Abstract
Voltage-gated calcium channels play a key role in chemical synaptic transmission by providing the calcium trigger for regulated neurotransmitter release. Genes encoding the primary structural subunit, α1, as well as accessory subunits of presynaptic calcium channels have now been identified in a variety of organisms. Thecacophony (cac) gene in Drosophila, also known as nightblind A, encodes a voltage-gated calcium-channel α1 subunit homologous to vertebrate α1 subunits implicated in neurotransmitter release. A recent genetic screen in our laboratory isolated cacTS2, a conditional cac mutant exhibiting rapid paralysis at elevated temperatures. This mutant has allowed synaptic electrophysiology after acute perturbation of a specific calcium-channel gene product, demonstrating that cacencodes a primary calcium channel functioning in neurotransmitter release. Here we report the molecular lesion incacTS2, a missense mutation within a calcium-dependent regulatory domain of the α1 subunit, as well as phenotypic rescue of temperature-sensitive and lethalcac mutations by transgenic expression of a wild-typecac cDNA. Notably, rescue of rapid, calcium-triggered neurotransmitter release was achieved by neural expression of a single cDNA containing a subset of alternative exons and lacking any conserved synaptic-protein interaction sequence. Possible implications of these findings are discussed in the context of structure–function studies of synaptic calcium channels, as well as alternative splicing and mRNA editing of the cac transcript.
Keywords: synapse, calcium channel, Drosophila, temperature-sensitive, alternative splicing, SYNPRINT
Calcium channels implicated in neurotransmitter release are composed of a primary structural subunit, α1, as well as β, α2/δ, and possibly γ subunits (for review, see Catterall, 1998). The α1 subunit alone may form voltage-gated calcium channels in heterologous expression systems; however, coexpression of accessory subunits is thought to more closely reproduce the function and regulation of endogenous channels (for review, seeWalker and De Waard, 1998; Ikeda and Dunlap, 1999). The α1 subunit polypeptide is organized into four repeating domains, each including six transmembrane segments (Fig.1A). Vertebrate genes encoding primary α1 subunits functioning in neurotransmitter release include α1A (Cav2.1) and α1B (Cav2.2), which encode P/Q- and N-type channels, respectively. Both of these channel types have been localized to presynaptic terminals (for review, see Stanley, 1997; Catterall, 1998), and the pharmacology of these channels when expressed in heterologous systems is similar to that observed for neurotransmitter release.
Fig. 1.
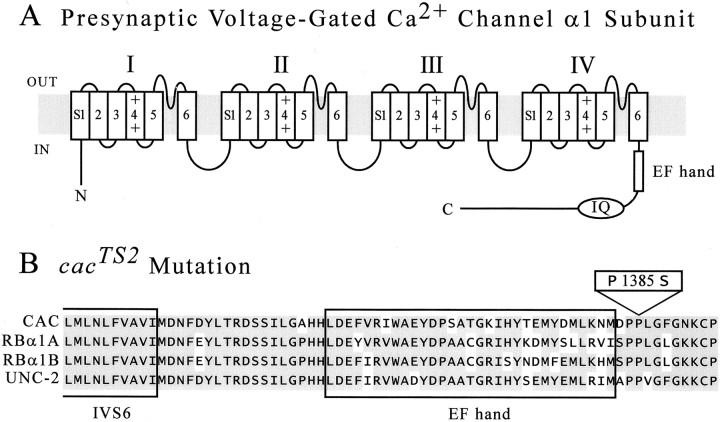
A missense mutation incacTS2. A, Presynaptic voltage-gated calcium-channel α1 subunit topology.Shading represents the plasma membrane. Roman numerals refer to the four repeating domains, each including six transmembrane segments (S1–S6). A charged S4 segment is thought to serve as a voltage sensor. An intramembranous “P loop” between the S5 and S6 segments of each repeat contributes to formation of the channel pore. Both the N and C termini of the α1 subunit are intracellular, and the four repeats are linked by three major intracellular loops. Also represented are calcium-binding (EF hand) and calmodulin-binding (IQ) domains within the C-terminal cytoplasmic tail. B, ThecacTS2 mutation. Alignment of CAC with related calcium-channel α1 subunit polypeptide sequences is shown. Amino acid identities with CAC are shaded. Boxed sequences correspond to the EF hand and a portion of the IVS6 transmembrane segment. The cacTS2mutation, P1385S, maps to an invariant proline residue adjacent to the EF hand. The aligned sequences correspond to CAC (U55776), rat brain α1A and α1B (M64373 and M92905, respectively), andCaenorhabditis elegans UNC-2 (U25119).
Synaptic calcium channel diversity
The structural diversity of presynaptic calcium channels is provided by different α1 subunit genes, as well as assembly of α1 with different combinations of accessory subunits. In contrast to the closely related α1 subunit genes encoding vertebrate presynaptic calcium channels, cacophony (cac) appears to be the only homologous gene in Drosophila (Smith et al., 1996;Littleton and Ganetzky, 2000). Fly genes encoding L-type (Gielow et al., 1995; Zheng et al., 1995; Ren et al., 1998) and T-type calcium channels, as well as each of the known accessory subunits, have been identified as well (Littleton and Ganetzky, 2000).
Another mechanism for generating presynaptic calcium-channel variants involves alternative splicing of α1 subunit mRNAs. This process acts on mammalian α1A and α1B transcripts to produce a variety of mRNAs encoding different α1 subunit proteins (Lin et al., 1997, 1999;Bourinet et al., 1999; Hans et al., 1999; Krovetz et al., 2000; Pan and Lipscombe, 2000). Similarly, alternative splicing of the cacα1 subunit mRNA contributes to calcium-channel diversity inDrosophila (Smith et al., 1996, 1998b; Peixoto et al., 1997). Several alternative exons have been described in cac, including two mutually exclusive exon pairs, IS4a/IS4b and I-IIa/I-IIb, as well as a 3, 6, or 9 bp insertion adding one to three amino acids to the IVS3–IVS4 loop (Smith et al., 1996; results reported here). The latter splicing site appears to be conserved in mammalian α1 subunit genes. At the equivalent position within the α1A and α1B transcripts, inclusion of a 6 bp alternative exon adds two amino acids to the IVS3–IVS4 loop and alters functional properties of the channels (Lin et al., 1997, 1999; Bourinet et al., 1999; Hans et al., 1999;Krovetz et al., 2000). Additional mRNA splicing variants ofcac are reported in the present study.
