Abstract
Hypocretins (Hcrts), or orexins, are a recently described set of hypothalamic peptides that have been implicated in feeding, neuroendocrine regulation, sleep-wakefulness, and disorders of sleep, such as narcolepsy. Hcrt-containing neurons, which are located exclusively in the lateral hypothalamic area, provide a dense innervation to the medial septum/diagonal band of Broca (MSDB), a sleep-associated brain region that has been suggested to show intense axonal degeneration in canine narcoleptics. The MSDB, via its cholinergic and GABAergic projections to the hippocampus, controls the hippocampal theta rhythm and associated learning and memory functions that occur during exploratory behavior and rapid eye movement sleep. Neurons of the MSDB express the Hcrt receptor 2, which is mutated in canine narcoleptics, but lack the Hcrt receptor 1 mRNA. In the present study, we investigated the electrophysiological effects of Hcrt2 on MSDB neurons from rat brain slices. We report that Hcrt2 produces a reversible, reproducible, concentration-dependent and direct postsynaptic excitation of GABA-type neurons of the MSDB with an EC50 of 207 nm. This effect is sodium dependent but not potassium or chloride dependent and is attenuated by blockers of the Na+–Ca+ exchanger. Hcrt2 also increases impulse-dependent release of GABA within the MSDB. Using recordings from retrogradely labeled septohippocampal neurons, we found that Hcrt2-excited MSDB neurons project to the hippocampus and have a GABAergic physiological signature. Double-immunolabeling studies confirmed the presence of Hcrt receptor-2 immunoreactivity in septohippocampal GABAergic neurons, as well as the presence of Hcrt fibers adjacent to these neurons. Based on these results, we speculate that Hcrt2-induced activation of septohippocampal GABAergic neurons will, by engaging disinhibitory mechanisms in the hippocampus, promote generation of the hippocampal theta rhythm and associated behaviors.
Keywords: theta rhythm, hypocretin, orexin, sleep, memory, cognition, disinhibition, cataplexy, narcolepsy
The hypocretins (Hcrts) are a newly described set of hypothalamic peptides that bind to two different G-protein-coupled receptors, the Hcrt receptor 1 (Hcrt-R1) and the Hcrt-receptor 2 (Hcrt-R2) (de Lecea et al., 1998; Sakurai et al., 1998). The Hcrts have been implicated in various physiological functions, such as feeding (Yamada et al., 2000), neuroendocrine regulation (van den Pol et al., 1998; Date et al., 1999), normal sleep-wakefulness (Piper et al., 2000), and disorders of sleep, such as narcolepsy. Human narcoleptics have an 85–100% reduction in the number of Hcrt neurons (Peyron et al., 2000; Thannickal et al., 2000), and Hcrt null mutant mice (Chemelli et al., 1999) exhibit a phenotype strikingly similar to human narcolepsy patients, as well as to canarc-1 mutant dogs (Lin et al., 1999). Lesions in the area of Hcrt neurons in adult rats produce hypersomnolence and increase rapid eye movement (REM) sleep (Gerashchenko et al., 2001b). Hcrt-containing neurons, which are located exclusively in the lateral hypothalamic area, provide innervation to most brain structures associated with sleep–wake regulation, which includes a dense innervation of the medial septum/diagonal band of Broca (MSDB) in the rat (de Lecea et al., 1998;Peyron et al., 1998; Nambu et al., 1999) and in other species (Galas et al., 2001). The MSDB, via its cholinergic and GABAergic projections to the hippocampus, controls the hippocampal theta rhythm and associated learning and memory functions that occur during exploratory behavior and REM sleep (Vanderwolf, 1969; Winson, 1976). Pronounced hippocampal theta activity similar to that seen in REM sleep also occurs during cataplectic attacks in canine narcoleptics (Kushida et al., 1985; Wu et al., 1999).
Neurons of the MSDB express the Hcrt-R2 but not the Hcrt-R1 mRNA (Trivedi et al., 1998); a disruption of the Hcrt-R2 gene underlies canine narcolepsy (Lin et al., 1999). Intense axonal degeneration of unidentified fibers has been suggested in the MSDB of canine narcoleptics and in patients with symptomatic narcolepsy (Siegel et al., 1999).
In a recent study, a complete loss of hippocampal theta, during both wakefulness and REM sleep, was found to occur after lesioning of Hcrt receptor-bearing neurons of the MSDB but not after selective lesioning of MSDB cholinergic neurons, suggesting that Hcrt receptors may be present on the noncholinergic (presumably, GABAergic) neurons of the MSDB (Gerashchenko et al., 2001a). Infusion of Hcrt into the medial septum has also been reported to increase behavioral, electroencephalographic, and electromyographic indices of arousal (Espana et al., 2001). Thus, Hcrt inputs to the basal forebrain may be important for the regulation of sleep-wakefulness (Kilduff and Peyron, 2000). As such, the goal of the present study was to begin investigations into the effects of Hcrt on MSDB neurons, with special reference to the hippocampally projecting noncholinergic, i.e., GABAergic, neurons. This goal was accomplished by testing the electrophysiological effects of Hcrt2 on retrogradely identified septohippocampal neurons (SHNs) in rat brain slices using extracellular and whole-cell recording techniques. In addition, double-immunolabeling studies were used to determine the innervation pattern of Hcrt-containing fibers and the distribution of Hcrt-R2 immunoreactivity (IR) with respect to the septohippocampal GABAergic neurons.
MATERIALS AND METHODS
Slice preparation for electrophysiological recordings
Brain slices containing the MSDB were prepared from male Sprague Dawley albino rats (2–4 weeks old) using methods detailed previously (Alreja and Liu, 1996). Briefly, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and killed by decapitation. The artificial CSF (ACSF), pH 7.35–7.38, equilibrated with 95%O2–5% CO2, contained the following (in mm): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 10d-glucose, 25 NaHCO3, 2 CaCl2, and 2 MgSO4. After decapitation, the brain was removed and placed in a Petri dish containing ACSF and trimmed to yield a small block containing the MSDB. Coronal slices of 300–600 μm thickness containing the MSDB were cut with a vibrating-knife microtome (Frederick Haer Co., Bowdoinham, ME) and transferred to a Plexiglas recording chamber (1.5 ml volume) on the fixed stage of anOlympus Optical (Tokyo, Japan) BX50WI scope or to an interface-type chamber. The slices were maintained at 33 ± 0.5°C. One to 2 hr later, the slice was used for recording. The chamber was continuously perfused with normal ACSF at a rate of 2–3 ml/min. Extracellular and intracellular recordings were performed using blind recordings in slices maintained in the interface chamber wherein the recording site was visualized using a dissection microscope (Alreja and Liu, 1996). Visualized whole-cell patch-clamp recordings on identified septohippocampal projection neurons were performed using the infrared differential interference contrast (IR-DIC) setup (see below).
Retrograde labeling of SHNs
In 10- to 14-d-old anesthetized rats (see above), retrograde labeling of SHNs was performed by pressure injecting 50–100 nl of rhodamine-labeled fluorescent latex microspheres (Lumafluor, Naples, FL) at several sites within the hippocampus using a glass micropipette (40–50 μm tip diameter) (Alreja et al., 2000b; Wu et al., 2000). The stereotaxic coordinates were as follows (anteroposterior, lateral, and ventral, respectively): −2.8, −1.4, and −2.8; −4, −1.4, and −2.8; and −5.8, −4.5, and −3.5 to −6 mm track. Two or more days later, the injected rats were used to prepare brain slices. Injection sites were confirmed for each experiment.
Fluorescence and infrared imaging
IR-DIC was performed to visualize neurons for extracellular or patch-clamp recording using an Olympus Optical BX-50 microscope using methods described in previous studies (Alreja et al., 2000b; Wu et al., 2000). Rhodamine-labeled neurons were visualized using the appropriate fluorescence filter. A neuron viewed with infrared optics was considered to be the same as that viewed with fluorescence optics when the infrared image and the fluorescent image of the neuron had the same position and orientation with the two imaging systems.
Electrophysiology recordings
Extracellular and intracellular recordings.Extracellular and intracellular recordings were performed, and cells were identified as per previously described methods (Alreja and Liu, 1996; Alreja et al., 2000b). Briefly, extracellular potentials were recorded with glass micropipettes filled with 2 mNaCl (5–10 MΩ), and intracellular recordings were performed using sharp microelectrodes (25–35 MΩ resistance) filled with 2m KCl using an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) in either the bridge mode or the discontinuous single-electrode voltage-clamp mode. In current-clamp recordings, the output signal was filtered at 10 kHz. The cells selected for study had spike amplitudes of 70–100 mV. Spike durations were measured at half-spike amplitude. The current and voltage signals were amplified and displayed on storage oscilloscopes and also continuously recorded on a chart recorder (Gould 2200; Gould Instruments, Valley View, OH). Spike analysis and input measurements were performed either on-line using Clampex or off-line using Clampfit modules of pClamp 7 (Axon Instruments).
