Abstract
Critical learning periods are common in vertebrate development. In many birds, song learning is limited by a critical period; juveniles copy songs from adult birds by forming memories of those songs during a restricted developmental period and then using auditory feedback to practice their own vocalizations. Adult songs are stable over time regardless of exposure to other birds, but auditory feedback is required for the maintenance of stable adult song. A technique was developed to reversibly deafen Bengalese Finches by destruction and regeneration of inner ear auditory hair cells. With this approach, we asked two questions about the plasticity of song information stored in the adult brain. First, do adult birds store memories or “templates” of their songs that exist independent of auditory reinforcement? Such memories could be used to control vocal output by acting as fixed models of song to which ongoing vocalizations are matched. Second, can adult song learning, which does not normally occur in this species, be induced by removing and then restoring hearing? Studying changes in adult song behavior during hair cell loss and regeneration revealed two findings: (1) adult birds store memories or templates of their songs that exist independent of auditory input and can be used to restore normal vocal behavior when hearing is restored; (2) under experimental circumstances, adult birds can be induced to acquire song material from other birds. Results suggest that, in Bengalese Finches, the degree of behavioral and neural plasticity in juvenile and adult birds may be less distinct that previously thought.
Keywords: songbird, song, plasticity, auditory feedback, hair cell, vocalization
Song learning in birds is a process of sensory memorization and motor practice limited by a critical (or sensitive) learning period (Marler and Tamura, 1964; Konishi, 1965;Marler and Peters, 1987). Juvenile birds memorize the songs of adult conspecific birds and practice their own vocalizations until they match the memorized song. In Bengalese Finches Lonchura striata domestica, this process occurs between ∼25 and 90 d of age (Immelmann, 1969; Dietrich, 1980; Clayton, 1987, 1988, 1989). At sexual maturity, a Bengalese Finch's song behavior stabilizes (a process called crystallization) and does not normally change in adulthood. The stability of adult song is dependent on hearing, however. Songs begin to degrade soon after deafening, indicating that normal adults use auditory feedback to maintain stable song over time (Okanoya and Yamaguchi, 1997; Woolley and Rubel, 1997, 1999). The effects of deafening are similar in other songbirds but have a different time course (Nordeen and Nordeen, 1992; Brainard and Doupe, 2000; Lombardino and Nottebohm, 2000).
We developed a technique to study the effects of deafening and then restoration of hearing on adult song behavior in Bengalese Finches. This approach allowed us to address two fundamental questions about the plasticity of song information stored in the adult brain. First, does a bird store a memory of its own song that is retained independent of both the song motor output and auditory experience? For example, does the bird remember its own original song even when it is unable to hear its song because of severe hearing loss and that song has become degraded? If so, then the original song may be restored when hearing recovers. Second, can the adult brain be induced to learn new song elements; can the critical period for song learning be reopened after deprivation of the appropriate sensory stimulation? Because deafening is thought to force song behavior and its motor circuitry into a plastic state (Brainard and Doupe, 2000), hearing restoration might induce or reveal the ability to learn new song elements during a recovery period of vocal practice that is reminiscent of motor learning in juveniles.
We took advantage of the ability of adult birds to regenerate auditory hair cells after loss of original hair cells caused by ototoxic drugs or sound exposure (Cotanche, 1987; Cruz et al., 1987; Corwin and Cotanche, 1988; Ryals and Rubel, 1988). We have shown previously that auditory hair cell loss in Bengalese Finches results in song degradation comparable to that seen after surgical deafening (Woolley and Rubel, 1999). Because hearing is restored to near-normal sensitivity when auditory hair cells regenerate (Tucci and Rubel, 1990;Marean et al., 1993; Woolley et al., 2001), we were able to make detailed comparisons between original songs, songs of the same birds after deafening, and then during and after hair cell regeneration.
MATERIALS AND METHODS
Animals and experimental design. Forty adult male Bengalese Finches were used. All birds were obtained from a supplier (Magnolia Bird Farm, Anaheim, CA) as young adults. To minimize age differences among subjects, birds were kept in our colony for a minimum of 3 months and a maximum of 6 months before the initiation of the experiment. Stable adult song was recorded from 15 birds on two occasions that were between 4 and 10 weeks apart. Those birds were then deafened using a treatment that destroys sensory hair cells throughout nearly the entire hearing organ (basilar papilla) and causes a profound high- to low-frequency hearing loss in ∼50% of the subjects (Woolley and Rubel, 1999). After treatment, song was recorded from each bird. At this recovery time (1 d from the end of treatment, 8 d from the beginning of treatment), eight birds showed profound hearing losses and were singing degraded song. The other seven treated birds maintained stable song that matched the pretreatment song despite treatment. Auditory thresholds for those birds were determined 1 d after the end of treatment by electrophysiologically recording auditory brainstem responses (ABRs) to tone stimuli (see below). Their basilar papillae were then prepared for scanning electron microscopy (EM) to determine the extent and location of hair cell loss in each bird.
The eight birds singing degraded songs after treatment were then divided into two groups for the recovery period. Three recovering birds were housed together during 8 weeks of hair cell regeneration. Each of the other five birds was individually housed with one untreated adult male singing stable song. Three of these birds were allowed to recover for 8 weeks, and two birds recovered for 14 weeks. During recovery, song was recorded weekly. At the end of the recovery period, auditory thresholds were measured to confirm hearing recovery by recording ABRs. Immediately after this procedure, birds' papillae were prepared for scanning EM to ensure that each bird had regenerated a full complement of new auditory hair cells. Normal auditory thresholds were determined using an additional eight birds from which song was not recorded. In a separate group of 12 adult birds with stable song, we tested the effects of the treatment inducing hair cell loss on plasma levels of testosterone (T). Six birds were treated to induce hair cell loss, and six birds received no treatment. Immediately after treatment, blood from all 12 birds was collected and analyzed for circulating plasma T levels.
Treatment. To induce the maximum hair cell loss that is required for song degradation (Woolley and Rubel 1999), we used intramuscular injections (alternating doses of 150 mg · kg−1 · d−1 and 300 mg · kg−1 · d−1) of the ototoxic aminoglycoside, Amikacin (Faulding Puerto Rico Inc.) in combination with nightly exposures to low-pass-filtered white noise for 12 hr at an intensity of 126 dB sound pressure level (SPL). This treatment regimen was continued for 7 d for all but one bird that was treated for 9 d. The white noise stimulus was filtered at 60 dB per octave above 1.0 kHz. This filtering was used to eliminate the frequency content of the noise that fell above 1 kHz because the intent of the noise stimulus was to target only the hair cells encoding low frequencies. Amikacin, like all aminoglycosides, preferentially targets hair cells encoding higher frequencies (Woolley and Rubel, 2001). Amikacin was chosen for treatment because it is particularly toxic to auditory hair cells and less toxic to renal function than other aminoglycosides (Lenoir and Puel, 1987; Kitasato et al., 1990; Beaubien et al., 1995; Vago et al., 1998). Treatment with a combination of ototoxic drugs and intense sound exposure causes larger hair cell lesions than either drugs or sound presentation alone (Bone and Ryan, 1978; Collins, 1988; Brummett et al., 1990, 1992; Pye and Collins, 1991). Previous studies have indicated this to be the optimum treatment schedule for consistently killing as many hair cells as possible without compromising a bird's health (Woolley and Rubel, 1999).
For sound exposures, birds were placed in a cylindrical wire mesh cage with food and water available ad libitum. The cage was then placed inside a custom sound-attenuated chamber, and a low-frequency loudspeaker (JBL 2206H) was placed 8 cm above the wire cage. The chamber and the speaker were housed inside a sound isolation booth (Industrial Acoustics). Calibration of sound intensity and analysis of the harmonic content of the sound were performed using a dynamic signal analyzer (Hewlett-Packard) at the beginning and end of each exposure period.
Testosterone measurement. Plasma testosterone levels were measured using standard procedures according to Tramontin et al. (2000).
Song recording. “Undirected” (not in the presence of a female) song was recorded as described previously (Woolley and Rubel, 1997, 1999). Each male was alone in a 6 inch × 6 inch × 8 inch cylindrical wire mesh cage within a sound isolation booth (Industrial Acoustics). A low impedance microphone (Sony, F-98) was placed 25 cm above the bird's perch. The microphone was connected to a digital audiotape recorder (Sony TCD-100). For each recording date, 10–20 singing bouts composed of several motifs were recorded. Song bouts were defined as episodes of continuous singing surrounded by 2 or more seconds of silence.
Terminology for song units. Terminology for the units and organization of bird song in this study is from Nottebohm and Konishi (1969). Bengalese Finch songs are composed of between 2 and 10 syllables that are normally sung in a stereotyped order and can be composed of one or more notes (see Fig. 3A). Notes are continuous sounds surrounded by short (∼20–60 msec) silences. Syllables are defined as groups of notes that are sung sequentially and consistently as units and are separated from other sound units by longer (more than ∼60 msec) silences than appear between notes. Notes composing a syllable may be very different in spectral structure as long as they are sung consistently as a unit and show only a short (less than ∼60 msec) silence between them (see Fig. 7A,notes B, C, D, and E). One repeat of a stereotyped sequence of syllables is called a motif. Motifs are repeated several times in succession to form a singing bout. Both notes and syllables have characteristic acoustic structures that are normally stable over time. These methods for defining the levels of song organization have been used in previous work examining the composition of Bengalese Finch song (Woolley and Rubel, 1997,1999).
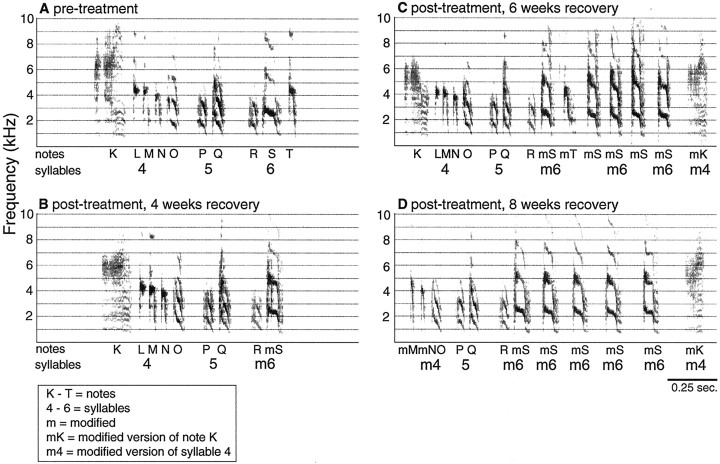
Fig. 3.
