Abstract
In brain development, neurons have to be connected with specific postsynaptic neurons to establish functional neuronal circuits. Cadherins are cell adhesion molecules, which mark developing neuronal circuits. Each member of this class of molecules is expressed only on a restricted set of fiber fascicles that connect gray matter structures to form functional neural circuits. In view of their expression patterns, cadherins have been postulated to play a functional role in the proper establishment of fiber connections. We chose the chicken optic tectum to analyze the instructive potential of cadherins in axonal pathfinding. Three tectofugal pathways, the tectothalamic, tectobulbar, and tectoisthmic tracts, exit the dorsal mesencephalon via the brachium of the superior colliculus, a large fiber structure, which can be divided in specific subtracts that are characterized by the selective expression of N-cadherin, cadherin-7, cadherin-6B, or R-cadherin. By using in vivo electroporation, we overexpressed each of the cadherins in tectal projection neurons between embryonic days 6 and 11. Cotransfection with green fluorescent protein expression plasmid allowed us to assess the pathway choice, which the transgenic axons had made. Quantification based on confocal laser scanning microscopic images revealed that transgenic axons selectively fasciculated with tectofugal tracts specified by the matching type of cadherin. This is the first direct evidence that cadherins mediate differential axonal pathfinding in vivo, possibly by a preferentially homotypic adhesive mechanism.
Keywords: cadherin-6B, cadherin-7, N-cadherin, R-cadherin, chicken embryo, tectum, axonal pathfinding, selective fasciculation, in ovo electroporation, gain-of-function, green fluorescent protein, tracing, neural development
During the formation of neural circuits in the developing CNS, axons often need to navigate over long distances to reach their target cells. Because many neurons of a particular functional system share a highly stereotyped path to the same destination in the developing brain, the concept of pioneer axons was proposed. Early-generated pioneer axons are guided by various molecular mechanisms to their targets. They thereby generate a scaffold that can be used by the axons from later-born neurons to selectively fasciculate with the pioneer axons to reach the same target (for review, see Tessier-Lavigne and Goodman, 1996).
One candidate class of molecules proposed to take part in selective fasciculation are the cadherins. For most members of the classic cadherins, it was shown that they preferentially mediate homophilic interactions between cells and that this interaction can result in cells segregating from each other according to the type of cadherin they express (Nose et al., 1988). Most intriguingly, detailed analyses of cadherin expression during brain development revealed that specific neuronal circuits are often characterized by the expression of a particular type of cadherin (Redies et al., 1993) (for review, seeRedies, 2000). Thus, it was proposed that cadherins not only selectively label specific fascicles but also provide an adhesive recognition code that has an instructive function during the establishment of fiber connections, similar to the concept proposed by Sperry in his chemoaffinity hypothesis (Sperry, 1963). Functional studies so far concentrated mainly on N-cadherin (Ncad). Purified Ncad is a potent inducer for axon outgrowth in vitro (Bixby and Zhang, 1990). Moreover, loss of function studies in vivousing interfering antibodies in chick and Xenopus (Honig and Rutishauser, 1996; Stone and Sakaguchi, 1996; Inoue and Sanes, 1997), overexpression of dominant negative mutants in Xenopus(Riehl et al., 1996), or genetic analysis in Drosophila(Iwai et al., 1997; Lee et al., 2001) and Caenorhabditis elegans (Broadbent and Pettitt, 2002) all resulted in impaired axon outgrowth, defasciculation, pathfinding errors, and/or axonal mistargeting.
In the study presented here, we chose a gain-of-function approach and extended the analysis to include three more cadherins in addition to Ncad: R-cadherin (Rcad), cadherin-6B (cad6B), and cadherin-7 (cad7). Previous immunohistochemical analyses have shown that each of these four cadherins selectively labels specific neuronal connections of the chicken tectum with the diencephalon, the hindbrain, and the isthmic region (Wöhrn et al., 1999). If a particular cadherin, or particular combination of cadherins, is responsible for the correct establishment of these tectofugal connections, changing the cadherin composition on the growth cones of the outgrowing axons should change their projection accordingly. Here we demonstrate that, by in vivo electroporation, we can selectively alter the cadherin composition of a small number of tectofugal neurons in an otherwise wild-type context. Colabeling of transgenic axons with green fluorescent protein (GFP) allows to trace the projection route of the transgenic axons to their target. Image analysis based on laser scanning microscopic images of immunostained cryostat sections revealed that the selective overexpression of each of the four cadherins directed transgenic axons to preferentially choose tectofugal pathways that express the same cadherin. These results indicate that cadherins provide instructive cues that tell a neuron to which neural circuit it belongs and how to find its proper target.
MATERIALS AND METHODS
Animals. Fertilized eggs from White Leghorn chicken were obtained from a local breeder (Sörries-Trockels, Möhnesee-Hewingsen, Germany) and incubated at 37°C and 65% humidity in a forced-draft incubator.
Axonal tracing with biotinylated dextran amine. Embryonic day 17 (E17) chick embryos were anesthetized by cooling on ice and killed by decapitation, according to institutional and national guidelines for the use of animals in research. Whole brains were prepared in ice-cold HBSS containing the following (in mm): 100 HEPES, 140 NaCl, 5 KCl, 5 glucose, 0.4 Na2HPO4, and 0.04 phenol red supplemented with 1 CaCl2 and 1 MgCl2 (all reagents from Merck, Darmstadt, Germany). Biotinylated dextran amine (BDA-3000) (molecular weight of 3000 Da; Molecular Probes, Leiden, The Netherlands) was solubilized in 1% Fast Green (Serva, Heidelberg, Germany) in water and dried on the tip of a tungsten needle. Tracer was applied to the lateral part of the brachium of the superior colliculus (BCS) under microscopic control with the needle held in place for ∼20 sec. Brains were cultivated in constantly oxygenized HEPES-buffered Ringer's solution supplemented with 2 mm Ca2+ and 1 mm Mg2+ for 24 hr at room temperature (Glover et al., 1986), followed by fixation in 4%formaldehyde–HBSS for 3 hr on ice and submersion in an ascending series of sucrose solutions [12 (w/v), 15, and 18% sucrose in HBSS] for 1–3 hr each. Whole mounts were embedded in Tissue-Tek O.C.T. Compound (Sakura, Zoeterwoude, The Netherlands) and stored at −80°C.
Plasmids. The full-length cDNA for each cadherin (kind gift from Dr. Masatoshi Takeichi, Kyoto University, Kyoto, Japan) was cloned into blunted EcoRI or XhoI sites of the pCAGGS vector (Niwa et al., 1991). Plasmid was purified from Top10 (Invitrogen, Groningen, The Netherlands) or XL1-blue strains ofEscherichia coli (Stratagene, La Jolla, CA) using Qiagen (Hilden, Germany) columns. pCAGGS-cad7 and pCAGGS-GFP (Nakagawa and Takeichi, 1998; Momose et al., 1999) were kindly provided by Dr. Shinchi Nakagawa (Kyoto University, Kyoto, Japan) and Dr. Hidesato Ogawa (Dana Farber Cancer Institute, Boston, MA), respectively.
In vivo electroporation. The method of in ovoelectroporation (Momose et al., 1999) was applied to late-stage embryos in shell-less egg cultures (Auerbach et al., 1974; Thanos and Bonhoeffer, 1983). In brief, fertilized eggs of White Leghorn chicken were incubated in shell-less cultures until E6, when 0.2–1 μl of plasmid solution [1 μg/μl pCAGGS-cadherin, 0.2 μg/μl pCAGGS-GFP, and 0.1% Fast Green solubilized in Gey's buffered salt solution (Invitrogen) were injected into the right tectal ventricle, followed by immediate electroporation (CUY21 electroporator; TR Tech Co. Ltd., Tokyo, Japan). Electrodes were placed around the midbrain. Pulses of 50 msec length at 25 V were repeated six times with 100 msec intervals. Embryos were then incubated until E11 and killed by decapitation. Brains were isolated, fixed, and embedded as described previously (Redies et al., 1992).