Whole-cell recordings from visualized neurons. The image of the cells in the slice was displayed on a video monitor, and glass pipettes used for electrophysiological recordings were visually advanced through the slice to the surface of the cell from which recordings were made. Whole-cell patch-clamp recordings were performed using previously described methods (Alreja and Liu, 1996). In brief, low-resistance (2.5–3.5 MΩ) patch pipettes were filled with a solution containing the following (in mm): 120 K-methylsulfonate, 10 HEPES, 5 BAPTA K4, 20 sucrose, 2.38 CaCl2, 1 MgCl2, 1 K2ATP, and 0.1 GTP, pH 7.32–7.35. Voltage-clamp recordings were performed using the continuous single-electrode voltage-clamp mode.
Current–voltage plots (I–V curves) were obtained before and during drug application using slow ramps (6 mV/sec) to allow for attainment of steady-state conditions. The ramps were generated using pClamp software and filtered at 10 Hz. In experiments related to the study of ionic mechanisms, Na substitution was performed by replacing 80% of the NaCl equiosmolarly with choline chloride. BaCl2 (1 mm), CsCl (3 mm), NiCl2 (250 μm or 3 mm), and KB-R7943 (10–80 μm; Tocris Cookson, Ellisville, MO) were added to the ACSF in the individual experiments.
Acquisition and analysis of synaptic currents
Synaptic currents were recorded with whole-cell electrodes containing CsCl using the continuous single-electrode voltage-clamp mode. The series resistance was continually monitored, and cells were used for recording only if the series resistance was <6 MΩ. Series resistance compensation was not done. If the series resistance increased during the course of the experiment and caused significant reductions in the synaptic current amplitudes, efforts were made to improve access by applying one of several maneuvers (such as applying additional suction or slight positive pressure), failing which the experiment was discontinued.
Spontaneously occurring synaptic currents were filtered at 3 kHz, amplified 100×, and digitized at 20 kHz (to minimize distortions in the fast rising phase of the synaptic currents) using Digidata 1200 (Axon Instruments). Synaptic currents were acquired at a holding potential of −80 mV. Synaptic currents were collected over 30–60 sec for each experimental condition. The pharmacological identity of the fast synaptic currents was assessed using bath-applied NMDA and non-NMDA receptor antagonists AP-5 (50 μm) and CNQX (20 μm) and the GABAA receptor antagonists picrotoxin (100 μm) and bicuculline (10 μm).
Off-line analysis of synaptic currents was performed using the commercially available software package Minianalysis (version 5.3.1; Synaptosoft, Decatur, GA). Synaptic events were screened automatically using an amplitude threshold of 3 pA. Events were then visually screened to ensure that the analysis was not corrupted by any slight change in the noise level or by membrane fluctuations. If the background noise increased during the recording, the data from that cell was discarded. The data generated from these measurements were used to plot cumulative probability amplitude and interevent interval graphs, with each distribution normalized to a maximal value of 1. Cumulative probability plots obtained under different experimental conditions were compared using the nonparametric Kolmogorov–Smirnov test (K–S test), which estimates the probability that two cumulative distributions differ from each other by chance alone (Van Der Kloot, 1991; Lupica, 1995). The significance level for the K–S test was set at a conservative value of p < 0.01. All numerical values are plotted as mean ± SEM.
Colocalization of Hcrt receptor 2 and parvalbumin
The calcium-binding protein parvalbumin (PA) is uniquely expressed by the septohippocampal GABAergic neurons in the MSDB (Freund, 1989) and was thus used as a marker for these neurons. To determine the possible coexistence of Hcrt-R2 and PA, the very specific “mirror” colocalization technique of Kosaka (Kosaka et al., 1985) was used. Pairs of 50-μm-thick consecutive vibratome sections of the septum (20 pairs of sections per animal; a total of three animals) were placed in alternate wells of a 24-well tissue culture plate. Every other section was immunostained for PA, whereas the others were immunolabeled for Ηcrt-R2. To visualize immunoreactivity for PA and Ηcrt-R2, a monoclonal mouse anti-PA antibody (1:5000) and a rabbit anti-Ηcrt-R2 antiserum (1:250) were used (both from Chemicon, Temecula, CA). These primary immunoreagents were diluted in phosphate buffer (PB) containing 0.1% sodium azide and 1% normal horse (for PA) or normal goat (for Hcrt-R2) serum. Sections were incubated in the primary antisera overnight at room temperature. To control for specificity, sections were incubated in normal sera lacking the primary antibody. To limit the extent of immunostaining to the surface of the sections, no Triton X-100 was used. This was followed by incubation in the secondary antisera, biotinylated anti-mouse IgG (1:250 in PB) for PA and biotinylated anti-rabbit IgG (1:250 in PB) for Hcrt-R2 (Vector Laboratories, Burlingame, CA), and then in avidin–biotin–peroxidase (ABC Elite, 1:50 in PB; Vector Laboratories), each for 2 hr at room temperature. The tissue-bound peroxidase was visualized with a brown diaminobenzidine (DAB) reaction (15 mg of DAB and 165 μl of 0.3% H2O2 in 30 ml of PB, for 4–6 min). The sections were washed (four times, 10 min) between each incubation step. Consecutive sections were mounted on gelatin-coated slides (two sections per slide; one immunostained for PA and the other for Ηcrt-R2) in such a way that the posterior side of the first section and the anterior side of the second section were face up. Slides were dehydrated and coverslipped in Permount. Light microscopic examination and photographs were taken from consecutive sections of the same identified perikarya to determine colocalization of the two substances.
Double-immunolabeling studies for Hcrt and parvalbumin
Light microscopic double immunostaining was performed to determine the distribution of Hcrt peptide-containing fibers in relation to the parvalbumin-containing septohippocampal GABAergic neurons. Sections were incubated in a mixture containing equal amounts of primary antisera against the two antigens [goat anti-Hcrt (1:1000; Phoenix Pharmaceuticals, Mountain View, CA) and mouse anti-PA (1:1000) diluted in PB containing 0.1% Triton X-100] for 48 hr at 4°C. Similar patterns of immunostaining for Hcrt were found with a rabbit antiserum against Hcrt2, as described previously (van den Pol et al., 1998). After several washes with PB, sections were incubated in a mixture containing equal amounts of the secondary antibodies [biotinylated rabbit anti-goat IgG (1:125) and horse anti-mouse IgG (1:25)] for 4 hr at room temperature. After adequate rinsing, the slices were incubated for 2 hr at room temperature in ABC Elite (1 ml of PB plus 20 μl of component A and 20 μl of component B). Hcrt fibers were visualized using a nickel–DAB reaction (40 ml of PB, 15 mg of DAB, 12 mg of ammonium chloride, 0.12 mg of Ni-ammonium sulfate, 600 μl of 0.05 m ammonium sulfate, and 600 μl of 10% β-d-glucose in double-distilled water) that resulted in a black staining of Hcrt fibers. After several rinses, the sections were incubated in a mouse anti-peroxidase–antiperoxidase (1:100) for 24 hr at 4°C and then visualized using a DAB reaction (4–5 min at room temperature in 30 ml of PB containing 15 mg of DAB and 156 μl of 0.1% hiperol) that resulted in a light brown staining of PA neurons. After rinsing, sections were mounted on gelatin-coated slides and allowed to dry. The slides were dehydrated in increasing concentrations of ethanol (25, 50, 70, 90, 100%; 10 min in each), cleared in xylene, and coverslipped with Permount.
Materials
Antibodies to Hcrt-R2 and the calcium binding protein parvalbumin were obtained from Chemicon. Antibody to the Hcrt peptide was obtained from Phoenix Pharmaceuticals. Acetylcholine chloride–muscarine chloride was obtained from Research Biochemicals (Natick, MA). KB-R7943 mesylate was obtained from Tocris Cookson. The Hcrt2 peptide used was synthesized at Stanford University (Stanford, CA). All drugs were diluted in ACSF from previously prepared stock solutions that were prepared in water and stored at −20°C. Rhodamine microspheres were obtained from Lumafluor.