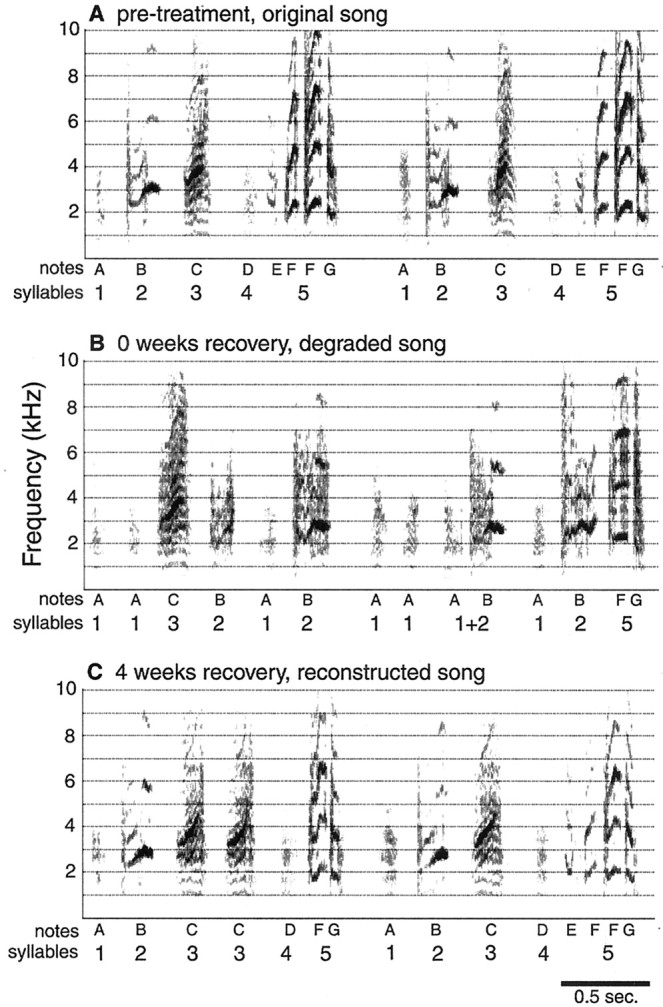
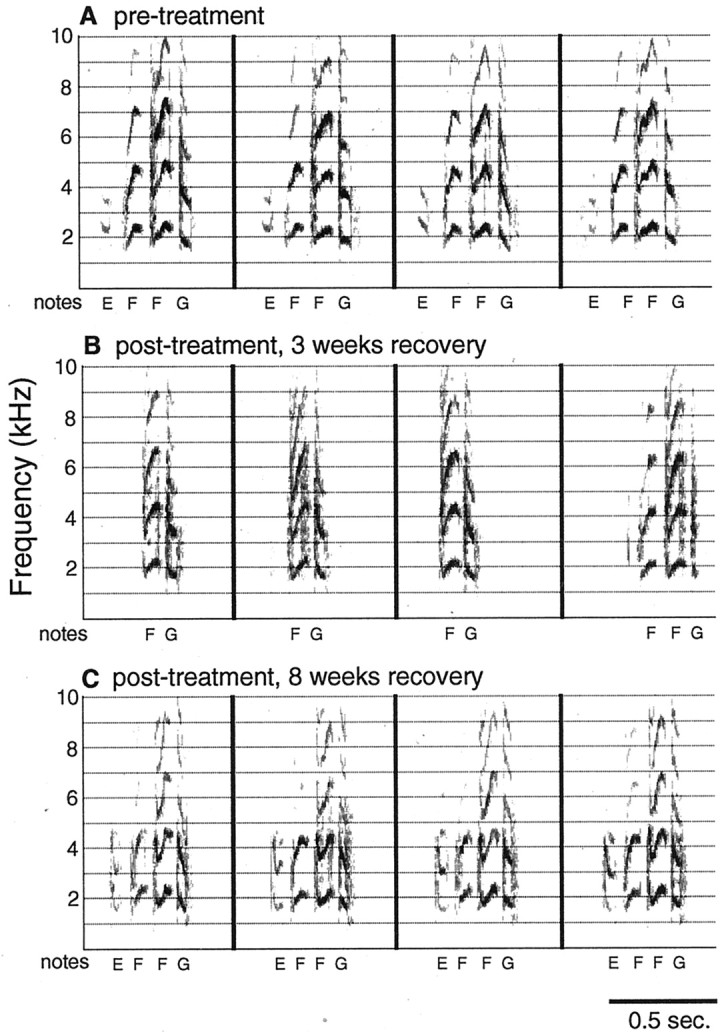
Eight of fifteen birds showed degraded song after treatment to cause hair cell loss. Syllable sequences returned to the original, pretreatment order, and some aspects of syllable structure recovered toward that of the original song by 4 weeks after treatment. A, Spectrograph (frequency over time plot) of two song motifs recorded before treatment.B, Spectrograph of a syllable sequence from the same bird as in A but recorded 1 d after the end of treatment. Comparison of A and B shows that syllable order and the acoustic structure of syllables degraded in this bird after treatment. C, Two song motifs from the same bird as in A and B recorded 4 weeks after treatment. Comparison of the three panels shows that the song recorded after 4 weeks of recovery appears similar to the pretreatment song and dissimilar to the song recorded immediately after treatment. Individual syllables are labeled withnumbers, and notes are labeled withletters below the x-axis.Darkness indicates the intensity of vocalizations.
Fig. 7.
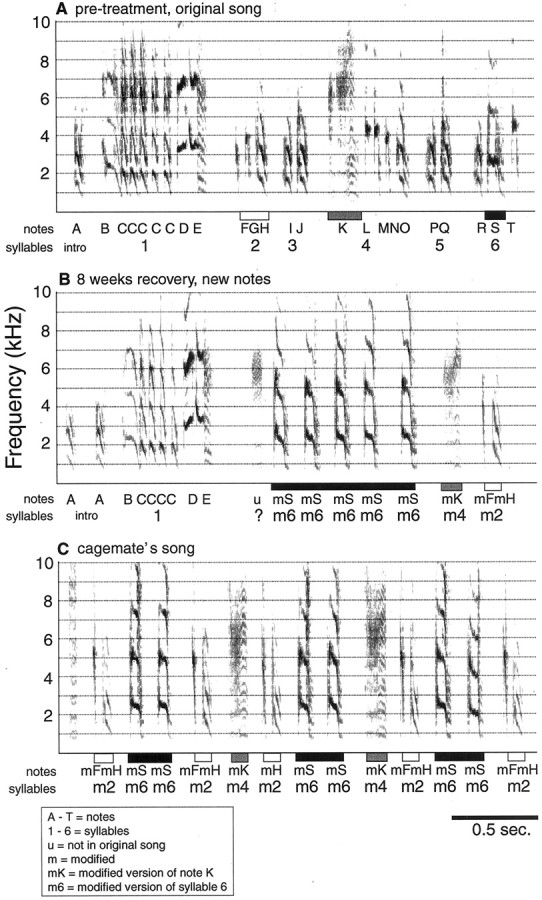
By 8 weeks of recovery, 52% of syllables in the recovered songs of three birds did not match their pretreatment versions. Some notes were deleted, whereas other notes were structurally modified and placed in new locations within a motif, forming new single or multinote syllables. In all cases, new notes and syllables matched the notes and syllables of their cage mates' songs.A, Spectrograph of one song motif recorded before treatment. B, Spectrograph of one motif from the same bird as in A but recorded after 8 weeks of recovery. This motif shows that the introductory note (A) and the first syllable (composed of notes B,C, D, and E) recovered to their original structures, but the following notes are modified versions of notes from original syllables. These modified notes immediately follow the first syllable, and the rest of the motif is skipped. C, Spectrograph of the song sung by the cage mate of the bird shown in A and B. The similarities between B and C are stronger than between A and B. Barsbelow the x-axis indicate the similar song portions among A, B, and C. Syllables are labeled with numbers, and notes are labeled with letters below the x-axis. Pretreatment and pretreatment-like notes are labeled withuppercase letters(A–T). A note for which the pretreatment versions could not be determined is labeled with a lowercase letter (u). The lowercase letter m indicates a modified version of a pretreatment note. InC, letters indicate which of the recovering bird's syllables and notes match the syllables and notes above. They do not identify the cage mate's own syllables and notes.
Song analysis. Song recordings were quantitatively analyzed for temporal stereotypy or the stability of the order in which syllables were sung over time and for stability of the spectral content or acoustic structure of individual syllables over time. Analyses were done blind as to the timing of the recordings.
Temporal stereotypy/syllable sequences. Analysis of the syllable order composing each bird's song used methods described in previous work on song production in Bengalese Finches (Woolley and Rubel, 1997, 1999). Briefly, hard copies of song spectrographs and amplitude waveforms were made for the three longest bouts from each bird's song recordings on each day. For each bird, a single pretreatment recording served as a reference recording for normal song with which all other recordings were compared. This reference recording was the longest singing bout found in the recordings of normal song and contained an average of eight motifs. From this reference, “typical” syllables and syllable transitions were defined (see below). All other song records from that bird were coded and randomized so that during the analysis the experimenters were blind with respect to the recording time of a particular set of spectrographs and waveforms. Syllables and transitions between syllables were labeled on all records. Syllables were given identifying numbers in their corresponding order of appearance in a bird's normal song. Song notes were labeled with letters in their corresponding order of appearance. For example, one bird's song (see Fig. 3A) was composed of five syllables and seven notes. Syllable 1 was composed of note A; syllable 2 was composed of note B; syllable 3 was composed note C; syllable 4 was composed of note D; and syllable 5 was composed of notes E, F, and G.
