Abstract
We have investigated the intracellular signaling mechanisms underlying the release of nitric oxide (NO) evoked by β-adrenoceptor (AR) agonists in urinary bladder strips and cultured bladder urothelial cells from adult rats. Reverse transcription-PCR revealed that inducible NO synthase and endothelial NOS but not neuronal NOS genes were expressed in urothelial cells. NO release from both urothelial cells and bladder strips was decreased (37–42%) in the absence of extracellular Ca2+ (100 μmEGTA) and was ablated after incubation with BAPTA-AM (5 μm) or caffeine (10 mm), indicating that the NO production is mediated in part by intracellular calcium stores. NO release was reduced (18–24%) by nifedipine (10 μm) and potentiated (29–32%) by incubation with the Ca2+ channel opener BAYK8644 (1–10 μm). In addition, β-AR-evoked NO release (isoproterenol; dobutamine; terbutaline; 10−9 to 10−5m) was blocked by the NOS inhibitors NG-nitro-l-arginine methyl ester (30 μm) orNG-monomethyl-l-arginine (50 μm), by β-adrenoceptor antagonists (propranol, β1/β2; atenolol, β1; ICI 118551; β2; 100 μm), or by the calmodulin antagonist trifluoperazine (50 μm). Incubating cells with the nonhydrolyzable GTP analog GTPγS (1 μm) or the membrane-permeant cAMP analog dibutyryl-cAMP (10–100 μm) directly evoked NO release. Forskolin (10 μm) or the phosphodiesterase IBMX (50 μm) enhanced (39–42%) agonist-evoked NO release. These results indicate that β-adrenoceptor stimulation activates the adenylate cyclase pathway in bladder epithelial cells and initiates an increase in intracellular Ca2+ that triggers NO production and release. These findings are considered in light of recent reports that urothelial cells may exhibit a number of “neuron-like” properties, including the expression of receptors/ion channels similar to those found in sensory neurons.
Keywords: nitric oxide, adrenergic, urothelium, urinary bladder, eNOS, iNOS
There is increasing evidence that nitric oxide (NO) produced by the enzyme NO synthase (NOS) is involved in both physiologic and pathologic processes in the lower urinary tract (LUT) (Andersson, 1993; Ehren et al., 1994; Folkerts and Nijkamp, 1998;de Groat and Yoshimura, 2001). The putative physiological functions of NO in the LUT include the relaxation of urethral smooth muscle, modulation of transmitter release from efferent nerves, regulation of urothelial permeability, and modulation of afferent nerve activity (Ehren et al., 1994; Andersson and Persson, 1995; Smet et al., 1996;Ozawa et al., 1999; Lewis, 2000; Mumtaz et al., 2000; Yoshimura et al., 2001). A pathological role of NO has also been suggested because injury or chronic inflammation can upregulate the expression of inducible NOS (iNOS) (Moncada et al., 1991; Kubes and McCafferty, 2000; Colansanti and Suzuki, 2001). This raises the possibility that the transmitter function of NO is plastic and can be altered by chronic pathological conditions (Olsson et al., 1998; Kubes and McCafferty, 2000; Alfierei et al., 2001; Morcos et al., 2001).
Stimulation of β-adrenoceptors (ARs) in the urinary bladder (UB) induces detrusor relaxation (Lefkowitz, 1996; Longhurst and Levendusky, 1999; Takeda et al., 2000; Igawa et al., 2001). Although this effect clearly involves a direct action on smooth muscle, it may also be mediated by release of “epithelium-derived relaxing factors” (which include NO) from the urothelium (Hawthorn et al., 2000). Although there are several possible sources of NO production, including endothelial cells, nerves, smooth muscle, and urothelium, our studies demonstrated that major sites of NO release were the urothelium and afferent nerves (Birder et al., 1997, 1998, 2001). In the urinary bladder, NO can also influence smooth muscle activity activated by reflex mechanisms. Intravesical administration of NO donors suppresses detrusor hyperactivity (Ozawa et al., 1999), whereas intravesical administration of oxyhemoglobin, an NO scavenger, stimulates bladder activity (Pandita et al., 2000). However, the effects of urothelial NO may not be mediated by a direct action on smooth muscle, because bladder smooth muscle cells (SMC) lack soluble guanylate cyclase, an important component in NO-mediated relaxation (Smet et al., 1996; Fathian-Sabet et al., 2001). Instead, NO may modulate bladder reflexes by altering afferent nerve activity. Localization of afferent nerves next to the urothelium suggests that chemicals released by these cells may modulate afferent excitability. Thus, information about the mechanisms of β-adrenoceptor-evoked NO release from bladder urothelium may provide insights into the role of urothelial cells (UCs) in bladder sensory functions.
To better understand the mechanisms underlying β-AR-induced urothelial NO production, the present studies were designed to evaluate the signaling pathway involved in NO release. In addition, to determine the site of NO release, experiments evaluated β-AR-evoked release from innervated and denervated bladder strips and cultured urothelial cells. Although cells in the urothelium of the rat urinary bladder have been shown to exhibit NADPH-diaphorase (used as a marker for NOS) (Persson et al., 1999), the identity of the NOS isoform present in the urothelium has not been established. To explore this issue, we examined the expression of the three NOS isoforms [neuronal NOS (nNOS), iNOS, and endothelial NOS (eNOS)] in rat bladder strips, urothelial cells, and bladder smooth muscle tissue (SMT) or cells.
MATERIALS AND METHODS
All procedures involving rats were conducted in accordance with Institutional Animal Care and Use Committee policies.
Reverse transcription-PCR characterization. Tissue or pelleted cultured cells were homogenized in TRIzol to isolate total RNA; poly(A+) RNA was further isolated using Oligotex columns. Twenty nanograms of poly(A+) RNA were reverse transcribed with an oligo-dT primer in 50 mm Tris-Cl, 75 mm KCl, 3 mmMgCl2, 0.01 m DTT, 20 U of RNase inhibitor, and 0.5 mm dNTPs using 200 U of Superscript II and then treated with 1 U of RNase H. PCR amplification was performed in 20 mm Tris-Cl, 50 mm KCl, 1.5 mmMgCl2, 0.2 mm dNTPs, and 0.4 μm primer pairs using 1.25 U of platinumTaq. PCR cycling was initiated at 94°C for 2 min, followed by: 94°C for 30 sec; annealing at 55°C for 30 sec (for iNOS) or 65°C for 30 sec (for eNOS and nNOS); 72°C for 1 min, and a final extension step at 72°C for 2 min. Negative results were confirmed using a 50 cycle reaction. Amplification primers were as follows: iNOS, 5′-atggcttgcccttggaagtttctc-3′ and 5′-cctctgatggtgccatcgggcatctg-3′; eNOS, 5′-ccttccggctgccacctgatcct-3′ and 5′-aacatgtgtccttgctcgaggca-3′ (Thuringer et al., 2000); nNOS, 5′-gaataccagcctgatccatggaa-3′ and 5′-tccaggagggtgtccaccgcatg-3′ (Shin et al., 2000). Amplified products were visualized on a 1% agarose gel using ethidium bromide. The presence or absence of a given product was confirmed by transferring the agarose gels to nylon membranes, prehybridization for 2 hr at 42°C, and overnight hybridization at 42°C with a 32P-labeled internal oligonucleotide for each of the three isoforms. Probe sequences were as follows: iNOS, 5′-ggacaagctgcatgtgactc-′3′; eNOS, 5′-caactggaccatctctaccg-3′; and nNOS, 5′-ggctgtgctttaatggagat-3′. Probes exhibited no cross-reactivity between isoforms. Further confirmation of the NOS isoform amplification was achieved by gel extraction and sequencing of the PCR products.