RESULTS
Hypocretin-2 excites neurons of the medial septum/diagonal band of Broca
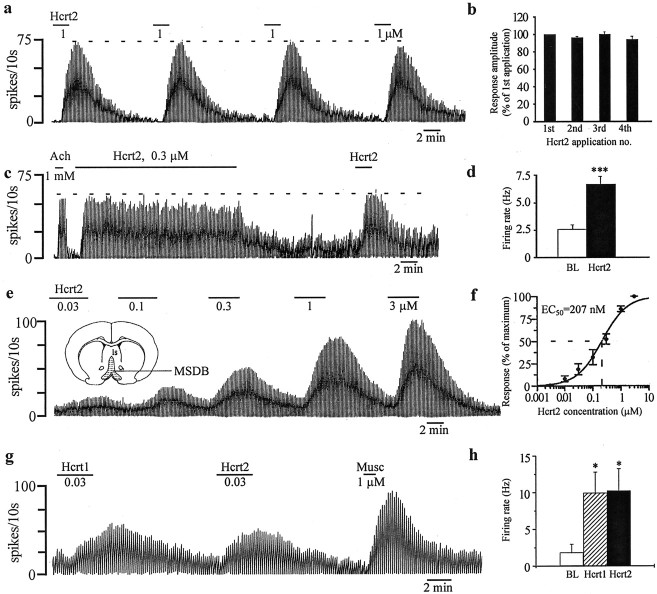
As a first step, we tested the effect of bath-applied Hcrt2 peptide (300 nm to 1 μm) on unidentified MSDB neurons using extracellular recordings. Unlike the Hcrt1 peptide, which has similar affinity for both Hcrt-R1 and Hcrt-R2, the Hcrt2 peptide has a much higher affinity for the Hcrt-R2 compared with the Hcrt-R1 (Sakurai et al., 1998). Hcrt2 peptide produced profound excitatory effects in 90% of the neurons tested (55 of 61) and had no effect in the remaining 10% neurons (Fig. 1). The excitatory response to Hcrt2, as tested in 41 neurons using a 300 nm concentration, was reversible and resulted in a 17–2700% increase in basal firing rates (median increase, 186%; mean increase, 852 ± 395%); in two outlier cells, the increase in firing rate exceeded 8000%. The mean basal firing rates for this cell population were 2.6 ± 0.4 Hz, and Hcrt2 increased the rates to 6.7 ± 0.7 Hz (p < 0.001;n = 41) (Fig. 1d).
Fig. 1.
Neurons of the medial septum/diagonal band region are excited by the Hcrt peptides in a reversible, reproducible, concentration-dependent manner. a, Response of an MSDB neuron to repeated applications of Hcrt2. Note the lack of desensitization. This cell was also excited by muscarine (data not shown). b, Bar chart summarizes the reproducibility of the Hcrt2 response. c, The response of an ACh-excited MSDB neuron to a 20 min application of 0.3 μm Hcrt2. Only a minimal reduction in the response was observed. Note that a second 2 min application of Hcrt2 produced a similar response. d, Bar chart summarizes the magnitude of the Hcrt2 excitation in MSDB neurons. e, The concentration dependence of the Hcrt response in an MSDB neuron. The response of this neuron to 3 and 10 nm Hcrt2 is not shown. f, Concentration–response curve summarizes the effect of Hcrt2 on three neurons. An EC50 value of 207 nm was obtained.g, h, Chart record shows the response of muscarine-excited MSDB neuron to equimolar concentrations of Hcrt1 and Hcrt2 peptides. Note that both peptides induced responses of a similar magnitude. BL, Baseline.
To determine the reproducibility of the Hcrt response, the agonist was applied up to four times at intervals of 3–40 min, and the magnitude of the response was recorded. In 72% of the neurons tested (12 of 18 cells), the amplitude of the response to repeated applications (compared with the first response) exhibited a <5% reduction (Fig.1a,b). The remaining neurons showed a 9–17% reduction in the response to successive applications of Hcrt2. Thus, the response of MSDB neurons to Hcrt2 was highly reproducible.
The concentration dependence of the Hcrt2 response was determined by plotting a seven-point concentration curve (3 nm to 3 μm). Detectable changes in firing rate could be recorded at a concentration of 10 nm Hcrt2 that produced a 13–25% change in basal firing rate. An EC50 value of 207 nm was obtained for the three neurons tested (Fig.1e,f). Thus, a vast majority of MSDB neurons are strongly excited by Hcrt2 in a concentration-dependent manner.
We also tested the effect of the Hcrt1 peptide on MSDB neurons. Hcrt1 peptide has a similar affinity for both Hcrt-R1 and Hcrt-R2 (Sakurai et al., 1998). In the three neurons tested, an equimolar concentration of Hcrt1 peptide produced a response similar in magnitude to the Hcrt2 (Fig. 1g,h), suggesting a similar affinity of both peptides for the Hcrt-R2, which is consistent with the reported literature (Sakurai et al., 1998).
In a preliminary attempt to identify the neurons recorded, we also tested the effects of bath-applied ACh–muscarine on the neurons mentioned above. Muscarinic agonists are a good neuropharmacological marker of MSDB neurons because the two main neuronal subpopulations of the MSDB, the cholinergic and the noncholinergic neurons, respond differently to these two agonists. Thus, whereas cholinergic MSDB neurons are never excited by muscarinic receptor agonists, >90% of noncholinergic MSDB neurons (presumably, GABAergic) are strongly excited after application of muscarinic receptor agonists (Wu et al., 2000). In the present study, we found that 90% of the Hcrt2-excited neurons also responded to ACh–muscarine with an excitation (45 of 50 neurons tested), whereas only 4% of the Hcrt2-excited neurons were inhibited by muscarine. These results, therefore, strongly suggested that MSDB neurons that are profoundly excited by Hcrt2 may belong to the noncholinergic, i.e., primarily GABAergic, subpopulation of MSDB neurons.
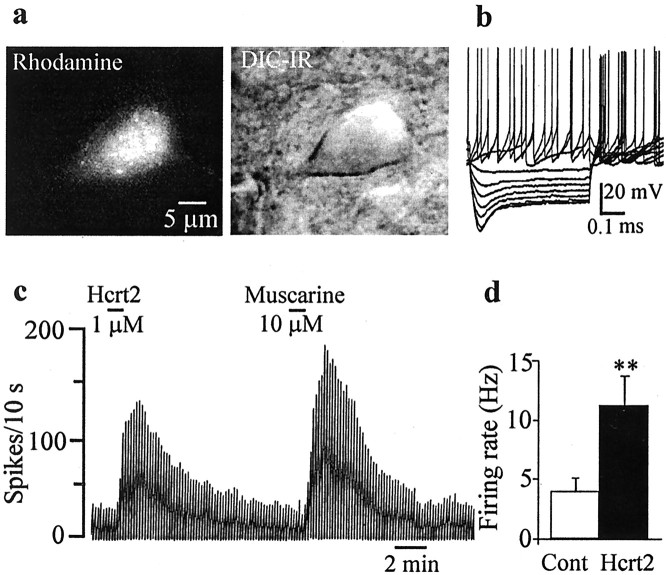
As a next step, we performed intracellular recordings on MSDB neurons that exhibited electrophysiological characteristics of GABAergic MSDB neurons. These characteristics included the presence of short-duration spikes (0.3–0.7 msec) and a depolarizing sag in response to a hyperpolarizing current (Wu et al., 2000). Of the 27 MSDB neurons so characterized, 26 responded to Hcrt2 with a clear-cut excitatory effect as tested using either current-clamp (Fig.2a–d) or discontinuous voltage-clamp (Fig. 2e,f) recordings. In the nine neurons recorded in the current-clamp mode using KCl-containing electrodes, 300 nm Hcrt2 produced a 2–12 mV depolarization (mean, 5.1 ± 1.0 mV; n= 9) (Fig. 2a,b). Mean basal firing rates of 3.2 ± 0.34 Hz (range, 0–9 Hz) increased to 12.6 ± 0.30 Hz (range, 1–30 Hz; p < 0.001) after application of Hcrt2. During these intracellular recordings, we also noted a dramatic increase in synaptic activity after bath application of Hcrt in 76% (13 of 17) of neurons tested (Fig. 2c).
Fig. 2.
