Abstract
The mammalian neocortex is organized into subdivisions referred to as areas that are distinguished from one another by differences in architecture, axonal connections, and function. The transcription factors EMX1, EMX2, and PAX6 have been proposed to regulate arealization. Emx1 and Emx2 are expressed by progenitor cells in a low rostrolateral to high caudomedial gradient across the embryonic neocortex, and Pax6 is expressed in a high rostrolateral to low caudomedial gradient. Recent evidence has suggested that EMX2 and PAX6 have a role in the genetic regulation of arealization. Here we use a panel of seven genes (Cad6, Cad8, Id2,RZRβ, p75, EphA7, and ephrin-A5) representative of a broad range of proteins as complementary markers of positional identity to obtain a more thorough assessment of the suggested roles for EMX2 and PAX6 in arealization, and in addition to assess the proposed but untested role for EMX1 in arealization. Orderly changes in the size and positioning of domains of marker expression in Emx2 andPax6 mutants strongly imply that rostrolateral areas (motor and somatosensory) are expanded, whereas caudomedial areas (visual) are reduced in Emx2 mutants and that opposite effects occur in Pax6 mutants, consistent with their opposing gradients of expression. In contrast, patterns of marker expression, as well as the distribution of area-specific thalamocortical projections, appear normal in Emx1mutants, indicating that they do not exhibit changes in arealization. This lack of a defined role for EMX1 in arealization is supported by our finding of similar shifts in patterns of marker expression inEmx1; Emx2 double mutants as in Emx2 mutants. Thus, our findings indicate that EMX2 and PAX6 regulate, in opposing manners, arealization of the neocortex and impart positional identity to cortical cells, whereas EMX1 appears not to have a role in this process.
Keywords: area identity, cadherins, corticogenesis, EphA7, ephrin-A5, Id2, p75, patterning mechanisms, positional identity, RORβ, RZRβ, thalamocortical projections, transcription factors
The neocortex, a dorsal telencephalic structure unique to mammals, is the largest region of the cerebral cortex and is responsible for sensory perception, cognition, and volitional control of movements. In its tangential dimension, the neocortex is organized into subdivisions referred to as areas. Areas are distinguished from one another by major differences in their architecture and axonal connections, which in large part determine the functional specializations that characterize areas in the adult. It has been assumed that the specification and differentiation of neocortical areas during development are controlled by an interplay between genetic regulation intrinsic to the neocortex and extrinsic influences arising from outside the neocortex (Rakic, 1988; O'Leary, 1989).
Until recently, most experimental evidence has implicated extrinsic mechanisms in arealization, in particular the influences of thalamocortical axons (TCAs), the major afferent projections to the cortex that define the modality of the primary sensory areas (Chenn et al., 1997). Evidence for the genetic regulation of arealization has only begun to emerge over the last 3 years (Monuki and Walsh, 2001;Ragsdale and Grove, 2001; O'Leary and Nakagawa, 2002). The initial evidence was indirect and limited to descriptions of graded or restricted patterns of gene expression across the ventricular zone or the cortical plate before TCAs enter the neocortex (Donoghue and Rakic, 1999; Mackarehtschian et al., 1999; Miyashita-Lin et al., 1999;Nakagawa et al., 1999). Analyses of Gbx2 andMash1 mutant mice, which fail to develop a TCA projection (Miyashita-Lin et al., 1999; Tuttle et al., 1999), have shown that these differential patterns of gene expression are established and maintained in the embryonic neocortex independent of TCA input (Miyashita-Lin et al., 1999; Nakagawa et al., 1999) and are therefore likely controlled by mechanisms intrinsic to the dorsal telencephalon.
The closely related homeodomain transcription factors EMX1 and EMX2, and the paired-box domain transcription factor PAX6, have been proposed to be genetic regulators of arealization (O'Leary et al., 1994). Emx1 and Emx2 are expressed in a low rostrolateral to high caudomedial gradient (Gulisano et al., 1996;Mallamaci et al., 1998) and Pax6 in a high rostrolateral to low caudomedial gradient (Stoykova and Gruss, 1994) across the embryonic neocortex. The expression of Emx2 and PAX6 is restricted primarily to cortical progenitors cells, whereasEmx1 is expressed in both progenitors and their postmitotic progeny. If involved in arealization, EMX1 and EMX2 should preferentially impart caudal and medial area identities (such as visual), whereas PAX6 should preferentially impart rostral and lateral area identities (such as motor and somatosensory).
Recent studies have presented evidence for a role for EMX2 (Bishop et al., 2000; Mallamaci et al., 2000a) and PAX6 (Bishop et al., 2000) in arealization by analyzing Emx2 and Pax6 Small eye (Sey/Sey) mutant mice. Because Emx2 and Pax6 mutants die on the day of birth, before areas become anatomically and functionally distinct, these analyses relied mainly on the use of genes expressed in differential patterns across the neocortex as putative molecular markers of area identity. Changes in marker expression and patterns of area-specific TCA projections suggested that rostrolateral areas are expanded, whereas caudomedial areas are reduced in Emx2mutants. The analysis of the Pax6 mutants was limited to the patterned expression of Cad6 and Cad8 (Bishop et al., 2000), which exhibit changes opposite to those in Emx2mutants, suggesting that rostrolateral areas are reduced and caudomedial areas are expanded in the Pax6 mutants.
Because the interpretations in these studies were based on very limited markers, not only in number but also in the classes of genes represented and in their patterns of expression, we have performed a more thorough marker analysis of arealization in Emx2 andPax6 mutants, using a battery of seven complementary markers representative of a broad range of genes. These genes encode a broad range of proteins, including cell adhesion molecules (Cad6and Cad8), an HLH transcription factor (Id2), an orphan nuclear receptor (RZRβ, also termed RORβ), and a neurotrophin receptor (p75), as well as receptors and ligands involved in axon guidance and cell migration (EphA7 andephrin-A5). This more extensive analysis is especially critical for substantiating the role of PAX6 in arealization, becausePax6 mutants lack a TCA projection, and therefore the area-specific patterning of this projection cannot be studied. In addition, we analyze sectioned material that reveals more complex and informative expression patterns than observed in the previous whole-mount in situ analysis.
The similarities in the graded expression of Emx1 andEmx2, and their close sequence homologies, have prompted the proposal that EMX1 also may regulate arealization of the neocortex (Cecchi and Boncinelli, 2000). Therefore, we have also addressed its role in arealization by analyzing in Emx1 mutant mice the patterned expression of the set of seven gene markers, as well as area-specific TCA projections. To our surprise, the Emx1mutants do not exhibit defects in arealization. To assess whether this may be attributable to Emx2 compensating for the loss ofEmx1, we performed a marker analysis of Emx1; Emx2 double mutants. Our findings indicate that EMX2 and PAX6 disproportionately regulate arealization of the neocortex in opposing manners, whereas EMX1 has no apparent role in this process.
MATERIALS AND METHODS
Mice. Single-mutant Emx1 andEmx2 mice used for this study were generated from heterozygous breeding pairs, which allowed for a direct comparison of gene expression patterns between +/+, +/−, and −/− littermates for each mutant studied. Emx2 mice were maintained on a C57/BL6 background, and Emx1 mice were maintained on a 129/Sv background. To generate Emx1; Emx2 double mutant embryos,Emx1+/− mice were crossed with Emx2+/− mice. Offspring were genotyped and Emx1+/−; Emx2+/− andEmx1−/−; Emx2+/− mice were bred with each other to obtain double mutant embryos and their littermates. Emx2(Pelligrini et al., 1996), Emx1 (Qiu et al., 1996), andEmx1; Emx2 double mutant mice were genotyped by PCR as described. PAX6 mutants were obtained from mating heterozygous Sey mice (Hill et al., 1991) maintained on a C57BL/6J × DBA/2J background. Sey mice were genotyped by eye morphology: Sey/Sey mice lack eyes, and +/Sey mice have a pronounced reduction in the external size of the eye and the lens at embryonic day (E) 18.5 (Hill et al., 1991). Because Emx2−/−, Sey/Sey, and Emx1−/−; Emx2−/− mice die within a few hours after birth, embryos were removed and fixed at E18.5, just before birth, which for these mouse strains occurs at approximately E19.0. Although Emx1 null mice live to adulthood and are fertile (Qiu et al., 1996), we used newborn [postnatal day (P) 0] Emx1 mice to facilitate comparisons of gene expression patterns between the three mutants analyzed in this study. Analyses were done blinded to genotype. Midday of the day of vaginal plug detection was considered E0.5, and the first 24 hr after birth is termed P0. Animal care was in accordance with institutional guidelines.
