Abstract
The present study determined whether the serotonin2A (5-HT2A) receptors in the hypothalamic paraventricular nucleus mediate the neuroendocrine responses to a peripheral injection of the 5-HT2A/2Creceptor agonist (−)DOI [(−)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane]. The 5-HT2A receptor antagonist MDL100,907 ((±)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidinemethanol), the 5-HT2C receptor antagonist SB-242084 (6-chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-indoline), or vehicle were microinjected bilaterally through a chronically implanted double-barreled cannula into the hypothalamic paraventricular nucleus 15 min before a peripheral injection of (−)DOI in conscious rats. (−)DOI significantly elevated plasma levels of oxytocin, prolactin, ACTH, corticosterone, and renin. Neither the 5-HT2A receptor antagonist nor the 5-HT2Creceptor antagonist, injected alone, altered the basal levels of these hormones. MDL100,907 (0.748, 7.48, and 18.7 nmol) dose dependently inhibited the (−)DOI-induced increase in all of the hormones except corticosterone. In contrast, SB-242084 (10 nmol) did not inhibit (−)DOI-increased hormone levels. To confirm the presence of 5-HT2A receptors in the hypothalamic paraventricular nucleus, 5-HT2A receptors were mapped using immunohistochemistry. Densely labeled magnocellular neurons were observed throughout the anterior and posterior magnocellular subdivisions of the hypothalamic paraventricular nucleus. Moderately to densely labeled cells were also observed in parvicellular regions. Thus, it is likely that 5-HT2A receptors are present on neuroendocrine cells in the hypothalamic paraventricular nucleus. These data provide the first direct evidence that neuroendocrine responses to a peripheral injection of (−)DOI are predominantly mediated by activation of 5-HT2A receptors in the hypothalamic paraventricular nucleus.
Keywords: serotonin; ACTH; oxytocin; MDL100,907; PVN; prolactin; renin; corticosterone
Serotonin2A/2C(5-HT2A/2C) receptors are involved in the regulation of many physiological functions and are the cellular targets of drugs used to treat psychiatric disorders, such as depression and schizophrenia (Price et al., 1997; Sargent et al., 1998; Blier and de Montigny, 1999; Aghajanian and Marek, 2000). 5-HT2A and 5-HT2C receptors share a large sequence homology and both are coupled via Gq/11-proteins to phosphoinositide signaling cascades (Martin and Humphrey, 1994; Barnes and Sharp, 1999). However, their affinities for serotonin and patterns of distribution in the brain are different (Hoffman and Mezey, 1989; Appel et al., 1990; Zifa and Fillion, 1992). Recent data suggest that their signal transduction mechanisms can be distinguished from each other (Berg et al., 2001).
(±)DOI [(±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane], the most selective 5-HT2A/2C agonist to date, has been used to study 5-HT2A/2C receptor-mediated behavioral and physiological functions (McCall and Harris, 1988; Bagdy et al., 1989; Kozuru et al., 2000). MDL100,907 ((±)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidinemethanol) is a 5-HT2A antagonist (pKi = 9.07) with a much lower affinity for 5-HT2C(pKi = 7.06) and adrenergic α1 receptors (pKi = 6.89) (Kehne et al., 1996). SB-242084 (6-chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl] carbamoyl]-indoline) is a 5-HT2C antagonist (pKi = 9.0) with a considerably lower affinity for other serotonergic and nonserotonergic receptors (5-HT2B, pKi = 7.0; 5-HT2A, pKi= 6.8; α1 adrenergic, pKi < 5.0) (Kennett et al., 1997). These two receptor subtype antagonists can be used to dissect 5-HT2A versus 5-HT2C receptor-mediated effects of (−)DOI.
The hypothalamic paraventricular nucleus plays a crucial role in mediating neuroendocrine responses to serotonergic activation (Rittenhouse et al., 1993, 1994; Bagdy, 1996). The hypothalamic paraventricular nucleus receives serotonergic projections from the raphe nuclei, which also send collaterals to other limbic structures, such as amygdala (Swanson and Sawchenko, 1983; Liposits et al., 1987;Petrov et al., 1994). The hormone responses to serotonergic stimulation reflect serotonergic function in the hypothalamus.
(±)DOI increases the release of oxytocin, prolactin, ACTH, corticosterone, and renin (Van de Kar et al., 1995a; Bagdy, 1996) and also increases c-Fos expression in oxytocin and CRF cells in the hypothalamic paraventricular nucleus (Van de Kar et al., 2001). Both of the effects of (±)DOI (intraperitoneally) can be blocked by the 5-HT2A antagonist MDL100,907 (subcutaneously) (Van de Kar et al., 2001). Whether these effects of (±)DOI result from a direct activation of 5-HT2A receptors in the hypothalamic paraventricular nucleus is not clear.
Autoradiographic and in situ hybridization studies have found moderate densities of 5-HT2A/2C binding sites and 5-HT2A transcripts in the hypothalamic paraventricular nucleus (Appel et al., 1990; Wright et al., 1995;Gundlah et al., 1999). However, because of a limited resolution of these approaches, the detailed cellular distribution of 5-HT2A receptors in the paraventricular nucleus remains unknown.
The present study, using immunohistochemistry, examined the cellular distribution of 5-HT2A receptors in the hypothalamic paraventricular nucleus. Moreover, by microinjecting MDL100,907 or SB-242084 into the paraventricular nucleus, the relative contribution of 5-HT2A versus 5-HT2C receptors in the nucleus to (−)DOI-induced neuroendocrine responses was examined. Our study provides the first evidence that the neuroendocrine responses to (−)DOI results from direct activation of 5-HT2Areceptors in the hypothalamic paraventricular nucleus.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (225–275 gm) were purchased from Harlan Sprague Dawley (Indianapolis, IN). Before surgery, the rats were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on from 7:00 A.M. to 7:00 P.M.). After surgery, the rats were housed one per cage in the same room. Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by Loyola University Institutional Animal Care and Use Committee.
