Abstract
Recent reviews highlight a longstanding controversy about how different taste qualities are coded in the CNS. To address this issue, we have analyzed gustatory coding in the relatively simple and accessible nervous system of the locust, in which neural responses and gustatory elicited behavior are readily comparable. The intracellular responses of a population of spiking local interneurons in the metathoracic ganglion that receive monosynaptic inputs from chemosensory afferents were analyzed in response to stimulation with droplets of four behaviorally relevant chemicals: sodium chloride, sucrose, lysine glutamate, and nicotine hydrogen tartrate. There was a significant positive correlation between chemical concentration and response duration and the number of spikes evoked in 81% of interneurons sampled. The threshold of sensitivity to different chemicals varied but was consistent between all interneurons tested, being most sensitive to nicotine hydrogen tartrate and least sensitive to sucrose. Each interneuron responded similarly to specific chemicals at single concentrations. Interneurons that responded phasically to one chemical responded similarly to others, whereas interneurons that responded phasotonically to one stimulus also did so to others. Hindleg motor neurons also responded in a concentration-dependent manner to all test chemicals. Therefore, we found no interneurons or motor neurons that responded only to specific chemicals. We discuss the responses of the local circuit neurons in relation to the known chemically evoked behavioral responses of locusts and suggest that the aversiveness of a chemical, rather than its identity, is encoded directly in the local circuits.
Keywords: chemosensory processing, taste, local circuits, spiking local interneuron, motor neuron, withdrawal reflex, grasshopper
The sense of taste has a vital role in the selection of appropriate foods, in the decision to ingest food, and in regulating dietary intake (Scott and Verhagen, 2000; Spector, 2000). All animals must solve the same basic problem of how to categorize, process, and represent different taste qualities in the CNS. A number of recent reviews highlight a longstanding controversy of how different chemicals are coded between two contrasting coding models (Smith and St John, 1999; Scott and Giza, 2000; Smith et al., 2000). Across-fiber pattern coding (Pfaffmann, 1959) contends that individual chemosensory neurons vary from each other in sensitivity but are broadly tuned, with each responding to a number of different chemicals and with the range of sensitivity of any individual neuron overlapping with that of several others. Chemical identity is decoded by the CNS by comparing the population response of many neurons. In contrast, the labeled-line theory asserts that there are distinct neuron classes that each code for different taste qualities with no overlap in sensitivity (Pfaffmann, 1974; Pfaffmann et al., 1976).
Studies at peripheral and central levels have shown that gustatory neurons in general lack strong stimulus specificity (Pfaffmann, 1959), with central neurons being broadly tuned to different taste stimuli (Smith and Travers, 1979; Spector, 2000). A major difficulty with understanding precisely how different animals code tastes is that the behavioral significance of different gustatory qualities in influencing feeding decisions remains largely unknown. Nor is it clear how and where gustatory information is further processed in the CNS before reaching motor centers controlling behavior. In recent years, we have been analyzing the central pathways responsible for taste processing in the comparatively simple nervous system of the locust (Newland, 1999), in which the taste receptors, or basiconic sensilla, are distributed over most of the body surface (Chapman, 1982). At the sensory level, the consensus has been that across-fiber pattern coding is used (Chapman, 1995). We have been able to focus our analyses on readily accessible taste receptors that project to regions of the CNS (Newland et al., 2000) that we know in detail (Burrows, 1996) and to which we have developed a model of chemosensory-elicited behavior (Rogers and Newland, 2000).
In vertebrates, neurons in the nucleus of the solitary tract (NST) are broadly tuned to more than one stimulus quality and represent the initial stage in the central coding and processing of taste signals (Smith and Travers, 1979). In insects, spiking local interneurons serve a similar role, receiving monosynaptic inputs from gustatory afferents innervating basiconic sensilla on a leg (Newland, 1999). In turn, these local interneurons make output connections with leg motor neurons that activate the leg musculature. Spiking local interneurons and motor neurons in the thoracic nervous system are readily accessible to intracellular recording. Here, we analyze their responses to a number of stimulus qualities ranging from nutrient to toxic in a range of concentrations and show how they are encoded in local circuits.
MATERIALS AND METHODS
Adult desert locusts, Schistocerca gregaria(Forskål), of both sexes were taken from a colony maintained at the University of Southampton. Animals were secured ventral side uppermost in modeling clay to prevent movement. The right hindleg was positioned laterally and restrained securely with the anterior surface uppermost and accessible. The mesothoracic and metathoracic ganglia were exposed by removing a window of cuticle in the ventral thorax and overlying air sacs. The abdominal connectives were severed, and a small platform, made from a wax-coated chloridated silver wire, was inserted underneath the ganglia for support. In most experiments, all peripheral nerves were cut except nerve 5 of the metathoracic ganglion that contains axons of sensory neurons innervating receptors on and in the hindleg. The ganglion sheath was softened by direct application of a protease (Sigma type XIV) for 45–60 sec, and thereafter the thorax was continuously superperfused with locust saline at 20–25°C. A more detailed description of this procedure has been described by Burrows et al. (1989).
Recording. Intracellular recordings were made from the somata of leg motor neurons and midline spiking local interneurons of local circuits producing and controlling hindleg movements (Fig.1A) using glass microelectrodes filled with 2 m potassium acetate and with direct current resistance of 50–80 MΩ. Recordings were made with either an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) or an amplifier designed and built at the University of Cambridge (Cambridge, UK). For motor neuron recordings, a pair of insulated 50 μm copper wires, exposed only at their tips, was implanted in the tibial extensor muscle of the hindleg and used to stimulate the muscle to induce antidromic spikes in the fast extensor tibia motor neuron (FETi). Flexor tibia motor neurons, which occur in three groups, each consisting of a fast, intermediate, and slow neuron, were identified according to a number of criteria as detailed by Burrows et al. (1989). These criteria were: the occurrence of short and constant latency depolarizing potentials (monosynaptic connection) after antidromic spikes in FETi, relative position of the somata in the metathoracic ganglion, and spiking rates and tibial movements when depolarizing current was injected into a flexor motor neuron. Other motor neurons were identified on the basis of the part of the leg that moved, and the speed of movement, when depolarizing current was injected into the neuron. Spiking local interneurons were all from a population with somata in a ventral midline location, which have GABAergic synaptic outputs (Siegler and Burrows, 1983; Burrows and Siegler, 1984). Interneurons were characterized according to exteroceptive or proprioceptive input and by receptive field, as determined by lightly touching different parts of the leg with a paintbrush (Siegler and Burrows, 1983; Burrows and Siegler, 1984; Burrows, 1985). Spiking local neurons were injected with hyperpolarizing current just sufficient to stop spontaneous spike activity before the beginning of experiments.