In situ hybridization. For in situhybridization on sections, brains were fixed with 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (PB), cryoprotected with 30% sucrose in PFA/PB, and cut at 20 μm in the sagittal plane on a cryostat. In situ hybridization using35S-labeled riboprobes and counterstaining with bisbenzimide were performed as described previously (Liu et al., 2000). The following riboprobes were synthesized from cDNA templates:Cad6 (from S. Mah and C. Kintner); Cad8;Id2 (from M. Israel), RZRβ (from M. Becker-Andre, Serono Pharmaceutical Research Institute, Plan-Les-Ouates, Switzerland), p75 (from K.-F. Lee, Salk Institute, LaJolla, CA), EphA7 (from A. Brown and G. Lemke, Salk Institute, LaJolla, CA) and ephrin-A5.Whole-mount in situ hybridization of the intact forebrain and midbrain was performed with digoxygenin-labeled riboprobes forCad6 and Cad8 using a modification of the protocol of Henrique et al. (1995), described in Nakagawa et al. (1999).
Retrograde axon labeling. Newborn mice were perfused transcardially with 4% PFA in PB, and their brains were removed and postfixed overnight. Thalamocortical projection neurons were retrogradely labeled using crystals of the lipophilic dyes 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) (Molecular Probes, Eugene, OR) and 4-[4-(dihexadecylamino)stryryl]-N-methylpyridinium iodide (DiA) (Molecular Probes) (Honig and Hume, 1989a,b) placed into the neocortex of Emx1+/+, +/−, and −/− littermates. In the same cortical hemisphere, a single small crystal of DiI was placed at a site that in a wild-type mouse would be near the center of primary visual area, and a single small crystal of DiA was placed at a site that in a wild-type mouse would be near the center of the primary somatosensory area. Care was taken to equate crystal size and placement between littermate sets of brains. In addition, we attempted to place the DiA crystal in the cortical plate and not involve the underlying subplate, the intracortical pathway of TCAs, to avoid the possible labeling of dorsal lateral geniculate thalamic nucleus (dLG) axons en route to the primary visual area. Brains were stored for 2 weeks at 30°C in fixative, which was sufficient time to completely fill thalamocortical projection neurons. The brains were then cut sagitally at 100 μm on a vibratome. Sections were counterstained with bisbenzimide, and every section through the thalamus and cortical crystal sites was serially mounted. Sections were analyzed and photographed with a Nikon upright fluorescence microscope using RITC (DiI), FITC (DiA) and UV (bisbenzimide) filter cubes. Before sectioning, the dorsal surface of the cortical hemisphere was photographed to document the locations of the DiI and DiA crystal placements; the placement sites and their sizes were further documented in the sections.
RESULTS
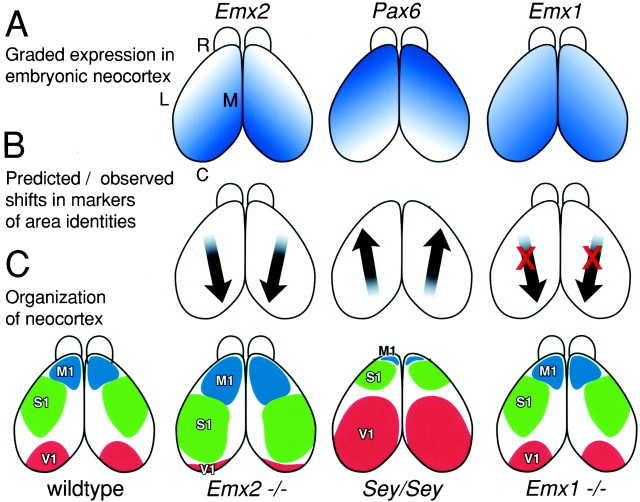
In the major component of this study, we examined in wild-type mice, in Pax6, Emx2 and Emx1 mutant mice, and in Emx1; Emx2 double mutant mice, the expression of seven genes (Cad8, Cad6, Id2,p75, RZRβ, ephrin-A5, andEphA7) that in wild-type mice exhibit restricted or differential patterns of expression across the embryonic neocortex (see introductory remarks). Therefore, these genes are useful as molecular markers of position-dependent or areal properties of neocortical cells. Our analyses were done on E18.5 (hours before birth) or P0, the dayEmx2 and Pax6 mutants die. Figure1 presents in a schematic manner the graded expression of Emx1, Emx2, andPax6 and summarizes our predictions and findings from loss-of-function mutant analyses. For simplicity, we use the terms motor, auditory, somatosensory, and visual areas to describe the areal expression patterns that we observed.
Fig. 1.
Hypotheses, predicted results, and interpretations of analyses in this study. Diagrams are of dorsal views of the mouse neocortex. A, Graded expression patterns of the transcription factors Emx2, Pax6, andEmx1 across the embryonic neocortex. Emx2and Emx1 are expressed in a high caudomedial to low rostrolateral gradient, whereas Pax6 is expressed in an opposing gradient. B, Arrows indicate the direction of the predicted shifts in markers of area identity inEmx2, Pax6 (Sey/Sey), andEmx1 loss-of-function mutants, if these genes are involved in regulating arealization of the neocortex. The predicted shifts are observed in Emx2 and Pax6mutants but not in Emx1 mutants (indicated by red X marks). C, Organization of the mouse neocortex into areas predicted by our findings. These diagrams are not intended to show the exact sizes and shapes of the primary neocortical areas but rather to depict the disproportionate changes in area size and positioning, or no changes, in arealization in the different mutants. These predicted organizations suggested by our analyses of gene markers and area-specific thalamocortical projections are limited because the Emx2 and Pax6 mutants die on the day of birth, before areas become anatomically and functionally distinct, and thalamocortical projections do not develop inPax6 mutants. For simplicity, only the primary visual (V1), motor (M1), and somatosensory (S1) areas are shown. C, Caudal;L, lateral; M, medial; R, rostral; Sey, small eye mutant.
Genes with expression patterns restricted to one area have yet to be identified (Liu et al., 2000), but the layer-specific expression domains of several of the genes examined here approximate the locations of cortical areas or boundaries between them; citations are provided for instances in which these relationships have been corroborated in previous studies. However, even genes with restricted or graded expression patterns that do not directly relate to a specific cortical area or a boundary between areas can be used as markers for position-dependent molecular properties of neocortical cells that differ across the tangential or “areal” extent of the neocortex and presumably are part of a repertoire of molecular markers that define the area identity and characteristics of neocortical cells. Expansions and contractions of the expression domains in the mutant neocortex, in particular if the changes are consistent across the set of markers, are evidence for changes in the position-dependent molecular properties of neocortical cells.
Areal patterns of gene expression in the neocortex are altered in opposing manners in Emx2 and Pax6mutants
In the first part of this study, we examine in Emx2 andPax6 mutant mice the expression of seven genes that in wild-type mice are expressed in restricted or graded patterns that relate to the organization of the neocortex into areas. To facilitate this comparison, we have divided the seven genes into three groups (Cad8, Cad6, and Id2;RZRβ and p75; ephrin-A5 andEphA7) on the basis of how effectively their expression patterns complement or corroborate one another. The expression of these genes is also layer-specific, and for most of them, their areal expression patterns differ between layers. AlthoughEmx2 mutants exhibit minor lamination defects (Mallamaci et al., 2000b), at a qualitative level of analysis, the changes in areal patterning of the marker genes appears to be consistent across layers; it is difficult to comment on this issue in the Pax6 mutants because of the significant defects in lamination (Caric et al., 1997).
Emx2 and Pax6 mutants:Cad8, Cad6, and Id2
As we showed recently using whole-mount in situanalysis (Bishop et al., 2000), the rostral domain of Cad8expression observed in wild-type cortex exhibits a caudal expansion inEmx2 mutants and a rostral restriction in Pax6mutants (Fig.2A–D). However, these whole-mount in situ analyses of the cortical hemisphere reveal only the superficial rostral expression domain ofCad8, which corresponds to the high level of expression in layers 2/3 of the motor area (Suzuki et al., 1997; Inoue et al., 1998). Therefore, we have confirmed and extended the finding of Bishop et al. (2000) by doing an analysis of the more complex, and revealing, expression pattern of Cad8 obtained by in situhybridization on sections from E18.5 wild-type and mutant cortex.
Fig. 2.
