Abstract
The dendritic and axonal arbors of developing retinal ganglion cells (RGCs) are exposed to two sources of BDNF: RGC dendrites are exposed to BDNF locally within the retina, and RGC axons are exposed to BDNF at the target, the optic tectum. Our previous studies demonstrated that increasing tectal BDNF levels promotes RGC axon terminal arborization, whereas increasing retinal BDNF levels inhibits RGC dendritic arborization. These results suggested that differential neurotrophic action at the axon versus dendrite might be responsible for the opposing effects of BDNF on RGC axonal versus dendritic arborization. To explore this possibility, we examined the effects of altering BDNF levels at the optic tectum on the elaboration of RGC dendritic arbors in the retina. Increasing tectal BDNF levels resulted in a significant increase in dendritic branching, whereas neutralizing endogenous tectal BDNF with function-blocking antibodies significantly decreased dendritic arbor complexity. Thus, RGC dendritic arbors react in opposing manners to retinal- versus tectal-derived BDNF. Alterations in retinal BDNF levels, however, did not affect axon terminal arborization. Thus, RGC dendritic arborization is controlled in a complementary manner by both local and target-derived sources of BDNF, whereas axon arborization is modulated solely by neurotrophic interactions at the target. Together, our results indicate that developing RGCs modulate dendritic arborization by integrating signals from discrete sources of BDNF in the eye and brain. Differential integration of spatially discrete neurotrophin signals within a single neuron may therefore finely tune afferent and efferent neuronal connectivity.
Keywords: brain-derived neurotrophic factor, retinal ganglion cell, retina, dendrite, arborization, Xenopus laevis, visual system development, neurotrophin
Neuronal morphogenesis is a critical process in the development of neuronal connectivity. The shape and extent of the dendritic arbor of a neuron profoundly influence its potential to receive and transmit synaptic information. The differentiation of highly branched, morphologically complex dendritic arbors is influenced by numerous intrinsic and environmental signals, which include local afferent input as well as target interactions (Rakic and Sidman, 1973; Purves, 1988; Montague and Friedlander, 1991;McAllister, 2000; Cline, 2001; Scott and Luo, 2001). In contrast, the elaboration of axon terminal arbors is thought to be modulated primarily by interactions occurring at the target. The neurotrophin family of molecular cues has been widely implicated in regulating many aspects of neuronal development, including morphological differentiation (McAllister et al., 1999; Schuman, 1999; Thoenen, 2000;Poo, 2001). Neurotrophins are known to shape dendritic morphology (Purves et al., 1988; Snider, 1988; Cohen-Cory et al., 1991; McAllister et al., 1995; Schwartz et al., 1997; Morrison and Mason, 1998; Xu et al., 2000) and to be potent modulators of axonal arborization, primarily by acting as target-derived trophic factors (Zhang et al., 1994; Cohen-Cory and Fraser, 1995; Inoue and Sanes, 1997). The expression patterns of neurotrophins and their receptors indicate that developing neurons are exposed to multiple neurotrophic sources that can exert both spatial and temporal control over their differentiation (Lewin and Barde, 1996; Huang and Reichardt, 2001).
In the vertebrate visual system, retinal ganglion cells (RGCs) provide a uniquely accessible model to investigate the spatial control that neurotrophins exert during the morphological differentiation of axons and dendrites. RGCs elaborate their axonal and dendritic arbors within two spatially segregated CNS regions that each contain neurotrophin-expressing cells (for review, see von Bartheld, 1998). Dendrites arborize locally within the retina, whereas axons arborize distally at the contralateral midbrain target, the optic tectum. Of the neurotrophins, brain-derived neurotrophic factor (BDNF) plays particularly important roles in RGC development, survival, and differentiation (Cui et al., 1998; von Bartheld, 1998; Bahr, 2000). RGCs are exposed to two distinct sources of BDNF that spatially and temporally coincide with the differentiation of their axonal and dendritic arbors. During development, BDNF is expressed in the optic tectum as well as locally within the retina. Within the developing retina, a subpopulation of neurons in the ganglion cell layer expresses BDNF (Perez and Caminos, 1995; Cohen-Cory et al., 1996; Hallbook et al., 1996), whereas RGCs as well as a subset of neurons of the inner nuclear layer of a retina express TrkB, the specific BDNF receptor (Cohen-Cory et al., 1996; Garner et al., 1996). Thus, the temporal and spatial expression patterns of BDNF and TrkB receptors within the developing visual system indicate that BDNF is available to influence the morphological differentiation of both axons and dendrites of developing RGCs.
Using the Xenopus laevis visual system as an in vivo model, we demonstrated previously that retinal and tectal BDNF influence RGC arborization in dramatically different ways. Altering BDNF levels in the tectum rapidly influenced RGC axonal arborization. RGC axon terminals responded to increased tectal BDNF by extending more complex axon terminals, adding more branches, and increasing their total arbor length (Cohen-Cory and Fraser, 1995). In contrast, altering BDNF levels within the Xenopus retina exerted a distinct response on RGC dendritic arborization (Lom and Cohen-Cory, 1999). RGCs responded to exogenous BDNF within the developing retina by extending dendritic arbors that were significantly less complex, contained fewer primary dendrites, and branched less than controls. Thus, developing RGCs respond differentially to tectal- and retinal-applied BDNF. This differential response to BDNF raised the intriguing possibility that BDNF action at the RGC axon terminal may differ from BDNF action at its dendrites. Here, we examined whether alterations in tectal BDNF levels influenced RGC dendritic arborization and compared these effects with those resulting from alterations in retinal BDNF levels. Our results indicate that RGCs respond in opposite manners to retinal- versus tectal-derived BDNF to modulate the morphology of their dendritic arbors. Consequently, differential spatial integration of neurotrophic signals, which originate locally and within the target, may fine-tune dendritic morphology and connectivity.
MATERIALS AND METHODS
Reagents. All reagents were obtained from Sigma (St. Louis, MO) unless otherwise indicated. Recombinant human BDNF (rhBDNF) was kindly provided by Amgen (Thousand Oaks, CA), recombinant human neurotrophin-4 (NT-4) was generously provided by Genentech (South San Francisco, CA), and anti-rhBDNF neutralizing antibody (mouse IgG1) was obtained from R & D Systems (Minneapolis, MN).
X. laevis tadpoles. X. laevis embryos were obtained by in vitro fertilization of eggs obtained from adult females (Xenopus One, Dexter, MI) primed with human chorionic gonadotropin. Embryos were reared in 20% modified Steinberg's solution [60 mm NaCl, 0.67 mm KCl, 0.34 mmCa(NO3)2, 0.83 mm MgSO4, 10 mm HEPES, and 40 mg/l gentamycin, pH 7.4] (Keller, 1991). Embryos were developmentally staged according toNieuwkoop and Faber (1967). A percentage (0.001%) of phenylthiocarbamide was included in the rearing solution to reduce pigmentation. Animals were anesthetized for experimental manipulation by immersion in rearing solution that contained 0.05% tricane methanesulfonate (Finquel; Argent Labs, Remond, WA).
Neurotrophin-treated microspheres. Green fluorescent microspheres (50–200 nm in diameter) (Lumafluor, Naples, FL) were prepared as described by Lom and Cohen-Cory (1999). Briefly, deionized microspheres were incubated overnight at 4°C in a 1:4 mix of microspheres to 1, 10, or 100 ng/μl neurotrophin or control protein (cytochrome c). Microspheres were then centrifuged and resuspended in sterile water. Neurotrophin-coated microspheres have been shown to exert neurotrophic activity comparable with that of free neurotrophins for at least 4 d (Riddle et al., 1997). Anti-BDNF or a control antiserum (anti-peroxidase mouse IgG1) was mixed with deionized microspheres to a concentration of 250–375 mg/ml immediately before injection. Microspheres were then microinjected into the retina or tectum of anesthetized tadpoles at stage 38 and/or 42. Tadpoles were subsequently reared in darkness. The control antiserum had no distinguishable effects on any parameter of RGC dendritic arborization measured (see below) versus control protein (data not shown).