Fig. 1.
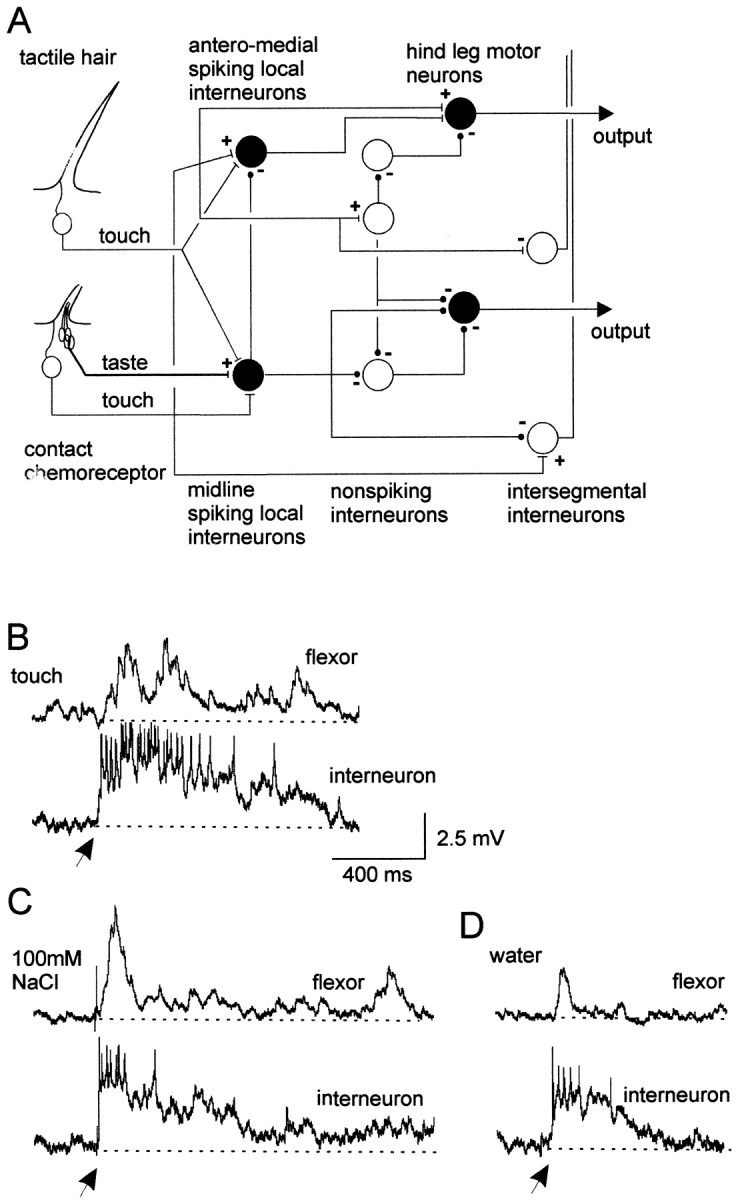
Spiking local interneurons and hindleg motor neurons receive convergent mechanosensory and chemosensory inputs.A, Summary of the basic organization of chemosensory and mechanosensory processing pathways in local circuits. Cell types recorded are shown in black.B, Simultaneous dual recording of a midline spiking local interneuron and a posterior flexor tibia motor neuron. Gently deflecting receptors on a hindleg with a paint brush elicited a sustained depolarization and spikes in an interneuron and depolarizing potentials in a flexor tibia motor neuron. C, Applying a droplet of 100 mm NaCl to the leg also elicited a sustained depolarization and spikes in the same interneuron and a long-lasting depolarization in the same flexor tibia motor neuron. D, A droplet of water applied to the same site on the hindleg evoked briefer depolarizing responses in both neurons. All recordings are from the same pair of neurons. Arrows indicate onset of droplets. In this and subsequent figures of intracellular recordings, thedotted lines represent the membrane potential before stimulation. Flexor, Flexor tibia motor neuron;interneuron, spiking local interneuron of a ventral midline population.
Test protocol. Chemical stimuli were applied as droplets of aqueous solutions to taste receptors, or basiconic sensilla, with Pasteur pipettes in a region of the leg identified as having the strongest mechanosensory input onto sampled spiking local interneurons. It has been established previously that the mechanosensory and chemosensory receptive fields are coincident for most spiking local interneurons (Newland, 1999). Droplets were applied to the distal dorsal tibia and tarsus in experiments characterizing the properties of motor neurons, unless otherwise specified. Single droplets were extruded onto the target site with the tips of pipettes held ∼10 mm above the target site. There were no detectable differences in the volume of droplets of different chemical solutions (Rogers and Newland, 2000). To rule out variability caused by the viscosity of the test chemical acting on the mechanosensory afferent of a receptor that provides convergent input onto interneurons (for example, caused by high concentrations of sucrose), the droplets of chemicals were left on the leg for 10 sec, after which time all responses ceased. The droplets were then wiped off. The chemicals used in the study, water, NaCl, sucrose, nicotine hydrogen tartrate (NHT), and lysine glutamate, were chosen because they represent a variety of qualities to locusts and were the same chemicals used in a previous behavioral study by Rogers and Newland (2000). Sucrose and lysine glutamate are representative of the two classes of macronutrient, carbohydrate and protein, and are known to actively promote feeding (Simpson and Raubenheimer, 1993). Salts are required in small quantities in the diet but become feeding deterrents at higher concentrations (Simpson, 1994), whereas NHT is a potent feeding deterrent, even at low concentrations, and may provoke active avoidance responses (White and Chapman, 1990).