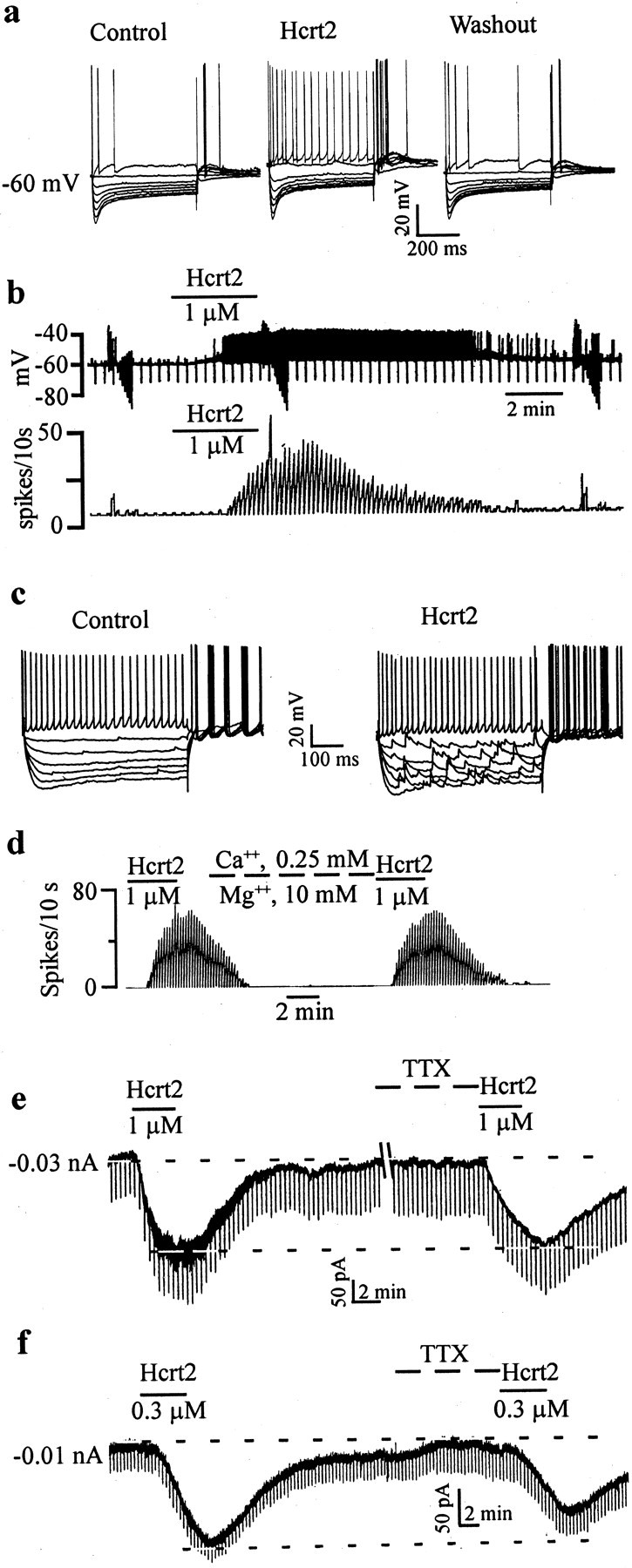
GABA-type MSDB neurons are excited by Hcrt2 via a direct postsynaptic effect. a, Intracellular recordings from a GABA-type MSDB neuron showing the reversible, excitatory effect of bath-applied Hcrt2. Note the depolarizing sag in response to the hyperpolarizing pulses and the presence of an anode-break excitation after termination of the hyperpolarizing pulses, features that are characteristic of GABA-type MSDB neurons. This cell had a spike duration of 0.43 msec and was also excited by muscarine (data not shown). b, Chart records showing the membrane potential (top) and firing rate (bottom) of a GABA-type MSDB neuron recorded intracellularly. Note that Hcrt2 depolarized the cell and induced firing [action potentials clipped, see rate record (bottom trace)]. c, A current-clamp recording from a GABA-type MSDB neuron that also showed a clear-cut increase in synaptic activity after bath application of Hcrt2. d, The effect of Hcrt2 in normal ACSF and after blockade of synaptic transmission using ACSF containing 0.5 mm Ca2+ and 10 mm Mg2+. e,f, Voltage-clamp recordings from two GABA-type neurons (holding potential, 65 mV; step command, −5 mV every 20 sec) show that Hcrt2 produced inward currents, 210 and 140 pA, respectively. Also note the increase in synaptic noise in e. Note that, although the Hcrt2 effect persisted even after bath application of 2 μm TTX in both neurons, the response to Hcrt was reduced in the cell shown in f (before TTX, 140 pA; after TTX, 80 pA) but not in the cell shown in e; five of nine neurons tested showed a reduction in the Hcrt response after application of TTX. Also note that TTX blocked the Hcrt-induced increase in synaptic noise. This may indicate the presence of a TTX-sensitive sodium component and/or an indirect component (see Discussion).
An increase in synaptic noise could also be observed in voltage-clamp recordings (Fig. 2e); TTX completely eliminated the increase in synaptic noise. In 17 neurons voltage clamped at −65 mV, Hcrt2 (300 nm to 1 μm) produced a 129 ± 17.2 pA inward current (range, 50–250 pA; median, 120 pA); an increase in synaptic noise was observed in 73% (19 of 26) of the neurons tested. As such, measurements of the changes in input conductance after application of Hcrt were performed only after blockade of synaptic activity using TTX (see below).
Hcrt-2 excites MSDB neurons via a direct postsynaptic effect
To determine whether the effect of Hcrt2 on MSDB neurons had a direct postsynaptic component, we tested the effects of Hcrt2 in the presence of TTX. In all of the eight cells tested with TTX, the Hcrt-2-induced inward current persisted in the presence of TTX, suggesting presence of a direct postsynaptic effect (Fig.2e,f). However, five of eight neurons showed a 20–43% reduction in the response amplitude after TTX. This is in contrast to the <20% reduction seen after repeated applications of Hcrt2 in control ACSF at time intervals similar to those used in the TTX experiments (see above). This reduction in the response could reflect the presence of an indirect component, such as a TTX-dependent increase in synaptic activity (Fig. 2e) (see below). The effect of Hcrt also persisted in ACSF containing GABAA and glutamate receptor antagonists (bicuculline, CNQX, and AP-5; n = 5; data not shown), as well as in low-Ca2+, high-Mg2+-containing ACSF in all cells examined (Fig. 2d) (n = 7). However, similar to TTX, the Hcrt response in low-Ca2+, high-Mg2+-containing ACSF was reduced by 22–55% in three of seven neurons tested. These experiments, therefore, suggested that the actions of Hcrt on MSDB neurons are mediated via direct, as well as indirect, effects.
Hcrt2-induced excitation is mediated via activation of the sodium–calcium exchanger
We next performed a series of experiments to determine the possible ionic mechanisms that might contribute to the Hcrt-induced direct excitation of MSDB neurons. As a first step, we measured theI–V relationship of MSDB neuron recordings voltage clamped at a holding potential of −65 mV using slow steady-state ramps to −125 mV in the absence and presence of Hcrt2. I–V curves were recorded in a total of 26 neurons, 17 of which were recorded using K-methylsulfonate-containing patch electrodes, and the remaining nine neurons were recorded with KCl-containing sharp microelectrodes. Hcrt2 induced an inward current in the −65 to −125 mV range, resulting in a parallel I–V relationship in all of the 26 neurons tested, regardless of whether a chloride-containing electrode was used or not, suggesting lack of involvement of chloride channels (Fig.3).
Fig. 3.
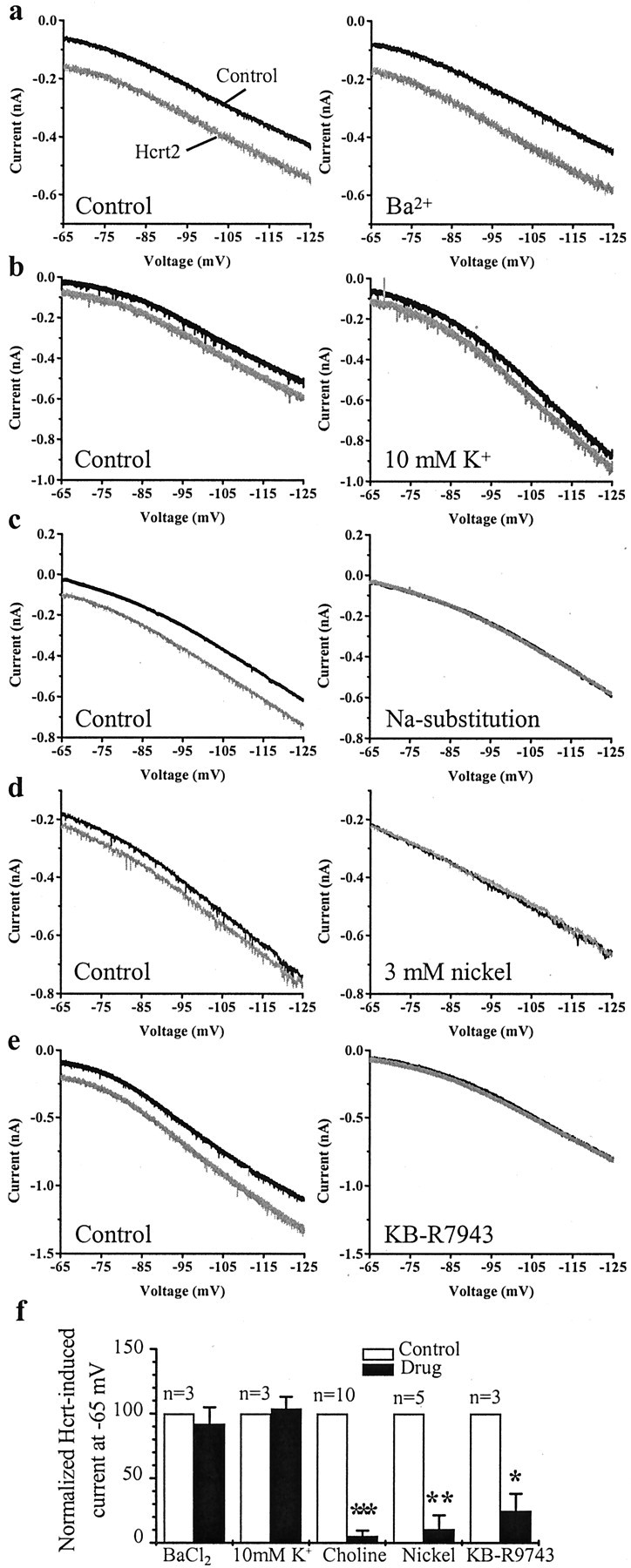
Hcrt excites MSDB neurons via activation of the sodium–calcium exchanger. Voltage-clamp recordings from MSDB neurons showing the response of the cells to a slow steady-state ramp in the absence and presence of Hcrt. a, Hcrt induces an inward current between −65 and −125 mV. External Ba2+ (1 mm) does not alter the response to Hcrt. bshows that enhancing external potassium concentration from 3 to 10 mm also does not alter the response to Hcrt.c, Substitution of sodium in the ACSF with choline, however, completely blocks the Hcrt-induced inward current.d, Nickel chloride at 3 mm blocks the Hcrt-induced inward current. e, KB-R7943 (80 μm), a Na+–Ca+exchange blocker, also blocks the Hcrt-induced inward current.f, Bar chart summarizes the effect of all treatments.
