Abstract
Children and adolescents are increasingly exposed to psychostimulants, either illicitly or for the treatment of common neuropsychiatric conditions, such as attention deficit disorder with and without hyperactivity. Despite the widespread use of psychomotor stimulants in younger age groups, little is known regarding the chronic molecular neuroadaptive responses to these agents in the immature brain. Here we demonstrate that, after chronic administration of the psychostimulants cocaine and amphetamine, the transcription factor ΔFosB is upregulated in the nucleus accumbens of periadolescent mice but not in post-weanling or adult mice. Induction of ΔFosB also occurs exclusively in the caudate putamen of periadolescent mice after amphetamine administration. These results demonstrate the unique plasticity in the adolescent brain of a critical molecule that regulates psychostimulant action and suggest that these neuroadaptive changes may be involved in the mediation of enhanced addictive tendencies in the adolescent relative to the adult.
Keywords: cocaine, amphetamine, development, ΔFosB, mice, psychostimulants, nucleus accumbens, caudate putamen, periadolescent
Psychostimulants are used in the treatment of common childhood disorders, such as attention deficit hyperactivity disorder. In addition, abuse of stimulants, including amphetamine and cocaine, is common among adolescents, an age at which there is evidence for enhanced addictive tendencies relative to adults (Estroff et al., 1989; Myers and Anderson, 1991). Despite data indicating developmentally regulated behavioral effects, little is known regarding the molecular neuroadaptive responses in the immature brain that occur during the time of administration of these agents. Cocaine and amphetamine may effect long-lasting behavioral changes partially via stimulation of dopamine D1receptors and increases in levels of transcription factors, including ΔFosB, in the dorsal striatum (i.e., caudate putamen) and the ventral striatum (i.e., nucleus accumbens) (Chen et al., 1997). Increases in levels of ΔFosB, perhaps via stabilization of protein products, is sustained for several weeks after chronic exposure to cocaine or amphetamine and is regulated at least in part by the dopamine signal transduction pathway (Chen et al., 1997; Nestler et al., 2001).
The central dopaminergic system of young animals is very much in flux as a result of the changing levels of critical molecules during normal development, including the dopamine D1receptor DARPP-32 (dopamine and cAMP regulated phosphoprotein; Mr of 32 kDa) and cAMP (Ehrlich et al., 1990;Teicher et al., 1993; Perrone-Capano et al., 1996; Tarazi et al., 1999;Andersen, 2002). Exposure during this period to psychostimulants, which enhance dopaminergic neurotransmission, may therefore result in quantitatively and/or qualitatively different molecular responses, including alterations in ΔFosB expression. To test the hypothesis that there are age-dependent neuroadaptive responses during chronic exposure to psychostimulants, three groups of mice were analyzed in serial experiments: adults (60 d old at onset of injections), periadolescent (33 d old at onset of injections), and post-weanling (24 d old at onset of injection). This is the first direct comparison of molecular neuroadaptive responses to chronic psychostimulant exposure in these three age groups. We found that, after identical treatment paradigms, periadolescent mice show enhanced ΔFosB upregulation in response to both cocaine and amphetamine.
