Abstract
Transmitter release at the hippocampal mossy fiber (MF)–CA3 synapse exhibits robust use-dependent short-term plasticity with an extremely wide dynamic range. Recent studies revealed that presynaptic kainate receptors (KARs), which specifically localized on the MF axons, mediate unusually large facilitation at this particular synapse in concert with the action of residual Ca2+. However, it is currently unclear how activation of kainate autoreceptors enhances transmitter release in an activity-dependent manner. Using fluorescence recordings of presynaptic Ca2+ and voltage in hippocampal slices, here we demonstrate that paired-pulse stimulation (with 20–200 msec intervals) resulted in facilitation of Ca2+ influx into the MF terminals, as opposed to other synapses, such as the Schaffer collateral–CA1 synapse. These observations deviate from typical residual Ca2+hypothesis of facilitation, assuming an equal amount of Ca2+ influx per action potential. Pharmacological experiments reveal that the facilitation of presynaptic Ca2+ influx is mediated by activation of KARs. We also found that action potentials of MF axons are followed by prominent afterdepolarization, which is partly mediated by activation of KARs. Notably, the time course of the afterdepolarization approximates to that of the paired-pulse facilitation of Ca2+influx, suggesting that these two processes are closely related to each other. These results suggest that the novel mechanism amplifying presynaptic Ca2+ influx may underlie the robust short-term synaptic plasticity at the MF–CA3 synapse in the hippocampus, and this process is mediated by KARs whose activation evokes prominent afterdepolarization of MF axons and thereby enhances action potential-driven Ca2+ influx into the presynaptic terminals.
Keywords: hippocampus, kainate receptor, mossy fiber, paired-pulse facilitation, presynaptic Ca2+ influx, short-term plasticity
A prominent feature of transmission at chemical synapses involves modifiability of the strength of information transfer depending on the previous firing history of presynaptic neurons. Both short- and long-lasting forms of use-dependent modifications, referred to as short- and long-term synaptic plasticity, have been described among many central and peripheral synapses (Zucker, 1989; Bliss and Collingridge, 1993;Malenka and Nicoll, 1999). Long-term plasticity might underlie information storage in the CNS, whereas short-term plasticity plays pivotal roles in coding temporal patterns of the activity of the neuronal networks.
In the hippocampus, three principal excitatory connections [perforant path–dentate gyrus synapse, mossy fiber (MF)–CA3 synapse, and Schaffer collateral–CA1 synapse] display very different forms of short- and long-term plasticity (Nicoll and Malenka, 1995; Salin et al., 1996), suggesting that the specific functional roles of each of these synapses in hippocampal information processing may differ. Typically, the amount of transmitter released from hippocampal MF terminals is highly dependent on the frequency of afferent stimulation, and extremely large paired-pulse facilitation (PPF) is an experimental hallmark of MF synaptic transmission (Henze et al., 2000). Because short-term plasticity at this particular synapse displays an unusually wide dynamic range, we hypothesized that some additional mechanism other than the action of residual Ca2+ (Zucker, 1989; Zucker and Regehr, 2002) might be involved in activity-dependent tuning of the synaptic strength. The recent demonstration that presynaptic kainate receptors (KARs) (Kamiya and Ozawa, 2000; Kullmann, 2001; Lerma et al., 2001;Schmitz et al., 2001a; Kamiya, 2002) are specifically involved in the frequency facilitation (Schmitz et al., 2001b) prompted us to search for additional mechanisms underlying the unusually large PPF at the MF–CA3 synapse.
In the present study, we used optical measurement of presynaptic Ca2+ (Regehr and Tank, 1991; Wu and Saggau, 1994; Kamiya and Ozawa, 1999) and membrane potentials (Sabatini and Regehr, 1996, 1997) in hippocampal slices to elucidate precise cellular mechanisms underlying the robust short-term plasticity at the MF–CA3 synapse. We found that unusually large PPF at this synapse was accompanied by facilitation of stimulus-dependent presynaptic Ca2+ influx, as opposed to other synapses, such as Schaffer collateral–CA1 synapses in the hippocampus (Wu and Saggau, 1994; Kamiya and Ozawa; 1998) or parallel fiber synapses in the cerebellum (Regehr and Atluri, 1995; Kreitzer and Regehr, 2000). Pharmacological analysis revealed that this novel mechanism amplifying presynaptic Ca2+ influx is mediated by kainate autoreceptors specifically localized on the MF axons (Kullmann, 2001; Schmitz et al., 2001a; Kamiya, 2002). It should be noted that this unique autoreceptor system operates substantially by only a single preceding stimulus, as demonstrated by the prominent afterdepolarization of presynaptic axons revealed using voltage-sensitive dye. The evidence for activation of presynaptic kainate receptors by a single stimulus contrasts sharply with the fact that postsynaptic kainate receptors at this synapse are activated substantially only by repeated stimuli (Castillo et al., 1997; Vignes and Collingridge, 1997). Our results suggest that activation of kainate autoreceptors evokes prominent afterdepolarization and thereby modulates action potential-driven Ca2+ influx into the presynaptic terminals in an activity-dependent manner.
