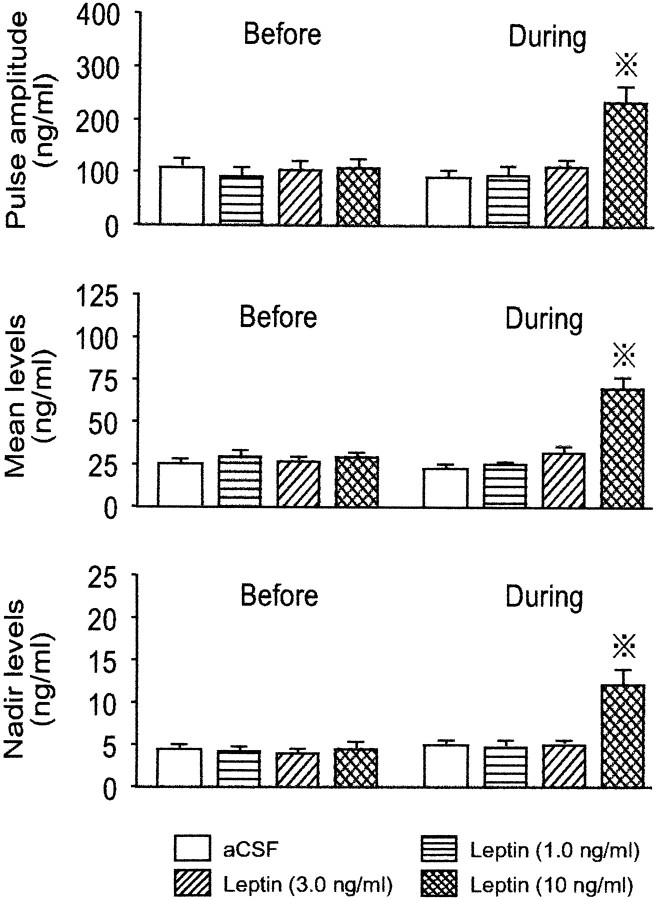
Figure 1 shows representative profiles of plasma GH in four fed rats that underwent different treatments and group average data of GRF, SRIH, α-MSH, and NPY in perfusates from the four fed groups. In all animals, pulsatile GH secretion with two major peaks was observed during each of the first (9 A.M. to 2 P.M.) and second (2–7 P.M.) periods of perfusion. In the control animal that received the vehicle only throughout the experiment, no apparent difference was observed in either the amplitude or frequency of GH pulses between the first and second periods of sampling. This lack of alterations in the GH pulse parameters was also true of the other two rats that received 1.0 or 3.0 ng/ml leptin, respectively. In contrast, the infusion of 10 ng/ml leptin caused an apparent increase in the GH pulse amplitude, albeit without changing the pulse frequency. Analysis of the group data revealed that this effect of leptin was significant (Fig. 2). The GH pulse amplitude was not affected by 1.0 or 3.0 ng/ml leptin but was significantly increased by its 10 ng/ml dose to an approximately twofold higher value (p < 0.05) than those in the remaining three groups. Similarly, the mean and nadir GH levels during the perfusion of 10 ng/ml leptin were 2–2.5 times higher (p < 0.02–0.05) than those in the remaining three groups. However, the GH pulse frequency, interpulse interval, and pulse width were not significantly affected even by the highest concentration of leptin (data not shown).
Fig. 1.
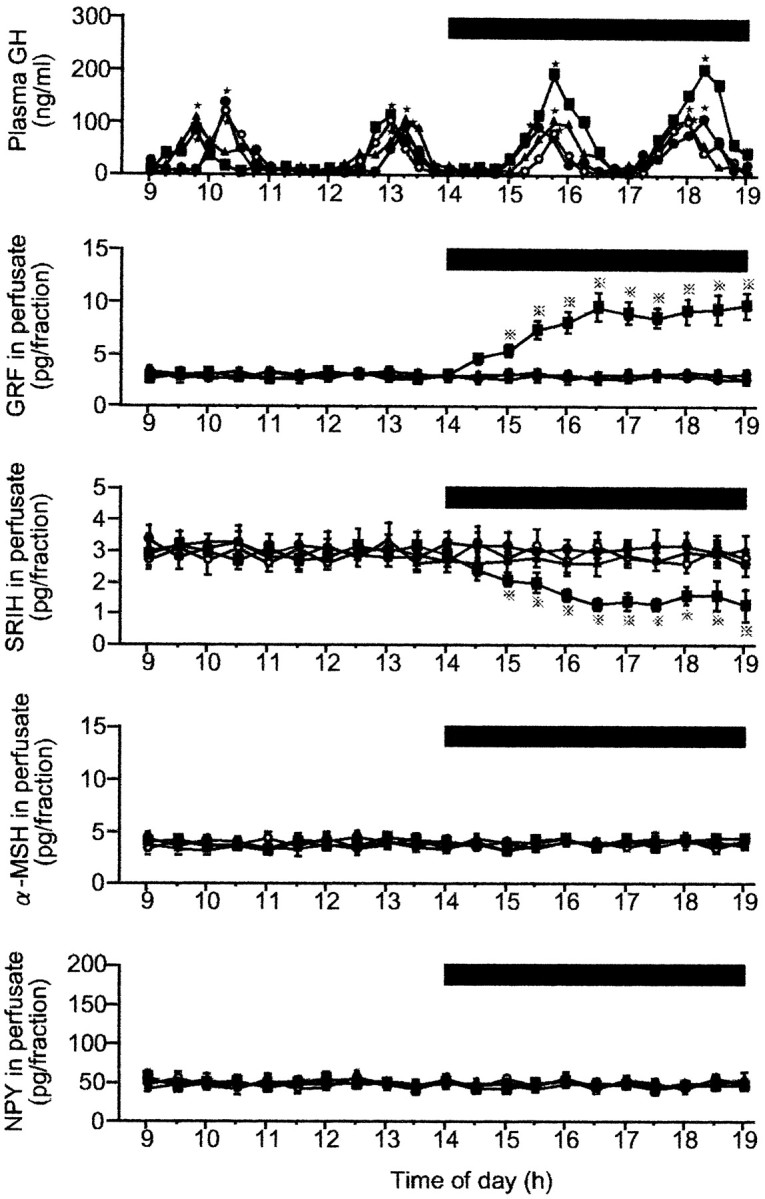
Representative profiles of plasma GH in four fed male rats and group data of neurohormones in the ME–ARC perfusates in the four fed groups before and during the leptin infusion. The number of animals in each group was 9–11. In this figure and Figure 3, (1) the time of the perfusate collection for neuropeptide assays is shifted 15 min ahead of the actual time of perfusion, because the dead space of the pull system (225 μl) corresponds to a 15 min period of perfusion (flow rate, 15 μl/min); (2) data of the four neuropeptides in perfusates are expressed as point values at the center of their collection periods; and (3) where SE values are not shown, they were smaller than the symbols. Black bar, Period during which leptin or aCSF (vehicle) was infused; filled squares, leptin (10 ng/ml); filled triangles, leptin (3.0 ng/ml);filled circles, leptin (1.0 ng/ml); open circles, aCSF (control); stars, significant GH pulses as detected by Cluster analysis; dotted crosses, statistically significant versus the other three groups.
Fig. 2.
Characteristics of GH secretion before and during the leptin infusion into the ME–ARC region of fed male rats. The number of animals in each group was 9–11. Dotted crosses, Statistically significant versus theBefore value of the same group and also theDuring values of the other three groups.
With regard to the release of the neuropeptides in the ME–ARC region, the infusion of the vehicle or 1.0 or 3.0 ng/ml leptin did not cause any significant change in any of the four peptides (GRF, SRIH, α-MSH, and NPY) during the entire period of observation. In contrast, as in the case of GH secretion, 10 ng/ml leptin induced a significant increase or decrease in GRF or SRIH, respectively. Compared with the values in the vehicle-infused group, GRF and SRIH started to significantly increase or decrease, respectively, on and from 3 P.M. (60 min after the commencement of the leptin infusion). Thereafter, the levels of GRF and SRIH were gradually increased or decreased, respectively, up to 4:30 P.M., after which almost consistent levels were maintained for both peptides until the perfusion was over. The outputs of α-MSH and NPY were not significantly affected even by the highest concentration of leptin. Cluster analysis in any individual animal did not reveal any significant pulsatile release of any of the four neuropeptides throughout the sampling period (Fig. 1).