The song spectrographs from each bird at each recording time were analyzed for changes in syllable order/sequence stereotypy compared with syllable order in pretreatment recordings using a standard method (Scharff and Nottebohm, 1991; Woolley and Rubel, 1997, 1999). This method calculates a sequence stereotypy score for each singing bout by taking the average of two stereotypy ratios: sequence linearity and sequence consistency. The maximum value for each of these scores is 1. The calculations are expressed as follows: sequence linearity = # different syllables per bout/# transition types per bout; sequence consistency = sum typical transitions per bout/sum total transitions per bout; sequence stereotypy = S linearity + S consistency/2.
Sequence linearity measures how many different ways syllables are ordered. Sequence consistency measures how often syllable sequences are unlike those in the pretreatment song.
Sequence stereotypy scores were calculated for each of the three song bouts analyzed per recording date, per bird, and averaged to give one final score for each bird at each recording date. Those scores were then averaged over all treated birds separately for each recording time and statistically analyzed for changes in sequence stereotypy over time.
Spectral content/syllable structure. To measure changes in syllable structure over time, we used the software Sound Analysis (Tchernichovski et al., 2000), which generates a percent similarity score for two sounds by comparing the pitch, frequency modulation, spectral continuity, and Wiener entropy within a sound sample (in this case, a syllable). Sound features were scaled specifically for Bengalese Finch (dark chocolate and white morph) song. The similarity threshold was set at 92%, and intervals were set at 50 msec. The minimum duration required for a similarity measure of 100% to be called meaningful (similarity section) was 4 msec. Scoring was done using the overall mode. To measure the degradation of syllable structure after hair cell loss and the subsequent changes in syllable structure during hair cell regeneration, we calculated the percent similarity of three randomly selected examples of each syllable type between two pretreatment song recordings and recordings made after 1, 4, and 8 weeks of recovery.
Note structure. Analysis of the changes in song that lead to the production of modified/new syllables showed that such changes were initiated at the level of notes (see Results). We therefore used Sound Analysis to generate percent similarity scores using notes as the units of analysis by comparing (1) song notes from the pretreatment song with the same notes from the recovering/recovered song and (2) notes from the recovering/recovered song with notes from the cage mate's song that were visually judged to match notes from the recovering bird's song. For each note, three iterations from each of the three longest singing bouts (nine total) from each recording date were analyzed and compared with a matching number of notes from other recordings and the cage mate's song. We calculated the percent similarity between modified/new notes and original notes that were visually most similar to the modified/new notes from that bird. Percent similarity for those notes was measured after 4, 6, and 8 weeks of recovery. Percent similarity between modified/new notes and a cage mate's song notes was determined in the same way, by comparing the notes from a bird and its cage mate that were qualitatively most similar. Although this analysis may seem biased toward finding high similarities, it should be noted that it is equivalently biased for comparisons between notes from the same bird over time and notes between an experimental bird and its cage mate. Four control analyses were done using Sound Analysis. To estimate a baseline percent similarity among Bengalese Finch notes in general, randomly chosen notes from all the recovered birds' songs and all cage mates' songs were compared. To estimate the general similarity among notes within a birds' song, we computed the average percent similarity score for randomly chosen notes within each bird's song and then averaged those scores across birds. To get the baseline similarity between notes that were visually similar regardless of exposure to each other's songs, the percent similarity between visually similar notes between all recovering birds' songs and all the cage mates' songs was calculated. Finally, the percent similarity between visually similar notes from a recovered bird's song and the songs of cage mates that did not tutor that bird was calculated. With this analysis, the difference between coincidentally similar notes between recovered birds and cage mates and the similarity resulting from song imitation could be quantified.
Auditory brainstem responses. Evoked potential thresholds were determined by electrophysiologically recording from auditory brainstem nuclei during presentation of sound stimuli as described inWoolley and Rubel (1999). Briefly, sound stimuli were pure tone bursts at test frequencies of 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, and 6.0 kHz. Tone bursts with 1 msec rise/fall and 10 msec total duration were delivered at a rate of nine per second. Stimuli were delivered with a free field speaker (Realistic Minimus-7) placed at a 90° angle to the midline of the head and at a distance of 7 cm from the head. The stimulus delivery system was calibrated at the beginning of each experiment using an ER-10 microphone (Etymotic Research) placed at the ear, and custom software was used to acquire and analyze evoked potential traces. All threshold data were recorded in decibels SPL.
Animals were anesthetized with urethane (0.002 mg/kg). Body temperature was maintained at 38°C. Birds were placed on a flat platform, and their heads were stabilized in a specially designed holder. Two pin electrodes (Grass Instruments Co., Quincy, MA) were implanted bilaterally through the cranium into the brain. One electrode was placed in the cerebellum just above the auditory brainstem nuclei (active electrode), and a second electrode was placed in the telencephalon (active electrode). A third electrode was inserted into leg muscle (ground electrode). Responses were amplified, filtered (0.03–3.0 kHz bandpass), and digitized at a rate of 200 kHz. Responses were averaged over 200 stimulus presentations above threshold and 500 presentations at and around threshold. Stimulus presentations for each frequency were begun at 90 dB and decreased or increased in intensity by 10 dB steps to past threshold and then in 5 dB steps to determine threshold. Threshold was defined as the intensity at which the averaged peak-to-peak response was at least twice the amplitude of baseline variation.
Scanning electron microscopy. The extent and patterns of hair cell loss and regeneration were examined by scanning EM according to procedures described in Woolley and Rubel (1999). Briefly, birds were euthanized by an intramuscular injection of sodium pentobarbital (Anpro Pharmaceuticals) and then decapitated. Basilar papillae were perfused with 2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.1m PBS. The epithelial surfaces of the hair cells were exposed and prepared for scanning EM using standard dissection and histological procedures. Scanning EM was performed with a JEOL 6300F electron microscope (accelerating voltage of 15 kV) to document the extent and location of original hair cell damage and the extent of hair cell regeneration in each animal.
Quantification of hair cell damage was done by digitally acquiring images of each papilla onto a Powermac 8100/80 and measuring total papilla area and area of damaged papilla using image analysis software (NIH Image v.1.61b7). The damaged area of papilla was defined as the region showing hair cells with expanded surfaces that were missing stereocilia, showing no hair cells, or regenerating hair cells. Regenerated hair cells and original hair cells were distinguished by their morphological differences. Regenerated hair cells are identified by their misshapen luminal surfaces and misaligned stereocilia bundles (Duckert and Rubel, 1990, 1993; Marean et al., 1993, 1995). Therefore, the area of papilla originally damaged but then repopulated with new hair cells could be measured accurately.
Statistical analysis. Statistical analyses on sequence stereotypy scores and percent similarity were performed using repeated measures, one-factor ANOVAs. Post hoc comparisons were made with the Scheffé F test. Student's t tests (two-tailed) were used to compare the percent similarity between recovered song notes (4 weeks) and modified/new (8 weeks) notes within a bird and to compare the percent similarity of new notes with their original versions and with their cage mates' notes. Differences in hearing thresholds between normal birds and treated birds that had recovered for either 8 or 14 weeks were tested using Student'st tests at each test frequency.
RESULTS
Hair cell regeneration and recovery of auditory function
In all birds used to study song recovery after hair cell loss, we measured the extent of hair cell loss and regeneration at 8 or 14 weeks after treatment using scanning EM (Figs. 1A,2) and the recovery of hearing thresholds at those same times using ABRs (Fig. 1B). These procedures were done on each bird to confirm (1) the extent of hair cell loss, (2) the extent and completeness of hair cell regeneration, and (3) the recovery of hearing.
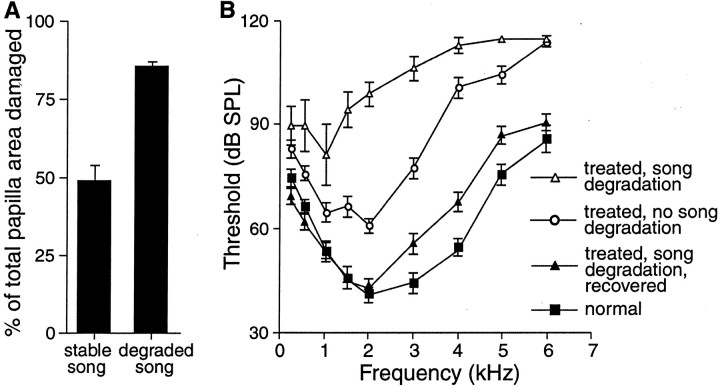
Fig. 1.
Birds that sang degraded song after treatment had greater hair cell loss than birds that sang stable (like pretreatment) song after treatment. Birds that were allowed to recover from treatment for 8 or 14 weeks regained normal hearing in the low frequencies and near normal hearing in the higher frequencies. A, Percent of total papilla area missing original hair cells is plotted for birds with stable song after treatment and birds with degraded song after treatment. B, Threshold curves showing hearing thresholds for birds with profound hearing loss and degraded song immediately after treatment [▵; data from Woolley and Rubel (1999)], thresholds for birds that were also treated but maintained stable song (○), thresholds for birds that were treated, showed song degradation, and recovered for 8 or 14 weeks (▴), and normal birds (▪). Error bars represent ± SEM.
Fig. 2.