Structural diversity of synaptic calcium channels may also be generated through editing of α1 subunit mRNAs. A-to-I mRNA editing results in conversion of adenosine residues to inosine, which may behave as guanosine during translation (for review, see Reenan, 2001). Editing of the cac transcript by this mechanism alters thecac coding sequence at a number of positions (Smith et al., 1998a; Palladino et al., 2000) (see Figure 3); however, the functional consequences of this editing remain to be determined. To date, editing of calcium-channel transcripts has been described only inDrosophila; however, editing of other mammalian ion-channel mRNAs and its functional consequences have been reported previously (Higuchi et al., 2000; Reenan, 2001).
Fig. 3.

Diversity of cac head mRNA transcripts. Nucleotide and amino acid positions correspond to those of the cac cDNA sequence (U55776). Most variants were identified previously in the analysis of whole-body mRNA, with the following exceptions: *1, The variant lacking both I-IIa and I-IIb. *2, The C-terminal variable alternative exons. *3, Editing of nucleotide 936; however, this has been documented by others in recent analysis of whole body mRNA (see Discussion).
Mechanisms of calcium-channel regulation
The gating of presynaptic calcium channels is regulated by several mechanisms, including direct α1 subunit interactions with G-proteins, calcium/calmodulin, and components of the neurotransmitter release apparatus. Inhibition of neurotransmitter release by G-protein-linked receptor agonists occurs through direct interactions between the calcium-channel α1 subunit and βγ subunits of heterotrimeric G-proteins (De Waard et al., 1997; Dunlap, 1997; Zamponi et al., 1997; Mirotznik et al., 2000; Colecraft et al., 2001). Regulation by G-proteins is antagonized by protein kinase C-mediated phosphorylation of the α1 subunit (Zamponi et al., 1997; Herlitze et al., 2001), which has also been reported to increase basal calcium current (Yang and Tsien, 1993). Another regulatory mechanism involves direct binding of calcium/calmodulin to the IQ motif within the C-terminal cytoplasmic domain of the α1 subunit and is thought to mediate calcium-dependent channel gating, including facilitation and inactivation (Lee et al., 1999; DeMaria et al., 2001; Erickson et al., 2001). An EF hand calcium-binding motif within the same C-terminal region of the α1 subunit may also contribute to calcium-dependent inactivation (Peterson et al., 2000). Finally, interaction of presynaptic calcium-channel α1 subunits with syntaxin, a core protein of the neurotransmitter release apparatus, has been shown to regulate channel gating (Bezprozvanny et al., 1995, 2000;Degtiar et al., 2000) and also to promote regulation by G-proteins (Stanley, 1997; Jarvis and Zamponi, 2001; Lü et al., 2001). Binding of syntaxin and several other synaptic proteins to calcium channels led to identification of a synaptic-protein interaction (SYNPRINT) domain within the intracellular loop linking domains II and III of α1A (P/Q-type) and α1B (N-type) subunits (for review, seeSheng et al., 1998). This domain is proposed to mediate fast coupling of calcium influx to synaptic vesicle fusion by tethering calcium channels and the release apparatus and by participating in calcium-channel regulation (Mochida et al., 1996; Sheng et al., 1998;Wu et al., 1999; Zhong et al., 1999). Although our previous work has shown that cac-encoded calcium channels function in fast, calcium-triggered neurotransmitter release (Kawasaki et al., 2000), no sequence homologous to known calcium-channel synaptic-protein interaction domains is present in cac or elsewhere in the fly genome (Kawasaki et al., 2000; Littleton and Ganetzky, 2000). These findings suggest either a novel synaptic-protein interaction domain or an alternative mechanism for the fast coupling of calcium influx to synaptic vesicle fusion.
The central importance of presynaptic calcium channels has motivated genetic analysis to investigate the in vivo functions of specific calcium-channel proteins at native synapses (Schafer and Kenyon, 1995; Dove et al., 1998; Lorenzon et al., 1998; Jun et al., 1999; Saegusa et al., 2000; Ino et al., 2001). This presents several challenges, including the long-term compensatory changes that may occur in null or hypomorphic mutant animals (Jun et al., 1999; Saegusa et al., 2000; Ino et al., 2001). Thus, temperature-sensitive (TS) paralytic mutants of Drosophila provide an important and complementary tool allowing acute perturbation of specific gene products for analysis of the molecular mechanisms underlying physiological processes. Our previous genetic (Dellinger et al., 2000) and electrophysiological (Kawasaki et al., 2000) studies of the TS paralytic mutant cacTS2 demonstrated thatcac encodes a primary calcium-channel α1 subunit functioning in neurotransmitter release. Here we report characterization of the molecular lesion underlying the TS paralytic and synaptic phenotypes of cacTS2, as well as rescue of cac mutants by transgenic expression of a specific cac-encoded α1 subunit. These studies further characterize a TS paralytic calcium-channel mutant and further define the molecular determinants governing in vivo function of presynaptic calcium channels.
MATERIALS AND METHODS
Fly stocks
cacTS2 was from our laboratory stock collection. The cac lethal mutantsl(1)L1320-3 andl(1)L13HC129 were generously provided by Jeffrey C. Hall (Brandeis University, Waltham, MA). These were established in the following balanced lines:l(1)L1320-3/In(1)FM7i, y93j sc8w1 oc1ptg1 B1P{w+mC= ActGFP}JMR3 andl(1)L13HC129/In(1)FM7i, y93j sc8w1 oc1ptg1 B1P{w+mC= ActGFP}JMR3. The X-linked elav-GAL4 enhancer trap line, P(w+) elavC155, was obtained from the Bloomington Stock Center (Indiana University, Bloomington, IN). This driver will be referred to as elav-GAL4. elav-GAL4 cacTS2, elav-GAL4 l(1)L1320-3, and elav-GAL4 l(1)L13HC129 recombinant chromosomes were generated in our laboratory. Wild-type (WT) flies were Canton S.
Molecular analysis
Preparation of head RNA. Fifty flies were placed in a microcentrifuge tube, frozen in liquid nitrogen, and vortexed to remove the heads, which were collected and homogenized in 3m LiCl and 6 m urea for total RNA preparation by conventional methods.
Reverse transcriptase PCR. Total RNA from 50 heads was used for first-strand cDNA synthesis by conventional methods using Moloney murine leukemia virus reverse transcriptase (RT) (Invitrogen, Carlsbad, CA) and random oligonucleotide primers (Invitrogen). A previously reported cac sequence (Smith et al., 1996) was used to design primers for RT-PCR using the head cDNA preparations as a template. The entire cac open reading frame (ORF) was amplified in three segments using six primers. Two different primer sets were used to generate slightly different PCR fragments for sequencing (primer set 1) and cloning (primer set 2). Primer set 1 included the following primer pairs (forward and reverse primers in a pair are separated by a slash):cac11-catcaacaggactccttagg/cac21-caccaccctgagatatgatg;cac12-cccgatagcactgttgactg/cac22-gaattttccaccgtacctag; andcac13-ggcacttccctatgtctgtt/cac23-cctgatgctatacccagatc. Primer set 2 included the following:cac11-catcaacaggactccttagg/cac22B-cggtgaatcccatgttaatg;cac12-cccgatagcactgttgactg/cac22-gaattttccaccgtacctag; andcac12A-ccaaccaatcccatacgacg/cac23-cctgatgctatacccagatc. For direct sequencing of PCR products, PCRs were performed withTaq polymerase (PGC Scientific, Gaithersburg, MD), whereas Pfu polymerase (Stratagene, La Jolla, CA) was used in reactions for cloning.