Urothelial cell culture and cAMP accumulation. Preparation and characterization of urothelial cultures have been described in previous reports (Birder et al., 1998; Truschel et al., 1999; Birder et al., 2001). Briefly, bladders were excised from deeply anesthetized (urethane, 1.2 gm · kg−1, i.p.) Sprague Dawley rats (of either sex), cut open, and gently stretched (urothelial side down). Anesthesia was determined to be adequate for surgery by periodically testing for the absence of a withdrawal reflex to a strong pinch of a hind paw and absence of an eye-blink reflex to tactile stimulation of the cornea. After tissue removal, all animals were killed via overdose of anesthetic. The tissue was incubated overnight in minimal essential medium (Cellgro; Mediatech, Herndon, VA), penicillin/streptomycin/fungizone, and 2.5 mg · ml−1 dispase (Invitrogen, Rockville, MD). The urothelium was gently scraped from underlying tissue, treated with 0.25% trypsin, and resuspended in keratinocyte medium (Invitrogen). The dissociated cell suspension (0.1 ml, 50,000–150,000 cells ml−1) was plated on the surface of collagen-coated dishes and maintained in culture for 1–3 d. Because long-term maintenance of cells in culture could significantly change the properties of some types of cells (Bevan and Winter, 1995), the cells in this study were examined after a short time in culture. In general, all cells were used within the first 3 d after plating. All cells in these cultures were cytokeratin positive (Dako Corp., Carpinteria, CA) and therefore were presumably of epithelial origin. For cAMP accumulation, urothelial cells were aliquoted to contain 1 × 106cells/ml and incubated with vehicle, isoproterenol (10 μm), or forskolin (10 μm). cAMP was quantified by radioimmunoassay (cAMP Direct Biotrak System; Amersham Biosciences, Piscataway, NJ) and expressed as picomoles of cAMP accumulated (increase over basal levels) using a standard curve run in parallel.
Measurement of NO release. Urothelial cells and tissue strips (bladder and urethra) obtained from deeply anesthetized rats were maintained in vitro in a temperature-regulated oxygenated bath (37°C). They were perfused (1 ml · min−1) with a solution containing (in mmol/l): 4.8 KCl, 120 NaCl, 1.2 NaH2PO4, 1.1 MgSO4, 15.5 NaHCO3, 2 CaCl2, and 11 glucose, pH 7.4. The tip of an NO-specific sensor (NO detection limit, 1 nm; response time, 1 msec; tip diameter, 10–20 μm) was placed directly onto the luminal surface of isolated urinary bladder or urethral strips or onto the surface of isolated urothelial cells (Kanai et al., 1995,1997; Birder et al., 1998). The sensor was prepared by inserting carbon strands (one to five fibers; Amoco, Greenville, SC) into glass capillary tubes (1 mm internal diameter) pulled to a 100 μm opening at one end. Copper wire was connected to the carbon fiber, with electrically conductive epoxy at the blunt end, whereas the strand protruded at the pulled end. The fiber was cut to the desired length and coated with tetrakis (3-methoxy-4-hydroxyphenyl)-nickel(II)porphyrin (TMHPPNi; Frontier Scientific, Logan, UT). Monomeric TMHPPNi was dissolved in 0.1N NaOH and deposited, as a polymeric film, on the carbon fiber using a multiple-potential scanning cyclic voltammeter (−0.2 to +1 V, model 283 Potentiostat/Galvanostat; PerkinElmer Instruments–Princeton Applied Research, Oakridge, TN). Polymeric TMHPPNi catalyzes the oxidation of NO to NO+. The cation exchanger Nafion (Sigma, St. Louis, MO) was applied to the microsensors by dipping in a 1% ethanol solution. The microsensors were characterized by differential pulse voltammetry to determine the redox potential of the oxidation of NO to NO+. Chronoamperometry, performed at a constant potential 50 mV more positive than the redox potential, was used to determine the NO concentration. High-purity (>99.99%) NO standards were prepared daily to accurately calibrate the electrodes. The currents generated by the oxidation of NO to NO+ at the porphyrinic interface were amplified and converted to voltages and then digitized for analysis. Before drug application, controls were evaluated without external stimuli and after the direct application of perfusate to ensure that flow did not cause cellular disruption. In general, urinary bladder strips were used within 2–4 hr after tissue removal, without any appreciable decrease in ability to release NO. All compounds were applied locally (at a distance 100 μm from cell) using a microperfusion system (ALA Instruments, Westbury, NY) with 10 min (agonists) or 20 min (antagonists) washout periods between applications to prevent desensitization to repeated exposure. Agonists were applied locally (1 sec pulse duration) under constant flow (1 ml · min−1), and antagonists were bath applied for 5 min. In view of the apparent lack of desensitization between agonist applications, paired comparisons (either between agonists or after varying concentrations of the same agonist) were made using the same urinary bladder strips.
Denervation. In a separate group of deeply anesthetized (halothane, 2%) animals, the major pelvic ganglia (MPG) were removed bilaterally (n = 5) to denervate the urinary bladder. Five animals served as sham-operated controls in which the urinary bladder was exposed via an abdominal incision. All animals survived an additional 4 d before tissue collection. Urinary bladder tissue strips were removed as described above and used to measure endogenous NO production.
Removal of the mucosa. To study the effect of agents on the isolated urinary bladder mucosa containing the urothelial layer, the mucosa was selectively removed from the urinary bladder smooth muscle by pinning the isolated urinary bladder strips on a Sylgard-coated plate, then gently peeling the muscle layer away.
Materials. All reagents used for reverse transcription (RT)-PCR were obtained from Invitrogen. Unless otherwise stated, all other chemical compounds were obtained from Sigma.
Data analysis. For all experiments, data (mean ± SEM) represent measurements from a minimum of four to five strips or six to seven cells per experimental treatment obtained from a minimum of three to six animals. ANOVA and the Student–Newman–Keuls test were used for multigroup comparisons. p values of <0.05 were considered statistically significant.