Visualizing RGC dendritic arbors. RGC dendritic arbors were fluorescently labeled with rhodamine–dextran (3 kDa; Molecular Probes, Eugene, OR) as described by Lom and Cohen-Cory (1999). Tectal injections of rhodamine–dextran randomly filled a subpopulation of RGCs at sufficiently sparse densities so that individual dendritic arbors were easily discriminated. Because RGC axons are the sole connection from the retina to the brain, this technique specifically and exclusively labeled RGCs. Briefly, rhodamine–dextran was microinjected into the tecta of anesthetized stage 42 tadpoles (when RGC axons begin to arborize). Tadpoles were then reared to stage 45 and fixed in 4% paraformaldehyde. The retinas were prepared as whole mounts and visualized with a high-resolution cooled CCD camera (Photometrics, Tucson, AZ) on a Nikon (Tokyo, Japan) E800 fluorescent microscope with a 100× oil-immersion objective. Images were collected through the entire extent (z-axis) of each dendritic arbor at 0.5 μm intervals using MetaMorph software (Universal Imaging, Corp., West Chester, PA). The dendritic arbor of each RGC was reconstructed plane-by-plane from the three-dimensional image stack, and the reconstructed image was then analyzed with MetaMorph. Only RGCs with at least one primary dendrite ≥10 μm long were analyzed. Branch tips were identified as the terminal ends of primary dendrites. Primary dendrites were defined as direct extensions from the soma of ≥10 μm in length. To calculate total dendritic arbor length and soma area, images of dendritic arbors were thresholded, binarized, and skeletonized with the MetaMorph software so that soma perimeter and dendrites were represented as a single pixel in width. Dendritic arbor morphology was statistically compared using ANOVA with Tukey's post hoc test or two-sample ttest (Systat; Statistical Program for the Social Sciences, Chicago, IL). Significance was assigned when ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001.
Visualizing RGC axonal arbors. To analyze the effects of retinal-derived BDNF during RGC axon arborization, RGC axon arbors were visualized by anterograde labeling with the fluorescent vital dye DiI (Molecular Probes) or by expression of the yellow fluorescent protein (YFP). In brief, retinas of anesthetized stage 41 tadpoles were iontophoretically injected with minute amounts of the DiI to label RGC axon arbors as described by Cohen-Cory and Fraser (1995). Alternatively, RGC axon arbors were labeled by lipofection with YFP cDNA (Clontech, Palo Alto, CA) at stage 20 of development (before experimentation) as described by Alsina et al. (2001). Tadpoles with one to two distinguishable axonal arbors branching in the optic tectum were imaged with a PCM2000 Nikon laser-scanning confocal microscope at stage 43 of development. Immediately after imaging, tadpoles were anesthetized and intraocularly injected with BDNF, cytochromec, or anti-BDNF either in soluble form (including 0.04% fast green as a tracer of injection site) or coupled to green fluorescent microspheres (as described above). Axon morphologies were imaged again 24 and 48 hr later. Axon arbor morphologies were then compared to determine the effects of altered retinal BDNF on axon arbor complexity and to compare them with the effects of altered tectal BDNF levels (Cohen-Cory and Fraser, 1995; Alsina et al., 2001). For each individual arbor, total branch number and total arbor length were compared at 0, 24, and 48 hr to determine the changes in both branch number and total arbor length over time (increase in arbor complexity). After the last imaging session, tadpoles were anesthetized and fixed, and the retina was whole mounted to confirm that the DiI- or YFP-labeled RGCs were directly exposed to the treatment, as determined by the presence of fluorescent microspheres in the vicinity of the labeled RGC (Fig. 1B). Statistical analysis was performed as described by Cohen-Cory and Fraser (1995). No significant differences in axon arbor morphology or axon branch dynamics were observed between axons labeled with DiI or YFP, or between animals that received retinal injections of soluble BDNF or BDNF-treated microspheres (data not shown). Thus, DiI- and YFP-labeled axons and soluble- and microsphere-treated animals were grouped together for statistical analysis.
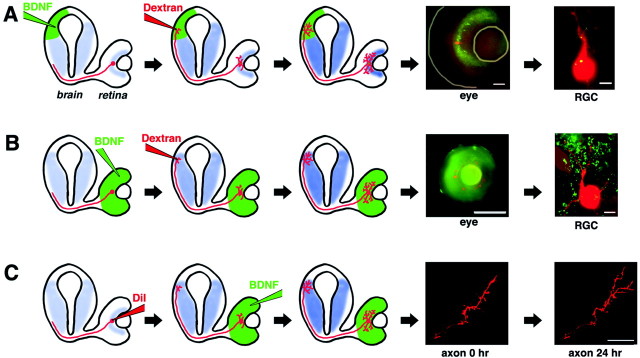
Fig. 1.
Altering endogenous retinal and tectal BDNF levelsin vivo. Diagrams representing a transverse view of aXenopus tadpole brain and eye illustrate experimental procedures (see Materials and Methods). RGCs are depicted inred, relative endogenous BDNF expression levels (Cohen-Cory et al., 1996) are depicted in blue, and exogenously applied factors are depicted in green.A, Effects of altered tectal neurotrophins on RGC dendritic arborization. Control, anti-BDNF, or BDNF-treated green fluorescent microspheres were injected into the stage 38 tadpole tectum. At stage 42, RGCs were retrogradely labeled by injecting rhodamine–dextran in the contralateral tectum. At stage 45, dendritic morphologies of double-labeled RGCs were evaluated. A low-power view of a tadpole eye shows green fluorescent microspheres retrogradely transported to the retinal ganglion cell layer, where a rhodamine–dextran-labeled RGC soma can also be visualized (lines denote lens and eye periphery). Scale bar, 50 μm. A single-plane, high-power view of a stage 45 retina reveals a rhodamine–dextran-labeled RGC with internalized green fluorescent microspheres. Scale bar, 5 μm. B, Effects of altered retinal neurotrophins on RGC dendritic arborization. Control, anti-BDNF, or BDNF-treated microspheres were injected into the stage 38 tadpole retina, and then RGCs were retrogradely labeled at stage 42. The low-power view shows rhodamine–dextran-labeled RGCs and green fluorescent microspheres restricted within the tadpole eye. Scale bar, 200 μm. The single-plane, high-power view of a stage 45 retina reveals the morphology of a rhodamine–dextran-labeled RGC surrounded by green fluorescent microspheres. Scale bar, 5 μm. C, Effects of altered retinal neurotrophins on RGC axonal arborization in the tectum. Control, anti-BDNF, or BDNF-treated microspheres were injected into the stage 43 tadpole retina, and the morphology of DiI- or YFP-labeled RGC axon arbors was visualized 24 and 48 hr later. Confocal microscope images of a control RGC axon at 0 and 24 hr demonstrate normal RGC axon arborization dynamics. Scale bar, 20 μm.
RESULTS
Tectal BDNF promotes RGC primary dendrite extension
In the developing tadpole, RGC dendritic differentiation begins at approximately stage 38, when RGCs begin to initiate short, unbranched primary dendrites. At the same time, RGCs actively extend their axons through the midbrain en route to the tectum (Sakaguchi et al., 1984;Holt, 1989; Chien and Harris, 1994). To begin to understand whether differential spatial integration of local versus target-derived neurotrophin signals may be responsible for the differential effects of BDNF on axons versus dendrites, we used three experimental approaches to determine local versus target-derived effects of BDNF on axonal and dendritic arborization (Fig. 1). First, we examined the effects of altering BDNF levels within the developing target (optic tectum) during RGC dendritic arborization (Fig. 1A). Fluorescent microspheres (Riddle et al., 1997) treated with BDNF, control protein (cytochrome c), control antiserum, or a function-blocking BDNF antibody were microinjected in the optic tectum of stage 38 tadpoles, before the first axons reach the tectum, and then again at stage 42, when RGC axons begin to arborize in the optic tectum and dendrites begin to differentiate within the retinal inner plexiform layer. Fluorescent microspheres, in addition to serving as neurotrophin delivery vehicles, provided a visible marker of neurotrophin treatment, because axon terminals that contacted the microspheres internalized and retrogradely transported the microspheres to the soma (Katz and Iarovici, 1990; Riddle et al., 1995, 1997). We visualized RGC dendritic arbor morphology by microinjecting rhodamine–dextran into the optic tectum at stage 41/42 of development, when axons begin to arborize. At stage 45, the dendritic morphology of individual RGCs that encountered altered BDNF levels at their axon termini (as determined by the retrograde transport of green fluorescent microspheres) (Fig.1A) was analyzed.