Sequencing. Direct sequencing of PCR products and sequencing of cac clones was performed at the Penn State Nucleic Acids Facility. Sequencing primers for each of the three cac ORF fragments were designed on the basis of a previously reportedcac sequence (Smith et al., 1996). BecausecacTS2 was isolated as an extragenic enhancer of comatoseST53 (Dellinger et al., 2000), the cacTS2 mutation was identified by comparing the cac ORF sequence fromcacTS2 and thecomatoseST53 parent chromosome. Any apparent sequence differences were re-examined in independent RNA preparations. With respect to the published cac coding sequence (U55776), three silent polymorphisms were detected incac ORF sequences; these were present in bothcacTS2 and the parentcomatoseST53 line. Sequence alignments were performed by the Clustal method, using the Megalign feature of the Lasergene Software Package (DNAStar, Madison, WI).
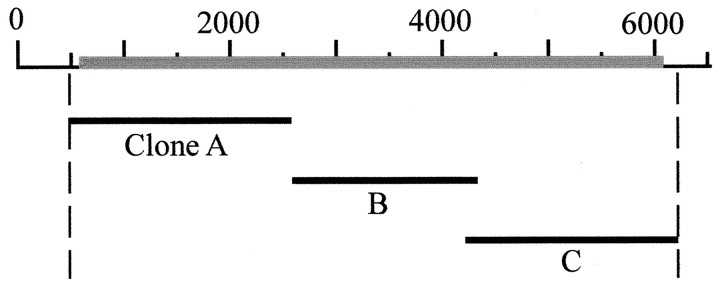
Cloning. Each of the three cac ORF fragments generated with primer set 2 was cut at unique restriction sites and cloned separately into the pBluescript SK− vector. These overlapping segments (Fig. 2) were then assembled into a single clone containing a complete ORF, which was subsequently shuttled into the KpnI and NotI sites of the transformation vector, pUAST (Brand and Perrimon, 1993). Clones of ORF segments B and C (Fig. 2) were toxic to several different strains of bacteria. These could be propagated successfully in JM109 cells (Promega, Madison, WI); however, only small colonies were obtained, and liquid cultures grew poorly. The presence of specific splice variants among the population of clones for each segment was determined by sequencing and restriction mapping. As described in Results, two novel alternative exons of 12 and 60 bp were found in the region of the transcript encoding the C-terminal cytoplasmic tail of the α1 subunit. These were identified as contiguous exons in thecac gene and have the following nucleotide sequences (60 bp, CGGAAGAAGCTGGAGCACGATGATGAGCATAAATATAGCCCAACGGCAGTCGAGGAGCCG; 12 bp, AACTGGAAGGAG).
Fig. 2.
Three overlapping cDNA clones assembled into a clone containing the entire cac ORF. Details regarding the generation and cloning of these segments are provided in Materials and Methods. Nucleotide positions (in base pairs) correspond to those of the cac cDNA sequence (U55776). The ORF is represented as a gray bar.
Transgenic lines were generated essentially as described previously (Karess and Rubin, 1984). Briefly, the transformation constructUAS-cac1 in pUAST was prepared for injection by column purification (Plasmid Maxi Kit; Qiagen, Valencia, CA). DNA (0.8 μg/μl) was injected into the posterior pole ofw1118 embryos before cellularization. Coinjection of the pπ25.7wc plasmid (0.2 μg/μl) provided transient expression of the P element transposase. Thew+ marker carried by pUAST was used to select transformed progeny.
Transformation rescue
Rescue data were obtained using the third chromosome insertion line UAS-cac1 256B. For rescue of both TS and lethalcac phenotypes, eight independent UAS-cac1insertion lines were tested; seven produced results similar to those ofUAS-cac1 256B. The remaining line was somewhat less effective, producing rescue of cacTS2paralysis but not full rescue of cac lethal mutants to adult viability.
Rescue of temperature-sensitive paralysis. All flies used for behavioral tests were raised and maintained at room temperature (22–25°C). Paralysis at a given temperature was monitored as described previously (Dellinger et al., 2000). Briefly, groups of six flies were introduced into preheated vials and the time at which 50% of the flies were no longer standing was recorded (50% paralysis). For tests exceeding 5 min in duration, the vials were humidified at 5 min by adding water to the cotton plug sealing the vial.
Rescue of cac lethal phenotypes. Three different crosses were performed to determine the adult viability of each caclethal mutant and each rescued cac lethal mutant relative to that of males carrying a WT X chromosome. In rescue crosses, elav-GAL4 l(1)L1320-3/FM7i and elav-GAL4 l(1)L13HC129/FM7i females were mated to lines homozygous for a UAS-cac1 insertion. The extent of rescue to WT viability was measured by determining the percentage of total adult progeny represented by non-FM7i males (carrying the lethal mutation but rescued by transgene expression) relative to the percentage obtained from similar control crosses using +/FM7i females. These data were expressed as percentage viability values, which were calculated as follows: (% non-FM7i male progeny from the rescue cross/% non-FM7i male progeny from the control cross) × 100. Finally, viability of cac lethals in the absence of UAS-cac1 was determined similarly in crosses ofelav-GAL4 l(1)L1320-3/FM7i andelav-GAL4 l(1)L13HC129/FM7ifemales to WT (Canton S) males. All crosses were transferred frequently to prevent crowding of the vials.
Electrophysiology
Recordings of EPSCs from dorsal longitudinal muscle (DLM) neuromuscular synapses of the adult were obtained and analyzed as described previously (Kawasaki et al., 1998, 2000; Kawasaki and Ordway, 2000). Recordings were performed on 2- to 4-d-old flies raised at room temperature (22–25°C).
Data analysis
Graphing and analysis of numerical data were performed in Microsoft Excel (Microsoft, Seattle, WA). All data values throughout the text and bar graphs are given as means ± SEM. Statistical analysis was performed using the two-tailed Student'st test; significance was assigned to comparisons for whichp ≤ 0.05.