The concentration ranges used varied for each chemical and were determined from the data obtained by Rogers and Newland (2000). For each chemical, they spanned a range from concentrations little more likely to evoke local movements than water to concentrations that evoked movements in 60–70% of applications to the tarsus. All chemicals were dissolved in distilled water. The following concentrations were used (in mm): 0.05, 0.5, 2.5, and 5 NHT (Sigma, St. Louis, MO), pH 4.4–3.1; 25, 50, 75, and 100 NaCl (Fisher Scientific, Houston, TX), pH 6.3–6.6; 250, 500, 1000, and 2000 sucrose (BDH Chemicals, Poole, UK), pH 6.4–6.9; and 250, 500, 1000, and 2000 lysine glutamate (Sigma), pH 6.2–6.3.
The number of spikes elicited, the duration of the depolarization/hyperpolarization, and its peak amplitude in response to chemical stimulation were determined for all neural responses. The duration of response was calculated as the time from the onset of depolarization/hyperpolarization to a recovery of two-thirds the peak amplitude of depolarization/hyperpolarization.
Chemosensory receptors within the basiconic sensilla on a leg adapt rapidly to repeated stimulation with most chemicals (White and Chapman, 1990; Newland, 1998). For this reason, only a single droplet was applied every 2 min for tests of responsiveness of spiking local interneurons and every 3 min for tests on motor neurons to avoid adaptation. The droplet was applied for 5–10 sec and wiped off, and then a droplet of water was immediately applied and wiped off, after which the animal was left for the requisite time. Solutions were applied in ascending concentration series, normally starting with the most behaviorally ineffective solution. Behavioral effectiveness was derived from the study by Rogers and Newland (2000) and is defined as the frequency of locusts withdrawing their legs from the applied chemical stimulus. All data used in the results derive from experiments in which at least one ascending set of response data was gathered, followed by at least one further droplet from the weakest concentration. Wherever possible, two or more successive ascending concentration series were applied to control against underlying variation in recording quality and any possible long-lasting, concentration-independent variation in neuronal response.
RESULTS
Responses of spiking local interneurons to gustatory stimulation
Local circuits producing and controlling movements of the leg of locusts have been well studied, and we now know much about their general organization (Fig. 1A). Spiking local interneurons from the midline population receive convergent mechanosensory and chemosensory inputs from receptors on a hindleg (Fig. 1A). Touching hairs and basiconic sensilla with a fine brush evoked depolarizations and spikes in spiking local interneurons (Fig. 1B). The majority of spiking local interneurons that received exteroceptive inputs also received chemosensory inputs (see below). For example, a droplet of 100 mm NaCl applied to the hindleg evoked a long-lasting depolarization and spikes in the same interneuron that received mechanosensory inputs (Fig. 1C). The duration of the response to droplets containing chemicals was always longer than to droplets containing water only (Fig. 1C,D). Activity in the spiking local interneuron was rapidly followed by activity in a flexor tibia motor neuron, the amplitude of which paralleled that of inputs to the spiking local interneuron for both mechanosensory (Fig. 1B) and chemosensory (Fig. 1C) stimulation.
Concentration-dependent responses
Forty-five spiking local interneurons were tested with one of four test chemicals, each in a range of concentrations. Droplets of chemicals were applied to regions of a hindleg that were found to provide the strongest mechanosensory input to the recorded local interneuron. We measured the duration, amplitude, and number of spikes evoked in an interneuron by chemosensory stimulation. Thirty-six of the interneurons showed a statistically significant increase in the strength of the response with increasing chemical concentration (Table1; Figs. 2,3, and 4). The response characteristics most frequently correlated with chemical concentration were the duration of depolarization and the number of spikes evoked by chemical stimulation (Table 1). Response amplitude, however, was less reliably correlated with chemical concentration, reflecting the difficulty in measuring the amplitude of the underlying depolarization in a spiking neuron and vulnerability to variation in recording quality over the course of the experiments.
Table 1.
Correlation coefficients of chemical concentration and the duration, amplitude, and spike frequency of response for midline spiking local interneurons tested with four different chemicals