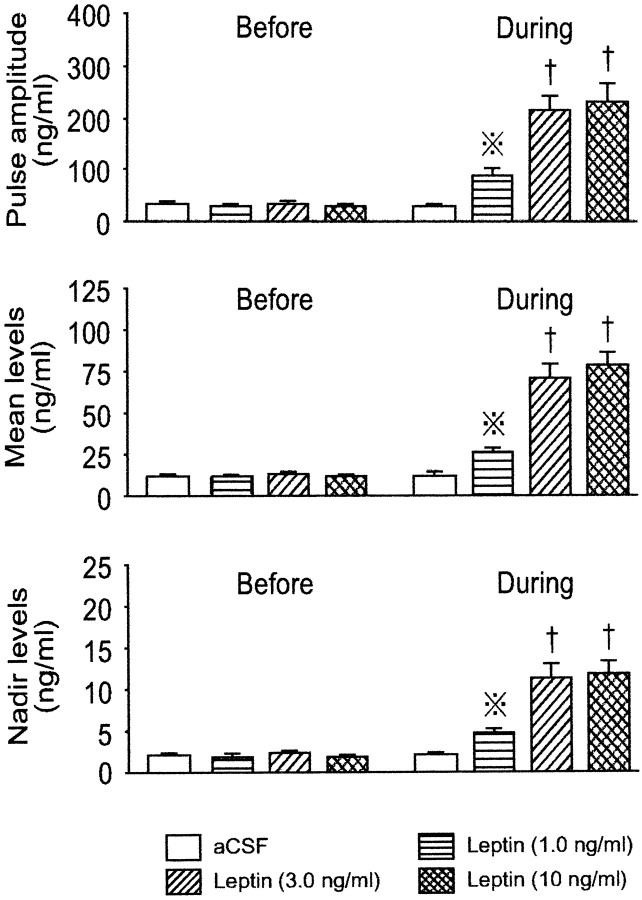
Figure 3 shows representative profiles of plasma GH in four fasted rats that underwent different infusions, and group average data of the hypothalamic peptides in perfusates from the four fasted groups. Cluster analysis disclosed that all four animals exhibited pulsatile GH secretion with two major peaks during each of the first and second periods of sampling. In the control animal that was perfused with the vehicle only, GH pulses with similar amplitude and frequency persisted throughout the experiment, although the pulse amplitude was markedly lower than that in the fed controls (Figs. 1,2). Interestingly, the administration of leptin to fasted rats, irrespective of its concentration, resulted in augmented amplitudes of GH pulses without affecting the pulse frequency. When evaluated as group data (Fig. 4), the 1.0 and 3.0 ng/ml concentrations of leptin dose-dependently increased the GH pulse amplitude, but this parameter was not further elevated by 10 ng/ml leptin. Similar intergroup differences were also observed for the mean and nadir levels of plasma GH. However, as in the case of fed animals, the GH pulse frequency, interpulse interval, and pulse width were not significantly altered by any concentration of leptin infused (data not shown).
Fig. 3.
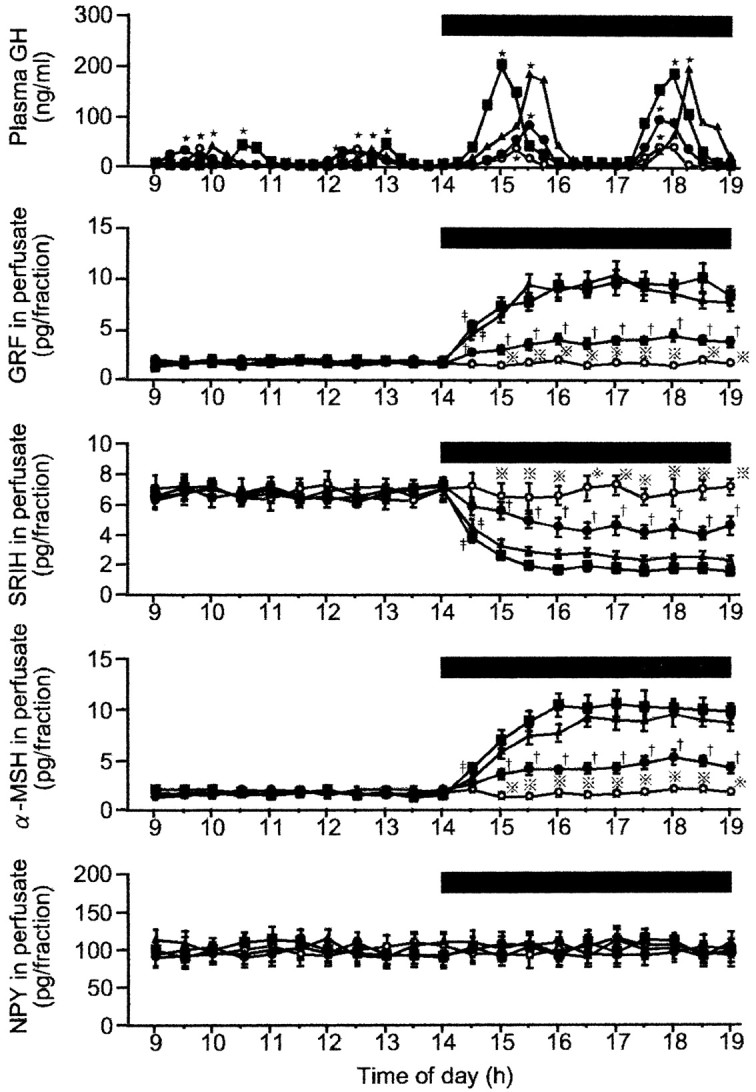
Representative profiles of plasma GH in four fasted male rats and group data of neurohormones in the ME–ARC perfusates in the four fasted groups before and during the leptin infusion. The number of animals in each group was 7–10. Black bar, Period during which leptin or aCSF (vehicle) was infused;filled squares, leptin (10 ng/ml); filled triangles, leptin (3.0 ng/ml); filled circles, leptin (1.0 ng/ml); open circles, aCSF (control);dotted crosses, statistically significant versus the other three groups; single daggers, statistically significant versus the leptin (3.0 ng/ml) and leptin (10 ng/ml) groups;double daggers, statistically significant versus the aCSF group.