RESULTS
A missense mutation in cacTS2
To examine whether the cacTS2mutation resides within coding sequence, the ORF ofcac was amplified by RT-PCR and sequenced. To distinguish the induced mutation from any background polymorphisms present in thecacTS2 strain, sequence fromcacTS2 was compared directly with thecac ORF sequence from the parent chromosome on which thecacTS2 mutation was generated (Dellinger et al., 2000) (see Materials and Methods). Putative sequence differences between cacTS2 and the parent line were re-examined using independent RNA preparations. The only nucleotide difference observed in cacTS2(c4714t) creates a proline-to-serine missense mutation at amino acid 1385. Thus, the cacTS2 mutation maps to the second proline of a highly conserved proline pair located adjacent to the EF hand domain within the C-terminal cytoplasmic tail of the α1 subunit (Fig. 1B). This region of the channel is known to participate in calcium-dependent inactivation, raising the possibility that cacTS2disrupts channel function by altering this regulatory process. By arrangement, the laboratory of Dr. Jeffrey C. Hall sequencedcacTS2 independently and identified the same missense mutation (B. Chan and J. C. Hall, Brandeis University, personal communication).
Generation of a cac transgene: head cDNAs reflect diversity of cac transcripts
As described in the introductory remarks, the cac gene is known to express diverse transcripts that differ with respect to mRNA splicing and editing, and possibly in their temporal and/or spatial expression patterns (Smith et al., 1996, 1998a,b; Peixoto et al., 1997). In light of this complexity, the in vivoexpression of specific cac-encoded protein variants will be an important tool in examining the structural determinants of calcium-channel function. Thus, molecular constructs were generated to allow in vivo expression of a specific caccDNA.
Transgenic expression studies required generation of caccDNA clones containing a complete ORF, which have not been reported previously. Thus, RT-PCR was performed to generate cac cDNAs for this purpose. On the basis of previous sequence analysis (Smith et al., 1996), PCR primers were designed for RT-PCR amplification ofcac from head RNA (see Materials and Methods). The resulting PCR products were used to generate three overlapping clones spanning the entire cac ORF (Fig. 2, clones A–C). As discussed in Materials and Methods, clones B and C were difficult to propagate in several bacterial strains. Multiple clones were generated and characterized for each ORF segment, revealing numerous cDNA variants that differed with respect to mRNA splicing and editing (Fig.3). Although previously published studies of cac mRNA processing were performed on whole-body mRNA (Smith et al., 1996, 1998a; Peixoto et al., 1997), most of the head cDNA variants observed in the present study have been reported previously. Several exceptions are described in Figure 3 (and see Discussion). Notably, a novel site of alternative splicing was identified in the region encoding the C-terminal cytoplasmic tail, and is referred to here as the “C-terminal variable” region (Fig.3A,B). In addition to clones corresponding to previously reported cac cDNAs covering this region, variants containing insertions of 12 and 60 bp were observed as well. Both of these nucleotide sequences were identified as single cac exons in the Drosophila genome sequence (see Materials and Methods) and were associated with canonical splice donor and acceptor sequences at the intron–exon boundaries (Mount et al., 1992). Inclusion of the 12 or 60 bp alternative exon results in the addition of 4 or 20 aa adjacent to the IQ binding domain for calmodulin (Fig. 3A). The protein sequences encoded by these exons are NWKE and RKKLEHDDEHKYSPTAVEEP, respectively. The latter amino acid sequence was used as a query in Basic Local Alignment Search Tool (BLAST) searches of nonredundant protein and translated databases (http://www.ncbi.nlm.nih.gov/BLAST); however, no clear matches outside of the Drosophila cac gene were found.
Clones for each of the three ORF segments were chosen to be assembled into a clone spanning the entire cac ORF. The specific clone selected for each segment represented the editing or splicing variant most abundant in the cDNA population and/or the most conserved with respect to vertebrate presynaptic calcium channels (Fig.4A). For example, alternative exon I-IIb was included in the transgene construct because it was more abundant than I-IIa among head cDNAs and includes protein binding motifs strongly conserved with those of mammalian presynaptic calcium channels (Smith et al., 1996) (see Discussion). In addition, previous work suggests that exon I-IIa may be eye-specific (Smith et al., 1998b).
Fig. 4.
Neural expression of a specific WTcac-encoded α1 subunit rescues TS paralysis ofcacTS2. A, Alternative exons and edited sequences included in the UAS-cac1transgene. B, Rescue ofcacTS2 paralytic behavior. Time for 50% paralysis was measured in WT,cacTS2, and elav-GAL4 cacTS2;;UAS-cac1/+(cacTS2 Rescue) at 38°C. Data and nvalues are provided in Results. Behavioral tests in WT animals were truncated after 50 min. The time for 50% paralysis values obtained incacTS2Rescueexperiments was significantly different from that obtained forcacTS2 alone (p ≤ 0.05).
Transformation rescue of temperature-sensitive behavioral and synaptic phenotypes in cacTS2
The clone including the entire cac ORF was shuttled into the pUAST transformation vector to produce the transgene referred to as UAS-cac1. The pUAST vector contains multiple binding sites for the yeast transcription factor GAL4; thus, controlled expression can be achieved using available drivers expressing GAL4 in different temporal and spatial expression patterns (Brand and Perrimon, 1993). Transgenic flies were generated to examine whether expression of the UAS-cac1 transgene could rescue thecacTS2 paralytic phenotype. In light of previous work in embryos indicating that cac is expressed in the nervous system (Smith et al., 1996), as well as our recent studies showing a presynaptic role for cac at adult neuromuscular synapses (Kawasaki et al., 2000), we chose to drive expression specifically in the nervous system. A GAL4 enhancer trap element in the pan-neurally expressed elav gene (Robinow and White, 1988; Lin and Goodman, 1994) was combined with aUAS-cac1 transgene in a cacTS2mutant background. Neural expression of UAS-cac1 produced striking rescue of cacTS2 paralysis at 38°C (Fig. 4B), increasing the time for paralysis from 0.27 ± 0.01 min (n = 5) incacTS2 alone to 39.19 ± 2.16 min (n = 6) in the presence of the transgene. Neither theelav-GAL4 driver nor the UAS-cac1 transgene alone produced rescue, as expected from the requirement for both elements to drive expression.
To examine rescue of cacTS2 in more detail, voltage-clamp analysis of synaptic currents at DLM neuromuscular synapses was performed to determine whether the synaptic phenotype of cacTS2 was also rescued. As described previously (Kawasaki et al., 2000),cacTS2 exhibited a WT synaptic current at 20°C and a marked reduction in synaptic current amplitude with respect to WT at 36°C (Fig.5A). In contrast,cacTS2Rescue flies carrying theelav-GAL4 driver and UAS-cac1 transgene exhibited marked rescue of this synaptic phenotype (Figs.5A,B). With respect to WT, synaptic current amplitudes incacTS2 andcacTS2Rescue flies were 38.1 ± 2.6% (n = 4) and 80.2 ± 5.6% (n = 4), respectively. The above results confirm the presynaptic nature of the cacTS2 phenotype (Kawasaki et al., 2000) and show that the specific α1 subunit variant expressed from the UAS-cac1 transgene can support fast, calcium-triggered neurotransmitter release (see Discussion).