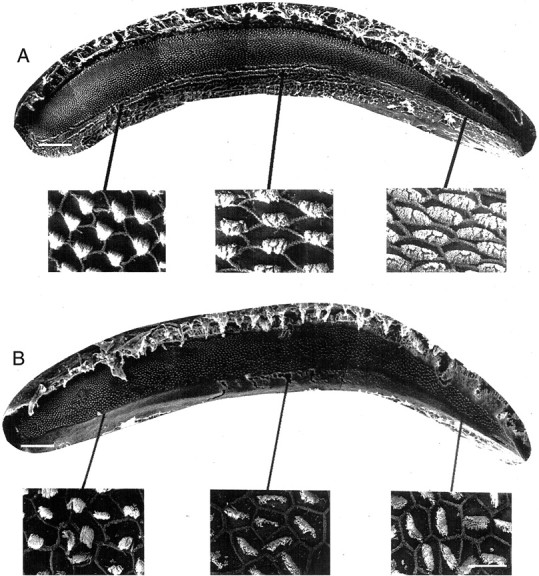
Scanning electron photomicrographs showing the epithelial surface of a basilar papilla from a normal bird and a papilla from a bird that received treatment to kill hair cells and sang degraded song after treatment. This bird recovered from treatment for 8 weeks before these images were collected. A, Montage of photomicrographs showing a normal basilar papilla. The wider, apical end encoding low frequencies is to the left. The narrower, basal end where high frequencies are encoded is to theright. Three higher magnification micrographs showing the well organized, hexagonal array of normal hair cells and the alignment of stereocilia bundles in the low-, mid-, and high-frequency regions of the papilla are below. B, Montage of photomicrographs showing a basilar papilla from a recovered bird with regenerated hair cells. New hair cells cover the entire epithelial surface, except along the superior edge of the apical region, where hair cells are original. Below are higher-magnification views of regenerated hair cells. They are distinguished from original hair cells by their misshapen luminal surface structures and misaligned stereocilia bundles. For the papillas, scale bar = 100 μm; for the higher-magnification micrographs, scale bar = 10 μm.
Thresholds for birds with regenerated hair cells were compared with thresholds for normal adult male Bengalese Finches with intact hearing. Figure 1B shows the hearing threshold curves for (1) normal birds, (2) birds that were treated to induce hair cell loss, showed song degradation, and were tested immediately after treatment [profound hearing loss, no recovery; data from Woolley and Rubel (1999)], (3) birds that were treated and showed no song degradation (less hearing loss), and (4) treated birds that showed song degradation and were allowed to regenerate new hair cells (thresholds recovered). In recovered birds, thresholds at 2 kHz and below were normal by 8–14 weeks after treatment (p > 0.1). Thresholds were still significantly higher than thresholds for normal birds at 3, 4, and 5 kHz (p < 0.01) (Fig.1B). Residual hearing losses at higher frequencies are typically observed in birds with regenerated hair cells (Tucci and Rubel, 1990; Marean et al., 1993; Woolley et al., 2001). For the Bengalese Finch, previous work has shown that only low-frequency auditory feedback is required for maintenance of normal song and therefore may be the most important auditory information for song production. If so, then higher-frequency threshold shifts may have little effect on a bird's ability to shape its own song. Furthermore, in recovered birds, the hearing losses above 2 kHz were 6–11 dB. This magnitude of loss would not be expected to interfere with a bird's ability to hear its own vocalizations because the intensity of song output at the head is loud enough to overcome such shifts and bone conduction would not be compromised. Because it was possible that high-frequency threshold shifts did affect a recovering bird's ability to copy song from a cage mate, we examined the differences between the threshold curves for recovering birds that learned and those that did not learn. There were no threshold differences between these two groups.
A normal Bengalese Finch papilla is characterized by a highly organized array of hair cells covering the luminal surface of the sensory epithelium. Figure 2A shows a scanning EM low-magnification, photomicrographic montage of a normal papilla. Bone surrounds a curvilinear sheet of hair cells. The epithelium progressively widens from the basal, high-frequency end (right) to the apical, low-frequency end (left). The bottom three panels show higher-magnification views of the highly organized hexagonal pattern formed by normal hair cell luminal surfaces and the alignment of stereocilia bundles across neighboring cells. In birds that sang degraded song after treatment, scanning EM analysis showed that original hair cells had been destroyed over nearly the entire basilar papilla and replaced by regenerated hair cells. Figure2B shows a papilla from a representative bird that sang degraded song after treatment and then recovered for 8 weeks after the end of treatment. A low-magnification view of the entire papilla shows that regenerated hair cells cover nearly the entire epithelial surface. On average, 85.1 ± 1.7% (mean ± SEM) of the total papilla area had been damaged, and original hair cells had been replaced by regenerated hair cells (Fig. 1A). The high-magnification panels below the papilla show regenerated hair cells from the apical, mid, and basal regions of the array. Comparison of the high-magnification panels in A and B shows the morphological differences between original and regenerated hair cells. In contrast to normal hair cells, regenerated hair cells are characterized by slightly misshapen luminal surfaces and stereocilia bundles that are not precisely aligned with respect to those on neighboring cells (Girod et al., 1991; Hashino et al., 1992; Cotanche et al., 1994; Woolley et al., 2001). In contrast to the birds that sang degraded song, birds that maintained stable pretreatment-like song despite the same treatment showed damage to only 49 ± 4.7% of the total papilla area (Fig. 1A). In those birds, hair cells in the apical one-third of the papilla appeared normal.
Song
Analysis of the two sets of song recordings made before treatment (4–10 weeks apart) showed that syllable order was stereotyped and stable over time. Song bouts began with introductory notes and were followed by repeating motifs. The number of motifs per bout ranged between 2 and 19, with an average of 5 motifs. Syllable sequence stereotypy scores averaged 0.80 ± 0.02 (mean ± SEM) and 0.81 ± 0.02 for the first and second pretreatment recordings, respectively (see Fig. 4A). Syllable structure was also stable over time. Individual iterations of syllable types (e.g., 1, 2, and 3) and note types (e.g., A, B, and C) were consistent in structure across pretreatment recordings made 4–10 weeks apart. The average acoustic percent similarity (comparing pitch, frequency modulation, spectral continuity, and Wiener entropy) between different iterations of a particular syllable type from the first and second pretreatment song recordings was 84.1 ± 0.9% (see Fig.4B).
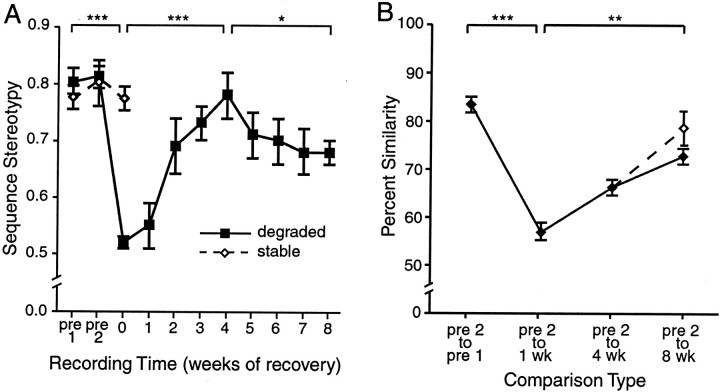
Fig. 4.
In all deafened birds, syllable order degraded and then recovered to match the original song by 4 weeks after treatment. Syllable structure recovered more slowly and less completely. A, Sequence stereotypy scores for the eight birds that sang degraded song after treatment (filled symbols) and the seven birds that maintained low-frequency hearing and sang stable song that matched the pretreatment song after treatment (open symbols). Scores for birds singing degraded song after hair cell loss decreased significantly by 1 d after treatment. By 4 weeks after treatment, scores had increased significantly and were no longer significantly different from scores for recordings made before treatment. Between 4 and 8 weeks after treatment, scores decreased again. B, Average percent similarity between pretreatment syllables and between pretreatment syllables and syllables recorded after treatment show that syllables significantly degraded by 1 week after treatment. Percent similarity between syllables from pretreatment recordings and recordings made 8 weeks after recovery was improved significantly. Theopen symbol and dashed line indicate percent similarity between pretreatment syllables and syllables recorded at 8 weeks of recovery with modified/new syllables removed (see Results). For A, numbersbelow the x-axis indicate the number of weeks after treatment. Pre 1 indicates the first pretreatment recording, and pre 2 indicates the second pretreatment recording. For B, 1 wk indicates 1 week after treatment. Error bars represent ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001.
Effects of hair cell loss on song
The aminoglycoside and sound exposure treatment used in this study have been shown to destroy hair cells throughout nearly the entire hearing organ (basilar papilla) and cause a profound hearing loss in 50% of the subjects (Woolley and Rubel, 1999). After 1 week of this treatment, song was recorded from each bird. At this time, 1 d, 0 weeks recovery time (8 d after the initiation of treatment), 8 of the 15 birds were singing degraded song (Fig.3, compare A, B). Syllables were stuttered and sung in atypical sequences. In these birds, sequence stereotypy scores were calculated for songs recorded 1 d and 1–8 weeks after the end of the deafening treatment and compared with scores for the two stable pretreatment song recordings. A total of 7895 syllables were analyzed. Syllable sequence stereotypy changed significantly over time (F(10,77) = 7.148; p< 0.001). The stereotypy of syllable sequences recorded 1 d after treatment was significantly decreased (0.52 ± 0.01) compared with stereotypy before treatment (0.81 ± 0.02; p < 0.001) (Fig. 4A).
The structure of syllables also changed after inducing hair cell loss throughout the papilla (Figs. 3, 4B,5). Syllables exhibited frequency modulations, spectral spread, and variability in duration that were not typical of pretreatment syllables (Figs. 3, 5). We calculated the percent similarity of three randomly selected examples of each syllable type between the second pretreatment song recordings and the other recordings made before treatment and those made after 1, 4, and 8 weeks of hair cell regeneration. A total of 1478 syllable pairs were analyzed. Quantification of the similarity between syllables from pretreatment song recordings and the same syllables sung after treatment showed that syllables changed significantly over time (F(3,28) = 10.4; p < 0.001). Syllable structure had changed significantly by 1 week after treatment (Fig. 4B). The overall percent similarity between pretreatment and post-treatment syllables was significantly decreased to a mean of 57.0 ± 1.9% from a mean of 84.1 ± 0.9% by 1 week after the end of treatment (p < 0.001). There was considerable variability in the amount of structural change among syllables, even within a bird. Although 83% of the total number of syllables became severely degraded (Fig. 3, syllable 5), 17% of syllables did not appear to change markedly after treatment (Fig. 3, syllable 3).
Fig. 5.