RESULTS
Type of NOS in urothelium, smooth muscle
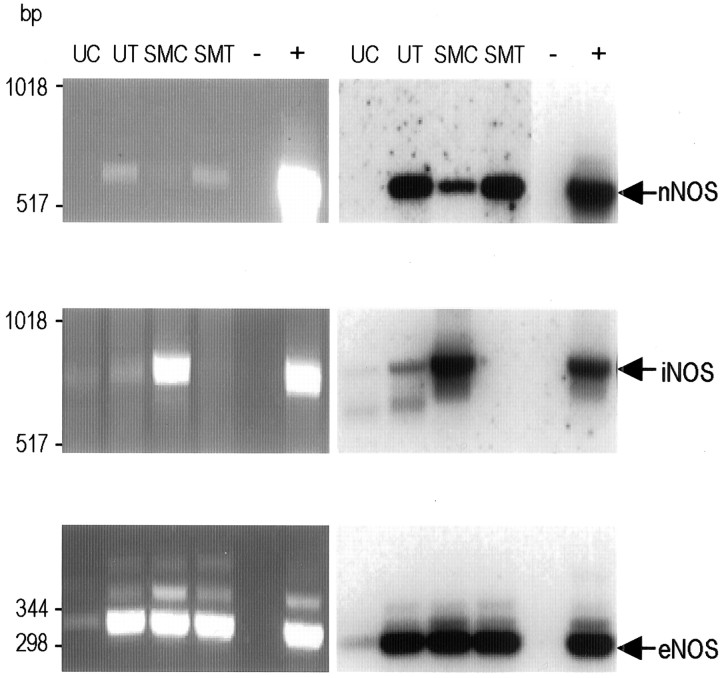
RT-PCR revealed the expression of both iNOS and eNOS in isolated urinary bladder UC (Fig. 1) as well as urothelial tissue (UT). The latter contains urothelial cells, along with connective, vascular, and nervous tissue. Although nNOS RNA was identified in urothelial tissue, there was no evidence of nNOS expression in cultured UC (Fig. 1). In contrast, all three NOS isoforms (nNOS, iNOS, and eNOS) were expressed in cultured bladder SMC (Fig. 1) and SMT (i.e., bladder strips in which the urothelium was removed).
Fig. 1.
Identification of NOS isoforms in bladder tissue and cultured cells. Ethidium bromide-stained agarose gels of RT-PCR products (left column) and Southern blots (right column) indicate the presence or absence of NOS isoforms in cultured UC; UT containing urothelial cells, along with connective, vascular, and nervous tissue; cultured SMC; and de-epithelialized bladder strip (removal of epithelium from underlying smooth muscle) SMT, containing smooth muscle cells and connective, vascular, and nervous tissue. − indicates no template control; + indicates rat brain cDNA as a template. Expected product sizes: eNOS, 343 bp; iNOS, 827 bp; nNOS, 701 bp. For nNOS, the positive and negative control lanes for the Southern blot are from a shorter exposure to prevent masking of fainter signals.
Adrenoceptor agonist evoked NO release from isolated bladder and urethral strips
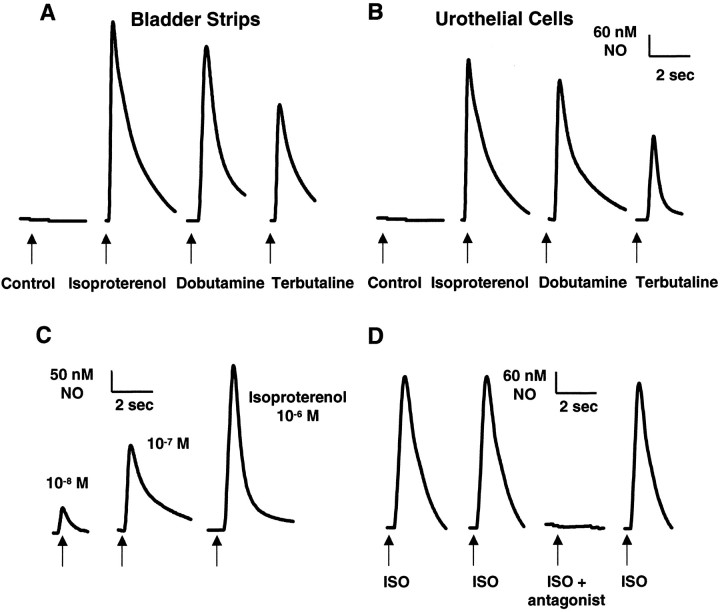
Tissue strips were studied within 1–5 hr after isolation from the urinary bladder or urethra. In the absence of chemical stimulation, basal NO release was not detectable (sensitivity, 1–5 nmNO). In addition, application of perfusate did not elicit NO release. Isoproterenol (a nonselective β-adrenoceptor, β-AR agonist), dobutamine (β1-AR agonist), and terbutaline (selective β2-AR agonist) at a concentration of 10−9m did not elicit detectable NO release; however, higher concentrations (10−8 to 10−5m) evoked a transient concentration-dependent release of NO from the luminal surface of urinary bladder strips (Table1; Fig.2A). For isoproterenol and dobutamine, the magnitude of peak NO release was similar (490–650 nm NO), whereas the peak NO release after application of terbutaline was significantly less (410–470 nm NO) (Table1). The β-AR agonists evoked a similar NO release (range, 400–650 nm NO) from urethral strips (data not shown). Paired comparisons (either between agonists or after multiple applications of the same agonist) were made using the same urinary bladder or urethral strips (n = 4–5 strips). Successive application of agonists (four to five applications with a 5 min washout period between applications) elicited reproducible results (Fig.2D). NO release began 100–200 msec after application of agonist and continued for 2–6 sec.
Table 1.
Magnitude of peak NO release (nm) induced by increasing concentrations of the adrenoceptor agonists isoproterenol, dobutamine, or terbutaline recorded in UB strips or UCs
| Isoproterenol | Dobutamine | Terbutaline | ||
|---|---|---|---|---|
| 10−5m | UB | 590 ± 35 | 560 ± 52 | 440 ± 30 |
| UC | 547 ± 42 | 510 ± 55 | 390 ± 40 | |
| 10−6m | UB | 450 ± 63 | 410 ± 46 | 300 ± 45 |
| UC | 391 ± 38 | 340 ± 31 | 230 ± 28 | |
| 10−7m | UB | 265 ± 24 | 240 ± 17 | 190 ± 22 |
| UC | 225 ± 24 | 199 ± 29 | 150 ± 25 | |
| 10−8m | UB | 120 ± 15 | 95 ± 35 | 78 ± 14 |
| UC | 79 ± 22 | 65 ± 22 | 55 ± 8 |
For all experiments, data (mean ± SEM) are from three to six independent experiments (n = 3–6 animals). Each paradigm represents a minimum of four to five strips or six to seven cells per treatment. Peak NO release induced by terbutaline (10−6 to 10−5m) was significantly less than that evoked by isoproterenol or dobutamine.
