Abstract
Diets high in antioxidant properties are known to reverse some deficits in neuronal and cognitive function that occur in aging animals. Antioxidants are also known to reduce levels of proinflammatory factors in the CNS. We report here that 6 weeks of a spinach-enriched diet ameliorates deficits in cerebellar-dependent delay classical eyeblink learning and reduces the proinflammatory cytokines tumor necrosis factor α (TNFα) and TNFβ in the cerebelli of eyeblink-trained animals. Eighteen-month-old Fischer 344 rats were given spinach-enriched lab chow or regular lab chow for 6 weeks. The rats were then given 6 d of 30 trials per day training using a 3 kHz tone conditioned stimulus and airpuff unconditioned stimulus. Rats were killed 3 weeks after eyeblink training. Cytokine expression was measured using RNase protection assay analysis in the eyeblink-trained animals and in a group of young control animals given regular lab chow diet. Old animals on the spinach-enriched lab chow diet learned delay eyeblink conditioning significantly faster than old animals on the regular diet. Cerebelli from older animals on the spinach-enriched diet had significantly less TNFα and TNFβ than cerebelli from older animals on the control diet.
Keywords: diet, antioxidant, TNFα, TNFβ, classical conditioning, cytokines
Oxidative stress has been postulated to play a leading role in age-related declines in neuronal function and neurodegenerative diseases of aging such as Alzheimer's disease (AD) and Parkinson's disease (Harman, 1956; Leibovitz and Siegel, 1980;Ames et al., 1993). Administration of antioxidants has been used as an approach to ameliorate some of these changes (Cao et al., 1998; Chen et al., 2001).
Increases in by-products of oxidative stress, such as protein carbonyls, lipid peroxides, and damaged DNA are observed in the aged brain. Treatment of older subjects with specific antioxidant agents has had some success in ameliorating disease processes. For example, vitamin E has been shown to slow some aspects of the progression of AD (Sano et al., 1997). Ginkgo biloba has also been shown to improve performance on certain cognitive tests in patients with AD (Rai et al., 1991).
Cao et al. (1996) have identified fruits and vegetables with high antioxidant potential based on an in vitro assay of oxygen radical absorbance capacity (ORAC). Experiments using dietary intervention with fruit and vegetable supplements (blueberry, strawberry, spinach, and vitamin E) high in ORAC activity have resulted in successful amelioration of such age-related declines as muscarinic receptor sensitivity, noradrenergic modulation of cerebellar Purkinje neurons, calcium regulation, and Morris water maze performance, among others (Joseph et al., 1998, 1999; Bickford et al., 2000).
Chronic inflammation has also been implicated as an underlying factor in the pathogenesis of many age-related diseases. Inflammation is a process accompanied by the release of several products, including cytokines. Cytokines are mediators and inhibitors of diverse forms of neurodegeneration, are locally induced in response to brain injury, and have diverse actions that can mediate cellular injury and repair. Proinflammatory cytokines such as interleukin-1 (IL-1), IL-1 receptor antagonist, tumor necrosis factor (TNF), and transforming growth factor-β increase with aging (Lynch, 1998; Knoblach et al., 1999). An age-related increase in IL-1β has been associated with a deficit in long-term potentiation (Murray and Lynch, 1997, 1998), and the level of IL-1β in the hippocampus of aged rats is decreased by treatment with antioxidants (Murray et al., 1997).
We have now begun to examine how antioxidant-rich diets affect the expression of proinflammatory cytokines in the cerebellum of aging rats (Gemma et al., 2002). We demonstrated that TNFα and TNFβ are significantly elevated in the cerebellum of 20-month-old animals. When aged rats were given diets enriched with spirulina or apple, foods with elevated ORAC activity, there was a significant reduction in the expression of these cytokines and of malondialdehyde, suggesting that these diets lowered markers of oxidative stress and neuroinflammation.
Given these results, we were interested in investigating further the roles of reactive oxygen species and inflammatory processes in the cerebellum of aging animals that were fed the spinach-enriched diet. We used classical eyeblink conditioning, the delay paradigm, as a measure of cerebellar-dependent procedural learning in older rats, and we examined the expression of the proinflammatory cytokines TNFα and TNFβ in cerebellar tissue from animals given 6 weeks of the spinach-enriched diet.