Immunofluorescence. Consecutive series of 20-μm-thick cryostat sections (30 μm for tracing) were collected on gelatinized glass slides and stained for indirect immunofluorescence using monoclonal antibodies NCD-2 (Hatta and Takeichi, 1986), RCD-2 (Redies et al., 1992), NK-2, and NK-7 (Nakagawa and Takeichi, 1998), followed by appropriate Cy3-labeled secondary antibodies (Dianova, Hamburg, Germany) and bisbenzimide nuclear staining with the Hoechst 33258 dye (Sigma, Deisenhofen, Germany). BDA-3000 was visualized by applying the fluorochrome Alexa647 (Molecular Probes) conjugated to streptavidin together with the antibodies. Stained sections were scanned with a laser scanning microscope (LSM 510; Zeiss, Oberkochen, Germany), and the scanned images were processed and analyzed with the Photoshop computer program (Adobe Systems, Mountain View, CA).
Analysis of tracing experiments. For the tracing experiments, all cells identified on printouts of tectal hemispheres were counted, and colabeling by tracer filling was determined. Three hemispheres were counted for each case. Data analysis was performed by counting traced cells in defined regions of the traced hemisphere, calculating the percentage for each of the four cadherins, and comparing it with percentages retrieved from nontraced control specimens treated the same way as described above.
Analysis of axonal pathway selection. The four consecutive sections representing the largest number of transgenic fibers at the BCS of a specimen were selected for analysis. Sections were scanned using a Plan Neofluar 10×/0.3 objective in combination with electronic zoom of 0.7 at a 1024 × 1024 pixel resolution. Three channels were tracked with excitations of 364 nm (Enterprise UV laser), 488 nm (argon laser), and 543 nm (helium neon laser) yielding a blue, green, and red scan corresponding to cell nuclei, GFP-expressing transgenic neurons, and cadherin staining, respectively. Nuclear staining was used to determine the borders of the BCS. Surrounding tectal and midbrain areas were electronically cut away. Trimmed scans were then exported to Photoshop format (Adobe Systems), and channels were separated. Analysis performed with NIH Image analysis software (Scion, Frederick, MD) is explained in detail in Results (see Quantification of cadherin-specific tract selection).
RESULTS
Short introduction to the tectofugal system
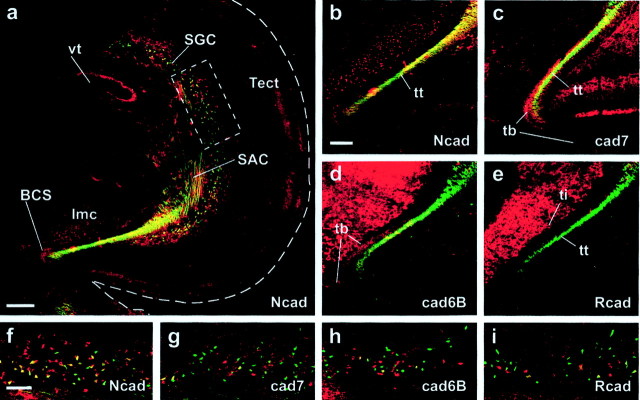
In birds, visual information is processed in a multilayered dorsal midbrain structure, the optic tectum (Fig.1a, Tect), in which it is eventually conveyed to tectofugal neurons that project their output fibers along at least seven different tectofugal pathways to diverse brain nuclei in the thalamus, midbrain, and brainstem (Hunt and Künzle, 1976). A large portion of the tectofugal axons originates from multipolar neurons in a deeper layer of the tectum, the stratum griseum centrale (SGC) (Fig. 1b). These axons grow via the underlying stratum album centrale (SAC) (Fig. 1b) toward the caudal pole of the tectum. Here, the fibers collect in a large fiber bundle, the brachium of the superior colliculus (Fig.1a,b, BCS). At least three major output fascicles projecting to three different parts of the brain can be distinguished in the BCS: the tectothalamic tract (tt) (Fig.1a,c) running to the diencephalon (Fig.1a, Di, light red area), the tectobulbar tract (tb) (Fig. 1a,c) projecting to the hindbrain (Fig. 1a, Hb, light blue), and the tectoisthmic tract (Fig.1a,c, ti) reaching to the isthmic region (Fig. 1a, Ist, yellow). Each of these different tectofugal pathways, as well as their targets, is characterized by its combinatorial expression of four different cadherins: Ncad, Rcad, cad6B, and cad7 (Redies et al., 1993;Wöhrn et al., 1999; Redies, 2000). Whereas the cell bodies of these SGC neurons were shown to be intermingled and evenly distributed over the entire tectal hemisphere, their axons fasciculate and sort out according to the cadherin or combination of cadherins they express on their route to the BCS (Fig. 1b). When reaching the BCS, the axons have segregated into different fascicles with specific projection patterns (for details, see Fig. 1a,c).
Fig. 1.
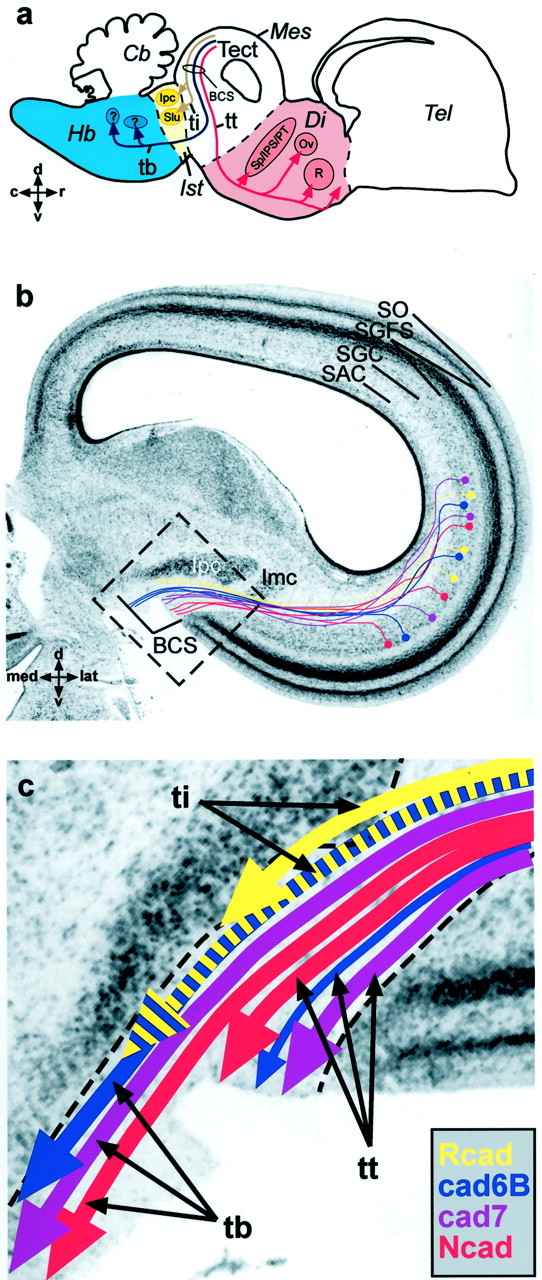
Schematic overview of tectofugal projections.a, Parasagittal schematic view of three axon pathways connecting the tectum opticum (Tect) of the dorsal mesencephalon (Mes) with other areas of the brain. Shown in red is the tectothalamic tract (tt) projecting to several nuclei in the diencephalon (Di), in blue is the tectobulbar tract (tb) projecting to nuclei in the hindbrain (Hb), and inyellow is the tectoisthmic tract (ti) projecting to the nuclei of the isthmic region (Ist). Rostral (r) is to the right, and dorsal (d) is to the top.b, Nissl-stained frontal section through the right half of the dorsal chicken midbrain with a schematic overlay of the tectofugal neurons. Incoming fibers from the retina form the most superficial tectal layer, the stratum opticum (SO), and terminate in the retinorecipient layers of the stratum griseum et fibrosum superficiale (SGFS), establishing a retinotopic projection on the entire tectal hemisphere. Different combinations of cadherins (shown in color) mark different classes of tectoefferent neurons in the stratum griseum centrale (SGC). These neurons project via the stratum album centrale (SAC) toward the brachium of the superior colliculus (BCS), in which they segregate into different fascicles according to their cadherin expression and leave the tectum. Cadherins thereby label specific subfascicles of the axon tracts depicted in the scheme above. These tracts eventually separate to innervate different nuclei and subnuclei. Medial (med) is to the left, and dorsal (d) is to the top. (Note that the use of similar colors ina and b should not implicate a simple 100% match of one tract system with only one cadherin. It is rather the combination of cadherins, which specify certain tracts.)c, Schematic illustration of cadherin-specific fascicles found in the BCS. In the tectothalamic tract, the Ncad-positive fibers (in red) project to pretectal nuclei (Sp/IPS/PT in a) and to the nucleus rotundus (R in a) of the thalamus (Redies et al., 1993). The cad7-positive fibers (pink) can be followed to the nucleus ovoidalis complex (Ov ina) and to a subregion of the nucleus rotundus (Wöhrn et al., 1999). cad6B-positive neurons give rise to a small but distinct fiber fascicle (in blue) projecting alongside the tectothalamic tract to the anterior nucleus of the ventral supraoptic commissure (not depicted in a) (Wöhrn et al., 1999). In