Abstract
Receptor-mediated feedback control plays an important role in dopamine (DA) neurotransmission. Recent evidence suggests that release and uptake, key mechanisms determining brain extracellular levels of the neurotransmitter, are governed by presynaptic autoreceptors. The goal of this study was to investigate whether autoreceptors regulate both mechanisms concurrently. Extracellular DA in the caudate–putamen and nucleus accumbens, evoked by electrical stimulation of the medial forebrain bundle, was monitored in the anesthetized rat by real-time voltammetry. Effects of the D2 antagonist haloperidol (0.5 mg/kg, i.p.) on evoked DA levels were measured to evaluate autoreceptor control mechanisms. Two strategies were used to resolve individual contributions of release and uptake to the robust increases in DA signals observed after acute haloperidol challenge in naive animals: pretreatment with 3β-(p-chlorophenyl)tropan-2β-carboxylic acidp-isothiocyanatophenylmethyl ester hydrochloride (RTI-76; 100 nmol, i.c.v.), an irreversible inhibitor of the DA transporter, and kinetic analysis of extracellular DA dynamics. RTI-76 effectively removed the uptake component from recorded signals. In RTI-76-pretreated rats, haloperidol induced only modest increases in DA elicited by low frequencies and had little or no effect at high frequencies. These results suggest that D2 antagonism alters uptake at all frequencies but only release at low frequencies. Kinetic analysis similarly demonstrated that haloperidol decreasedVmax for DA uptake and increased DA release at low (10–30 Hz) but not high (40–60 Hz) stimulus frequencies. We conclude that presynaptic DA autoreceptors concurrently downregulate release and upregulate uptake, and that the mechanisms are also independently controlled during neurotransmission.
Keywords: dopamine, autoreceptors, release, uptake, striatum, voltammetry
Release and uptake are primary components of dopamine (DA) neurotransmission. The release of DA into the synaptic cleft and its subsequent diffusion to target cells initiates signaling, which is terminated by a transporter clearing the neurotransmitter from extracellular space (Cooper et al., 1991). Autoreceptors provide important feedback control during DA signaling by governing firing rate, synthesis, and release (Starke et al., 1989;Wolf and Roth, 1990; Bunney et al., 1991). The efficacy of release-regulating autoreceptors is clearly demonstrated in vitro by potent D2 inhibition of DA levels elicited by a single electrical pulse (Palij et al., 1990; Kennedy et al., 1992) and rapid (<100 msec) response after receptor activation (Mayer et al., 1988; Cejna et al., 1990). More recent identification of uptake-regulating autoreceptors (Meiergerd et al., 1993; Wieczorek and Kruk, 1994; Rothblat and Schneider, 1997; Dickinson et al., 1999;Hoffman et al., 1999; Mayfield and Zahniser, 2001) suggests complex presynaptic control of DA neurotransmission. Indeed, autoreceptors may contribute to regional variation in extrasynaptic communication determined by differential dopamine transporter (DAT) activity (Garris et al., 1994; Cline et al., 1995; Cragg et al., 2001). DAT gene deletion also reveals an intimate association among transporter, autoreceptor, and terminal homeostasis (Jones et al., 1998, 1999).
Extracellular DA dynamics elicited by pulse train stimulation reflect the balance between the opposing actions of release and uptake (Wightman and Zimmerman, 1990). Because individual components are resolved by real-time voltammetry and mathematical means (Wightman et al., 1988; Peters and Michael, 2000), these evoked signals are ideally suited for investigating autoreceptor interactions. Interestingly, only release-regulating autoreceptors have been identified using this approach in vivo (May and Wightman, 1989; Kawagoe et al., 1992; Wiedemann et al., 1992). Whether this result reveals properties of integrative autoreceptor control of DA neurotransmission in the intact brain or experimental limitations requires additional investigation.
The present study examined whether autoreceptors govern DA release and uptake concurrently. Extracellular DA was monitored in the striatum of anesthetized rats by real-time voltammetry and evoked by electrical stimulation of the medial forebrain bundle (Garris and Wightman, 1995a). The D2 antagonist haloperidol was used to evaluate autoreceptor function. Although feedback control of DA neurons is achieved at multiple levels, the combination of electrical stimulation and voltammetry permits investigation of presynaptic autoreceptor function in vivo after systemic drug administration. Dopamine neurons faithfully track the exogenous pulse train but become quiescent during the period immediately after (Kuhr et al., 1987). Consequently, haloperidol-induced changes in the voltammetric record primarily reflect presynaptic autoreceptor blockade (Benoit-Marand et al., 2001). Two new strategies were used to resolve the evoked signals into the individual components of DA release and uptake. The first pharmacologically isolated release from uptake by pretreatment with 3β-(p-chlorophenyl)tropan-2β-carboxylic acidp-isothiocyanatophenylmethyl ester hydrochloride (RTI-76), an irreversible DAT inhibitor (Fleckenstein et al., 1996). The second exploited kinetic analysis permitting evaluation of release and uptake without identifying drug mechanism a priori (Wu et al., 2001a). Thus, these experiments sought to investigate autoregulation of DA neurotransmission on a fundamental level.
MATERIALS AND METHODS
Animals. Male Sprague Dawley rats (weighing 300–450 gm) were purchased from Harlan Sprague Dawley (Indianapolis, IN) and housed under controlled lighting, temperature, and humidity. Food and water were available ad libitum. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 86-23) and was approved and monitored by the Institutional Animal Care and Use Committee of Illinois State University.
Surgery. The surgery to prepare animals for in vivo voltammetry has been described previously (Bergstrom and Garris, 1999). After anesthesia with urethane (1.5 gm/kg, i.p.), animals were placed on a Deltaphase isothermal pad (Braintree Scientific, Braintree, MA) to maintain body temperature and immobilized in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Skin and muscle layers covering the skull were retracted, and holes were drilled through the skull for placement of reference, stimulating, and working electrodes. All stereotaxic coordinates described herein are given in millimeters according to the atlas of Paxinos and Watson (1986). Anteroposterior (AP) and mediolateral (ML) coordinates are referenced from bregma, and dorsoventral (DV) coordinates are referenced from dura. A working electrode was implanted into both the caudate–putamen (CP; +1.2 AP, +2.0 ML, and −4.5 to −5.5 DV) and the nucleus accumbens (NAc; +1.4 AP, +2.0 ML, and −6.6 to −7.5 DV). To accommodate two working electrodes on one side of the brain, the microsensor in the CP was angled at 12° (Wu et al., 2001a,b). The working electrodes were also offset slightly in the AP direction so that the tip of the microsensor in the CP would never contact the shaft of the microsensor implanted in the NAc. At a minimum, the microsensor tips differed by 1.1 mm dorsoventrally; typically, this distance was much larger, and sensors were also located at different ML coordinates. A stimulating electrode was lowered to a position just dorsal to the medial forebrain bundle (−4.6 AP, +1.4 ML, and −7.0 DV) and incrementally lowered until a signal, voltammetrically identified as DA (Baur et al., 1988), was observed in both the CP and NAc. The position of the stimulating electrode was then optimized to elicit a maximum response. The reference electrode was situated in superficial cortex contralateral to stimulating and working electrodes.
Electrochemistry. Fast-scan cyclic voltammetry at carbon fiber microelectrodes was used to monitor DA (Garris and Wightman, 1995a). Cylinder microelectrodes were fabricated as described previously (Cahill et al., 1996). Approximately 50–100 μm of the carbon fiber (radius = 2.5 μm) extended beyond the glass insulation. Electrochemistry was computer-controlled using an EI 400 potentiostat (Ensman Instruments, Bloomington, IN). The potential of the working electrode, which rested at a bias of −400 mV, was linearly scanned at 100 msec intervals to 1000 mV and back at a rate of 300 V/sec. The analog output of the potentiostat was digitized (DMA Labmaster; Scientific Solutions, Solon, OH) and stored in a computer file using locally written software. The DA concentration was calculated from the current at the peak oxidation potential for DA (typically 500–700 mV) using a calibration factor determined for each working electrode after the experiment. Background-subtracted voltammograms were obtained by subtracting voltammograms collected during baseline recording from those during electrical stimulation. All voltages were referenced to a silver/silver chloride electrode. After each experiment, the working electrode was removed from the brain and calibrated in vitro using flow injection analysis.