Most degraded syllables returned to the structure of original syllables. By 8 weeks, syllable structure was more similar to pretreatment song than to song recorded earlier in recovery.A, Spectrographs of the first four iterations of the same syllable type taken from one randomly selected song bout in pretreatment records. The variability of several iterations of the same syllable type in normal song can be seen. B, Spectrographs of the first four iterations of the same syllable type from the same bird as in A taken from one randomly selected song bout recorded after 3 weeks of recovery.C, The first four iterations of the same syllable type from the same bird as in A and B taken from one randomly selected bout recorded after 8 weeks of recovery.A and C are more similar thanB is to either A or C. These syllables were taken from the song of a bird that did not learn new song elements. Notes are labeled with letters below each syllable.
The remaining seven treated birds maintained normal, pretreatment-like song despite treatment. In contrast to the eight birds showing degraded song after treatment, these birds' songs showed normal syllable order and structure. Sequence stereotypy scores for these birds' songs were not significantly different before and after treatment (F(2,12) = 3.33; p > 0.05). Scores were 0.78 ± 0.02 and 0.80 ± 0.04 for the two sets of pretreatment recordings and 0.78 ± 0.02 after treatment (Fig. 4A). Scanning EM analysis and ABR recordings showed that these birds maintained apical hair cells (Fig.1A) and low-frequency hearing (Fig.1B) despite treatment. This difference between birds with degraded song and birds that maintained stable song after the same treatment confirms previous work showing that birds retaining original hair cells in the apical (low frequency) one-third of the basilar papilla and residual low-frequency hearing after this treatment maintain stable song that matches pretreatment song (Woolley and Rubel, 1999). These birds' songs were not studied further.
Song recovery during hair cell regeneration
In all recovering birds, the ordering of song syllables gradually returned toward the ordering of syllables in the original, pretreatment song. The instances of syllable stuttering that were commonly observed immediately after treatment were less frequent as recovery time increased. This recovery of the original syllable order and the increase in order stereotypy occurred between 1 d and 4 weeks after treatment. Figure 3 shows one bird's normal song (A), the song of the same bird immediately after treatment (B), and the same bird's song again after 4 weeks of recovery from treatment (C). The normal song and the song recorded immediately after treatment are dissimilar in syllable order and structure. The same bird's song recorded after 4 weeks of recovery matches the syllable ordering of the pretreatment song. Quantitative analysis of changes in syllable sequence stereotypy showed that by 4 weeks after treatment, sequence stereotypy in recovering birds' songs was the same as the sequence stereotypy in pretreatment recordings (Fig. 4A). At this time, stereotypy scores had recovered to normal levels (0.78 ± 0.04). Scores for song bouts recorded after 4 weeks of recovery were significantly higher than those for recordings made at 0 weeks of recovery (0.52 ± 0.01; p < 0.001) and were no longer significantly different from pretreatment scores (0.81 ± 0.02; p > 0.1).
Although syllable ordering returned to match that of pretreatment songs by 4 weeks after treatment, syllable structure returned toward that of pretreatment song more slowly. Syllable structure was severely degraded after 1 week of recovery. Mean percent similarity scores between syllables recorded 1 week after treatment and their respective pretreatment versions were significantly decreased to 57.0 ± 1.9% from a mean percent similarity of 84.1 ± 0.9% between pretreatment recordings (p < 0.001) (Fig.4B). By 4 weeks after treatment, syllable structure had significantly improved toward that of pretreatment song (Figs. 3,4B). Percent similarity scores were significantly improved to a mean of 66.7 ± 1.8% between recovering and pretreatment syllables (p < 0.05). This percent similarity, however, was still significantly lower than pretreatment percent similarity (p < 0.05) (Fig.4B). This persistence of syllable structure degradation was in contrast to the recovery of syllable order, which appeared complete by 4 weeks (Fig. 4A).
By 8 weeks after treatment, most syllables appeared to have recovered to their original structures. Notes composing syllables were less noisy than they had been earlier in recovery, and the fine structure within a note was evident again. The note sequence within a syllable had become more stereotyped and matched well with the pretreatment song. The number of note repetitions within a syllable returned to match that of the pretreatment iterations. Figure 5 shows the recovery of normal, pretreatment-like syllable morphology. Four iterations of the same syllable type from a pretreatment song bout are shown in A. Figure 5B shows four iterations of the same syllable from a song bout recorded 3 weeks after treatment. Figure 5C shows four iterations of that same syllable again, but recorded after 8 weeks of recovery. The structures of syllables recorded after 3 weeks of recovery appear degraded, whereas the structure of the same syllables recorded after 8 weeks of recovery match better with the pretreatment structure. Overall, the mean percent similarity between pretreatment and recovered syllables recorded after 8 weeks of recovery (73.1 ± 1.4%) was significantly higher than the percent similarity between pretreatment syllables and syllables recorded only 1 week after treatment (57.0 ± 1.9%; p < 0.01) and the syllables recorded after 4 weeks of recovery (66.7 ± 1.8%;p < 0.05). Mean scores at 8 weeks were still significantly lower (p < 0.05), however, than the 84.1 ± 0.9% similarity between pretreatment syllables.
Some exceptions to the general pattern of syllables returning to their original structures were evident starting ∼5 weeks after treatment. Some syllables in three of the eight birds' songs were dismantled, and the notes from those syllables were aberrantly (i.e., unlike pretreatment song) placed within a motif. In addition to changes in the placement of these notes, the acoustic structure of such notes changed over time. These modifications to song occurred gradually and are described in detail below. The mean percent similarity between pretreatment syllables and syllables sung 8 weeks after treatment with those exceptional syllables/notes removed was 77.4 ± 3.6% (Fig.4B, dashed line and open symbol). This average was close to but still significantly different from the pretreatment similarities (p< 0.05). In the two birds that recovered for 14 weeks, syllables did not appear to change between 8 and 14 weeks after treatment.
Between 5 and 8 weeks after treatment, songs were occasionally sung in shortened sequences, with middle syllables omitted. These shortened syllable sequences were not typical of normal songs and therefore contributed to a decrease in stereotypy scores between 4 and 8 weeks after treatment (Fig. 4A).
Modifications to song after initial song recovery
After the full recovery of pretreatment-like syllable order and partial recovery of syllable structure in eight of eight birds with regenerating hair cells, three of those eight birds began to modify their songs away from the pretreatment-like composition. In these birds, like the other five, the recovery of songs during the first 4 weeks after treatment progressively approximated the bird's original song, but during the subsequent 4–8 weeks, significant deviations occurred. In these three birds, 52% of syllables changed markedly after songs had recovered toward their original compositions (Figs. 6,7, 8,9). The remaining syllables (48% of the total syllables) in each bird's song remained intact and continued to recover toward a match with the original song. Three of six syllables were modified in one bird's song (Figs. 6, 7), and two of five were altered in another bird's song. A third bird modified four of six syllables (Fig. 9). One of these birds was from the group in which three recovering birds were housed together. The other two birds were from the group in which an individual recovering bird was housed in isolation with one untreated male. Therefore, birds recovering in both social housing conditions showed these additional song modifications.
Fig. 6.
Some syllables were gradually altered away from their original structure between 4 and 8 weeks of recovery.A, Spectrograph of the last three syllables and their corresponding notes in a bird's song, showing the original pretreatment order and structure of both notes and syllables.B, Spectrograph of the same notes and syllables as inA but recorded after 4 weeks of recovery.C, The same notes as in A andB but recorded after 6 weeks of recovery. A note from the sixth syllable (S) appears structurally modified (mS) and is repeated several times. A modified version (mK) of the first note of the fourth syllable (K) is placed at the end of the motif.D, The same syllables/notes as inA–C recorded 8 weeks after treatment. Notes K and L are deleted. The modified notes (mK and mS) appear at the end of the motif, and mS is repeated five times. Syllables are labeled with numbers, and notes are labeled withletters below the x-axis. Thelowercase letter m indicates a modified version of a pretreatment note (e.g., mS designates a modified version the note S).
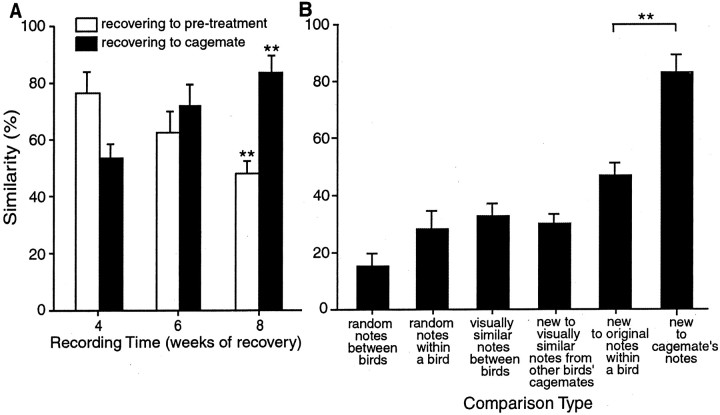
Fig. 8.
Individual notes were compared in terms of pitch, frequency modulation, spectral continuity, and Wiener entropy to calculate the structural similarity between notes. A, Percent similarity scores between modified notes and their original versions (open bars) decreased significantly (p < 0.01) between 4 and 8 weeks after treatment. Percent similarity between modified notes and the cage mate's notes (filled bars) increased significantly (p < 0.01) between 4 and 8 weeks of recovery. Similarities are higher at 4 weeks than would be expected on the basis of data shown in Figure 4 because notes deleted from syllables during song modification could not be included in this analysis. B, By 8 weeks after treatment, modified notes were more similar to a cage mate's notes than to a bird's own original notes. The mean percent similarity scores between recovered birds modified notes and their cage mate's notes (sixth bar from left) was significantly higher than the percent similarity between those same modified notes and their original version in the pretreatment song (fifth bar). These scores were higher than four control analyses: the mean percent similarity between modified notes and notes from other birds' cage mates' songs that were visually similar (fourth bar); percent similarity of visually similar notes between birds (third bar); randomly selected notes within a bird (second bar); and randomly selected notes between birds (first bar). Error bars represent ± SEM; **p< 0.01.
Fig. 9.