the tectobulbar tract, three cadherins (Ncad, cad7, and cad6B) label different but partially overlapping subsets of fiber fascicles projecting to hindbrain targets that cannot be determined with certainty (question marks ina). The tectoisthmic tract divides into two subtracts: one is characterized by Rcad expression (inyellow) and has the isthmic nucleus as its target (e.g.,Ipc in a and b orImc in b), and one expresses Rcad and cad6B (blue with yellow stripes) and seems to terminate in the nucleus semilunaris (Slu). Not all subfascicles are present at all levels of the BCS. Some leave more rostrally than others. They also show overlap with each other at their margins. The area boxed in b is shown at higher magnification in c. c, Caudal;Cb, cerebellum; Imc, nucleus isthmi, pars magnocellularis; Ipc, nucleus isthmi, pars parvocellularis; lat, lateral; Sp/IPS/PT, nuclei of the pretectal area, nucleus subpretectalis/nucleus interstitio-pretecto-subpretectalis/nucleus pretectalis;Tel, telencephalon; v, ventral.
Tracing of tectothalamic axons
To further substantiate these previous findings based on immunocytological studies, we used retrograde tracing with biotinylated dextran amines and chose to visualize the Ncad-positive part of the tectothalamic tract as an example. In Figure2, the traced fibers are shown ingreen and cadherin staining in red. The yellow additive color of fibers labeled green and red in the case of Ncad immunostaining reveals that the traced, retrogradely stained axons primarily coincide with the Ncad-positive fiber fascicle in the BCS (Fig. 2a,b). Only a smaller portion of the traced fibers in the tectothalamic tract expresses also cad7 and cad6B (Fig. 2c,d). Almost no fibers expressing Rcad were labeled (Fig. 2d). Other tracts, such as the tectobulbar tract (Fig. 2c,d, tb) or the tectoisthmic tract (Fig. 2e, ti), are not traced. Accordingly, the majority of SGC cell somata retrogradely filled with tracer is Ncad positive (Fig. 2, compare f withg, h, i). Moreover, counting of all traced cells shows that the percentage of Ncad-positive SGC cell somata retrogradely filled with tracer (69%) is larger than the overall percentage of Ncad-positive neurons among all SGC neurons (40%). In contrast, the percentage of traced neurons expressing the other cadherins remains the same or decreases (Rcad, 13% of traced neurons vs 14% of all SGC neurons; cad6B, 5 vs 27%; and cad7, 14 vs 35%). Therefore, via tracing, we identified a portion of the tectothalamic tract, in which the majority of axons is positive for Ncad. This result confirms by direct axonal tracing that specific cadherins mark different subfascicles of different tectofugal systems (Wöhrn et al., 1999).
Fig. 2.
Axonal tracing of the Ncad-positive portion of tectothalamic tract at E17. a, Overview of tectal hemisphere (Tect) with traced fibers (green) and Ncad immunostaining (red). Yellow indicates costaining.Imc, Nucleus isthmi, pars magnocellularis;vt, tectal ventricle. Scale bar, 200 μm.b–e, Traced fibers of the tectothalamic tract (tt) in the stratum album centrale (SAC) and the brachium of the superior colliculus (BCS) stained for different cadherins (Ncad in b; cad7 inc; cad6B in d; and Rcad ine). Note that the tectobulbar tract (tbin d) and the tectoisthmic tract (ti ine) are not traced. Scale bar (in b):b–e, 100 μm. f–i, Representative details (see box in a) of stratum griseum centrale (SGC) neurons stained for different cadherins showing that the majority of traced SGC neurons is Ncad positive (f) but only a small percentage of cells show immunoreactivity for cad7 (g), cad6B (h), and Rcad (i). Scale bar (in f): f–i, 100 μm.
In vivo electroporation to overexpress different cadherins on SGC neurons
Based on these results, we wanted to test directly whether the cadherins contributed to the segregation of the fiber fascicles in the BCS. If pathway choices by SGC axons depend on the type of cadherin expressed on their growth cones, altering the cadherin expression on a small subset of SGC neurons in an otherwise wild-type context should alter the pathfinding decisions of their axons according to the altered cadherin composition. A recently developed method to achieve such restricted transgenic overexpression in the chick is in ovoelectroporation (Momose et al., 1999). We developed a variation of this technique by applying it to shell-less cultures of chicken embryos. This modification allows ready access to the dorsal midbrain of the chicken embryo at 6 d of incubation, the chosen time point for electroporation. Injection of an expression plasmid for GFP into the posterior tectal ventricle and subsequent electroporation resulted in transfected ventricular progenitor cells, easily identified by their GFP labeling (Fig. 3a,arrows, dorsolateral view of a specimen at 24 hr after electroporation). As the progenitor cells at this stage are dividing rapidly, the cells deriving from them (including SGC neurons) can also be identified by their GFP expression, even 5 d after electroporation (Fig. 3c, see columnar distribution of GFP signal in all layers of the tectal hemisphere). Moreover, the GFP expression from the cytomegalovirus–chicken β-actin promoter was high enough to trace the axonal projections of the transgenic neurons to the BCS and beyond (Fig. 3c, arrows). Next, we overexpressed selectively each cadherin from coinjected plasmids featuring the same promoter. Whereas GFP showed an even cytoplasmic distribution, cadherins were found to be expressed in a more patchy pattern on the surface of cell bodies and axons (Fig.3b, see confocal laser scanning image of double transgenic neurons; Rcad expression is shown in red, and GFP is shown in green). Indirect immunofluorescence demonstrates that the vast majority of GFP-labeled neurons overexpressed the coelectroporated cadherin (Fig. 3d,g,k,n). Results showed that the levels of transgenic cadherin expression were always as high as or higher than the endogenous expression levels in the SGC layer, and they were similar for each cadherin, with the exception of Ncad that showed weaker expression levels, i.e., the percentage of cells overexpressing high levels of cadherin is smaller for Ncad than for the other cadherins, as is reflected in the smaller number of yellow labeled somata in double stainings (Fig. 3, compared with g, k, n). Cadherin overexpression was not confined to the cell somata but was also prominent on axons and their growth cones (Fig.3e,h,l,o). In conclusion, we are able to overexpress each of the four cadherins on SGC neurons. Via GFP labeling, we are able to trace the axons of the transgenic neurons.