| NaCl | Sucrose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuron | RF | n | Dur | Amp | Sp | Neuron | RF | n | Dur | Amp | Sp |
| 1 | Ta | 16 | 0.681-160 | −0.06 | 0.61* | 14 | dFe | 24 | 0.48* | 0.36 | 0.36 |
| 2 | dTi-Ta | 6 | 0.72 | 0.73 | 0.961-160 | 15 | dFE | 10 | 0.68* | 0.37 | 0.20 |
| 3 | Ta | 141-a | 0.59* | 0.50* | 0.54* | 16 | dTi | 22 | 0.671-160 | 0.17 | 0.32 |
| 4 | dTi | 28 | 0.841-160 | 0.63* | 0.891-160 | 17 | Ta | 8 | 0.83* | 0.72* | 0.01 |
| 5 | Ti-Ta | 10 | 0.54 | 0.18 | 0.70* | 18 | Ta | 211-a | 0.741-160 | −0.03 | 0.36 |
| 6 | vFe | 6 | 0.62 | 0.91* | 0.77 | 19 | dTi-Ta | 4 | 11-160 | 0.40 | 11-160 |
| 7 | Cp | 4 | 0.95* | 0.74 | 0.95* | 20 | dTi-Ta | 4 | 11-160 | 0.20 | 0.80 |
| 8 | Cp | 8 | 0.78* | −0.39 | 0.71* | 21 | dTi-Ta | 6 | 0.79 | −0.09 | 0.85* |
| 9 | dFe | 10 | 0.941-160 | 0.54 | 0.28 | 22 | dTi-Ta | 7 | 0.66 | 0.81* | 0.40 |
| 10 | dFe | 4 | 0.95* | 0.74 | 0.95* | 23 | Cp | 8 | 0.76 | 0.951-160 | 0.76* |
| 11 | vFe | 14 | 0.60* | 0.26 | 0.16 | 24 | dTi | 21 | 0.09 | 0.27 | 0.41 |
| 12 | dFe | 10 | 0.871-160 | 0.70* | 25 | dFe | 4 | 0.95* | −0.95* | 0.21 | |
| 13 | Ti-Ta | 7 | 0.27 | 0.11 | 0.03 | 26 | dFe | 9 | 0.75* | 0.04 | 0.20 |
| NHT | Lysine glutamate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuron | RF | n | Dur | Amp | Sp | Neuron | RF | n | Dur | Amp | Sp |
| 27 | Cp | 9 | 0.72* | 0.37 | 0.71* | 35 | dTi | 22 | 0.751-160 | 0.22 | 0.561-160 |
| 28 | dFe | 14 | 0.02 | −0.21 | 0.64* | 36 | Ta | 4 | 0.74 | −0.32 | |
| 29 | vTi | 14 | 0.941-160 | 0.52 | 0.66* | 37 | PFe | 10 | 0.08 | 0.66* | 0.20 |
| 30 | dTi | 19 | 0.83 | 0.03 | 0.46* | 38 | dTi-Ta | 8 | 0.03 | 0.18 | 0.57 |
| 31 | dTi | 13 | 0.781-160 | 0.781-160 | 0.781-160 | 39 | Cp | 28 | 0.67* | 0.31 | 0.48* |
| 32 | dTi-Ta | 18 | 0.72* | 0.27 | 0.791-160 | 40 | vFe | 13 | 0.48 | 0.18 | 0.63* |
| 33 | dTi | 18 | 0.691-160 | 0.47 | 0.45 | 41 | dTi-Ta | 17 | 0.681-160 | 0.31 | 0.53* |
| 34 | dFe | 20 | 0.701-160 | 0.54* | 0.29 | 42 | dFe | 23 | 0.581-160 | 0.35 | 0.43* |
| 43 | dFe | 6 | 0.85* | 0.44 | 0.79 | ||||||
| 44 | dTi-Ta | 9 | 0.831-160 | 0.18 | 0.951-160 | ||||||
| 45 | dFe | 14 | 0.801-160 | 0.64* | 0.761-160 | ||||||
Results of experiments in which individual spiking local interneurons were tested with increasing concentrations of one of the four test chemicals, unless indicated (
), when the dose–response relationship of an individual neuron to more than one chemical was obtained. The duration (Dur), maximum amplitude of EPSP (Amp in mV), and number of spikes (Sp) in the responses to each application of a test solution were measured, and the Spearman's ρ correlation coefficients calculated, with n indicating the number of droplets applied. Significant correlations, in bold, are marked as follows:
p ≤0.05;
Fig. 2.
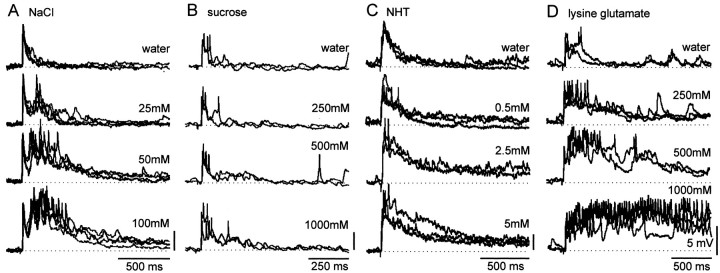
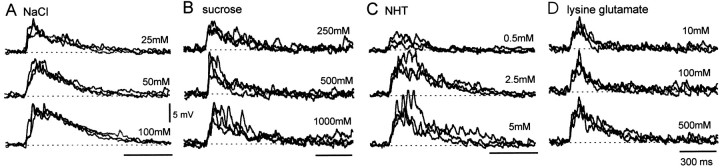
Responses of midline spiking local interneurons to chemosensory stimulation. A, Responses to NaCl. B, Responses to sucrose. C, Responses to NHT. D, Responses to lysine glutamate. Recordings from four different spiking local interneurons showing their responses to repeated applications of increasing concentrations of the four test chemicals. The greater the chemical concentration, the greater the response in an interneuron. Chemical droplets were applied once every 2 min in an ascending concentration series; each application was followed by water, and the entire sequence was repeated.
Fig. 3.
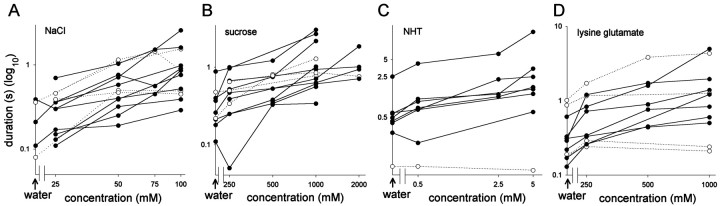
Relationship between chemical concentration and response in interneurons. Summaries of the mean duration of depolarization evoked in response to a concentration series of four test chemicals are shown. A, Responses to NaCl.B, Responses to sucrose. C, Responses to NHT. D, Responses to lysine glutamate. In general, the greater the concentration of a test chemical, the greater the duration of response elicited in an interneuron. Each linerepresents data from a different interneuron, with solid lines indicating significant correlations. Data detailing the number of droplets applied and the significance of the correlations between concentration and duration of response are given for each interneuron in Table 1. Arrows indicate responses to water alone.
Fig. 4.
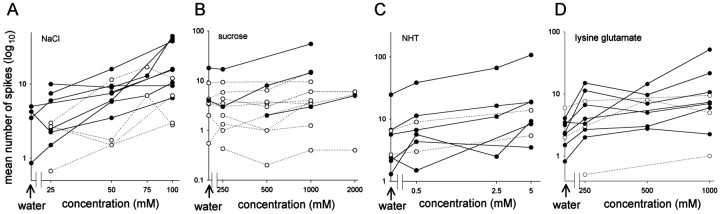
Relationship between chemical concentration and interneuron spike number. Summaries of the number of spikes evoked by different concentrations of the test chemicals applied to the leg are shown. A, Responses to NaCl. B, Responses to sucrose. C, Responses to NHT. D, Responses to lysine glutamate. Each line represents data from a different interneuron, with solid linesindicating significant correlations. In general, the greater the concentration of a chemical, the greater number of spikes evoked in an interneuron. Arrows indicate responses to water alone. Neuron identities are the same as in Figure 3 and Table 1.