Electrical stimulation. Biphasic stimulus pulses (4 msec and 300 μA for each phase) were computer-generated, passed through a constant current and optical isolation device (NL 800, Neurolog; Medical Systems, Great Neck, NY), and applied to a twisted, bipolar stimulating electrode (MS 303/2; Plastics One, Roanoke, VA). Tips of the stimulating electrode (0.2 mm diameter) were separated by ∼1 mm. For each pulse train, the duration was 2 sec, and the frequency randomly varied from 10 to 60 Hz.
Experimental design. Autoreceptors governing DA release and uptake were evaluated by examining the effects of haloperidol on electrically evoked levels of DA measured by in vivovoltammetry. Two strategies were used to determine whether the D2 antagonist altered the release or uptake component, or both, of these signals. The first was pretreatment with the RTI-76, an irreversible inhibitor of DAT (Fleckenstein et al., 1996; Wang et al., 2000). The rationale was that RTI-76 nearly completely removes the component of uptake (Wu et al., 2001b). Consequently, the effects of haloperidol on evoked DA levels after pretreatment primarily reflected disinhibition of release-regulating autoreceptors. These results were then compared with haloperidol effects in naive animals, in which both mechanisms were fully operational, to determine the respective contribution of release- and uptake-regulating autoreceptors.
The second strategy for resolving release and uptake components was kinetic analysis, based on the neurochemical model developed byWightman et al. (1988). As described below in more detail, the model characterizes measured signals in terms of one parameter for DA release and two parameters for DA uptake. The constants are determined by fitting experimental data to curves simulated by the model (Wu et al., 2001a). Thus, observed haloperidol-induced increases in evoked DA levels are reduced to a change in parameters for either DA release or uptake. Kinetic analysis in the present study differs from earlier attempts to evaluate the effects of D2antagonists on the voltammetric measurements, because all three parameters were determined in drug-naive animals and after drug administration. Previously, a Km for DA uptake and a partial drug mechanism were assumed a priori(May and Wightman, 1989; Kawagoe et al., 1992; Wiedemann et al., 1992).
Kinetic analysis. Temporal changes in brain extracellular DA during transient electrical stimulation are described as a balance between the opposing processes of DA release and uptake (Wightman et al., 1988):
| Equation 1 |
where f is the frequency of the stimulation, [DA]p is the concentration of extracellular DA released per stimulus pulse, and Vmaxand Km are Michaelis–Menten parameters for DA uptake. The model does not describe release caused by the transporter-mediated efflux of DA (Eshleman et al., 1994; Johnson et al., 1998), but this amount most likely does not appreciably contribute to measured electrically evoked levels. After the electrical pulse train, the rate of [DA] change is solely described by DA uptake:
| Equation 2 |
Vmax was calculated by measuring the linear clearance rate of DA concentrations evoked by a frequency of 60 Hz. At high concentrations, at which [DA] ≫Km, Equation 2 reduces to:
| Equation 3 |
After fixing Vmax to this value, [DA]p andKm were obtained by simultaneously fitting recordings describing the frequency-dependent changes in DA levels to Equations 1 and 2. Curve fitting was accomplished by nonlinear regression using simplex minimization (Wu et al., 2001a).
Pretreatment with RTI-76. RTI-76 was microinjected intracerebroventricularly either 1 or 2 d before voltammetric experiments (Wu et al., 2001b). For the injection procedure, rats were anesthetized with Equithesin (3 ml/kg, i.p.) and placed in a stereotaxic apparatus as described above. A single hole was drilled through the skull for placement of the injection needle (30 ga hypodermic tubing sharpened at the tip; Small Parts Inc., Miami Lakes, FL). The needle was lowered to −0.25 AP, 1.4 ML, and −4 to −5 DV, and 100 nmol of RTI-76, dissolved in 10 μl of sterile saline, was infused at a flow rate of 0.5 μl/min using a microsyringe pump (KD Scientific model 100; Fisher Scientific, Fair Lawn, NJ). The injection site was ipsilateral to sites for voltammetric recordings. After injection, the needle remained at the injection site for an additional 5 min. The needle was then retracted, the hole in the skull was sealed with bone wax, and the scalp was sutured. Animals were returned to housing only after the effects of anesthesia had worn off and used for experiments 1–2 d later.
Radioligand binding assay. Binding of RTI-76 to D2 receptors was investigated in vitroin rat striatal homogenates according to the method of Walker et al. (1990). Coronal slices (2.0 mm) were cut from freshly dissected rat brain using an ice-cold block, and the dorsal striatum was excised (Heffner et al., 1980). Striatal tissue was frozen at −80°C until used. Frozen tissue was homogenized with seven manual strokes in 8 ml of buffer (in mm: 50 HEPES and 4.0 MgCl2, pH 7.4) in a Teflon–glass homogenizer. The homogenate was spun for 10 min at 27,000 × g, and the supernatant was discarded. The pellet was resuspended in buffer using 5 strokes and centrifuged again. The resulting pellet was resuspended at 2 mg wet weight/ml, and 1 mg of tissue was added to each assay tube. D2 receptors were labeled with [3H]spiperone (0.07 nm). Ketanserin (40 nm) was added to each tube to mask [3H]spiperone binding to serotonin receptors, and nonspecific binding was determined by the addition of 1 μm chlorpromazine. Assay tubes (1 ml final volume) were incubated for 15 min at 37°C. Binding was terminated by filtering with 15 ml of ice-cold buffer across glass fiber filter mats using a Brandel (Gaithersburg, MD) cell harvester. Radioactivity left on the filters was determined on a Packard (Meridian, CT) Tri-Carb scintillation counter.
Data were normalized by expressing the average disintegrations per minute at each competitor concentration as a percentage of total binding. The percent inhibition values were averaged across all experiments (n = 5). An IC50value was calculated from each specific binding curve using a nonlinear regression algorithm for sigmoid curves (Prism 3.0; Graph Pad, San Diego, CA). The goodness of fit to the equation (r2 value) was typically >0.99.
Statistical analysis. Where possible, data are expressed as the mean ± SEM, where n is the number of rats. Statistical analysis was performed by SAS (Cary, NC) and used either at test or ANOVA (Sokal and Rohlf, 1995). Linear regression was performed by SigmaPlot (SPSS, Chicago, IL). The significance of the regression coefficient (r) was determined by calculating at statistic (ts):
| Equation 4 |
The significance level was set at p < 0.05 for all tests.
Drugs and reagents. Unless indicated, all chemicals and drugs were used as received and purchased from Sigma or Research Biochemicals–Sigma (St. Louis, MO). [3H]Spiperone was purchased from PerkinElmer Life Sciences (Emeryville, CA). RTI-76 was synthesized at Research Triangle Institute. Aqueous solutions were prepared in doubly distilled, deionized water (Barnstead/Themolyne, Dubuque, IA).