Fig. 3.
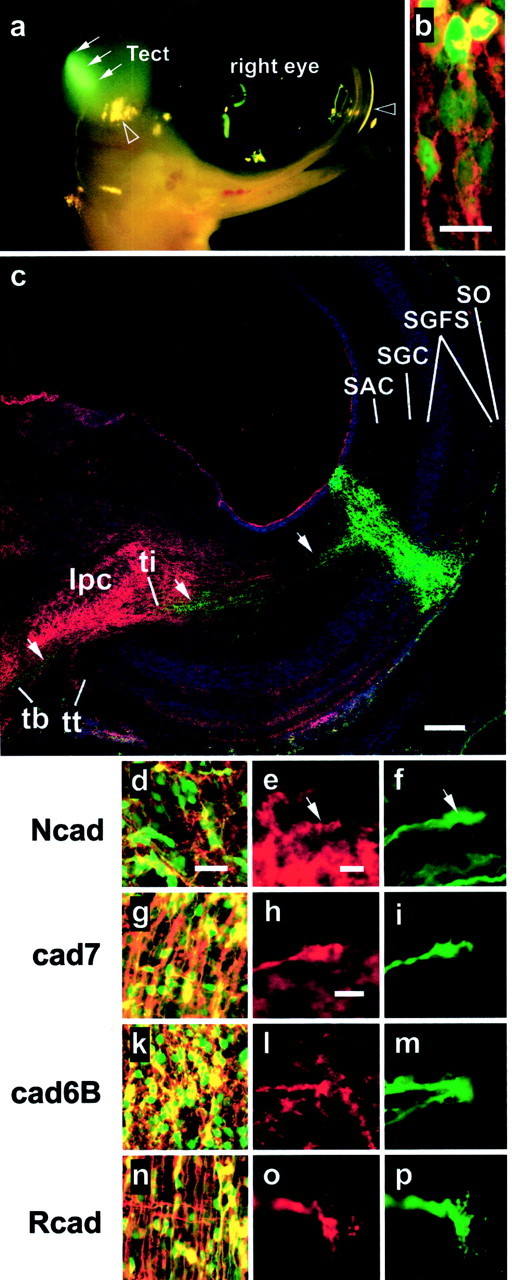
In vivo electroporation of embryonic chicken midbrain. a, Epifluorescence photography of a chicken embryo at E7, i.e., at 24 hr after electroporation with an expression plasmid for GFP showing expression in the dorsocaudal region of the tectum. Arrows point to the transgenic GFP expression domain; note that the yellowish dots are merely reflections of light, and two examples of these artifacts are marked by open arrowheads.b, Confocal laser scanning image of double transgenic tectal neurons showing cytoplasmic localization of GFP in their cell somata and transgenic R-cadherin labeling on their surface. Scale bar, 10 μm. c, Laser scanning image of an immunostained frontal section of a transgenic tectal hemisphere at 5 d after electroporation (E11). Transgenic neurons are identified by their expression of GFP. Axonal projections of labeled stratum griseum centrale (SGC) neurons can be followed (arrows) via the stratum album centrale (SAC) to the brachium of the superior colliculus (BCS), in which the tectoefferent fascicles segregate into the tectothalamic tract (tt), tectobulbar tract (tb), and tectoisthmic tract (ti). Tectal layering is revealed by nuclear staining (in blue). Cadherin staining (Rcad, in red) marks the tectoisthmic projection to the nuclus isthmi, pars parvocellularis (IPC). SO, Stratum opticum;SGFS, stratum griseum et fibrosum superficiale. Scale bar, 100 μm. d–p, Coelectroporation of different expression plasmids for GFP and a cadherin leads to respective overexpression of GFP (d, f,g, i, k, m,n, p) and the cadherin (Ncad ind and e, cad7 in g andh, cad6B in k and l, and Rcad in n and o) by tectal neurons (d, g, k,n; yellow indicates costaining) and their axonal growth cones (e, f,h, i, l, m,o, p; red andgreen scans are shown separately). Arrowsin e and f point to matching areas of the red and the green scan. Scale bars: (in d)d, g, k, n, 20 μm ; (in e) e, d,l, m and (in h)h, i, o, p, 5 μm.
Cadherins direct pathway selection in the BCS
The next step was to ask whether ectopic expression of the cadherins had altered the way in which the transgenic neurites associate with specific BCS fascicles. Five days after electroporation (at E11), GFP-labeled fibers (Fig. 4,green) were analyzed by laser scanning microscopy in consecutive frontal sections through the caudal tectum immunostained for all four cadherins (Fig. 4, red). In five to seven specimens for each cadherin and the GFP control, a sufficiently large number of transgenic SGC axons had reached the BCS to be analyzed. Because the BCS is a three-dimensional structure stretching over several hundred micrometers at E11 and the transgenic axons have made different pathway choices depending on the experimental condition, the sections analyzed were chosen according to the number of axons found that were labeled strongly by GFP. Therefore, the sections represented here are at different levels of the midbrain. For example, the Ncad-positive portion of the tectobulbar tract is not visible at the level shown in Figure 4a but can be easily identified on the other sections.
Fig. 4.