Ten of 13 spiking local interneurons tested with droplets of NaCl showed statistically significant correlations (Spearman's ρ) between the concentration of the chemical in the droplet and the duration (Figs. 2A, 3A) and/or the number of spikes (Fig. 4A) evoked in response to the stimulation (Table 1). Another 13 spiking local interneurons were tested with sucrose, and of these 11 showed significant correlations between concentration and neural response, either duration and/or the number of spikes evoked (Table 1; Figs. 2B, 3B,4B). All eight neurons tested with NHT (Table 1; Figs. 2C, 3C, 4C) and 7 of 11 neurons tested with lysine glutamate (Table 1; Figs. 2D,3D, 4D) had responses that were significantly correlated with chemical concentration. We found no neurons exhibiting significantly smaller responses with increasing concentration for any of the test chemicals. Moreover, there were no significant correlations between interneuron response and the pH of the test chemicals.
The responses of individual spiking local interneurons were clearly dose dependent, but there was considerable variation in their absolute excitability. This is reflected in the control response to water, in which the duration of the depolarization after stimulation of the leg ranged from ∼0.1 to 1 sec or from 0.2 to >5 sec for the highest concentrations of stimulating chemicals (Fig. 3). This variability is clearly seen in the representative traces of recordings from interneurons shown in Figure 2. The responses of the interneuron in Figure 2B were very short to all droplets but nevertheless increased in amplitude with chemical concentration, whereas the interneuron in Figure 2D exhibited considerably more prolonged responses. The interneurons shown in Figure2A and C are typical of interneurons with response durations intermediate to these two extremes. The chemical used had no consistent effect on excitability; responses ranging from extremely rapid to long lasting were found in interneurons tested with any one of the four chemicals (Fig. 3).
In summary, 81% of spiking local interneurons tested with one of the four test chemicals showed a significant relationship between chemical concentration and duration of response, which varied from phasic to phasotonic.
Evidence for the convergence of chemosensory inputs onto spiking local interneurons
Many studies have shown that the different sensory neurons within a single basiconic sensillum encode a wide range of different chemicals (Blaney, 1975; White and Chapman, 1990).
Our results show that there is a high probability of any sampled spiking local interneuron responding in a dose-dependent manner to increasing concentrations of any of the test chemicals. This suggests that each of these interneurons may be sensitive to a large number of different chemical stimuli. It is not clear, however, whether an interneuron giving long-lasting phasotonic responses to one chemical concentration series would respond similarly to a different test chemical or whether it could respond more phasically. If this were so, different chemical identities could be encoded in the ensemble response of several different neurons. To test whether individual interneurons showed different response characteristics to different chemicals, experiments were performed on spiking local interneurons in which the leg was tested with single concentrations of three different chemicals and water. To balance between the need to sample several different chemicals and to ensure that each chemical could be applied repeatedly during the course of a single recording, not all of the test chemicals were used. Two solutions, water and 250 mm sucrose, were chosen because they led to comparatively weak responses and two more, 250 mm NaCl and 5 mm NHT, were chosen because they evoked relatively strong responses (Figs. 2, 3). Thirteen interneurons were tested repeatedly with each of these solutions. Statistically significant differences in response duration were found in 8 of the 13 interneurons (Kruskal–Wallis tests; significance taken at p < 0.05) (Fig.5A). For each interneuron, the responses to 250 mm NaCl and 5 mm NHT were stronger than to 250 mm sucrose and water; there were no sugar-best interneurons (Fig. 5A). As with the previous experiments, there were considerable differences in the absolute excitability of different recorded neurons, but we found that interneurons that responded phasically to 250 mm NaCl also did so to 5 mm NHT and similarly when interneurons that responded phasotonically to one stimulus also did so to the other (Fig.5A,B). The relative strength of response correlates with the response expected to particular chemicals based on concentration series experiments (Fig. 3). Whether the interneurons show a concentration-dependent response or an apparent sensitivity to particular chemicals, however, depends entirely on experimental design, because the actual response of any one of the interneurons depends on both chemical concentration (Fig. 3) and identity (Fig. 5).
Fig. 5.
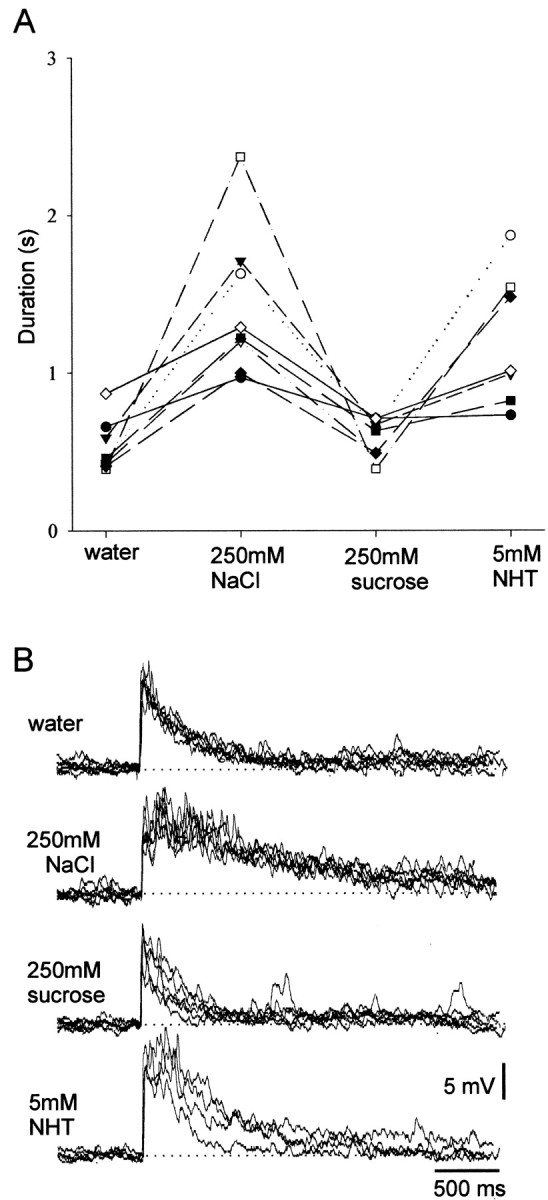
Individual spiking local interneurons respond to stimulation with different chemicals. The response characteristics of different local interneurons were all similar when tested with different chemicals at fixed concentrations. Neurons were repeatedly tested with single concentrations of four different chemicals at 2 min intervals. A, In experiments where significant differences in response duration occurred, interneurons always responded most strongly to 5 mm NHT and 250 mmNaCl than to 250 mm sucrose and water. Eachsymbol/line represents data from a different interneuron.B, Typical recordings of the responses obtained during an experiment showing the longer-duration responses to NHT and NaCl. Five responses to each test chemical are superimposed. Note the consistency of the responses to each test chemical.