Drugs
(−)DOI was purchased from Research Biochemicals (Natick, MA). MDL100,907 was generously donated by Hoechst Marion Roussel Research Institute (Cincinnati, OH). SB-242084 was generously donated by GlaxoSmithKline Pharmaceuticals (Harlow, UK). Xylazine was purchased from Phoenix Pharmaceuticals (St. Joseph, MO). Ketamine was purchased from Fort Dodge Animal Health (Fort Dodge, IA). (−)DOI was dissolved in 0.9% saline at a concentration of 1 mg/ml. Both MDL100,907 and SB-242084 were prepared by sonicating in a minimal volume of 0.01N HCl containing 10% of 2-hydroxypropyl-β-cyclodextrin (Sigma, St. Louis, MO) and further diluted to their final concentrations with saline (0.748, 7.48, and 18.7 mm for MDL100,907; 10 mm for SB-242084). The vehicle was the solvent used to dissolve the highest concentration of MDL100,907 (18.7 mm). Ampicillin (Sigma) was dissolved in saline at a concentration of 50 mg/ml. Sulfamethoxazole and trimethoprim (Alpharma USPD, Baltimore, MD) was suspended in drinking water (4.2 mg of sulfamethoxazole and 0.85 mg of trimethoprim in 250 ml of water). All solutions were made fresh before administration.
Surgery
Rats were anesthetized with a mixture of ketamine and xylazine (100 mg/kg ketamine plus 7 mg/kg xylazine, 1.4 ml/kg, i.p.). A double-barreled guide cannula (26 gauge, 1.2 mm center-to-center distance) with its corresponding dummy cannula inserted inside (Plastic One, Roanoke, VA) was implanted into the brain above both sides of the paraventricular nucleus according to the following stereotaxic coordinates: 5.7 mm rostral, ±0.6 mm lateral with respect to lambda, and 6.1 mm ventral from the skull surface. Dehydration was prevented by injecting 1 ml of saline (subcutaneously) after surgery. To prevent postsurgery infection, all rats received ampicillin (50 mg/kg, s.c.) immediately after surgery, followed by 6 d of administration of sulfamethoxazole and trimethoprim suspended in the drinking water. Rats were not treated further for a minimum of 10 d.
Experimental protocol
Rats were handled for 4 consecutive days before the experiment. On the day of the experiment, rats were randomly assigned to different experimental groups [eight rats for saline-challenged groups and 13–15 rats for (−)DOI-challenged groups]. After removal of the dummy cannula, an injection cannula (33 gauge, 1.2 mm center-to-center distance, 1.5 mm projection from the tip of the guide cannula) was inserted through the implanted double-barreled guide cannula to a position 1.5 mm ventral to the tip of the guide cannula. Rats received an intra-paraventricular microinjection (0.5 μl/side) of the vehicle, increasing doses of MDL100,907 (0.748, 7.48, and 18.7 nmol), or SB-242084 (10 nmol). The drugs were microinjected over 1 min. The injection cannula was left in situ for an additional 1 min before removal. Fifteen minutes after the intra-paraventricular microinjections, the rats received an injection of (−)DOI (1 mg/kg, s.c.) or saline (1 ml/kg, s.c.). The rats were killed by decapitation 30 min after (−)DOI injection. The blood was collected in centrifuge tubes containing a 0.5 ml solution of 0.3m EDTA, pH 7.4. After centrifugation, the plasma was stored at −70°C for radioimmunoassays of plasma hormones. The brains were frozen on dry ice and saved for a histological verification of the positions of the cannulas.
Histology
Coronal sections (30 μm) were cut on a cryostat and mounted on gelatin-coated slides. The slides were stained with cresyl violet, dehydrated, and coverslipped. The positions of the tips of the double-barreled injection cannulas were inspected under a light microscope and photographed. Only animals with cannula positions immediately dorsal to the hypothalamic paraventricular nucleus and with intact neurons in the nucleus were used for data analysis (see Fig.1).
Fig. 1.
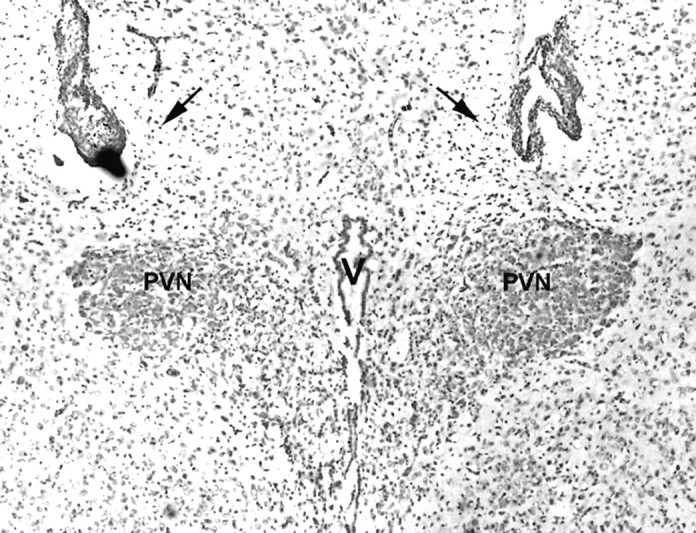
Histological verification of the position of the tips of the double-barreled injection cannula (30 μm; cresyl violet staining). Arrows, The tips of a double-barreled cannula. PVN, The hypothalamic paraventricular nucleus;V, the 3rd ventricle.
Radioimmunoassay
Plasma ACTH, corticosterone, oxytocin, and prolactin concentrations were determined by radioimmunoassays as described in detail previously (Li et al., 1993, 1997). Plasma renin activity (PRA) was determined as the ability of renin to generate angiotensin I from endogenous substrate. Plasma renin concentration (PRC) was determined as the generation of angiotensin I by endogenous renin from a saturating amount of added renin substrate. Both PRA and PRC were measured as described previously (Richardson Morton et al., 1989).
Immunohistochemical labeling of 5-HT2A receptors
Tissue preparation. Three Sprague Dawley rats (225–275 gm) were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and then perfused intracardially through the ascending aorta first with 0.01 m PBS, pH 7.4, followed by 0.1 m phosphate-buffered 4% formaldehyde, pH 7.4. The brains were removed and postfixed for 2 hr at 4°C in the above fixative solution and then cryoprotected for 24 hr in 0.01 m PBS buffer, pH 7.4, containing 30% sucrose before sectioning. Serial coronal sections of the hypothalamus (30 μm) were cut with a freezing microtome and transferred into 0.01 m PBS buffer, pH 7.4, and stored at 4°C.