MATERIALS AND METHODS
Animals. Twenty 18-month-old male Fischer 344 rats were housed in the Colorado Veterans Administration Medical Center (Denver, CO) animal colony under the supervision of the animal care staff, the institutional animal care and use committee, and the veterinarians of the University of Colorado Health Science Center (Denver, CO). Animals were housed in pairs before surgery and singly after surgery and were kept on a 12 hr light/dark schedule withad libitum access to food and water. Ten animals were given NIH-31 (TD 00365; Harlan Teklab, Madison, WI) rodent diet and 10 animals were given NIH-31 rodent diet supplemented with 0.02% w/w dry spinach (freeze-dried powder; Van Drunen Farms, Momence, IL). Animal weight and food intake were monitored every other day to determine general health and the ingestion of chow. No differences in these indexes were observed between groups.
Surgery. All animals were given 1 mg/ml acetaminophen analgesic in their drinking water 24 hr before surgery. Surgeries began 34 d after the beginning of the diet. At the time the surgeries were performed, the animals were 19 months of age. One spinach-fed animal and three control animals had died of natural causes during the intervening month. The remaining seven control animals and nine spinach-fed animals were anesthetized for surgery using ketamine/xylazine (80 mg/kg, 12 mg/kg). The cranium was exposed, and three stainless-steel skull screws were placed in the bone to anchor the headstage. Two EMG wires were run under the skin of the upper eyelid so that they protruded at the distal edge of the eyelid. The distal end of the wire was stripped of the Teflon coating to a point ∼3 mm under the skin of the eyelid. The two wires were placed at least 6 mm apart on the eyelid. A third wire was fixed to one of the skull screws to serve as a ground wire. Headstages were coupled to a cable from a commutator in the training chamber. The headstage was fixed to the head of the animal using dental acrylic. On day 10 after surgery, the animals were handled for 15 min and habituated to the training chamber for 0.5 hr. On the day after habituation, training began.
Data collection. The recording chamber was a melamine-coated box (22 × 22 × 45 cm) with a plastic door that allowed the rat to be observed from the front. This box was set inside a larger sound-attenuating chamber. The larger box was outfitted with a ventilation fan and a speaker for delivery of the tone. The airpuff pressure was controlled with a two-stage regulator. The EMG signal was directed through a field effect transistor and amplified 1000 times. The EMG signal was rectified, integrated, and monitored using a digital storage oscilloscope. The tone was a 3 kHz, 90 dB sine wave signal. Delivery of the training trials and collection of EMG data were accomplished using a Data Wave Technologies Control Box (Loveland, CO). Data collected from the rats were analyzed using the DataMunch (J. Tracy, D. King, and J. E. Steinmetz, Indiana University, Bloomington, IN) software program.
Training parameters. For each of the treatment groups, daily training sessions consisted of 30 training trials. The training trials were grouped into three blocks of 10 trials, each block consisting of one tone-alone trial and nine paired trials. Trials were separated by randomized 10–30 sec intertrial intervals.
Data analysis. The percentage of conditioned responses (CRs) that animals made in a daily training session was used as a measure of learning. A conditioned response was defined as movement of the eyelid that was significantly greater than baseline and that occurred in the last 200 msec of the conditioned stimulus (CS) period and before the unconditioned stimulus period. Our criterion for identifying conditioned responses was 10 SDs above the mean pre-CS EMG baseline. A discrimination window of 50 msec after the tone was used to avoid having spontaneous blinks to the tone recorded as conditioned responses. Performance of the learned behavior was evaluated by examining the amplitude of the conditioned response, the unconditioned response (UR), and the latency-to-peak of the conditioned response. Bad trials were identified as any trial during which baseline EMG activity in the last 80 msec of the pre-CS period deviated by >10 SDs from the mean of the activity recorded during that period. Bad trials were removed before data analysis. All parameters were analyzed for each daily training session.
