Abstract
It was found recently that locomotor and rewarding effects of psychostimulants and opiates were dramatically decreased or suppressed in mice lacking α1b-adrenergic receptors [α1b-adrenergic receptor knock-outs (α1bAR-KOs)] (Drouin et al., 2002). Here we show that blunted locomotor responses induced by 3 and 6 mg/kgd-amphetamine in α1bAR-KO mice [−84 and −74%, respectively, when compared with wild-type (WT) mice] are correlated with an absence of d-amphetamine-induced increase in extracellular dopamine (DA) levels in the nucleus accumbens of α1bAR-KO mice. Moreover, basal extracellular DA levels in the nucleus accumbens are lower in α1bAR-KO than in WT littermates (−28%;p < 0.001).
In rats however, prazosin, an α1-adrenergic antagonist, decreasesd-amphetamine-induced locomotor hyperactivity without affecting extracellular DA levels in the nucleus accumbens, a finding related to the presence of an important nonfunctional release of DA (Darracq et al., 1998). We show here that locald-amphetamine releases nonfunctional DA with the same affinity but a more than threefold lower amplitude in C57BL6/J mice than in Sprague Dawley rats. Altogether, this suggests that a trans-synaptic mechanism amplifies functional DA into nonfunctional DA release.
Our data confirm the presence of a powerful coupling between noradrenergic and dopaminergic neurons through the stimulation of α1b-adrenergic receptors and indicate that nonfunctional DA release is critical in the interpretation of changes in extracellular DA levels. These results suggest that α1b-adrenergic receptors may be important therapeutic pharmacological targets not only in addiction but also in psychosis because most neuroleptics possess anti-α1-adrenergic properties.
Keywords: α1b-adrenergic receptor, d-amphetamine, dopamine, microdialysis, rats, mice
d-amphetamine is generally assumed to exert its locomotor and rewarding effects through an increased release of dopamine (DA) in a subcortical structure, the nucleus accumbens (Wise, 1996). d-amphetamine acts on both vesicular storage of DA and directly by reversing the DA transporter (DAT) located on dopaminergic terminals (Heikkila et al., 1975; Seiden et al., 1993; Sulzer et al., 1995). d-amphetamine acts also on noradrenergic terminals (Nakamura et al., 1982), and numerous studies in mice or rats have shown that prazosin, a specific α1-adrenergic antagonist, hampers d-amphetamine-induced locomotor hyperactivity (Dickinson et al., 1988; Blanc et al., 1994;Darracq et al., 1998). This suggested that the stimulation of α1-adrenergic receptors was necessary to obtaind-amphetamine-induced DA release in the nucleus accumbens. However, microdialysis experiments performed in freely moving rats indicated that the partial inhibiting effects of prazosin ond-amphetamine-induced locomotor hyperactivity were not associated with a significant modification of thed-amphetamine-induced increase in extracellular DA levels in the nucleus accumbens (Darracq et al., 1998). This was explained by showing that d-amphetamine-induced increase in extracellular DA levels in the nucleus accumbens could be divided into two components: a major one, caused by the local effect ofd-amphetamine in the nucleus accumbens and that does not cause locomotor hyperactivity (nonfunctional DA), and a minor one, caused by an effect of d-amphetamine distal from the nucleus accumbens and correlated with the development of locomotor hyperactivity (functional DA). Two sequential administrations ofd-amphetamine, first a local injection into the nucleus accumbens by reverse microdialysis inducing a nonfunctional DA release, and then a second, systemic injection, inducing locomotor hyperactivity, allowed to reach these conclusions (Darracq et al., 1998). Pretreatment with prazosin had no effect on nonfunctional DA release but inhibited the functional part of the DA release, suggesting that only the minor component of the d-amphetamine-induced DA release was under the control of α1-adrenergic receptor stimulation.