MATERIALS AND METHODS
Animals and drug administration. Male CD-1 mice (Charles River Laboratories, Kingston, NY) were housed on a 12 hr light/dark cycle (6:00 A.M. to 6:00 P.M.) with ad libitumaccess to food and water. Animals were allowed to accommodate to the animal room for a minimum of 10 d before the initiation of injections. Animals were handled by two investigators who performed all injections in the same room in which the animals were housed. All animals were weaned at 21 d of age. Injections began at 24 (post-weanling), 33 (periadolescent), or 60 (adult) d of age. Animals received 20 mg/kg cocaine (Sigma, St. Louis, MO), 5 mg/kg amphetamine (Sigma), or an equal volume of saline intraperitoneally between 4:00 and 5:00 P.M. daily for 7 d. Animals were killed by decapitation after brief exposure to CO2 at 10:00 A.M. on the day after the final injection. Brains were immediately removed from the skull, and the caudate putamen and nucleus accumbens were rapidly dissected on ice. All dissections were performed from coronal brain slices by a single investigator, and protein extracts were prepared from fresh tissue without freezing. All animal procedures were approved by the Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Western blot analysis. For Western blot analyses, equal amounts of protein (40 μg for caudate putamen and 20 μg for nucleus accumbens) from each sample were loaded in each lane of a 10% SDS-polyacrylamide gel after measurement of protein concentrations with the BCA assay (Pierce, Rockford, IL). Equal protein loading was also verified by visualization of total protein by Ponceau Red after transfer to nitrocellulose and/or blotting with anti-actin antibody (1:500; Sigma). The Fos-related antigen (FRA) antiserum, which recognizes the ΔFosB isoforms, was generously provided by Dr. M. Iadarola (National Institutes of Health, Bethesda, MD) and used at a concentration of 1:4000. Previous studies (Chen et al., 1997; Hiroi et al., 1997), including preadsorption of the FRA antiserum with the M-peptide immunogen, demonstrated the specificity of this antiserum. The DARPP-32 5a monoclonal antibody, used at 1:10,000, was generously provided by Drs. Hugh Hemmings and Paul Greengard (The Rockefeller University, New York, NY). The dopamine transporter (DAT) antibody was from Chemicon (Temecula, CA). Blots were reacted with NEN-DuPont (Boston, MA) chemiluminescence system and exposed to film. Densitometric values for ΔFosB immunoreactivity were obtained using ScanAnalysis for Apple (Biosoft, Ferguson, MO). Statistical significance was determined using a one-way ANOVA, followed by post hoc Tukey's multiple comparison test or an unpaired, two-tailed Student's t test as indicated in the figure legends. For the drug treatment experiments, analysis of each age group was performed on a separate blot, and, therefore, each saline group was arbitrarily assigned a 100% value for comparison between age groups. For the ontogeny studies, samples from all age groups were analyzed together on a single blot.
RESULTS
Induction of ΔFosB after cocaine and amphetamine occurs in the nucleus accumbens of only periadolescent mice
The expression of ΔFosB was measured in the nucleus accumbens and caudate putamen of post-weanling, periadolescent, and adult mice after 7 d of amphetamine or cocaine administration. The nucleus accumbens is the brain region believed to be most critical for mediating the rewarding effects of psychostimulants. ΔFosB immunoreactivity (35 kDa) was selectively induced in the nucleus accumbens of periadolescent animals after chronic administration of amphetamine (Fig. 1A) or cocaine (Fig. 1B). In contrast, levels of ΔFosB (35 kDa) were not significantly altered in the nucleus accumbens of post-weanling or adult animals (Fig.1A,B). In the caudate putamen, ΔFosB levels (35 kDa) were also significantly upregulated after chronic amphetamine administration only in periadolescent animals (Fig.2A). All three age groups showed significant increases in ΔFosB (35 kDa) expression in the caudate putamen after chronic administration of cocaine (Fig.2B). The magnitude of induction, however, was greatest in the periadolescent animals, particularly compared with post-weanlings (Fig. 2B). Other FRA and Fos isoforms were unaltered in all age groups (data not shown).
Fig. 1.