Immunohistochemistry. Free-floating sections were exposed to microwave radiation (400 W, 15 sec). After cooling, sections were treated with 0.2% Triton X-100 (Fisher Scientific, Hanover Park, IL) for 40 min and rinsed three times in PBS buffer (Fritschy et al., 1998). The endogenous hydrogen peroxidase was inactivated by treating the sections with 3% hydrogen peroxide for 10 min. Nonspecific binding of the antibodies was reduced by preincubating the sections in 5% normal horse serum diluted in PBS for 1 hr at room temperature. The blocking serum was also used as diluents for antibody preparations. The sections were incubated overnight at room temperature with a monoclonal mouse anti-5-HT2A IgG (1:150 dilution; PharMingen, San Diego, CA). After washing in PBS, the sections were incubated with biotinylated horse anti-mouse IgG (1:2000 dilution; Vector Laboratories, Burlingame, CA) for 1 hr at room temperature, followed by PBS washing. Subsequently, the sections were incubated with avidin–biotinylated peroxidase complex (ABC; dilution, 1:200; Vector Laboratories) for 40 min, followed by PBS washing. Before the peroxidase reaction, the sections were transferred for 5 min into 0.05m Tris-buffered saline (TBS), pH 7.4. The peroxidase reaction was performed by incubating the sections in 0.05m Tris-HCl containing 0.02% (w/v) 3,3′-diaminobenzidine tetrahydrochloride (DAB) in the presence of ammonium nickel sulfate (0.25%) and 0.002% (v/v) hydrogen peroxide. When the intensity of the signal was optimal, the reaction was stopped by rinsing the sections in TBS buffer. The resulting nickel-DAB polymer was further silver intensified in a solution containing 0.1% silver nitrate, 0.1% ammonium nitrate, 1% silicotungstic acid, and 0.2% formaldehyde for 2–3 min, and the reaction was stopped by rinsing in 1% acetic acid. Finally, the sections were rinsed, mounted on gelatin-coated slides, dehydrated, and coverslipped for light microscopy and photomicrography. The images were scanned and analyzed using Adobe Photoshop software (Adobe Systems, San Jose, CA). Four rostrocaudal levels of the hypothalamic paraventricular nucleus were examined. These rostrocaudal levels are based on the book by Swanson (1992).
Control experiment. The control sections were treated exactly in the same way as the experimental sections, except that the primary antibody was replaced with the diluents of the primary antibody.
Statistical analyses
The hormone data are presented as group means and the SEM and analyzed by two-way ANOVA. Post hoc tests were conducted using Newman–Keuls multiple-range test. A computer program (GB STAT; Dynamic Microsystems, Silver Spring, MD) was used for the statistical analyses.
RESULTS
Histological verification of the position of double-barreled injection cannula
Figure 1 is a representative picture showing the double-barreled cannula tracts terminating bilaterally at the dorsal border of the hypothalamic paraventricular nucleus. The border of the hypothalamic paraventricular nucleus and the neurons in the hypothalamic paraventricular nucleus were intact, indicating no damage in the nucleus. Histological examination of the (−)DOI-challenged rats indicated that 85% (63 of 74) had correct cannula positions and no damage to the neurons of the hypothalamic paraventricular nucleus. The saline-challenged rats and the (−)DOI-challenged rats with verified cannula positions were used for the statistical evaluation of hormone data.
Microinjection of MDL100,907 into the hypothalamic paraventricular nucleus inhibits oxytocin and prolactin responses to (−)DOI
Microinjection of increasing doses of MDL100,907 into the hypothalamic paraventricular nucleus did not affect basal levels of oxytocin and prolactin but inhibited (−)DOI-induced oxytocin and prolactin release in a dose-dependent manner (Fig.2). For plasma oxytocin, the two-way ANOVA indicated a significant main effect of (−)DOI (F(1,68) = 61.00; p < 0.0001) and a significant main effect of MDL100,907 (F(3,68) = 11.04; p < 0.0001). There was a significant interaction between (−)DOI and MDL100,907 (F(3,68) = 13.11;p < 0.0001). The post hoc Newman–Keuls test indicated that (−)DOI significantly increased plasma oxytocin levels in rats receiving vehicle or the lowest dose of MDL100,907. All three doses of MDL100,907 (0.748, 7.48, and 18.7 nmol) significantly inhibited (26, 72, and 92%, respectively) the oxytocin response to (−)DOI.
Fig. 2.
Dose-dependent inhibition of oxytocin (A) and prolactin (B) responses to (−)DOI by MDL100,907 microinjected into the hypothalamic paraventricular nucleus. The data represent the mean ± SEM of 7–14 rats per group. *p < 0.05, **p < 0.01, significant effect of (−)DOI compared with the saline challenge group. #p < 0.05, ##p < 0.01, significant effect of MDL100,907 compared with the vehicle–(−)DOI group (two-way ANOVA and Newman–Keuls multiple range test). Veh, Vehicle;PVN, the hypothalamic paraventricular nucleus.
For plasma prolactin, the two-way ANOVA indicated a significant main effect of (−)DOI (F(1,68) = 22.65;p < 0.0001) and MDL100,907 (F(3,68) = 10.24; p < 0.0001). There was a significant interaction between (−)DOI and MDL100,907 (F(3,68) = 9.60;p < 0.0001). The post hoc Newman–Keuls test indicated that (−)DOI significantly increased the plasma level of prolactin in rats receiving vehicle or the lowest dose of MDL100,907. MDL100,907 inhibited (−)DOI-induced prolactin release by 56% at the dose of 0.748 nmol, 98% at the dose of 7.48 nmol, and 100% at the dose of 18.7 nmol.