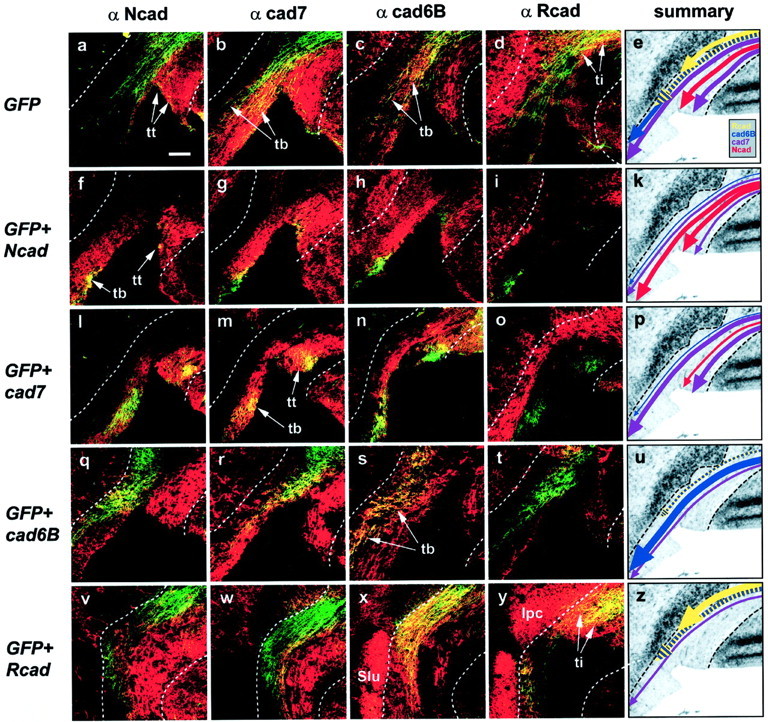
Pathway selection of transgenic stratum griseum centrale (SGC) axons at the brachium of the superior colliculus (BCS). GFP-labeled axons (green) are assayed for their tract selection based on cadherin immunostaining (red; Ncad infirst column from the left; cad7 in thesecond column; cad6B in the third column; Rcad in the fourth column). Dashed linesrepresent the borders of the BCS. a–d, Control axons transgenic for GFP alone can be detected in all tracts.e, Schematic summary depicting the result shown ina–d: fibers are found to follow the Rcad-positive (yellow) and cad6B/Rcad-positive (yellow–blue) axons constituting the tectoisthmic tract (ti), the cad6B-positive (blue), and cad7-positive (pink) portion of the tectobulbar tract (tb) and also the cad7-/Ncad-positive tectothalamic tract (tt).f–i, Axons overexpressing Ncad choose an Ncad-positive portion of the tt that overlaps with the cad7-positive portion. In the tb, transgenic axons are detected in the Ncad-positive portion of the tb (f) that partially overlaps with the cad7-positive portion (g) and very little with the cad6B-positive portion (h). No transgenic axons are found in the Rcad-positive tectoisthmic tract (i). k, Summary: the majority of axons select the Ncad-positive portion of the tb and tt (red). l–o, cad7-transgenic fibers show a similarly selective distribution but choose the cad7-positive portion of the tb instead of the Ncad-positive portion (comparel with m). As can be seen also ing and h, this cad7-positive portion has some overlap with the cad6B-positive portion of the tb.p, Summary: fibers prefer cad7-positive tracts (pink) and overlap with other cadherins only where there is endogenous overlap. q–t, Cad6B-overexpressing axons choose the respective cad6B-positive portion of the tb and show only overlap with Ncad (q) and cad7 (r) where there is endogenous overlap. Some fibers can be seen also in the ti (t). u, Summary: most axons are found in cad6B-positive fascicles of the tb (blue) and ti (yellow–blue). v–y, The majority of Rcad transgenic axons projects through the Rcad-positive isthmic tract (ti) to the isthmic nucleus, pars parvocellularis (IPC, y) or together with cad6B toward the nucleus semilunaris (Slu). Only a minority of axons follows a pathway in tb that is devoid of Rcad but characterized by cad6B (x) and cad7 coexpression (w). z, Summary: most fibers follow tracts labeled by Rcad (yellow andyellow–blue). Scale bar (in a):a–d, f–i, l–o,q–t, v–y, 100 μm.
GFP alone
In control specimens, in which only the GFP plasmid had been electroporated, no clear preference for particular subtracts by the transgenic axons was observed. For example, in the specimen shown in Figure 4, fibers can be detected in the tectothalamic tract (Fig.4a, tt), the tectobulbar tract (Fig.4b,c, tb), and the tectoisthmic tract (Fig. 4d, ti). Within the tracts, the SGC axons have chosen different subtracts: in the tb, the axons are found in cad7- and cad6B-positive portions, whereas in the tt, they comigrate with cad7 and Ncad. Transgenic fibers are also found in the tectoisthmic tract with its Rcad-positive and cad6B/Rcad-positive portions. In summary, axons from SGC neurons transfected with GFP expression plasmid fasciculate with different fiber tracts when leaving the tectum via the BCS (Fig. 4e, see schematicarrows with different colors representing different cadherins; compare with Fig. 1c).
Ncad
The pattern described above for axons transfected only with GFP clearly changes when N-cadherin is overexpressed (Fig.4f–k). The majority of transgenic fibers joins fiber fascicles of the tectothalamic and tectobulbar pathways that express Ncad (Fig. 4f, tt, tb) but not the tectoisthmic tract specified by Rcad expression (Fig. 4i). In the tectothalamic tract, in which there is substantial endogenous overlap of the Ncad-positive portion with the cad7-positive portion of the tt, yellow fiber color indicates comigration with the cad7-positive portion (Fig. 4g). Similarly, in the tb, some fibers can be found at the margin of endogenous Ncad-positive portion overlapping with cad6B- and cad7-positive subtracts (Fig.4g,h, yellow fibers). In summary, Ncad-overexpressing neurons fasciculate predominantly with axons expressing Ncad endogenously (Fig. 4k, arrows). Analyzed at the section level shown here, more fibers choose the tectobulbar pathway than the tectothalamic tract. However, at more rostral levels of the same specimen, GFP-labeled axons are found predominantly in the tt (data not shown).
cad7
Axons overexpressing cad7 show a different pathway selection, matching well with the cad7-positive portions of the tt and tb (Fig.4m). Again, in the tectothalamic tract but not in the tectobulbar tract, there is also comigration with Ncad-positve fibers as a result of endogenous coexpression or intermixing (Fig.4l). No fasciculation can be detected with Rcad-positive fibers (Fig. 4o) and only little with the cad6B-positive portion of the tectobulbar tract at the small overlapping margin between the cad6B- and cad7-positive portions (Fig.4n). In summary, cad7-overexpressing fibers prefer to grow alongside cad7-labeled subtracts (Fig. 4p).
cad6B
In embryos, in which cad6B expression was overexpressed, transgenic fibers choose the cad6B-labeled subfascicle projecting toward the hindbrain (Fig. 4s). Overlap with other pathways is confined to the marginal borders of the neighboring subtracts that are marked by a different cadherin (Fig.4q,r). Axons, which seem to terminate in the nucleus semilunaris, a cad6B-positive nucleus of the isthmic region found more posterior to the level shown here, express Rcad in addition to cad6B (Fig. 4t). In summary, cad6B transgenic neurons prefer cad6B-positive tracts.
Rcad
Finally, the great majority of axons from neurons, which are electroporated with Rcad, target the isthmic nuclei. To better visualize this portion, a slightly more dorsal detail of the sections is presented in Figure 4v–y compared with Figure4a–o. Note also that the level of the sections is more caudal, as indicated by the sectioning of the cad6B- and Rcad-positive portion of nucleus semilunaris (Fig. 4x,y,Slu). Only little comigration with Ncad- or cad7-positive fibers is found. Transgenic fibers either project via the tectoisthmic tract (Fig. 4y, ti) to the Rcad-positive isthmic nucleus, pars parvocellularis (Fig. 4y, Ipc), or to the nucleus semilunaris. Because this nucleus and its projection are also cad6B positive, overlap with fibers positive for this cadherin is expected (Fig. 4x). In summary, Rcad-overexpressing axons choose pathways characterized by Rcad expression (Fig.4z, yellow andyellow–blue).
Quantification of cadherin-specific tract selection
From the above results, it is evident that projection patterns differ depending on the choice of expression plasmid that has been electroporated. To quantify these effects, we developed an image analysis algorithm to assess the degree of fasciculation of GFP-labeled transgenic axons (Fig. 5,green) with the respective cadherin-specific subtracts (Fig.5, red). Because the majority of green fluorescent axons also coexpresses the transfected cadherin, we were interested only in the cadherin expression profile of the regions immediately adjacent to the transgenic green fibers, because they contain the fibers with which the transgenic axons fasciculate.
Fig. 5.