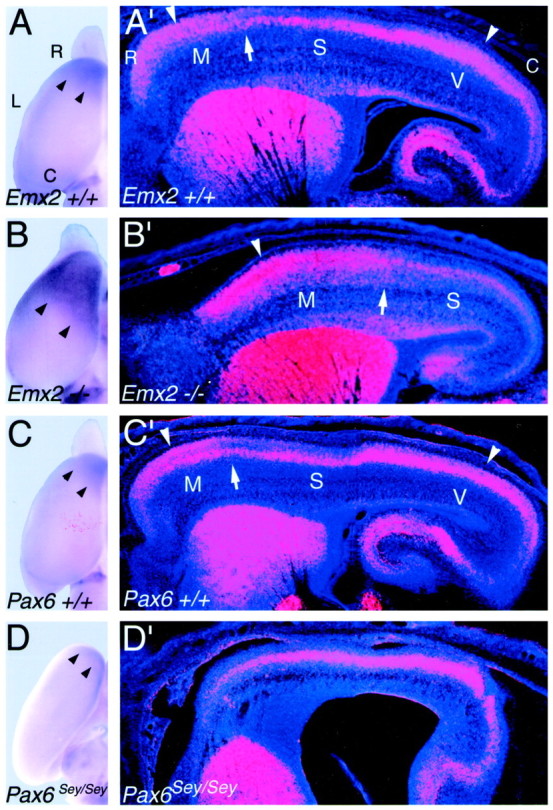
Opposing changes in the expression domains of the cadherin, Cad8 in Emx2, andPax6 (Sey/Sey) mutants.A–D, Dorsal views of whole mounts of P0 cortical hemisphere of Emx2 wild-type (+/+) (A), Emx2 mutant (−/−) (B), Pax6 wild-type (+/+) (C), and Pax6(Sey/Sey) mutant (D) processed forin situ hybridization using digoxygenin-labeled riboprobes for Cad8. Arrowheads mark the caudal limit of the rostral expression domain ofCad8 in the superficial layers, which is characteristic of motor areas. A′–D′, Sagittal sections through E18.5 brains of mice of the corresponding genotypes as inA–D, processed for in situ hybridization using S35-labeled riboprobes for Cad8. Sections were later counterstained with bisbenzimide. Sections are taken from similar mediolateral positions; rostral is to the left and dorsal is to thetop. Each panel is a montage of single-exposure photos using dark-field illumination with a red filter to view the silver grains and with UV fluorescence to view the counterstain. Marked are the approximate locations of the motor (M), somatosensory (S), and visual (V) areas in the wild-type cortex and their shifted locations in theEmx2−/− cortex suggested by the expansion and caudal shift in patterns of Cad8 expression, which are unique in each of these areas in wild-type mice. Arrowheads inA′–C′ mark rostral and caudal expression domains in the superficial layers characteristic of motor and visual areas; in comparison, expression is substantially diminished in the superficial layers of the intervening somatosensory area.Arrows in A′–C′ mark the presumed border between motor and somatosensory areas. TheCad8 expression rostral to these arrowsis the expression that is evident in the whole mounts shown inA–C. This superficial rostral expression domain characteristic of motor areas is essentially absent inPax6 (Sey/Sey) mutants (D,D′). The wild type and mutants in each pair are age-matched littermates. See Results for details. C, Caudal; L, lateral; R, rostral.
In wild-type mice, Cad8 is broadly expressed across the neocortex within layer 5; however, throughout rostral neocortex,Cad8 is also highly expressed in layers 2/3 (Suzuki et al., 1997; Nakagawa et al., 1999) (Fig.2A′,C′). The caudal boundary of this superficial expression domain (i.e., layers 2/3) has been reported to correspond to the boundary between motor and somatosensory areas (Suzuki et al., 1997). In Emx2 mutants, this superficial rostral expression domain is expanded, and its caudal boundary is shifted caudally (Fig. 2, compare A′, B′). An opposite and dramatic change is observed in Pax6 mutants in which the superficial rostral expression domain is absent (Fig. 2, compare C′, D′).
Similar to its rostral superficial expression domain, in caudal neocortex corresponding to the visual area, Cad8 is also expressed in layers 2/3 and deep to them, whereas in contrast, expression in the somatosensory area positioned between the motor and visual areas is very low in the superficial layers and limited mainly to layer 5 (Suzuki et al., 1997; Nakagawa et al., 1999) (Fig.2A′,C′). In Emx2 mutants, the expression pattern characteristic of the somatosensory area also shifts caudally and is found within caudal neocortex in the location normally occupied by the caudal superficial Cad8 expression domain characteristic of the visual area, which appears to be absent (Fig.2B′).
Figure 3 illustrates the expression ofId2 and Cad6 in Emx2 andPax6 mutants and their wild-type littermates. In wild-type mice, both genes are broadly expressed across the neocortex, but each exhibits differential laminar patterns of expression characterized by abrupt changes in expression that are layer specific. Id2exhibits strong expression in layer 5 in intermediate and caudal parts of the neocortex but weak expression in rostral parts. The change in layer 5 from strong to weak expression occurs abruptly at a position that corresponds to the boundary between motor and somatosensory areas (Rubenstein et al., 1999). This boundary between strong and weak layer 5 expression is shifted caudally in Emx2 mutants and rostrally in Pax6 mutants. In addition, wild-type mice exhibit another differential expression pattern specific for layers 2/3. Id2 is strongly expressed in layers 2/3 of rostral neocortex, but in intermediate parts of the neocortex (e.g., the somatosensory area), the expression level declines rapidly to low or nondetectable levels characteristic of caudal neocortex (i.e., the visual area). The rostral domain of strong expression in layers 2/3 expands caudally in Emx2 mutants, leaving only the extreme caudal pole of the cortical hemisphere with a low level ofId2 expression. In Pax6 mutants, the domain of strong expression characteristic of layers 2/3 of rostral neocortex appears to be absent throughout the neocortex.
Fig. 3.
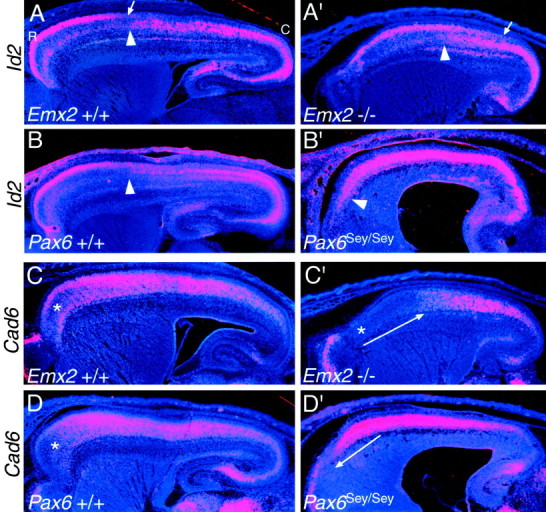
Id2 and Cad6, used as markers of rostral neocortical areas, show opposing shifts inEmx2 and Pax6 mutants. In situ hybridizations on sagittal sections through the forebrain of E18.5 mice using S35-labeled riboprobes for the HLH transcription factor, Id2(A–B′) or the cadherin,Cad6 (C–D′), and counterstained with bisbenzimide are shown. Sections are fromEmx2 wild-type (+/+) and mutant (−/−) littermates orPax6 wild-type (+/+) and mutant (Sey/Sey) littermates and are taken from similar medial–lateral positions. Eachpanel is a montage of single-exposure photos using dark-field illumination with a red filter to view the silver grains and UV fluorescence to view the counterstain. Id2 exhibits a graded expression in superficial layers of rostral areas in wild-type mice; the arrows in A and A′ mark the position where the expression declines to very low levels. Thearrowheads in A–B′ mark the transition from low to high expression reported in layer 5; this transition corresponds to the border between motor and somatosensory areas. The asterisks in C, C′, andD mark a domain of low Cad6 expression normally characteristic of far rostral neocortex. This domain of low expression expands and shifts caudally in Emx2 mutants, whereas inPax6 mutants it shifts rostrally and essentially disappears (C′, D′, long arrows). See Results for details. C, Caudal; R, rostral.
In wild-type mice, Cad6 is expressed prominently in dorsal and lateral parts of the neocortex corresponding to somatosensory and auditory areas and exhibits a domain of low expression, especially deficient in layers 4 and 5, in extreme rostral neocortex (Suzuki et al., 1997; Nakagawa et al., 1999). In Emx2 mutants, the domain of low Cad6 expression normally confined to extreme rostral neocortex is greatly expanded and covers virtually the rostral half of the neocortex. In contrast, this domain is essentially absent in Pax6 mutants, and high levels of Cad6expression continue to the rostral pole of the neocortex.
These data on changes in the expression of Cad8,Cad6, and Id2 are consistent with an expansion of rostral neocortical areas and a reduction of caudal areas inEmx2 mutants and a contraction of rostral neocortical areas and an expansion of caudal areas in Pax6 mutants.