MATERIALS AND METHODS
Transverse hippocampal slices (∼400 μm thick) were prepared from BALB/c mice (14–20 d of age). All experiments were performed according to the guidelines laid down by the Animal Care and Experimentation Committee of Gunma University and Kobe University. Mossy fibers were stimulated at the stratum granulosum in the dentate gyrus, and the resultant field EPSPs were recorded from the stratum lucidum in the CA3 region. Slices were continuously superfused with the solution composed of the following (in mm):127 NaCl, 1.5 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 26 NaHCO3, and 10 glucose. The solution was equilibrated with 95% O2 and 5% CO2. Fluorescence recordings of presynaptic Ca2+ were made as described previously (Kamiya and Ozawa, 1999). Briefly, rhod-2 AM (Dojindo Laboratory, Kumamoto, Japan), a membrane-permeable Ca2+ indicator, was loaded into the MF terminals without severing the axons (Regehr and Tank, 1991). The dye was injected locally into the stratum lucidum, resulting in selective labeling of the mossy fibers (Kamiya and Ozawa, 1999). The fluorescence (excitation at 510–560 nm and monitoring above 580 nm) from the area (∼100 μm diameter) containing the labeled terminals was measured with a single photodiode (S2281–01; Hamamatsu Photonics, Hamamatsu, Japan). The ΔF/F value evoked by a single electrical stimulus was used as a measure of [Ca2+]i increase during an action potential. For the optical measurement of presynaptic voltage, fluorescent voltage-sensitive dye (di-8-ANEPPS; Molecular Probes, Eugene, OR) was injected locally into the axon bundles (stratum lucidum) (see Fig. 7A). Four to 6 hr after the injection, the fluorescence transient was also measured with the photodiode. The fluorescence (monitored in the same wavelength range as noted above) decreased transiently in response to the stimulation of MF. In Figures7B and 8A, the decrease in fluorescence was illustrated as an upward deflection. The output of the photodiode was I–V converted, amplified, and filtered at 500 Hz with an eight-pole Bessel filter (FLA-1; Cygnus Technology, Delaware Water Gap, PA). The signal was then digitized with a 12 bit analog-to-digital converter (Digidata 1200A; Axon instruments, Foster City, CA) and acquired at 10 kHz using pClamp8 software (Axon Instruments). The values in the text and figures are expressed as mean ± SEM (the number of experiments). Statistical analysis was performed using the paired t test, and p < 0.05 was accepted for statistical significance.
Fig. 7.
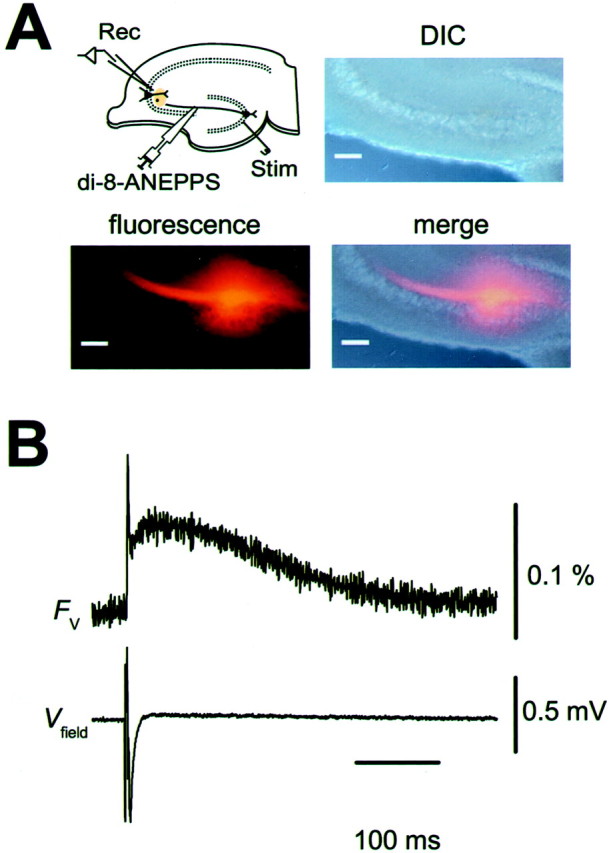
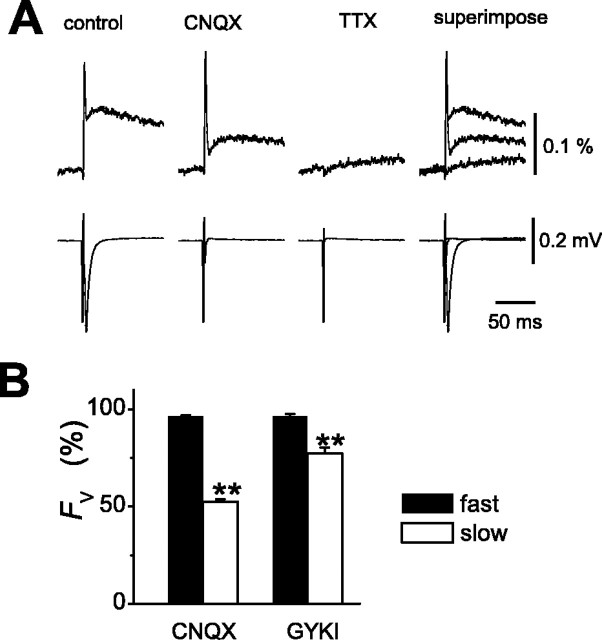
Optical recordings of presynaptic voltage transient (Fv) at MF–CA3 synapse.A, Selective loading of MF with the voltage-sensitive dye di-8-ANEPPS. Locally injected di-8-ANEPPS diffused along MF axons in the stratum lucidum. Changes in fluorescence intensity (Fv) were measured at the synaptic area distant from the injection site. Scale bars, 100 μm.Rec, Recording electrode; Stim, stimulating electrode. B, Representative records of presynaptic voltage transient (Fv) and simultaneously recorded Vfield.Traces are the average of 16 trials.Fv consisted of fast and slow components, possibly representing action potential and afterdepolarization of MF axons.
Fig. 8.
Evidence for activation of kainate autoreceptors by a single stimulus. A, Effects of CNQX onFV. Application of 10 μm CNQX selectively reduced the amplitude of the slow but not the fast components of Fv. B, Summary graph for the effects of 10 μm CNQX (n = 12) and 100 μm GYKI 52466 (n = 5) on the fast and slow components ofFv.
RESULTS
Short-term plasticity of presynaptic Ca2+transients at the MF–CA3 synapse
First we addressed why the MF synapse exhibits unusually large PPF. One obvious possibility is that the amount of presynaptic Ca2+ influx per action potential is modified in an activity-dependent manner (Jackson et al., 1991; Borst and Sakmann, 1998; Cuttle et al., 1998). To test this possibility directly, we optically measured the amount of presynaptic Ca2+ influx at the MF–CA3 synapse (Kamiya and Ozawa, 1999). Figure1A shows representative presynaptic Ca2+ transients (FCa) when single or paired stimulation was given to the mossy fibers. The amplitude of the fluorescence transient elicited in response to the second stimulus was considerably larger than that elicited by the first stimulus (Fig.1B). This effect lasted for several hundreds of milliseconds (Fig.2A,B). At interstimulus intervals (ISI) of 50 msec, the ratio of the second response to the first one (F2/F1) was 121 ± 2% (n = 22). This result was in contrast with that found for the Schaffer collateral–CA1 synapse (Wu and Saggau, 1994; Kamiya and Ozawa; 1998), in which the ratio never exceeds 1. It should be noted that EPSPs showed substantial PPF at ISIs longer than 300 msec, whereas the facilitation of Ca2+ transients disappeared completely at the time. This finding suggests that facilitation is attributable, at least in part, to mechanisms other than the increase in FCa.