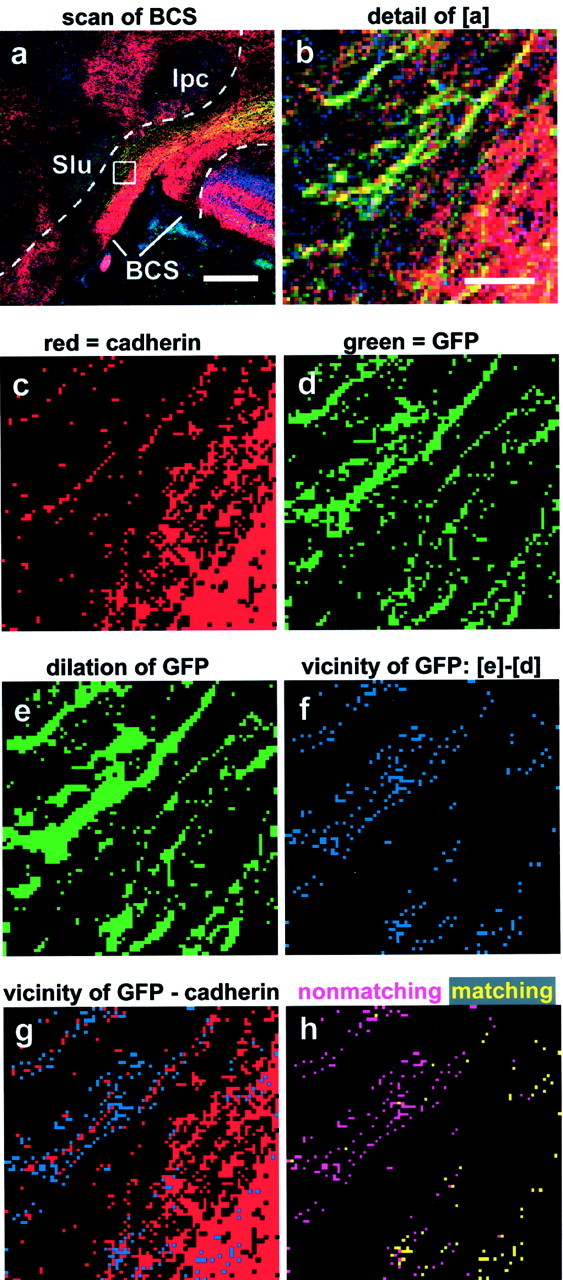
Outline of the quantitative image analysis procedure. a, Laser scanning image of cad7/GFP double transgenic axons reaching the BCS (cad7 staining in red, GFP in green, and nuclear staining inblue). The dashed lines mark the boundaries of the BCS used to electronically cut out the region of interest. Scale bar, 200 μm. b, Enlargement ofregion boxed in a to visualize single pixels. Scale bar (in b): b–h, 10 μm.c, Binarized image of the cadherin immunostaining.d, Binarized image of the GFP labeling.e, GFP labeling after one round of dilation to generate pixels covering the immediate neighborhood of the transgenic axons.f, Subtraction of the original binarized GFP image (d) from e generates pixels (blue) that represent the close vicinity of the transgenic fibers. g, Overlay of the vicinity pixels with the binarized pixels of the cadherin immunostaining (in red) to reveal those of the vicinity pixels, which match with the cadherin immunostaining.h, Yellow and pink pixels represent matching and nonmatching pixels, respectively. Note that cad7-overexpressing fibers outside the cad7-positive portion of the tectobulbar tract are correctly revealed as nonmatching. The quantification analysis was performed on scans of the whole BCS region like that outlined in a, and counting of pixels was performed by the computer.
In Figure 5, we give a short outline of the algorithm used. Shown is a scan of a cad7-immunostained frontal section through the BCS from a GFP/cad7 electroporation experiment (Fig. 5a). As a first step, the BCS region was outlined (Fig. 5a, dashed lines) on the basis of the nuclear staining (Fig. 5a,blue channel); only this region was analyzed further. For a better understanding of the subsequent steps and to visualize single pixels, we show a detail comprising both matching and nonmatching fibers in the next panels (Fig. 5, b is an enlargement of the area boxed in a). To determine how many of the fibers have chosen the matching pathway and how many did not, the red channel (cadherin expression) and the green channel (GFP expression) were separated and binarized (Fig.5c,d). Then, a close vicinity outline of the green fibers was generated by one round of dilation of green fluorescent pixels (Fig. 5e), followed by subtraction of the original green pixels (i.e., those shown in Fig. 5d). The resulting “vicinity pixels” (Fig. 5f) represent the immediate neighborhood of the transgenic GFP fibers. We then determined whether or not these vicinity pixels coexpressed the respective cadherin (here, cad7) by comparing the vicinity pixels (Fig.5f) with the binarized red scan (Fig. 5c) on a pixel-by-pixel basis (Fig. 5g). The matching of two pixels in the corresponding images, vicinity pixels and the binarized red scan were determined by two rounds of image subtractions. In the first round, cadherin pixels were subtracted from vicinity pixels (here, Fig. 5g minus Fig. 5c). This deletes all matching pixels and generates the nonmatching pixels as a result (Fig.5h, pink pixels). Subsequent subtraction of the nonmatching pixels from all vicinity pixels (here, Fig. 5fminus pink pixels) then yields the matching pixels (Fig.5h, yellow pixels). Matching pixels were counted as a measure of comigration and expressed in percentage of the overall number of vicinity pixels.
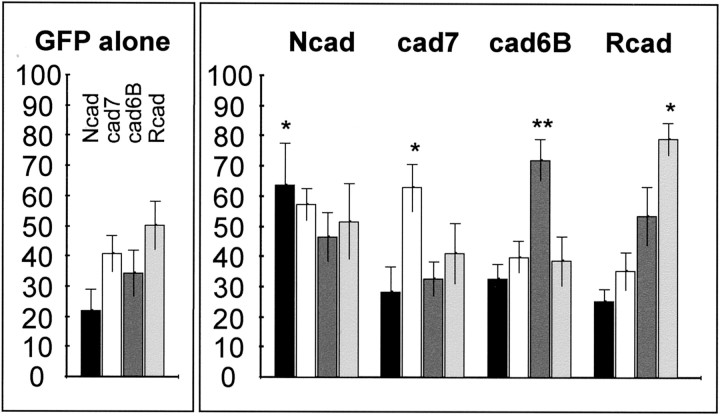
Analysis of 29 separate electroporation experiments yielded the results shown in Figure 6. In the control case (GFP alone), the Ncad-positive fibers seem somewhat underrepresented. This is most likely the consequence of the fact that the majority of Ncad-labeled axons in the BCS constitutes the tectothalamic tract, which is almost completely established at the time of electroporation (E6) (Wu et al., 2000). During electroporation with specific cadherins, the transgenic fibers show a clear tendency to grow alongside the fiber tracts, which express the respective cadherin subtype. For all cadherins, the majority of fibers selects this path over the other choices. This preference leads to a statistically significant change of tract usage by the transgenic fibers compared with the control.
Fig. 6.
Quantification of cadherin-dependent tract selection. Results are based on image analysis performed on four different cadherin immunostainings in five different experimental series (±SEM). Bars represent the average percentage of vicinity pixels of GFP-labeled transgenic axons that colocalize with cadherin immunostaining (black for Ncad,white for cad7, dark gray for cad6B, andlight gray for Rcad). The left(small box) shows results for control embryos (GFP alone, n = 7). The right(large box) summarizes results for the experimental embryos overexpressing one particular cadherin (Ncad,n = 5; cad7, n = 5; cad6B,n = 7; and Rcad, n = 5). *p < 0.05; **p < 0.005; two-tailed unpaired Student's t test comparing average of experimental with control conditions (GFP alone).
DISCUSSION
Cadherins direct pathway selection
Our study provides the first direct evidence that the differential and restricted expression of different cadherins mediates specificity of axonal pathfinding. It has been shown previously that the expression pattern of these molecules divides a class of chicken midbrain neurons, the SGC neurons of the tectum, into several subclasses (Redies et al., 1993; Miskevitch et al., 1998) that follow different projection pathways to their target regions (Wöhrn et al., 1999). By usingin vivo electroporation, we selectively overexpressed four different cadherins on a small group of SGC neurons. As a result, their axons preferred to choose tectofugal axon pathways, which were specified by the respective cadherin. This demonstrates that a change of cadherin expression on the surface of SGC neurons can alter axonal pathfinding decisions and, consequently, neuronal connectivity.