Fig. 2.
Adrenoceptor agonists (isoproterenol, dobutamine, terbutaline; 10−5m) evoke NO release from isolated bladder strips (A) or isolated urothelial cells (B) from the rat urinary bladder. Control, Basal NO release was undetectable before drug application. A similar response was detected from urethral strips. C, Peak NO response to increasing concentrations (10−8 to 10−6m) of isoproterenol in isolated urothelial cells. Arrows indicate start of drug application. D, Peak NO response to repeated application of isoproterenol (ISO; 10−5m) in isolated urothelial cells. Tracing is typical of that seen in 10 separate recordings. Response was blocked by incubation with the antagonist propranolol (10−4m), and response to isoproterenol recovered after washout. Each trace is typical of the data obtained from three to six experiments (n = 3–6 animals) containing a total of 40–60 cells.
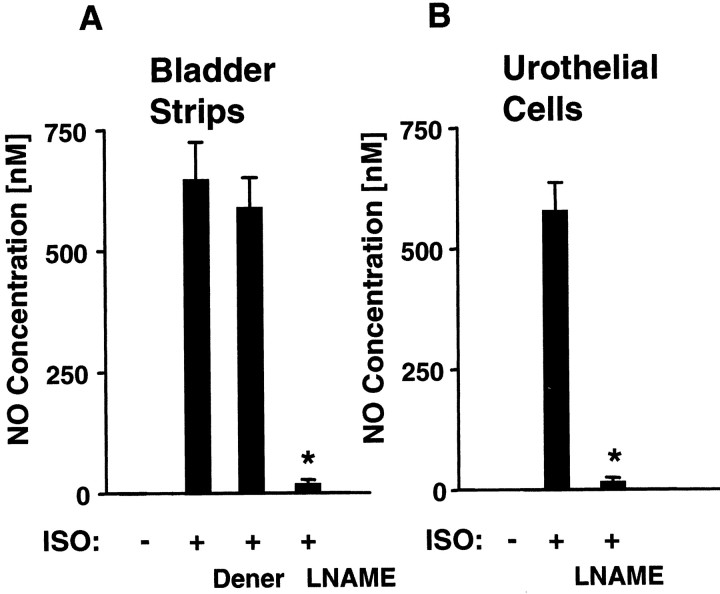
The responses to adrenoceptor agonists were ablated by application of the NOS inhibitorsNG-nitro-l-arginine methyl ester (l-NAME) (30 μm) (Fig. 3A) orNG-monomethyl-l-arginine (l-NMMA) (50 μm; data not shown). Adrenoceptor agonist-evoked NO release was also blocked by application (10−6 to 10−4) of β-AR antagonists (propranolol, β1β2,; atenolol, β1; ICI 118551, β2; data not shown). Although atenolol and ICI 118551 selectively blocked the response to dobutamine and terbutaline, respectively, neither antagonist completely ablated the response to isoproterenol. After a washout period (10 min), the response to agonists fully recovered (Fig. 2D).
Fig. 3.
Effect of NOS antagonists on isoproterenol-evoked NO release. The NO release evoked by isoproterenol (ISO; 10−5m) in both normal urinary bladder strips (A), and urothelial cells (B) is reduced to a similar extent after incubation with the NOS antagonists l-NAME (30 μm) or l-NMMA (50 μm; data not shown). Dener indicates isoproterenol-evoked NO release from denervated urinary bladder strips after bilateral removal of MPG (4 d before). Similar effects were obtained with other adrenoceptor agonists. Each bar is typical of the data obtained from three to six experiments containing a total of 35–50 cells. Values represent mean ± SEM; *significantly different from ISO alone.
NO release after chronic denervation
To evaluate the contribution of nervous tissue to NO release, the urinary bladder was denervated by bilateral removal of the MPG 4 d before harvesting of the tissue. The urinary bladder appeared grossly overdistended (mean urinary bladder weight, 272 ± 16 mg) compared with bladders in sham-operated or neurally intact animals (mean urinary bladder weight, 57 ± 7 mg). The lesioned animals' behavior was unremarkable during their survival, with no visible signs of distress. In isolated, denervated urinary bladder strips, basal release of NO was not detected, and the NO release induced by application of adrenoceptor agonists was not significantly different from release in tissues from sham-operated (data not shown) or intact animals (Fig.3A).
NO release in urothelial cells
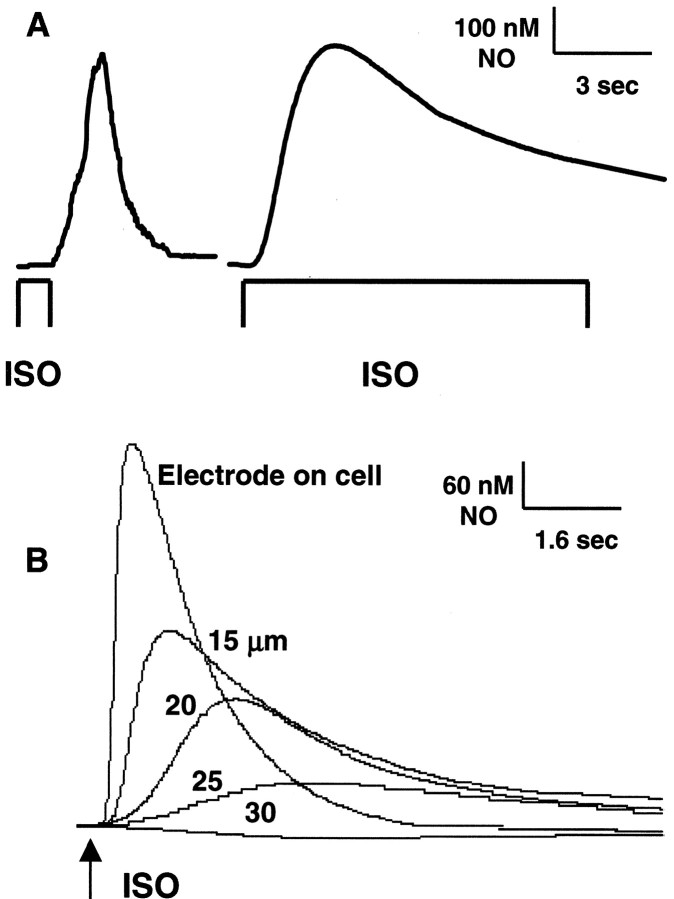
To establish the urothelium as a possible source of NO, the effects of β-AR agonists were examined on cultured urinary bladder urothelial cells. Similar to results in bladder strips, basal NO release was not detectable in the absence of stimuli. A transient concentration-dependent peak NO release was recorded in urothelial cells after application (10−8 to 10−5m) of the β-AR agonist isoproterenol (79–547 nm NO), dobutamine (65–510 nm NO), and terbutaline (55–390 nm NO) (Table1; Fig. 2B,C). The peak magnitude of the NO concentration elicited by adrenoceptor stimulation was not significantly different from that evoked in urinary bladder strips (maximal response: 650 nm NO in strips; 610 nm NO in cells). When the influence of stimulus duration on NO release was evaluated, it was found that a brief (1 sec) agonist pulse elicited a transient NO release (2–6 sec duration), whereas a sustained (10 sec pulse) application of agonist elicited a more prolonged (10–25 sec duration) NO release (Fig.4A). Verification that a measured NO response was from an individual urothelial cell was demonstrated by raising the tip of the microsensor from the cell surface and applying the agonist. As the distance from the cell surface increased, there was a gradual decrease in the measured NO concentration, and NO was undetectable beyond a distance of 30 μm (Fig. 4B).