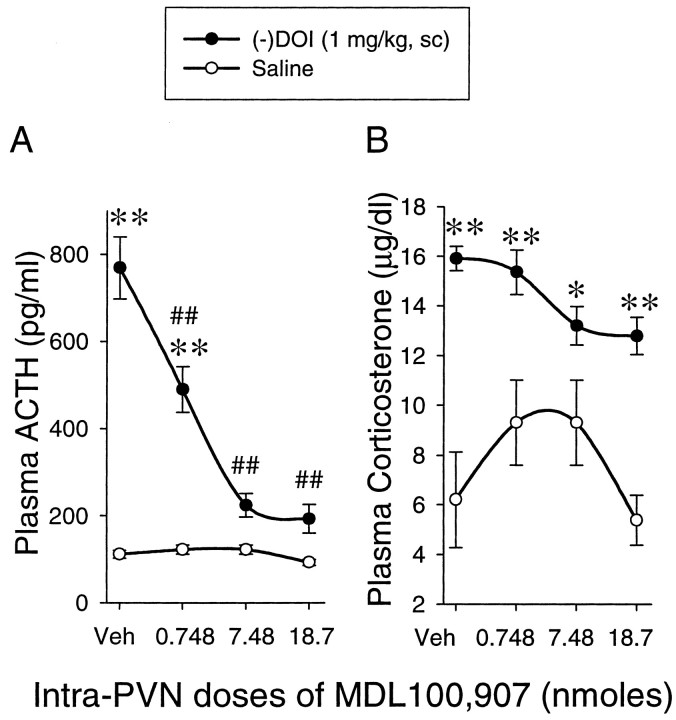
Microinjection of MDL100,907 into the hypothalamic paraventricular nucleus inhibits ACTH but not corticosterone response to (−)DOI
Injection of MDL100,907 into the hypothalamic paraventricular nucleus did not produce a significant change in basal plasma ACTH level. (−)DOI-induced ACTH release was dose-dependently inhibited by MDL100,907 microinjected into the hypothalamic paraventricular nucleus (Fig. 3A). The two-way ANOVA indicated a significant main effect of (−)DOI (F(1,68) = 82.58; p < 0.0001) and a significant main effect of MDL100,907 (F(3,68) = 16.33; p < 0.0001). The interaction between (−)DOI and MDL100,907 was also significant (F(3,68) = 15.48;p < 0.0001). The post hoc Newman–Keuls test indicated that (−)DOI significantly increased plasma ACTH levels in rats receiving vehicle or the lowest dose of MDL100,907. MDL100,907 inhibited (−)DOI-induced ACTH release by 42% at 0.748 nmol, 83% at 7.48 nmol, and 88% at 18.7 nmol.
Fig. 3.
Microinjection of MDL100,907 into the hypothalamic paraventricular nucleus dose-dependently inhibits ACTH (A) but not corticosterone (B) responses to (−)DOI. The data represent the mean ± SEM of 7–14 rats per group. *p < 0.05, **p < 0.01, significant effect of (−)DOI compared with the saline challenge group. ##p < 0.01, significant effect of MDL100,907 compared with the vehicle–(−)DOI group (two-way ANOVA and Newman–Keuls multiple range test). Veh, Vehicle; PVN, the hypothalamic paraventricular nucleus.
For plasma corticosterone, the two-way ANOVA indicated a significant main effect of (−)DOI (F(1,68) = 73.65; p < 0.0001) and a significant main effect of MDL100,907 (F(3,68) = 2.95; p < 0.05). The interaction between (−)DOI and MDL100,907 was not significant (F(3,68) = 2.38;p = 0.0771). The post hoc Newman–Keuls test indicated that microinjection of MDL100,907 into the hypothalamic paraventricular nucleus did not change the basal corticosterone level. Whereas (−)DOI significantly elevated plasma corticosterone, the corticosterone response to (−)DOI was not inhibited by any of the three doses of MDL100,907 microinjected into the hypothalamic paraventricular nucleus (Fig. 3B).
Microinjection of MDL100,907 into the hypothalamic paraventricular nucleus inhibits the renin response to (−)DOI
Injection of MDL100,907 into the hypothalamic paraventricular nucleus did not alter basal renin activity or renin concentration. Both plasma renin activity and renin concentration were increased after (−)DOI challenge. This effect of (−)DOI was dose-dependently inhibited by increasing doses of MDL100,907 microinjected into the hypothalamic paraventricular nucleus (Fig.4). The two-way ANOVA indicated a significant main effect of (−)DOI on renin activity (F(1,68) = 42.13; p < 0.0001) and renin concentration (F(1,68) = 62.06; p < 0.0001). The main effect of MDL100,907 was also significant for both renin activity (F(3,68) = 10.37;p < 0.0001) and renin concentration (F(3,68) = 9.16; p < 0.0001). There was a significant interaction between (−)DOI and MDL100,907 for renin activity (F(3,68)= 6.66; p = 0.0005), as well as for renin concentration (F(3,68) = 8.23; p < 0.0001). The post hoc Newman–Keuls test indicated that the three doses of MDL100,907 (0.748, 7.48, and 18.7 nmol) inhibited the (−)DOI-induced increase in plasma renin activity by 58, 90, and 92%, respectively. Similarly, the (−)DOI-induced increase in renin concentration was inhibited by 37, 77, and 87%, respectively (Fig.4).
Fig. 4.
Microinjection of MDL100,907 into the hypothalamic paraventricular nucleus dose-dependently inhibited (−)DOI-induced increase in renin activity (A) and renin concentration (B). The data represent the mean ± SEM of 7–14 rats per group. **p < 0.01, significant effect of (−)DOI compared with the saline challenge group. ##p < 0.01, significant effect of MDL100,907 compared with the vehicle–(−)DOI group (two-way ANOVA and Newman–Keuls multiple range test). Veh, Vehicle;PVN, the hypothalamic paraventricular nucleus.
Microinjection of SB-242084 into the hypothalamic paraventricular nucleus does not inhibit neuroendocrine responses to (−)DOI
Microinjection of SB-242084 (10 nmol) into the hypothalamic paraventricular nucleus did not alter basal plasma levels of oxytocin, prolactin, ACTH, corticosterone, renin activity, or renin concentration (Fig. 5). The plasma levels of all of the hormones were elevated by (−)DOI challenge (Fig. 5).