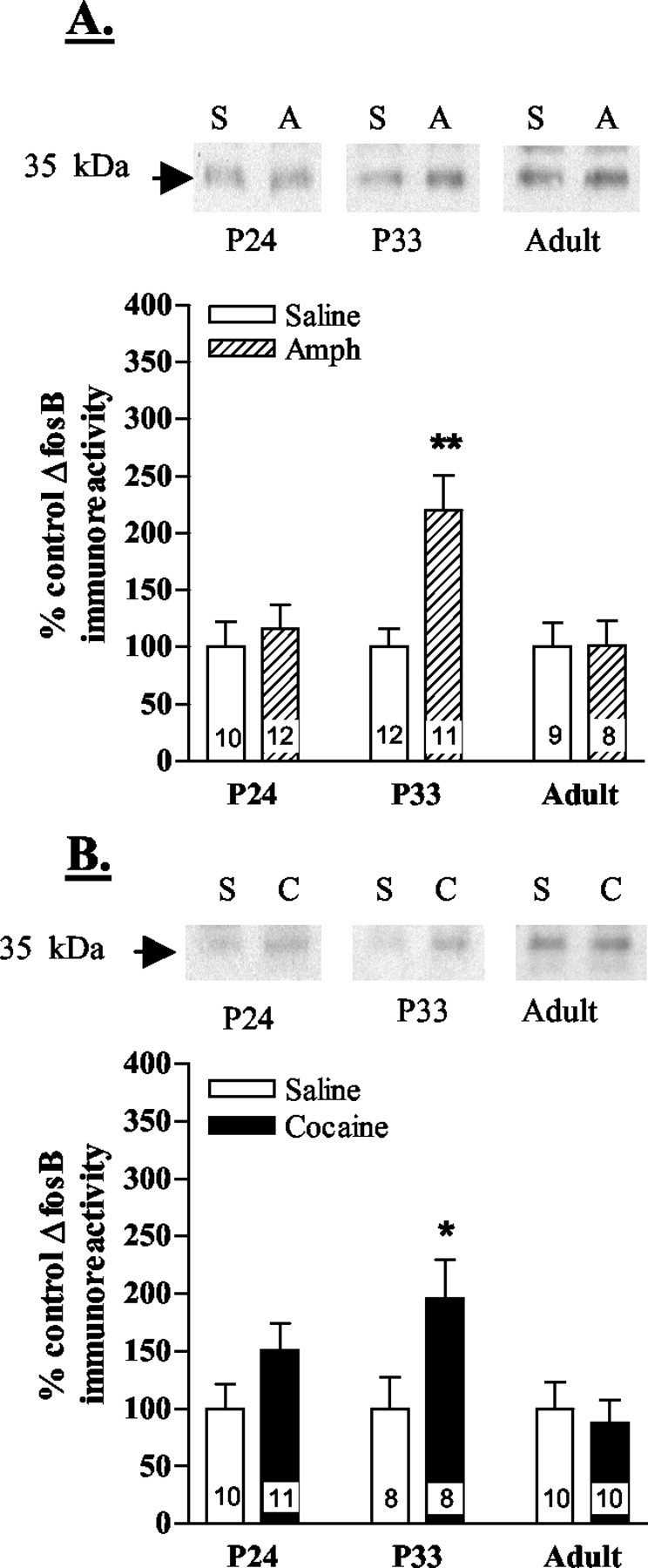
ΔFosB immunoreactivity in the nucleus accumbens after chronic psychostimulant administration. CD-1 mice were injected once daily with saline, amphetamine, or cocaine for 7 d beginning on day 24 (P24; post-weanling), day 33 (P33; periadolescent), or day 60 (Adult). Levels of ΔFosB (35 kDa) immunoreactivity in the nucleus accumbens are shown after chronic amphetamine (A) or cocaine (B) administration. Representative immunoblots from saline- (S), amphetamine- (A), and cocaine- (C) injected post-weanling (P24), periadolescent (P33), and adult mice are shown in the top panels. Bottom panels show mean ± SEM percentage of basal ΔFosB expression. n values for each group are shown in the bars. Significant increases in ΔFosB were found in the nucleus accumbens of only the periadolescent mice. *p < 0.05; **p < 0.01 (Student's t test; saline vs drug).
Fig. 2.