Fig. 1.
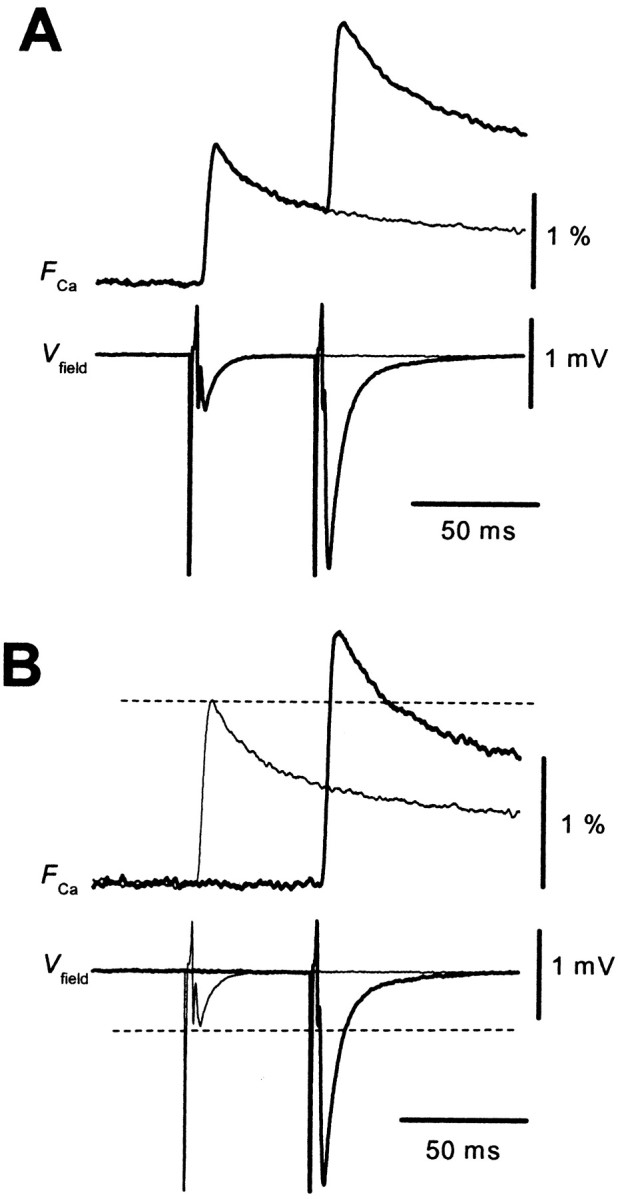
PPF of presynaptic Ca2+transients at the MF–CA3 synapse. A, Representative records of presynaptic Ca2+ transients (FCa) and simultaneously recorded field EPSPs (Vfield) evoked by single (thin traces) or paired-pulse stimulation (thick traces) with an ISI of 50 msec. Traces are the average of 10 sweeps. B, The second response to the paired stimulation was extracted by subtracting that evoked by the single stimulus and superimposed with the single response for comparison. Note that the second response was considerably larger than the first one.
Fig. 2.
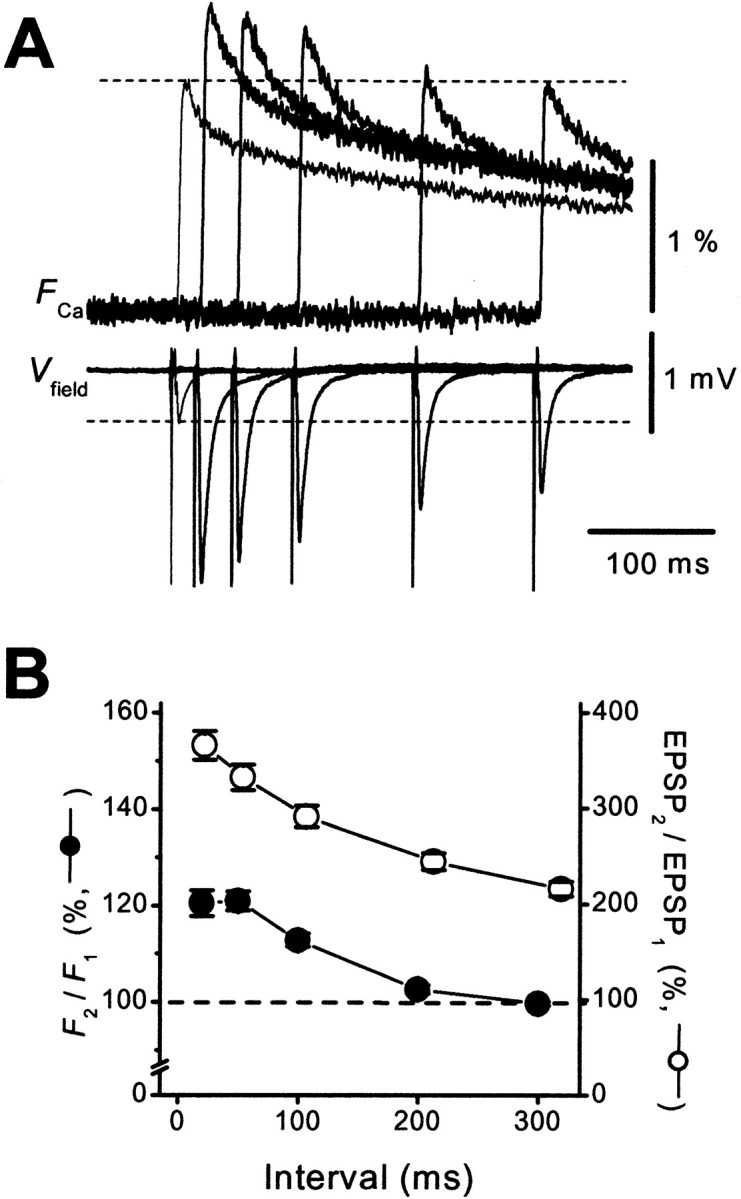
Time course of facilitation ofFCa. A, The first and second responses were calculated and displayed as in Figure1B. B, Ratios ofFCa(F2/F1; ●) and field EPSPs (EPSP2/EPSP1; ○) were plotted against ISI (20–300 msec; n = 22).