RESULTS
RTI-76 binding to D2 receptors
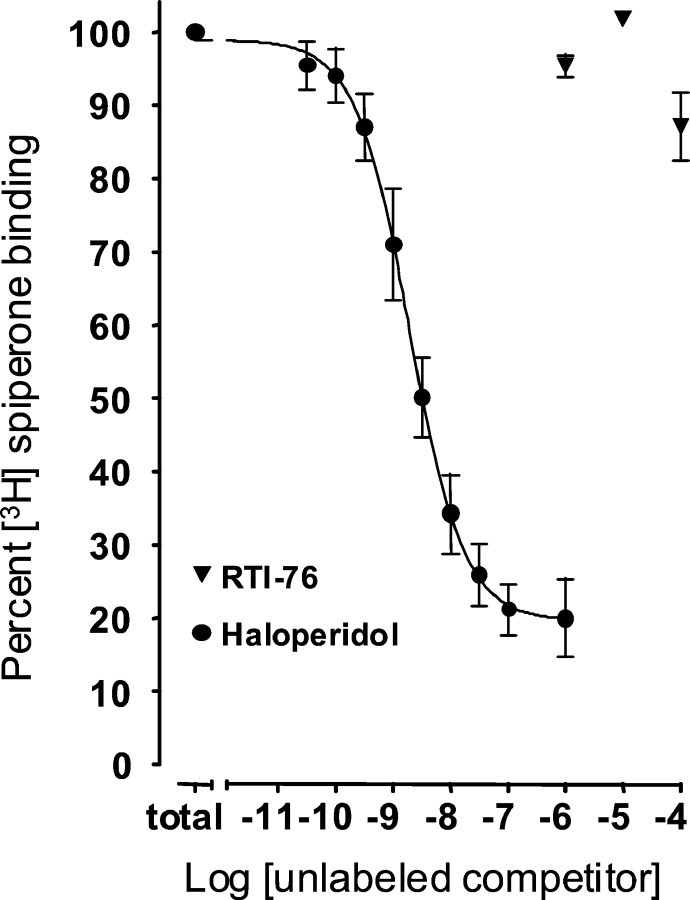
Figure 1 shows the average of all competition binding experiments for D2 receptors labeled with [3H]spiperone. Unlike haloperidol, RTI-76 did not prove to be an effective antagonist of [3H]spiperone binding to D2 receptors. Haloperidol (1 μm) blocked ∼80% of specific [3H]spiperone binding, whereas RTI-76 was ineffective at 1 and 10 μm and blocked no more than 12% of [3H]spiperone binding at 100 μm. The IC50 for haloperidol was 2.0 ± 0.4 nm (n = 5), indicating high potency, and the r2 for the regression line was 0.999. By contrast, 1 μmRTI-76 decreases Bmax for [3H]2β-carbomethoxy-3β-(4-fluorophenyl) tropane binding to human DAT expressed in human embryonic kidney 293 cells by ∼55% (Wang et al., 2000). RTI-76 decreasedVmax for dopamine uptake in the present studies to a similar level (see Figs. 7 and 8), suggesting that a similar concentration of RTI-76 may have been present in the tissue surrounding the microsensor. At this tissue concentration of RTI-76 (or even at 10 or 100 μm), the present in vitro results indicate that few D2 receptors would be occupied by RTI-76.
Fig. 1.
Haloperidol and RTI-76 competition for D2 receptors labeled by [3H]spiperone. Data for each experiment were expressed as a percentage of total binding, which is the lowest concentration point on the haloperidol curve. Data were compiled from five separate experiments. Eachpoint is the mean; error bars indicate SEM.
Fig. 7.
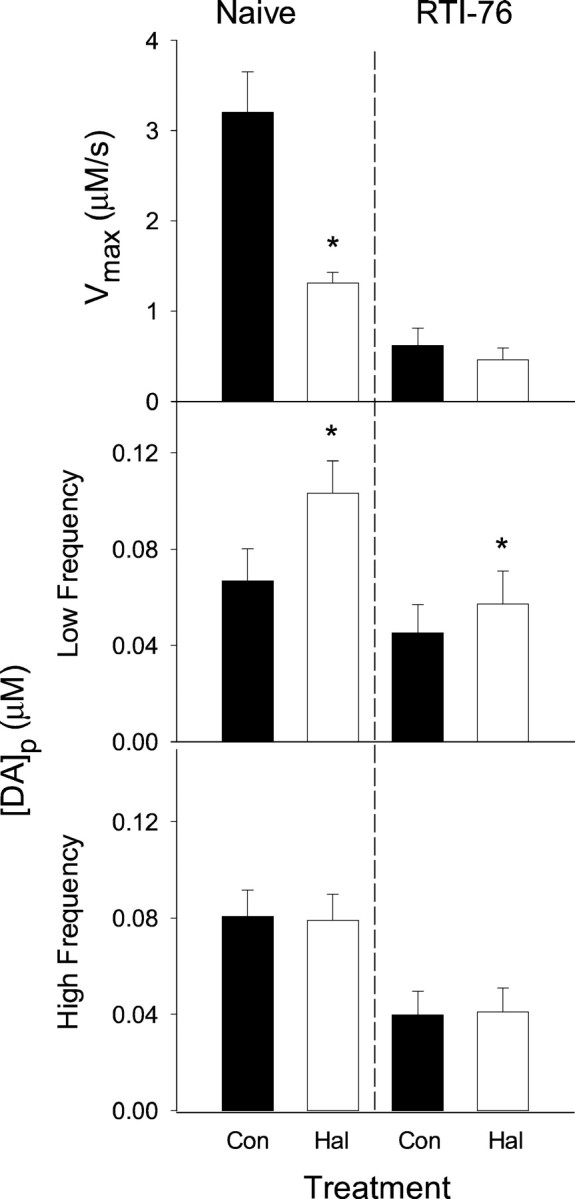
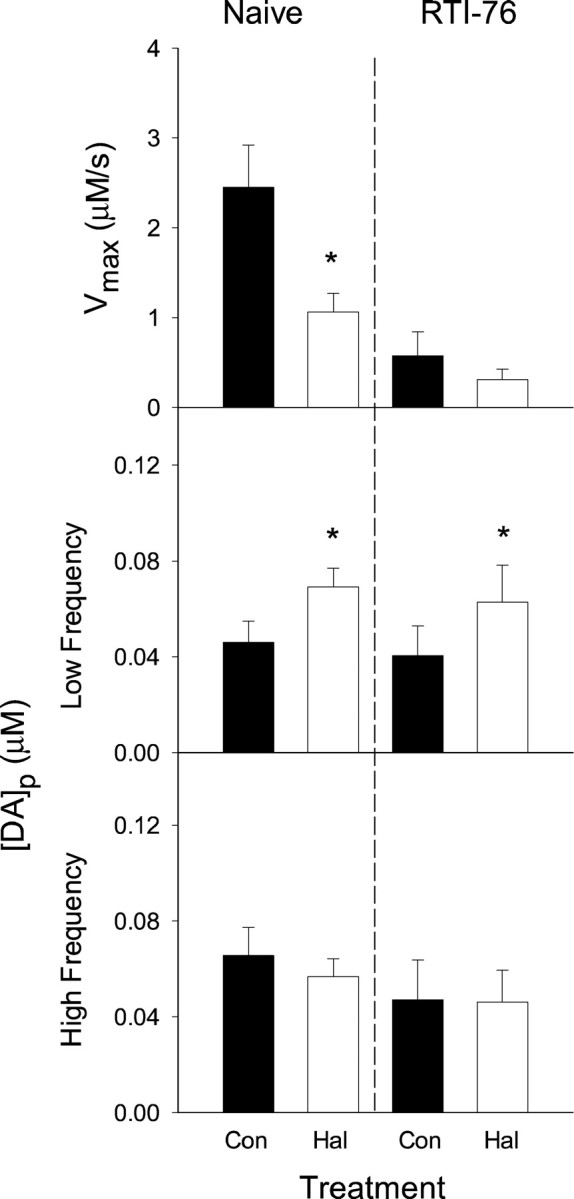
Parameters for DA release and uptake in the CP of naive and RTI-76-pretreated animals. Evoked DA responses shown representatively in Figures 2 and 4 were kinetically evaluated to determine parameters for DA release and uptake. Data are mean ± SEM (n = 8–9). Parameters calculated in naive and RTI-76-pretreated animals are shown in the left andright graphs, respectively. These were calculated from baseline recordings (either naive or RTI-76) or from recordings collected after haloperidol (Hal) administration (either Hal or RTI-76 and Hal), and are described by thefilled and open bars, respectively.Top graphs, Vmax.Middle graphs, [DA]p calculated from curves evoked by frequencies between 10 and 30 Hz. Haloperidol significantly increased [DA]p in naive animals (t = 4.35; p < 0.002) and in animals after RTI-76 pretreatment (t = 3.73;p < 0.01). Bottom graphs, [DA]p calculated from curves evoked by frequencies between 40 and 60 Hz. Because Km was found to be similar at low and high frequencies, an average was taken to represent all frequencies. The resulting values are as follows: naive, 0.39 ± 0.07 μm; Hal, 0.89 ± 0.21 μm; RTI-76, 0.53 ± 0.09 μm; and RTI-76 and Hal, 0.83 ± 0.16 μm. No significant differences were found among the Km values of the four experimental groups (F(3,28) = 2.78; p> 0.06; ANOVA). Con, Control.
Fig. 8.
Parameters for DA release and uptake in the NAc of naive and RTI-76 pretreated animals. Evoked DA responses shown representatively in Figures 3 and 5 were kinetically evaluated to determine parameters for DA release and uptake. Data are mean ± SEM (n = 5–9). See legend to Figure 7 for details. In the middle graphs, haloperidol (Hal) significantly increased [DA]pin naive animals (t = 4.21; p< 0.014) and in animals after RTI-76 pretreatment (t = 3.53; p < 0.008).Km was calculated as described in Figure 7and is as follows: naive, 0.42 ± 0.17 μm; Hal, 0.52 ± 0.18 μm; RTI-76, 0.32 ± 0.06 μm; and RTI-76 and Hal, 0.58 ± 0.09 μm. No significant differences were found among theKm values of the four experimental groups (F(3,28) = 0.357; p> 0.70; ANOVA). Con, Control.