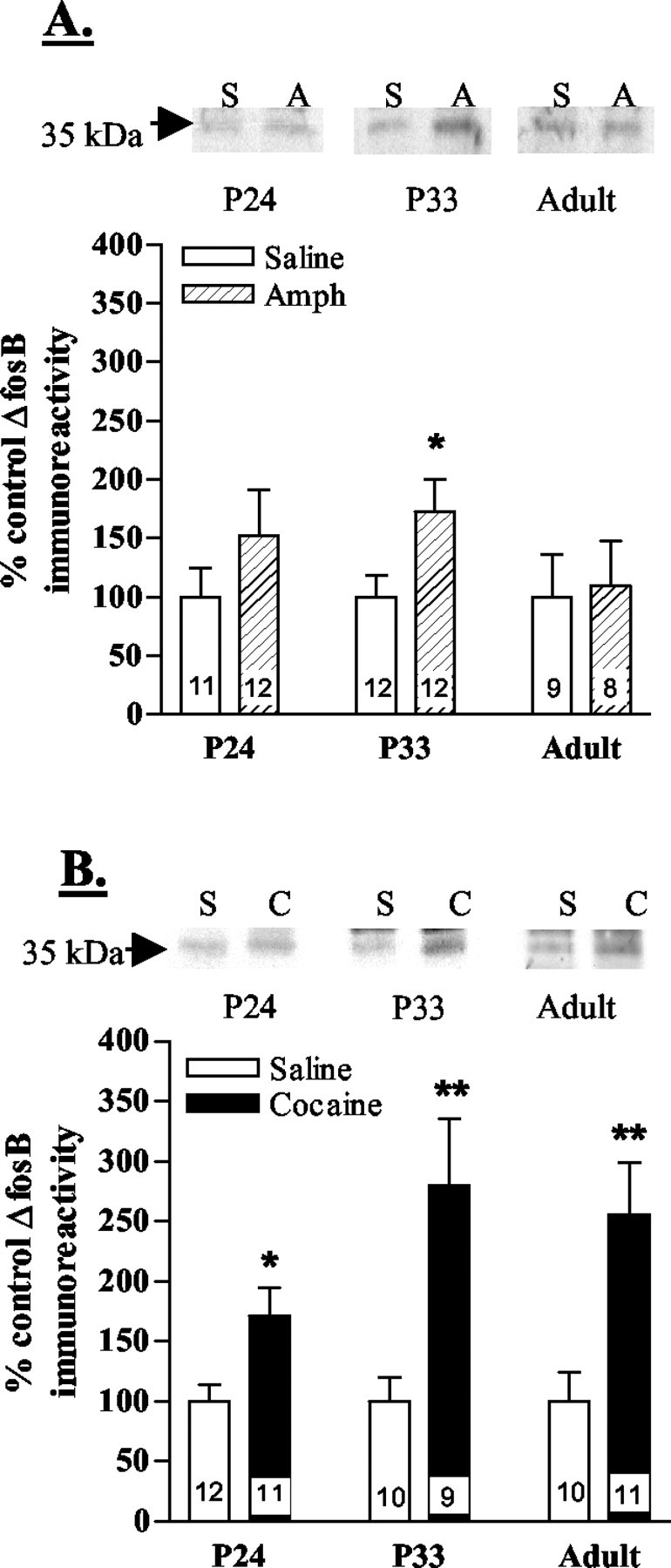
ΔFosB immunoreactivity in the caudate putamen after chronic psychostimulant administration. CD-1 mice were injected once daily with saline, amphetamine, or cocaine for 7 d beginning on day 24 (P24; post-weanling), day 33 (P33; periadolescent), or day 60 (Adult). Levels of ΔFosB (35 kDa) immunoreactivity in the caudate putamen are shown after chronic amphetamine (A) or cocaine (B) administration. Representative immunoblots from saline- (S), amphetamine- (A), and cocaine- (C) injected periadolescent mice (P33) are shown in thetop panels. Bottom panels show mean ± SEM percentage of basal ΔFosB expression. n values for each group are shown in the bars. Significant amphetamine-induced increases in ΔFosB immunoreactivity were found in the caudate putamen of only periadolescent mice (A). Chronic cocaine administration produced increases in ΔFosB in all three age groups (B). *p < 0.05; **p < 0.01 (Student's t test; saline vs drug).