RNA isolation and RNase protection assay. The five living control diet-fed rats and the six living spinach diet-fed rats were killed 3 weeks after eyeblink training. In addition, five completely naive 4-month-old rats were killed for comparison of baseline cytokine levels. All animals were killed by decapitation. The brains were removed, dissected, frozen in liquid nitrogen, and then stored at −80°C until total RNA was extracted (Gemma et al., 2000). Useable tissue samples with sufficient RNA were obtained from four spinach-fed rats, five control diet-fed rats, and five naive young rats.
Total RNA was extracted from whole cerebellar dissections using the Qiagen Rneasy minikit (Qiagen, Valencia, CA). A total of 20 μg of total RNA from each sample was hybridized with antisense, radiolabeled probes. Remaining single-stranded RNA was digested with RNaseA/T1. Double-stranded RNase-protected fragments were resolved on 5% denaturing polyacrylamide gels, and a PharMingen (San Diego, CA) probe template was used for identification of TNFα and TNFβ. A positive control transcript was made using a probe specific for the ribosomal protein L32, a housekeeping gene, to calculate the specific activity and achieve a sufficient excess of probe over target for L32. L32 probe was added to the probe template before the hybridization reaction started. Yeast transfer RNA and rat mRNA were used as negative and positive controls, respectively. Dried gels were placed on a phosphorimager screen for 16–20 hr. The screen was scanned with a phosphorimager (Molecular Image System GS-363; Bio-Rad, Hercules, CA), and the images were processed using Molecular Analyst software (Bio-Rad). Optical density values obtained from each band were normalized against the optical density obtained from the L32 band in each sample using the following expression: (optical density of sample band/optical density of the L32 band × 100).
RESULTS
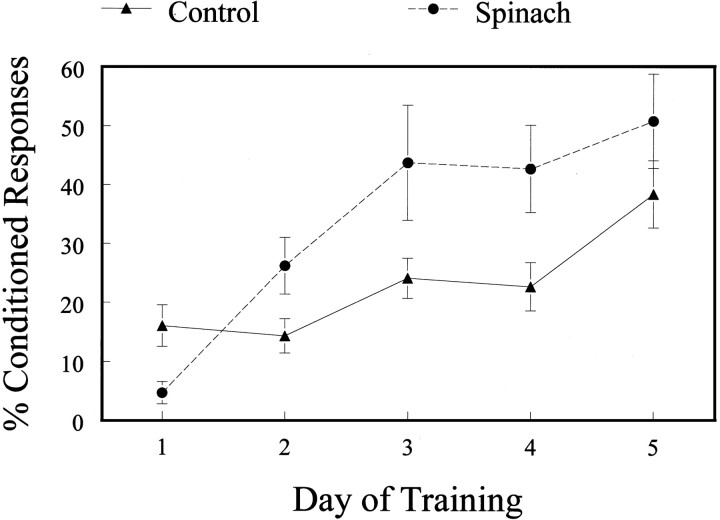
The two groups of aged rats were tested for delay eyeblink conditioning. The aged spinach-fed rats showed significantly faster learning than aged rats fed the control diet (standard mixed-effects model with repeated measures; F(3,15)= 6.12; p < 0.002) (Laird and Ware, 1982) (Fig.1). Conversely, measures of behavioral performance, including conditioned response amplitude and timing and reflexive response amplitude and timing, were not different between groups (ANOVA with repeated measures: CR amplitude,F(1,14) = 0.701, NS; CR timing,F(1,14) = 0.241, NS; UR amplitude, F(1,14) = 2.394, NS; UR timing, F(1,14) = 1.215, NS), but there was a significant interaction for days of training and CR amplitude (F(1,4) = 5.275;p > 0.001), with conditioned responses becoming larger over days of training in both groups.
Fig. 1.
Learning is expressed as the percentage of conditioned responses made in each daily training session. Daily training sessions consisted of 27 paired trials and three CS-alone trials. Rats fed a spinach-enriched diet showed significantly faster learning (analysis with a standard mixed-effects model and repeated measures).