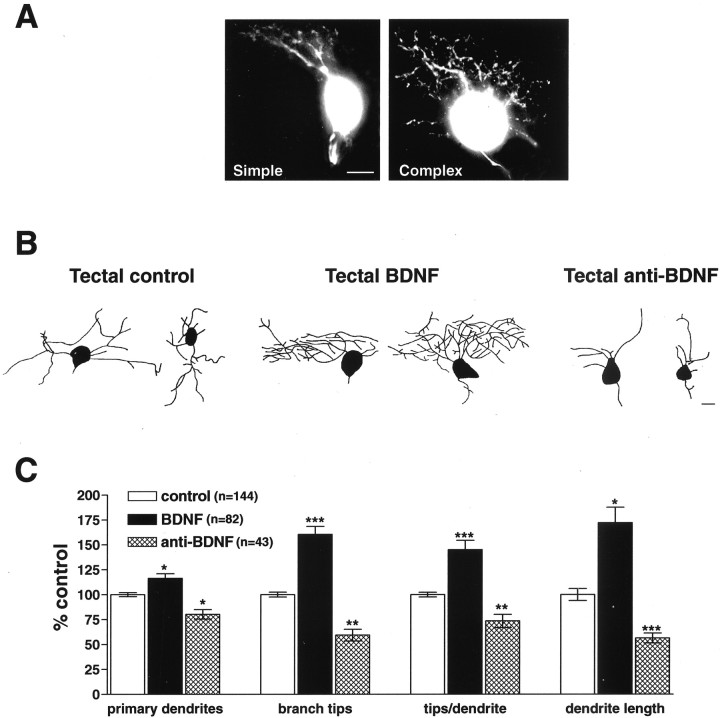
Qualitatively, the dendritic arbors of RGCs exposed to increased BDNF levels at the tectal target were more complex than controls (Fig.2B). Correspondingly, RGCs exposed to BDNF-neutralizing antibodies exhibited less complex dendritic arbors (Fig. 2B), extending significantly fewer primary dendrites and branch tips per RGC. To examine the influence of altered tectal BDNF levels on RGC dendritic arborization, we quantified several morphological parameters (Fig. 2C). The effects of altering tectal BDNF levels on primary dendrites were evaluated by counting the number of dendrites that extended directly from the soma of each double-labeled RGC. RGCs exposed to increased tectal BDNF extended significantly more primary dendrites when compared with control RGCs (116.4 ± 4.7% of control; p = 0.014). Conversely, neutralizing endogenous tectal BDNF with function-blocking antibodies specifically decreased the number of RGC primary dendrites versus controls (80.3 ± 4.8% of control;p = 0.02). Thus, altering endogenous tectal BDNF significantly altered the number of RGC primary dendrites. These results indicate that BDNF can act at a distance to modulate RGC primary dendrite extension.
Fig. 2.
Tectal BDNF retrogradely enhances RGC dendritic arborization. To determine whether tectal BDNF influences RGC dendritic arborization within the retina, tadpoles received tectal injections of microspheres treated with control, BDNF, or anti-BDNF function-blocking antibodies. Microsphere-containing neurons colabeled with rhodamine–dextran were analyzed morphologically (Fig.1A). A, Image reconstructions of two rhodamine-labeled RGCs with simple and complex dendritic arbors illustrate differences in dendritic arbor morphologies.B, Images of RGC dendritic arbors reveal that increasing tectal BDNF enhances RGC dendritic arborization, whereas neutralizing endogenous tectal BDNF with function-blocking antibodies reduces RGC dendritic arborization. C, Quantitative analysis reveals that primary dendrite number, branch tip number, branch tips per primary dendrite, and overall dendritic length were significantly enhanced by increasing tectal BDNF and reduced by injecting anti-BDNF into the optic tectum. Scale bar, 5 μm. Error bars indicate SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Tectal BDNF promotes RGC dendritic branching
Qualitative observations suggested that tectal BDNF modulates not only RGC primary dendrite extension but also branching (Fig.2B). To determine tectal BDNF effects on RGC dendritic branching, we quantified total dendritic branch tip number per RGC in tadpoles exposed to control, BDNF-, or anti-BDNF-treated microspheres (Fig. 2C). RGCs exposed to increased tectal BDNF had significantly more branch tips than controls (160.5 ± 8% of control; p < 0.001). Neutralizing endogenous tectal BDNF, similar to its effects on primary dendrite extension, significantly reduced branch tip number per RGC (59.4 ± 5.9% of control; p = 0.001). These results indicate that altering BDNF levels at the distal tectal target during active dendritic and axonal arborization significantly alters dendritic branching. Together, these results reveal that endogenous tectal BDNF plays a significant role in modulating RGC primary dendrite extension and dendritic branching within the retina.
To determine whether the tectal BDNF-elicited increase in RGC branching was a consequence of the increase in primary dendrite number or whether tectal BDNF increases dendrite branching in addition to increasing primary dendrite number, we calculated the average number of branch tips per primary dendrite (Fig. 2C). RGCs that were exposed to elevated tectal BDNF levels had significantly more branch tips per primary dendrite than did control RGCs (145 ± 9.4% of control;p < 0.001). Correspondingly, when endogenous tectal BDNF was neutralized with function-blocking antibodies, primary dendrites branched significantly less than controls (73.5 ± 6.6% of control; p = 0.002). Thus, tectal BDNF regulates RGC dendritic branching by enhancing both primary dendrite extension and the secondary branching of these dendrites.
Tectal BDNF increases overall RGC dendritic arbor complexity
We observed that increasing tectal BDNF levels increased the number of RGC primary dendrites as well as dendritic branching, thus increasing dendritic arbor complexity. It is possible that RGCs, in an attempt to achieve a targeted total dendritic arbor surface input area, could compensate for the increased dendritic complexity by extending shorter dendrites. To determine whether alterations in dendritic arbor length correlate with the increase in dendritic arbor complexity, we compared the total arbor length of RGCs exposed to BDNF-treated, control, or anti-BDNF-treated microspheres at the tectum (Fig.2C). Tectal BDNF significantly increased RGC total dendritic arbor length (172.1 ± 15.4% of control; p = 0.03), whereas anti-BDNF significantly decreased dendritic arbor length (56.4 ± 5% of control; p < 0.001). Total dendritic arbor length was increased by exogenous BDNF and was decreased by neutralizing endogenous tectal BDNF in manners similar to those observed for total branch number and primary dendrites. Thus, modulating tectal BDNF levels enhances primary dendrite extension and secondary branching, and these increases correlate with an increase in the total RGC dendritic arbor length.
Direct exposure to BDNF at the axon terminal is required for BDNF to affect RGC dendritic arborization
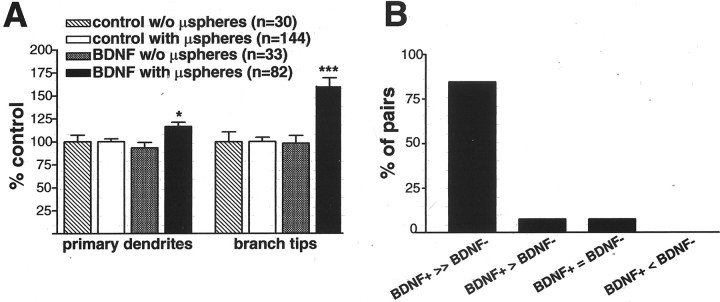
Tectal BDNF may exert its influence on RGC dendritic arborization directly or indirectly. Tectal BDNF in the tectum may initiate a retrograde signal that directly controls RGC dendritic arborization, or BDNF may modulate RGC dendritic morphology indirectly by promoting alterations in the target optic tectum that in turn influence RGC dendritic complexity and afferent connectivity. To begin to differentiate between these possibilities, we determined whether direct exposure of axon terminals to the BDNF treatment was necessary for BDNF to influence RGC dendritic complexity. We compared dendritic morphologies of RGCs that did not transport microspheres retrogradely to their cell bodies (and, therefore, their axons were not in direct contact with the microspheres) with those of RGCs that transported the microspheres (rhodamine–dextran and green fluorescent microsphere double-labeled RGCs) in tadpoles with tectal injections of control or BDNF-coupled microspheres (Fig. 3). For RGCs that did not transport the fluorescent microspheres back to their cell bodies, both the number of primary dendrites (93.4 ± 5.7% of control; p > 0.05; n = 33 for BDNF and n = 30 for control; three independent experiments) and total branch tip number (98.28 ± 8.2% of control;p > 0.05) were indistinguishable between BDNF-treated and control animals. These results are in contrast to the significant effects we observed on RGCs that retrogradely transported microspheres (primary dendrites, 116.4 ± 4.7% of control, p< 0.02; total branch tip number, 160.5 ± 8% of control,p < 0.001). The number of primary dendrites and branch tip number for RGCs in control tadpoles with and without retrogradely transported microspheres was indistinguishable (data not shown). Thus, these results indicate that RGCs required direct exposure to BDNF at their axon terminal in order for tectal BDNF to enhance their dendritic complexity back in the retina.