Fig. 4.
Influence of stimulus duration on peak NO release from isolated urothelial cells. A, The left trace depicts NO release after a brief 1 sec pulse isoproterenol (ISO; 10−5m) application; the right trace depicts NO release after a sustained (10 sec duration) isoproterenol (10−5m) application. B, NO release from an individual urothelial cell, as demonstrated by raising the microsensor (from 15 to 30 μm) and reapplying isoproterenol. Response was lost at a distance of 30 μm from the cell surface. All NO recordings in this report were made from urothelial cells with a ≥30 μm cell-free radius surrounding them.
NO release from cultured cells by adrenoceptor agonists was blocked by the NOS inhibitors l-NAME (30 μm) (Fig.3B) or l-NMMA (50 μm; data not shown) as well as by the β-AR antagonists (10−5 to 10−4m) propranolol, atenolol, or ICI 118551 (data not shown). In addition, release from urothelial cells was completely blocked by preincubation (5 min) with the calmodulin antagonist trifluoperazine (50 μm; data not shown).
Involvement of calcium
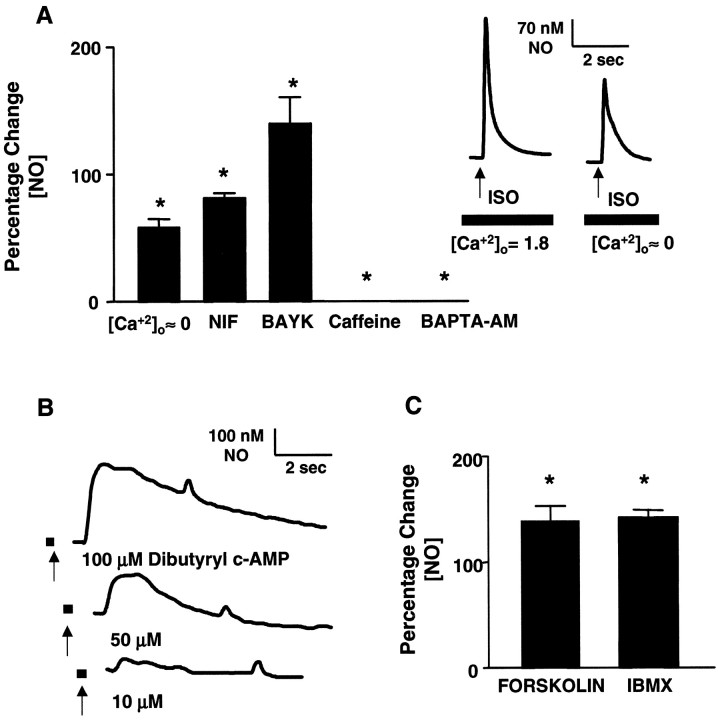
Adrenoceptor-evoked NO release was dependent on both extracellular and intracellular Ca2+. The dependence on extracellular Ca2+ was demonstrated in both bladder strips and isolated urothelial cells in three ways. Agonist-evoked NO production was decreased (37 ± 5% in strips; 42 ± 7% in cells) after removal and chelation (100 μm EGTA) of extracellular Ca2+, decreased (20–24 ± 5% in strips; 18–21 ± 4% in cells) in the presence of the dihydropyridine (DHP) Ca2+-channel antagonist nifedipine (10 μm), and increased (29 ± 8% in strips; 32 ± 9% in cells) in the presence of the DHP agonist BAYK8644 (1–10 μm) (Fig.5A). Application of BAYK8644 alone did not evoke NO release, nor did an increase in the [K+] in the medium to 50 mm (data not shown). In urothelial cultures, the dependence on intracellular Ca2+ was further demonstrated when agonist-evoked NO production was blocked either after depletion of intracellular Ca2+ stores with repeated applications of caffeine (10 mm) or after ablation by incubation with 5 μm BAPTA-AM in the absence of extracellular calcium (100 μm EGTA) (Fig.5A).
Fig. 5.
β-adrenoceptor agonist activates the adenylate cyclase pathway and initiates increased intracellular Ca2+ and NO release in rat urothelial cells.A, Inset, Transient NO release from urothelial cells elicited by application of isoproterenol (ISO; [Ca2+]o = 1.8 mm) was diminished in the absence ([Ca2+]o ≈ 0) of extracellular calcium (100 μm EGTA). The graph depicts isoproterenol-elicited NO production in urothelial cells (percentage change in NO): [Ca2+]o ≈ 0; isoproterenol-evoked NO release is diminished in the absence of extracellular Ca2+. NIF, Reduction of isoproterenol-evoked NO release after incubation with the L-type Ca2+ channel antagonist nifedipine (10 μm); BAYK, increase in isoproterenol-evoked NO release after incubation with BAYK8644 (10 μm); Caffeine, significant reduction in isoproterenol-evoked NO release after repeated (n = 3) incubations with caffeine (10 mm);BAPTA-AM, block of transient isoproterenol-evoked NO release after incubation with BAPTA-AM (5 μm). A similar response was detected in bladder strips (data not shown).B, Transient NO release elicited by increasing concentrations (10–100 μm) of the membrane-permeant cAMP analog dibutyryl-cAMP. Arrows indicate the start of drug application, which occurred 3 sec before the onset of NO release.C, Isoproterenol-evoked NO release from isolated urothelial cells is enhanced (percentage change in NO) after incubation with either forskolin (10 μm) or the phosphodiesterase inhibitor IBMX (50 μm). Data are based on calculations from 30 to 50 cells per treatment, recorded in a minimum of three independent experiments. Values represent mean ± SEM; *significantly different from isoproterenol alone.