Very recently, experiments indicated that locomotor effects ofd-amphetamine are dramatically decreased in mice lacking the α1b subtype of adrenergic receptors [α1b subtype of adrenergic receptor knock-outs (α1bAR-KOs)] when compared with their wild-type (WT) littermates (Drouin et al., 2002). Complementary experiments showed that catecholamine tissue levels, D1 and D2 receptors, DA reuptake sites, and the locomotor response to a D1 agonist were not modified in α1bAR-KO mice, suggesting that global dopaminergic transmission was not affected by the α1b-AR gene deletion. It seemed therefore interesting to test whether d-amphetamine still induced increases in extracellular DA levels in the nucleus accumbens of α1bAR-KO mice, or whether, as observed in rats after a pretreatment with prazosin, d-amphetamine-induced locomotor hyperactivity is inhibited in α1bAR-KO mice without any change in extracellular DA levels in the nucleus accumbens.
MATERIALS AND METHODS
Animals
Mice. Experiments were performed in α1bAR-KO and WT adult male mice bred at the Institut de Pharmacologie et Toxicologie (Lausanne, Switzerland), weighing 30–40 gm at the time of the surgery. Their genetic background was a 129/SvXC57BL/6J mixture for both the WT and α1bAR-KO, as described by Cavalli et al. (1997) and Drouin et al. (2002). Adult male C57BL/6J mice (Iffa-Credo, Lyon, France), weighing 25–35 gm at the time of surgery, were used in reverse dialysis experiments.
Rats. Male Sprague Dawley rats (Iffa-Credo) were used as subjects in reverse dialysis experiment. They weighed 280–300 gm at the time of surgery.
All animals were housed in plastic cages with food and water ad libitum. The colony rooms were maintained under constant temperature and humidity on a 12 hr light/dark cycle (7:00 A.M. to 7:00 P.M.). Experiments were conducted in accordance with the guidelines for care and use of experimental animals of the European Economic Community (86/809; DL27.01.92, Number 16). All efforts were made to minimize the number of animals used and their suffering.
Surgery
Mice were anesthetized with sodium pentobarbital (60 mg/kg; Sanofi Santé Animale) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The head was positioned by means of a mouse nose-clamp adaptor (Kopf model 922) supplemented by rat ear bars placed lightly in the external auditory meatus. Unilateral permanent cannula (CMA/7 guide cannula; Microdialysis AB) was implanted into the nucleus accumbens and was secured on the skull with screw and dental cement. The coordinates for the guide cannula tip were anteroposterior (AP): +1.3 relative to bregma, mediolateral (ML): +0.8, and dorsoventral (DV): −2,4 mm from dura (Paxinos and Franklin, 2001).
Rats were anesthetized with sodium pentobarbital (60 mg/kg; Sanofi Santé Animale). Unilateral permanent cannula (CMA/11 guide cannula; Microdialysis AB) was implanted into the nucleus accumbens. The coordinates for the guide cannula tip were AP: +1.7 relative to bregma, ML: +1.1, and DV: −5.7 mm from dura (Paxinos and Watson, 1986).
After surgery, animals were placed in individual plastic cages and allowed to recover for at least 4 d.
Drug
d-amphetamine sulfate was purchased from Sigma Aldrich (L'Isle d'Abeau-Chesne, France) and was prepared in saline or in CSF and either injected intraperitoneally or perfused into the nucleus accumbens by reverse dialysis. Doses are expressed as salt.
Microdialysis experiment
The day of the experiment, the microdialysis probe was inserted (CMA/7; membrane length, 2 mm; diameter, 0.24 mm; cutoff, 6000 Da; Microdialysis AB; for mice or CMA/11 with identical probe characteristics for rats). Artificial CSF (in mm: NaCl: 147; KCl: 3,5; CaCl2: 1; MgCl2: 1,2, NaH2PO4: 1; NaHCO3: 25, pH 7.6) was perfused with a CMA100 microinjection pump through the probe at a rate of 1 μl/min (2 μl/min for rats) via a Teflon (fluoroethylene propylene) catheter (internal diameter 0.12 mm for mice) or polyethylene catheter (internal diameter 0.3 mm for rats) connected to a fluid swivel. Adequate steady state of DA levels in perfusate samples was reached 140 min after probe insertion for mice and rats, and samples were collected in 300 μl vials placed into a refrigerated computer-controlled fraction collector (CMA/170). Samples (20 μl every 20 min for mice and 10 μl every 5 min for rats) were collected for 100 and 90 min for mice and rats, respectively, to determine basal extracellular DA values. After d-amphetamine injection, samples were collected for 2 hr 40 min. For reverse dialysis experiments, d-amphetamine (3, 5, 10, and 100 μm) was infused 1 hr after determination of the basal extracellular DA level.