To ascertain the magnitude of the conductance change in response to Hcrt, we measured the conductance change in the presence of TTX. In eight of 10 neurons tested, Hcrt produced no apparent change in input conductance. As measured between −65 and −70 mV, in seven of 10 cells tested, two neurons showed a small increase in apparent input conductance, and one neuron showed a small decrease in conductance (input conductance: control, 8.8 ± 2.4 nS; Hcrt, 9.2 ± 2.5 nS; not significant as measured by paired Student's ttest).
The nature of the curves and lack of a reversal potential in the vicinity of the potassium equilibrium potential (calculatedEk of −103 mV) suggested possible lack of involvement of K+ channels. To confirm this finding, we tested the effect of external Ba2+ on the Hcrt2-induced inward current. As shown in Figure 3, a and f, 1 mm external Ba2+ had no effect on the Hcrt2-induced response in the entire voltage range tested (n = 3). Similarly, the Hcrt2-induced inward current remained completely unaffected by alterations in external K+ concentrations from 3 mm (control) to either 6 mm(data not shown) or 10 mm (Fig.3b,f) (n = 3).
We next determined the effect of sodium substitution on theI–V relationships of the Hcrt2 response. In 10 of 10 neurons tested, Na+ substitution with choline completely blocked the Hcrt2 response in the entire voltage range tested (Fig. 3c,f), strongly suggesting involvement of an Na+-dependent mechanism. Because the Hcrt-induced inward current was found not to be dependent on external K+ (see above), the involvement of the Na+–K+ pump was ruled out. The involvement of Na+ and K+-dependent H-current was also ruled out by testing the effect of external Cs+ that did not alter the response to Hcrt in the nine neurons tested (data not shown). Moreover, in current-clamp recordings, Hcrt did not produce any apparent change in the depolarizing sag that is associated with the H-current (Fig. 2a,c)
We next tested for the involvement of the Na+–Ca2+exchanger, which brings Na+ from extracellular space and extrudes intracellular Ca2+ with a 3:1 Na+/Ca2+stoichiometry, resulting in a net influx of one positive charge for each exchange. First, we tried to test the effects of substituting external NaCl with LiCl, because similar to choline, Li+ cannot substitute for Na+ in the Na+–Ca2+exchange mechanism and would therefore be expected to block the Hcrt response if it was dependent on the Na+–Ca2+exchanger. LiCl can, however, replace Na+as a charge carrier for a cationic channel and would not therefore block Na+-dependent cationic currents. However, both 100 and 50% NaCl substitution with LiCl destabilized MSDB neurons by producing massive inward currents (∼300–400 pA), as has been reported previously for rat amygdala neurons (Keele et al., 1997), precluding testing of the Hcrt response. Next, we tested the effects of external Ni2+ on the Hcrt-induced inward current because high concentrations of Ni2+ reversibly block the Na+–Ca2+exchanger. High concentrations of external NiCl (3 mm;n = 5) (Fig. 3d) significantly and reversibly reduced the Hcrt-induced inward current; low concentrations of Ni2+ (250 μm), which block T-type Ca2+ channels but not high-voltage-activated channels or the Na+–Ca2+exchanger, did not block the Hcrt response (n = 2; data not shown). Furthermore, KB-R7943 (10–80 μm;n = 3) (Fig. 3e,f), a selective blocker of the forward mode of the Na+–Ca2+exchanger (Iwamoto et al., 1996), also significantly blocked the Hcrt-induced inward current, suggesting that the Hcrt-induced inward current in MSDB neurons is mediated via the Na+–Ca2+exchanger.
Hcrt2 increases synaptic activity in MSDB neurons
Next we examined the indirect synaptic effects of Hcrt2 on MSDB neurons. Whole-cell recordings were performed with CsCl-containing patch electrodes and synaptic currents recorded under voltage-clamp at a holding potential of −80 mV. The low noise enabled recording of synaptic currents as low as 5 pA in amplitude. Hcrt2 produced a profound increase in the frequency of spontaneously occurring synaptic currents in 14 of 15 neurons tested. In accordance with the results of our previous studies, the spontaneously occurring synaptic currents were blocked by the GABAA receptor antagonists bicuculline (n = 5) or picrotoxin (n = 2) (Alreja and Liu, 1996; Alreja et al., 2000a). Moreover, the GABAA receptor antagonists also prevented the Hcrt2-induced increase in synaptic activity, suggesting that Hcrt2 increases the release of GABA within the MSDB (Fig.4a,c).
Fig. 4.
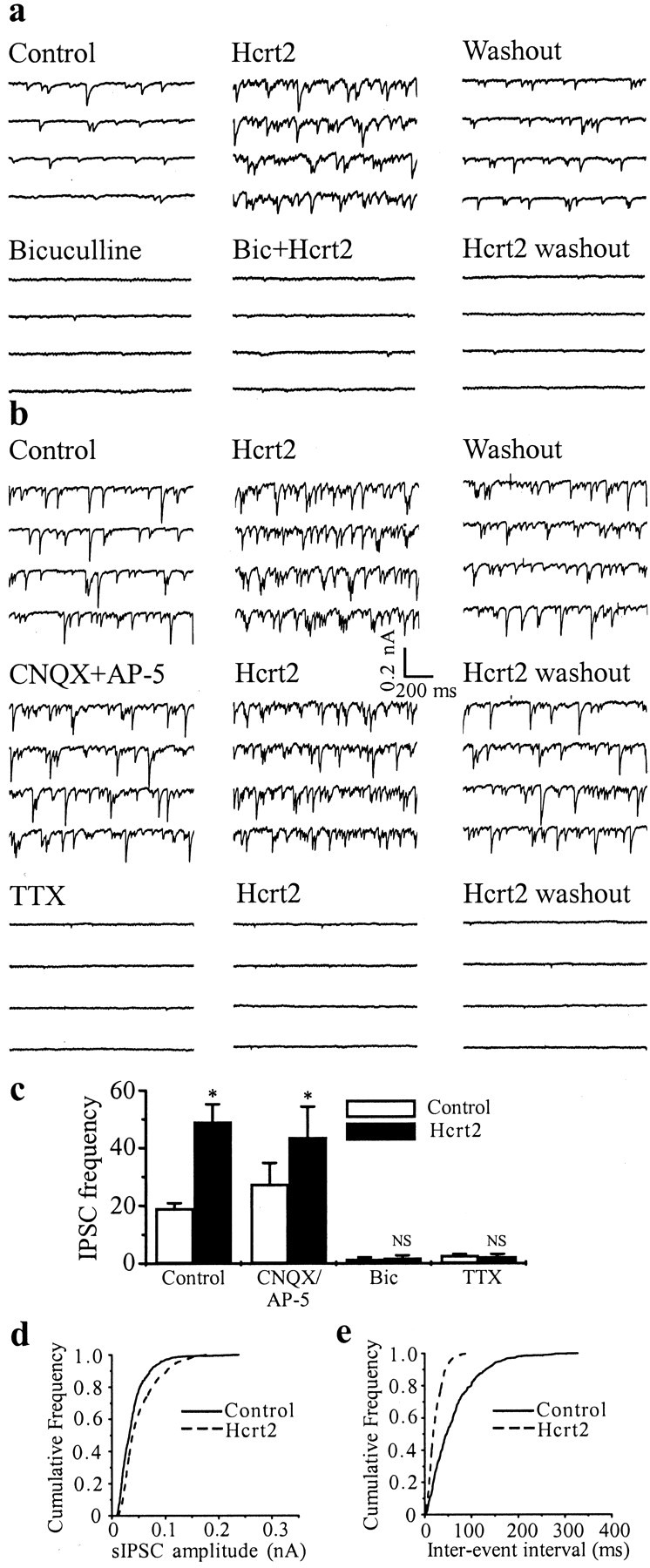
Hcrt increases impulse-dependent but not impulse-independent release of GABA. a, Whole-cell voltage-clamp recording from an MSDB neuron with a CsCl-containing patch electrode. Consecutive 0.5 sec traces show spontaneously occurring synaptic currents recorded at −80 mV in the absence and presence of Hcrt. Note that the GABAA receptor antagonist bicuculline blocked ongoing synaptic activity, as well as the Hcrt-induced increase in synaptic activity. b shows that glutamate receptor antagonists do not block basal or Hcrt-induced increase in synaptic activity. TTX, however, completely blocks ongoing and Hcrt-induced increase in synaptic activity. c summarizes the effects of all treatments on sIPSC frequency. d,e, Cumulative amplitude and frequency distributions of sIPSCs constructed from data shown in a. Both the amplitude and frequency distribution were statistically different under the two experimental conditions (p < 0.0001 using K–S test; 493 events analyzed for control and 1327 events for Hcrt).