Fig. 5.

Microinjection of SB-242084 into the hypothalamic paraventricular nucleus does not inhibit the neuroendocrine responses to (−)DOI. The data represent the mean ± SEM of 7–14 rats per group. **p < 0.01, significant effect of (−)DOI compared with the saline challenge groups (two-way ANOVA and Newman–Keuls multiple range test). Veh, Vehicle;SB, SB-242084; PVN, the hypothalamic paraventricular nucleus.
(−)DOI-induced oxytocin (Fig. 5A) and prolactin (Fig.5B) release were not inhibited by an intra-paraventricular microinjection of SB-242084. The two-way ANOVA indicated a significant main effect of (−)DOI on oxytocin (F(1,36) = 42.16; p < 0.0001) and prolactin (F(1,36) = 41.86; p < 0.0001). There was no significant main effect of SB-242084 on oxytocin (F(1,36) = 0.1039; p = 0.7491) or prolactin (F(1,36) = 0.90;p = 0.3499). The interaction between (−)DOI and SB-242084 was not significant for oxytocin (F(1,36) = 0.00162; p= 0.681) or prolactin (F(1,36) = 0.63;p = 0.4327).
SB-242084 microinjected into the hypothalamic paraventricular nucleus did not inhibit the ACTH (Fig. 5C) or corticosterone (Fig.5D) responses to (−)DOI challenge. The two-way ANOVA indicated a significant main effect of (−)DOI on ACTH (F(1,36) = 54.28; p < 0.0001) and corticosterone (F(1,36) = 62.6; p < 0.0001). No significant main effect of SB-242084 was observed for ACTH (F(1,36) = 1.16; p = 0.2889) or corticosterone (F(1,36) = 2.59; p = 0.1164). There was no significant interaction between (−)DOI and SB-242084 for ACTH (F(1,36) = 1.67; p = 0.2044) or corticosterone (F(1,36) = 0; p = 0.9996).
Similarly, SB-242084 did not inhibit the renin response to (−)DOI when microinjected into the hypothalamic paraventricular nucleus (Fig.5E,F). The two-way ANOVA indicated a significant main effect of (−)DOI on renin activity (F(1,36) = 63.52; p < 0.0001) and renin concentration (F(1,36) = 68.06; p < 0.0001). There was no significant main effect of SB-242084 on renin activity (F(1,36) = 0.90;p = 0.3494) or on renin concentration (F(1,36) = 0.00342; p= 0.9537). The interaction between (−)DOI and SB-242084 was not significant for renin activity (F(1,36) = 0.40; p = 0.5325) or renin concentration (F(1,36) = 0.32; p = 0.5767).
Immunohistochemical staining of 5-HT2A receptors in the hypothalamic paraventricular nucleus
In the hypothalamic paraventricular nucleus, 5-HT2A receptor-immunoreactive perikarya were heterogeneously distributed throughout the nucleus (Fig.6A–D). A high density of the labeling was found in the anterior and posterior magnocellular regions. A moderate density of labeling was found in the medial and dorsal parvicellular regions. As a positive control, we observed a typical distribution of 5-HT2A receptor immunoreactivity in the cortex; the majority of the staining was localized to pyramidal cells and the apical dendrites of pyramidal cells (Fig. 6F). No staining was observed in the absence of primary anti-5-HT2A receptor antibody (Fig. 6G).
Fig. 6.

5-HT2A receptor immunoreactivity in the hypothalamic paraventricular nucleus (PVN).V denotes the 3rd ventricle. The numbersindicate the rostrocaudal distance from bregma (Swanson, 1992).A–D, Distribution of 5-HT2A receptor immunoreactivity in the hypothalamic paraventricular nucleus. Scale bar, 100 μm. E, High magnification ofC. Scale bar, 10 μm. F, 5-HT2A receptor immunoreactivity in cerebral cortex.Arrows, Pyramidal neurons; arrowheads, apical dendrites of pyramidal neurons. Scale bar, 10 μm.G, No 5-HT2A receptor-like immunoreactivity was observed in the hypothalamic paraventricular nucleus in the absence of primary antibody. Scale bar, 100 μm.
DISCUSSION
The present study provides immunohistochemical and pharmacological evidence demonstrating that peripheral administration of the 5-HT2A/2C agonist (−)DOI primarily activates 5-HT2A receptors in the hypothalamic paraventricular nucleus, leading to the release of oxytocin, ACTH, prolactin, and renin into the circulation.
The hypothalamic paraventricular nucleus is an integral part of the limbic system (Saphier and Feldman, 1986; Herman and Cullinan, 1997) and participates in mood modulation (Legros, 1992; Bernstein et al., 1998; Scott and Dinan, 1998). Many depressed patients have high plasma levels of cortisol, suggesting a disrupted regulation of the hypothalamic–pituitary–adrenal axis (Sherman and Pfohl, 1985; Barden et al., 1995). A larger density of oxytocin- and vasopressin-expressing neurons was found in the hypothalamic paraventricular nucleus in postmortem brains of depressed patients (Purba et al., 1996). For many years, psychiatrists have administered serotonin agonists to depressed patients to determine whether the hormone responses can provide a peripheral diagnostic tool of the functioning of serotonin receptors in the hypothalamus. Our study demonstrates in rats that hormone responses to a 5-HT2A/2C agonist are primarily mediated by activation of 5-HT2A receptors in the hypothalamic paraventricular nucleus.
Injection of 2.5 mg/kg (±)DOI increases plasma levels of oxytocin, prolactin, ACTH, corticosterone, and renin (Van de Kar et al., 2001). This effect of (±)DOI can be blocked by the 5-HT2A antagonist MDL100,907 but not by SB-242084, indicating an activation of 5-HT2Areceptors (Van de Kar et al., 2001; Hemrick-Luecke and Evans, 2002). A dose of 1 mg/kg of the bioactive isomer (−)DOI is equivalent to 2.5 mg/kg (±)DOI in producing a similar degree of elevation of plasma hormones (M. Shankaran, G. Battaglia, D. D'Souza, Y. Zhang, and L. D. Van de Kar, unpublished observations). Thus, we chose 1 mg/kg (subcutaneously) as the challenge dose of (−)DOI to examine the function of 5-HT2A receptors.