Fig. 3.
Tectal BDNF promotes RGC dendritic arborization by direct interaction with axon terminals. To determine whether RGC dendritic arborization requires direct axonal interaction with tectal BDNF, the dendritic arbors of RGCs from tadpoles treated with control or BDNF-treated microspheres (μspheres) were analyzed. The presence of green fluorescent microspheres in the soma of rhodamine-labeled RGCs (A, with μspheres) indicated that the axon termini of these neurons interacted directly with exogenous BDNF in the tectum, whereas the absence (A, w/o μspheres) indicated that RGC did not retrogradely transport microspheres and therefore did not interact directly with tectal BDNF. A, Quantitative analysis of dendritic morphology revealed that primary dendrites and branch tip numbers were increased by tectal BDNF only when RGC axons internalized and retrogradely transported BDNF-treated microspheres. Error bars indicate SEM. ∗p < 0.05; ∗∗∗p < 0.001. B, Analysis of neighboring RGC pairs (separated by 1–2 soma diameters) with and without retrogradely transported BDNF-treated microspheres revealed that double-labeled RGCs directly exposed to BDNF (BDNF+) had more than twice as many dendrite branches than their neighboring RGCs without microspheres (BDNF−) (in x-axis, >> equals >200%, > equals >150%, and = equals same number of total branch tips; n = 13 pairs).
By analyzing pairs of neighboring rhodamine-labeled RGCs (separation of one to two soma in diameter) with and without retrogradely transported microspheres, we observed that RGCs directly exposed to BDNF (double labeled) were significantly more complex than their neighboring RGCs without microspheres (single labeled) (Fig. 3B). This analysis provided an additional measure of the spatially restricted effects of BDNF. In tadpoles that received tectal injections of BDNF-treated microspheres, the RGC that retrogradely transported microspheres was significantly more complex in 84.6% of RGC pairs (>200% of the number of branch tips) than the neighboring RGC without microspheres. In 7.6% of the pairs, the RGC with microspheres was slightly more complex (150% of the number of branch tips) than the RGC without microspheres, and in 7.6% of the pairs, the complexity of the two RGCs was similar (n = 13 pairs). This difference in complexity between RGCs that directly encountered BDNF at their axon terminals versus neighboring RGCs that did not was also highlighted by the difference in the total number of branch tips per RGC. RGCs that interacted with BDNF-treated microspheres had 22.2 ± 2.7 branch tips per neuron, whereas RGCs in those retinas that did not transport microspheres had only 9.9 ± 1.3 branch tips per neuron (p < 0.005). Comparing the complexity in neighboring RGCs that that did and did not transport control microspheres revealed no significant difference (n = 10 pairs; data not shown). Thus, axon terminals must be directly exposed to BDNF for the neurotrophin to influence RGC dendritic complexity.
The effects of tectal BDNF on dendritic arborization are specific
The TrkB tyrosine kinase receptor recognizes both BDNF and NT-4 ligands and interacts with the p75 low-affinity neurotrophin receptor to transduce its signals (Friedman and Greene, 1999; Kaplan and Miller, 2000; Patapoutian and Reichardt, 2001). In Xenopus, as in other species, NT-4 has been shown to exert effects different from those of BDNF (Cohen-Cory and Fraser, 1995; Riddle and Katz, 1995). For example, we have shown previously that NT-4 does not alter the complexity of RGC dendritic arbors when applied to the retina but can significantly increase RGC soma size (Lom and Cohen-Cory, 1999). Thus, to determine the specificity of target-derived BDNF during RGC dendritic arborization, microspheres treated with NT-4 were microinjected into the tecta of tadpoles during active RGC arborization. RGCs exposed to exogenous NT-4 elaborated dendritic arbors that were similar to controls. None of the morphological characteristics of the dendritic arbors of RGCs exposed to NT-4 at the target were significantly altered. Analysis of NT-4-treated RGCs revealed that primary dendrite branch number (94.9 ± 8.7% of control; p > 0.05), branch tip number (106.5 ± 8% of control; p > 0.05), and total dendritic arbor length (102.2 ± 12.6% of control; p > 0.05) did not differ significantly from controls (data not shown graphically). These results support our previous observations that neurotrophins other than BDNF exert distinct effects on RGC dendritic arborization (Lom and Cohen-Cory, 1999). Thus, BDNF acting both at the retina and at the target optic tectum specifically modulates RGC dendritic elaboration.
The differential effects of retinal- and tectal-derived BDNF are specific and not attributable to concentration differences
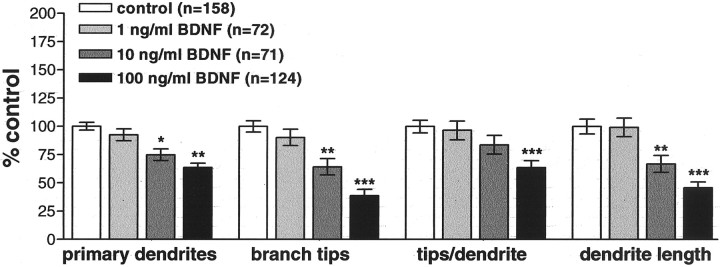
To determine whether the differential response in dendritic elaboration by RGCs to retinal- and tectal-derived BDNF was caused by neurotrophin concentration differences at the cell body versus axon terminal, we experimentally altered endogenous retinal BDNF levels in stage 38 Xenopus tadpoles, at the onset of dendritogenesis. Microinjecting BDNF-treated microspheres at three different concentrations allowed us to make a more direct comparison between the effects of altered tectal BDNF and the effects of alterations in retinal BDNF levels that we had observed previously (Lom and Cohen-Cory, 1999). Microspheres treated with BDNF or control protein (cytochrome c) at three different concentrations (1, 10, or 100 ng/μl) (Fig. 1B) were intraocularly injected into stage 38 anesthetized tadpoles, and RGCs were fluorescently labeled by tectal injection of rhodamine–dextran at stage 42 of development (Fig. 1B). Tadpoles were reared to stage 45, the developmental stage at which endogenous retinal BDNF levels peak (Cohen-Cory and Fraser, 1994) and RGC dendrites actively arborize (Sakaguchi et al., 1984; Holt, 1989). Dendritic arbors of RGCs exposed to microspheres within the retina were visualized by fluorescence microscopy, and their arbor morphologies were analyzed (Fig.1B). The inhibitory effects of increasing BDNF levels in the retina were dose dependent (Fig.4). As we observed previously (Lom and Cohen-Cory, 1999), the highest dose of BDNF significantly inhibited all dendritic morphological parameters evaluated. Retinal injection of 100 ng/μl BDNF resulted in RGCs with significantly fewer primary dendrites than controls (63.5 ± 3.9% of control;p < 0.001). This concentration of BDNF also significantly decreased dendritic branching as measured by the total number of branch tips per neuron (39 ± 6%; p < 0.001) and the average number of branch tips per primary dendrite (63.5 ± 6.3%; p < 0.001). As a consequence, total dendritic arbor length was also significantly reduced versus controls (45.7 ± 5.4%; p < 0.001). Microspheres treated with intermediate concentrations of BDNF (10 ng/μl) caused more moderate yet significant effects on dendritic morphology. BDNF (10 ng/μl) reduced primary dendrite number to 74.9 ± 4.6% of control (p = 0.001), branch tip number to 64.3 ± 6.4% of control (p = 0.001), tips per dendrite to 84% of control (p > 0.05), and total dendritic arbor length to 66.9 ± 7.5% of control (p = 0.007). The lowest dose of BDNF (1 ng/μl) had no significant effects on any measured parameter of dendritic arborization (90–99% of control; p > 0.05). Thus, RGC responses to altered retinal BDNF levels are concentration dependent, with BDNF limiting dendritic elaboration in a dose-dependent manner. These results suggest that RGCs interpret retinal BDNF signals in a specific, concentration-dependent manner that differs from tectal-derived BDNF.