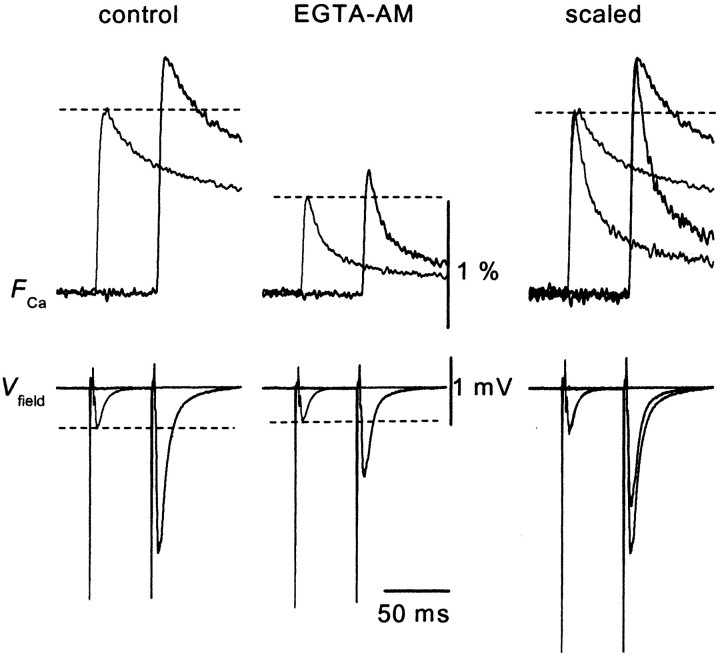
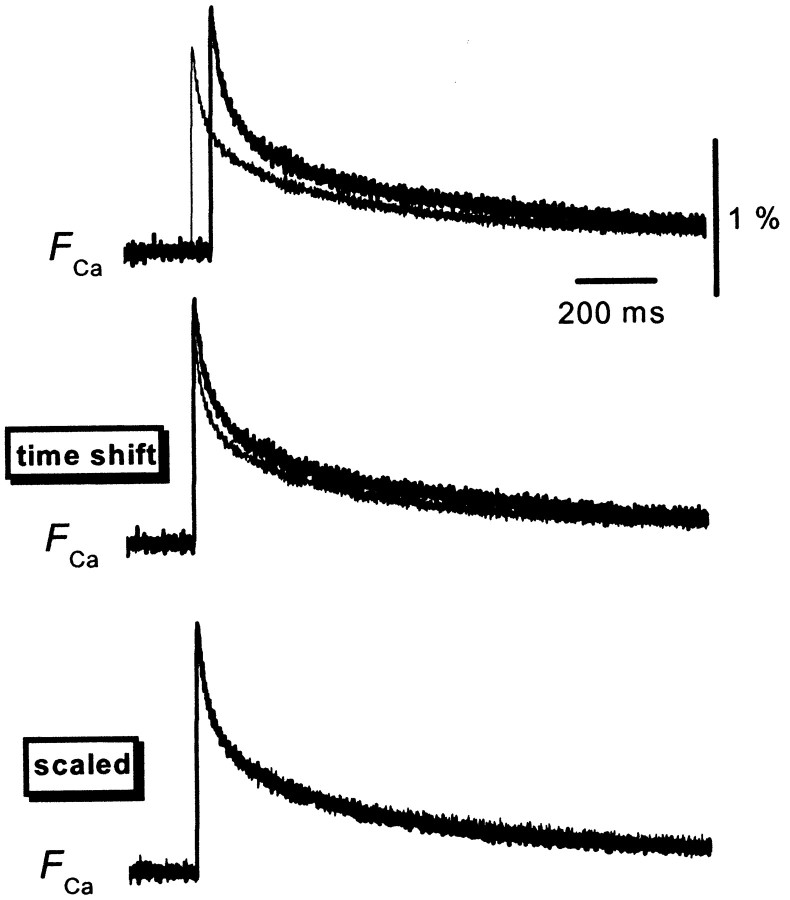
We next explored the mechanism of PPF ofFCa. This phenomenon may reflect increased Ca2+ influx during paired stimuli (Jackson et al., 1991; Borst and Sakmann, 1998; Cuttle et al., 1998; Brody and Yue, 2000; Lee et al., 2000; DeMaria et al., 2001;Currie and Fox, 2002; Tsujimoto et al., 2002). Alternatively, it may be attributable to saturation of the endogenous mobile high-affinity Ca2+ buffer (Neher, 1998). Modulation of the degree of saturation of endogenous Ca2+ buffers by the Ca2+ influx elicited by the first action potential could result in enhanced transmitter release simply by an increased increment of intraterminal Ca2+. In fact, it has been demonstrated that such a supralinear summation of Ca2+ transients in response to repetitive depolarizing pulses occurs in cerebellar Purkinje cells (Maeda et al., 1999), which express a high-affinity Ca2+binding protein calbindin D28k. This possibility of saturable Ca2+ buffer must be carefully explored, because calbindin D28k is exclusively expressed in hippocampal MF terminals, and knock-out mice of this protein exhibit reduced PPF at MF–CA3 synapses but not at CA1 synapses (Klapstein et al., 1998). Altered short-term plasticity has also been reported recently in knock-out mice of parvalbumin (Caillardet al., 2000), which is another Ca2+ binding protein with an EF-hand motif. To test the possibility of Ca2+ buffer saturation, we used the membrane-permeable slow Ca2+ chelator EGTA AM (Atluri and Regehr, 1996; Salin et al., 1996) to perturb intraterminal Ca2+ buffering. Bath application of 100 μm EGTA AM for 20 min reduced the amplitude of the EPSP andFCa to 81 ± 8 and 63 ± 4% of the control levels (n = 8) (Fig.3), respectively. The slight inhibition of the first EPSP might suggest that the release sites are not in the immediate vicinity of the Ca2+ channels at this particular synapse (Salin et al., 1996). On the other hand, the ratioF2/F1was not changed significantly by application of EGTA AM (121 ± 4 and 119 ± 3% in the absence and presence of EGTA AM, respectively; n = 8). This result suggests that saturation of endogenous Ca2+ buffer during PPF is not significant at this synapse, and observed facilitation of FCa is likely to be explained by genuine facilitation of presynaptic Ca2+ influx. In line with this notion, the time course of the facilitated FCa was not significantly different from that of the unconditioned responses (Fig. 4).