In contrast, bath application of NMDA and non-NMDA glutamate receptor antagonists (AP-5, 50 μm; CNQX, 25 μm;n = 4) did not produce any significant difference in either the frequency of the ongoing synaptic activity or the effects of Hcrt2 on synaptic activity (Fig. 4b,c). Thus, Hctr2 increases the release of GABA but not glutamate in MSDB slices.
To determine whether the Hcrt-induced increase in synaptic activity was impulse dependent, experiments were done in the presence of the fast sodium channel blocker TTX (n = 5). Again, consistent with our previous findings, TTX suppressed ongoing synaptic activity (Alreja and Liu, 1996; Alreja et al., 2000a), and it also prevented the Hcrt-induced increase in synaptic activity (Fig.4b,c), suggesting that Hcrt-induced increase in GABA release within the MSDB is impulse dependent and is therefore attributable to an increase in the firing rate of GABAergic neurons present within the slice preparation. These results are consistent with our findings presented above, wherein GABA-type MSDB neurons responded to Hcrt with an increase in firing (Figs. 1, 2). Additionally, the failure of Hcrt2 to increase synaptic activity after application of TTX suggests that Hcrt increases impulse-dependent GABA release but does not increase the release of GABA at the terminals. It should be mentioned that, under the experimental conditions used to measure synaptic currents, changes in miniature synaptic currents could not have gone undetected in this study.
An analysis of the amplitude and frequency distribution of the synaptic currents after bath application of Hcrt revealed a highly significant change (p < 0.0001) in frequency distribution in 14 of 15 neurons tested wherein the interevent interval distribution was shifted toward shorter interevent intervals (Fig.4d,e). The amplitude distribution was also significantly altered in 9 of 15 neurons tested (p < 0.001; mean amplitude: control, 0.08 ± 0.028 nA; Hcrt, 0.14 ± 0.04 nA), presumably attributable to Hcrt-induced firing of previously quiescent neurons.
Hcrt-2-excited MSDB neurons project to the hippocampus and are GABAergic
Having confirmed the presence of Hcrt-induced excitation using both synaptic recordings and direct recordings from GABA-type MSDB neurons, we next determined whether the Hcrt2-excited neurons project to the hippocampus. It should be mentioned that, in the MSDB, GABAergic neurons can belong to either the local subpopulation or the subpopulation that projects to the hippocampus. To answer this question, we tested the effect of Hcrt2 on nine neurons identified to be septohippocampal using the technique of retrograde tracing, wherein the retrograde tracer rhodamine beads was injected into the hippocampus 2 d before slicing (Fig.5a). The noncholinergic, i.e., GABAergic, nature of the recorded neuron was confirmed by its electrophysiological responsiveness to depolarizing and hyperpolarizing steps and also by testing its responsiveness to muscarine as already mentioned above (Fig. 5b). Eight of nine neurons so identified responded to Hcrt2 with an excitation. The Hcrt2-induced excitation resulted in a 60–1260% increase in firing rate (mean, 315 ± 124%; median, 237%) (Fig. 5c,d). These neurons had basal firing rates of 4.1 ± 1 Hz (range, 0.2–7.8 Hz); Hcrt (1 μm) increased the rates to 11.2 ± 2.5 Hz (range, 3.2–26.3 Hz). These results, therefore, suggested that Hcrt2 excites septohippocampal GABAergic-type neurons that are excited by muscarine.
Fig. 5.
Retrogradely labeled septohippocampal GABAergic-type neurons are excited by Hcrt2. a shows an SHN that was retrogradely labeled using rhodamine beads (left); IR-DIC image of the same neuron (right). b, Electrophysiological signature of a septohippocampal GABAergic-type neuron. Note the depolarizing sag in response to hyperpolarizing steps (step size, 0.5 nA; maximum step, +1.0 nA). c, Chart record shows the profound excitatory effect of bath-applied Hcrt2 on a GABAergic-type SHN. This neuron was also excited by muscarine; muscarine excites septohippocampal GABAergic but not cholinergic neurons.d, Bar chart summarizes the excitatory effect of Hcrt2 on septohippocampal GABAergic-type neurons, which excited all of the nine neurons tested.
Septohippocampal GABAergic neurons contain Hcrt receptor 2
To corroborate the above electrophysiological findings, we performed a colocalization study to determine whether Hcrt-R2 is present on septohippocampal GABAergic neurons of the MSDB. The GABAergic neurons that project to the hippocampus from the MSDB are distinct from other neurons in this area (cholinergic and local GABAergic) in that they exclusively express the calcium-binding protein PA (Freund, 1989). Because of this unique property, PA has become a well established marker of septohippocampal GABA neurons. As such, we used the mirror colocalization technique (Kosaka et al., 1985) to determine whether PA-containing MSDB neurons cocontain Hcrt-R2. The mirror colocalization technique has the advantage of being specific because immunostaining for the two tissue antigens is performed on separate sections. It is therefore devoid of the disadvantages of the double-immunofluorescence technique, which can lead to errors in interpretation attributable to the possibility of an overlap in the emission spectra of the fluorochromes, uncontrolled cross-reactivity between immunoreagents, and the quickness with which the fluorochromes fade under light microscopic examination (which also makes an in-depth analysis difficult). Figure6, a and b, shows that Hcrt-R2-IR is indeed present in all PA-containing septohippocampal GABAergic neurons. However, not all of the Hcrt-R2-IR neurons contain PA. These non-PA-containing cells are situated more laterally in the MSDB.
Fig. 6.
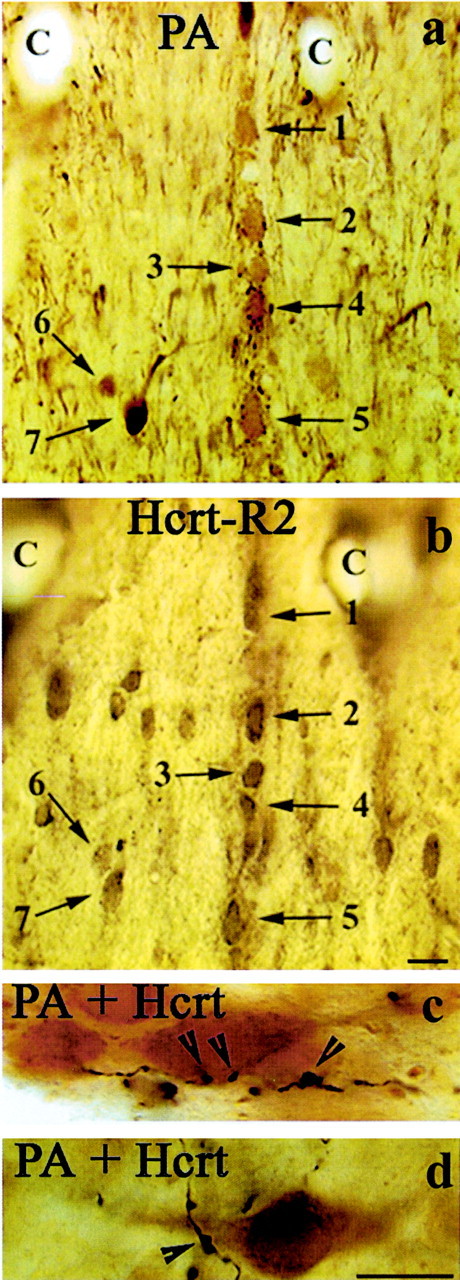
Double-immunolabeling studies demonstrating the relationship between Hcrt receptor 2 and Hcrt-containing fibers with septohippocampal GABAergic neurons. a, b, Light micrographs show the result of a mirror colocalization experiment for PA (a) and Hcrt-R2 (b) on a pair of consecutive vibratome sections of the MSDB. PA is uniquely expressed by the septohippocampal GABAergic neurons of the MSDB. Note that every PA-immunoreactive neuron (numbered arrows) also exhibits immunoreactivity for Hcrt-R2. Also, note the presence of Hcrt-R2-IR in the more laterally situated PA-negative neurons, suggesting that Hcrt-R2 is also present on additional neuronal subpopulations of the MSDB. C labels identical capillaries in the two sections. c, d, Color light micrographs demonstrate the result of a double-immunostaining experiment for PA and the peptide Hcrt in the rat MSDB. Immunoreactivity for PA is labeled by a brown diaminobenzidine reaction, whereas Hcrt-immunopositive axons were labeled by a dark blue to black nickel–diaminobenzidine reaction. Note that Hcrt-immunoreactive axons and presynaptic boutons and axonal swellings (arrowheads) are in close proximity of the dendrites (c) and the somata (d) of the PA-containing neurons. Scale bars, 10 μm.