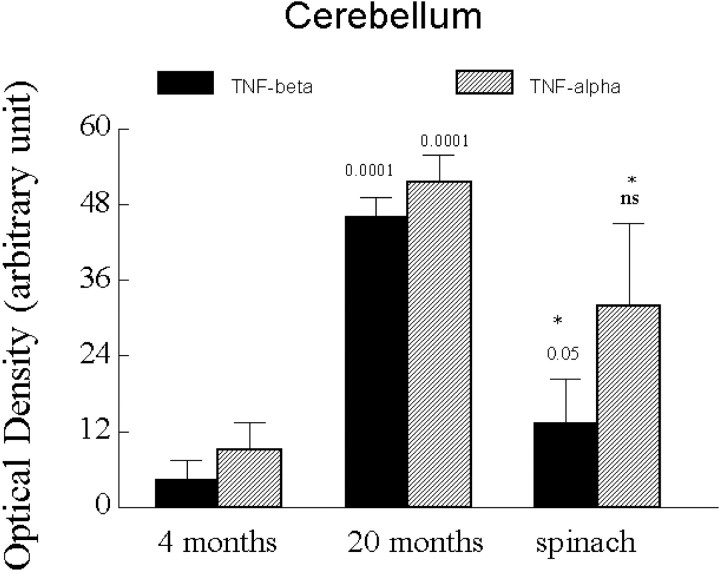
Results of the RNase protection assay (RPA) indicate significant differences between age and diet groups for both TNFα and TNFβ (ANOVA; F(2,11) = 31.812;p > 0.001). Post hoc analysis using Fisher's PLSD indicates significant differences between 4-month-old and 20-month-old control diet-fed rats for both TNFα and TNFβ (p = 0.0001). In addition, the spinach-fed animals were significantly different from the control diet-fed animals for each cytokine (TNFα, p = 0.05; TNFβ,p = −0.001). Finally, although TNFα mRNA levels in spinach diet-fed rats remained significantly higher than in 4-month-old naive rats (p = 0.05), mRNA for TNFβ in the spinach diet-fed rats was not significantly different from that seen in 4-month-old naive rats (Fig. 2).
Fig. 2.
RPA results show that older rats fed a spinach-enriched diet for 6 weeks have a significantly reduced level of TNFα and TNFβ compared with aged rats fed a standard control diet (asterisk denotes significance to 0.05). The levels of TNFα mRNA in the spinach-fed older animals remain significantly elevated over levels of TNFα in 4-month-old animals fed the control diet (significant to 0.05). However, levels of TNFβ mRNA in old spinach-fed rats are not significantly different from levels of TNFβ in young rats. ns, Not significant. Levels of TNFα and TNFβ in older control diet-fed rats were significantly different from 4-month-old animals (significant to 0.0001).
DISCUSSION
Our results indicate that older animals show an increased rate of learning of the delay eyeblink conditioning paradigm after 6 weeks of receiving a diet enriched with 2% w/w spinach. In the same animals, levels of the proinflammatory cytokines TNFα and TNFβ were significantly reduced in the spinach-fed animals compared with the control diet-fed animals. In fact, in the spinach-fed animals, the levels of TNFβ were not different from levels found in 4-month-old control rats. This study shows improvement in eyeblink learning after dietary intervention with an antioxidant-rich supplement. These results indicate that there may be a link between the reduction of inflammatory processes and improvement in learning in older animals.
Spinach is a proven antioxidant. Studies conducted in our lab and in collaboration with others (Joseph et al., 1998) have shown that older rats fed a spinach-enriched diet show improvement on varying measures of age-related decline including: modulation of GABA by isoproteronol in cerebellar Purkinje neurons, Morris water maze reference memory tests, calcium recovery or sequestration after neuronal action potentials, and dichlorofluorescein fluorescence measures of reactive oxygen species production. Spinach is clearly an important agent for some kinds of change that benefit physiological processes.