Emx2 and Pax6 mutants:RZRβ and p75
Figure 4 illustrates the expression of RZRβ (RORβ) and p75 at E18.5 inEmx2 and Pax6 mutants and their wild-type littermates. RZRβ and p75 exhibit strongly graded patterns of expression across the neocortex, but in opposing directions (Mackarehtschian et al., 1999; Rubenstein et al., 1999). In wild-type mice, RZRβ exhibits a graded expression within layer 4 that extends across most of the rostrocaudal extent of the neocortex; expression is strong rostrally and progressively declines to very low levels near the extreme caudal pole of the neocortex. In addition to this reported graded expression within layer 4 (Rubenstein et al., 1999), we find that RZRβ is also differentially expressed within layer 5, with a domain of high expression restricted to the rostral pole of the neocortex that exhibits a rapid decline of expression to reach very low levels of expression at about the midpoint of the neocortex; expression is very low or nondetectable in the caudal half of the neocortex.
Fig. 4.
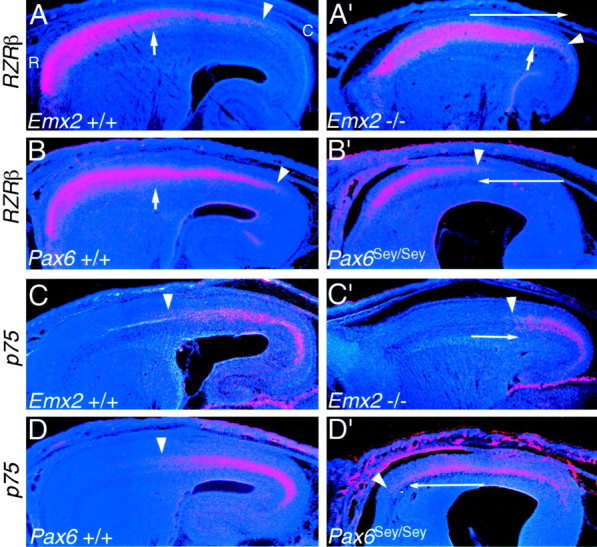
The complementary expression patterns ofRZRβ and p75 show opposing shifts inEmx2 and Pax6 mutants. In situ hybridizations on sagittal sections through the forebrain of E18.5 mice using S35-labeled riboprobes for either the nuclear receptor RZRβ (A–B′) or the neurotrophin receptorp75 (C–D′) and counterstained with bisbenzimide are shown. Sections are fromEmx2 wild-type (+/+) and mutant (−/−) littermates orPax6 wild-type (+/+) and mutant (Sey/Sey) littermates and are taken from similar medial–lateral positions. Eachpanel is a montage of single-exposure photos using dark-field illumination with a red filter to view the silver grains and UV fluorescence to view the counterstain. RZRβ shows two distinct high rostral to low caudal gradients across the wild-type neocortex (A, B), a gradient in superficial layers that extends farther caudally (marked byarrowheads) than a gradient within the deeper layers (marked by short arrows). Both gradients ofRZRβ expression expand caudally in Emx2mutants (A′) and constrict rostrally inPax6 mutants (B′). p75 is expressed in the deep layers in roughly the caudal half of the wild-type neocortex (C, D).p75 expression constricts caudally inEmx2 mutants (C′) and expands rostrally in Pax6 mutants (D′). Thearrowheads mark the rostral limit of expression. Thelong arrows in A′–D′ indicate the opposing shifts in expression in the mutants. See Results for details. C, Caudal; R, rostral.
Each of these layer-specific graded expression patterns ofRZRβ exhibit opposing changes in Emx2 andPax6 mutants. In Emx2 mutants, RZRβis highly expressed in layer 4 across virtually the entire neocortex, and the domain of RZRβ layer 5 expression characteristic of rostral neocortex is substantially expanded and exhibits high expression throughout intermediate parts of the neocortex and well into caudal neocortex. In Pax6 mutants, we observe the opposite changes in expression, with virtually no expression detected in any layers in the caudal half of the neocortex.
In wild-type neocortex, p75 expression is confined to the subplate and layer 6 of caudal neocortex and tapers off sharply to nondetectable levels of expression rostrally (Mackarehtschian et al., 1999). In Emx2 mutants, the domain of p75expression is contracted and shifted caudally. The changes inp75 expression are more impressive in the Pax6mutants, in which the domain of p75 expression expands rostrally to such an extent that it covers virtually the entire rostrocaudal axis of the neocortex.
These data on changes in the expression of RZRβ andp75 are consistent with an expansion of rostral neocortical areas and reduction of caudal areas in Emx2 mutants and contraction of rostral neocortical areas and expansion of caudal areas in Pax6 mutants.
Emx2 and Pax6 mutants:ephrin-A5 and EphA7
Figure 5 illustrates the expression of ephrin-A5 and EphA7 at E18.5 inEmx2 and Pax6 mutants and their wild-type littermates. In wild-type mice, ephrin-A5 is expressed most highly in dorsolateral parts of the neocortex that correspond to the somatosensory area (Mackarehtschian et al., 1999). The domain of highephrin-A5 expression is shifted and restricted caudally inEmx2 mutant neocortex, whereas in Pax6 mutants, the domain of high ephrin-A5 expression is shifted and restricted rostrally. In wild-type mice, EphA7 is expressed throughout the neocortex but at much lower levels in intermediate parts of the neocortex corresponding to the somatosensory area (Rubenstein et al., 1999). In Emx2 mutants, this domain of lowerEphA7 expression is reduced in extent and shifted caudally, whereas in Pax6 mutants a domain of lower EphA7expression similar to that observed in wild type is not evident, but a small domain of reduced expression is apparent and restricted to a more rostral position. These data on changes in the expression ofephrin-A5 and EphA7 are consistent with an expansion of rostral neocortical areas and reduction of caudal areas inEmx2 mutants and contraction of rostral neocortical areas and expansion of caudal areas in Pax6 mutants.
Fig. 5.
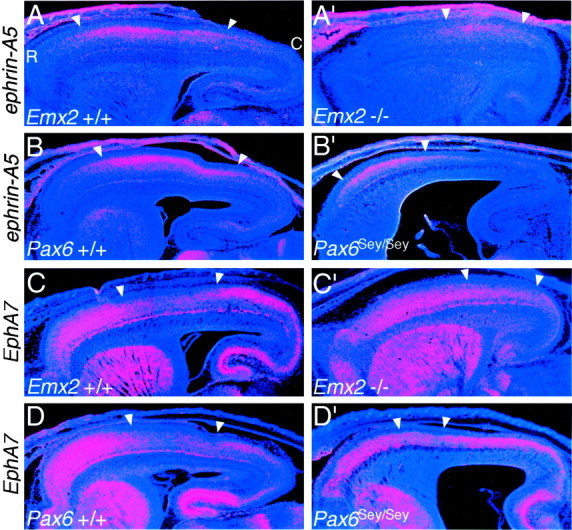
The complementary expression patterns ofephrin-A5 and EphA7, used as markers of intermediate neocortical areas, show opposing shifts inEmx2 and Pax6 mutants. In situ hybridizations on sagittal sections through the forebrain of E18.5 mice using S35-labeled riboprobes for the axon guidance ligand, ephrin-A5, and one of its receptors, EphA7, and counterstained with bisbenzimide are shown. Sections are from Emx2 wild-type (+/+) and mutant (−/−) littermates (A–B′) orPax6 wild-type (+/+) and mutant (Sey/Sey) littermates (C–D′) and are taken from similar medial–lateral positions. Each panel is a montage of single-exposure photos using dark-field illumination with a red filter to view the silver grains and UV fluorescence to view the counterstain. In wild-type mice, ephrin-A5 has high expression centered on the somatosensory area (A,B), whereas EphA7 has low expression centered on the somatosensory area (C,D). These domains shift caudally in Emx2mutants (A′–B′) and rostrally inPax6 mutants (C′–D′).Arrowheads mark the domains of highephrin-A5 or low EphA7 expression. See Results for details. C, Caudal; R, rostral.
Areal patterns of gene expression in the neocortex ofEmx1 mutants resemble those in wild-type mice
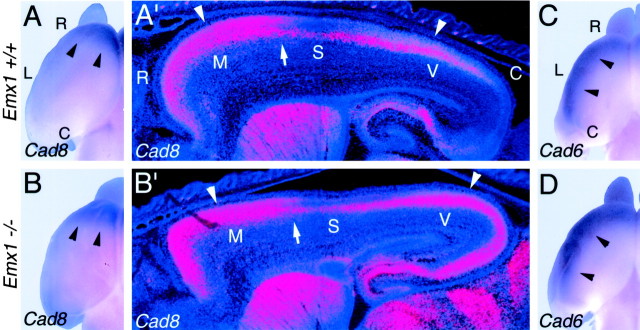
The changes in the patterns of gene markers described above indicate that EMX2 and PAX6 are involved in regulating the areal patterning of molecular correlates of neocortical arealization. In the second part of this study, we have performed a set of analyses to determine whether EMX1 may also confer position-dependent or area-specific properties to neocortical cells. First, we have used the same panel of seven genes as markers for potential changes in arealization of Emx1 mutant neocortex by doing a comparison of gene expression patterns inEmx1+/+, +/−, and −/− littermates. As illustrated in Figures 6 and7, we observe no significant alterations in the areal expression patterns of the panel of seven genes analyzed (data from the Emx1 heterozygous mice are not shown).