Fig. 4.
Retinal BDNF inhibits RGC dendritic arborization in a dose-dependent manner. To determine whether RGCs are sensitive to the concentration of BDNF in the retina, Xenopus retinas were microinjected with 1–100 ng/μl BDNF or control microspheres at the onset of dendritic arborization. Quantitative measures of dendritic arbor morphology revealed a dose-dependent response to BDNF. The highest concentration of BDNF most dramatically decreased primary dendrite number, branch tip number, tips per dendrite, and dendrite length versus control. Error bars indicate SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
RGC dendritic arborization is temporally sensitive to retinal BDNF
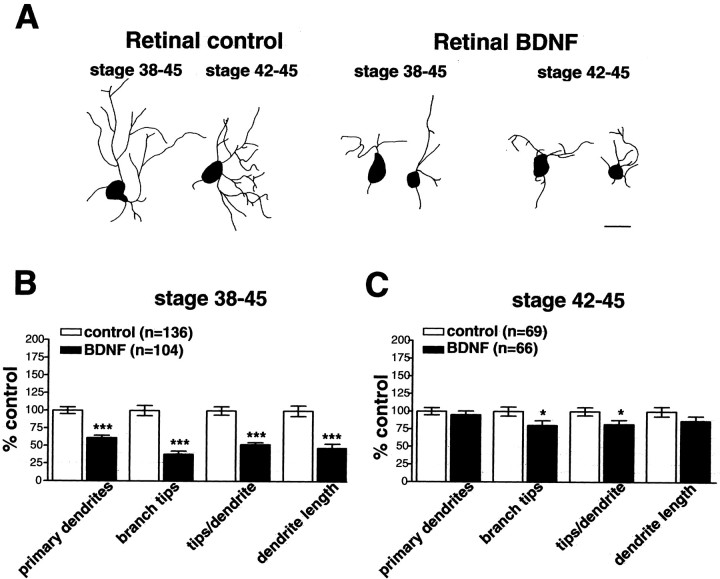
In the Xenopus retina, BDNF mRNA expression is first detected in RGCs at stage 39/40 of development and peaks at stage 45 (Cohen-Cory and Fraser, 1994). RGCs express BDNF, whereas RGCs and amacrine cells express TrkB (Cohen-Cory et al., 1996). In our previous studies, we investigated the role of retinal-derived BDNF beginning at stage 38, the onset of RGC dendritic differentiation, before peak BDNF expression. In the present studies, tectal treatment with BDNF also began at stage 38, the time that the earliest RGC axons are en route to the optic tectum, and when TrkB protein expression is first detected on RGC axons along the optic nerve (S. Cohen-Cory, unpublished observations). Because RGC axons may encounter altered tectal BDNF levels past stage 38, once dendritogenesis is ongoing, it is possible that differences in the maturation state of RGC are responsible for the differential effects of altering retinal and tectal BDNF levels. To examine this possibility, we compared the effects of altering retinal BDNF levels beginning at stage 42 of development with the effects of altering BDNF levels beginning at stage 38. BDNF-treated microspheres (100 ng/μl) microinjected into the retina at stage 42 of development decreased dendritogenesis (Fig. 5), although the effects were less pronounced than altering retinal BDNF levels beginning at stage 38. Primary dendrites of RGCs exposed to BDNF from stages 38–45 were significantly reduced to 61 ± 3.6% of control (p < 0.001), whereas primary dendrites of RGCs exposed to BDNF between stages 42 and 45 were not significantly altered (95.3 ± 5.2% of control; p > 0.05). Thus, primary dendritic extension is most sensitive to retinal BDNF during the initial phases of dendritogenesis that occur before stage 42.
Fig. 5.
RGC dendritic arborization is temporally sensitive to increased retinal BDNF levels. To determine whether RGCs were sensitive to enhanced retinal BDNF in a stage-dependent manner, control or BDNF-treated microspheres were injected into Xenopus retinas at stage 38 or 42.A, The morphology of RGC dendritic arbors revealed a stage-dependent response to increased retinal BDNF levels.B–C, Quantitative analysis of dendritic differentiation indicates that earlier exposure to exogenous BDNF (stages 38–45) inhibited dendritic arborization more dramatically than later exposure to BDNF (stages 42–45). Primary dendrite number as well as dendritic branching was significantly decreased by altering retinal BDNF starting at stage 38 (B), whereas altering retinal BDNF levels from stage 42 onward (C) selectively reduced dendritic branching without affecting primary dendrite number. Error bars indicate SEM. ∗p < 0.05; ∗∗∗p < 0.001. Scale bar, 10 μm.
Analysis of other parameters of dendritic elaboration revealed that altering endogenous retinal BDNF levels beginning at stage 42 significantly altered secondary branching. Total branch tip number per RGC in retinas exposed to BDNF from stages 42–45 of development was significantly decreased versus control (80.4 ± 6.6% of control;p = 0.032). Correspondingly, branch tip number per primary dendrite was significantly reduced in RGCs exposed to BDNF at stage 42 (82 ± 5.9% of control; p = 0.028). Exposing RGCs to BDNF from stages 38–45 had more pronounced effects on secondary branching. BDNF significantly reduced branch tip number to 38.2 ± 4.8% and branch tip number per primary dendrite to 52 ± 3% of control; p = 0.001 (Lom and Cohen-Cory, 1999). Thus, secondary branching is affected when altering retinal BDNF levels both before and after the onset of RGC dendritogenesis.
Our observations of the inhibitory effects of BDNF on primary dendrite number and total branch tip number indicated that retinal BDNF decreases dendritogenesis, but the effects of BDNF are more moderate when neurons are exposed to altered BDNF levels after the onset of RGC dendritic differentiation. We observed moderate yet nonsignificant reductions in total dendritic arbor complexity measured as total arbor length. Exposing RGCs to BDNF-treated microspheres from stages 42–45 decreased total dendritic arbor length to 86.7 ± 6.9% of control (p > 0.05), whereas exposure to BDNF beginning at stage 38 significantly decreased total arbor length (45.7 ± 5.4% of control; p < 0.001). Thus, our observation that RGCs are less sensitive to altered retinal BDNF levels at stage 42 of development may be attributable to the fact that primary dendritogenesis is well underway at the onset of treatment. Furthermore, our finding that altering BDNF once dendrite differentiation is ongoing (from stage 42 onward) affected secondary branching but not primary dendrite number suggests that retinal BDNF prevents dendrite initiation rather than eliciting branch retraction. Thus, developing RGCs show stage-specific reductions in dendritic arborization in response to altered retinal BDNF levels, further suggesting that the differential effects of tectal- and retinal-derived BDNF are caused by differential neurotrophin actions initiated at the axon versus dendrite.