Fig. 3.
Effect of 100 μm EGTA AM, a membrane-permeable Ca2+ chelator, on PPF ofFCa (50 msec ISI). Application of EGTA AM reduced the amplitude and accelerated the decay time course of the Ca2+ signal, confirming the loading of the presynaptic terminals with EGTA in these experimental conditions. However, the facilitation ratio did not change significantly, as demonstrated by the peak-scaled traces in the right panels.
Fig. 4.
Comparison of the time course of theFCa evoked by the first and second stimuli delivered at 50 msec ISI. The top traces are the superimposition of the first and second responses calculated as in Figure 1B. In the middle traces, the second response is shifted for 50 msec to adjust for the timing of the stimulus. Note the lack of obvious difference in the time course of these signals, as demonstrated in the bottom traces, in which the peak amplitudes were scaled.
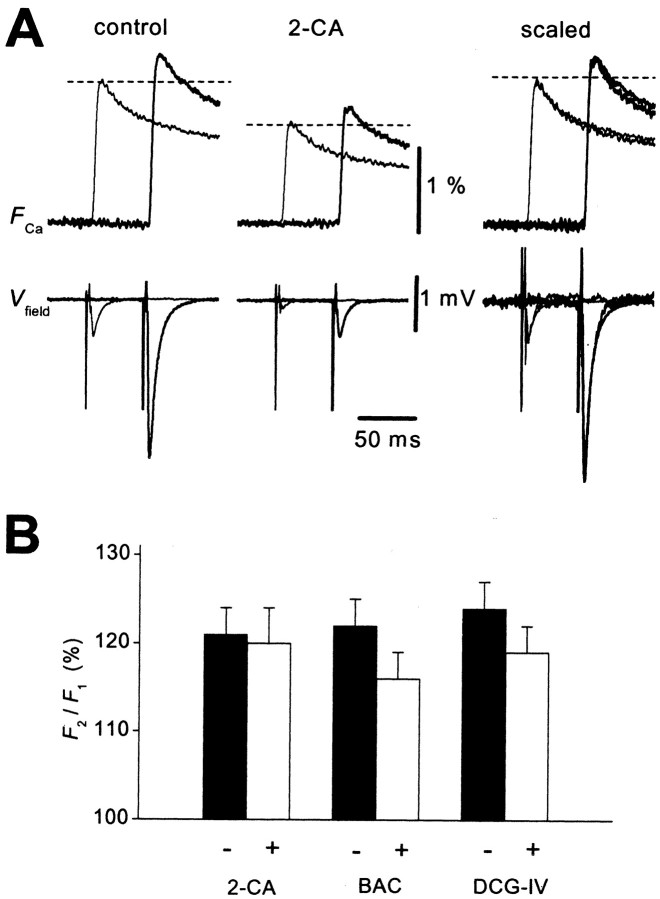
Another possible mechanism is the facilitation of the Ca2+ current attributable to either the acceleration of activation (Borst and Sakmann, 1998; Cuttle et al., 1998; Tsujimoto et al., 2002) or the relief of G-protein inhibition (Park and Dunlap, 1998; Brody and Yue, 2000). MF terminals express several G-protein-coupled autoreceptors whose activation leads to inhibition of presynaptic Ca2+ currents. Among them, adenosine A1 and GABAB receptors are activated tonically, whereas group II metabotropic glutamate (mGlu) receptors are not (Yamamoto et al., 1993; Kamiya et al., 1996; Vogt and Nicoll, 1999). Therefore voltage-dependent relief of tonic inhibition of Ca2+ channels through A1 or GABAB receptors might occur during paired stimuli. To test this possibility, we next examined the effect of pharmacological activation of these G-protein-coupled autoreceptors. Application of a selective agonist of A1 receptors, 2-chloro-adenosine (2-CA) at 10 μm, reduced the amplitude of the first EPSP andFCa to 22 ± 4 and 62 ± 4% of the control value (n = 7), respectively. However, the ratio of the second response to the first one (F2/F1) did not change significantly (121 ± 3 and 120 ± 4% in the absence and presence of 2-CA, respectively; n = 7) (Fig.5A,B). Similar results were obtained for the GABABreceptor agonist baclofen and the group II mGlu receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV). Baclofen at 10 μm decreased the first EPSP and FCa to 14 ± 4 and 60 ± 3% of the control value (n = 6), respectively, but the ratioF2/F1did not change significantly (122 ± 2 and 116 ± 3% in the absence and presence of baclofen, respectively; n = 6) (Fig. 5B). DCG-IV at 1 μm also decreased the first EPSP and FCa to 11 ± 5 and 64 ± 3% of the control value (n= 6), but the ratio did not change significantly (124 ± 3 and 119 ± 3% in the absence and presence of DCG-IV, respectively;n = 6) (Fig. 5B). Thus, it is unlikely that relief of G-protein inhibition of Ca2+channels (Park and Dunlap, 1998; Brody and Yue, 2000) is involved in the PPF of FCa at this synapse.
Fig. 5.
PPF of FCa is unchanged during inhibition of Ca2+ channels via G-protein-coupled metabotropic receptors. A, Representative records of FCa evoked by paired-pulse stimulation (50 msec ISI) before (left) and after (middle) application of 10 μm 2-CA, an agonist of adenosine A1 receptor. 2-CA reduced both the first and second responses to a similar degree, whereas the facilitation ratio did not change significantly (scaled traces;right). B, Summary graph for the effects of 2-CA (10 μm; n = 7), the GABAB receptor agonist baclofen (Bac; 10 μm; n = 6), and the group II metabotropic glutamate receptor agonist DCG-IV (1 μm;n = 6) on the facilitation ratio of theFCa(F2/F1).