Fig. 4.
Characteristics of GH secretion before and during the leptin infusion into the ME–ARC region of fasted male rats. The number of animals in each group was 7–10. Dotted crosses, Statistically significant versus theBefore value of the same group and also theDuring values of the other three groups; single daggers, statistically significant versus theBefore values of the respective groups and also theDuring values of the aCSF and leptin (1.0 ng/ml) groups.
With respect to the neuropeptides in the ME–ARC perfusates, it may be worth noting that fasting augmented the responsiveness of GRF and SRIH to leptin. In analogy with its effect on GH secretion in fasted rats, 1.0 ng/ml leptin was already effective to alter the secretion of both GRF and SRIH with an increase or a decrease, respectively. These changes in GRF and SRIH were further augmented by the 3.0 and 10 ng/ml doses of leptin, but the influences of these two concentrations were of a similar magnitude. In addition, as a finding observed only in the fasted animals, leptin was also able to stimulate the release of α-MSH, which showed a dose-dependent response to leptin as with GRF and SRIH. The lack of change in NPY outputs during the leptin infusion was the same as in the fed animals. Cluster analysis in every animal did not detect any significant pulsatile secretion of any of the four neuropeptides throughout the experiment (Fig. 3).
A comparison of the basal outputs of the four neurohormones between the fed and the fasted animals suggested that both GRF and α-MSH were approximately twofold higher in the fed rats, and conversely, both SRIH and NPY were approximately twofold higher in the fasted animals. When all the individual rat data from 9 A.M. to 2 P.M. were subjected to a statistical analysis, these intergroup differences proved to be statistically significant (p < 0.05).
DISCUSSION
In this study, it is worth noting that the fed and the fasted rats showed a clear difference in the neuroendocrine GH axis responses to leptin. Compared with the fed rats, the fasted animals were more sensitive to leptin, with larger changes in GH, GRF, and SRIH secretion. This may be in accord with the previous data that fasting increases leptin receptor concentrations in the ARC at both its mRNA and protein levels (Baskin et al., 1998, 1999a). The present findings that GH secretion was augmented above normal by the supraphysiological concentrations of leptin in both the fed and the fasted rats appear to be consistent with three recent studies in sheep (Nagatani et al., 2000; Henry et al., 2001; Morrison et al., 2001). Because GH secretion in sheep is known to increase on food restriction in contrast to decreased GH levels in undernourished rodents (Gluckman et al., 1987;Thissen et al., 1994), all these data from sheep and rats allow us to suggest that leptin actions on the neuroendocrine GH axis may be basically stimulatory. The other two studies in rats (Tannenbaum et al., 1998) and pigs (Barb et al., 1998) also support this possibility.
With respect to the basal release of GRF, SRIH, α-MSH, and NPY in the ME–ARC region, we found that the fed rats had approximately twofold higher levels of GRF and α-MSH and ∼50% lower outputs of SRIH and NPY than the fasted animals, with all these differences reaching statistical significance. These results are consistent with previous studies that examined the effects of fasting on the synthesis of these hypothalamic peptides (Bruno et al., 1990; Stephens et al., 1995;Schwartz et al., 1996, 1997, 1998; Ishikawa et al., 1997; Thornton et al., 1997; Mizuno et al., 1998). Several previous studies in vivo and in vitro implicated a significant participation of both GRF and SRIH in the leptin-stimulated GH secretion in rats (Quintela et al., 1997; LaPaglia et al., 1998; Carro et al., 1999; Cocchi et al., 1999). In support of these reports, we found in the present study that the enhanced GH release during leptin infusion was clearly associated with a concomitant increase or decrease in GRF or SRIH, respectively, in both the fed and fasted rats. These effects of leptin on the neuropeptide release may be in agreement with the neuroanatomical evidence that the ME–ARC region contains a high concentration of leptin receptors at both its gene and protein levels (Mercer et al., 1996; Schwartz et al., 1996; Fei et al., 1997; Elmquist et al., 1998; Hakansson et al., 1998). Because most cell bodies and nerve endings of GRF neurons are located in the ARC and the ME, respectively, and a large part of SRIH-containing axon terminals exists in the ME (Lantos et al., 1995), our present results suggest that leptin may directly act on both GRF and SRIH neurons to modulate the release of these neurohormones. This may be in agreement with the immunohistochemical evidence that at least part of both GRF and SRIH neurons express leptin receptors (Hakansson et al., 1998; Iqbal et al., 2000b). Because leptin is a blood-borne peptide like many other hormones in the general circulation, the present data that leptin can directly act in the ME–ARC region may be of physiological significance from a teleological point of view. Because the ME is one of the structures called the circumventricular organs that lack the blood–brain barrier (Broadwell and Brightman, 1976), our present results make it plausible that circulating leptin may enter the brain through the ME, bind directly to its receptors in the ME–ARC region, and subsequently influence the release of GRF and SRIH.