DAT and DARPP-32 levels are not altered after chronic cocaine or amphetamine
Several key molecules expressed by dopaminergic and/or dopaminoceptive neurons, including DARPP-32, the D1 dopamine receptor, and DAT, contribute to acute and chronic responses to psychostimulants (Moratalla et al., 1996; Fienberg et al., 1998; Sora et al., 1998; Gainetdinov et al., 2001). Data from DARPP-32, D1 receptor, and DAT null and DAT knock-down mice indicate a complicated relationship among their levels, the regulation of dopaminergic activity, and responses to psychostimulants. In fact, ΔFosB induction does not occur in DARPP-32 null mice that receive chronic cocaine (Fienberg et al., 1998). In adult mice, however, 7 d exposure to 20 mg/kg cocaine does not alter total levels of DARPP-32 (Fienberg et al., 1998). DAT protein regulation has not been reported previously in mice exposed chronically to psychostimulants, although alterations in radioligand binding to the dopamine transporter after exposure to psychostimulants has been reported in some species (Letchworth et al., 2001). Here we measured the levels of DARPP-32 and DAT protein to determine whether the expression of these proteins is altered after chronic psychostimulant administration in any of the three ages of mice. Our findings indicate that there were no significant changes in levels of total DARPP-32 or DAT in the entire caudate putamen or nucleus accumbens after chronic administration of either cocaine or amphetamine in any of the three age groups (Table 1).
Table 1.
Relative densitometry values for DARPP-32 and DAT in amphetamine- and cocaine-treated P24, P33, and adult mice relative to control, saline values, arbitrarily set at 100%
| Amphetamine | Cocaine | |||||
|---|---|---|---|---|---|---|
| P24 | P33 | Adult | P24 | P33 | Adult | |
| DARPP-32 | ||||||
| NA | 94.7 ± 23.4 (7) | 87.5 ± 13.0 (10) | 99.9 ± 5.4 (6) | 92.9 ± 11.3 (7) | 93.5 ± 10.4 (8) | 81.2 ± 24.4 (4) |
| CPu | 95.0 ± 3.8 (12) | 97.9 ± 7.9 (12) | 106.9 ± 9.5 (10) | 95.5 ± 6.4 (12) | 98.9 ± 6.8 (8) | 105.9 ± 9.7 (11) |
| DAT | ||||||
| NA | 125.2 ± 37.3 (6) | 106.2 ± 27.4 (5) | 84.6 ± 23.9 (7) | 83.6 ± 9.9 (12) | 104.6 ± 11.9 (11) | 94.4 ± 11.3 (5) |
| CPu | 82.9 ± 12.8 (12) | 102.5 ± 16.8 (12) | 69.2 ± 24.4 (8) | 102.8 ± 25.3 (12) | 114.3 ± 34.1 (8) | 123.8 ± 19.3 (11) |
Changes in DARPP-32 and DAT immunoreactivity after amphetamine and cocaine administration in post-weanling (P24), periadolescent (P33), and adult (P60) mice. Values shown represent percentage of control values determined for animals of the same age injected with saline. Numbers of animals in the amphetamine and cocaine groups are given in parentheses, and n values for saline-injected animals were similar in each case. p > 0.05 in all cases. NA, Nucleus accumbens; CPu, caudate putamen.
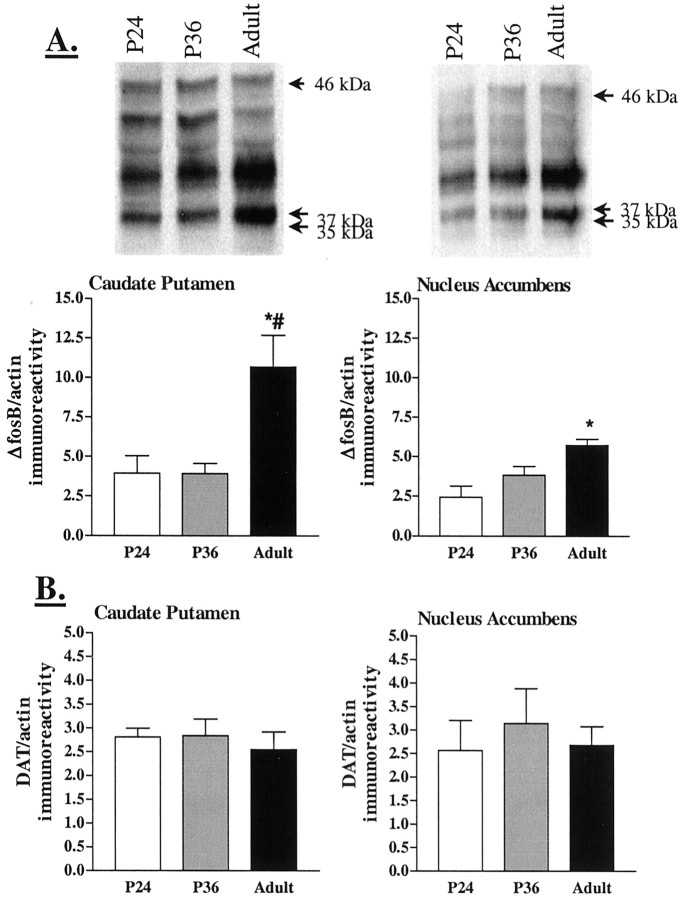
Baseline levels of ΔFosB are developmentally regulated