Involvement of kainate autoreceptors in PPF of presynaptic Ca2+ transients
Schmitz et al. (2001b) reported that presynaptic KARs (Kullmann, 2001; Schmitz et al., 2001a; Kamiya, 2002), unique autoreceptors whose activation leads to the enhancement of transmitter release (Turecek and Trussell, 2001), might specifically contribute to frequency facilitation at this synapse. We therefore examined the involvement of KARs in PPF of presynaptic Ca2+ influx. For this purpose, we tested the effect of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a non-NMDA receptor antagonist. As reported previously (Kamiya and Ozawa, 1999), CNQX at 10 μm, which suppressed field EPSPs completely, did not affect the FCa in response to a single stimulus (97 ± 2% of control; n = 10), suggesting that the fluorescence signals originated exclusively from the presynaptic structure in these measurements. In contrast, PPF ofFCa was selectively reduced by application of CNQX (121 ± 2 and 108 ± 2% in the absence and presence of CNQX, respectively; n = 10;p < 0.01) (Fig.6A,B). The AMPA receptor-selective blocker 1-(4-aminophenyl)-4-methyl-7,8-methylene-dioxy-5H-2,3-benzodiazepine (GYKI 52466) hydrochloride at 100 μm, which also abolished field EPSP completely, did not mimic this effect (120 ± 2 and 115 ± 3% in the absence and presence of GYKI 52466; n = 7; p = 0.10) (Fig.6B), suggesting that activation of KARs underlies PPF of presynaptic Ca2+ influx. The observation that blocking KARs affect PPF of theFCa raised the question of whether presynaptic KARs are activated substantially during paired-pulse protocols. To examine whether the preceding “single” stimulus is able to activate presynaptic KARs and to cause substantial axonal depolarization, we used optical measurement after selective labeling of MF with the voltage-sensitive dye di-8-ANEPPS (Sabatini and Regehr, 1996, 1997) as in Figure 7A. The presynaptic voltage transient (Fv) consists of fast and slow components (Fig. 7B), possibly representing action potential and afterdepolarization of MF axons (Geiger and Jonas, 2000). Application of 10 μmCNQX reduced the amplitudes of the slow but not the fast components (52 ± 2 and 96 ± 1% of control, respectively;n = 12; p < 0.01) (Fig.8A,B), whereas 1 μm TTX blocked both components of the signal. GYKI 52466 at 100 μm suppressed the slow component to 77 ± 3%, whereas the fast component was little affected (96 ± 1%; n = 5; p < 0.01) (Fig. 8B). The effect of GYKI 52466 on the slow component of Fv was weaker than that of CNQX (77 ± 3 and 52 ± 2%, respectively;p < 0.01). These results indicate that kainate autoreceptors mediate a prominent part of afterdepolarization of MF axons and thereby modulate the amount of presynaptic Ca2+ influx elicited by a subsequent stimulus. In support of this notion, the time course of the slow component of Fv (Fig. 7B) approximates to that of the PPF of FCa(Fig. 2B, filled circles).
Fig. 6.
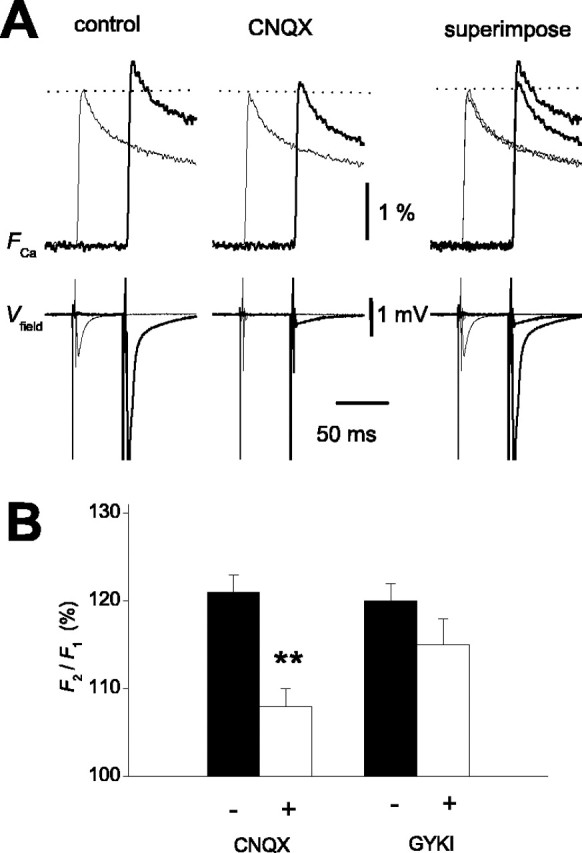
KAR involvement in PPF ofFCa. A, Effect of CNQX on PPF of FCa. Application of 10 μmCNQX suppressed the second response, whereas the first one was little affected. Selective inhibition of the facilitation ratio by CNQX is revealed in the superimposed (right) traces.B, Summary graph for the effect of 10 μmCNQX (n = 10) and the AMPA receptor-selective antagonist GYKI 52466 (100 μm; n = 7; **p < 0.01).
DISCUSSION
Activity-dependent plasticity of presynaptic Ca2+ influx at the MF-CA3 synapse
Although the residual Ca2+ hypothesis has often been postulated to explain PPF (Zucker, 1989), recent evidence suggests that considerable revision of this hypothesis may be needed for some synapses (Zucker and Regehr, 2002). One aspect of the revision is involvement of a different Ca2+-dependent process from exocytosis (Kamiya and Zucker, 1994; Atluri and Regehr, 1996; Zucker and Regehr, 2002). Another consideration is whether the amount of presynaptic Ca2+ influx per action potential is modified in an activity-dependent manner (Jackson et al., 1991; Borst and Sakmann, 1998; Cuttle et al., 1998; Brody and Yue, 2000; Lee et al., 2000; DeMaria et al., 2001; Currie and Fox, 2002; Tsujimoto et al., 2002). We demonstrated here that unusually large PPF at the hippocampal MF–CA3 synapse was accompanied by facilitation of presynaptic Ca2+ transients (FCa), in contrast to the Schaffer collateral–CA1 synapse (Wu and Saggau, 1994; Kamiya and Ozawa, 1998).