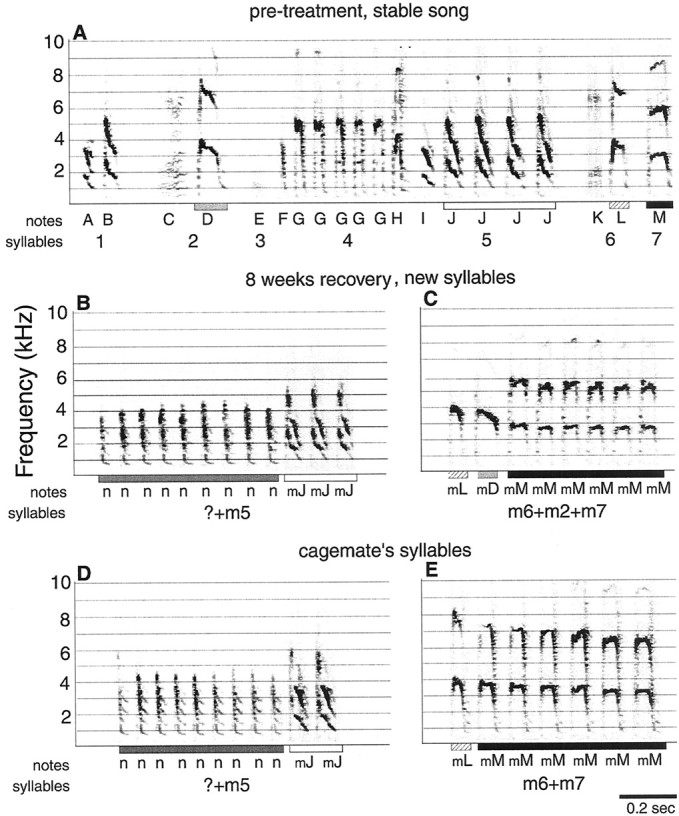
New notes were arranged into new syllables that matched a cage mate's syllable. A, Spectrograph of one motif recorded before treatment. B, One syllable (composed of notes n and mJ) from the same bird's song recorded 8 weeks after treatment. Note that the first repeated note (n) does not match any note from the original song, but the second repeated note (mJ) matches a note sequence in the originalsyllable 5. C, Another example from the same bird. The notes (mL, mD, andmM) are similar to notes (L,D, and M) from three different syllables in the original song. One note (mM) is repeated six times. D, Syllable from the cage mate's song that matches the syllable in B. E, Syllable from the cage mate's song that matches the syllable inC. Bars below the x-axis indicate the similar elements among A, B,C, D, and E. Syllables are labeled with numbers, and notes are labeled withletters below the axis. Pretreatment and pretreatment-like notes are labeled with uppercase letters. A note for which the pretreatment match was undetermined (n) is labeled with alowercase letter. The lowercase letter mindicates a modified version of a pretreatment note. InD and E, numbers andletters indicate which syllables and notes from the recovered bird's song match the cage mate's syllables and notes shown.
The time sequence and patterns of syllable and note modifications were similar in the three birds. Seven of the nine total syllables that were eventually modified began to change by 5 weeks. Slight modifications were evident in two of nine syllables by 4 weeks after treatment (Fig.6B, note S). In all three birds, syllables were gradually altered over several weeks in five ways: (1) original syllables were broken into parts, separating notes that normally formed a unit; (2) some notes from those syllables were dropped; (3) the structures of remaining notes from those syllables were modified away from their original morphology; (4) modified notes were repeated and/or combined to form new syllables; and (5) modified syllables were sung at the beginning or end of the song motif, thereby creating a new sequence of syllables (Figs. 6, 7).
Figure 6 shows the gradual modification of the last three syllables from one bird's song. These changes occurred between 4 and 8 weeks after treatment. A complete motif from this same bird is shown in Figure 7A. Figure 6 shows the dismantling of recovered syllables, deletion of some notes, structural changes in other notes, repetition of one note type, and placement of the modified notes/syllables at the end of the motif. At 4 weeks after treatment (Fig. 6B), the last three syllables of this bird's song have recovered to be similar to their pretreatment versions (A), except for some structural differences in the last syllable composed of notes R, S, and T. Figure 6C shows those same syllables recorded 6 weeks after treatment. The structures of notes S and T are modified (mS, mT). The modified version of note S (mS) is repeated four times after the syllable that had been the last syllable in the motif. A modified version of note K (mK) is placed at the end of the motif. Figure 6D shows the same syllables recorded after 8 weeks. Note K is missing from its original location. The mS notes are spread out in time, and mK remains at the end of the motif. By examining the spectrographs of weekly song recordings, gradual note/syllable modifications were tracked over time such that the original, pretreatment versions of even highly modified notes could be identified. For all modified notes but one (Fig. 9,note n), we were able to determine which original notes had been modified to result in the unique notes/syllables recorded 8 weeks after treatment. These changes in the notes composing syllables and their placement in a motif contributed to the decrease in sequence stereotypy scores seen in Figure 4A between 4 and 8 weeks after treatment because they were not typical of normal, pretreatment songs.
Matching song between recovered birds and their cage mates
For each bird, the song changes that occurred between 4 and 8 weeks after treatment resulted in the production of modified/new notes and syllables that visually and quantitatively matched the syllables of its cage mate's song (Figs. 7, 8, 9,10). All modified/new notes and syllables across all three birds (nine syllables total) matched specific notes and syllables in the cage mate's song. The other notes in each recovering bird's song were not modified in that they recovered to match their original versions and did not change further. Therefore, no spontaneous (i.e., without a match in the cage mate's song) changes in song were evident in our recordings. One of the new notes, however, did not appear to match any notes in that bird's original song (Fig. 9, note n). This could be an example of the generation of a novel note that matches a cage mate note but was not modified from an original note. Because this was observed in only one case, it is noted anecdotally but does not appear to be a common process by which new syllables are generated in these adult birds.
Fig. 10.
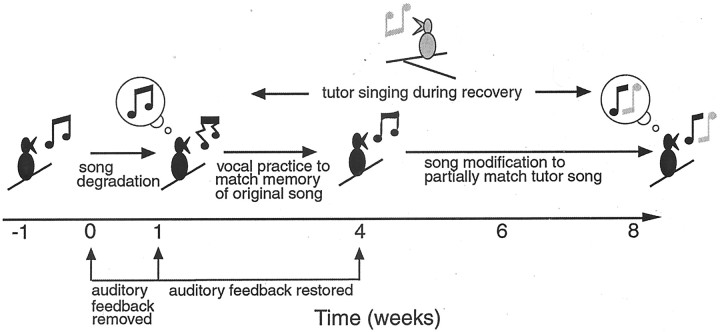
A working model showing the proposed sequence of changes in song, changes in auditory feedback, and effects of exposure to another singing bird over the course of hair cell loss and regeneration. The model begins with a bird (black) singing stable song. That bird is treated to cause hearing loss for 1 week, and song is degraded by the end of that week, indicated by thecrooked musical note. Our findings suggest that the bird stores a memory of its original song, indicated by the musical note in the bubble. As auditory feedback is restored during hair cell regeneration, the bird goes through a period of sensorimotor integration in which vocal output matches the song memory more closely over time such that the recovering song matches well with the original song by 4 weeks after the end of treatment. This is indicated by the musical notethat is similar but not identical to the original note. If a tutor bird (gray) is singing during the recovery of the treated bird (black), the recovering bird's song may be modified to partially match the tutor's song once auditory feedback is maximally restored. This is indicated by the combination of parts of the original musical note (black) and the tutor's note (gray) in the bubble and to theright of the recovered bird at 8 weeks of recovery.
Examination of the spectrographs for songs recorded between 4 and 8 weeks of recovery suggested that the syllables that were dismantled into smaller units and modified were those in which the structure of some notes already shared some similarity with the notes of a cage mate's syllables. Notes that did not resemble a cage mate's note(s) were often dropped, whereas those notes that shared at least some similarity with a cage mate's notes were retained and moved to the end or beginning of the song. Notes were also structurally modified and arranged with other notes or repeated such that the final resulting syllable matched a cage mate's syllable (Figs. 7, 9). Therefore, it appeared that some level of similarity between the treated bird's syllables and its cage mate's syllables facilitated the modifying of those recovering syllables until they were highly similar to the cage mate's note and no longer highly similar to their original structures. This preexisting similarity between some recovering notes and the cage mate's notes can be seen quantitatively by comparing the similarity between recovering birds' notes and their cage mates' notes that were eventually copied (Fig. 8A, first black bar) with the similarity of randomly selected notes between birds (Fig. 8B, first bar). The difference between those two bars indicates that the notes that were eventually copied were more similar to cage mate notes than randomly chosen notes between those same birds.
Quantitative analyses supported the observation that the similarity between modified/new notes from the recovered birds' songs and the notes from their cage mates' songs that were judged to have been copied was higher than the similarities in the control analyses. Figure 8A shows the decrease in similarity between notes under modification and their original versions between 4 and 8 weeks (open bars). The qualitative example of this effect is shown in Figure 6 (see above). The simultaneous increase in percent similarity between notes under modification and the cage mate's notes (black bars) is also shown. Figure 8Bshows the mean percent similarity scores between modified/new notes and their pretreatment versions. This percent similarity is significantly lower than the percent similarity scores between those same new notes and the cage mate's notes (p < 0.01). Four control analyses showed that the high similarities between new notes and cage mate notes was not caused by general acoustic similarities among notes and syllables in Bengalese Finch song (Fig.8B). Percent similarity among randomly selected song notes between birds was only 14.3 ± 5.0%. Randomly selected notes within a bird scored 32.6 ± 6.2% similarity. Visually similar notes between birds showed 36.8 ± 4.7% similarity. Finally, the visually similar notes between recovered birds' songs and those of the other birds' cage mates (i.e., tutors that could not have tutored that recovering bird) scored 34.5 ± 3.5% similarity. All of these percent similarity scores were significantly lower than the percent similarity scores between cage mates' notes that appeared to have been copied and the modified/new notes from the recovered birds' songs (84 ± 5.1%).
Effects of treatment on testosterone levels
In a separate group of 12 birds, we tested the effects of the treatment inducing hair cell loss on circulating levels of T. This was done because the stability of developing song in juvenile birds can be manipulated by changing plasma T levels (for review, seeBottjer and Johnson, 1997). Testosterone levels in six treated birds and six untreated controls were not significantly different. Measured T levels were 1.94 ± 0.4 ng/ml (mean ± SEM) in birds with hair cell loss and 1.95 ± 0.3 ng/ml in controls.