In previous studies we have examined in some detail how oxidative stress and aging affect the cerebellum, and more particularly, how they affect the role of norepinephrine in cerebellar physiology and cerebellar-dependent learning. We have shown in older rats that the β-noradrenergic signal transduction cascade in Purkinje cells is compromised (Gould and Bickford, 1997) and that performance of a cerebellar-dependent motor learning task is also compromised (Gould and Bickford, 1996). We have examined the role of oxidative stress in bringing about the changes we observe in the noradrenergic system. The results of our investigations have shown that vitamin E, the spin-trapping agents α-phenyl-N-tert-butylnitrone and 3,4-dihydro-3,3-dimethyl-isoquinoline-2-oxide, and diets supplemented with antioxidant-rich foods restore function at the level of the β receptor in older rats (Gould and Bickford, 1996; Gould et al., 1998; Bickford et al., 2000).
Our current results support the theory that cerebellar physiology is intrinsic to learning in some contexts, and that oxidative stress and neuroinflammation play a role in cerebellar physiology that is intrinsic to learning and memory. The delay paradigm of classical eyeblink conditioning is of interest to us as a measure of cerebellar-dependant learning. Deficits in eyeblink learning with age are well documented in cats, rats, rabbits, and humans (Powell et al., 1981; Harrison and Buchwald, 1983; Graves and Solomon, 1985;Woodruff-Pak and Thompson, 1988; Weiss and Thompson, 1991; Knuttinen et al., 2001). The delay eyeblink classical conditioning paradigm has proven to be an important and accurate measure of age-related deficits in cerebellar function in humans. Poor performance on delay eyeblink conditioning is being suggested as a predictor of Alzheimer's disease, well in advance of cognitive signs of the disease (Woodruff-Pak et al., 1990; Woodruff-Pak and Papka, 1996). As yet we know too little about the cellular changes that encode eyeblink learning and the processes of aging that break it down. The results from our spinach study suggest that immunological factors could play a role in that breakdown.
Beneficial effects on inflammatory processes of a high intake of fruits and vegetables may not be related just to antioxidants such as vitamins E, C, A, and β-carotene (Martin et al., 2000), but rather to some antioxidant or nonantioxidant phytochemicals, and is likely to be the combination of more than one component (Cao et al., 1998). Flavonoids and other phenolic compounds appear to be antioxidants that contribute to the high antioxidant capacity observed in certain fruits and vegetables. There are studies that have shown that flavonoids inhibit the production of nitric oxide and the expression of inducible nitric oxide synthase mRNA by murine macrophages (Chen et al., 2001).
A study from our lab (Gemma et al., 2002) has demonstrated for the first time that the beneficial effect of antioxidant-rich diets on cytokine expression is dependent on the ORAC dose present in each diet. Either apple- or spirulina-enriched diets (both high in ORAC activity) but not cucumber (a food with low ORAC activity) completely reversed the overexpression of proinflammatory cytokines observed in the cerebellum of old rats and improved β-adrenergic receptor function in aged rats as well, suggesting an ORAC dose-dependent effect.
The results of our spinach study show that an increase in mRNA expression of the proinflammatory cytokines TNFα and TNFβ in the cerebellum of old rats was significantly reversed when aged animals were fed for 6 weeks with the spinach-supplemented diet, suggesting that one mechanism by which the enriched antioxidant diet works is to modulate an age-related increase in inflammatory responses. Further study will be required, however, to determine what mechanisms are at work in bringing about changes in inflammatory processes, and whether these changes are related to increased learning capacity in older subjects.
Footnotes
This research was supported by National Institutes of Health Grant AG04418 and National Science Foundation Grant NSFIBN0196474.
Correspondence should be addressed to Paula C. Bickford, Center for Aging and Brain Repair MDC-78, University of South Florida, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612. E-mail: pbickfor@hsc.usf.edu.