Biochemistry
Dialysate samples were completed to 30 μl with the mobile phase and placed into a refrigerated automatic injector (Triathlon; Spark Holland, Emmen, The Netherlands). Twenty five microliters of the sample was injected every 15 min through a rheodyne valve in the mobile phase circuit. HPLC was performed with a reverse-phase column (80 × 4.6 mm; 3 μm particle size; HR-80; ESA, Chelmsford, MA). Mobile phase (NaH2PO475 mm, EDTA 20 μm, octane sulfonic acid 2.75 mm, triethylamine 0.7 mm, acetonitrile 6%, and methanol 6%, pH 5.2) was delivered at 0.7 ml/min by an ESA-580 pump. Electrochemical detection was performed with an ESA coulometric detector (Coulochem II 5100A; with a 5014B analytical cell; Eurosep, Cergy, France). The conditioning electrode was set at −0.175 mV, and the detecting electrode was set at +0.175 mV, allowing a good signal-to-noise ratio of the DA oxidation current. External standards were regularly injected to determine the stability of the sensitivity (0.3–0.4 pg of DA).
Locomotor activity
The locomotor activity of mice injected systemically withd-amphetamine was measured with a video camera. Movements were accounted each time mice crossed a quarter of the cylinder.
Histology
At the end of the experiment, brains of mice or rats were conserved into formaldehyde solution and cut on a microtome in serial coronal slices according to the atlas of Paxinos and Franklin (2001) (mice) or Paxinos and Watson (1986) (rats). Histological examination of cannula tip placement was subsequently made on 100 μm safranine-stained coronal sections (Fig.1).
Fig. 1.
Illustrations of the localization of dialysis probes in the nucleus accumbens. Mouse (A) and rat slices (B) (100 μm thick) were stained with safranine.
Statistics
Results presented are means ± SEM of data obtained with five to nine animals. Statistical analysis was performed using GraphPad Prism 3.0 software (San Diego, CA). Data from microdialysis experiments were expressed as a percentage of the respective mean basal value to equate for between-subject differences. The extracellular DA levels obtained before and after the d-amphetamine intraperitoneal injection (3 and 6 mg/kg,) were compared and analyzed with repeated measures ANOVA (two-way and one-way ANOVA followed by a Dunnett's multiple comparison test). Locomotor activities afterd-amphetamine were compared with the locomotor basal activity with a two-way ANOVA and between doses with a Student'st test. The effects of the concentration of locald-amphetamine and of rodent species on the increase in extracellular DA levels were tested with a two-way ANOVA. Log EC50 values were compared after fitting curves with a Student's t test. Pharmacological treatments correspond to independent groups of animals. Significant differences were set at p < 0.05.