Until recently, differentiation between 5-HT2Aand 5-HT2C receptor-mediated effects was hindered by a lack of receptor subtype-selective antagonists. Many available 5-HT2 antagonists, such as ketanserin and ritanserin, have a high affinity for both 5-HT2Aand 5-HT2C receptors (Leysen et al., 1985; Van Wijngaarden et al., 1990). MDL100,907 is a 5-HT2Aantagonist with 100-fold higher affinity for 5-HT2A than 5-HT2Creceptors and a low affinity for dopamine D2receptors (Kehne et al., 1996). SB-242084 is a 5-HT2C antagonist with a low affinity for other serotonin and nonserotonin receptors (Kennett et al., 1997). Because of its low solubility, the highest dose of SB-242084 injected into the rats was 10 nmol. Still, 7.48 nmol of MDL100,907 produced a >70% inhibition of hormone responses to (−)DOI (Figs. 3-5), whereas no inhibition was observed by 10 nmol of SB-242084. Thus, the hormone responses to (−)DOI are mediated by 5-HT2Areceptors in the hypothalamic paraventricular nucleus.
The importance of the hypothalamic paraventricular nucleus in the neuroendocrine response to serotonergic stimulation has been established mainly via lesion studies (Rittenhouse et al., 1992; Bagdy and Makara, 1994; Bagdy, 1996). These lesions destroyed all of the cells (i.e., oxytocin and CRF cells) in the hypothalamic paraventricular nucleus and therefore cannot be used to examine the function of a particular type of receptor in the nucleus. In our experiment, a double-barreled cannula chronically implanted above both sides of the hypothalamic paraventricular nucleus produced no damage to the neurons in the nucleus. This microinjection technique allowed us to examine specifically which receptor subtype in the hypothalamic paraventricular nucleus mediates hormone responses to (−)DOI.
One concern regarding microinjection technique is the accuracy of injection and the degree of drug diffusion from the injection site. To ensure the accuracy of injection, the tips of injection cannulas were histologically verified. In a preliminary experiment, the diffusion pattern of fast green dye microinjected using the same experimental protocol was examined. The green dye primarily localized in the hypothalamic paraventricular nucleus, with limited diffusion to surrounding nuclei. Moreover, the nuclei neighboring the hypothalamic paraventricular nucleus are not involved in the serotonergic stimulation of hormone release (Richardson Morton et al., 1989;Rittenhouse et al., 1992, 1993; Bagdy, 1996). Thus, diffusion of the antagonist into regions surrounding the paraventricular nucleus would not confound the results of the present study. For example, the supraoptic nucleus has a high density of oxytocin cells and 5-HT2A receptor immunoreactivity. However, lesions in the supraoptic nucleus do not inhibit the release of oxytocin by serotonergic stimulation (Van de Kar et al., 1995b). Lesions in the ventromedial nucleus and dorsomedial nucleus do not affect the release of prolactin and renin after serotonergic stimulation (Rittenhouse et al., 1992). Although stimulation of neurons in the dorsomedial nucleus increases ACTH release (Stotz-Potter et al., 1996; Bailey and DiMicco, 2001), we did not detect 5-HT2A receptor labeling in the dorsomedial nucleus. Thus, it is not likely that the dorsomedial nucleus contributes to (−)DOI-induced release of ACTH.
In this study, we measured a wide range of hormones that use different regulatory mechanisms and signaling cascades. The oxytocin cells in the hypothalamic paraventricular nucleus send their axons to the posterior lobe of the pituitary, directly releasing oxytocin into the bloodstream. ACTH and prolactin are released by corticotrophs and lactotrophs, respectively, in the anterior lobe of the pituitary and are under the regulation of releasing and/or inhibiting hormones from the hypothalamus. Thus, compared with oxytocin, ACTH and prolactin represent amplified indices subsequent to hypothalamic activation. Corticosterone, released from the adrenal cortex via stimulation by ACTH, is the most amplified signal of hypothalamic activation. Renin is released from the kidney by stimulation of the sympathetic nervous system and an endocrine renin-releasing factor (Urban et al., 1992). The hypothalamic paraventricular nucleus is a part of the complex system that regulates renin release (Van de Kar et al., 1987; Van de Kar and Blair, 1999). Because a majority of the hormones released by (−)DOI was inhibited by intra-paraventricular MDL100,907, activation of 5-HT2A receptors in the hypothalamic paraventricular nucleus represents the common mechanism underlying hormone responses to (−)DOI.
A difference in amplification of various hormones can be observed by comparing their degrees of inhibition by MDL100,907. A dose of 18.7 nmol of MDL100,907 completely blocked the oxytocin and prolactin responses to (−)DOI, whereas the same dose of MDL100,907 produced ∼90% inhibition of renin activity, renin concentration, and ACTH. Corticosterone is most amplified through the hypothalamus–pituitary–adrenal axis. A plasma concentration of ACTH ∼200 pg/ml would be sufficient to drive a maximal release of corticosterone (Rittenhouse et al., 1994; Levy et al., 1994; Bagdy, 1996). We found that the highest dose of MDL100,907 (18.7 nmol) lowered (−)DOI-increased plasma level of ACTH from 769 to 193 pg/ml. Accordingly, the corticosterone response to (−)DOI was not inhibited by intra-paraventricular injection of MDL100,907.
Another reason why intra-paraventricular injection of MDL100,907 produced a lower degree of inhibition on plasma ACTH response to (−)DOI is that (−)DOI may activate neurons in other nuclei, such as the amygdala, that are also involved in ACTH release (Beaulieu et al., 1986; Feldman et al., 1998). In addition, the corticosterone response to an acute injection of DOI may also involve direct effects on the adrenal gland (Alper, 1990; Rittenhouse et al., 1994). Thus, it is not unexpected that the inhibitory effect of intra-paraventricular injection of MDL100,907 on (−)DOI-induced hormone release was observed for all of the other hormones except corticosterone.