Fig. 6.
Patterned expression ofCad8 and Cad6 appears normal inEmx1 mutants. A–D, Dorsal views of whole mounts of E18.5 cortical hemispheres ofEmx1 wild-type (+/+) and mutant (−/−) mice processed for in situ hybridization using digoxygenin-labeled riboprobes for Cad8 (A, B) or Cad6 (C, D).Arrowheads in A and B mark the caudal limit of the rostral Cad8 expression domain characteristic of motor areas and mark the lateral expression domain ofCad6 in C and D.A′, B′, Sagittal sections through E18.5 brains of Emx1 wild-type (+/+) and mutant (−/−) mice processed for in situ hybridization using S35-labeled riboprobes for Cad8.Sections were later counterstained with bisbenzimide. Sections are taken from similar medial–lateral positions; rostral is to theleft and dorsal is to the top. Eachpanel is a montage of single-exposure photos using dark-field illumination with a red filter to view the silver grains and UV fluorescence to view the counterstain. Marked are the approximate locations of the motor (M), somatosensory (S), and visual (V) areas suggested by the patterns ofCad8 expression, which are unique in each of these areas in wild-type mice. Arrowheads inA′–B′ mark rostral and caudal expression domains in the superficial layers characteristic of motor and visual areas; in comparison, expression is substantially diminished in the superficial layers of the intervening somatosensory area. Arrows inA′–B′ mark the presumed border between motor and somatosensory areas. The Cad8 expression rostral to these arrows is the expression that is evident in the whole mounts shown in A andB. The wild-type and mutants in each pair are age-matched littermates. See Results for details. C, Caudal; L, lateral; R, rostral.
Fig. 7.
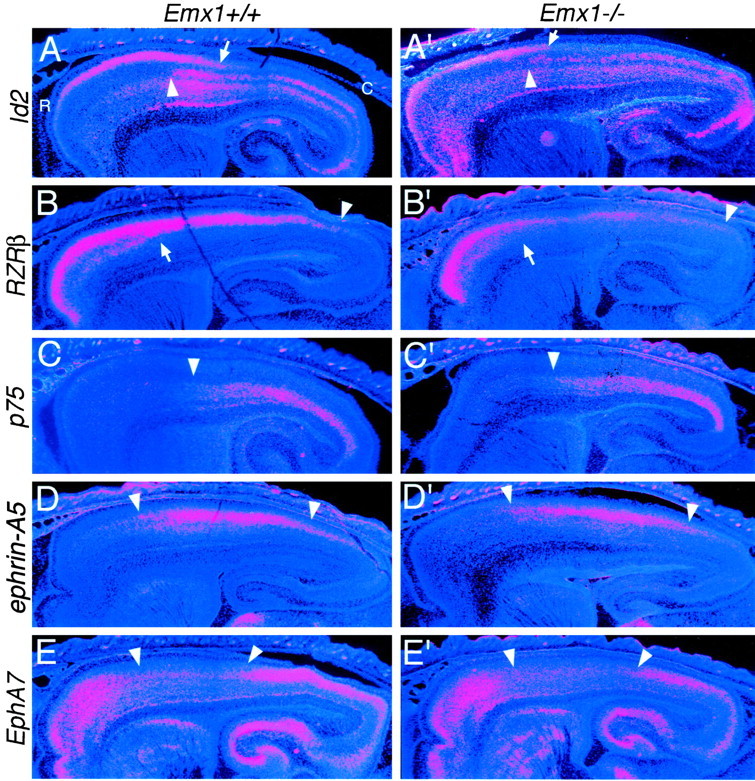
The expression domains of genes that mark rostral, caudal, or intermediate areas of the neocortex appear normal in Emx1 mutants. In situ hybridizations on sagittal sections through the forebrain of E18.5 Emx1 wild-type (+/+) and mutant (−/−) littermates using S35-labeled riboprobes forId2, RZRβ, p75,ephrin-A5, and EphA7 and counterstained with bisbenzimide are shown. Sections are taken from similar medial–lateral positions. Each panel is a montage of single-exposure photos using dark-field illumination using a red filter to view the silver grains and UV fluorescence to view the counterstain.Id2 exhibits a graded expression in superficial layers of rostral areas in wild-type mice; the arrows inA and A′ mark the position where the expression declines to very low levels. The arrowheadsin A–B′ mark the transition from low to high expression reported in layer 5; this transition corresponds to the border between motor and somatosensory areas. RZRβ is expressed in two distinct high rostral to low caudal gradients across the neocortex (B, B′); a gradient in superficial layers that extends farther caudally (marked byarrowheads) than a gradient within the deeper layers (marked by short arrows). p75 is expressed in the deep layers in roughly the caudal half of the neocortex (C, C′). Thearrowheads mark the rostral limit of expression.ephrin-A5 has high expression centered on the somatosensory area (D, D′), whereasEphA7 has low expression centered on the somatosensory area (E, E′). Arrowheadsmark the domains of high ephrin-A5 or lowEphA7 expression. Overall, the areal expression patterns of this panel of marker genes are similar in wild-type and mutant neocortex. See Results for details. C, Caudal;R, rostral.
Expression analysis of Cad8 and Cad6 was done using in situ hybridization on both whole mounts and sagittal sections of E18.5 in wild-type and Emx1 mutants (Fig. 6). In whole mounts of wild-type brains, the expression domain ofCad8 is restricted to rostral neocortex, which corresponds to the high level of expression in layers 2/3 of the motor area (Suzuki et al., 1997; Inoue et al., 1998). This rostral expression domain ofCad8 is unaltered in Emx1 mutants compared with wild type (Fig. 6A,B). In whole mounts of wild-type brains, Cad6 is expressed in a domain in lateral neocortex corresponding to somatosensory and auditory areas (Suzuki et al., 1997; Inoue et al., 1998). This expression domain ofCad6 appears unaltered in Emx1 mutants compared with wild type (Fig. 6C,D). Expression analyses show that the more complex expression patterns observed in sagittal sections are also similar between wild-type andEmx1 mutants for both Cad8 (Fig.6A′,B′) and Cad6 (data not shown). Expression analyses of Id2, RZRβ,p75, ephrin-A5, and EphA7, on sagittal sections also reveal very similar patterns in the cortex ofEmx1 mutants compared with wild type (Fig. 7). Taken together, these findings indicate that molecular markers of neocortical areas are expressed normally in Emx1 mutant neocortex.
Area-specific thalamocortical projections appear normal inEmx1 mutants
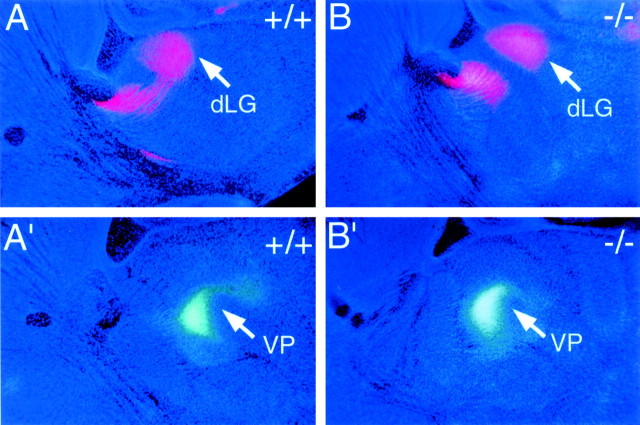
As an additional assessment of the potential role for EMX1 in regulating neocortical area identity, we used DiI and DiA as retrograde axon tracers to assess area-specific thalamocortical projections in newborn Emx1 mutant mice and their wild-type littermates. In wild-type mice, placements of DiI crystals confined to the cortical plate of occipital cortex, the location of the primary visual area, retrogradely label neurons in the dLG (Fig. 8A). Placement of DiI crystals at the same site in Emx1mutants results in a distribution of retrogradely labeled neurons (Fig.8B) similar to that observed in wild type (Fig.8A). In wild-type mice, placements of DiA crystals confined to the cortical plate of parietal cortex, the location of the somatosensory area, retrogradely label neurons in the ventroposterior thalamic nucleus (Fig. 8A′, VP). Placement of DiA crystals at the same site in Emx1 mutants results in a distribution of retrogradely labeled neurons (Fig.8B′) similar to that observed in wild-type mice (Fig.8A′). Thus, retrograde labeling from somatosensory and visual areas of the neocortex indicates that thalamocortical connections in Emx1 mutants exhibit normal area-specific patterns of connections. Taken together, our analyses using molecular markers and axon tracing suggest that the Emx1 null mutation does not affect arealization in embryonic or neonatal mice.