RESULTS
Effects of d-amphetamine on extracellular DA levels in the nucleus accumbens and on locomotor activity of α1bAR-KO and WT mice
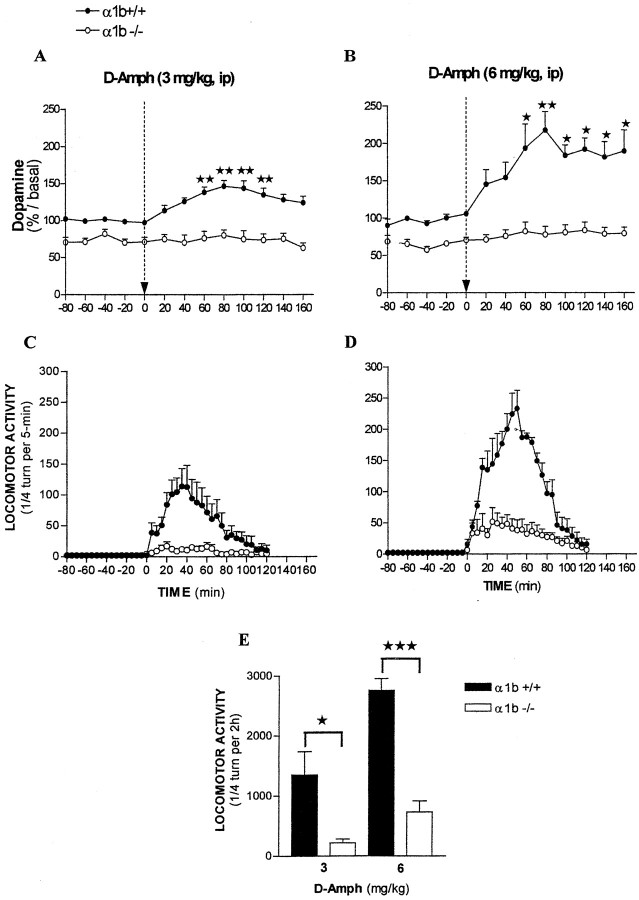
Basal DA dialysate from the nucleus accumbens of α1bAR-KO mice was significantly lower (−28%) than that of WT (1.26 ± 0.01 and 1.86 ± 0.02 pg of DA/20 min, respectively) (F(1,119) = 67.20; p< 0.001; two-way ANOVA).
As expected, d-amphetamine (3 and 6 mg/kg, i.p.) enhanced extracellular DA levels in the nucleus accumbens of WT mice (F(1,80) = 82.89, p < 0.001 and F(1,37) = 59.34,p < 0.001, two-way ANOVA, for 3 and 6 mg/kg, respectively) (Fig.2A,B). In α1bAR-KO mice, 3 mg/kg d-amphetamine did not modify basal extracellular DA levels (F(1,55) = 0.655; p = 0.421; two-way ANOVA). After 6 mg/kgd-amphetamine, however, a slight mean increase (+25%) in α1bAR-KO extracellular DA levels was noticed (F(1,64) = 7.1; p < 0.01; two-way ANOVA), but no individual point was significantly different from mean basal DA values (p > 0.05; Dunnett's multiple comparison test) (Fig. 2A,B).
Fig. 2.
Effects of systemicd-amphetamine on extracellular DA levels in the nucleus accumbens and on locomotor activity in WT and α1bAR-KO mice.d-amphetamine was injected 240 min after the introduction of the probe. A, B, Extracellular DA levels are expressed in function of WT mice basal DA values. *p < 0.05; **p < 0.01, significantly different from respective basal DA values (Dunnett's multiple test). C, D, Locomotor activities before and after d-amphetamine injections.E, Histograms of locomotor activities for 120 min after the d-amphetamine injections. *p < 0.05; ***p < 0.001, significantly different from WT mice (Student's t test) (N = 5–9 mice per group).
Recording of locomotor activities indicated significant effects ofd-amphetamine both in WT (F(1,135) = 141.5, p< 0.001 and F(1,16) = 718.2,p < 0.001; for 3 and 6 mg/kgd-amphetamine, respectively) and in α1bAR-KO mice (F(1,135) = 71.54,p < 0.001 andF(1,136) = 84.23, p < 0.001; for 3 and 6 mg/kg d-amphetamine, respectively) (Fig. 2C,D). However, locomotor hyperactivities of WT mice were significantly higher than those of α1bAR-KO mice (1352 ± 389 vs 219 ± 62; p< 0.05; t(1,4) = 2.876; Student'st test with Welch's correction; 2758 ± 199 vs 734 ± 184; p < 0.001;t(1,8) = 7.459; Student'st test for 3 and 6 mg/kg d-amphetamine and for WT and α1bAR-KO mice, respectively) (Fig.2E).