It is interesting to note that in the fasted rats, leptin infusion also caused a significant release of α-MSH in the ME–ARC region, although this was not the case with the fed animals. These results are in agreement with a recent study in vitro by Kim et al. (2000)that leptin increased α-MSH release from hypothalamic explants from fasted rats but did not do so in the tissue from fed animals. The leptin-stimulated α-MSH release seems to be consistent with the reports that the ARC POMC neurons abundantly express leptin receptors (Cheung et al., 1997; Finn et al., 1998) and also that leptin upregulates POMC mRNA levels in the ARC (Schwartz et al., 1997;Thornton et al., 1997; Mizuno et al., 1998; Cowley et al., 2001). We found that α-MSH, GRF, and SRIH all showed dose-dependent alterations of similar magnitudes to increasing concentrations of leptin. However, because of the following reasons, we think it unlikely that the stimulated release of α-MSH was causally related to the concurrent changes in GH, GRF, or SRIH. First, in the fed animals 10 ng/ml leptin was without effect on α-MSH, although this treatment exerted significant influences on the release of GH, GRF, and SRIH. Second,Koegler et al. (2001) recently reported that fasting decreases the gene expression of hypothalamic POMC in the monkey, which is a species exhibiting enhanced but not depressed GH secretion on food deprivation (Gluckman et al., 1987; Thissen et al., 1994). Because our finding that leptin increased α-MSH release only in the fasted rats seems to agree with the fasting-induced increase in the ARC sensitivity to leptin (Baskin et al., 1998, 1999a), it is more likely that the enhanced secretion of the anorectic α-MSH from the ARC is associated with feeding behavior. Indeed, the ARC is known to play a crucial role in the hypothalamic regulation of food intake and energy balance through synthesizing and integrating a number of appetite-regulating factors (Kalra et al., 1999), including the orexigenic NPY and agouti-related peptide (Hahn et al., 1998) and the anorectic α-MSH and cocaine- and amphetamine-regulated transcript (Elias et al., 1998).
Several previous studies suggested that NPY may mediate at least part of the leptin stimulatory effects on GH secretion in rats (Stephens et al., 1995; Schwartz et al., 1996, 1998; Quintela et al., 1997; Carro et al., 1998; Giustina and Veldhuis, 1998; Vuagnat et al., 1998; Cocchi et al., 1999). We thus hypothesized that leptin infusion would alter NPY release in the ME–ARC region if leptin could stimulate GH secretion. However, we found that the leptin-induced stimulation of GH secretion was not associated with a concomitant change in NPY secretion in either the fed or the fasted rats. The significant difference in the basal NPY release between these two nutritional states that we observed may endorse the sufficient sensitivity of our PPP method, which thus lends credence to the negative NPY data during leptin infusion. Therefore, our present results may suggest that leptin does not exert any acute effects on hypothalamic NPY release, and NPY is unlikely to mediate the acute effects of leptin on GRF, SRIH, and GH secretion, although leptin is known to modulate the NPY neuronal activity and its gene expression in a more chronic manner (Stephens et al., 1995; Schwartz et al., 1996,1998; Baskin et al., 1999b). Our data seem to be consistent with findings in recent in vitro studies in normal mice and rats that leptin does not acutely affect NPY release from the hypothalamus (Jang et al., 2000; King et al., 2000). In addition, the findings from NPY-deficient mice that these animals normally respond to exogenous leptin indicate that the ARC NPY neurons are not the sole target of leptin (Erickson et al., 1996, 1997).
The existence of leptin receptors in the pituitary gland, most importantly its functioning long form, has been reported by several studies (Zamorano et al., 1997; Shimon et al., 1998; Cai and Hyde, 1999; Jin et al., 1999, 2000; Iqbal et al., 2000a; Lin et al., 2000). Furthermore, it was demonstrated that leptin receptors are most abundantly expressed in somatotrophs among various cell populations in the pituitary (Cai and Hyde, 1999; Iqbal et al., 2000a). It is thus possible that part of the changes in GH secretion we observed resulted from intrapituitary actions of leptin diffusing from the hypothalamus.
In summary, in this study we obtained the first in vivoevidence that leptin can alter the secretion of both GRF and SRIH in the ME–ARC region with an increase or a decrease, respectively, in association with concomitant stimulation of GH secretion. The sensitivity of these hormonal responses to leptin was higher in the fasted than in the fed rats. Leptin also stimulated α-MSH release in the ME–ARC region in the fasted but not fed, rats. Unexpectedly, NPY release in the ME–ARC region was not significantly affected by leptin in either the fed or fasted rats, which suggests that NPY may not mediate the acute effects of leptin on GRF, SRIH, or GH. Inasmuch as supraphysiological concentrations of leptin stimulated GH secretion even surpassing the levels in normally fed rats, we suggest that the influence of leptin on the neuroendocrine GH axis may be basically stimulatory.