We examined the ontogeny of ΔFosB because adult mice with genetically engineered increased expression of ΔFosB in the striatum have a heightened behavioral response to psychostimulants (Kelz et al., 1999). We found that baseline levels of ΔFosB were significantly lower in younger animals compared with adult in both the caudate putamen and nucleus accumbens (Fig.3A). Levels of functional markers of the dopamine system, including DARPP-32 (Ehrlich et al., 1990), DAT (Perrone-Capano et al., 1996), and dopamine receptors (Teicher et al., 1993; Tarazi et al., 1999) are also developmentally regulated. Previous reports in CD-1 mice indicate a peak in striatal DARPP-32 at postnatal day 28 (P28) (Ehrlich et al., 1990). In rat caudate putamen and nucleus accumbens, D1receptor levels peak from P28 to P40 (Teicher et al., 1993; Tarazi et al., 1999), but similar studies have not been performed in the mouse. In contrast, here we found that DAT protein levels in the caudate putamen and nucleus accumbens were constant between postnatal day 24 and adulthood (Fig. 3B). Thus, the relative ratios between D1 receptors, DAT, DARPP-32, and ΔFosB differ between age groups, potentially resulting in differences in D1 receptor activity that could influence the degree of ΔFosB induction.
Fig. 3.
Developmental expression of ΔFosB and DAT. A, ΔFosB (35–37 kDa) immunoreactivity in the caudate putamen and nucleus accumbens of naive CD-1 mice as a function of age. Representative immunoblots are shown in the top panels.Bottom panels show means ± SEM of three mice per group. *p < 0.05, adult versus P24; #p < 0.05, adult versus P36 (Tukey's multiple comparison test after ANOVA). B, Densitometric values of DAT immunoreactivity in the caudate putamen and nucleus accumbens for naive CD-1 mice as a function of age. Levels of DAT did not differ among the three age groups.
DISCUSSION
Behavioral effects of psychomotor stimulants are age dependent. Addictive tendencies are highest in adolescence, when use of illicit substances escalates (Estroff et al., 1989; Myers and Anderson, 1991). In fact, younger children frequently become dysphoric when exposed to psychostimulants, whereas adolescents and adults experience euphoria (Rapoport et al., 1980). In rodent models, some studies suggest that periadolescent animals have higher baseline levels of activity (Spear and Brake, 1983) and altered responses to psychostimulants relative to younger and older animals. Thus, they show less locomotor stimulation and novelty seeking in response to acute low-dose administration of psychostimulants relative to weanling and adult animals but increased hyperactivity after high-dose treatment. With chronic administration, sensitization to cocaine-induced locomotion is greater in periadolescent rats compared with adults, whereas sensitization to stereotypy is lower. Also, microdialysis data have revealed differences between periadolescent and adult rats in regard to sensitization to amphetamine-induced dopamine release (Laviola et al., 1995; Adriani et al., 1998; Adriani and Laviola, 2000;Laviola et al., 2001). However, there are conflicting studies as to the long-term reactivity to cocaine after methylphenidate administration in adolescent rats (Brandon et al., 2001; Andersen et al., 2002). These latter two reports highlight the difficulty in comparing studies when different experimental paradigms are used. Attempts to compare behavioral studies in younger animals are further confounded by use of different species and strains.