Differences in d-amphetamine-induced increases in dialysate DA levels between α1bAR-KO and WT mice were not expected because, in rats, an α1-adrenergic antagonist, prazosin, partly inhibitsd-amphetamine-induced locomotor hyperactivity without modifying extracellular DA responses in the nucleus accumbens (Darracq et al., 1998). Experiments were therefore conducted to quantify in mice nonfunctional DA release.
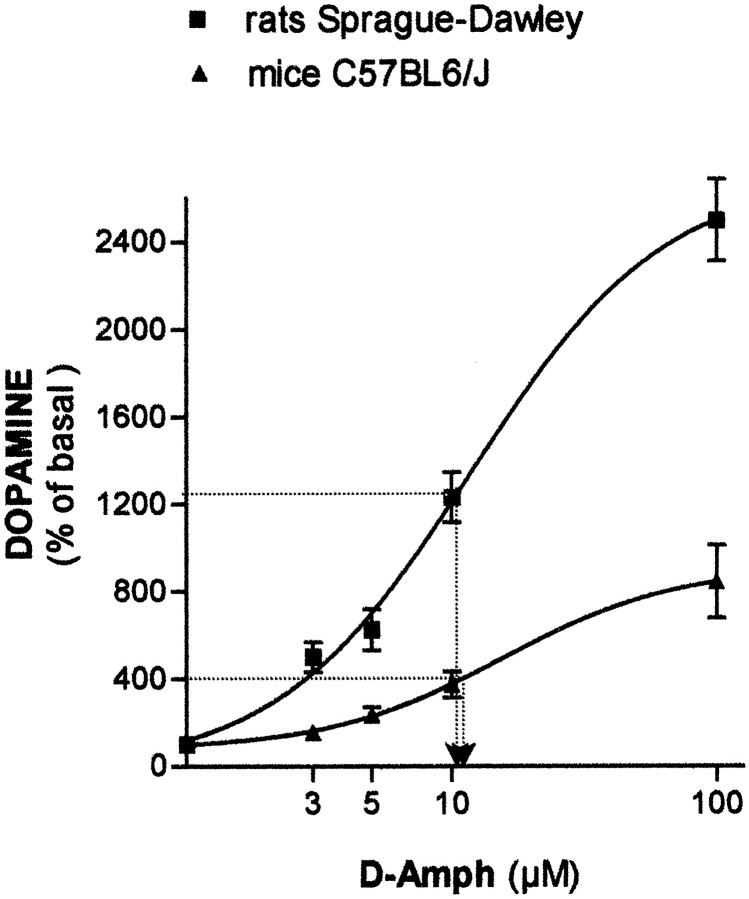
Effects on DA levels of the local perfusion of d-amphetamine in the nucleus accumbens of C57BL6/J mice and Sprague Dawley rats
Local perfusion of d-amphetamine in the nucleus accumbens was used to quantify nonfunctional DA release. Initial experiments indicated that 3 μmd-amphetamine induced a DA release in WT mice more than fivefold lower than previously found in rats (data not shown; Darracq et al., 1998). Because of the mixed genetic background of WT and α1bAR-KO mice,d-amphetamine dose–response curves were performed in C57BL6/J mice and compared in the same experimental conditions with those of Sprague Dawley rats. As found in rats, perfusion ofd-amphetamine in mice nucleus accumbens up to 100 μm did not induce any locomotor hyperactivity (data not shown). Figure 3 indicates that DA release is concentration-dependent and more than threefold lower in C57BL6/J mice than in Sprague Dawley rats (F(4,185) = 63.19, p< 0.001 for d-amphetamine concentrations andF(1,185) = 87.63, p < 0.001 for comparison between rodent species). However, EC50 values were found not significantly different (11.8 ± 1.3 and 15.6 ± 1.1 μm, for rats and mice, respectively;p > 0.05, Student's t test).
Fig. 3.