Differential adhesion as guidance mechanism
Classic cadherins bind preferentially in a homotypic manner, i.e., juxtaposed cell surface membranes, which express the same cadherin subtype, and selectively associate with each other (for review, seeTakeichi, 1988; Redies, 2000). This mechanism has been proposed to regulate various aspects of brain development, such as neuromere formation, gray matter regionalization and morphogenesis, target recognition, synapse formation, and plasticity (Redies et al.,1993;Yamagata et al., 1995; Fannon and Colman, 1996; Uchida et al., 1996;Inoue and Sanes, 1997; Manabe et al., 2000; Inoue et al., 2001; Price et al., 2002). Cadherin-mediated adhesiveness may thus be a general mechanism to generate specificity of neural interaction. The present results suggest that this mechanism is also used for the tracking of axons along preexisting fibers. Once the first fiber connections have been established, a differential adhesive code may lead to a selective association of growth cones with preexisting axons, which guides the following axons to their targets. A match of cadherin expression provides the appropriate cue for guidance and fasciculation. Differential adhesiveness is the most straightforward assumption for how the cadherins might mediate specificity based on their homophilic interaction mechanism.
A previous in vitro study has demonstrated the existence of specific adhesive guidance cues for tectofugal axons. E6 tectal explants placed on top of E14 tectal slices preferred to grow on SAC and SGC, whereas explants of the retina exhibited a different selectivity (Yamagata and Sanes, 1995). In the present study, we did not measure actual adhesion. Therefore, we cannot rule out more indirect effects of cadherin overexpression on axon guidance, involving other molecules. Indeed, the importance of differential adhesion for growth cone steering was disputed based on in vivomeasurements of filopodial retraction rates in the grasshopper (Isbister and O'Connor, 1999). Furthermore, evidence was presented that cadherin-mediated cell sorting does not rely on differential adhesiveness in vitro (Niessen and Gumbiner, 2002). Thus, we cannot exclude the possibility that there are other signaling mechanisms that facilitate the growth on axons by matching of cadherin expression.
One such alternative explanation is the selective survival of axons that have associated with membranes of matching cadherin subtype. Because cadherins were proposed to take part also in the establishment of synapses by stabilizing presynaptic and postsynaptic membranes (Fannon and Colman, 1996; Uchida et al., 1996), a cadherin match at the synapse may cause selective survival of those fibers, which have successfully established synaptic connections. However, synapses tend to form later (e.g., at approximately E11 for the retinotectal system) (Mey and Thanos, 2000), and we were not able to identify any rise in cell death indicating such a mechanism (as judged by the terminal deoxynucleotidyl transferase-mediated tetramethyl rhodamine dUTP nick end labeling assay) (data not shown).
Effect of the amount of cadherin
Why do we also find transgenic fibers that do not project according to the cadherin they overexpress? Transfection of more than one plasmid at the same time is one of the major advantages of the electroporation method and yields rates of coexpression as high as 92% (Haas et al., 2001). However, it is still possible that not all GFP-positive axons overexpress the coelectroporated cadherin to the same degree. Consequently, in weakly overexpressing cells, the endogenous cadherin expression or other factors may dominate pathway selection. Another result from this study points to this direction. With the plasmid constructs used, Ncad is expressed relatively weakly by the transgenic fibers when compared with the endogenous expression (Fig. 3d,e). Accordingly, although a significant change in tract selection can be observed with Ncad transfection, this effect seems weaker than for the other cadherins.
Combinatorial use of cadherins
It is also likely that more molecules than the four cadherins investigated are required for proper pathfinding in the tectofugal system. For example, although cad7 overexpression is sufficient to direct axon growth to the matching cad7-positive subtracts, it cannot distinguish between such different projections as the tectothalamic and tectobulbar tracts. Rather, some SGC axons take the matching cad7-positive portion of one tract and some pick the cad7-positive portion of the other. Most likely, additional receptors for guidance cues are required to discriminate between these two projections. Other factors may include other cadherins that were not investigated here. Single SGC neurons were shown to be able to coexpress more than one cadherin (Wöhrn et al., 1999), and our results corroborate the concept of combinatorial cadherin action in single cells. For example, fibers expressing cad6B and Rcad seem to specify the projection to the nucleus semilunaris. This combinatorial use of cadherins to specify distinct neural circuits may also reflect the fact that there are many more circuits in the vertebrate CNS than genes in the genome. In this respect, it is remarkable that the cadherins are a still growing family of >100 molecules, with most members expressed in the developing CNS (for review, see Nollet et al., 2000; Redies, 2000). Moreover, for the more recently identified cadherin-related neuronal receptor protocadherins, a mechanism to generate great molecular diversity similar to that in the immune system was hypothesized (Wu and Maniatis, 1999).
Other factors working together with cadherins
There are many other candidate molecules that may cooperate with the cadherins to mediate axonal guidance decisions. Members of the Ig superfamily are expressed in the chicken tectobulbar tract (Kröger and Schwarz, 1990), and antibody perturbation experimentsin vivo implicated these molecules in target specification of sensory afferents in chicken spinal cord (Perrin et al., 2001). Another class of molecules acting in axonal pathfinding and fasciculation are the receptor protein tyrosine phosphatases. Remarkably, in Drosophila, a mutation in the gene DLAR leads to targeting errors of photoreceptor axons very similar to those found in the Ncad mutation (Clandinin et al., 2001; Lee et al., 2001;Maurel-Zaffran et al., 2001). However, to date, the epistatic relationship of the two genes is not clear. There is also growing evidence for a functional link of cadherins with more general growth-promoting receptors, such as the β1-integrins. Injections of antibodies against Ncad and β1-integrins at the same time caused pathfinding errors in the retinotectal projection (Stone and Sakaguchi, 1996). More recently, both molecules were found to be coordinately regulated during signaling in response to the outgrowth inhibiting proteoglycan neurocan (Li et al., 2000). Moreover, the protein tyrosine kinase Fer was identified as an epigenetic factor that passes from the Ncad adhesion complex to the β1-integrin machinery (Arregui et al., 2000).
Integrating cadherin-mediated selective fasciculation into other guidance mechanisms
Selective fasciculation depends on preexisting axons that have already reached the target region. Investigations of the molecular guiding mechanisms for these early fibers identified several evolutionary highly conserved guidance systems, such as ephrins, netrins, slits, and semaphorins. These molecules either establish attractive or repulsive gradients, mediate topographic information, or form barriers preventing specific axons to proceed and, in result, channel them toward their destination (for review, see Chisholm and Tessier-Lavigne, 1999; Yu and Bargmann, 2001; Knöll and Drescher, 2002). These molecules are all present at the same time, and, consequently, cadherin signaling has to converge with their inputs, as was shown for example for members of the Ig superfamily (Winberg et al., 1998; Stein and Tessier-Lavigne, 2001). Future studies will have to combine genetic manipulation with the observation of growth conesin vivo (for review, see Mason and Erskine, 2000) to reveal how growth cones integrate the different signals simultaneously (Rose and Chiba, 1999) or in a temporal order (Diefenbach et al., 2000). In the end, all of the different signals might converge at the level of second messengers, such as intracellular Ca2+, that regulate the turning of growth cones (Zheng, 2000; Gomez et al., 2001; Ming et al., 2001).
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grant Re 616/4-3 (C.R.). We thank Masatoshi Takeichi, Shinichi Nakagawa, and Hidesato Ogawa for their generous gifts of reagents, Kenji Shimamura for technical advice, Ulrike Laub for technical assistance, and Kirsten Arndt for valuable suggestions on this manuscript.