Retinal BDNF does not influence RGC axonal arborization
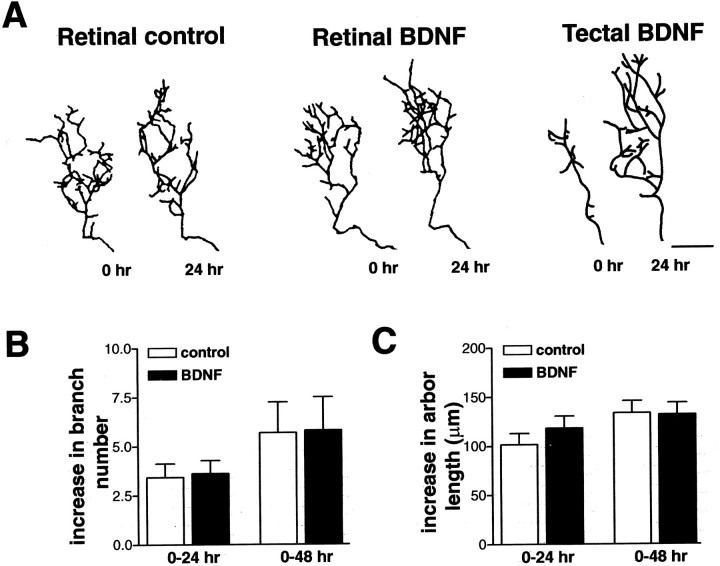
Our observations that the interaction of an RGC axon terminal with BDNF in the tectum can modify RGC dendritic arborization after 2–3 d suggested that trophic signals were retrogradely transmitted along the axon length to affect dendritic arborization at a distance. These observations consequently raised the possibility that retinal BDNF might also anterogradely influence RGC axon arborization in the tectum. To determine whether altering retinal BDNF levels also influences RGC axon arborization, control, BDNF-, and anti-BDNF-treated microspheres were microinjected into the retina of stage 43 tadpoles, and axon arbor morphology of individual, fluorescently labeled RGC axons branching in the optic tectum was visualized by confocal microscopy (Fig.1C). Determining the number of total axon branches and total axon arbor length at 0, 24, and 48 hr provided a comparison of axon arbor complexity before and after retinal BDNF treatment (Cohen-Cory and Fraser, 1995; Cohen-Cory, 1999; Cogen and Cohen-Cory, 2000; Alsina et al., 2001). RGC axon arbors labeled with DiI or YFP in control, BDNF-, and anti-BDNF-treated tadpoles increased their complexity by adding branches and increasing their total arbor length by 24 and 48 hr (Fig. 6). Altering endogenous BDNF levels in the developing retina by microinjecting BDNF- or anti-BDNF-coupled microspheres did not alter axon arbor complexity at the tectum (Fig.6). The increase in branch number (Fig. 6B) and in total arbor length (Fig. 6C) in BDNF-treated tadpoles was statistically indistinguishable from control at 24 hr [new branches: control, 3.43 ± 0.7; BDNF, 3.66 ± 0.67; increase in total arbor length (in μm): control, 101.82 ± 11.4; BDNF, 118.25 ± 12.3; n = 23 for control; n = 40 for BDNF; p > 0.05] and at 48 hr (new branches: control, 5.75 ± 1.5; BDNF, 5.8 ± 1.7; increase in total arbor length: control, 133.6 ± 12.4; BDNF, 132.1 ± 11.9;n = 10 for control; n = 11 for BDNF;p > 0.05). Similarly, no significant differences were observed after retinal treatment with anti-BDNF (5.14 ± 1.4 μm new branches and 123.3 ± 26.9 μm increase in branch length at 24 hr; n = 7). These results are in contrast to our previous observations that BDNF within the optic tectum significantly promotes axon arborization within 2 hr of treatment (Cohen-Cory and Fraser, 1995; Alsina et al., 2001). Thus, RGC axon arbors are unaffected by retinal BDNF and solely affected by tectal BDNF.
Fig. 6.
RGC axon arbor complexity is unaffected by retinal BDNF levels. To determine whether retinal BDNF influences RGC axon arborization at a distance, tadpoles were intraocularly injected with control, BDNF-, or anti-BDNF-treated microspheres, and the resulting changes in RGC axon arbor dynamics were compared with tectally applied BDNF (Cohen-Cory and Fraser, 1995; Lom and Cohen-Cory, 1999). A, Individual RGC axon arbor morphologies of control, retinal BDNF, and tectal BDNF at 0 and 24 hr after treatment demonstrate that only tectally applied BDNF significantly alters RGC axon arborization. B, C, Altering retinal BDNF levels had no significant effects on RGC axon arbor complexity as measured by the increase in total branch number (B) and total arbor length (C) 24 and 48 hr after treatment (p > 0.05). Error bars indicate SEM. Scale bar, 20 μm.
DISCUSSION
In the developing Xenopus visual system, both retinal and tectal neurons express BDNF transcripts during active RGC arborization, suggesting that BDNF is available to exert both local and target-derived effects on RGC axonal and dendritic arborization (Cohen-Cory and Fraser, 1994; Cohen-Cory et al., 1996). We previously investigated the local effects of altered BDNF levels on RGC axonal and dendritic arborization in vivo and found that that exogenous tectal BDNF enhanced RGC axon arborization, whereas retinal BDNF limited RGC dendritic arborization (Cohen-Cory and Fraser, 1995;Cohen-Cory, 1999; Lom and Cohen-Cory, 1999). Thus, BDNF applied locally, at the site of branching, exerted differential effects on axons versus dendrites. Here, we report the influence of target-derived BDNF on RGC dendritic arborization. Increasing BDNF levels within the optic tectum enhanced RGC dendritic arborization. Correspondingly, tectal applications of neutralizing BDNF antibodies reduced RGC dendritic arbors, further indicating that tectal BDNF influences RGC dendritic arborization in the retina. Collectively, our studies indicate that RGCs respond to signals initiated by both retinal- and tectal-derived BDNF to regulate the elaboration of their dendritic arbors. Moreover, our results indicate that retinal BDNF and tectal BDNF impart opposing effects on RGC dendritic arborization, providing both positive and negative regulation of dendritic arborization during a critical period of neuronal differentiation.
The extent and form of the dendritic arbor of a neuron results from interactions between intrinsic developmental programs and environmental cues, which include local cell-mediated interactions as well as interactions with target cells (Voyvodic, 1989; McAllister 2000; Cline, 2001; Scott and Luo, 2001). RGC dendritic arborization is known to be influenced locally within the retina by afferent input mediated through classic neurotransmitters (for review, see Sernagor et al., 2001) and by RGC density (Perry and Maffei, 1988; Bahr et al., 1992; Troilo et al., 1996). The influence of target-derived factors on RGC dendritic arborization is considerably less well understood. Here, we demonstrated that alterations in target levels of BDNF significantly influence RGC dendritic arbor complexity. Altering tectal BDNF levels had slight yet significant effects on the number of primary dendrites that RGCs extend but had dramatic effects on total dendritic length and branch tip number, suggesting that BDNF signals generated at the target have the ability to regulate dendritic branching back in the retina. Previous studies suggested that Xenopus RGCs initiate primary dendrites and elaborate dendritic arbors by target-independent mechanisms. RGCs initiate short, unbranched primary dendrites before RGC axons reach the optic tectum (Holt, 1989; Sakaguchi, 1989). Active dendritic elaboration, including subsequent dendrite branching, continues after RGC axons contact the tectum (Sakaguchi et al., 1984). These observations suggested that initial primary dendrites might escape the influence of target-derived cues. Our results, however, indicate that RGC primary dendrite extension can be modulated by target-derived BDNF, presumably after axonal contact with the tectum.
Early studies investigating how peripheral targets regulate dendritic morphology revealed that the size of the target profoundly influences dendritic geometry (Voyvodic, 1989; Yin and Oppenheim, 1992). Although these and other studies suggested that retrograde trophic signals derived from the target regulate dendritic morphology (Purves, 1988), the identity of such signals was unknown. Our current studies demonstrate that BDNF acting at the target modulates RGC dendritic differentiation. Tectal BDNF may directly control RGC dendritic arborization. It is also possible that tectal BDNF modulates dendritogenesis indirectly, by regulating axon branching and connectivity (Cohen-Cory and Fraser, 1995; Alsina et al., 2001). RGCs, by increasing their axonal arborization and connectivity, may encounter or generate and retrogradely transmit other signals that in turn influence dendritic morphology. Although our studies do not rule out this possibility, we show that direct interaction between BDNF and the RGC axon terminal is necessary for tectal BDNF to modulate dendritic arborization. To differentiate between direct effects at the axon terminal versus retrograde effects on dendritic terminals, thorough dissection of the intracellular signaling mechanisms evoked by BDNFin vivo (Miller and Kaplan, 2001; Watson et al., 2001;Heerssen and Segal, 2002) is still required.
The present study demonstrates that a single neurotrophin can exert differential effects on RGC dendrites in vivo by acting locally versus at the axon target. BDNF has also been shown to exert differential effects during the elaboration of basal versus apical dendrites in cultured cortical neurons (McAllister et al., 1995, 1997). Thus, neurotrophins can simultaneously elicit signals that a developing neuron can interpret differentially to modulate dendritic arbor morphology. Our current study reveals a critical role for the location of neurotrophin action (axon vs dendrite) during the elaboration of both axons and dendrites in vivo. The ability to alter neurotrophin levels discretely at two locations in the live animal and then evaluate the consequences of these alterations on the same parameter of morphological differentiation revealed that BDNF can spatially modulate afferent and efferent neuronal connectivity. Our observation that retinal BDNF did not influence RGC axon elaboration is not surprising, because previous studies have demonstrated that axon terminals must be directly exposed to neurotrophins to elicit axon elaboration. Using cultured sympathetic neurons, Campenot (1977, 1982,1987, 1994) showed that NGF acts locally to modulate neurite elaboration, and exposure of neuronal cell bodies to NGF does not influence distant neurite arborization. Thus, although developing neurons are capable of transporting neurotrophins anterogradely (von Bartheld et al., 1996, 2001), direct effects of anterogradely transported BDNF on axon terminals that release the neurotrophin remain to be established.