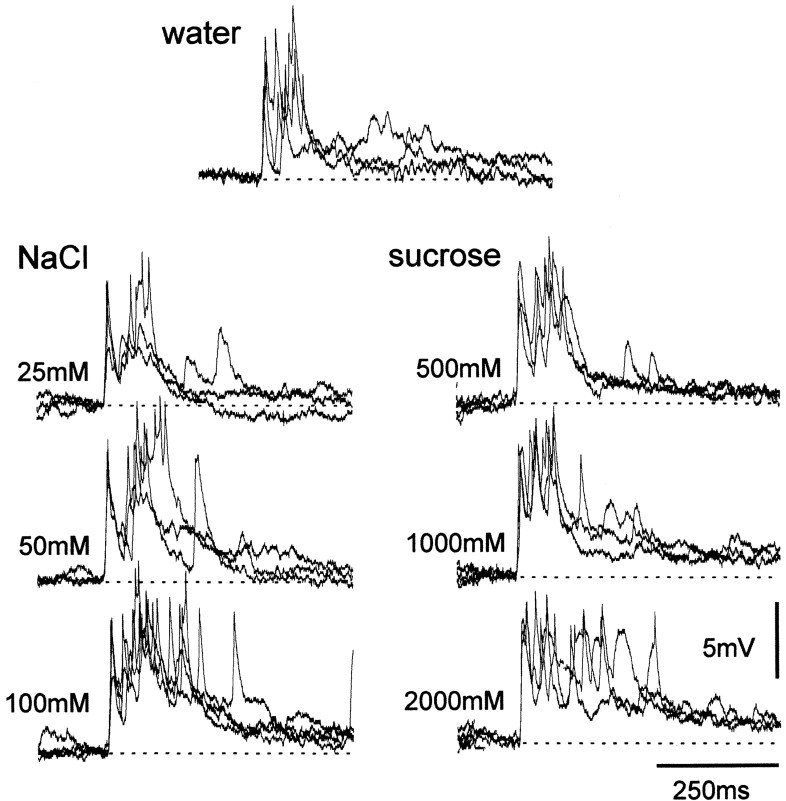
Additional evidence that individual interneurons respond in a similar manner to different chemicals of similar behavioral effectiveness is shown in Figure 6, in which a spiking local interneuron responded in a similar phasic manner to a concentration series of both NaCl and sucrose.
Fig. 6.
Responses of spiking local interneurons to concentration series of two different chemicals. For both NaCl and sucrose, increasing the test concentration resulted in an increase in response duration. Three traces are superimposed for each chemical concentration. The durations of the response for any test concentration were similar.
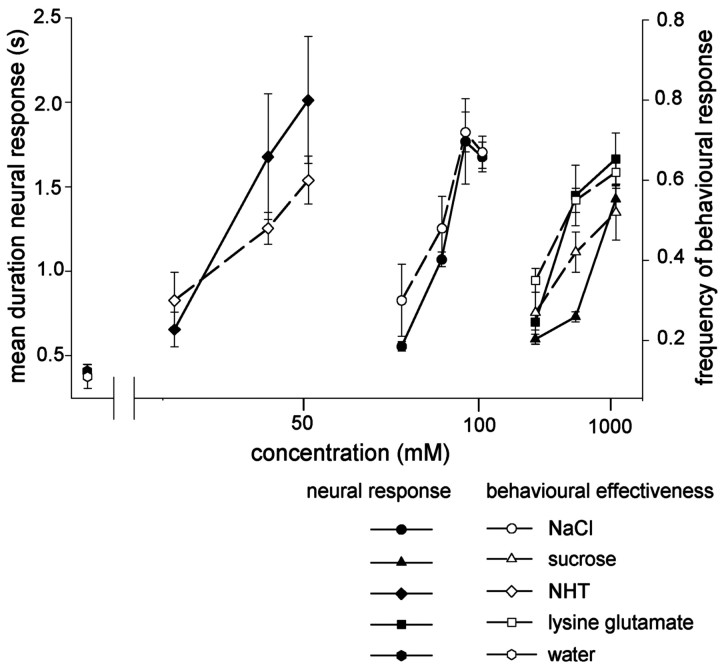
In summary, the results suggest that spiking local interneurons in the midline population receive inputs from chemosensory neurons sensitive to many, if not all, test chemicals, and that the response durations of these neurons are a function of both chemical concentration and identity. The data suggest that any individual interneuron will respond with similar relative duration to an input of similar behavioral effectiveness (Rogers and Newland, 2000) regardless of chemical type. This is illustrated in Figure 6, where 50 mm NaCl and 1000 mm sucrose, both of which elicit withdrawal movements of the leg in ∼50% of cases, evoke similar numbers of spikes in the interneuron (see Fig. 11).
Fig. 11.
The response durations of spiking local interneurons correspond closely with the behavioral effectiveness of different chemical stimuli at different concentrations. Thefilled symbols show the mean ± SEM response durations (left ordinate) of spiking local interneurons to each of the test chemical stimuli at different concentrations (abscissa). Data were normalized so as to give the same mean response to water to minimize the variability associated with the different phasotonic response characteristics of individual neurons (Fig. 2). The behavioral effectiveness (right ordinate), as measured by the frequency of locusts withdrawing their legs from droplets of chemical stimuli at different concentrations, are shown by the open symbols (mean ± SEM) (data from Rogers and Newland, 2000). There is a Pearson correlation of 0.91 (p < 0.001;n = 14) between behavioral effectiveness and duration of interneuron response.