Although the presence of 5-HT2A receptors in the hypothalamic paraventricular nucleus has been demonstrated by in situ hybridization, autoradiographic studies, and Western blot analysis (Appel et al., 1990; Wright et al., 1995; Gundlah et al., 1999), conventional immunohistochemistry failed to label 5-HT2A receptors in the nucleus. This suggests that 5-HT2A receptor epitopes in the hypothalamic paraventricular nucleus are not readily accessible in situ. In the present study, we adapted antigen retrieval procedures based on microwave irradiation to enhance the immunohistochemical staining of 5-HT2A receptors (Fritschy et al., 1998). The microwave irradiation procedure did not affect the immunostaining pattern of 5-HT2A receptors reported in other brain regions, such as the cortex (Willins et al., 1997;Cornea-Hébert et al., 1999). The negative control sections, as defined by the lack of primary antibody, did not exhibit 5-HT2A receptor immunoreactivity. Therefore, the 5-HT2A receptor immunopositive perikarya in the hypothalamic paraventricular nucleus most likely represent specific labeling of 5-HT2A receptors.
5-HT2A receptor immunopositive perikarya were heterogeneously distributed in the hypothalamic paraventricular nucleus, including the regions that are known to express oxytocin and CRF cells (Sawchenko and Swanson, 1985). Our recent immunofluorescence studies show that 5-HT2A receptors are present on oxytocin-containing cells (Zhang et al., 2001). Thus, it is possible that 5-HT2A receptors are located on oxytocin, CRF, and/or other neurons in the hypothalamic paraventricular nucleus.
In summary, 5-HT2A receptor immunoreactivity was found in the hypothalamic paraventricular nucleus in which CRF and oxytocin cells are located. Neuroendocrine responses to (−)DOI were dose-dependently inhibited by intra-paraventricular injection of the 5-HT2A antagonist MDL100,907 but not by the 5-HT2C antagonist SB-242084. Thus, neuroendocrine responses to (−)DOI can be used as a peripheral indicator of the function of 5-HT2A receptors in the hypothalamic paraventricular nucleus. This study provides valuable information for the possible development of neuroendocrine challenge tests for patients suffering from mood disorders.
Footnotes
This work was supported by United States Public Health Service Grants NS34153 and DA13669. We are grateful to Dr. Lanny C. Keil from the National Aeronautics and Space Administration Ames Research Center (Moffat Field, CA) for the oxytocin antiserum, to Hoechst Marion Roussel Research Institute (Cincinnati, OH) for the sample of MDL100,907, and to SmithKline Beecham Pharmaceuticals (Harlow, UK) for the sample of SB-242084. We are thankful to K. A. Haskins and K. Waimey for technical assistance.
Correspondence should be addressed to Dr. Louis D. Van de Kar, Department of Pharmacology, Loyola University of Chicago, Stritch School of Medicine, 2160 South First Avenue, Maywood, IL 60153. E-mail:lvandek@lumc.edu.
REFERENCES
- 1.Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Alper RH. Evidence for central and peripheral serotonergic control of corticosterone secretion in the conscious rat. Neuroendocrinology. 1990;51:255–260. doi: 10.1159/000125347. [DOI] [PubMed] [Google Scholar]
- 3.Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teiteler M, de Souza EB. Autoradiographic characterization of (±)-1-(2, 5-dimethoxy-4-[125I]iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- 4.Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- 5.Bagdy G, Makara GB. Hypothalamic paraventricular nucleus lesions differentially affect serotonin-1A (5-HT1A) and 5-HT2 receptor agonist-induced oxytocin, prolactin, and corticosterone responses. Endocrinology. 1994;134:1127–1131. doi: 10.1210/endo.134.3.8119151. [DOI] [PubMed] [Google Scholar]
- 6.Bagdy G, Calogero AE, Murphy DL, Szemeredi K. Serotonin agonists cause parallel activation of the sympathoadrenomedullary system and the hypothalamo-pituitary-adrenocortical axis in conscious rats. Endocrinology. 1989;125:2664–2669. doi: 10.1210/endo-125-5-2664. [DOI] [PubMed] [Google Scholar]
- 7.Bailey TW, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- 8.Barden N, Reul JMHM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system. Trends Neurosci. 1995;18:6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]
- 9.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu S, DiPaolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- 11.Berg KA, Stout BD, Maayani S, Clarke WP. Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J Pharmacol Exp Ther. 2001;299:593–602. [PubMed] [Google Scholar]
- 12.Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867–875. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- 13.Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 14.Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Feldman S, Newman ME, Gur E, Weidenfeld J. Role of serotonin in the amygdala in hypothalamo-pituitary-adrenocortical responses. NeuroReport. 1998;9:2007–2009. doi: 10.1097/00001756-199806220-00017. [DOI] [PubMed] [Google Scholar]
- 16.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- 17.Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- 18.Hemrick-Luecke SK, Evans DC. Comparison of the potency of MDL 100, 907 and SB 242084 in blocking the serotonin (5-HT)2 receptor agonist-induced increases in rat serum corticosterone concentrations: evidence for 5-HT2A receptor mediation of the HPA axis. Neuropharmacology. 2002;42:162–169. doi: 10.1016/s0028-3908(01)00166-6. [DOI] [PubMed] [Google Scholar]
- 19.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman BJ, Mezey E. Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett. 1989;247:453–462. doi: 10.1016/0014-5793(89)81390-0. [DOI] [PubMed] [Google Scholar]
- 21.Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100, 907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacology. 1996;15:116–124. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- 22.Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 23.Kozuru T, Kagaya A, Takebayashi M, Horiguchi J, Yamawaki S. Chronic electroconvulsive shock decreases (±)1-(4-iodo-2, 5-dimethoxyphenyl)-2-aminopropane hydrochloride (DOI)-induced wet-dog shake behaviors of dexamethasone-treated rats. Life Sci. 2000;66:1271–1279. doi: 10.1016/s0024-3205(00)00431-8. [DOI] [PubMed] [Google Scholar]