Hcrt-immunoreactive axons can be seen in the vicinity of PA-containing septohippocampal GABAergic neurons
Having demonstrated the presence of Hcrt-R2-IR in the PA-containing septohippocampal GABAergic neurons, we next performed double-immunolabeling studies to determine whether PA-containing neurons are contacted by Hcrt peptide-containing fibers. Light microscopic examination of double-labeled tissue indeed confirmed the presence of Hcrt-containing fibers adjacent to the PA-containing septohippocampal GABAergic neurons (Fig.6c,d).
In conclusion, septohippocampal GABAergic neurons that are strongly excited by Hcrt2 express Hcrt-R2 and are also surrounded by Hcrt-containing fibers.
DISCUSSION
The chief findings of this study are that hypocretin 2 produced a direct postsynaptic excitatory effect on septohippocampal GABAergic neurons via activation of the Na+–Ca2+exchanger, presumably via the Hcrt receptor 2, immunoreactivity for which was found in the parvalbumin-containing septohippocampal GABAergic neurons. In addition, Hcrt peptide-containing fibers were also found in the vicinity of the septohippocampal GABAergic neurons. Hcrt also increased impulse-dependent release of GABA locally within the MSDB. These findings may be significant in terms of understanding the role played by Hcrts in arousal, normal sleep-wakefulness, and disorders of sleep.
Hcrt1 and Hcrt2 excite septohippocampal GABAergic neurons of the MSDB
The induction of excitatory effects by Hcrt in the MSDB is consistent with the previously reported neuroexcitatory effects of Hcrts on CNS neurons. Thus, excitatory effects of Hcrt have been described in the locus ceruleus (Hagan et al., 1999; Horvath et al., 1999; Ivanov and Aston-Jones, 2000) and the dorsal raphe nucleus (Brown et al., 2001). In the locus ceruleus, the effects of Hcrt are mediated via Hcrt-R1 (Bourgin et al., 2000). Excitatory effects of Hcrt2/orexin B have been reported previously in slice and culture preparations of the hypothalamus, but not in hippocampal neurons, in which immunoreactive axons are rare (de Lecea et al., 1998; van den Pol et al., 1998). In the present study, Hcrt2 induced excitatory effects in MSDB neurons with an EC50 of 207 nm. Although EC50 values have not been reported in the above mentioned in vitroelectrophysiological studies, the concentrations used in this study are comparable with the 1 μm concentration used in some of the published studies. In addition, we report that the excitatory responses to Hcrt2 are reproducible in the MSDB, showing little or no desensitization with repeated applications. Equimolar concentrations of Hcrt1 also excited MSDB neurons to a similar magnitude. This is consistent with previous findings that suggest that both Hcrt1 and Hcrt2 peptides have a similar affinity for Hcrt-R2, whereas Hcrt1 has an ∼10 times higher affinity than Hcrt2 for Hcrt-R1 (Sakurai et al., 1998).
In the present study, septohippocampal GABAergic projection neurons were identified and found to be excited by Hcrt. In double-labeling studies, Hcrt-R2-IR neurons were clearly detectable in the MSDB. As mentioned earlier, the presence of Hcrt-R2 but not Hcrt-R1 mRNA has been reported previously in the MSDB (Trivedi et al., 1998). Additionally, our colocalization studies demonstrate that the Hcrt-R2 immunoreactivity colocalizes with the parvalbumin-containing septohippocampal GABAergic neurons. However, Hcrt effects on other neuronal subpopulations of the MSDB cannot be ruled out. In fact, our double-immunolabeling studies also indicate the presence of Hcrt-R2-IR in parvalbumin-negative neurons (Fig. 6a,b), suggesting that Hcrt-R2 is also present on MSDB neurons other than those identified in this study. In fact the increase in GABAergic synaptic activity within the MSDB strongly indicates that GABAergic interneurons are also strongly excited by Hcrt.
Hcrt-R2 immunoreactivity colocalizes with septohippocampal GABAergic neurons
In addition to demonstrating the presence of Hcrt-R2 immunoreactivity in the septohippocampal GABAergic neurons, we also confirmed the presence of Hcrt-containing fibers in the MSDB (Peyron et al., 1998; Nambu et al., 1999). Additionally, we report that Hcrt-containing fibers are present in the vicinity of the parvalbumin-positive septohippocampal GABAergic neurons, which, as mentioned above, also express the Hcrt-receptor 2. Thus, septohippocampal GABAergic neurons could be a likely target of locally released Hcrts.
Hcrt excites MSDB GABAergic neurons via activation of the sodium–calcium exchanger
Another important finding of this study is that Hcrt-induced excitation of MSDB GABAergic neurons is mediated via activation of the Na+–Ca+exchanger. This conclusion is based on the following evidence. An analysis of the I–V relationship of the Hcrt response revealed a parallel relationship in the −65 to −125 mV region and a lack of dependence on external potassium as well as insensitivity to external barium, suggesting a lack of dependence on potassium. Because recordings with both chloride-containing and chloride-free electrodes also revealed similar I–V relationships, a lack of dependence on chloride is also indicated. A complete abolition of the response in low-Na+-containing external solutions but a lack of dependence on external potassium excluded involvement of the Na+–K+ATPase. Similarly, a lack of sensitivity to external Cs+ ruled out involvement of the Na+-dependent H-current, which is present in all septohippocampal GABAergic neurons. However, the involvement of the Na+–Ca+exchanger is strongly indicated by the fact that high but not low concentrations of nickel, as well as the selective Na+–Ca+exchange inhibitor KB-R7943, blocked the Hcrt-induced inward current. A recent study in histaminergic neurons of the tuberomammillary nucleus has also suggested involvement of the Na+–Ca+exchanger in mediating the Hcrt response (Eriksson et al., 2001). In rat basolateral amygdala neurons, the metabotropic agonist quisqualate induces an inward current that involves activation of the Na+–Ca+exchanger and has properties similar to the Hcrt-induced inward current obtained in the present study (Keele et al., 1997). Thus, the quisqualate-induced current also persists in TTX, low-Ca2+-containing external solutions and is insensitive to external barium and cesium but is completely blocked after replacement of external sodium. In addition, the quisqualate-induced inward current is also not associated with a change in conductance and has an I–V relationship similar to the Hcrt-induced inward current observed in the present study. In locus ceruleus neurons, Hcrt excitation involves a potassium-dependent (Ivanov and Aston-Jones, 2000), as well as a sodium-dependent (van den Pol et al., 2002) mechanism.
Hcrt2 increases impulse-dependent release of GABA within the MSDB
Additionally, although our electrophysiological experiments clearly demonstrate the presence of a direct postsynaptic effect of Hcrt2 on septohippocampal GABAergic neurons, the presence of an indirect effect of Hcrt2 on septohippocampal GABAergic neurons is also indicated. The indirect effect of Hcrt involves an increase in impulse-dependent GABA release within the MSDB, because Hcrt-induced synaptic activity was completely blocked by TTX and by bicuculline–picrotoxin but not by CNQX and AP-5. Furthermore, Hcrt did not alter the frequency of TTX-insensitive miniature synaptic currents in the MSDB. Indirect effects of Hcrt on septohippocampal GABAergic neurons could also result from Hcrt effects on septohippocampal cholinergic neurons, which also establish physiologically significant synaptic contacts with the septohippocampal GABAergic neurons (Alreja and Liu, 1996; Alreja et al., 2000a,b). Indeed, light microscopy studies have suggested the presence of Hcrt fibers in the vicinity of cholinergic neurons in the diagonal band region (Chemelli et al., 1999). Future studies will focus on the effects of Hcrts on these other neuronal subpopulations of the MSDB. Recently, cholinergic neurons of the magnocellular preoptic nucleus have been reported to be excited by Hcrt (Eggermann et al., 2001).
Functional implications
In a recent study, fos expression in Hcrt-containing neurons of the hypothalamus correlated positively with the amount of wakefulness and negatively with the amounts of non-REM and REM sleep, suggesting that activation of Hcrt neurons may contribute to the promotion or maintenance of wakefulness. Conversely, relative inactivity of Hcrt neurons may allow the expression of sleep (Estabrooke et al., 2001). Our results suggest that a release of Hcrts in the vicinity of the septohippocampal GABAergic neurons during wakefulness would, via activation of Hcrt-R2, lead to increased impulse flow in the septohippocampal GABAergic pathway. In this regard, Hcrts exert an effect similar to that exerted by muscarinic agonists, which, when infused into the MSDB, enhance impulse flow in the septohippocampal GABAergic pathway, promote the development of theta rhythm, and improve performance in learning and memory tasks (Wu et al., 2000). In narcoleptic dogs, infusions of muscarinic agonists into the diagonal band region and the magnocellular preoptic area induces cataplexy (Nishino et al., 1995), possibly via M3receptors (Reid et al., 1998), a receptor subtype that we showed to mediate the excitatory responses of muscarine in septohippocampal GABAergic neurons (Liu et al., 1998; Wu et al., 2000). These effects are the opposite of what muscarinic antagonists such as scopolamine–atropine do. Atropine reduces cataplectic signs in narcoleptic dogs (Reid et al., 1998). In normal rats, monkeys, and humans, atropine–scopolamine treatment disrupts impulse flow in the septohippocampal GABAergic pathway via M3receptors, blocks theta rhythm, and produces amnesia (Alreja et al., 2000b). In conclusion, the hippocampal electroencephalographic changes observed after intraseptal infusions of Hcrt (Espana et al., 2001) can be explained by the findings of the present study.