Fig. 8.
Area-specific thalamocortical projections appear normal in Emx1 mutant mice. Sagittal sections through P0Emx1 wild type (+/+) and mutant (−/−) brains showing retrograde DiI (red) and DiA (green-bluish) labeling and bisbenzimide counterstain (dark blue). Rostral is to theleft and dorsal is to the top in allpanels. A, B, An injection of DiI into visual (occipital) cortex retrogradely labels neurons in the dorsal lateral geniculate nucleus (dLG) in bothEmx1+/+ and Emx1−/− mice.C, D, An injection of DiA into somatosensory (parietal) cortex of the same set of brains retrogradely labels neurons in the ventroposterior thalamic nucleus (VP) in both Emx1+/+ andEmx1−/− mice. Each pair of sections is from the same brain; the dLG-labeled sections are lateral to those with the VP labeling. See Results for details.
Areal patterns of gene expression in the neocortex ofEmx double mutants resemble those inEmx2 single mutants
The coincident graded expression of Emx1 andEmx2 in the cortical ventricular zone, and their high sequence homology, suggests the possibility that the lack of an arealization phenotype in the Emx1 mutants may be caused byEmx2 compensating for the loss of Emx1. To investigate this possibility, we examined the expression patterns of the panel of seven gene markers in sagittal sections throughEmx1−/−; Emx2−/− double mutant and wild-type (i.e.,Emx1+/−; Emx2+/+) littermates at E18.5. Because Emx double mutants lack a TCA projection (K. M. Bishop, S. Garel, J. L. R. Rubenstein, and D. D. M. O'Leary, unpublished observations), we were not able to examine the areal patterning of this projection.
In Figure 9, we illustrate the expression patterns for four of the seven genes analyzed (Cad6,p75, ephrin-A5, and EphA7), and for ease of comparison, we include panels showing the expression of these genes in Emx2 single mutant neocortex. The findings from these four markers are representative of the findings from the full panel of seven markers. In the areal dimension, the expression domains of each of these markers are shifted caudally in Emx1; Emx2 double mutants compared with wild type. Because the tangential extent of the neocortex is substantially reduced in theEmx1; Emx2 double mutants compared with wild-type mice (by ∼50% in its surface area), one needs to be somewhat cautious in interpreting patterns of gene expression. However, the relative shift and positioning of the expression domain of each of these marker genes in the Emx1; Emx2 double mutants appears to be similar to that in the Emx2 single mutants. Thus, the lack of a defective arealization phenotype in Emx1 mutants is not caused by Emx2 compensating for the loss of Emx1. Therefore, these findings support the conclusion from our analyses of the Emx1 mutants that EMX1 has no apparent role in regulating arealization of the neocortex.
Fig. 9.
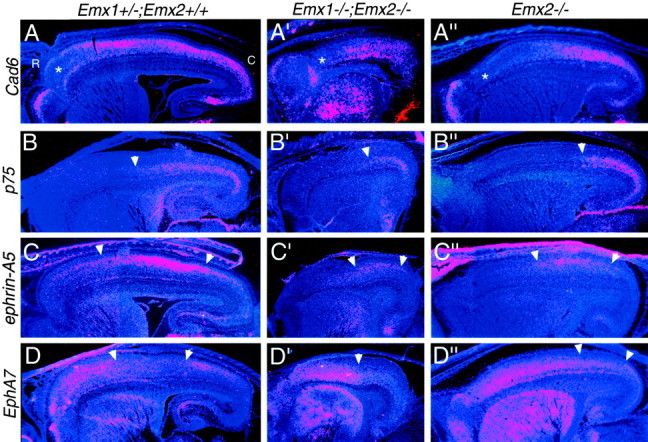
The relative positioning of expression domains of gene markers in Emx1; Emx2 double mutant neocortex resembles that in Emx2 single mutant neocortex. Shown are in situ hybridizations on sagittal sections through the forebrain of E18.5 (A–D) wild-type (i.e.,Emx1+/−; Emx2 +/+) (A′–D′) Emx double mutant (Emx1−/−; Emx2−/−), and (A′′–D′′) Emx2 single mutant (Emx2−/−) mice using S35-labeled riboprobes for Cad6,p75, ephrin-A5, or EphA7and later counterstained with bisbenzimide. Note that the neocortex of the Emx double mutant is reduced to approximately half of the wild-type area. Sections are taken from similar medial–lateral positions. Each panel is a montage of single-exposure photos using dark-field illumination and a red filter to view the silver grains and UV fluorescence to view the counterstain. Theasterisks in A–A′′ mark a domain of low Cad6 expression normally characteristic of far rostral neocortex. This domain of low expression expands and shifts caudally in Emx double mutants and Emx2 mutants.p75 is expressed in the deep layers in roughly the caudal half of the wild-type neocortex (B); this expression domain constricts caudally in Emx double mutants andEmx2 mutants (B′, B′′). The arrowheads mark the rostral limit of expression. In wild-type mice, ephrin-A5 has high expression centered on the somatosensory area (A, B), whereasEphA7 has low expression centered on the somatosensory area (C, D). These domains shift caudally in Emx double mutants and Emx2 mutants (C′, D′, C′′,D′′). Arrowheads mark the domains of highephrin-A5 or low EphA7 expression. See Results for details. C, Caudal; R, rostral.
DISCUSSION
Defining roles for Emx1, Emx2, andPax6 in arealization
We have presented evidence that strengthens the claim that EMX2 and PAX6 regulate arealization of the developing neocortex and confer positional identities to cortical cells. In addition, we show that EMX1 has no apparent role in regulating arealization. Figure 1 summarizes our predictions and interpretations. Because Emx2 and Pax6 (Sey/Sey) mutant mice die on the day of birth before areas become anatomically and functionally distinct, we have used genetic markers to assess changes in positional, or area, identity of cortical cells and presumably associated changes in arealization of the neocortex. We are confident in basing our interpretations on these data because each of the seven marker genes analyzed exhibits opposing expansions or contractions, and border shifts, in its expression domain in Emx2 andPax6 mutants, as predicted by the opposing gradients ofEmx2 and Pax6 expression (Fig. 1). The changes in marker expression indicate that arealization of the neocortex is disproportionately altered in Emx2 and Pax6mutant mice in opposing manners: in Emx2 mutants, rostrolateral areas are expanded compared with wild type, whereas caudomedial areas are reduced, and in Pax6 mutants the opposite changes occur. We conclude that EMX2 and PAX6 act in opposing manners to regulate arealization and to confer positional, or area, identity to neocortical cells. EMX2 appears to preferentially impart caudomedial area identities, and PAX6 preferentially imparts rostrolateral area identities.
We expected to observe similar changes in neocortical arealization inEmx1 mutants as in Emx2 mutants, because in the ventricular zone, Emx1 has a graded expression similar to that of Emx2 (Fig. 1). However, we find that each of the seven marker genes exhibits a normal expression pattern in the neocortex of Emx1 mutants. In addition, the area-specific organization of TCA projections is normal in Emx1 mutants. These findings suggest that unlike EMX2, EMX1 is not involved in arealization. An alternative is that Emx function is redundant in arealization and that EMX2 in particular can more fully compensate for the loss of EMX1 than vice versa. This is suggested by the coincident expression of the Emx genes in the cortical ventricular zone, their high sequence homology, and that in the dorsal telencephalon Emx2 expression begins at E8.0–8.5, whereasEmx1 expression begins at E9.5 (Simeone et al., 1992). However, our marker analysis of Emx1; Emx2 double mutants reveals that the changes in patterns of gene expression are similar to those observed in Emx2 single mutants. Thus, unlike EMX2, EMX1 does not appear to play a role in regulating arealization of the neocortex.
Emx genes may function in the patterning of neocortical areas in the same way that their Drosophila ortholog, the empty spiracle (ems) gene, specifies in the developing head early populations of neuroblasts that at later stages form specific brain structures (Younossi-Hartenstein et al., 1997; Hartmann et al., 2000). If the Emx genes act in this manner, we would predict that a deletion of both would result in the complete loss of caudomedial neocortical tissue, along with areas that differentiate within it, such as visual areas. However, our findings are inconsistent with this scenario, because rather than a complete loss of tissue in which caudal areas would form, our marker analyses indicate that rostral neocortical areas expand and shift caudally into domains that would normally develop caudal area identities and that caudal areas are present but contracted. It seems more likely that EMX2 confers positional identity to cortical neurons around the time they are generated. Further support for this interpretation is our finding that the patterned expression of the marker genes and the positioning of their expression domains retain the same relative relationships with each other, and with the neocortex as a whole, betweenEmx2 single mutants and Emx1; Emx2 double mutants, although the double mutant cortex is approximately half the normal size.