Footnotes
This study was supported in part by Japan Society for the Promotion of Science Grant-in-Aid 12671072 and grants-in-aid from the Foundation for Growth Science and the International University of Health and Welfare. We thank the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases and Dr. A. F. Parlow for the generous donation of reagents for rat growth hormone RIA.
Correspondence should be addressed to Dr. Hajime Watanobe, Division of Internal Medicine, Clinical Research Center, International University of Health and Welfare, 2600-1 Kitakanemaru, Otawara, Tochigi 324-8501, Japan. E-mail: watah@iuhw.ac.jp.
REFERENCES
- 1.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 2.Barb CR, Yan X, Azain MJ, Kraeling RR, Rampacek GB, Ramsay TG. Recombinant porcine leptin reduces feed intake and stimulates growth hormone secretion in swine. Domest Anim Endocrinol. 1998;15:77–86. doi: 10.1016/s0739-7240(97)00064-7. [DOI] [PubMed] [Google Scholar]
- 3.Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW. Increased expression of mRNA for the long from of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes. 1998;47:538–543. doi: 10.2337/diabetes.47.4.538. [DOI] [PubMed] [Google Scholar]
- 4.Baskin DG, Breininger JF, Bonigut S, Miller MA. Leptin binding in the arcuate nucleus is increased during fasting. Brain Res. 1999a;828:154–158. doi: 10.1016/s0006-8993(99)01252-4. [DOI] [PubMed] [Google Scholar]
- 5.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999b;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 6.Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976;166:257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- 7.Bruno JF, Olchovsky D, White JD, Leidy JW, Song J, Berelowitz M. Influence of food deprivation in the rat on hypothalamic expression of growth hormone-releasing factor and somatostatin. Endocrinology. 1990;127:2111–2116. doi: 10.1210/endo-127-5-2111. [DOI] [PubMed] [Google Scholar]
- 8.Cai A, Hyde JF. The human growth hormone-releasing hormone transgenic mouse as a model of modest obesity: differential changes in leptin receptor (OBR) gene expression in the anterior pituitary and hypothalamus after fasting and OBR localization in somatotrophs. Endocrinology. 1999;140:3609–3614. doi: 10.1210/endo.140.8.6925. [DOI] [PubMed] [Google Scholar]
- 9.Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology. 1997;138:2203–2206. doi: 10.1210/endo.138.5.5238. [DOI] [PubMed] [Google Scholar]
- 10.Carro E, Seoane LM, Senaris R, Considine RV, Casanueva FF, Dieguez C. Interation between leptin and neuropeptide Y on in vivo growth hormone secretion. Neuroendocrinology. 1998;68:187–191. doi: 10.1159/000054365. [DOI] [PubMed] [Google Scholar]
- 11.Carro E, Senaris RM, Seoane LM, Frohman LA, Arimura A, Casanueva FF, Dieguez C. Role of growth hormone (GH)-releasing hormone and somatostatin on leptin-induced GH secretion. Neuroendocrinology. 1999;69:3–10. doi: 10.1159/000054397. [DOI] [PubMed] [Google Scholar]
- 12.Carro E, Seoane M, Senaris R, Casanueva FF, Dieguez C. Leptin increases in vivo GH responses to GHRH and GH-releasing peptide-6 in food-deprived rats. Eur J Endocrinol. 2000;142:66–70. doi: 10.1530/eje.0.1420066. [DOI] [PubMed] [Google Scholar]
- 13.Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20:317–363. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Roh SG, Nie GY, Loneragan K, Xu RW, Ruan M, Clarke LJ, Goding JW, Gertler A. The in vitro effect of leptin on growth hormone secretion from cultured ovine somatotrophs. Endocrine. 2001;14:73–78. doi: 10.1385/endo:14:1:073. [DOI] [PubMed] [Google Scholar]
- 15.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi D, De Gennaro Colonna V, Bagnasco M, Bonacci D, Muller EE. Leptin regulates GH secretion in the rat by acting on GHRH and somatostatinergic functions. J Endocrinol. 1999;162:95–99. doi: 10.1677/joe.0.1620095. [DOI] [PubMed] [Google Scholar]
- 17.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Dano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 18.Dieguez C, Casanueva FF. Influence of metabolic substrates and obesity on GH secretion. Trends Endocrinol Metab. 1995;6:55–59. doi: 10.1016/1043-2760(94)00206-j. [DOI] [PubMed] [Google Scholar]
- 19.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 20.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distribution of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 21.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 22.Erickson JC, Ahima RS, Hollopeter G, Flier JS, Palmiter RD. Endocrine function of neuropeptide Y knockout mice. Regul Pept. 1997;70:199–202. doi: 10.1016/s0167-0115(97)01007-0. [DOI] [PubMed] [Google Scholar]