Our previous observations indicated that exogenous retinal BDNF limits RGC dendritogenesis (Lom and Cohen-Cory, 1999). By applying retinal BDNF at stage 38, well before peak endogenous retinal BDNF expression at stage 45 (Cohen-Cory and Fraser, 1994), we prematurely exposed RGCs to the limiting effects of retinal BDNF. In Xenopus, the onset of RGC dendritic arborization occurs at stage 38, when low levels of BDNF can first be detected in the retina and tectum. Thus, early BDNF exposure may have caused an enhanced response that reduced primary dendritogenesis and dendritic branching. This enhanced response is supported by our observation that later BDNF exposure (starting at stage 42) had more moderate effects, reducing dendritic branching without altering primary dendrite number. Consequently, our results suggest that BDNF selectively limits dendritic branch extension without affecting branch elimination, similar to the actions of BDNF on axon branching (Cohen-Cory and Fraser, 1995). In vivo time-lapse imaging of RGC dendritic arborization, however, is necessary to directly demonstrate the selective ability of BDNF to inhibit dendritic branch extension.
RGCs simultaneously experience maximal BDNF levels both locally within the retina as well as at the target at stage 45, during active dendritic and axonal arborization (Cohen-Cory et al., 1996). Our current results indicate that, in contrast to retinal-derived BDNF, target-derived BDNF promotes dendritic arborization. These results, together with the dynamic BDNF expression patterns in theXenopus visual system, suggest that RGCs that have successfully reached the tectum simultaneously experience opposing BDNF stimuli from the tectum and retina that promote and inhibit dendritic development, respectively. These simultaneous, opposing stimuli may balance each other, allowing other local retinal cues to regulate dendritic development. Alternatively, RGCs may integrate the relative strengths of contrary retinal- versus tectal-derived BDNF signals to upregulate or downregulate dendritic development programs. RGCs with axons that did not reach the tectum (or did not effectively compete for tectal BDNF) would only experience the limiting retinal BDNF signals and consequently elaborate simpler dendritic arbors than the RGCs that experienced both retinal and tectal BDNF signals. Simultaneous BDNF expression in the retina and tectum may therefore represent a coordinated mechanism that selectively enhances the dendritic arborization of RGCs that innervate the tectum. The relative amount of retinal- versus tectal-derived BDNF that an RGC experiences at stage 45 could also potentially contribute to its differentiation into one of three distinct morphological categories, which reflect receptive field differences. Interestingly, after stage 45, RGCs begin assuming one of the three morphological subtypes, characterized by soma size and dendritic branching patterns (Sakaguchi, 1989). Endogenous retinal BDNF may also play a role in fine-tuning RGC dendritic arbor morphology by limiting dendrite branching during the later dendritic arbor refinement. It is also interesting to note that a mechanism of RGC-mediated contact inhibition, perhaps through dendrodendritic contacts, also influences RGC dendritic arborization (Sernagor et al., 2001). Within the developing retina, RGCs themselves express BDNF (Perez and Caminos, 1995; Cohen-Cory et al., 1996). Thus, RGCs may use BDNF as an autocrine/paracrine factor to control dendritic arbor size as the retina grows and matures. Alternatively, retinal BDNF may act on TrkB-expressing amacrine cells that in turn influence RGC dendritic connectivity via their afferent input to RGC dendrites.
Several recently described neurotrophin-signaling mechanisms may begin to explain the cellular basis by which retinal- and tectal-derived BDNF differentially modulates RGC dendritic arborization. DevelopingXenopus RGCs express both trkB and p75 transcripts, indicating that these high- and low-affinity receptors are available to transduce neurotrophic signals (Cohen-Cory and Fraser, 1994; Cohen-Cory et al., 1996; Hutson and Bothwell, 2001). TrkB receptor protein is first detected on RGC axons along the optic nerve, as they begin to travel to their target, and is only detected on RGC soma after their axons reach and begin to arborize in the optic tectum (Cohen-Cory, unpublished observations). Thus, it is possible that differential distribution of neurotrophin receptors on RGC dendrites versus axon terminals (Tongiorgi et al., 2000) could potentially underlie the differential responses we observed in RGC dendritic morphology. Furthermore, differential expression of truncated TrkB (lacking the intracellular kinase domain) by RGC dendrites and axons could modulate relative levels of available BDNF and/or BDNF signaling at these two locations. Alterations in truncated TrkB expression alter cortical neuron dendritic arborization (Yacoubian and Lo, 2000), implicating truncated TrkB as a potential mechanism for the differential effects of BDNF on dendritic arborization (McAllister et al., 1995, 1997). RGCs are capable of retrogradely transporting BDNF (Herzog and von Bartheld, 1998), but whether receptor-mediated internalization of BDNF is required for BDNF to influence RGC dendrites remains to be elucidated. Differential activation of signal transduction molecules at the axon versus dendrite may also contribute to the differential BDNF effects we observed. A growing body of work demonstrates that neurotrophins can signal through several intracellular signal transduction cascades that may or may not involve retrograde transport of neurotrophins and/or their receptors (Miller and Kaplan, 2001; Heerssen and Segal, 2002;MacInnis and Campenot, 2002). Recently, Watson et al. (2001)demonstrated that local versus target-derived neurotrophic stimulation of sensory neurons in vitro engages distinct mitogen-activated protein kinase (MAPK) signal transduction pathways at the cell body versus axon terminal. Thus, differential activation of distinct MAPK signaling pathways may enable tectal versus retinal BDNF to exert opposing effects on RGC dendritic arborization in vivo. Additionally, altering cyclic nucleotide second messengersin vitro can invert growth cone responses to specific molecules (including BDNF) from attractive into repulsive (Song and Poo, 1999; McFarlane, 2000). Thus, the differential effects of BDNF on RGC dendritic morphology may occur by differentially influencing afferent and efferent connectivity or by differentially activating receptor and/or intracellular signaling cascades locally at the dendrites versus distally at the axon termini. Although the precise nature of the differential signaling of BDNF has yet to be determined, our observations that RGCs translate retinal and tectal BDNF cues into opposing outcomes for dendritic arborization provide a novel mechanism for fine-tuning the morphological differentiation of a neuron during the wiring of the embryonic CNS.
Footnotes
This work was supported by National Eye Institute (NEI) postdoctoral fellowships (B.L. and J.C.), an American Association of University Women publication grant (B.L.), and Alfred P. Sloan, Stein/Oppenheimer, Arnold and Mabel Beckman Foundation, and NEI awards (S.C.-C.). We thank Allan Leung, Berta Alsina, and Adam French for assistance. Amgen and Genentech generously provided the neurotrophins used in this study.
Correspondence should be addressed to Susana Cohen-Cory, Mental Retardation Research Center, University of California, Los Angeles, 760 Westwood Plaza, NPI 58-258, Los Angeles, CA 90095. E-mail:scohenco@ucla.edu.