One may argue that recruitment of more fibers with the second stimulus underlies the observed facilitation ofFCa. Afterdepolarization of MF axons (Fig. 7) (Geiger and Jonas, 2000) may lower threshold for the second stimulus. However, Schmitz et al. (2000) demonstrated that the site of activation of KA autoreceptors are restricted in the stratum lucidum (MF termination zone) but not in the granule cell layer. Because the stimulating electrode was placed in the granule cell layer in the present study, recruitment of subthreshold fibers is less likely to contribute to the results. In support of this idea, the amplitude of presynaptic fiber volley was not augmented by paired stimuli, as illustrated in Figure 1A.
Other possible artifacts, e.g., saturation of the Ca2+ indicator or polarization of stimulating electrode, would be expected to counteract the facilitation of FCa. Consistent with this notion, presynaptic Ca2+ transients at the CA1 synapse, which exhibits smaller PPF than at the MF–CA3 synapse, decrease slightly in response to paired stimuli attributable to saturation of the high-affinity dye (Wu and Saggau, 1994).
The effect of EGTA AM was complicated by the fact that it affects both the time course and the amplitude of the fluorescence transients. Bath application of 100 μm EGTA AM reduced the amplitude ofFCa by 37% and that of EPSPs by 19% on average. Analysis of the quantitative relationships between EPSPs and FCa by changing extracellular Ca2+ concentrations (Kamiya and Ozawa, 1999) revealed a supralinear relationship at this particular synapse. Therefore, we suppose that sublinear dependency of EPSPs onFCa during EGTA AM treatment does not imply that transmitter release at this synapse is very weakly sensitive to Ca2+ but rather reflects the limited spatial and temporal resolution of the recording system. Our methods do not allow detection of the localized peak Ca2+ transients at active zone that is responsible for transmitter release but only provide a measure for the volume-averaged Ca2+ changes within the whole presynaptic terminals. Because of this limitation, a slow Ca2+ chelator such as EGTA would be expected to preferentially suppress the volume-averaged Ca2+ transient (which we measured in this study) with relatively small effects on the large, brief calcium increases that trigger release. In support of this idea, Atluri and Regehr (1996) also reported sublinear dependency (decrease in peak Ca2+ by 45% and reduction of EPSC by 42%) during EGTA AM application at 100 μm (the same concentration as in this study) in the similar multifiber presynaptic Ca2+ measurement in cerebellar synapses using lower affinity dyes, which is expected to reflect undistorted Ca2+ transients. More importantly, EGTA did not affect the facilitation ratio of theFCa (Fig. 3), suggesting that PPF of the FCa is less likely attributable to the possible saturation of the endogenous Ca2+ buffer.
With these considerations, we conclude that the activity-dependent short-term plasticity of FCa at the MF–CA3 synapses is most likely interpretable by facilitation of presynaptic Ca2+ influx. This novel mechanism of amplifying Ca2+ signaling within the presynaptic MF terminals supports an extremely wide dynamic range of activity-dependent regulation of the synaptic efficacy (Salin et al., 1996; Henze et al., 2000) in concert with the action of residual Ca2+ (Regehr et al., 1994).
Kainate autoreceptor involvement in PPF of presynaptic Ca2+ influx
We demonstrated pharmacologically that KARs are involved in PPF ofFCa and prominent afterdepolarization of MFs. However, it should be noted that GYKI 52466 at 100 μm weakly reduced both PPF of Ca2+ signals (although statistically not significant) and presynaptic afterdepolarization, as shown in Figures6B and 8B. These findings may reflect relatively poor selectivity of this antagonist for AMPA versus KA receptors. Although GYKI 52466 is the most selective commercially available AMPA receptor-selective antagonist, it was reported that 100 μm GYKI 52466 weakly inhibited KARs (to ∼70–80% of control) while almost completely blocking AMPA receptors in cultured hippocampal neurons (Paternain et al., 1995).
One missing link in this study is whether facilitation ofFCa mediates synaptic PPF. The suppression of facilitation of FCa by CNQX does, however, strongly support a causal relationship. Although CNQX blocked field EPSPs and therefore may not be used to examine the effect on synaptic PPF, Schmitz et al. (2001b) bypassed this problem by measuring NMDA receptor-mediated EPSCs (EPSCNMDA) at positive membrane potential and found that CNQX reduces facilitation of EPSCNMDA during 25 Hz (40 msec ISI) train (close to our conditions of 50 msec ISI) (Fig. 6) without affecting the first responses. The similar (but not identical) time course between them (Fig. 2B) also strongly suggests thatFCa facilitation underlies synaptic PPF.
How does activation of kainate autoreceptors lead to facilitation of presynaptic Ca2+ influx? It is possible that depolarization of MF axons (Geiger and Jonas, 2000) may inactivate K+ channels shaping repolarization of presynaptic action potentials, thereby increasing Ca2+ influx. However, the results obtained by direct whole-terminal recordings from MF boutons (Geiger and Jonas, 2000) suggests that broadening of action potentials is minimal with the PPF protocol used in this study (e.g., 1.3% prolongation per action potential at 50 Hz). In fact, the duration of the fast component ofFV was not prolonged by paired stimuli delivered at 50 msec ISI (H. Kamiya, unpublished observation).
Another possible mechanism is the facilitation of presynaptic Ca2+ channels. Whole-cell recordings from the calyx-type presynaptic terminals in the brainstem have revealed that depolarizing prepulses resulted in shot-term facilitation of the presynaptic Ca2+ current (Borst and Sakmann, 1998; Cuttle et al., 1998; Currie and Fox, 2002). It was demonstrated that calmodulin (Lee et al., 2000; DeMaria et al., 2001) or neuronal calcium sensor 1 (Tsujimoto et al., 2002) is involved in this action. Because fluorescence measurement of presynaptic voltage revealed prominent afterdepolarization of MF axons after a single stimulus (Fig. 7B) (Geiger and Jonas, 2000), the first action potential as well as the subsequent afterdepolarization may modify the state of Ca2+ channels and thereby facilitate Ca2+ current in response to the second action potential. It should be noted that, although it has been proposed that relief of G-protein inhibition of Ca2+ channels is involved in short-term plasticity in cultured hippocampal neurons (Brody and Yue, 2000), this mechanism was not responsible for facilitation of presynaptic Ca2+ influx observed in this study, because the pharmacological activation of G-protein-coupled metabotropic receptors failed to affect this phenomenon significantly (Fig. 5). Slight decrease in theF2/F1ratio by 2-CA, baclofen, or DCG-IV, although statistically insignificant, might be explained by the reduction in glutamate release and subsequent activation of KA autoreceptors.