Effects of local perfusion ofd-amphetamine in the nucleus accumbens on extracellular DA levels in C57BL6/J mice and Sprague Dawley rats. Extracellular DA levels are expressed in percentage of basal DA values (3.50 ± 0.021 and 4.71 ± 0.015 pg of DA per 20 min for mice and rats, respectively). d-amphetamine concentrations correspond to those perfused into the probe (N = 5 animals per group).
DISCUSSION
The first finding of this study is that basal extracellular DA levels are almost 30% lower in the nucleus accumbens of α1bAR-KO mice when compared with that of WT littermates. In agreement with previous hypothesis, this suggests that stimulation of α1b subtype of adrenergic receptors exerts a tonic excitatory effect on subcortical DA release (Drouin et al., 2002).
The second finding is that systemic d-amphetamine fails to increase extracellular DA levels in the nucleus accumbens of α1bAR-KO mice. It is very likely that this lack ofd-amphetamine-induced release of DA in α1bAR-KO mice is related to their blunted locomotor response to different doses ofd-amphetamine (this paper and Drouin et al., 2002). The presence of a weak increase in DA levels in α1bAR-KO mice for the highest dose of d-amphetamine tested is in agreement with the observation of a significant locomotor hyperactivity at this dose. Because bursting activities of DA neurons in the ventral tegmental area are either blocked by prazosin (Grenhoff and Svensson, 1993) or increased by a specific inhibitor of the noradrenergic transporter reboxetine (Linner et al., 2001), it can be proposed thatd-amphetamine exerts, at least partly, its effects on subcortical DA release through the stimulation of α1b-adrenergic receptors. We have already discussed that such a stimulation could be attributable to an increased release of norepinephrine byd-amphetamine in the prefrontal cortex (Florin et al., 1994), a structure containing a high density of α1b-adrenergic receptors (Drouin et al., 2002) and possibly responsible for the regulation of DA release in the nucleus accumbens (Murase et al., 1993;Darracq et al., 1998).
The third finding of this study is that nonfunctional DA release is likely to be caused by a trans-synaptic mechanism. The lower amplitude of DA release evoked by local d-amphetamine in mice when compared with rats cannot be related to differences in DA reuptake systems or vesicular monoamine storage because affinities ford-amphetamine were found identical in both species. Moreover, nonfunctional DA release in mice nucleus accumbens does not seem limited by the quantity of DA stored in DA neurons because extracellular DA levels in mice could reach up to 800% of the basal DA values. Finally, because probes are too large to distinguish between shell and core in mice and were located in the rat at the edge of the two substructures, it can be excluded that the observed differences reflect a shell–core localization. Numerous studies have indicated that glutamate could increase DA release in the nucleus accumbens through cortical afferents that form synaptic contacts with the same target neurons (Cheramy et al., 1986; Sesack and Pickel, 1990; Berendse et al., 1992; Taber and Fibiger, 1995). It has also been found that DA inhibits glutamate reuptake (Kerkerian et al., 1987) and thatd-amphetamine increases extracellular glutamate levels in the ventral striatum (Gray et al., 1999). Altogether, a synergistic effect between glutamate and DA may amplify trans-synaptically thed-amphetamine-induced increase in extracellular DA levels, any one of these steps being probably less efficient in mice than in rats.
If one considers that, in α1bAR-KO mice, there is an absence of both functional and nonfunctional d-amphetamine-induced DA releases and that, in rats, the blockade of functional DA release by an antagonist of metabotropic glutamatergic receptors located in rat nucleus accumbens also inhibits nonfunctional DA release (Darracq et al., 2001), it is tempting to speculate that nonfunctional DA release is the result of a trans-synaptic amplification of functional DA release.
Interestingly, in WT mice, dialysate DA levels after systemicd-amphetamine stay significantly increased even when mice recover normal locomotor activity, especially for the higher dose ofd-amphetamine (Fig. 2A,B), suggesting a shift in the occurrence of nonfunctional DA release.