Correspondence should be addressed to Ullrich Treubert-Zimmermann, Institute of Anatomy, University of Essen Medical School, Hufelandstrasse 55, D-45122 Essen, Germany. E-mail:ullrich.treubert@uni-essen.de.
REFERENCES
- 1.Arregui C, Pathre P, Lilien J, Balsamo J. The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J Cell Biol. 2000;149:1263–1274. doi: 10.1083/jcb.149.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- 3.Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broadbent ID, Pettitt J. The C. elegans hmr-1 gene can encode a neuronal classic cadherin involved in the regulation of axon fasciculation. Curr Biol. 2002;12:59–63. doi: 10.1016/s0960-9822(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- 6.Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 7.Diefenbach TJ, Guthrie PB, Kater SB. Stimulus history alters behavioral responses of neuronal growth cones. J Neurosci. 2000;20:1484–1494. doi: 10.1523/JNEUROSCI.20-04-01484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 9.Glover JC, Petursdottir G, Jansen JK. Fluorescent dextran amines used as axonal tracers in the nervous system of the chicken embryo. J Neurosci Methods. 1986;18:243–254. doi: 10.1016/0165-0270(86)90011-7. [DOI] [PubMed] [Google Scholar]
- 10.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 11.Haas K, Sin WC, Javaherian A, Li Z, Cline HT. Single-cell electroporation for gene transfer in vivo. Neuron. 2001;29:583–591. doi: 10.1016/s0896-6273(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 12.Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- 13.Honig MG, Rutishauser US. Changes in the segmental pattern of sensory neuron projections in the chick hindlimb under conditions of altered cell adhesion molecule function. Dev Biol. 1996;175:325–337. doi: 10.1006/dbio.1996.0118. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SP, Künzle H. Observations on the projections and intrinsic organization of the pigeon optic tectum: an autoradiographic study based on anterograde and retrograde, axonal and dendritic flow. J Comp Neurol. 1976;170:153–172. doi: 10.1002/cne.901700203. [DOI] [PubMed] [Google Scholar]
- 15.Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Tanaka T, Takeichi M, Chisaka O, Nakamura S, Osumi N. Role of cadherins in maintaining the compartment boundary between cortex and striatum during development. Development. 2001;128:561–569. doi: 10.1242/dev.128.4.561. [DOI] [PubMed] [Google Scholar]
- 17.Isbister CM, O'Connor TP. Filopodial adhesion does not predict growth cone steering events in vivo. J Neurosci. 1999;19:2589–2600. doi: 10.1523/JNEUROSCI.19-07-02589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- 19.Knöll B, Drescher U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 2002;25:145–149. doi: 10.1016/s0166-2236(00)02093-2. [DOI] [PubMed] [Google Scholar]
- 20.Kröger S, Schwarz U. The avian tectobulbar tract: development, explant culture, and effects of antibodies on the pattern of neurite outgrowth. J Neurosci. 1990;10:3118–3134. doi: 10.1523/JNEUROSCI.10-09-03118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Leung TC, Hoffman S, Balsamo J, Lilien J. Coordinate regulation of cadherin and integrin function by the chondroitin sulfate proteoglycan neurocan. J Cell Biol. 2000;149:1275–1288. doi: 10.1083/jcb.149.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci. 2000;15:534–546. doi: 10.1006/mcne.2000.0849. [DOI] [PubMed] [Google Scholar]
- 24.Mason C, Erskine L. Growth cone form, behavior, and interactions in vivo: retinal axon pathfinding as a model. J Neurobiol. 2000;44:260–270. doi: 10.1002/1097-4695(200008)44:2<260::aid-neu14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Maurel-Zaffran C, Suzuki T, Gahmon G, Treisman JE, Dickson BJ. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001;32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 26.Mey J, Thanos S. Development of the visual system of the chick. I. Cell differentiation and histogenesis. Brain Res Brain Res Rev. 2000;32:343–379. doi: 10.1016/s0165-0173(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 28.Miskevitch F, Zhu Y, Ranscht B, Sanes JR. Expression of multiple cadherins and catenins in the chick optic tectum. Mol Cell Neurosci. 1998;12:240–255. doi: 10.1006/mcne.1998.0718. [DOI] [PubMed] [Google Scholar]
- 29.Momose T, Tonegawa A, Takeuchi J, Ogawa H, Umesono K, Yasuda K. Efficient targeting of gene expression in chick embryos by microelectroporation. Dev Growth Differ. 1999;41:335–344. doi: 10.1046/j.1440-169x.1999.413437.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 31.Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–400. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 33.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 34.Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 35.Perrin FE, Rathjen FG, Stoeckli ET. Distinct subpopulations of sensory afferents require F11 or axonin-1 for growth to their target layers within the spinal cord of the chick. Neuron. 2001;30:707–723. doi: 10.1016/s0896-6273(01)00315-4. [DOI] [PubMed] [Google Scholar]
- 36.Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 37.Redies C. Cadherins in the central nervous system. Prog Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 38.Redies C, Inuzuka H, Takeichi M. Restricted expression of N- and R-cadherin on neurites of the developing chicken CNS. J Neurosci. 1992;12:3525–3534. doi: 10.1523/JNEUROSCI.12-09-03525.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redies C, Engelhart K, Takeichi M. Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J Comp Neurol. 1993;333:398–416. doi: 10.1002/cne.903330307. [DOI] [PubMed] [Google Scholar]
- 40.Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 41.Rose D, Chiba A. A single growth cone is capable of integrating simultaneously presented and functionally distinct molecular cues during target recognition. J Neurosci. 1999;19:4899–4906. doi: 10.1523/JNEUROSCI.19-12-04899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–709. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 44.Stone KE, Sakaguchi DS. Perturbation of the developing Xenopus retinotectal projection following injections of antibodies against beta1 integrin receptors and N-cadherin. Dev Biol. 1996;180:297–310. doi: 10.1006/dbio.1996.0302. [DOI] [PubMed] [Google Scholar]
- 45.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 46.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 47.Thanos S, Bonhoeffer F. Investigations on the development and topographic order of retinotectal axons: anterograde and retrograde staining of axons and perikarya with rhodamine in vivo. J Comp Neurol. 1983;219:420–430. doi: 10.1002/cne.902190404. [DOI] [PubMed] [Google Scholar]
- 48.Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winberg ML, Mitchell KJ, Goodman CS. Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell. 1998;93:581–591. doi: 10.1016/s0092-8674(00)81187-3. [DOI] [PubMed] [Google Scholar]
- 50.Wöhrn JC, Nakagawa S, Ast M, Takeichi M, Redies C. Combinatorial expression of cadherins in the tectum and the sorting of neurites in the tectofugal pathways of the chicken embryo. Neuroscience. 1999;90:985–1000. doi: 10.1016/s0306-4522(98)00526-0. [DOI] [PubMed] [Google Scholar]
- 51.Wu CC, Russell RM, Karten HJ. Ontogeny of the tectorotundal pathway in chicks (Gallus gallus): birthdating and pathway tracing study. J Comp Neurol. 2000;417:115–132. [PubMed] [Google Scholar]
- 52.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 53.Yamagata M, Sanes JR. Lamina-specific cues guide outgrowth and arborization of retinal axons in the optic tectum. Development. 1995;121:189–200. doi: 10.1242/dev.121.1.189. [DOI] [PubMed] [Google Scholar]
- 54.Yamagata M, Herman JP, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. J Neurosci. 1995;15:4556–4571. doi: 10.1523/JNEUROSCI.15-06-04556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu TW, Bargmann CI. Dynamic regulation of axon guidance. Nat Neurosci [Suppl] 2001;4:1169–1176. doi: 10.1038/nn748. [DOI] [PubMed] [Google Scholar]
- 56.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]