Because surface area of the cortex is reduced by ∼25% inEmx2 mutants and Pax6 mutants (Bishop et al., 2000), the disproportionate areal expansions and contractions in these mutants may be caused in part by a decreased growth of caudomedial cortex in Emx2 mutants and rostrolateral cortex inPax6 mutants (Muzio et al., 2002). However, several aspects of our findings cannot be accounted for by a regional decrease in cortical growth and therefore strongly argue that positional identity and arealization of the neocortex are altered in Emx2 andPax6 mutants (Bishop et al., 2000; present study). As discussed above, these include the similarities in changes in expression domains of areal markers between Emx2 single andEmx1; Emx2 double mutants, although cortical size in the double mutant is substantially decreased compared with the single mutant. Other aspects of our findings supporting this argument include that the expression domains of areal markers exhibit the following: (1) increases or decreases in their proportional size relative to that of the neocortex which significantly exceed that predicted by a region-specific decrease in growth, and for some markers, even increases in absolute size of domains of positive or negative expression, and (2) changes in relative positioning and coverage of the rostrocaudal axis of the neocortex that cannot be accounted for by a region-specific decrease in growth.
Potential mechanisms for EMX2 and PAX6 regulation of arealization
The details of how PAX6 and EMX2 regulate arealization are unknown. The loss of one or the other may result in a complete change or switch in area identity. PAX6, for example, has been implicated more generally in regulating the identity of ventrolateral regions of the cerebral cortex. In Pax6 mutants, progenitor cells in the ventrolateral regions lose expression of cortical regulatory genes and acquire expression of subcortical regulatory genes. As a result, ventral cortical regions (e.g., olfactory cortex) are hypoplastic, whereas adjacent subcortical areas expand (Stoykova et al., 2000;Toresson et al., 2000; Kim et al., 2001; Yun et al., 2001; Muzio et al., 2002). Evidence for cross-repression between EMX2 and PAX6 in the neocortex (Muzio et al., 2002) suggests that they may cooperate with each other, and possibly with other transcription factors (e.g., LHX2) (Nakagawa et al., 1999; Monuki et al., 2001), to confer positional or area identity to neocortical neurons. For example, EMX2 and PAX6 may participate in a combinatorial code of regulatory proteins that specifies the area identity of cortical neurons, as suggested for the specification of subtypes of motor neurons and interneurons in the spinal cord (Jessell, 2000). If so, the loss of either EMX2 or PAX6 may perturb the area identity of cortical neurons, resulting in an aberrant or chimera area identity, rather than a complete change from one area identity to another.
One protein that may collaborate with EMX2 and PAX6 in regulating arealization is COUP-TFI, an orphan nuclear receptor that has a high caudal to low rostral graded expression across the neocortex within the ventricular zone, subplate, and cortical plate (Liu et al., 2000). An analysis in CoupTfI mutants of the expression of the marker genes, Cad8, Id2, and RORβ(RZRβ), shows that they all lose their normally restricted, areal expression patterns and instead are broadly expressed across the neocortex (Zhou et al., 2001). This finding differs from that in Emx2 and Pax6 mutants, in which the marker genes retain specific patterns of expression, but the patterned expression is either expanded or contracted in a manner that opposes the graded expression of Emx2 or Pax6. In contrast, in CoupTfI mutants, Emx2 andPax6 show their normal graded patterns of expression. Thus,CoupTfI mutants have an apparent loss of areal specificity in patterns of marker expression, with the exception of Emx2and Pax6, suggesting that COUP-TFI does not directly regulate arealization but is required for the proper action of EMX2 and PAX6 in this process.
Establishment of graded expression of arealization genes across the neocortex
Evidence is emerging for a role for secreted signaling proteins with known patterning functions in establishing and maintaining the graded expression of regulatory genes, in particular Emx2, across the neocortical ventricular zone. One such signaling protein is FGF8, which is expressed in proximity to rostral parts of the cortical anlage at neural plate stages, in the anterior neural ridge, and later the rostrodorsal midline of the telencephalon (Crossley and Martin, 1995; Shimamura and Rubenstein, 1997; Crossley et al., 2001). Electroporation in E11.5 mouse cortex of vectors to overexpress FGF8 or a soluble FGF8 receptor body to diminish endogenous FGF8 results in shifts in areal markers (Fukuchi-Shimogori and Grove, 2001) similar to those expected if the graded expression of Emx2 was decreased or increased. That these effects are likely caused by FGF8 regulation of Emx2 is implied by the finding that FGF8-soaked beads implanted into dorsal telencephalon of embryonic chicks locally repress Emx2 expression (Crossley et al., 2001).
Other candidate regulators of Emx2 expression include members of the bone morphogenetic protein family, which along with Wnt family members are expressed in the cortical hem, a caudal midline structure adjacent to the hippocampal anlage (Furuta et al., 1997, Grove et al., 1998). Ectopic expression of Bmp4 in the dorsal telencephalon of embryonic chicks appears to enhanceEmx2 expression, either directly or through the repression of Fgf8 (Ohkubo et al., 2002). The zinc finger transcription factor Gli3, which is expressed broadly in the dorsal telencephalon, may be upstream to both Emx genes, because the expression of Emx1 is lost (Thiel et al., 1999; Tole et al., 2000), and Emx2 is lost (Thiel et al., 1999) or reduced (Tole et al., 2000) in the cortex of theextra-toesJ(XtJ) mutant mouse, a naturally occurringGli3 mutant (Franz, 1994). In contrast, Pax6expression is maintained in the XtJ mutant (Thiel et al., 1999; Tole et al., 2000). However, it is unclear whether Emx2 is normally regulated directly by GLI3, by signaling molecules produced in the cortical hem that are lost in theXt mutant, or by other molecular deficiencies inXtJ mutants (Grove et al., 1998;Thiel et al., 1999; Tole et al., 2000).
Both PAX6 and EMX2 are implicated in regulating members of the Wnt family, which in turn may influence corticogenesis. InPax6 mutants, expression of Wnt7b andSFRP2 in ventrolateral cortical progenitors is reduced (Kim et al., 2001), whereas Wnt3a andWnt8b expression in the dorsomedial cortex is expanded (Muzio et al., 2002). On the other hand, Wnt3a expression is reduced in dorsomedial cortex in Emx2 mutants (Muzio et al., 2002).
Defining area identity at the level of patterned gene expression
Many genes, including markers used in this study, exhibit abrupt transitions in their expression patterns within the cortical plate, and the areas themselves usually have abrupt borders. Therefore, the graded expressions of Emx2 and Pax6 are likely translated to generate downstream gene expression in restricted patterns with abrupt borders. Studies in Drosophila embryos have defined distinct mechanisms through which graded regulatory proteins can generate sharply bordered patterns of downstream gene expression, for example through concentration-dependent differences in binding efficacy to promoter and repressor elements (Rusch and Levine, 1996) or the combinatorial action of multiple activators and repressors of transcription (Stanojevic et al., 1991; Small et al., 1996). In the developing spinal cord, sonic hedgehog secreted by the notocord and floorplate represses or induces in the ventricular zone the expression of different classes of transcription factors in graded patterns, which are progressively converted into sharply bordered patterns through mutual repression (Jessell, 2000). This mechanism results in genetically distinct domains of progenitors, which generate different subtypes of spinal interneurons and motor neurons definable by their expression of unique subsets of transcription factors.
Mechanisms similar to those used in spinal cord are likely coopted to generate neocortical areas and area-specific identities of neurons, but some differences appear to exist. For example, at no time during corticogenesis are sharply bordered patterns of regulatory genes observed in the ventricular zone; all retain graded expression patterns. Initially, even genes differentially expressed in the cortical plate have graded patterns, although at later stages of development many acquire an expression pattern with abrupt changes that correlate with borders between areas (Rubenstein et al., 1999; Sestan et al., 2001).