- 23.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 25.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman PD, Breier BH, Davis SR. Physiology of the somatotropic axis with particular reference to the ruminant. J Dairy Sci. 1987;70:442–466. doi: 10.3168/jds.S0022-0302(87)80028-0. [DOI] [PubMed] [Google Scholar]
- 27.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 28.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansell A, Clarke IJ. Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function. Endocrinology. 1999;140:1175–1182. doi: 10.1210/endo.140.3.6604. [DOI] [PubMed] [Google Scholar]
- 30.Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Clarke IJ. Intracerebroventricular infusion of leptin elevates the secretion of luteinising hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of body weight. J Endocrinol. 2001;168:67–77. doi: 10.1677/joe.0.1680067. [DOI] [PubMed] [Google Scholar]
- 31.Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology. 2000a;141:1515–1520. doi: 10.1210/endo.141.4.7433. [DOI] [PubMed] [Google Scholar]
- 32.Iqbal J, Pompolo S, Murakami T, Clarke IJ. Localization of long-form leptin receptor in the somatostatin-containing neurons in the sheep hypothalamus. Brain Res. 2000b;887:1–6. doi: 10.1016/s0006-8993(00)02912-7. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa M, Mizobuchi M, Takahashi H, Bando H, Saito S. Somatostatin release as measured by in vivo microdialysis: circadian variation and effect of prolonged food deprivation. Brain Res. 1997;749:226–231. doi: 10.1016/S0006-8993(96)01163-8. [DOI] [PubMed] [Google Scholar]
- 34.Jang M, Mistry A, Swick A, Romsos DR. Leptin rapidly inhibits hypothalamic neuropeptide Y secretion and stimulates corticotropin-releasing hormone secretion in adrenalectomized mice. J Nutr. 2000;130:2813–2820. doi: 10.1093/jn/130.11.2813. [DOI] [PubMed] [Google Scholar]
- 35.Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- 37.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Small CJ, Stanley SA, Morgan DGA, Seal LJ, Kong WM, Edwards CMB, Abusnana S, Sunter D, Ghatei MA, Bloom SR. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effects of leptin. J Clin Invest. 2000;105:1005–1011. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King PJ, Widdowson PS, Doods H, Williams G. Regulation of neuropeptide Y release from hypothalamic slices by melanocortin-4 agonists and leptin. Peptides. 2000;21:45–48. doi: 10.1016/s0196-9781(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 40.Koegler FH, Grove KL, Schiffmacher A, Smith MS, Cameron JL. Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology. 2001;142:2586–2592. doi: 10.1210/endo.142.6.8198. [DOI] [PubMed] [Google Scholar]
- 41.Korbonits M, Chitnis MM, Gueorguier M, Norman D, Rosenfelder N, Suliman M, Jones TH, Fabbri KN, Besser GM, Burrin JM, Grossman AB. The release of leptin and its effect on hormone release from human pituitary adenomas. Clin Endocrinol (Oxf) 2001;54:781–789. doi: 10.1046/j.1365-2265.2001.01279.x. [DOI] [PubMed] [Google Scholar]
- 42.Lado-Abeal J, Hickox JR, Cheung TL, Veldhuis JD, Hardy DM, Norman RL. Neuroendocrine consequences of fasting in adult male macaques: effects of recombinant rhesus macaque leptin infusion. Neuroendocrinology. 2000;71:196–208. doi: 10.1159/000054537. [DOI] [PubMed] [Google Scholar]
- 43.Lantos TA, Gorcs TJ, Palkovits M. Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Res Brain Res Rev. 1995;20:209–249. doi: 10.1016/0165-0173(94)00013-f. [DOI] [PubMed] [Google Scholar]
- 44.LaPaglia N, Steiner J, Kirsteins L, Emanuele M, Emanuele N. Leptin alters the response of the growth hormone releasing factor-growth hormone-insulin-like growth factor-I axis to fasting. J Endocrinol. 1998;159:79–83. doi: 10.1677/joe.0.1590079. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Barb CR, Matteri RL, Kraeling RR, Chen X, Meinersmann RJ, Rampacek GB. Long form leptin receptor mRNA expression in the brain, pituitary, and other tissues in the pig. Domest Anim Endocrinol. 2000;19:53–61. doi: 10.1016/s0739-7240(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 46.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 47. Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 47 1998. 294 297 [Erratum (1998) 47:696] [DOI] [PubMed] [Google Scholar]
- 48.Morrison CD, Daniel JA, Holmberg BJ, Djiane J, Raver N, Gertler A, Keisler DH. Central infusion of leptin into well-fed and undernourished ewe lambs: effects on feed intake and serum concentrations of growth hormone and luteinizing hormone. J Endocrinol. 2001;168:317–324. doi: 10.1677/joe.0.1680317. [DOI] [PubMed] [Google Scholar]