Signaling pathways
In the presence of saponin (25 μg/ml), which increased membrane permeability, loading of the cells with the nonhydrolyzable GDP analog GDPβS (10 μm) completely blocked the response to agonist stimulation. However, after washout, the application of the nonhydrolyzable GTP analog GTPγS (1 μm) evoked direct NO release (410–480 nm NO). In addition, NO was released in a concentration-dependent manner (range, 50–290 nm NO) by application of the membrane-permeant cAMP analog dibutyryl-cAMP (10–100 μm) (Fig. 5B). Forskolin (10 μm), which directly activates adenylate cyclase, increased cAMP levels 20-fold over untreated controls (1.44 ± 0.5 pmol/ml to 20.5 ± 3.8 pmol/ml;n = 3) and enhanced adrenergic-evoked NO production (39 ± 12% increase) (Fig. 5C). Adrenergic-evoked NO production was also enhanced by the phosphodiesterase inhibitor IBMX (50 μm; 42 ± 7% increase) (Fig.5C). Neither forskolin nor IBMX exhibited any effect when administered alone.
DISCUSSION
This study provided information about the site of action, receptors, and signaling mechanisms underlying the release of NO in the urinary bladder by β-AR agonists. The analysis of NO release from bladder strips prepared from innervated or denervated bladders and from urothelial cells indicates that activation of β1 and β2adrenoceptors in the urothelium is primarily responsible for the effect of β-AR agonists. The intracellular signaling pathway involves cAMP- and Ca2+-dependent eNOS.
Because afferent and efferent nerves in the LUT express NOS and/or NADPH diaphorase, a commonly used marker for NOS (Persson et al., 1999), and because stimulation of afferent nerves with capsaicin releases NO in the bladder (Birder et al., 1997, 2001), it was important to determine the role of nervous tissue in the NO released by β-AR agonists. The contribution of nervous tissue to NO release was evaluated using bladder strips from denervated bladders. β-AR-evoked NO release in denervated preparations did not differ significantly from release in urinary bladder strips from sham-operated and neurally intact controls, suggesting that the urothelium was a major source of NO. This was confirmed by studying the effect of β-AR stimuli on cultured urothelial cells, which released NO in quantities similar to those released by denervated or intact bladder strips.
Multiple subtypes of β-ARs could mediate NO release, because β1, β2, and β3 receptors have been identified in the rat bladder (Morrison et al., 1986). In the present studies, both nonselective (isoproterenol) and selective (β1, dobutamine; β2, terbutaline) adrenoceptor agonists released NO from the urothelium. The maximal responses to isoproterenol and dobutamine were significantly larger than the response to terbutaline, suggesting that β1receptors might be more efficient than β2receptors in triggering NO production. Alternatively, because isoproterenol also activates β3-ARs (Trochu et al., 1999), we cannot discount an involvement of β3-ARs in mediating NO release from the urothelium. Additional studies using subtype-selective agents are needed to explore this possibility.
Several lines of evidence suggest that β-AR-evoked NO production is mediated via a cAMP/adenylate cyclase (AC) pathway involving both extracellular and intracellular Ca2+. Although the removal of extracellular Ca2+suppressed the NO response, chelation of free intracellular calcium with BAPTA-AM abolished it. In addition, NO production was potentiated by the L-type calcium agonist BAYK8644 and partially inhibited by the antagonist nifedipine. These results suggest that β-AR-mediated NO release is caused by both Ca2+ influx and release from intracellular stores, consistent with observations in vascular endothelium and airway epithelium (Kanai et al., 1995;Tamaoki, 1995). Cell-permeant cAMP analogs were also sufficient to release NO from urothelial cells, whereas forskolin and IBMX potentiated the isoproterenol-evoked NO release. These data suggest that cAMP production is responsible for the calcium-dependent activation of NOS in these cell types. Such a mechanism has also been proposed in cardiac muscle, where β-AR stimulation evokes NO production in a Ca2+/calmodulin-dependent manner, mediated through a cAMP/AC pathway (Kanai et al., 1997).
The basis of this apparent interaction between cAMP and Ca2+ signaling pathways is unclear. It has been shown that membrane-permeant cAMP analogs increase Ca2+ channel activity in various cell types (Kamp and Hell, 2000; Klein et al., 2000; Viard et al., 2000). In addition, phosphorylation of cardiac and neuronal L-type channels by cAMP-dependent protein kinases after β-AR stimulation may play a role in enhancing contractility or facilitating neurotransmitter release, respectively (Sculptoreanu et al., 1993; Lefkowitz, 1996; Colansanti and Suzuki, 2001). Moreover, the disinhibition of calcium channels in the sarcoplasmic reticulum by cAMP-dependent protein kinase accounts for cAMP-stimulated calcium release from intracellular stores in cardiac myocytes (Kiriazis and Kranias, 2000). Analogous mechanisms may be involved in β-AR-mediated NO release in the urinary bladder urothelium.
A number of recent studies suggest that in nonexcitable cells, DHP blocks store-operated channels that are depolarization insensitive (Dutta, 2000). This could initially result in less Ca2+ reuptake into cellular stores, leading to elevated cytoplasmic Ca2+levels. However, eventually this would lead to more Ca2+ being pumped out of the cell, resulting in a net decrease in cellular Ca2+ stores. Such store-operated channels appear to be opened after Ca2+ release into the cytoplasm via activation of IP3 or ryanodine receptors. Our finding that nifedipine partially reduced (18–24%) but did not completely block NO production is consistent with studies in other types of nonexcitable cells and suggests that block of store-operated channels rather than block of voltage-gated L-type Ca2+ channels may be involved in the observed DHP inhibition.
Our findings that NO release is blocked after removal of calcium and chelation of intracellular stores and by a calmodulin antagonist, trifluoperazine, are consistent with activation of a calcium-dependent NOS. Of the three known NOS isoforms, we have identified the expression of both the eNOS and iNOS forms in urinary bladder urothelial cells. This is in contrast to urinary bladder smooth muscle cells, which express all three NOS isoforms (eNOS, nNOS, and iNOS). These observations are consistent with previous investigations, which identified the eNOS isoform in the hamster urethral urothelium (Pinna et al., 1999).