Responses of leg motor neurons to gustatory stimulation
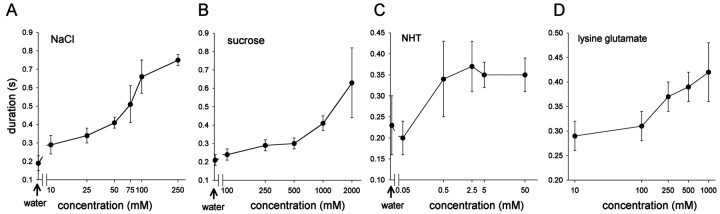
The responses of flexor tibia motor neurons resembled those of spiking local interneurons to the same four test chemicals. The amplitude and duration of responses in flexor tibia neurons after stimulation of the hind tibia and tarsus with droplets of chemical increased with concentration for all of the four test chemicals (Figs.7A–D,8A–D). The mean duration of depolarization increased from ∼200 msec after stimulation with water to 600–750 msec after stimulation with 2000 mm sucrose or 250 mm NaCl (Fig. 8). Flexor motor neurons occur in three distinct groups, each containing slow, intermediate, and fast members (Burrows, 1996). We found that responses were similar in motor neurons from all three groups and for fast and slow neurons within each group. For example, Figure 9 shows simultaneous responses from two flexor tibia motor neurons from the posterior and lateral groups to increasing concentrations of NaCl (Fig. 9A) and from the fast and slow flexor neurons in the posterior group to three concentrations of NHT (Fig. 9B). For any particular recording from a motor neuron, the duration of responses to repeated application of the same concentration of a test chemical was consistent.
Fig. 7.
Responses of flexor tibia motor neurons to chemosensory stimulation. Recording from a single posterior flexor tibia motor neuron showing responses to repeated applications of concentration series of each test chemical. A, Responses to NaCl. B, Responses to sucrose. C, Responses to NHT. D, Responses to lysine glutamate. The responses of the flexor tibia motor neuron showed a dose-dependent relationship with chemical concentration, with higher chemical concentrations evoking larger responses in the motor neuron. Droplets of chemical solutions were applied to the dorsal tibia and tarsus in ascending concentration at 3 min intervals. All recordings are from the same motor neuron tested with each chemical concentration at least five times. Three traces at each concentration are superimposed.
Fig. 8.
Mean dose–response relationships of flexor tibia motor neurons. A, Responses to NaCl. B, Responses to sucrose. C, Responses to NHT.D, Responses to lysine glutamate. Mean ± SEM response durations of flexor tibia motor neurons to concentration series of each of the test chemicals are shown. Response duration increased with higher concentrations of each test chemical. Data are from nine recordings each of motor neurons tested with NaCl and sucrose, six tested with NHT, and four tested with lysine glutamate.Arrows indicate responses to water alone.
Fig. 9.
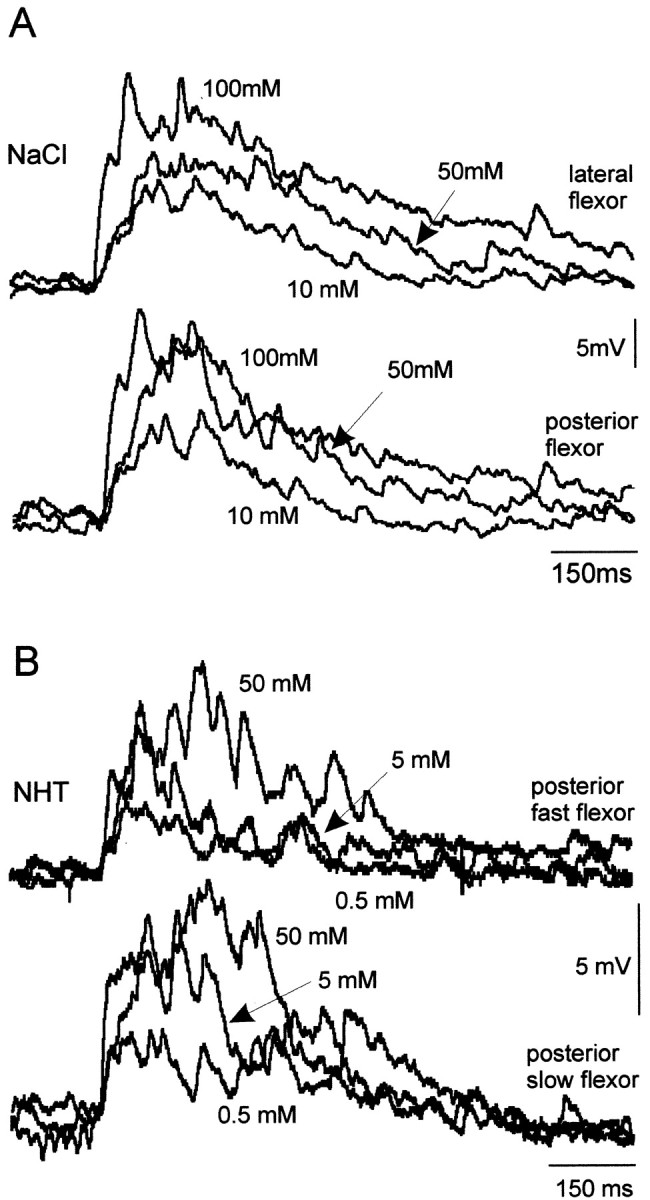
Chemosensitivity of flexor tibia motor neurons. All flexor tibia motor neurons tested in the three motor pools responded in a similar dose-dependent manner. Paired simultaneous recording of flexor tibia motor neurons showed responses to ascending concentrations of NaCl and NHT. A, A motor neuron from each of the lateral and posterior groups responds in a similar manner to each concentration of NaCl. B, The fast and slow flexor tibia motor neurons of the posterior group respond in a similar manner to a concentration series of NHT. Three traces, one at each concentration, are superimposed. Arrows indicate responses to the concentrations shown.