DISCUSSION
The induction of extensive auditory hair cell loss and regeneration in adult Bengalese Finches resulted in song degradation during hearing loss followed by an initial stage of song recovery in which the temporal organization (syllable ordering) within songs recovered to match the pretreatment song and the spectral content (syllable structure) within songs recovered toward a match with pretreatment song. This reconstruction of pretreatment-like song coincided with the restoration of hearing, which we estimate to occur over the first 4 weeks of hair cell regeneration (see below). In some (three of eight) birds, the initial song recovery was followed by a second stage of song changes in which significant temporal and spectral modifications were made such that the songs no longer matched their pretreatment versions. Birds dismantled recovered syllables, dropped notes from those syllables, altered the acoustic structure and number of repeats of the remaining notes, and rearranged those notes into new syllables. In each case, the new syllables or sequences of notes that resulted from these changes matched the existing syllables of a cage mate's song. Analysis of these changes in song during hair cell regeneration revealed two main findings with respect to the plasticity of the song information stored in the adult brain. First, the recovery of songs to match pretreatment songs suggests that adult birds store precise memories of their own songs that can be used to reshape vocal output once auditory feedback has been restored. Second, the subsequent song modifications that lead to matches with the songs of other adult birds to which recovering birds were exposed suggest that adult birds can be induced to copy song elements from other birds.
Hearing recovery and song changes over time
Figure 10 shows a working model of the potential correspondence between hearing loss and restoration, changes in song, and the effects of tutoring on song. This model is based on the findings of this study and previous work on Bengalese Finches with hair cell loss. The model shows profound hearing loss immediately after 1 week of treatment to kill hair cells. This effect is well documented in previous studies and is accompanied by the degradation of song structure (Woolley and Rubel, 1999). The model shows the restoration of hearing thresholds (i.e., auditory feedback) occurring during the first 4 weeks of recovery. This is an estimate based on a previous study examining the time courses of hearing restoration and hair cell regeneration after hair cell loss (Woolley et al., 2001). In that study, hearing restoration was complete by 4 weeks after treatment. However, the birds in that study had less hair cell loss than birds in this report. It is possible that the amount of hair cell loss affects the time course of hearing restoration. Therefore, the restoration of hearing thresholds occurring over the 4 weeks after treatment is an estimate here.
The time course of song recovery and, later, learning corresponds remarkably well with the estimated time course of hearing recovery. As the model shows, a bird's song progressively matches the prehearing loss song over the first 4 weeks after treatment. This would indicate that birds reconstruct their songs as auditory feedback becomes available, without delay. The modification of those recovered notes and syllables during exposure to a tutor appears to begin after 4 weeks of recovery, potentially at the time that hearing thresholds have reached plateau at normal (for low frequencies) or near normal (for higher frequencies) levels. This timing would suggest that copying song notes from other birds requires that hearing be restored to a stable condition (i.e., beyond a dynamic state of continual improvement). Another issue to consider when comparing hearing recovery and song recovery is that syllable structure did not recover perfectly. The residual hearing loss at frequencies >2 kHz that was observed in recovered birds may have hindered recovery of the fine acoustic structure found in individual notes.
Song memory
The formation and storage of song memory in juveniles has been clearly demonstrated in several songbird species (for review, seeMarler, 1997). The initial song recovery in this study was reminiscent of the sensory-motor integration seen during song development. Song behavior was shaped over time to (presumably) match a stored song model through the use of auditory feedback. These results demonstrate a memory of song that persists in the adult brain and can be maintained in the absence of auditory input. It is therefore possible that during normal adult singing, the song memory is used as a reference to which ongoing vocal output is continually matched using auditory feedback. This is a potential mechanism whereby song stability and stereotypy could be maintained normally. Vocalizations could be monitored continually via auditory feedback and compared with the stored song model. On the basis of this comparison, minor adjustments could be made to subsequent song output such that the result is a production of stable song over time.
The nature of the song memory and the song control nuclei participating in its storage could not be addressed by this study but are important issues to be investigated. It remains to be determined whether the neural mechanisms representing a stored song model are purely sensory (i.e., fully independent of motor commands), purely motor (i.e., a set of motor commands upstream of sensory feedback), or requiring interaction between sensory and motor structures similar to an efference copy model. The behavioral evidence for memory formation during song learning indicates that the juvenile song template is a sensory memory; the information appears to be encoded before birds develop song (Marler and Tamura, 1964; Konishi, 1965). The sensory memory assembled during song learning could persist as the adult song model against which sensory feedback of motor output is compared. Alternatively, it is possible that a sensory memory of song is translated into stored motor commands as the bird's own vocal behavior stabilizes at sexual maturity. Auditory feedback could then be used to compare ongoing modifiable vocal output with an upstream vocal motor program.
Song learning
We use the term “learning” here to mean the generation of new song behavior based on imitating the song of another bird. We do not consider self-imitation, as in the case of song recovery, to be new learning because it is possible that sensory-motor integration is ongoing throughout adulthood and could be a normal mechanism of stable song maintenance. The development of new song material that matched a cage mate's song suggests that processes somewhat like sensory acquisition and sensory-motor integration that normally occur only in juveniles were taking place in adult birds. Similar to song development, structured notes and syllables emerged from unstructured vocalizations under the influence of the vocalizations of other birds. Although the development of song from begging calls and immature vocalizations in young birds and development of new adult song by dismantling and reshaping existing songs are different, it is possible that the same neural circuitry is involved in both processes.
The production of copied song material was seen in three of eight birds with regenerating hair cells. The novelty of this finding suggests that adult song learning in “age-limited” song learners such as Bengalese Finches does not occur normally. Therefore, this effect does not tell us about how adult songbirds normally behave. Instead, this finding suggests that the as yet unidentified neural circuits required for song learning may be retained in the adult brain. This knowledge may then help in the characterization of the specific circuitry responsible for song learning. More work including studies using different methods to remove auditory feedback, different songbird species, and additional experimental groups should be done. For example, this study did not determine what plastic changes to adult song occur when birds recover from song degradation in complete isolation from other birds.
There are several practical possibilities as to why only some of the birds in this study learned new song material. First, birds that did not learn could have had poor hearing recovery attributable to incomplete hair cell regeneration. Our measurements of hearing recovery and hair cell regeneration indicate that all birds had similar auditory thresholds and complete hair cell regeneration. Threshold shifts >2 kHz were observed in the birds with regenerated hair cells. Previous studies, however, have shown that low-frequency (<2 kHz) auditory feedback is particularly important for song production in Bengalese Finches (Woolley and Rubel, 1999). Therefore, the threshold shifts were at higher frequencies than the frequencies that are most critical for song. Additionally, there were no differences between the threshold curves for birds that learned and for birds that did not learn. Therefore, it is unlikely that learning was prevented by the threshold shifts in recovering birds. Second, the motivation and opportunity to learn song from cage mates was likely different among recovering birds. Song learning in juveniles is greatly affected by the availability of a song model as well as the social dominance and social interactions between tutor and pupil (Marler and Tamura, 1964; Price, 1979; Marler and Peters, 1982; Eales, 1985, 1989; Clayton, 1987, 1988, 1989). These factors may have influenced learning differently for each bird in this study. For example, we observed that some cage mates of birds that did not learn sang less often than the cage mates of birds that did learn [although see Tchernichovski et al. (1999)]. Third, we observed that song learning did not appear to occur randomly. Instead, all but one of the modified/new notes that were copied from cage mates' songs had original versions that already shared at least some similarity in acoustic structure (but not repetition number or location) with song notes in the cage mates' songs. Therefore, it is possible that if very little acoustic similarity exists between the songs of the tutor and adult pupil, then no song copying occurs.
Implications for adult neural plasticity
Because vocal learning can be induced in adult birds formerly singing stable songs, it seems possible that the adult brain can reinstate a period of learning typically seen only during a critical developmental period. Reinstating the ability to learn song suggests that the basic neural circuits required for vocal learning are retained in the adult Bengalese Finch brain. Although it is unlikely that the degree of neural plasticity exhibited by the adult birds approaches the plasticity seen in the juvenile brain, circuits responsible for encoding and producing new song material appear to be present. Whether the activity of such circuitry was unmasked by destabilizing a normally stable motor pattern (by removing auditory feedback) or whether giving a bird a new population of auditory hair cells stimulated a level of neural plasticity or neurogenesis that normally is not present in the adult brain is unknown. It is possible that adult learning can be induced simply by destabilizing the motor circuitry controlling song.Eales (1985) and Clayton (1987) showed that Zebra and Bengalese Finches could learn song at ages beyond the normal critical period for song acquisition if development of stable song was prevented. These results suggest that instability of song output may be correlated with the ability to learn new song. Leonardo and Konishi (1999) showed that song degradation in adult Zebra Finches could be induced by presenting delayed auditory feedback overlays to singing Zebra Finches. Using this approach, the possibility of inducing song learning by destabilizing the song motor output but not disrupting auditory structures could be tested. Alternatively, it is possible that regeneration of peripheral sensory cells stimulates proliferative events or metabolic changes in the central auditory regions or regions that are regulated by auditory input (i.e., song control nuclei). It is known that hair cell loss and regeneration are accompanied by functional and structural changes in cochlear nucleus neurons (Lippe, 1991; Cohen and Saunders, 1994; Salvi et al., 1994; Chen et al., 1996; Durham et al., 2000). Such loss and recovery may involve molecular signaling mechanisms that promote plasticity in other neural circuits. The effects of hair cell regeneration on higher order auditory regions and forebrain structures involved in song production remain to be investigated.
Footnotes
This work was supported by National Institutes of Health Grants DC02854, DC03829, and GM07108. We thank M. Konishi for original discussions and continued interest in this work, O. Tchernichovski for assistance with data analysis, K. Lent for assistance with hormone assays, and E. Brenowitz, D. Perkel, E. Stevens, and F. Theunissen for their comments on earlier versions of this manuscript.