Effects of intraseptal Hcrt on learning and memory functions have yet to be tested. However, modafinil, which activates the hypothalamic hypocretin-containing neurons (Scammell et al., 2000), does enhance working memory in mice (Beracochea et al., 2001). It should, however, be mentioned that human narcolepsy, which has been attributed to a loss of Hcrt-containing neurons, is not necessarily accompanied by a loss in cognitive functioning, other than what can be attributed to a general lack of sleep. Thus, although a loss in memory functions is often reported by narcoleptic patients, objective testing in the laboratory has yielded conflicting results (Rogers and Rosenberg, 1990) and has led to a general consensus that supports a lack of memory deficits in narcoleptics (Hood and Bruck, 1997).
Preliminary studies in mice with targeted disruptions of the Hcrt-R1 and Hcrt-R2 genes show that, as expected, the Hcrt-R2 knock-out mice have characteristics of narcolepsy. However, the behavioral and electroencephalographic phenotype of the Hcrt-R2 null mice is less severe than that found in the Hcrt neuropeptide knock-out mice (Chemelli et al., 1999, 2000). Double receptor knock-outs (Hcrt-R1 and Hcrt-R2 null mice) appear to be a phenocopy of the ligand knock-out mice (Kisanuki et al., 2000). However, the Hcrt-R1 knock-outs do not have any overt behavioral abnormalities and exhibit only increased fragmentation of sleep-wakefulness cycles (Kisanuki et al., 2000). Interestingly, neurons of the locus ceruleus, which have been implicated in Hcrt-associated arousal functions, express Hcrt-R1 but not Hcrt-R2 (Bourgin et al., 2000). In this regard, it is interesting that, of the brain regions thus far known to be involved in the arousal enhancing effects of Hcrt, only the medial septum (Espana et al., 2001) and the pontine reticular formation (Thakkar et al., 2001) express Hcrt-R2. It should also be mentioned that, because the hippocampus is only sparsely innervated by Hcrt fibers (Peyron et al., 1998; Nambu et al., 1999), the effects of Hcrts on the septal component of the septohippocampal pathway could be pivotal in mediating the effects of Hcrt on septohippocampal functions.
Anatomically, the septohippocampal GABA neurons, which are activated by Hcrt2, are well positioned to exert indirect but strong effects on hippocampal pyramidal neurons. In contrast to the septohippocampal cholinergic neurons that innervate almost every type of neuron in the hippocampus (pyramidal cells, dentate granule cells, and inhibitory interneurons) (Frotscher and Leranth, 1985), the septohippocampal GABA neurons selectively innervate only the GABA interneurons of the hippocampus (Freund and Antal, 1988). Via this very selective connectivity, the septohippocampal GABAergic neurons can theoretically produce a powerful disinhibitory effect on hippocampal pyramidal neurons. In fact, a recent study performed using a novel combined septohippocampal slice preparation has provided electrophysiological evidence that activation of septohippocampal GABAergic fibers can lead to a disinhibition of pyramidal cells (Toth et al., 1997).
In conclusion, the observed effects of Hcrt2 on septohippocampal GABAergic neurons should contribute to an understanding of the cellular mechanisms by which Hcrts and Hcrt receptor 2 modulate arousal as well as sleep-wakefulness states both in normal and associated pathological states such as narcolepsy.
Footnotes
This work was supported by National Institutes of Health Grants MH61465, MH60858, and NS37788 to M.A., C.L., and A.N.v.d.P, respectively. We thank N. Margiotta for technical help and Leslie Rosello for secretarial help.
Correspondence should be addressed to Meenakshi Alreja, Department of Psychiatry, Connecticut Mental Health Center, 335A Yale University School of Medicine, 34 Park Street, New Haven, CT 06508. E-mail:meenakshi.alreja@yale.edu.
REFERENCES
- 1.Alreja M, Liu W. Noradrenaline induces IPSCs in rat medial septal/diagonal band neurons: involvement of septohippocampal GABAergic neurons. J Physiol (Lond) 1996;494:201–215. doi: 10.1113/jphysiol.1996.sp021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alreja M, Shanabrough M, Liu W, Leranth C. Opioids suppress IPSCs in neurons of the rat medial septum/diagonal band of Broca: involvement of mu-opioid receptors and septohippocampal GABAergic neurons. J Neurosci. 2000a;20:1179–1189. doi: 10.1523/JNEUROSCI.20-03-01179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: implications for learning and memory. J Neurosci. 2000b;20:8103–8110. doi: 10.1523/JNEUROSCI.20-21-08103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beracochea D, Cagnard B, Celerier A, le Merrer J, Peres M, Pierard C. First evidence of a delay-dependent working memory-enhancing effect of modafinil in mice. NeuroReport. 2001;12:375–378. doi: 10.1097/00001756-200102120-00038. [DOI] [PubMed] [Google Scholar]
- 5.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 7.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 8.Chemelli RM, Sinton CM, Yanagisawa M. Polysomnographic characterization of orexin-2 receptor knockout mice. Sleep [Abstr] 2000;23:A296–A297. [Google Scholar]
- 9.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 14.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund T. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- 16.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 17.Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- 18.Galas L, Vaudry H, Braun B, van den Pol AN, de Lecea L, Sutcliffe JG, Chartrel N. Immunohistochemical localization and biochemical characterization of hypocretin/orexin-related peptides in the central nervous system of the frog Rana ridibunda. J Comp Neurol. 2001;429:242–252. doi: 10.1002/1096-9861(20000108)429:2<242::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Gerashchenko D, Salin-Pascual R, Shiromani PJ. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001a;913:106–115. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- 20.Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001b;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood B, Bruck D. Metamemory in narcolepsy. J Sleep Res. 1997;6:205–210. doi: 10.1046/j.1365-2869.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- 23.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 24.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. NeuroReport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- 26.Keele NB, Arvanov VL, Shinnick-Gallagher P. Quisqualate-preferring metabotropic glutamate receptor activates Na+-Ca2+ exchange in rat basolateral amygdala neurones. J Physiol (Lond) 1997;499:87–104. doi: 10.1113/jphysiol.1997.sp021913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 28.Kisanuki YY, Chemelli RM, Sinton CM, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. The role of orexin receptor type-1 (OX1R) in the regulation of sleep. Sleep [Abstr] 2000;23:A91. [Google Scholar]
- 29.Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yamaihara M, Wu JY, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J Comp Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- 30.Kushida CA, Baker TL, Dement WC. Electroencephalographic correlates of cataplectic attacks in narcoleptic canines. Electroencephalogr Clin Neurophysiol. 1985;61:61–70. doi: 10.1016/0013-4694(85)91073-9. [DOI] [PubMed] [Google Scholar]
- 31.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Kumar A, Alreja M. Excitatory effects of muscarine on septohippocampal neurons: involvement of M3 receptors. Brain Res. 1998;805:220–233. doi: 10.1016/s0006-8993(98)00729-x. [DOI] [PubMed] [Google Scholar]
- 33.Lupica CR. δ and μ enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995;15:737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 35.Nishino S, Tafti M, Reid MS, Shelton J, Siegel JM, Dement WC, Mignot E. Muscle atonia is triggered by cholinergic stimulation of the basal forebrain: implication for the pathophysiology of canine narcolepsy. J Neurosci. 1995;15:4806–4814. doi: 10.1523/JNEUROSCI.15-07-04806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 38.Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 39.Reid MS, Nishino S, Tafti M, Siegel JM, Dement WC, Mignot E. Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines. Exp Neurol. 1998;151:89–104. doi: 10.1006/exnr.1998.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers AE, Rosenberg RS. Tests of memory in narcoleptics. Sleep. 1990;13:42–52. [PubMed] [Google Scholar]
- 41.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 42.Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;50:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel JM, Nienhuis R, Gulyani S, Ouyang S, Wu MF, Mignot E, Switzer RC, McMurry G, Cornford M. Neuronal degeneration in canine narcolepsy. J Neurosci. 1999;19:248–257. doi: 10.1523/JNEUROSCI.19-01-00248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- 45.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol (Lond) 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438 1998. 71 75[Erratum (1999) 442:122]. [DOI] [PubMed] [Google Scholar]
- 48.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol (Lond) 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- 51.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 52.Winson J. Hippocampal theta rhythm. II. Depth profiles in the freely moving rabbit. Brain Res. 1976;103:71–79. doi: 10.1016/0006-8993(76)90687-9. [DOI] [PubMed] [Google Scholar]
- 53.Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamada H, Okumura T, Motomura W, Kobayashi Y, Kohgo Y. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem Biophys Res Commun. 2000;267:527–531. doi: 10.1006/bbrc.1999.1998. [DOI] [PubMed] [Google Scholar]