REFERENCES
- 1.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 2.Bahr M. Live or let die: retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- 3.Bahr M, Wizenmann A, Thanos S. Effect of bilateral tectum lesions on retinal ganglion cell morphology in rats. J Comp Neurol. 1992;320:370–380. doi: 10.1002/cne.903200308. [DOI] [PubMed] [Google Scholar]
- 4.Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite growth by nerve growth factor. Dev Biol. 1982;93:1–12. doi: 10.1016/0012-1606(82)90232-9. [DOI] [PubMed] [Google Scholar]
- 6.Campenot RB. Local control of neurite sprouting in cultured sympathetic neurons by nerve growth factor. Brain Res. 1987;465:293–301. doi: 10.1016/0165-3806(87)90250-1. [DOI] [PubMed] [Google Scholar]
- 7.Campenot RB. NGF and the local control of nerve terminal growth. J Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- 8.Chien C-B, Harris WA. Axonal guidance from retina to tectum in embryonic Xenopus. Curr Top Dev Biol. 1994;29:135–169. doi: 10.1016/s0070-2153(08)60549-9. [DOI] [PubMed] [Google Scholar]
- 9.Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 10.Cogen J, Cohen-Cory S. Nitric oxide modulates retinal ganglion cell axon arbor remodeling in vivo. J Neurobiol. 2000;45:120–133. doi: 10.1002/1097-4695(20001105)45:2<120::aid-neu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Cory S. BDNF modulates, but does not mediate, activity-dependent branching and remodeling of optic axon arbors in vivo. J Neurosci. 1999;19:9996–10003. doi: 10.1523/JNEUROSCI.19-22-09996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Cory S, Fraser SE. BDNF in the development of the visual system of Xenopus. Neuron. 1994;12:747–761. doi: 10.1016/0896-6273(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Cory S, Dreyfus CF, Black IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J Neurosci. 1991;11:462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen-Cory S, Escandon E, Fraser SE. The cellular patterns of BDNF and trkB expression suggest multiple roles for BDNF during Xenopus visual system development. Dev Biol. 1996;179:102–115. doi: 10.1006/dbio.1996.0244. [DOI] [PubMed] [Google Scholar]
- 16.Cui Q, So KF, Yip HK. Major biological effects of neurotrophic factors on retinal ganglion cells in mammals. Biol Signals Recept. 1998;7:220–226. doi: 10.1159/000014546. [DOI] [PubMed] [Google Scholar]
- 17.Friedman WJ, Greene LJ. Neurotrophin signaling via trks and p75. Exp Cell Res. 1999;253:131–142. doi: 10.1006/excr.1999.4705. [DOI] [PubMed] [Google Scholar]
- 18.Garner AS, Menegay HJ, Boeshore KL, Xie XY, Voci JM, Johnson JE, Large TH. Expression of TrkB receptor isoforms in the developing avian visual system. J Neurosci. 1996;16:1740–1752. doi: 10.1523/JNEUROSCI.16-05-01740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallbook F, Backstrom A, Kullander K, Ebendal T, Carri NG. Expression of neurotrophins and trk receptors in the avian retina. J Comp Neurol. 1996;364:664–676. doi: 10.1002/(SICI)1096-9861(19960122)364:4<664::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Heerssen HM, Segal RA. Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci. 2002;25:160–165. doi: 10.1016/s0166-2236(02)02144-6. [DOI] [PubMed] [Google Scholar]
- 21.Herzog KH, von Bartheld CS. Contributions of the optic tectum and the retina as sources of brain-derived neurotrophic factor for retinal ganglion cells in the chick embryo. J Neurosci. 1998;18:2891–2906. doi: 10.1523/JNEUROSCI.18-08-02891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt CE. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J Neurosci. 1989;9:3123–3145. doi: 10.1523/JNEUROSCI.09-09-03123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutson LD, Bothwell MA. Expression and function of Xenopus laevis p75NTR suggest evolution of developmental regulatory mechanisms. J Neurobiol. 2001;49:79–98. doi: 10.1002/neu.1067. [DOI] [PubMed] [Google Scholar]
- 25.Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 27.Katz LC, Iarovici DM. Green fluorescent latex microspheres: a new retrograde tracer. Neuroscience. 1990;34:511–520. doi: 10.1016/0306-4522(90)90159-2. [DOI] [PubMed] [Google Scholar]
- 28.Keller R. Early embryonic development of Xenopus laevis. In: Kay BK, Peng HB, editors. Xenopus laevis: practical uses in cell and molecular biology. Academic; San Diego: 1991. pp. 102–116. [Google Scholar]
- 29.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 30.Lom B, Cohen-Cory C. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacInnis BL, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science. 2002;295:1536–1539. doi: 10.1126/science.1064913. [DOI] [PubMed] [Google Scholar]
- 32.McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- 33.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 34.McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 35.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 36.McFarlane S. Dendritic morphogenesis: building an arbor. Mol Neurobiol. 2000;22:1–9. doi: 10.1385/MN:22:1-3:001. [DOI] [PubMed] [Google Scholar]
- 37.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 38.Montague PR, Friedlander MJ. Morphogenesis and territorial coverage by isolated mammalian retinal ganglion cells. J Neurosci. 1991;11:1440–1457. doi: 10.1523/JNEUROSCI.11-05-01440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison ME, Mason CA. Granule neuron regulation of Purkinje cell development: striking a balance between neurotrophin and glutamate signaling. J Neurosci. 1998;18:3563–3573. doi: 10.1523/JNEUROSCI.18-10-03563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieuwkoop PD, Faber J. Normal table of Xenopus development. Elsevier; Amsterdam: 1967. [Google Scholar]
- 41.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 42.Perez MT, Caminos E. Expression of brain-derived neurotrophic factor and of its functional receptor in neonatal and adult rat retina. Neurosci Lett. 1995;183:96–99. doi: 10.1016/0304-3940(94)11123-z. [DOI] [PubMed] [Google Scholar]
- 43.Perry VH, Maffei L. Dendritic competition: competition for what? Brain Res. 1988;469:195–208. doi: 10.1016/0165-3806(88)90182-4. [DOI] [PubMed] [Google Scholar]
- 44.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 45.Purves D. Body and brain. Harvard UP; Cambridge MA: 1988. [Google Scholar]
- 46.Purves D, Snider WD, Voyvodic JT. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature. 1988;336:123–128. doi: 10.1038/336123a0. [DOI] [PubMed] [Google Scholar]
- 47.Rakic P, Sidman RL. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973;152:133–161. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- 48.Riddle DR, Lo DC, Katz LC. NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature. 1995;378:189–191. doi: 10.1038/378189a0. [DOI] [PubMed] [Google Scholar]
- 49. Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques 23 1997. 928 934,936–937. [DOI] [PubMed] [Google Scholar]
- 50.Sakaguchi DS. The development of retinal ganglion cells deprived of their targets. Dev Biol. 1989;134:103–111. doi: 10.1016/0012-1606(89)90081-x. [DOI] [PubMed] [Google Scholar]
- 51.Sakaguchi DS, Murphey RK, Hunt RK, Tompkins R. The development of retinal ganglion cells in a tetraploid strain of Xenopus laevis: a morphological study utilizing intracellular dye injection. J Comp Neurol. 1984;224:231–251. doi: 10.1002/cne.902240205. [DOI] [PubMed] [Google Scholar]
- 52.Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 54.Scott EK, Luo L. How do dendrites take their shape? Nat Neurosci. 2001;4:359–365. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- 55.Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Prog Retin Eye Res. 2001;20:139–174. doi: 10.1016/s1350-9462(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 56.Snider WD. Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. J Neurosci. 1988;8:2628–2634. doi: 10.1523/JNEUROSCI.08-07-02628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 58.Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- 59.Tongiorgi E, Armellin M, Cattaneo A. Differential somato-dendritic localization of TrkA, TrkB, TrkC and p75 mRNAs in vivo. NeuroReport. 2000;11:3265–3268. doi: 10.1097/00001756-200009280-00043. [DOI] [PubMed] [Google Scholar]
- 60.Troilo D, Xiong M, Crowley JC, Finlay BL. Factors controlling the dendritic arborization of retinal ganglion cells. Vis Neurosci. 1996;13:721–733. doi: 10.1017/s0952523800008609. [DOI] [PubMed] [Google Scholar]
- 61.von Bartheld CS. Neurotrophins in the developing and regenerating visual system. Histol Histopathol. 1998;13:437–459. doi: 10.14670/HH-13.437. [DOI] [PubMed] [Google Scholar]
- 62.von Bartheld CS, Byers MR, Williams R, Bothwell M. Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature. 1996;379:830–833. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- 63.von Bartheld CS, Wang X, Butowt R. Anterograde axonal transport, transcytosis, and recycling of neurotrophic factors: the concept of trophic currencies in neural networks. Mol Neurobiol. 2001;24:1–28. doi: 10.1385/MN:24:1-3:001. [DOI] [PubMed] [Google Scholar]
- 64.Voyvodic JT. Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci. 1989;9:1997–2010. doi: 10.1523/JNEUROSCI.09-06-01997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson FL, Heerssen HM, Bhattacharyya A, Kleese L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 66.Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000;26:233–245. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- 67.Yacoubian TA, Lo DC. Truncated and full-length trkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- 68.Yin QW, Oppenheim RW. Modifications of motoneuron development following transplantation of thoracic spinal cord to the lumbar region in the chick embryo: evidence for target-derived signals that regulate differentiation. J Neurobiol. 1992;23:376–395. doi: 10.1002/neu.480230405. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Schmidt RE, Yan Q, Snider WD. NGF and NT-3 have differing effects on the growth of dorsal root axons in developing mammalian spinal cord. J Neurosci. 1994;14:5187–5201. doi: 10.1523/JNEUROSCI.14-09-05187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]