- 24.Legros JJ. Neurohypophyseal peptides and psychopathology. Horm Res. 1992;37 [Suppl 3]:16–21. doi: 10.1159/000182396. [DOI] [PubMed] [Google Scholar]
- 25.Levy AD, Li Q, Van de Kar LD. Repeated cocaine exposure inhibits the adrenocorticotropic hormone response to the serotonin releaser d-fenfluramine and the 5-HT1A agonist, 8-OH-DPAT. Neuropharmacology. 1994;33:335–342. doi: 10.1016/0028-3908(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 26.Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PFM, Lauduron PM. Receptor-binding properties in vitro and in vivo of ritanserin. A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27:600–611. [PubMed] [Google Scholar]
- 27.Li Q, Brownfield MS, Battaglia G, Cabrera TM, Levy AD, Rittenhouse PA, Van de Kar LD. Long-term treatment with the antidepressants fluoxetine and desipramine potentiates endocrine responses to the serotonin agonists 6-chloro-2-[1-piperazinyl]-pyrazine (MK-212) and (±)-1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane HCI (DOI). J Pharmacol Exp Ther. 1993;266:836–844. [PubMed] [Google Scholar]
- 28.Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- 29.Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- 30.Martin GR, Humphrey PPA. Receptors for 5-hydroxytryptamine: current perspectives on classification and nomenclature. Neuropharmacology. 1994;33:261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 31.McCall RB, Harris LT. 5-HT2 receptor agonists increase spontaneous sympathetic nerve discharge. Eur J Pharmacol. 1988;151:113–116. doi: 10.1016/0014-2999(88)90698-x. [DOI] [PubMed] [Google Scholar]
- 32.Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- 33.Price LH, Malison RT, Mcdougle CJ, McCance-Katz EF, Owen KR, Heninger GR. Neurobiology of tryptophan depletion in depression: effects of m-chlorophenylpiperazine (mCPP). Neuropsychopharmacology. 1997;17:342–350. doi: 10.1016/S0893-133X(97)00084-5. [DOI] [PubMed] [Google Scholar]
- 34.Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 35.Richardson Morton KD, Van de Kar LD, Brownfield MS, Bethea CL. Neuronal cell bodies in the hypothalamic paraventricular nucleus mediate stress-induced renin and corticosterone secretion. Neuroendocrinology. 1989;50:73–80. doi: 10.1159/000125204. [DOI] [PubMed] [Google Scholar]
- 36.Rittenhouse PA, Li Q, Levy AD, Van de Kar LD. Neurons in the hypothalamic paraventricular nucleus mediate the serotonergic stimulation of renin secretion. Brain Res. 1992;593:105–113. doi: 10.1016/0006-8993(92)91270-o. [DOI] [PubMed] [Google Scholar]
- 37.Rittenhouse PA, Levy AD, Li Q, Bethea CL, Van de Kar LD. Neurons in the hypothalamic paraventricular nucleus mediate the serotonergic stimulation of prolactin secretion via 5-HT1C/2 receptors. Endocrinology. 1993;133:661–667. doi: 10.1210/endo.133.2.8344205. [DOI] [PubMed] [Google Scholar]
- 38.Rittenhouse PA, Bakkum EA, Levy AD, Li Q, Carnes M, Van de Kar LD. Evidence that ACTH secretion is regulated by serotonin2A/2C (5-HT2A/2C) receptors. J Pharmacol Exp Ther. 1994;271:1647–1655. [PubMed] [Google Scholar]
- 39.Saphier D, Feldman S. Electrophysiology of limbic forebrain and paraventricular nucleus connections. Brain Res Bull. 1986;17:743–750. doi: 10.1016/0361-9230(86)90085-7. [DOI] [PubMed] [Google Scholar]
- 40.Sargent PA, Quested DJ, Cowen PJ. Clomipramine enhances the cortisol response to 5-HTP: implications for the therapeutic role of 5-HT2 receptors. Psychopharmacology. 1998;140:120–122. doi: 10.1007/s002130050747. [DOI] [PubMed] [Google Scholar]
- 41.Sawchenko PE, Swanson LW. Localization, colocalization and plasticity of corticotropin- releasing factor immunoreactivity in rat brain. Fed Proc. 1985;44:221–227. [PubMed] [Google Scholar]
- 42.Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci. 1998;62:1985–1998. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 43.Sherman BM, Pfohl B. Rhythm-related changes in pituitary-adrenal function in depression. J Affect Disord. 1985;9:55–61. doi: 10.1016/0165-0327(85)90010-2. [DOI] [PubMed] [Google Scholar]
- 44.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- 45.Swanson LW. Brain maps: structure of the rat brain, pp 22, 25–27. Elsevier; Amsterdam: 1992. [Google Scholar]
- 46.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 47.Urban JH, Brownfield MS, Levine JE, Van de Kar LD. Distribution of a renin-releasing factor in the central nervous system of the rat. Neuroendocrinology. 1992;55:574–582. doi: 10.1159/000126170. [DOI] [PubMed] [Google Scholar]
- 48.Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 49.Van de Kar LD, Urban JH, Brownfield MS, Simmons WH. Partial characterization of a renin-releasing factor from plasma and hypothalamus. Hypertension. 1987;9:598–606. doi: 10.1161/01.hyp.9.6.598. [DOI] [PubMed] [Google Scholar]
- 50.Van de Kar LD, Rittenhouse PA, Li Q, Levy AD. Serotonergic regulation of renin and prolactin secretion. Behav Brain Res. 1995a;73:203–208. doi: 10.1016/0166-4328(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 51.Van de Kar LD, Rittenhouse PA, Li Q, Levy AD, Brownfield MS. Hypothalamic paraventricular, but not supraoptic neurons, mediate the serotonergic stimulation of oxytocin secretion. Brain Res Bull. 1995b;36:45–50. doi: 10.1016/0361-9230(94)00161-s. [DOI] [PubMed] [Google Scholar]
- 52.Van de Kar LD, Javed A, Zhang YH, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Wijngaarden I, Tulp MTM, Soudijn W. The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- 54.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 55.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Dudas B, Muma NA, Battaglia G, Van de Kar LD. Colocalization of 5-HT1A and 5-HT2A serotonin receptors on oxytocin cells in the hypothalamic paraventricular nucleus. Soc Neurosci Abstr. 2001;27:265.12. [Google Scholar]
- 57.Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992;44:401–458. [PubMed] [Google Scholar]