After damage to the urinary bladder, urothelium basal levels of NO can be detected even in the absence of agonist. In this context, NO generation is most likely caused by iNOS, which in other tissues is upregulated by inflammatory stimuli and whose activity is independent of calcium concentration (Moncada et al., 1991; Kubes and McCafferty, 2000; Colansanti and Suzuki, 2001). A role for iNOS in a normal, uninflamed urinary bladder urothelium is less well established. Our RT-PCR findings support the presence of iNOS in this tissue. However, we did not detect agonist-independent release of NO from a normal bladder urothelium. The reason for the discrepancy between iNOS mRNA expression and the apparent absence of basal NO release from the normal urothelium is unclear. It may reflect NO release below the sensitivity of our microsensor, failure of urothelial cells to produce functional iNOS protein from transcribed mRNA, and/or the presence of factors that inhibit iNOS activity. Nevertheless, the expression of iNOS mRNA in the normal urothelium suggests that NO production from iNOS may be rapidly induced by pathological stimuli, such as bacterial lipopolysaccharide (endotoxin). In this condition, NO release might be elicited by activation of constitutively produced iNOS followed by the upregulation of iNOS expression, which occurs after injury/inflammation. Basal release of NO has been reported in normal, uninflamed epithelium of the colon, where it has been speculated that iNOS-induced NO may play a role in host defense mechanisms (Roberts et al., 2001). In the placenta and fetal organs, iNOS is also normally expressed, producing substantial amounts of NO without apparent toxic consequences (Yoshiki et al., 2000). Thus, additional studies are necessary to evaluate the role of iNOS in normal urinary bladder urothelium.
Nitric oxide is thought to be an important neurotransmitter in the bladder neck and urethra (Andersson and Persson, 1995; Bennett et al., 1995; Garcia-Pascual and Triguero, 2000). Thus, β-AR-evoked NO release in the urethra may function as an inhibitory neurotransmitter to relax smooth muscle. Because the rat detrusor smooth muscle is insensitive to NO, the detrusor smooth muscle is an unlikely target for NO released from the bladder urothelium (Andersson and Persson, 1995;Fujiwara et al., 2000; Fathian-Sabet et al., 2001). However, the location of afferent nerves that terminate in close proximity to the urothelium suggests that urothelium-derived NO may influence afferent function. These nerves are poised to respond to neurotransmitters (NO, ATP) released by urothelial cells, raising the possibility of a chemical interaction between nerves and the urothelium (Namsivayam et al., 1999; Birder et al., 2001; Vlaskovska et al., 2001).
However, increasing evidence suggests that NO may have a number of complex effects that are dose dependent (Colansanti and Suzuki, 2001). In terms of urinary bladder function, high concentrations of NO produced by intravesical administration of NO donors depress bladder hyperactivity after bladder irritation (Ozawa et al., 1999). In addition, constitutively expressed NOS (nNOS and eNOS) is critical to normal physiology (Moncada et al., 1991; Andersson and Persson, 1995;Pandita et al., 2000). For example, NO production by constitutive NOS may regulate epithelial permeability (Moncada et al., 1991; Kone and Baylis, 1997; Lewis, 2000). Clinical studies have shown that NO levels are decreased in some patients with interstitial cystitis, a clinical syndrome characterized by chronic sensory symptoms, such as urinary urgency, frequency, and pain (Korting et al., 1999). Thus, NO may be both a beneficial and detrimental molecule, and alterations in NO levels could affect urethral smooth muscle tone as well as the excitability of sensory fibers within the urinary bladder (Yoshimura et al., 2001).
In summary, the results of this study raise the possibility that norepinephrine from adrenergic nerves in the bladder or catecholamines from the adrenal medulla could influence bladder function by acting on β-ARs in the urothelium to release NO and possibly other neurotransmitters, such as ATP (Namsivayam et al., 1999; Vlaskovska et al., 2001). Chemicals released from the urothelium could in turn affect afferent nerves or smooth muscle or alter urothelial permeability (Truschel et al., 1999; Cockayne et al., 2000; Hawthorn et al., 2000;Lewis, 2000; Yoshimura et al., 2001). These data provide further support for our previous speculation that the urothelium has neuron-like properties and that it may play a role in sensory mechanisms in the bladder (Birder et al., 2001).
Footnotes
This work was supported by National Institutes of Health Grants DK54824 and DK57284 (L.A.B.), HL57985 (A.J.K.), and DK54425 (G.A.), and by grants from the Blaustein Pain Research Fund and the American Cancer Society (M.J.C.).
Correspondence should be addressed to Lori A. Birder, Laboratory of Epithelial Cell Biology, Renal-Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, A1220 Scaife Hall, 3500 Terrace Street, Pittsburgh, PA 15213. E-mail:lbirder+@pitt.edu.
REFERENCES
- 1.Alfierei AB, Malave A, Cubeddu LX. Nitric oxide synthases and cyclophosphamide induced cystitis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:353–357. doi: 10.1007/s002100000371. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- 3.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol Suppl. 1995;175:43–53. [PubMed] [Google Scholar]
- 4.Bennett BC, Kruse MN, Roppolo JR, Flood HD, Fraser MO, de Groat WC. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153:2004–2009. [PubMed] [Google Scholar]
- 5.Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birder LA, Kanai AJ, de Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol. 1997;158:1989–1995. doi: 10.1016/s0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- 7.Birder LA, Apodaca G, de Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 8.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 10.Colansanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci. 2001;15:144–146. doi: 10.1016/s0165-6147(00)01499-1. [DOI] [PubMed] [Google Scholar]
- 11.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 12.Dutta D. Mechanism of store-operated calcium entry. J Biosci. 2000;25:297–404. doi: 10.1007/BF02703793. [DOI] [PubMed] [Google Scholar]
- 13.Ehren I, Iversen H, Jansson O, Adolfsson J, Widlund NP. Localization of nitric oxide synthase activity in the human lower urinary tract and its correlation with neuroeffector responses. Urology. 1994;44:683–687. doi: 10.1016/s0090-4295(94)80206-8. [DOI] [PubMed] [Google Scholar]
- 14.Fathian-Sabet B, Bloch W, Klotz T, Niggemann S, Jacobs G, Addicks K, Engelmann U. Localization of constitutive nitric oxide synthase isoforms and the nitric oxide target enzyme soluble guanylyl cyclase in the human bladder. J Urol. 2001;165:1724–1729. [PubMed] [Google Scholar]