Correspondence should be addressed to Edwin W Rubel, Virginia Merrill Bloedel Hearing Research Center, Box 357923, University of Washington, Seattle, WA 98195. E-mail: rubel@u.washington.edu.
S. M. N. Woolley's present address: Psychology Department, University of California, Berkeley, Berkeley, CA 94720.
REFERENCES
- 1.Beaubien AR, Karpinski K, Ormsby E. Toxicodynamics and toxicokinetics of Amikacin in the guinea pig cochlea. Hear Res. 1995;83:62–79. doi: 10.1016/0378-5955(94)00192-s. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC, Ryan AF. Audiometric and histologic correlates of the interaction between kanamycin and subtraumatic levels of noise in the chinchilla. Otolaryngology. 1978;86:400–404. doi: 10.1177/019459987808600305. [DOI] [PubMed] [Google Scholar]
- 3.Bottjer SW, Johnson F. Circuits, hormones and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Brainard M, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 5.Brummett RE, Fox KE, Jacobs F, Kempton JB, Stokes Z, Richmond AB. Augmented gentamicin ototoxicity induced by vancomycin in guinea pigs. Arch Otolaryngol Head Neck Surg. 1990;116:61–64. doi: 10.1001/archotol.1990.01870010065019. [DOI] [PubMed] [Google Scholar]
- 6.Brummett RE, Fox KE, Kempton JB. Quantitative relationships of the interaction between sound and kanamycin. Arch Otolaryngol Head Neck Surg. 1992;118:498–500. doi: 10.1001/archotol.1992.01880050044011. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Trautwein PG, Shero M, Salvi RJ. Tuning, spontaneous activity and tonotopic map in chicken cochlear ganglion neurons following sound-induced hair cell loss and regeneration. Hear Res. 1996;98:152–164. doi: 10.1016/0378-5955(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 8.Clayton NS. Song learning in Bengalese Finches: a comparison with Zebra Finches. Ethology. 1987;76:247–255. [Google Scholar]
- 9.Clayton NS. Song tutor choice in Zebra Finches and Bengalese Finches: the relative importance of visual and vocal cues. Behavior. 1988;104:281–299. [Google Scholar]
- 10.Clayton NS. The effects of cross-fostering on selective song learning in estrildid Finches. Behavior. 1989;109:163–175. [Google Scholar]
- 11.Cohen YE, Saunders JC. The effect of acoustic overexposure on the tonotopic organization of the nucleus magnocellularis. Hear Res. 1994;81:11–21. doi: 10.1016/0378-5955(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 12.Collins PW. Synergistic interactions of gentamicin and pure tones causing cochlear hair cell loss in pigmented guinea pigs. Hear Res. 1988;36:249–259. doi: 10.1016/0378-5955(88)90066-4. [DOI] [PubMed] [Google Scholar]
- 13.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 14.Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181–195. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- 15.Cotanche DA, Lee KH, Stone JS, Picard DA. Hair cell regeneration in the bird cochlea following noise damage or ototoxic drug damage. Anat Embryol. 1994;189:1–18. doi: 10.1007/BF00193125. [DOI] [PubMed] [Google Scholar]
- 16.Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich K. Model choice in the song development of young male Bengalese Finches. Z Tierpsychol. 1980;52:57–76. [Google Scholar]
- 18.Duckert LG, Rubel EW. Ultrastructural observations on regenerating hair cells in the chick basilar papilla. Hear Res. 1990;48:161–182. doi: 10.1016/0378-5955(90)90206-5. [DOI] [PubMed] [Google Scholar]
- 19.Duckert LG, Rubel EW. Morphological correlates of functional recovery in the chicken inner ear after gentamycin treatment. J Comp Neurol. 1993;331:75–96. doi: 10.1002/cne.903310105. [DOI] [PubMed] [Google Scholar]
- 20.Durham D, Park DL, Girod DA. Central nervous system plasticity during hair cell loss and regeneration. Hear Res. 2000;147:145–159. doi: 10.1016/s0378-5955(00)00128-3. [DOI] [PubMed] [Google Scholar]
- 21.Eales LA. Song learning in Zebra Finches: some effects of song model availability on what is learnt and when. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 22.Eales LA. The influences of visual and vocal interaction on song learning in Zebra Finches. Anim Behav. 1989;37:507–508. [Google Scholar]
- 23.Girod DA, Tucci DL, Rubel EW. Anatomical correlates of functional recovery in the avian inner ear following aminoglycoside ototoxicity. Laryngoscope. 1991;101:1139–1149. doi: 10.1288/00005537-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Hashino E, Tanaka Y, Salvi RJ, Sokabe M. Hair cell regeneration in the adult budgerigar after kanamycin ototoxicity. Hear Res. 1992;59:46–58. doi: 10.1016/0378-5955(92)90101-r. [DOI] [PubMed] [Google Scholar]
- 25.Immelmann K. Song development in the Zebra Finch and other estrildid Finches. In: Hinde RA, editor. Bird vocalizations: their relations to current problems in biology and psychology: essays presented by W. H. Thorpe. Cambridge UP; London: 1969. pp. 61–74. [Google Scholar]
- 26.Kitasato I, Yokota M, Inouye S, Igarashi M. Comparative ototoxicity of ribostamycin, doactimicin, kanamycin, amikacin, tobramycin, gentamicin, sisomicin and netilmicin in the inner ear of guinea pigs. Chemotherapy. 1990;36:155–168. doi: 10.1159/000238762. [DOI] [PubMed] [Google Scholar]
- 27.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z. Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 28.Lenoir M, Puel JL. Dose-dependent changes in the rat cochlea following aminoglycoside intoxication. II. Histological study. Hear Res. 1987;26:199–209. doi: 10.1016/0378-5955(87)90112-2. [DOI] [PubMed] [Google Scholar]
- 29.Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 30.Lippe WR. Reduction and recovery of neuronal size in the cochlear nucleus of the chicken following aminoglycoside intoxication. Hear Res. 1991;51:193–202. doi: 10.1016/0378-5955(91)90036-9. [DOI] [PubMed] [Google Scholar]
- 31.Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male Zebra Finches. J Neurosci. 2000;20:5054–5064. doi: 10.1523/JNEUROSCI.20-13-05054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marean GC, Burt JM, Beecher MD, Rubel EW. Hair cell regeneration in the European starling (Sturnus vulgaris): recovery of pure-tone detection thresholds. Hear Res. 1993;71:125–126. doi: 10.1016/0378-5955(93)90028-y. [DOI] [PubMed] [Google Scholar]
- 33.Marean GC, Cunningham D, Burt JM, Beecher MD, Rubel EW. Regenerated hair cells in the European starling: are they more resistant to kanamycin ototoxicity than original hair cells? Hear Res. 1995;82:267–276. doi: 10.1016/0378-5955(94)00183-q. [DOI] [PubMed] [Google Scholar]
- 34.Marler P. Three models of song learning. J Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- 35.Marler P, Peters S. Developmental overproduction and selective attrition: new processes in the epigenesis of birdsong. Dev Psychobiol. 1982;15:369–378. doi: 10.1002/dev.420150409. [DOI] [PubMed] [Google Scholar]
- 36.Marler P, Peters S. A sensitive period for song acquisition in the song sparrow, Melospiza melodia: a case of age-limited learning. Ethology. 1987;76:89–100. [Google Scholar]
- 37.Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in a sparrow. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- 38.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult Zebra Finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 39.Nottebohm F, Konishi M. Experimental studies in the ontogeny of avian vocalizations. In: Hinde RA, editor. Bird vocalizations: their relations to current problems in biology and psychology: essays presented by W. H. Thorpe. Cambridge UP; London: 1969. pp. 29–48. [Google Scholar]
- 40.Okanoya K, Yamaguchi A. Adult Bengalese Finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. J Neurobiol. 1997;33:343–356. [PubMed] [Google Scholar]
- 41.Price PH. Developmental determinants of structure in Zebra Finch song. J Comp Physiol Psychol. 1979;93:268–277. [Google Scholar]
- 42.Pye A, Collins P. Interaction between sound and gentamicin: immediate threshold and stereociliary changes. Hear Res. 1991;25:381–390. doi: 10.3109/03005369109076613. [DOI] [PubMed] [Google Scholar]
- 43.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 44.Salvi RJ, Saunders SS, Hashino E, Chen L. Discharge patterns of chicken cochlear ganglion neurons following kanamycin-induced hair cell loss and regeneration. J Comp Physiol [A] 1994;174:351–369. doi: 10.1007/BF00240217. [DOI] [PubMed] [Google Scholar]
- 45.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the Zebra Finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchernichovski O, Lints T, Mitra P, Nottebohm F. Vocal imitation in Zebra Finches is inversely related to model abundance. Proc Natl Acad Sci USA. 1999;96:12901–12904. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tchernichovski O, Nottebohm F, Ho CH, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 48.Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. J Neurosci. 2000;20:854–861. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tucci DL, Rubel EW. Physiologic status of regenerated hair cells in the avian inner ear following aminoglycoside ototoxicity. Otolaryngol Head Neck Surg. 1990;103:443–450. doi: 10.1177/019459989010300317. [DOI] [PubMed] [Google Scholar]
- 50. Vago P, Humbert G, Lenoir M. Amikacin intoxication induces apoptosis and cell proliferation in rat organ of Corti. NeuroReport 9 1998. 431 436167∴φ ∴ς 12. [DOI] [PubMed] [Google Scholar]
- 51.Woolley SMN, Rubel EW. Bengalese Finches Lonchura striata domestica depend on auditory feedback for the maintenance of adult song. J Neurosci. 1997;17:6380–6390. doi: 10.1523/JNEUROSCI.17-16-06380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolley SMN, Rubel EW. High-frequency auditory feedback is not required for adult song maintenance in Bengalese Finches. J Neurosci. 1999;19:358–371. doi: 10.1523/JNEUROSCI.19-01-00358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolley SMN, Wissmann AM, Rubel EW. Hair cell regeneration and recovery of auditory thresholds following aminoglycoside ototoxicity in Bengalese Finches. Hear Res. 2001;153:181–195. doi: 10.1016/s0378-5955(00)00217-3. [DOI] [PubMed] [Google Scholar]