Fig. 5.
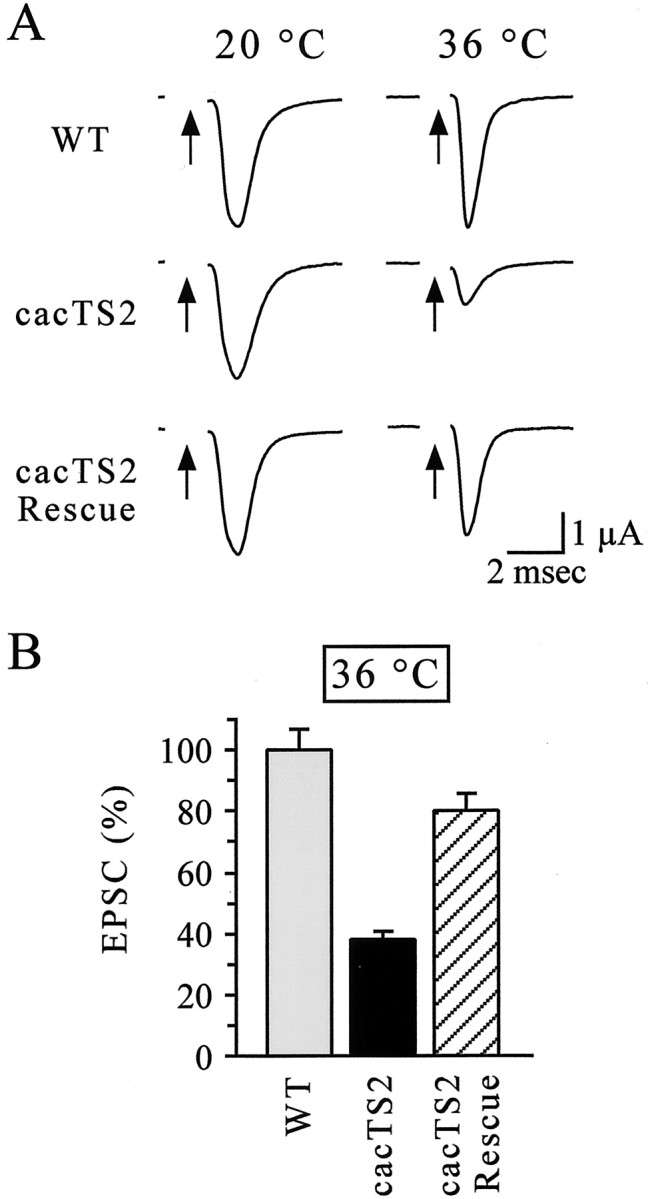
Rescue of the TS synaptic phenotype incacTS2. A, Representative synaptic current recordings from DLM neuromuscular synapses of WT, cacTS2, andelav-GAL4 cacTS2;;UAS-cac1/+ (cacTS2 Rescue). Arrows indicate stimulation of the cut motor axon. Stimulation artifacts were removed for clarity. B, Mean EPSC amplitudes relative to WT at 36°C. Data and n values are provided in Resutls. The synaptic current incacTS2Rescueexperiments was significantly different fromcacTS2 alone (p ≤ 0.05). Comparison of the synaptic current amplitudes incacTS2Rescue with those of WT resulted in a p value of 0.073.
Transformation rescue of cac lethal alleles
In addition to cacTS2 and other viable cac mutations, lethal alleles of cac have also been described. The lethal phase has been determined for severalcac lethal mutants, which exhibit lethality during late embryogenesis just before hatching (Perrimon et al., 1989; Smith et al., 1998b; our unpublished observations). This late embryonic lethal stage is consistent with analysis of cac expression during development, which increases dramatically during late embryogenesis (Smith et al., 1996); the similar lethal phenotypes of several mutants suggest that late embryonic lethality may reflect a complete loss of zygotic cac function. To further explore the molecular determinants of calcium-channel function, as well as the importance of calcium-channel diversity resulting from mRNA processing, we subsequently examined whether the specific α1 subunit expressed from the UAS-cac1 transgene could rescue cac lethal mutants.
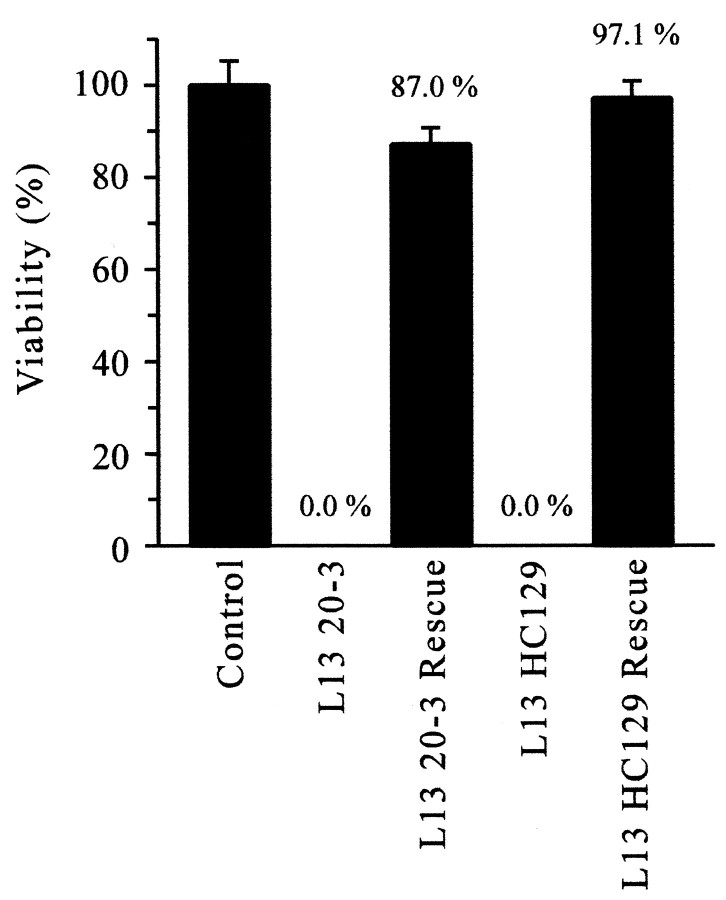
Rescue of two different cac lethals,l(1)L1320-3 andl(1)L13HC129, was attempted by driving neural expression of UAS-cac1 using the elav-GAL4driver. This resulted in striking rescue of the embryonic lethal phenotype in both mutants, producing viability similar to that of WT controls (Fig. 6). Routine observation of these rescued adults suggested that their motor behavior and activity were similar to WT, with the exception of wing function (see Discussion), and that they were fertile. These results indicate that neural expression of cac is sufficient to fulfill the essential functions of this gene. Furthermore, the specific α1 subunit expressed from UAS-cac1 appears to provide sufficient cac-encoded calcium-channel activity to support robust neural function. The strong rescue of cac lethal mutants is surprising given that the transgene was not expressed under the control of the native cac promoter and that only a single variant of many possible cac-encoded α1 subunits was expressed.