- 49.Nagatani S, Zeng Y, Keisler DH, Foster DL, Jaffe CA. Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep. Endocrinology. 2000;141:3965–3975. doi: 10.1210/endo.141.11.7762. [DOI] [PubMed] [Google Scholar]
- 50.Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum; New York: 1979. [Google Scholar]
- 51.Quintela M, Senaris R, Heiman ML, Casanueva FF, Dieguez C. Leptin inhibits in vitro hypothalamic somatostatin secretion and somatostatin mRNA levels. Endocrinology. 1997;138:5641–5644. doi: 10.1210/endo.138.12.5713. [DOI] [PubMed] [Google Scholar]
- 52.Roh S, Clarke IJ, Xu R, Goding JW, Loneragan K, Chen C. The in vitro effect of leptin on basal and growth hormone-releasing hormone-stimulated growth hormone secretion from the ovine pituitary gland. Neuroendocrinology. 1998;68:361–364. doi: 10.1159/000054385. [DOI] [PubMed] [Google Scholar]
- 53.Roh S, Nie G, Loneragan K, Gertler A, Chen C. Direct modification of somatotrope function by long-term leptin treatment of primary cultured ovine pituitary cells. Endocrinology. 2001;142:5167–5171. doi: 10.1210/endo.142.12.8559. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology. 1998;139:2629–2635. doi: 10.1210/endo.139.5.6000. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu H, Ohtani KI, Uehara Y, Abe Y, Takahashi H, Tsuchiya T, Sato N, Ibuki Y, Mori M. Orchiectomy and response to testosterone in the development of obesity in young Otsuka-Long-Evans-Tokushima Fatty (OLETF) rats. Int J Obes Relat Metab Disord. 1998;22:318–324. doi: 10.1038/sj.ijo.0800586. [DOI] [PubMed] [Google Scholar]
- 58.Shimon I, Yan X, Magoff DA, Friedman TC, Melmed S. Intact leptin receptor is selectively expressed in human fetal pituitary and pituitary adenomas and signals human fetal pituitary growth hormone secretion. J Clin Endocrinol Metab. 1998;83:4059–4064. doi: 10.1210/jcem.83.11.5273. [DOI] [PubMed] [Google Scholar]
- 59.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 60.Tannenbaum GS, Gurd W, Lapointe M. Leptin is a potent stimulator of spontaneous pulsatile growth hormone (GH) secretion and the GH response to GH-releasing hormone. Endocrinology. 1998;139:3871–3875. doi: 10.1210/endo.139.9.6206. [DOI] [PubMed] [Google Scholar]
- 61.Thissen J, Ketelslegers J, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 62.Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 63.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 64.Vuagnat B, Pierroz D, Lalaoui M, Englaro P, Pralong F, Blum W, Aubert M. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology. 1998;67:291–300. doi: 10.1159/000054326. [DOI] [PubMed] [Google Scholar]
- 65.Watanobe H, Suda T. A detailed study on the role of sex steroid milieu in determining plasma leptin concentrations in adult male and female rats. Biochem Biophys Res Commun. 1999;259:56–59. doi: 10.1006/bbrc.1999.0718. [DOI] [PubMed] [Google Scholar]
- 66.Watanobe H, Takebe K. Intrahypothalamic perfusion with interleukin-1-beta stimulates the local release of corticotropin-releasing hormone and arginine vasopressin and the plasma adrenocorticotropin in freely moving rats: a comparative perfusion of the paraventricular nucleus and the median eminence. Neuroendocrinology. 1993a;57:593–599. doi: 10.1159/000126412. [DOI] [PubMed] [Google Scholar]
- 67.Watanobe H, Takebe K. In vivo release of neurotensin from the median eminence of ovariectomized estrogen-primed rats as estimated by push-pull perfusion: correlation with luteinizing hormone and prolactin surges. Neuroendocrinology. 1993b;57:760–764. doi: 10.1159/000126434. [DOI] [PubMed] [Google Scholar]
- 68.Watanobe H, Takebe K. Effects of intravenous administration of interleukin-1-beta on the release of prostaglandin E2, corticotropin-releasing factor, and arginine vasopressin in several hypothalamic areas of freely moving rats: estimation by push-pull perfusion. Neuroendocrinology. 1994;60:8–15. doi: 10.1159/000126714. [DOI] [PubMed] [Google Scholar]
- 69.Watanobe H, Schiöth HB, Suda T. Stimulation of prolactin secretion by chronic, but not acute, administration of leptin in the rat. Brain Res. 2000;887:426–431. doi: 10.1016/s0006-8993(00)03019-5. [DOI] [PubMed] [Google Scholar]
- 70.Watanobe H, Yoneda M, Kohsaka A, Kakizaki Y, Suda T, Schiöth HB. Normalization of circulating leptin levels by fasting improves the reproductive function in obese OLETF female rats. Neuropeptides. 2001;35:45–49. doi: 10.1054/npep.2000.0842. [DOI] [PubMed] [Google Scholar]
- 71.Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology. 1997;65:223–228. doi: 10.1159/000127276. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]