Area specificity may differ among layers
Genes with an expression pattern restricted to one area have not been identified (Liu et al., 2000). The only genetic marker with an expression pattern restricted to one area is the H-2Z1 transgene, which marks the granular parts of postnatal mouse S1 (Cohen-Tannoudji et al., 1994). It seems reasonable to conclude then that a neocortical area and the area identity of the neurons that comprise it are defined by the expression of a unique subset of genes, each of which is also expressed in other areas. However, the actual scenario is more complex because each layer has a unique profile of gene expression: most genes reported to be differentially expressed in the neocortex and expressed in more than one layer, including markers that we use here, have different expression patterns in each layer. The expression ofId2 and RZRβ are clear examples described in this study (see Results). Thus, although neurons in different layers are generated by the same progenitors (Monuki and Walsh, 2001), they appear to have distinct positional or area identities, at least in terms of their expression of genes used as markers of these identities. This feature has significant implications for the genetic regulation of areas and how area identity is encoded in the ventricular zone and imparted by progenitors to their progeny. An understanding of these mechanisms will require the definition of areas at the level of gene expression, and defining the relationship between the specification of layer-specific and area-specific properties.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS31558 (D.O.) and NS34661 (J.R.), a McKnight Investigator Award (D.O.), National Institute of Mental Health Grant K02 MH01046 (J.R.), and postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes for Health Research (K.B.). We are grateful to P. Gruss for breeding pairs of Emx2 mutant mice, M. Goulding forSey mice, and various investigators for plasmids (see Materials and Methods). We thank Y. Nakagawa and N. Dwyer for helpful discussions and comments.
Correspondence should be addressed to Dr. Dennis D. M. O'Leary, Molecular Neurobiology Laboratory, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, CA 92037. E-mail:doleary@salk.edu.
REFERENCES
- 1.Bishop KM, Goudreau G, O'Leary DDM. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 2.Caric D, Gooday D, Hill RE, McConnell SK, Price DJ. Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development. 1997;124:5087–5096. doi: 10.1242/dev.124.24.5087. [DOI] [PubMed] [Google Scholar]
- 3.Cecchi C, Boncinelli E. Emx homeogenes and mouse brain development. Trends Neurosci. 2000;23:347–352. doi: 10.1016/s0166-2236(00)01608-8. [DOI] [PubMed] [Google Scholar]
- 4.Chenn A, Braisted JE, McConnell SK, O'Leary DDM. Development of the cerebral cortex: mechanisms controlling cell fate, laminar and areal patterning, and axonal connectivity. In: Dowan WM, Zipursky L, Jessell T, editors. Molecular and cellular approaches to neural development. Oxford UP; New York: 1997. pp. 440–473. [Google Scholar]
- 5.Cohen-Tannoudji M, Babinet C, Wassef M. Early determination of a mouse somatosensory cortex marker. Nature. 1994;368:460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- 6.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 7.Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 8.Donoghue MJ, Rakic P. Molecular evidence for the early specification of presumptive functional domains in the embryonic primate cerebral cortex. J Neurosci. 1999;19:5967–5979. doi: 10.1523/JNEUROSCI.19-14-05967.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franz T. Extra-toes (Xt) homozygous mutant mice demonstrate a role for the Gli-3 gene in the development of the forebrain. Acta Anat. 1994;150:38–44. doi: 10.1159/000147600. [DOI] [PubMed] [Google Scholar]
- 10.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- 11.Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 12.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 13.Gulisano M, Broccoli V, Pardini C, Boncinelli E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur J Neurosci. 1996;8:1037–1050. doi: 10.1111/j.1460-9568.1996.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann B, Hirth F, Walldorf U, Reichert H. Expression, regulation and function of the homeobox gene empty spiracles in brain and ventral nerve cord development of Drosophila. Mech Dev. 2000;90:143–153. doi: 10.1016/s0925-4773(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 15.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 16.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 17.Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labeling and pathway tracing. Trends Neurosci. 1989a;12:333–335. [PubMed] [Google Scholar]
- 18.Honig MG, Hume RI. Carbocyanine dyes. Novel markers for labeling neurons. Trends Neurosci. 1989b;12:336–338. [PubMed] [Google Scholar]
- 19.Inoue T, Tanaka T, Suzuki SC, Takeichi M. Cadherin-6 in the developing mouse brain: expression along restricted connection systems and synaptic localization suggest a potential role in neuronal circuitry. Dev Dyn. 1998;211:338–351. doi: 10.1002/(SICI)1097-0177(199804)211:4<338::AID-AJA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 21. Kim AS, Anderson SA, Rubenstein JLR, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci 21 2001. RC132(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Dwyer ND, O'Leary DDM. Differential expression of COUP-TF1, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20:7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackarehtschian K, Lau CK, Caras I, McConnell SK. Regional differences in the developing cerebral cortex revealed by ephrin-A5 expression. Cereb Cortex. 1999;9:601–610. doi: 10.1093/cercor/9.6.601. [DOI] [PubMed] [Google Scholar]
- 24.Mallamaci A, Iannone R, Briata P, Pintonello L, Mercurio S, Boncinelli E, Corte G. EMX2 protein in the developing mouse brain and olfactory area. Mech Dev. 1998;77:165–172. doi: 10.1016/s0925-4773(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 25.Mallamaci A, Muzio L, Chan C-H, Parnavelas J, Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2-/- mice. Nat Neurosci. 2000a;3:679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- 26.Mallamaci A, Mercurio S, Muzio L, Cecchi C, Pardini CL, Gruss P, Boncinelli E. The lack of Emx2 causes impairment of Reelin signaling and defects of neuronal migration in the developing cerebral cortex. J Neurosci. 2000b;20:1109–1118. doi: 10.1523/JNEUROSCI.20-03-01109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- 28.Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat Neurosci [Suppl] 2001;4:1199–1206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- 29.Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 30.Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Emx2 and Pax6 control regionalization of the pre-neuronogenic cortical primordium. Cereb Cortex. 2002;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa Y, Johnson JE, O'Leary DDM. Graded and areal expression patterns of regulatory genes and cadherins in embryonic cortex independent of thalamic innervation. J Neurosci. 1999;19:10877–10885. doi: 10.1523/JNEUROSCI.19-24-10877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohkubo Y, Chiang C, Rubenstein JLR. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 33.O'Leary DDM. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- 34.O'Leary DDM, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary DDM, Schlaggar BL, Tuttle R. Specification of neocortical areas and thalamocortical connections. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- 36.Pelligrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires Emx2. Development. 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- 37.Qiu M, Anderson S, Chen S, Meneses JJ, Hevner R, Kuwana E, Pedersen RA, Rubenstein JLR. Mutation of the Emx-1 homeobox gene disrupts the corpus callosum. Dev Biol. 1996;178:174–178. doi: 10.1006/dbio.1996.0207. [DOI] [PubMed] [Google Scholar]
- 38.Ragsdale CW, Grove EA. Patterning the mammalian cerebral cortex. Curr Opin Neurobiol. 2001;11:50–58. doi: 10.1016/s0959-4388(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 39.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 40.Rubenstein JLR, Anderson S, Shi L, Miyashita-Lin E, Bulfone A, Hevner RF. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–532. doi: 10.1093/cercor/9.6.524. [DOI] [PubMed] [Google Scholar]
- 41.Rusch J, Levine M. Threshold responses to the dorsal regulatory gradient and the subdivision of primary tissue territories in the Drosophila embryo. Curr Opin Genet Dev. 1996;6:416–423. doi: 10.1016/s0959-437x(96)80062-1. [DOI] [PubMed] [Google Scholar]
- 42.Sestan N, Rakic P, Donoghue MJ. Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol. 2001;11:39–43. doi: 10.1016/s0960-9822(00)00043-9. [DOI] [PubMed] [Google Scholar]
- 43.Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosphila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimamura K, Rubenstein JLR. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 45.Small S, Blair A, Levine M. Regulation of two pair-rule stripes by a single enhancer in the Drosophila embryo. Dev Biol. 1996;175:314–324. doi: 10.1006/dbio.1996.0117. [DOI] [PubMed] [Google Scholar]
- 46.Stanojevic D, Small S, Levine M. Regulation of a segmentation stripe by overlapping activators and repressors in the Drosophila embryo. Science. 1991;254:1385–1387. doi: 10.1126/science.1683715. [DOI] [PubMed] [Google Scholar]
- 47.Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 50.Thiel T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- 51.Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toesJ. Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- 52.Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 53.Tuttle R, Nakagawa Y, Johnson JE, O'Leary DDM. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. doi: 10.1242/dev.126.9.1903. [DOI] [PubMed] [Google Scholar]
- 54.Younossi-Hartenstein A, Green P, Liaw GJ, Rudolph K, Lengyel J, Hartenstein V. Control of early neurogenesis of the Drosophila brain by the head gap genes tll, otd, ems and btd. Dev Biol. 1997;182:270–283. doi: 10.1006/dbio.1996.8475. [DOI] [PubMed] [Google Scholar]
- 55.Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 56.Zhou C, Tsai SY, Tsai MJ. COUP-TF1: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]