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 3.Cao G, Sofic E, Prior RL. Antioxidant capacity of tea and common vegetables. J Agric Food Chem. 1996;44:3426–3431. [Google Scholar]
- 4.Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine, or vitamin C in elderly women. J Nutr. 1998;128:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-C, Shen S-C, Lee W-R, Hou W-C, Yang L-L, Lee TJF. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J Cell Biochem. 2001;82:537–548. doi: 10.1002/jcb.1184. [DOI] [PubMed] [Google Scholar]
- 6.Gemma C, Smith EM, Hughes TKJ, Opp MR. Human immunodeficiency virus glycoprotein 160 induces cytokine mRNA expression in the rat central nervous system. Cell Mol Neurobiol. 2000;20:419–431. doi: 10.1023/A:1007053129686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar β-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould TJ, Bickford PC. The effects of aging on cerebellar β-adrenergic receptor activation and motor learning in female F344 rats. Neurosci Lett. 1996;216:53–56. doi: 10.1016/0304-3940(96)13007-x. [DOI] [PubMed] [Google Scholar]
- 9.Gould TJ, Bickford PC. Age-related deficits in the cerebellar β adrenergic signal transduction cascade in Fischer 344 rats. J Pharmacol Exp Ther. 1997;281:965–971. [PubMed] [Google Scholar]
- 10.Gould TJ, Chadmam K, Bickford PC. Antioxidant protection of cerebellar β-adrenergic receptor function in aged F344 rats. Neurosci Lett. 1998;250:165–168. doi: 10.1016/s0304-3940(98)00477-7. [DOI] [PubMed] [Google Scholar]
- 11.Graves CA, Solomon PR. Age-related disruption of trace but not delay classical conditioning of the rabbit's nictitating membrane response. Behav Neurosci. 1985;99:88–96. doi: 10.1037//0735-7044.99.1.88. [DOI] [PubMed] [Google Scholar]
- 12.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:289–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 13.Harrison J, Buchwald J. Eyeblink conditioning deficits in the old cat. Neurobiol Aging. 1983;4:45–51. doi: 10.1016/0197-4580(83)90053-2. [DOI] [PubMed] [Google Scholar]
- 14.Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-α after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- 17.Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects on eyeblink conditioning in the F344 × Bn F1 hybrid rat. Neurbiol Aging. 2001;22:1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 19.Leibovitz BE, Siegel BV. Aspects of free radical reactions in biological systems: aging. J Gerontol. 1980;35:45–56. doi: 10.1093/geronj/35.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1β? Prog Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 21.Martin A, Prior R, Shukitt-Hale B, Cao G, Joseph JA. Effect of fruits, vegetables, or vitamin E-rich diet on vitamins and C distribution in peripheral and brain tissues: implication for brain function. J Gerontol A Biol Sci Med Sci. 2000;55:B144–B151. doi: 10.1093/gerona/55.3.b144. [DOI] [PubMed] [Google Scholar]
- 22.Murray C, Lynch MA. Impaired ability of aged animals to sustain long-term potentiation may result from increased hippocampal expression of interleukin-1β. J Physiol (London) 1997;501:87p. [Google Scholar]
- 23.Murray C, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell DA, Buchanan SL, Hernandez LL. Age-related changes in classical (Pavlovian) conditioning in the New Zealand albino rabbit. Exp Aging Res. 1981;7:453–465. doi: 10.1080/03610738108259824. [DOI] [PubMed] [Google Scholar]
- 25.Rai SS, Shovlin C, Wesnes KA. A double-blind placebo controlled study of Ginkgo biloba extract (“tanakan”) in elderly outpatients with mild to moderate memory impairment. Curr Med Res Opin. 1991;12:350–355. doi: 10.1185/03007999109111504. [DOI] [PubMed] [Google Scholar]
- 26.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, α-tocopherol, or both as treatment for Alzheimer's disease. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 27.Weiss G, Thompson RF. The effects of age on eyeblink conditioning in freely moving F344 rats. Neurobiol Aging. 1991;12:249–254. doi: 10.1016/0197-4580(91)90105-s. [DOI] [PubMed] [Google Scholar]
- 28.Woodruff-Pak DS, Papka M. Alzheimer's disease and eyeblink conditioning: 750 ms trace vs 400 ms delay paradigm. Neurobiol Aging. 1996;17:397–404. doi: 10.1016/0197-4580(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 29.Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18–83 years. Psychol Aging. 1988;3:1–11. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff-Pak DS, Finkbiner RG, Sasse DK. Eyeblink conditioning discriminates Alzheimer's patients from nondemented aged. NeuroReport. 1990;1:45–48. doi: 10.1097/00001756-199009000-00013. [DOI] [PubMed] [Google Scholar]