Fig. 6.
Rescue of cac lethal mutants. Three different crosses were performed to determine the adult viability of each cac lethal mutant and rescued caclethal mutant relative to males carrying a WT X chromosome (percentage viability; see Materials and Methods). +;;UAS-cac1/+ males (Control) served as a reference for WT viability. In the absence of aUAS-cac1 transgene, elav-GAL4 l(1)L1320-3 (L13 20-3) andelav-GAL4 l(1)L13HC129 (L13 HC129) males were never observed, indicating uniform lethality. In contrast, males of the genotypes elav-GAL4 l(1)L1320-3;;UAS-cac1/+ (L13 20-3 Rescue) and elav-GAL4 l(1)L13HC129;;UAS-cac1/+ (L13 HC129 Rescue) were clearly rescued and exhibited viability similar to that of WT controls. The mean percentage viability value obtained from five independent rescue experiments for each genotype (n = 5) is indicated. Viability ofL13 20-3 Rescue and L13 HC129 Rescue males was not significantly different from that of control males (p > 0.05).
DISCUSSION
The present study takes further advantage of theDrosophila genetic model system to investigate the molecular determinants of synaptic function. The findings reported here identify a missense mutation producing the TS paralytic and synaptic phenotypes in cacTS2, and show that caclethal and TS mutants can be rescued by neural expression of a specificcac cDNA. Together, these results further define the structural basis of presynaptic calcium-channel function in vivo.
The molecular lesion in cacTS2
A calcium-dependent regulatory domain within the C-terminal cytoplasmic tail of the α1 subunit includes a conserved calmodulin binding site (Fig. 1A, IQ) and a conserved EF hand calcium-binding domain. Together, the IQ and EF hand motifs are thought to mediate a form of channel inactivation that is dependent on calcium influx through the open channel (Peterson et al., 2000; DeMaria et al., 2001). The cacTS2 mutation (P1385S) substitutes serine for the second proline of a highly conserved proline pair adjacent to the EF hand (Fig.1B). Proline pairs are generally excluded from classical secondary structures, such as α helices and β strands, and instead are found in flexible loop and hinge regions (MacArthur and Thornton, 1991; Branden and Tooze, 1999). In light of recent models of calcium-dependent channel inactivation involving structural rearrangements of this regulatory domain (DeMaria et al., 2001;Erickson et al., 2001), it is possible that proline 1385 participates in folding transitions associated with calcium-dependent inactivation. In cacTS2, enhanced steady-state channel inactivation at elevated temperatures might account for reduced neurotransmitter release. Additional investigation of this issue will await direct analysis of WT and cacTS2channel behavior at permissive and restrictive temperatures.
Alternative splicing of cac head mRNA
The present study does not include a systematic analysis of alternative splicing; however, our survey of cac transcripts in head mRNA led to a few notable observations. One was the appearance of a novel site of alternative splicing producing variation within the C-terminal cytoplasmic tail of the α1 subunit (Fig. 3B). cDNA variants including either a 12 or 60 bp alternative exon at this position, as well as those lacking either exon, were observed. The close proximity of this C-terminal variable region to the IQ binding domain for calmodulin raises the possibility that the resulting channel variants may differ in their regulation by calmodulin. Given that theUAS-cac1 transgene did not include either the 12 or 60 bp exon, these sequences are not essential for rapid neurotransmitter release.
Another novel splice variant lacked both the I-IIa and I-IIb exons. Alternative exon I-IIb includes binding motifs for calcium-channel β subunits and heterotrimeric G-protein subunits that are highly conserved with respect to vertebrate presynaptic calcium-channel α1 subunits (Smith et al., 1998b). In contrast, exon I-IIa is less conserved and lacks a primary G-protein binding motif, QQxxRxLxGY. Thus, variation in these exons, including their absence in certain α1 subunit variants, creates the potential for differential regulation of distinct α1 subunits (Smith et al., 1998b). The conserved I-IIb alternative exon is present in the UAS-cac1 transgene.
A final consideration is the role of alternative exons that produce variation within the IVS3–IVS4 extracellular loop. In mammalian synaptic calcium-channel α1 subunits, inclusion of NP (α1A) or ET (α1B) sequences within this loop has been reported to alter channel activation, inactivation, and pharmacology (Lin et al., 1997,1999; Bourinet et al., 1999; Hans et al., 1999; Krovetz et al., 2000). Similar variation is generated by alternative splicing of thecac transcript, producing a one to three amino acid insertion at the same position. Interestingly, most cac mRNA variants in Drosophila heads lack any alternative exon in the sequence encoding the IVS3–IVS4 loop, perhaps consistent with work indicating that the analogous alternative exons in mouse α1A and α1B are enriched in the peripheral nervous system (Lin et al., 1999). It remains an open question whether variation in the IVS3–IVS4 loop of the cac-encoded α1 subunit serves a conserved functional role. However, the UAS-cac1 transgene lacks alternative exons at this site, suggesting that α1 subunit sequences encoded by these exons are not critical for calcium-channel function in neurotransmitter release.
A-to-I mRNA editing
As in the case of alternative splicing, previous studies ofcac mRNA editing have been performed using whole-fly RNA preparations. In the present study, only a qualitative survey of editing in head RNA was conducted; however, all of the reported sites were observed. In addition, sequence data were consistent with a novel editing event at nucleotide 936 (Fig. 3). On the basis of genomic and whole-fly cDNA sequence comparisons performed by others, position 936 has been confirmed as an mRNA editing site (Chan and Hall, personal communication). A subset of edited sites was present in theUAS-cac1 transgene. Although it is possible that the mRNA produced by this transgene is edited, we consider this unlikely because editing of mRNAs, including cac, may require intronic sequences (Smith et al., 1998a). Thus, edited sequences absent from the transgene are probably not required for fast, calcium-triggered neurotransmitter release. With respect to the general role of editing in Drosophila, recent studies of a mutant thought to lack A-to-I mRNA editing of all transcripts revealed a moderate phenotype (Palladino et al., 2000). Although this mutant exhibited behavioral phenotypes and progressive deterioration of the adult nervous system, A-to-I mRNA editing in general does not appear to be essential for basic neural development or function.