Haloperidol alters evoked DA dynamics measured in the intact brain of naive animals
Figure 2 shows that, in the CP of a urethane-anesthetized rat, systemic injection of haloperidol (0.5 mg/kg, i.p.) altered evoked DA dynamics monitored by a carbon fiber microelectrode. For example, haloperidol (filled circles) robustly increased the amplitude of evoked responses relative to those obtained in the naive animal at baseline (open circles). The increases were observed at all frequencies but were most pronounced at 20 and 30 Hz. Another effect of haloperidol was the slowed extracellular clearance of DA subsequent to its release. The reduction in clearance rate also occurred at all frequencies and is clearly shown in the inset to the responses evoked by 60 Hz. The inset compares curves for the poststimulation clearance of evoked extracellular DA for predrug and postdrug responses. Because the evoked DA levels are primarily reduced by transporter activity after completion of the pulse train (Wightman et al., 1988; Garris and Wightman, 1995b; Giros et al., 1996; Budygin et al., 1999), clearance curves would overlay if uptake rates are similar. Thus, the dissimilar curves indicated different kinetics and qualitatively suggested that haloperidol increased evoked DA levels by decreasing uptake. Figure3 shows qualitatively similar results obtained in the NAc.
Fig. 2.
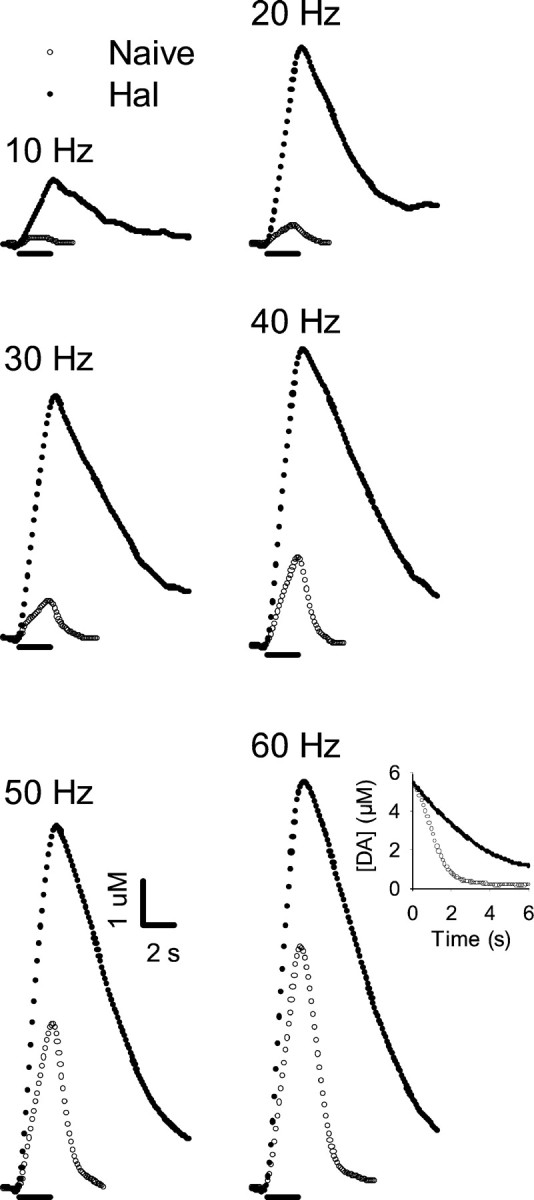
Effects of haloperidol on individual evoked responses collected in the CP of a naive rat. Data are from a single representative animal. After collection of a baseline frequency response (Naive, open circles), haloperidol (0.5 mg/kg i.p.) was injected, and after a 20 min wait, another set of frequency responses (Hal, filled circles) was collected. Each point represents the concentration of extracellular DA determined by the voltammetric microsensor at 100 msec intervals. The solid lineunderneath each set of curves demarcates the initiation and termination of the pulse train. The frequency of the pulse train is given at thetop left of each set of curves. Inset, Clearance portion of the curves evoked by 60 Hz beginning at the same concentration.
Fig. 3.
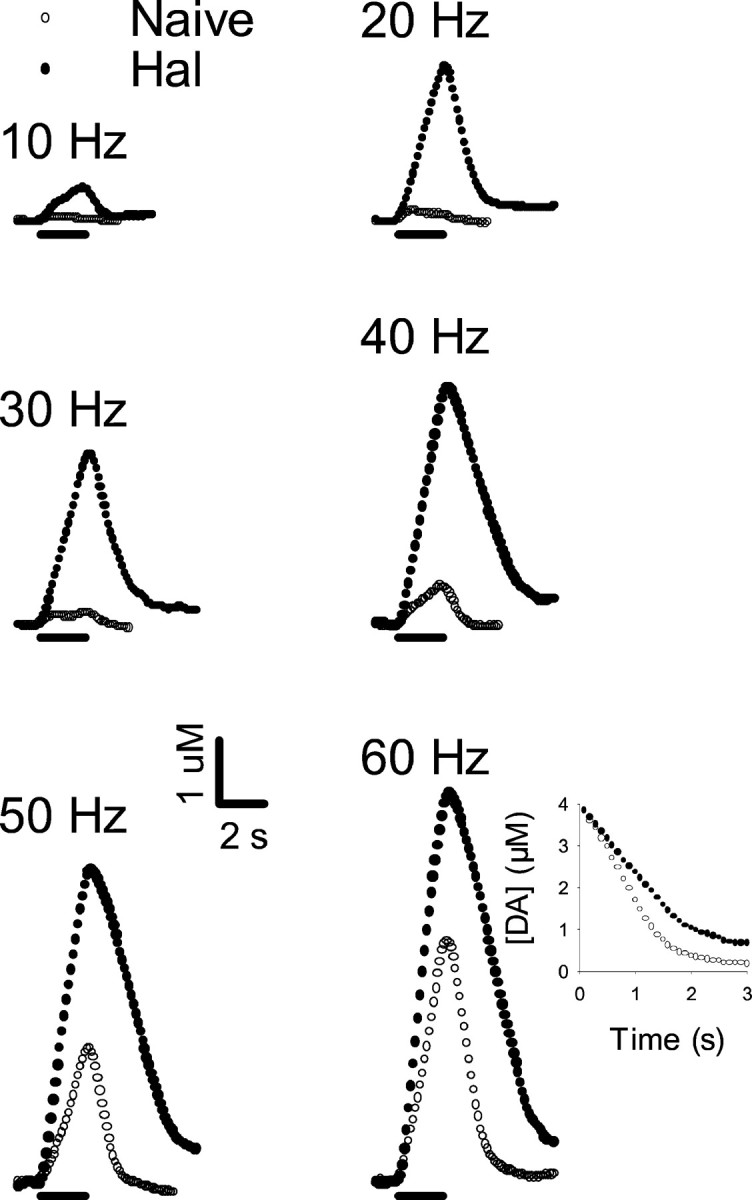
Effects of haloperidol on individual evoked responses collected in the NAc of a naive rat. Data are from a single representative animal. See legend to Figure 2 for details.