- 15.Folkerts G, Nijkamp FP. Airway epithelium: more than just a barrier! Trends Pharmacol Sci. 1998;19:334–337. doi: 10.1016/s0165-6147(98)01232-2. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara M, Andersson K, Persson K. Nitric oxide-induced cGMP accumulation in the mouse bladder is not related to smooth muscle relaxation. Eur J Pharmacol. 2000;401:241–250. doi: 10.1016/s0014-2999(00)00457-x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Pascual A, Triguero L. Relaxation mechanisms induced by stimulation of nerves and by nitric oxide sheep urethral muscle. J Physiol (Lond) 2000;476:333–347. doi: 10.1113/jphysiol.1994.sp020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawthorn M, Chapple C, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igawa Y, Yamazaki Y, Takeda H, Kaidoh K, Akahane M, Ajisawa Y, Yoneyama T, Nisizawa O, Andersson KE. Relaxant effects of isoproterenol and selective beta3-adrenoceptor agonists on normal, low compliant and hyperreflexic human bladders. J Urol. 2001;165:240–244. doi: 10.1097/00005392-200101000-00071. [DOI] [PubMed] [Google Scholar]
- 20.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 21.Kanai AJ, Strauss HC, Truskey GA, Crews AL, Grunfeld S, Malinski T. Shear stress induces ATP-independent transient nitric oxide release from vascular endothelial cells, measured directly with a porphyrinic microsensor. Circ Res. 1995;77:284–293. doi: 10.1161/01.res.77.2.284. [DOI] [PubMed] [Google Scholar]
- 22.Kanai AJ, Mesaros S, Finkel MS, Oddis CV, Birder LA, Malinski T. Beta-adrenergic regulation of constitutive nitric oxide synthase in cardiac myocytes. Am J Physiol. 1997;273:C1371–C1377. doi: 10.1152/ajpcell.1997.273.4.C1371. [DOI] [PubMed] [Google Scholar]
- 23.Kiriazis H, Kranias EG. Genetically engineered models with alterations in cardiac membrane calcium-handling proteins. Annu Rev Physiol. 2000;62:321–351. doi: 10.1146/annurev.physiol.62.1.321. [DOI] [PubMed] [Google Scholar]
- 24.Klein G, Drexler H, Schroder F. Protein kinase G reverses all isoproterenol induced changes of cardiac single L-type calcium channel gating. Cardiovasc Res. 2000;48:367–374. doi: 10.1016/s0008-6363(00)00194-2. [DOI] [PubMed] [Google Scholar]
- 25.Kone BC, Baylis C. Biosynthesis and homeostasis roles of nitric oxide in the normal kidney. Am J Physiol. 1997;272:F561–F578. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- 26.Korting GE, Smith SD, Wheeler MA, Weiss RM, Foster HE. A randomized double-blind trial of oral l-arginine for treatment of interstitial cystitis. J Urol. 1999;161:558–565. [PubMed] [Google Scholar]
- 27.Kubes P, McCafferty D. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz RJ. G protein-coupled receptors and receptor kinases: from molecular biology to potential therapeutic applications. Nat Biotechnol. 1996;14:283–286. doi: 10.1038/nbt0396-283. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 30.Longhurst PA, Levendusky M. Pharmacological characterization of beta-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br J Pharmacol. 1999;127:1744–1750. doi: 10.1038/sj.bjp.0702709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–141. [PubMed] [Google Scholar]
- 32.Morcos E, Jansson O, Adolfsson J, Ehren I, Widlund NP. Bacillus calmette-guerin induces long term local formation of nitric oxide in the bladder via the induction of nitric oxide synthase activity in urothelial cells. J Urol. 2001;165:682–684. doi: 10.1097/00005392-200102000-00093. [DOI] [PubMed] [Google Scholar]
- 33.Morrison JFB, Nimmo AJ, Whitaker EM. The localization of beta adrenoceptor subtypes in the rat urinary bladder. J Physiol (Lond) 1986;381:29P. [Google Scholar]
- 34.Mumtaz F, Khan M, Thompson C, Morgan R, Mikhailidis DP. Nitric oxide in the lower urinary tract. BJU Int. 2000;85:567–578. doi: 10.1046/j.1464-410x.2000.00459.x. [DOI] [PubMed] [Google Scholar]
- 35.Namsivayam S, Eardley I, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- 36.Olsson L, Wheeler M, Sessa W, Weiss RM. Bladder instillation and intraperitoneal injection of Escherichia coli lipopolysaccharide up-regulate cytokines and iNOS in rat urinary bladder. J Pharmacol Exp Ther. 1998;284:1203–1208. [PubMed] [Google Scholar]
- 37.Ozawa H, Chancellor NB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide induced cystitis. J Urol. 1999;162:191–202. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 38.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545–550. [PubMed] [Google Scholar]
- 39.Persson K, Poljakovic M, Johannson K, Larsson B. Morphological and biochemical investigation of nitric oxide synthase and related enzymes in the rat and pig urothelium. J Histochem Cytochem. 1999;47:739–750. doi: 10.1177/002215549904700603. [DOI] [PubMed] [Google Scholar]
- 40.Pinna C, Eberini I, Puglisi L, Burnstock G. Presence of constitutive endothelial nitric oxide synthase immunoreactivity in urothelial cells of hamster proximal urethra. Eur J Pharmacol. 1999;367:85–89. doi: 10.1016/s0014-2999(98)00981-9. [DOI] [PubMed] [Google Scholar]
- 41.Roberts PJ, Riley GP, Morgan K, Miller R, Hunter JO, Middleton SJ. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol. 2001;54:293–297. doi: 10.1136/jcp.54.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 43.Shin SJ, Lai FJ, Wen JD, Hsiao PJ, Hsieh MC, Tzeng TF, Chen HC, Guh JY, Tsai JH. Neuronal and endothelial nitric oxide synthase expression in outer medulla of streptozotocin-induced diabetic rat kidney. Diabetologica. 2000;43:649–659. doi: 10.1007/s001250051354. [DOI] [PubMed] [Google Scholar]
- 44.Smet PJ, Jonavicius J, Marshall JR, de Vente J. Distribution of nitric oxide-synthase immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 45.Takeda H, Yamazaki Y, Akahane M, Igawa Y, Ajisawa Y, Nisizawa O. Role of the beta 3 adrenoceptor in urine storage in the rat: comparison between the selective beta 3 adrenoceptor agonist, CL 316,243 and various smooth muscle relaxants. J Pharmacol Exp Ther. 2000;293:939–945. [PubMed] [Google Scholar]
- 46.Tamaoki J. Airway epithelium and nitric oxide. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:204–211. [PubMed] [Google Scholar]
- 47.Thuringer D, Rucker-Martin C, Frelin C. Cardiac capillary cells release biologically active nitric oxide at an early stage of in vitro development. Cardiovasc Res. 2000;47:726–737. doi: 10.1016/s0008-6363(00)00141-3. [DOI] [PubMed] [Google Scholar]
- 48.Trochu JN, Leblais V, Rautureau Y, Beverelli F, LeMarec H, Berdeaux A, Gauthier C. Beta 3 adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br J Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G. Primary uroepithelial cultures. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 50.Viard P, Macrez N, Coussin F, Morel JL, Mironneau J. Beta 3 adrenergic stimulation of L-type Ca2+ channels in rat portal vein myocytes. Br J Pharmacol. 2000;129:1497–1505. doi: 10.1038/sj.bjp.0703187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford APD, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshiki N, Kubota T, Aso T. Expression and localization of inducible nitric oxide synthase in human non-pregnant and early pregnant endometrium. Mol Hum Reprod. 2000;6:283–287. doi: 10.1093/molehr/6.3.283. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons in rat urinary bladder. J Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]