The novel mechanism of short-term plasticity revealed in this study may also be important for the induction of long-term potentiation (LTP) and long-term depression (LTD) at this synapse, because these forms of long-term plasticity depend on Ca2+accumulation within MF terminals (Castillo et al., 1994; Kobayashi et al., 1996) (but see Yeckel et al., 1999). In support of this notion, it has been demonstrated that MF–LTP is impaired in GluR6-deficient mice (Contractor et al., 2001) or by GluR5 antagonist LY 382884 (Bortolotto et al., 1999; Lauri et al., 2001), although there remains a substantial debate about this issue (Nicoll et al., 2000).
Activity-dependent regulation of signal transfer at the MF–CA3 synapse is extremely complex, i.e., homosynaptic and heterosynaptic activity-dependent presynaptic modulation mediated via mGlu- (Kamiya et al., 1996; Vogt and Nicoll, 1999), GABAB- (Vogt and Nicoll, 1999), and NMDA receptor-independent forms of LTP (Zalutsky and Nicoll, 1990) and LTD (Kobayashi et al., 1996). The novel mechanism of presynaptic plasticity involving the kainate autoreceptor system, as revealed in this study, must be also taken into account. The multiple autoreceptor systems, as well as the structural peculiarity of the MF–CA3 synapse (Henze et al., 2000), support an especially large dynamic range of activity-dependent tuning of the synaptic strength and therefore is important for information processing in the hippocampus.
Footnotes
This work was supported by Grants-in-Aid for Science Research (H.K., S.O., and T.M.), by Special Coordination Funds for Promoting Science and Technology (T.M.) from the Ministry of Education, Science, Sports, Culture and Technology of Japan, and by the grants from the Ichiro Kanehara Foundation and the Novartis Foundation (Japan) for the Promotion of Science (T.M.). We thank Prof. Atsu Aiba for reading this manuscript.
Correspondence should be addressed to Haruyuki Kamiya, Division of Cell Biology and Neurophysiology, Department of Neuroscience, Faculty of Medicine, Kobe University, Kobe, Hyogo 650-0017, Japan. E-mail:hkamiya-kob@umin.ac.jp.
REFERENCES
- 1.Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Borst JG, Sakmann B. Facilitation of presynaptic calcium currents in the rat brainstem. J Physiol (Lond) 1998;513:149–155. doi: 10.1111/j.1469-7793.1998.149by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- 5.Brody DL, Yue DT. Relief of G-protein inhibition of calcium channels and short-term synaptic facilitation in cultured hippocampal neurons. J Neurosci. 2000;20:889–898. doi: 10.1523/JNEUROSCI.20-03-00889.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA. 2000;97:13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 8.Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- 9.Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 10.Currie KP, Fox AP. Differential facilitation of N- and P/Q-type calcium channels during trains of action potential-like waveforms. J Physiol (Lond) 2002;539:419–431. doi: 10.1113/jphysiol.2001.013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol (Lond) 1998;512:723–729. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 13.Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 14.Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 15.Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA. 1991;88:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamiya H. Kainate receptor-dependent presynaptic modulation and plasticity. Neurosci Res. 2002;42:1–6. doi: 10.1016/s0168-0102(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol (Lond) 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiya H, Ozawa S. Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J Physiol (Lond) 1999;518:497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol (Lond) 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 21.Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapse. J Physiol (Lond) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klapstein GJ, Vietla S, Lieberman DN, Gray PA, Airaksinen MS, Thoenen H, Meyer M, Mody I. Calbindin-D28k fails to protect hippocampal neurons against ischemia in spite of its cytoplasmic calcium buffering properties: evidence from calbindin-D28k knockout mice. Neuroscience. 1998;85:361–373. doi: 10.1016/s0306-4522(97)00632-5. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 24.Kreitzer AC, Regehr WG. Modulation of transmission during trains at a cerebellar synapse. J Neurosci. 2000;20:1348–1357. doi: 10.1523/JNEUROSCI.20-04-01348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullmann DM. Presynaptic kainite receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 26.Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainite receptor in mossy fiber LTP. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerma J, Paternain AV, Rodríguez-Moreno A, López-García JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- 29.Maeda H, Ellis-Davies GC, Ito K, Miyashita Y, Kasai H. Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron. 1999;24:989–1002. doi: 10.1016/s0896-6273(00)81045-4. [DOI] [PubMed] [Google Scholar]
- 30.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 31.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 32.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 33.Nicoll RA, Mellor J, Frerking M, Schmitz D. Kainate receptors and synaptic plasticity. Nature. 2000;406:957. doi: 10.1038/35023075. [DOI] [PubMed] [Google Scholar]
- 34.Park D, Dunlap K. Dynamic regulation of calcium influx by G-proteins, action potential waveform, and neuronal firing frequency. J Neurosci. 1998;18:6757–6766. doi: 10.1523/JNEUROSCI.18-17-06757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 36.Regehr WG, Atluri PP. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995;68:2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regehr WG, Tank DW. Selective fura-2 loading of presynaptic terminals and nerve cell processes by local perfusion in mammalian brain slice. J Neurosci Methods. 1991;37:111–119. doi: 10.1016/0165-0270(91)90121-f. [DOI] [PubMed] [Google Scholar]
- 38.Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 40.Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2001a;98:11003–11008. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001b;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- 46.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- 47.Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- 48.Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci USA. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto C, Sawada S, Ohno-Shosaku T. Quantal analysis of modulating action of adenosine on the mossy fiber synapse in hippocampal slices. Hippocampus. 1993;3:87–92. doi: 10.1002/hipo.450030109. [DOI] [PubMed] [Google Scholar]
- 51.Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zalutsky RT, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 53.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 54.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]