RTI-76 pretreatment effectively blocks DA uptake and blunts haloperidol-induced increases in evoked responses
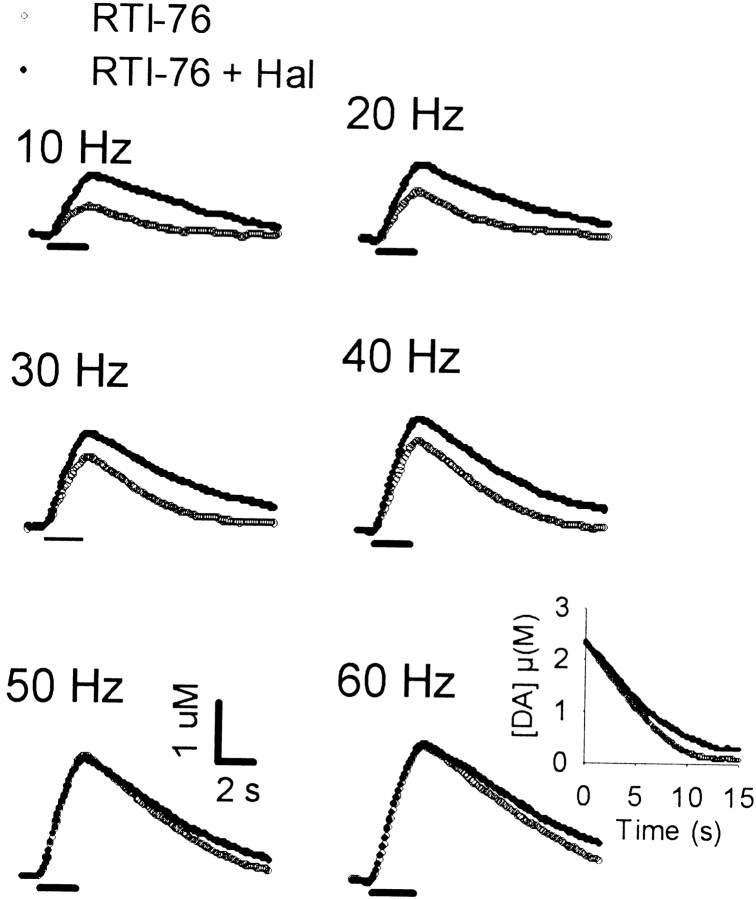
Individual responses, collected in the CP of a representative animal that received the irreversible inhibitor of DA uptake RTI-76 (100 nmol, i.c.v.) are shown in Figure 4(open circles). Evoked signals were qualitatively similar to those just described for haloperidol. Indeed, a prominent characteristic was the slowed extracellular clearance rate for DA. Compared with the effects observed in naive animals, haloperidol (0.5 mg/kg) administered to RTI-76-pretreated animals (filled circles) elicited relatively small changes in evoked DA levels. Increases in extracellular DA were most prominent at the low frequencies, similar to the frequency-dependent effects of haloperidol in drug-naive animals. Additionally, haloperidol did not appear to change the clearance rate of extracellular DA substantially after RTI-76 pretreatment (see inset to responses evoked by 60 Hz). As shown in Figure 5, qualitatively similar results were obtained in the NAc.
Fig. 4.
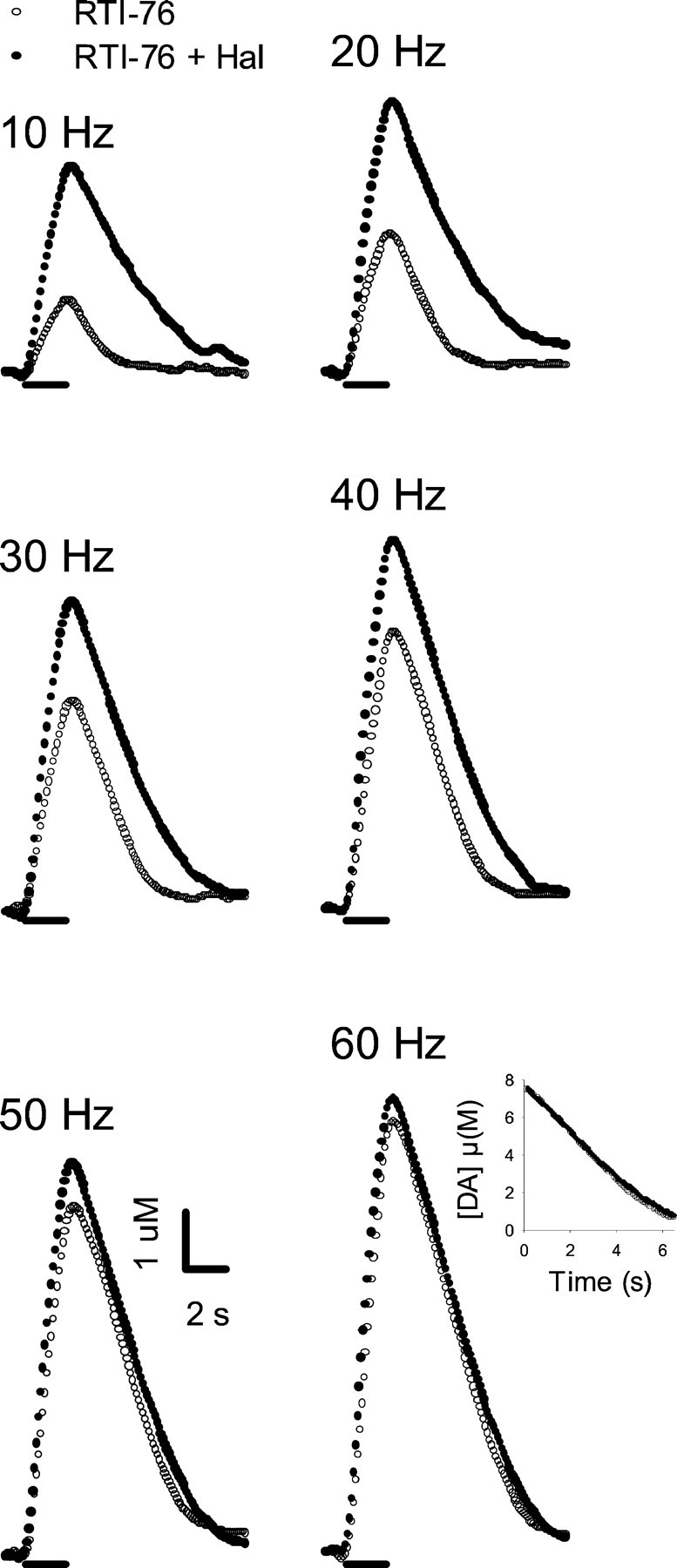
Effects of haloperidol on individual evoked responses collected in the CP of a rat pretreated with RTI-76. All data were collected in the same animal 1 d after injection of RTI-76 (100 nmol, i.c.v.). After collection of a baseline frequency response (RTI-76, open circles), haloperidol (0.5 mg/kg, i.p.) was injected, and after a 20 min wait, another set of frequency responses (RTI-76 + Hal,filled circles) was collected. See legend to Figure 2for details.
Fig. 5.
Effects of haloperidol on individual evoked responses collected in the NAc of a rat pretreated with RTI-76. All data were collected in the same animal 1 d after injection of RTI-76 (100 nmol, i.c.v.). See legend to Figure 4 for details.
Summary of frequency-dependent haloperidol effects
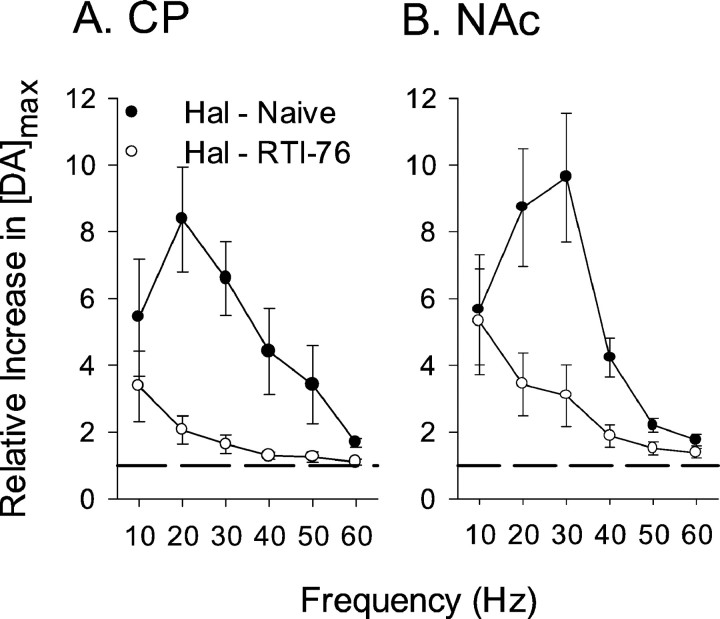
Frequency-dependent effects of haloperidol on evoked responses recorded in naive animals are summarized in Figure6 (filled circles). Data are expressed as the relative increase in maximal concentration of DA evoked ([DA]max). Marked haloperidol effects, present in individual responses (Figs. 2, 3), were also described by the averaged results. In both the CP (Fig.6A) and NAc (Fig. 6B), the frequency response was peak-shaped and reached a zenith at 20 or 30 Hz. Haloperidol-induced increases in [DA]max were greater at low compared with high stimulus frequencies, thereby confirming the frequency-dependent effects observed in the individual responses. The dashed line at a relative increase of 1 indicates the value estimated for drug-free conditions, during which DA signals are reproducibly evoked for at least 3 hr (Bergstrom and Garris, 1999). Average effects of haloperidol on evoked DA levels in RTI-76-pretreated animals are also shown in Figure 6 (open circles). Compiled results clearly showed the blunted effects of haloperidol in RTI-76-pretreated animals seen in individual recordings. RTI-76 significantly decreased haloperidol effects in both the CP (p < 0.001;F(1,5) = 29.71; ANOVA) and NAc (p < 0.001;F(1,5) = 12.38; ANOVA). The results also described the greater effects of haloperidol at lower frequencies, suggested by the individual responses, because relative increases in DA levels were near unity between 40 and 60 Hz, and apparent at and less than 30 Hz.