Nonfunctional DA release should be taken into account to interpret data obtained by microdialysis, especially in mice submitted to pharmacological treatments or gene deletion. For example, variations in the amplitude of nonfunctional DA release may explain why, in mice depleted in DA transporter (DAT −/−), d-amphetamine increases extracellular DA levels in the nucleus accumbens (Carboni et al., 2001), whereas it decreases locomotor hyperactivity (Gainetdinov et al., 1999; Spielewoy et al., 2001).
In conclusion, we show here that, in nucleus accumbens of mice lacking α1b-adrenergic receptors, basal DA release is lower than in WT littermates and d-amphetamine fails to increase extracellular DA levels. This is probably linked withd-amphetamine-induced blunted locomotor responses in α1bAR-KO mice and further confirms the existence of a powerful coupling between noradrenergic and dopaminergic neurons. In addition to potential consequences in the field of therapy of addiction to psychostimulants, this coupling may have some implications in mental diseases such as psychosis. Indeed, it is worth to recall that most antipsychotic compounds possess anti-α1-adrenergic properties.
Footnotes
A.A. has received a fellowship from Laboratoires Servier. We thank Gérard Blanc and Patricia Babouram for skillful technical assistance.
Correspondence should be addressed to Jean-Pol Tassin, Institut National de la Santé et de la Recherche Médicale U 114, Collège de France, 11, Place Marcelin Berthelot, 75231 Paris Cedex 05, France. E-mail: jean-pol.tassin@college-de-france.fr.
REFERENCES
- 1.Berendse HW, Galis-de-Graf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 2.Blanc G, Trovero F, Vezina P, Hervé D, Godeheu A-M, Glowinski J, Tassin JP. Blockade of prefronto-cortical α1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical d-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 3. Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci 21 2001. RC141 (1–4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, Michel MC, Yang M, Lembo G, Vecchione C, Mostardini M, Schmidt A, Beermann F, Cotecchia S. Decreased blood pressure response in mice deficient of the α1-b-adrenergic receptor. Proc Natl Acad Sci USA. 1997;94:11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheramy A, Romo R, Godeheu G, Baruch P, Glowinski J. In vivo presynaptic control of dopamine release in the cat caudate nucleus—II. Facilitatory or inhibitory influence of l-glutamate. Neuroscience. 1986;19:1081–1090. doi: 10.1016/0306-4522(86)90124-7. [DOI] [PubMed] [Google Scholar]
- 6.Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of d-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darracq L, Drouin C, Blanc G, Glowinski J, Tassin JP. Stimulation of metabotropic but not ionotropic glutamatergic receptors in the nucleus accumbens is required for the d-amphetamine-induced release of functional dopamine. Neuroscience. 2001;103:395–403. doi: 10.1016/s0306-4522(00)00578-9. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson SL, Gadie B, Tulloch F. α1- and α2-Adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology. 1988;96:521–527. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- 9.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. α1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florin SM, Kuczenski R, Segal DS. Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pre-treatment. Brain Res. 1994;654:53–62. doi: 10.1016/0006-8993(94)91570-9. [DOI] [PubMed] [Google Scholar]
- 11.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 12.Gray AM, Rawls SM, Shippenberg TS, McGinty J F. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- 13.Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- 14.Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of 3H-dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- 15.Kerkerian L, Dusticier N, Nieoullon A. Modulatory effect of dopamine on high-affinity glutamate uptake in the rat striatum. J Neurochem. 1987;48:1301–1306. doi: 10.1111/j.1471-4159.1987.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 16.Linner L, Endersz H, Ohman D, Bengtsson F, Schalling M, Svensson TH. Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J Pharmacol Exp Ther. 2001;297:540–546. [PubMed] [Google Scholar]
- 17.Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura S, Tepper JM, Young SJ, Groves PM. Changes in noradrenergic terminal excitability induced by amphetamine and their relation to impulse traffic. Neuroscience. 1982;7:2217–2224. doi: 10.1016/0306-4522(82)90132-4. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic; New York: 2001. [Google Scholar]
- 20.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 21.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;32:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 22.Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- 23.Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B. Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl) 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sulzer D, Chen T-K, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taber MT, Fibiger MC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]