Rescue of cac mutants by neural expression of theUAS-cac1 transgene
As discussed above, neural expression of the UAS-cac1transgene produced striking rescue of both TS paralytic and embryonic lethal cac mutants. Synaptic current recordings demonstrate that the specific α1 subunit variant encoded by the transgene can support fast neurotransmitter release at neuromuscular synapses and confirm previous findings demonstrating a presynaptic role for thecac gene product (Kawasaki et al., 2000). Becausecac encodes the only Drosophila homolog of vertebrate presynaptic calcium-channel α1 subunits and is expressed broadly in the embryonic CNS (Smith et al., 1996), it is likely that the same UAS-cac1 transgene product can support robust synaptic transmission in the CNS as well.
One surprising finding related to rescue of cac lethals was an apparent defect in the wing function of rescued adult flies. No formal behavioral analysis was performed; however, it was clear from routine observation that rescued flies were flightless and did not beat their wings under usual flight conditions. No gross morphological abnormalities were apparent in dissected preparations of caclethal mutants rescued to the adult stage; however, DLM neuromuscular synapses in these preparations produced only small synaptic currents ranging from 0 to 40% of WT amplitude (our unpublished results). The reason for the observed defect in wing function remains unclear. Rescue of the cacTS2 synaptic phenotype confirms that the pan-neural elav-GAL4 driver induces expression in the motor neurons innervating the DLMs. However, proper development of these connections may require precise temporal and spatial control ofcac expression, or perhaps a specific cac-encoded α1 subunit variant, that is not reproduced by expression of theUAS-cac1 transgene under the control ofelav-GAL4. Perhaps coincidentally, cac mutants were first identified in a phenotypic screen for alterations in the precise wing-beat patterns comprising the male courtship song (von Schilcher, 1976, 1977; Smith et al., 1998b). In combination with viablecac mutants exhibiting song phenotypes, the availability ofcac transgenes that may be driven in different temporal and spatial expression patterns may provide new insights into the cellular and molecular basis of the courtship song.
Absence of a conserved SYNPRINT domain within the calcium-channel α1 subunit does not prevent rapid coupling of calcium influx to neurotransmitter release
Previous work on the Drosophila cac gene indicated that the Drosophila genome contains no sequences related to characterized calcium-channel synaptic protein binding domains (Kawasaki et al., 2000; Littleton and Ganetzky, 2000). The present study extends this observation by demonstrating directly that a specific calcium-channel α1 subunit lacking these sequences functions in fast, calcium-triggered neurotransmitter release. These findings indicate either that the cac-encoded α1 subunit contains an analogous but distinct synaptic protein binding domain or that such domains do not play a critical role in synaptic transmission. Recent work in another system has shown that syntaxin-mediated enhancement of slow calcium-channel inactivation does not require the SYNPRINT domain, although this regulation was more effective on SYNPRINT-containing channels (Bezprozvanny et al., 2000). Together, these results suggest that multiple biochemical and functional interactions between calcium-channel α1 subunits and synaptic proteins may contribute to regulation of neurotransmitter release.
The present study further defines the determinants of synaptic calcium-channel function in Drosophila through molecular analysis of cacTS2, as well as rescue ofcac mutants by neural expression of a specificcac-encoded α1 subunit. These findings also serve as the basis for additional analysis in this model system, including direct studies of TS calcium-channel function produced by thecacTS2 mutation, analysis of second-sitecac mutations that either enhance or suppress thecacTS2 phenotype (Brooks et al., 2002), and a variety of investigations that require controlled expression of specific cac-encoded α1 subunits in vivo.
Footnotes
This work was supported by grants from the National Institutes of Health and the National Science Foundation and by The Pennsylvania State University President's Fund for Undergraduate Research. We thank Jeffrey C. Hall for helpful discussions and for sharing unpublished observations.
Correspondence should be addressed to Richard W. Ordway, Department of Biology, 208 Mueller Laboratory, Pennsylvania State University, University Park, PA 16802. E-mail: rwo4@psu.edu.
REFERENCES
- 1.Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 2.Bezprozvanny I, Zhong P, Scheller RH, Tsien RW. Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc Natl Acad Sci USA. 2000;97:13943–13948. doi: 10.1073/pnas.220389697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- 4.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 5.Branden C, Tooze J. Introduction to protein structure, Ed 2. Garland; New York: 1999. [Google Scholar]
- 6.Brooks IM, Felling R, Godeny MD, Ordway RW (2002) Genetic modifiers of a temperature-sensitive paralytic calcium channel mutant of Drosophila: genetic, molecular and phenotypic characterization. Paper presented at the 43rd AnnualDrosophila Research Conference, San Diego.