Fig. 6.
Averaged effects of haloperidol on evoked DA levels in naive and RTI-76-pretreated animals. All data are mean ± SEM (n = 5–9) and compiled from the individual curves shown representatively in Figures 2-5. The relative increase in [DA]EC after haloperidol (0.5 mg/kg, i.p.) administration, which is plotted along the y-axis, was calculated by taking the ratio of the maximum concentration of DA ([DA]max) evoked after and before haloperidol administration. This ratio was calculated for each frequency and averaged. The dashed line in each plot represents a relative increase of unity. The relative effects of haloperidol in the CP and NAc of naive (Hal - Naive, solid circles) and RTI-76-pretreated (Hal - RTI-76,filled circles) animals are shown in Aand B, respectively.
Kinetic evaluation of release- and uptake-regulating autoreceptors
The respective contributions of release and uptake to the haloperidol-induced increases in DA signals were evaluated by kinetic analysis. For these calculations, a release parameter, [DA]p, and two parameters for uptake,Km andVmax, were determined for each set of evoked responses using nonlinear regression (Wu et al., 2001a). Averaged values are shown in Figure 7 for the CP and Figure 8 for the NAc. Overall, similar results were found in both regions. Consistent with the qualitative observation of slowed clearance rate in naive animals, haloperidol significantly reduced Vmaxin both the CP and NAc (top left graphs; p< 0.01; paired t tests). In contrast,Vmax was unchanged in either region after haloperidol administration in RTI-76-pretreated animals (top right graphs). No significant differences inKm were found (see figure legends).
Because compiled results shown in Figure 6 indicated frequency-dependent effects of haloperidol, evoked responses were pooled into low- and high-frequency groups (10–30 and 40–60 Hz, respectively) and fit separately. In both the CP and NAc and in both naive animals and those pretreated with RTI-76, haloperidol significantly increased [DA]p at the low frequencies (Figs. 7, 8, middle graphs; see figure legends for statistical analysis). The relative increase in [DA]p was less than the relative decrease inVmax after administration of haloperidol in naive animals (CP, 59 and 35%, respectively; NAc, 57 and 37%, respectively). No effects of haloperidol on release were observed at the high frequencies (Figs. 7, 8, bottom graphs). The significant effects of haloperidol at the low stimulus frequency are surprising given the modest increases relative to the error term. It appears that interanimal variability masked ostensible haloperidol-induced changes in [DA]p. In fact, haloperidol increased the calculated release parameter in 29 of the 30 determinations compiled in Figures 7 and 8, middle graphs. The experimental design of the present study, in which predrug and postdrug determinations were made in the same animal, and statistical analysis of paired comparisons, however, allowed us to detect the haloperidol-induced changes in [DA]p despite the interanimal variability.
Recordings collected in RTI-76-pretreated animals show a dramatic slowing of the DA clearance rate, and kinetic analysis demonstrated a decrease in Vmax similar to that for responses collected after haloperidol administration in naive animals. This effect, seen in Figures 7 and 8, top right graphs, is better described by the analysis found in Table1, in which results from naive and drug-treated animals are directly compared. For simplicity, one value for [DA]p was determined for all frequencies. When compared with naive animals, RTI-76 was found to decreaseVmax in both the CP and NAc significantly (p < 0.05; t tests) but to have no effect on Km or [DA]p in either region. The modest decrease of [DA]p in the CP after RTI-76 pretreatment was not significant at the p = 0.058 level. The change inVmax, but notKm, is also consistent with the noncompetitive inhibition described for RTI-76 by in vitro(Wang et al., 2000) and ex vivo (Fleckenstein et al., 1996) studies and our previous characterization of the drug using in vivo voltammetry (Wu et al., 2001b).
Table 1.
Effects of RTI-76 on parameters for DA release and uptake
| Region | Group | [DA]p(nm) | Vmax(μm/sec) | Km (μm) |
|---|---|---|---|---|
| CP | Naive | 70 ± 8 | 3.21 ± 0.44 | 0.39 ± 0.07 |
| RTI-76 | 43 ± 11 | 0.62 ± 0.19* | 0.53 ± 0.09 | |
| NAc | Naive | 55 ± 9 | 2.45 ± 0.47 | 0.42 ± 0.17 |
| RTI-76 | 44 ± 14 | 0.57 ± 0.27* | 0.32 ± 0.06 |
All data are the mean ± SEM (n = 5–9).
p < 0.01 compared with value in naive group of same region.
DISCUSSION
Haloperidol and extracellular DA
Systemic administration of haloperidol increases striatal DA levels measured by microdialysis (Imperato and Di Chiara, 1985;Moghaddam and Bunney, 1990) and real-time voltammetry (Kawagoe et al., 1992; Wiedemann et al., 1992). The mechanism by which haloperidol exerts these effects is thought to be related to its high affinity for the D2 receptor (Vallone et al., 2000). Such receptors are located postsynaptically, in which haloperidol acts on DA neurons indirectly via long feedback loops (Hommer and Bunney, 1980), and on DA somatodentrites and terminals, where they function as autoreceptors (Starke et al., 1989; Wolf and Roth, 1990; Bunney et al., 1991). Although increases in dialysate DA reflect combined antagonism of D2 receptors, the effects of haloperidol on electrically evoked DA signals are primarily mediated by presynaptic autoreceptors, because the exogenous stimulus train strictly controls firing rate during the voltammetric measurement (Kuhr et al., 1987;Benoit-Marand et al., 2001). Large increases in evoked responses after administration of a low dose of haloperidol (Fig. 6) suggest that these presynaptic autoreceptors tightly control extracellular DA in the rat striatum.
Resolving DA release and uptake mechanisms
Observed increases in DA levels after acute haloperidol challenge are mediated by a change in release, uptake, or both (Wightman and Zimmerman, 1990). Distinguishing the contribution of each mechanism is accomplished in the present study by pharmacological and kinetic means. The pharmacological approach isolated release by pretreatment with RTI-76 to remove the uptake component of evoked signals. The marked effects of RTI-76 on electrically evoked DA levels are not mediated by DA receptors directly but rather by noncompetitive inhibition of DA uptake (Wang et al., 2000; Wu et al., 2001b). Indeed, RTI-76 exhibits little activity at D1 and D2 receptors as demonstrated by ex vivo (Fleckenstein et al., 1996) and in in vitrostudies (Fig. 1), respectively. The kinetic approach distinguishes release and uptake components mathematically. Although a theoretical framework for evaluating evoked DA signals measured by real-time voltammetry was established by Wightman et al. (1988), analysis has been limited by the availability of procedures for quantifying parameters. Early applications, for example, assumed aKm for DA uptake or a partial drug mechanism a priori (May et al., 1988; Wightman and Zimmerman, 1990; Garris and Wightman, 1994). More recently, a nonlinear regression, developed specifically for the model, was introduced to calculate the release parameter, [DA]p, and the Michaelis–Menten parameters for uptake,Km andVmax, simultaneously (Wu et al., 2001a). This tool is exploited for resolving presynaptic autoreceptor mechanisms.