The mouse is becoming an increasingly important animal model in the study of use and abuse of psychostimulants, and this is the first systematic analysis of molecular neuroadaptive responses in three different developmental ages in mouse or any other single species. Previous studies from which we derived our treatment paradigms have demonstrated an increase in ΔFosB in the isolated dorsal and ventral striatum of wild-type adult rats after chronic cocaine and amphetamine administration (Hope et al., 1994; Nye et al., 1995; Turgeon et al., 1997) but only in the combined dorsal and ventral striatum or isolated dorsal striatum of wild-type adult mice after chronic cocaine (Fienberg et al., 1998; Zachariou et al., 2001).
We now demonstrate a spatial and quantitative difference in psychostimulant-induced ΔFosB in post-weanling, periadolescent, and adult mice. The observation of a heightened response in the periadolescent animals compared with adults and post-weanlings is strengthened by the fact that the response is similar in cocaine- and amphetamine-treated mice. The psychostimulants cocaine and amphetamine both increase synaptic dopamine, as well as serotonin and norepinephrine, but by different mechanisms. Cocaine binds to the plasmalemma transporters for dopamine, serotonin, and norepinephrine and inhibits their reuptake into presynaptic terminals. In contrast, amphetamine promotes the release of these transmitters. The selective induction of ΔFosB in the nucleus accumbens of only the periadolescent age group after 7 d of stimulant administration and the relatively heightened induction of ΔFosB in the caudate putamen may be a neurobiological representation or cause of the previously noted increased tendency to abuse psychostimulants in this age group (Estroff et al., 1989; Myers and Anderson, 1991) and other long-term changes in gene expression, which differ between age groups (Andersen et al., 2002). Moreover, these differences may be intrinsically regulated by developmental alterations in levels of key molecules, including ΔFosB itself. The potential implications of differences in baseline levels of ΔFosB between age groups is analogous to that proposed regarding differences between rat strains (Haile et al., 2001). In fact, we anticipate that similar strain differences will be found among inbred mice. It is also possible that mice of different ages will show differing molecular adaptations in areas of the brain other than the nucleus accumbens. Additional analysis using periadolescent mice with genetically engineered alterations in levels of key molecules and concurrent behavioral observations will further test these hypotheses.
Footnotes
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant NS41871(M.E.E. and E.M.U.) and National Institute on Drug Abuse Grant P30-DA13429 (E.M.U.).
Correspondence should be addressed to Dr. Michelle E. Ehrlich, Thomas Jefferson University, Curtis 310, 1025 Walnut Street, Philadelphia, PA 19107. E-mail: michelle.ehrlich@mail.tju.edu.
REFERENCES
- 1.Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 2.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 3.Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD). Behav Brain Res. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- 5.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich ME, Rosen N, Kurihara T, Shalaby I, Greengard P. DARPP-32 development in the caudate nucleus is independent of afferent input from the substantia nigra. Dev Brain Res. 1990;54:257–263. doi: 10.1016/0165-3806(90)90148-r. [DOI] [PubMed] [Google Scholar]
- 8.Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse: addictive potential, behavioral and psychiatric effects. Clin Pediatr. 1989;12:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- 9.Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci USA. 2001;98:11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–190. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- 12.Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc Natl Acad Sci USA. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 14.Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 15.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–347. [PubMed] [Google Scholar]
- 16.Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to d-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 17.Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers DP, Anderson AR. Adolescent addiction: assessment and identification. Pediatr Health Care. 1991;5:86–93. doi: 10.1016/0891-5245(91)90096-9. [DOI] [PubMed] [Google Scholar]
- 20.Nestler EJ, Barrot M, Self DW. ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic Fos-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- 22.Perrone-Capano C, Tino A, Amadoro G, Pernas-Alonso R, Di Porzio U. Dopamine transporter gene expression in rat mesencephalic dopaminergic neurons is increased by direct interaction with target striatal cells in vitro. Proc Natl Acad Sci USA. 1996;39:160–166. doi: 10.1016/0169-328x(96)00022-8. [DOI] [PubMed] [Google Scholar]
- 23.Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine: its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- 24.Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7707. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spear LP, Brake SC. Periadolescene: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 26.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: an autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- 27.Teicher MH, Anderson SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res. 1993;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 28.Turgeon SM, Pollack AE, Fink S. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- 29.Zachariou V, Caldarone BJ, Wethers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]