- 7.Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 8.Colecraft HM, Brody DL, Yue DT. G-protein inhibition of N- and P/Q-type calcium channels: distinctive elementary mechanisms and their functional impact. J Neurosci. 2001;21:1137–1147. doi: 10.1523/JNEUROSCI.21-04-01137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degtiar VE, Scheller RH, Tsien RW. Syntaxin modulation of slow inactivation of N-type calcium channels. J Neurosci. 2000;20:4355–4367. doi: 10.1523/JNEUROSCI.20-12-04355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellinger BB, Felling R, Ordway RW. Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel α1 subunit gene, cacophony. Genetics. 2000;155:203–211. doi: 10.1093/genetics/155.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 12.De Waard M, Liu H, Walker D, Scott VES, Gurnett AA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 13.Dove LS, Abbott LC, Griffith WH. Whole-cell and single-channel analysis of P-type calcium currents in cerebellar Purkinje cells of leaner mutant mice. J Neurosci. 1998;18:7687–7699. doi: 10.1523/JNEUROSCI.18-19-07687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlap K. Integration hot-spot gets hotter. Nature. 1997;385:394–397. doi: 10.1038/385394a0. [DOI] [PubMed] [Google Scholar]
- 15.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 16.Gielow ML, Gu G-G, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci. 1995;15:6085–6093. doi: 10.1523/JNEUROSCI.15-09-06085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hans M, Urrutia A, Deal C, Brust PF, Stauderman K, Ellis SB, Harpold MM, Johnson EC, Williams ME. Structural elements in domain IV that influence biophysical and pharmacological properties of human α1A-containing high-voltage-activated calcium channels. Biophys J. 1999;76:1384–1400. doi: 10.1016/S0006-3495(99)77300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herlitze S, Zhong H, Scheuer T, Catterall WA. Allosteric modulation of Ca2+ channels by G proteins, voltage-dependent facilitation, protein kinase C, and Cavβ subunits. Proc Natl Acad Sci USA. 2001;98:4699–4704. doi: 10.1073/pnas.051628998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 21.Ino M, Yoshinaga T, Wakamori M, Miyamoto N, Takahashi E, Sonoda J, Kagaya T, Oki T, Nagasu T, Nishizawa Y, Tanaka I, Imoto K, Aizawa S, Koch S, Schwartz A, Niidome T, Sawada K, Mori Y. Functional disorders of the sympathetic nervous system in mice lacking the α1B subunit (Cav 2.2) of N-type calcium channels. Proc Natl Acad Sci USA. 2001;98:5323–5328. doi: 10.1073/pnas.081089398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis SE, Zamponi GW. Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J Neurosci. 2001;21:2939–2948. doi: 10.1523/JNEUROSCI.21-09-02939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin H-S. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A-subunit. Proc Natl Acad Sci USA. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karess RE, Rubin GM. Analysis of P transposable element functions in Drosophila. Cell. 1984;38:135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki F, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat Neurosci. 2000;3:859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki F, Mattiuz AM, Ordway RW. Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J Neurosci. 1998;18:10241–10249. doi: 10.1523/JNEUROSCI.18-24-10241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krovetz HS, Helton TD, Crews AL, Horne WA. C-terminal alternative splicing changes the gating properties of a human spinal cord calcium channel α1A subunit. J Neurosci. 2000;20:7564–7570. doi: 10.1523/JNEUROSCI.20-20-07564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 30.Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 31.Lin Z, Haus S, Edgerton J, Lipscombe D. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron. 1997;18:153–166. doi: 10.1016/s0896-6273(01)80054-4. [DOI] [PubMed] [Google Scholar]
- 32.Lin Z, Lin Y, Schorge S, Qian Pan J, Beierlein M, Lipscombe D. Alternative splicing of a short cassette exon in α1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci. 1999;19:5322–5331. doi: 10.1523/JNEUROSCI.19-13-05322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littleton TJ, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:36–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzon NM, Lutz CM, Frankel WN, Beam KG. Altered calcium channel currents in Purkinje cells of the neurological mutant mouse leaner. J Neurosci. 1998;18:4482–4489. doi: 10.1523/JNEUROSCI.18-12-04482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lü Q, AtKisson MS, Jarvis SE, Feng ZP, Zamponi GW, Dunlap K. Syntaxin 1A supports voltage-dependent inhibition of α1B Ca2+ channels by Gβγ in chick sensory neurons. J Neurosci. 2001;21:2949–2957. doi: 10.1523/JNEUROSCI.21-09-02949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacArthur MW, Thornton JM. Influence of proline residues on protein conformation. J Mol Biol. 1991;218:397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- 37.Mirotznik RR, Zheng X, Stanley EF. G-protein types involved in calcium channel inhibition at a presynaptic nerve terminal. J Neurosci. 2000;20:7614–7621. doi: 10.1523/JNEUROSCI.20-20-07614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mochida S, Sheng Z-H, Carl B, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 39.Mount SM, Burks C, Hertz G, Stormo GD, White O, Fields C. Splicing signals in Drosophila: intron size, information content, and consensus sequences. Nucleic Acids Res. 1992;20:4255–4262. doi: 10.1093/nar/20.16.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palladino MJ, Keegan LP, O'Connell MA, Reenan RA. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- 41.Pan JQ, Lipscombe D. Alternative splicing in the cytoplasmic II–III loop of the N-type Ca channel α1B subunit: functional differences are β subunit-specific. J Neurosci. 2000;20:4769–4775. doi: 10.1523/JNEUROSCI.20-13-04769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peixoto AA, Smith LA, Hall JC. Genomic organization and evolution of alternative exons in a Drosophila calcium channel gene. Genetics. 1997;145:1003–1013. doi: 10.1093/genetics/145.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrimon N, Engstrom L, Mahowald AP. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics. 1989;121:333–352. doi: 10.1093/genetics/121.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson BZ, Lee JS, Mulle JG, Wang Y, de Leon M, Yue DT. Critical determinants of Ca2+-dependent inactivation within an EF-hand motif of L-type Ca2+ channels. Biophys J. 2000;78:1906–1920. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reenan RA. The RNA world meets behavior: A to I pre-mRNA editing in animals. Trends Genet. 2001;17:53–56. doi: 10.1016/s0168-9525(00)02169-7. [DOI] [PubMed] [Google Scholar]
- 46.Ren D, Xu H, Eberl DF, Chopra M, Hall LM. A mutation affecting dihydropyridine-sensitive current levels and activation kinetics in Drosophila muscle and mammalian heart calcium channels. J Neurosci. 1998;18:2335–2341. doi: 10.1523/JNEUROSCI.18-07-02335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126:294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- 48.Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 50.Sheng Z-H, Westenbroek RE, Catterall WA. Physical link and functional coupling of presynaptic calcium channels and the synaptic vesicle docking/fusion machinery. J Bioenerg Biomembr. 1998;30:335–345. doi: 10.1023/a:1021985521748. [DOI] [PubMed] [Google Scholar]
- 51.Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel α1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith LA, Peixoto AA, Hall JC. RNA editing in the Drosophila Dmca1A calcium-channel α1 subunit transcript. J Neurogenet. 1998a;12:227–240. doi: 10.3109/01677069809108560. [DOI] [PubMed] [Google Scholar]
- 53.Smith LA, Peixoto AA, Kramer EM, Villella A, Hall JC. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel α1 subunit in Drosophila. Genetics. 1998b;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- 55.von Schilcher F. The behavior of cacophony, a courtship song mutant in Drosophila melanogaster. Behav Biol. 1976;17:187–196. doi: 10.1016/s0091-6773(76)90444-2. [DOI] [PubMed] [Google Scholar]
- 56.von Schilcher F. A mutation which changes courtship song in Drosophila melanogaster. Behav Genet. 1977;7:251–259. doi: 10.1007/BF01066278. [DOI] [PubMed] [Google Scholar]
- 57.Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- 58.Wu MN, Fergestad T, Lloyd TE, He Y, Broadie K, Bellen HJ. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- 60.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 61.Zheng W, Feng G, Ren D, Eberl DF, Hannan F, Dubald M, Hall LM. Cloning and characterization of a calcium channel α1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci. 1995;15:1132–1143. doi: 10.1523/JNEUROSCI.15-02-01132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong H, Yokoyama CT, Scheuer T, Catterall WA. Reciprocal regulation of P/Q-type Ca2+ channels by SNAP-25, syntaxin and synaptotagmin. Nat Neurosci. 1999;2:939–941. doi: 10.1038/14721. [DOI] [PubMed] [Google Scholar]