Autoreceptors governing DA release and uptake
The present results confirm DA autoreceptors downregulating release (May and Wightman, 1989; Kawagoe et al., 1992; Wiedemann et al., 1992) and upregulating uptake (Cass and Gerhardt, 1994; Rothblat and Schneider, 1997; Dickinson et al., 1999; Hoffman et al., 1999) in the intact brain. Autoreceptors governing release are demonstrated by D2 blockade-induced increases in [DA]p in naive animals (Figs. 7, 8) and in DA levels evoked by low frequencies after RTI-76 administration (Fig. 6). Haloperidol decreasing Vmax and its reduced efficacy after RTI-76 administration support the more recent postulate that DA uptake is governed by autoreceptors. AlteredVmax is consistent with the comparable effects of haloperidol and RTI-76 to slow extracellular DA dynamics (Figs. 2-5) and the increases in Vmaxfor [3H]dopamine uptake andBmax for DAT binding after D2 activation in oocytes coexpressing the D2 receptor and DAT (Mayfield and Zahniser, 2001). Although uptake parameters were not determined, an apparent slowing of the extracellular clearance of electrically evoked DA levelsin vivo by haloperidol systemically administered has also been demonstrated recently (Benoit-Marand et al., 2001). An intriguing question raised by these results is why previous studies using D2 antagonists and the techniques of in vivo voltammetry coupled to electrical stimulation and mathematical modeling failed to identify autoreceptors governing uptake (May and Wightman, 1989; Kawagoe et al., 1992; Wiedemann et al., 1992). One possible explanation is that the kinetic analysis used earlier made assumptions about the drug mechanism that emphasized changes in release over uptake. The excellent agreement between the pharmacological approach and kinetic analysis in the present study supports this supposition.
In addition to confirming previous notions about autoreceptors and DA neurotransmission, the present results are the first, to our knowledge, demonstrating concurrent autoreceptor regulation of DA release and uptake mechanisms. At low stimulation frequencies, for example, increased DA levels after haloperidol administration (Fig. 6) reflect a concomitant upregulation of release and downregulation of uptake (Figs. 7, 8). This finding was made possible by the capability for combined analysis of the two components constituting evoked signals. Most studies documenting uptake-regulating autoreceptors evaluated transporter activity by monitoring the extracellular clearance of exogenous DA (Meiergerd et al., 1993; Cass and Gerhardt, 1994; Rothblat and Schneider, 1997; Dickinson et al., 1999; Hoffman et al., 1999). Experiments using one-pulse or equivalent stimulation, moreover, typically characterized response amplitude (Palij et al., 1990;Limberger et al., 1991; Cragg and Greenfield, 1997). Although uptake information is present (Kennedy et al., 1992), one-pulse signals are only responsive to D2 agonists, not antagonists. Hence, quantifying uptake kinetics at the low DA concentrations resulting from autoreceptor activation is difficult (Jones et al., 1996). Pulse trains have been used in vitro, but contributions by release- and uptake-regulating autoreceptors were not resolved (Wieczorek and Kruk, 1994; Cragg and Greenfield, 1997), and a variable release rate complicates analysis (Kennedy et al., 1992).
The present study also demonstrates that autoreceptors governing DA release and uptake are unlinked at the high stimulation frequencies. Under these conditions, haloperidol alters rates for DA uptake only (Figs. 2, 4) and is mostly ineffective in RTI-76-pretreated animals (Fig. 6). An important question is whether the frequencies used in these experiments are physiological. Midbrain DA neurons are traditionally thought to fire at 5 Hz tonically and in bursts of up to 30 Hz when activated (Bunney et al., 1991). However, recordings in behaving animals demonstrate putative DA neurons firing at rates exceeding 100 Hz (Kiyatkin and Rebec, 1998). Identified DA neurons also track these high frequencies when antidromically activated (Grace and Bunney, 1983; Kuhr et al., 1987) and reliably release DA over a wide range of stimulus frequencies (Kawagoe et al., 1992; Garris and Wightman, 1994). Thus, the observed frequency-dependent regulation of DA release by autoreceptors may have a physiological basis.
Model for presynaptic autoreceptor control of DA neurotransmission
In summary, we propose that autoreceptors governing DA release and uptake act both concurrently and independently to lower functional levels of extracellular DA in the striatum. This postulate is based on our findings that downregulation of DA release by autoreceptors is linked to stimulation frequency but that uptake-regulating autoreceptors operate continuously. Given their unique characteristics of feedback control, the autoreceptors could regulate different phenomena of neurotransmission. Within the proposed framework, both autoreceptors oppose DA concentrations elevated by small increases in basal firing. When neuronal activation is more intense, feedback inhibition of DA release fails, and only an autoreceptor-mediated increase in transport activity is available to “reign in” high DA concentrations. The latter situation is mimicked by high-frequency stimulation, during which haloperidol causes a dramatic slowing of uptake. Because observed DA dynamics resemble those in the NAc of animals exposed to a novel environment (Rebec et al., 1997), uptake-regulating autoreceptors may be important for control of DA neurotransmission during intense synchronized burst firing elicited by salient behavioral stimuli (Bunney et al., 1991; Mirenowicz and Schultz, 1994; Overton and Clark, 1997).
The present results additionally suggest that autoreceptors governing DA uptake, rather than release, play the dominant role in regulating extracellular DA levels in the intact brain. For example, haloperidol altered uptake more than release, altered uptake at all frequencies but release only at low frequencies, and only modestly increased DA levels after RTI-76 pretreatment. A commanding role for uptake-regulating autoreceptors is consistent with DAT determining temporal and spatial dynamics of extrasynaptic DA signaling (Garris et al., 1994; Nirenberg et al., 1996; Cragg et al., 2001) and the profound changes in DA concentration and diffusion distance with altered uptake rates (van Horne et al., 1992; Garris and Wightman, 1994; Nicholson, 1995; Giros et al., 1996). The release of DA, on the other hand, is already tightly controlled by impulse flow (Wightman and Zimmerman, 1990). Extensive autoregulation may thus not be required to control this mechanism precisely. However, observed increases in DA release after autoreceptor blockade are modest (May and Wightman, 1989; Kawagoe et al., 1992;Wiedemann et al., 1992) in comparison with the near complete inhibition of one-pulse-evoked DA levels by D2 agonists (Palij et al., 1990; Kennedy et al., 1992). This result suggests that differences between the in vitro and in vivoexperiments should be considered further (Dugast et al., 1997). Haloperidol has been shown recently to increase DA levels electrically evoked by a three-pulse train in vivo (Benoit-Marand et al., 2001), indicating that stimulus parameters more closely resembling those applied in vitro can be used to investigate autoreceptors in the whole animal.
Footnotes
This research was supported by National Institutes of Health Grants NS 35298 (P.A.G.) and DA 08379 (M.E.A.R.). We thank Dr. Sara R. Jones for helpful discussion.
Correspondence should be addressed to Dr. Paul A. Garris, 245 Science Laboratory Building, Department of Biological Sciences, Illinois State University, Normal, IL 61790-4120. E-mail: pagarri@ilstu.edu.
REFERENCES
- 1.Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM. Fast-scan voltammetry of biogenic amines. Anal Chem. 1988;60:1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- 2.Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergstrom BP, Garris PA. Utility of a tripolar stimulating electrode for eliciting dopamine release in the rat striatum. J Neurosci Methods. 1999;87:201–208. doi: 10.1016/s0165-0270(99)00009-6. [DOI] [PubMed] [Google Scholar]
- 4.Budygin EA, Gainetdinov RR, Kilpatrick MR, Rayevsky KS, Mannisto PT, Wightman RM. Effect of tolcapone, a catechol-O-methyltransferase inhibitor, on striatal dopaminergic transmission during blockade of dopamine uptake. Eur J Pharmacol. 1999;370:125–131. doi: 10.1016/s0014-2999(99)00084-9. [DOI] [PubMed] [Google Scholar]
- 5.Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9:79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- 6.Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- 7.Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–263. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 8.Cejna M, Agneter E, Drobny H, Valenta B, Singer EA. Pulse-to-pulse modulation of transmitter release in the central nervous system. Basic and pharmacological aspects. Ann NY Acad Sci. 1990;604:211–221. doi: 10.1111/j.1749-6632.1990.tb31995.x. [DOI] [PubMed] [Google Scholar]
- 9.Cline EJ, Adams CE, Larson GA, Gerhardt GA, Zahniser NR. Medial dorsal striatum is more sensitive than lateral dorsal striatum to cocaine inhibition of exogenous dopamine clearance: relation to [3H]mazindol binding, but not striosome/matrix. Exp Neurol. 1995;134:135–149. doi: 10.1006/exnr.1995.1044. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JC, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. Oxford UP; New York: 1991. [Google Scholar]
- 11.Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci. 1997;17:5738–5746. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME. Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J Neurophysiol. 2001;85:1761–1771. doi: 10.1152/jn.2001.85.4.1761. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- 14.Dugast C, Brun P, Sotty F, Renaud B, Suaud-Chagny MF. On the involvement of a tonic dopamine D2-autoinhibition in the regulation of pulse-to-pulse-evoked dopamine release in the rat striatum in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:716–719. doi: 10.1007/pl00005004. [DOI] [PubMed] [Google Scholar]
- 15.Eshleman AJ, Henningsen RA, Neve KA, Janowsky A. Release of dopamine via the human transporter. Mol Pharmacol. 1994;45:312–316. [PubMed] [Google Scholar]
- 16.Fleckenstein AE, Pogun S, Carroll FI, Kuhar MJ. Recovery of dopamine transporter binding and function after intrastriatal administration of the irreversible inhibitor RTI-76 [3 beta-(3p-chlorophenyl) tropan-2 beta-carboxylic acid p-isothiocyanatophenylethyl ester hydrochloride]. J Pharmacol Exp Ther. 1996;279:200–206. [PubMed] [Google Scholar]
- 17.Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garris PA, Wightman RM. Regional differences in dopamine release, uptake, and diffusion measured by fast-scan cyclic voltammetry. In: Boulton A, Baker G, Adams RN, editors. Neuromethods: voltammetric methods in brain systems. Humana; Totowa, NJ: 1995a. pp. 179–220. [Google Scholar]
- 19.Garris PA, Wightman RM. Distinct pharmacological regulation of evoked dopamine efflux in the amygdala and striatum of the rat in vivo. Synapse. 1995b;20:269–279. doi: 10.1002/syn.890200311. [DOI] [PubMed] [Google Scholar]
- 20.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 22.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—3: evidence for electrotonic coupling. Neuroscience. 1983;10:333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- 23.Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman AF, Zahniser NR, Lupica CR, Gerhardt GA. Voltage-dependency of the dopamine transporter in the rat substantia nigra. Neurosci Lett. 1999;260:105–108. doi: 10.1016/s0304-3940(98)00951-3. [DOI] [PubMed] [Google Scholar]
- 25.Hommer DW, Bunney BS. Effect of sensory stimuli on the activity of dopaminergic neurons: involvement of non-dopaminergic nigral neurons and striato-nigral pathways. Life Sci. 1980;27:377–386. doi: 10.1016/0024-3205(80)90185-x. [DOI] [PubMed] [Google Scholar]
- 26.Imperato A, Di Chiara G. Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J Neurosci. 1985;5:297–306. doi: 10.1523/JNEUROSCI.05-02-00297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RA, Eshleman AJ, Meyers T, Neve KA, Janowsky A. [3H]substrate- and cell-specific effects of uptake inhibitors on human dopamine and serotonin transporter-mediated efflux. Synapse. 1998;30:97–106. doi: 10.1002/(SICI)1098-2396(199809)30:1<97::AID-SYN12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Jones SR, Lee TH, Wightman RM, Ellinwood EH. Effects of intermittent and continuous cocaine administration on dopamine release and uptake regulation in the striatum: in vitro voltammetric assessment. Psychopharmacology (Berl) 1996;126:331–338. doi: 10.1007/BF02247384. [DOI] [PubMed] [Google Scholar]
- 29.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 31.Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM. Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience. 1992;51:55–64. doi: 10.1016/0306-4522(92)90470-m. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- 33.Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recording and iontophoresis in awake, unrestrained rats. Neuroscience. 1998;85:1285–1309. doi: 10.1016/s0306-4522(98)00054-2. [DOI] [PubMed] [Google Scholar]
- 34.Kuhr WG, Wightman RM, Rebec GV. Dopaminergic neurons: simultaneous measurements of dopamine release and single-unit activity during stimulation of the medial forebrain bundle. Brain Res. 1987;418:122–128. doi: 10.1016/0006-8993(87)90968-1. [DOI] [PubMed] [Google Scholar]
- 35.Limberger N, Trout SJ, Kruk ZL, Starke K. “Real time” measurement of endogenous dopamine release during short trains of pulses in slices of rat neostriatum and nucleus accumbens: role of autoinhibition. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:623–629. doi: 10.1007/BF00174745. [DOI] [PubMed] [Google Scholar]
- 36.May LJ, Wightman RM. Effects of D-2 antagonists on frequency-dependent stimulated dopamine overflow in nucleus accumbens and caudate-putamen. J Neurochem. 1989;53:898–906. doi: 10.1111/j.1471-4159.1989.tb11789.x. [DOI] [PubMed] [Google Scholar]
- 37.May LJ, Kuhr WG, Wightman RM. Differentiation of dopamine overflow and uptake processes in the extracellular fluid of the rat caudate nucleus with fast-scan in vivo voltammetry. J Neurochem. 1988;51:1060–1069. doi: 10.1111/j.1471-4159.1988.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 38.Mayer A, Limberger N, Starke K. Transmitter release patterns of noradrenergic, dopaminergic and cholinergic axons in rabbit brain slices during short pulse trains, and the operation of presynaptic autoreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:632–643. doi: 10.1007/BF00165627. [DOI] [PubMed] [Google Scholar]
- 39.Mayfield RD, Zahniser NR. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol. 2001;59:113–121. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- 40.Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem. 1993;61:764–767. doi: 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 41.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 42.Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson C. Interaction between diffusion and Michaelis-Menten uptake of dopamine after iontophoresis in striatum. Biophys J. 1995;68:1699–1715. doi: 10.1016/S0006-3495(95)80348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 46.Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL, Humphrey PP. Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res. 1990;509:172–174. doi: 10.1016/0006-8993(90)90329-a. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 48.Peters JL, Michael AC. Changes in the kinetics of dopamine release and uptake have differential effects on the spatial distribution of extracellular dopamine concentration in rat striatum. J Neurochem. 2000;74:1563–1573. doi: 10.1046/j.1471-4159.2000.0741563.x. [DOI] [PubMed] [Google Scholar]
- 49.Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- 50.Rothblat DS, Schneider JS. Regionally specific effects of haloperidol and clozapine on dopamine reuptake in the striatum. Neurosci Lett. 1997;228:119–122. doi: 10.1016/s0304-3940(97)00377-7. [DOI] [PubMed] [Google Scholar]
- 51.Sokal RR, Rohlf FJ. Biometry. Freeman; New York: 1995. [Google Scholar]
- 52.Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 53.Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 54.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 55.van Horne C, Hoffer BJ, Stromberg I, Gerhardt GA. Clearance and diffusion of locally applied dopamine in normal and 6-hydroxydopamine-lesioned rat striatum. J Pharmacol Exp Ther. 1992;263:1285–1292. [PubMed] [Google Scholar]
- 56.Walker QD, Lewis MH, Crofton KM, Mailman RB. Triadimefon, a triazole fungicide, induces stereotyped behavior and alters monoamine metabolism in rats. Toxicol Appl Pharmacol. 1990;102:474–485. doi: 10.1016/0041-008x(90)90043-t. [DOI] [PubMed] [Google Scholar]
- 57.Wang LC, Berfield JL, Kuhar MJ, Carroll FI, Reith ME. RTI-76, an isothiocyanate derivative of a phenyltropane cocaine analog, as a tool for irreversibly inactivating dopamine transporter function in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:238–247. doi: 10.1007/s002100000289. [DOI] [PubMed] [Google Scholar]
- 58.Wieczorek WJ, Kruk ZL. A quantitative comparison on the effects of benztropine, cocaine and nomifensine on electrically evoked dopamine overflow and rate of re-uptake in the caudate putamen and nucleus accumbens in the rat brain slice. Brain Res. 1994;657:42–50. doi: 10.1016/0006-8993(94)90951-2. [DOI] [PubMed] [Google Scholar]
- 59.Wiedemann DJ, Garris PA, Near JA, Wightman RM. Effect of chronic haloperidol treatment on stimulated synaptic overflow of dopamine in the rat striatum. J Pharmacol Exp Ther. 1992;261:574–579. [PubMed] [Google Scholar]
- 60.Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- 61.Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- 62.Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann NY Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- 63.Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001a;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- 64.Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001b;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]