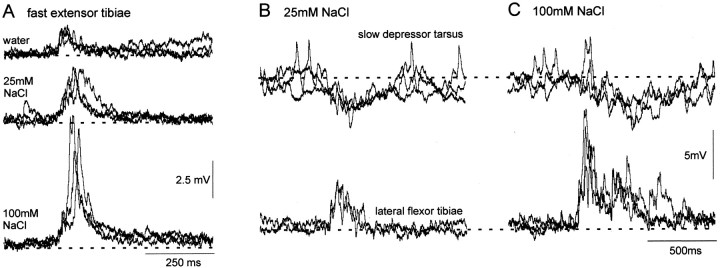
Other motor neurons controlling leg movement also exhibited dose-dependent responses after stimulation of basiconic sensilla on the leg with chemical solutions. The fast extensor tibia was depolarized after applications of droplets to the distal dorsal tibia (n = 3 animals), the duration and amplitude of which increased with greater concentrations of NaCl (Fig.10A). Compared with flexor tibia motor neurons, however, the depolarizations were both smaller and of shorter duration. For example, the duration of the response to 100 mm NaCl lasted for only 166 ± 15.9 msec (mean and SEM) compared with 640 ± 60 msec for the flexor tibia motor neurons. The slow depressor tarsus motor neuron, which receives inhibitory inputs from midline spiking local interneurons, was hyperpolarized after stimulation of the ventral tibia with droplets containing NaCl (n = 4) (Fig.10B,C). As with the flexor and extensor motor neurons, the duration of the response increased with increasing NaCl concentration.
Fig. 10.
Chemosensory responses of other hindleg motor neurons. A, Intracellular recording from a FETi showing superimposed traces of responses of increasing duration to higher concentrations of NaCl. Three traces at each concentration are superimposed. B, C, Paired recording of a lateral flexor tibia and the slow depressor tarsus motor neuron. Both neurons display longer-duration responses to the higher concentration of NaCl (C), but in this case, the depressor tarsus motor neuron is hyperpolarized by the chemosensory input.
DISCUSSION
Both spiking local interneurons and motor neurons receive synaptic inputs during stimulation with five chemicals that represent nutrient through to toxic qualities, but there is a consistent variation in the sensitivity of these neurons to the different chemicals. The duration of these inputs is significantly correlated with chemical concentration for neurons tested with any one chemical and is sufficiently large to give rise to action potentials whose frequencies are also related to chemical concentration and identity.
Overall, 81% of spiking local interneurons tested with one of the test chemicals showed a significant correlation between concentration and response. The high probability of any local interneuron receiving a chemosensory input from any one of the different test chemicals suggests that there is a convergence of a large number of taste qualities onto the same interneurons, either from many sensory neurons responding to a range of chemical qualities or from fewer, more broadly tuned sensory neurons. In addition, interneurons that responded phasically to one chemical responded phasically to all others tested, whereas interneurons that responded phasotonically to one chemical also did so to others. Although members of the same population of spiking local interneurons differed in their somatosensory receptive fields (Burrows and Siegler, 1984), they all shared similar relative sensitivities to different chemical qualities, being very sensitive to low concentrations of NHT but much less sensitive to sucrose, with other chemicals ranging in between. The duration of response, or mean spike number, of spiking local interneurons depended on both chemical identity and concentration. A neuron tested with an ascending concentration series of a single chemical displays a clear dose-dependent response. Conversely, for any single concentration, the relative strength of response of a neuron to different chemicals is highly dependent on the chemical presented (Fig.11).
Locust chemosensory afferents appear to lack a strict segregation into distinct classes of chemical sensitivity (Blaney, 1975; White and Chapman, 1990; Chapman et al., 1991) and show responses typical of vertebrate gustatory fibers (Sato and Beidler, 1997) by each responding to several different chemicals, but in common with other insects, there is some variation in the range of sensitivity between different sensory neurons (Dethier 1976; Schoonhoven et al., 1992). As many different afferents converge onto specific interneurons, however (Burrows and Newland, 1994; Newland and Burrows, 1994), it is not surprising that there appears to be little stimulus specificity at the central level. Furthermore, our previous anatomical studies have shown that chemosensory and mechanosensory afferents from basiconic sensilla on a specific region of the leg all project to the same region of neuropil, to which exteroceptive afferents from tactile hairs also project (Newland, 1991), with no visible anatomical segregation of afferent projections that could be related to either different modalities or chemical sensitivities (Newland et al., 2000). A similar convergence of different modalities is also evident in the taste pathways of vertebrates, with some taste-sensitive neurons also responding to tactile and temperature stimuli (Roper, 1989; Smith and Frank, 1993;Barry, 1999; Cruz and Green, 2000).
Spiking local interneurons are broadly tuned to many different chemical stimuli in a manner consistent with across-fiber pattern coding. Rodent peripheral taste fibers that converge on brainstem neurons in the NST are similarly broadly tuned and lack a high degree of stimulus specificity (Smith and Travers, 1979; Smith and St John, 1999). According to our data, however, there appears to be little evidence for variation in sensitivity range between interneurons in the population from which chemical identity could be extracted elsewhere in the CNS, a key tenet of the cross-fiber patterning hypothesis (Pfaffmann, 1959). Conversely, if individual chemical identities were encoded in labeled lines in the metathoracic ganglion, then we would expect to find at least some different neuron types specific for particular chemicals. All of the interneurons we encountered shared a similar broad response profile, being least sensitive to sucrose but more sensitive to NHT and NaCl. The seven interneurons we specifically tested with different chemicals showed some small variability in responses, but none could be described as defining different types.
These results suggest that local circuits of the metathoracic ganglion may not have separate chemosensory processing pathways for different chemicals or classes of chemical. How then could this system, broadly tuned but with all neurons apparently having the same range of sensitivity, be used to make effective behavioral decisions about chemicals in the environment? Clearly this kind of coding is inconsistent with a system that is concerned with establishing chemical identity, because a similar strength of response in an interneuron can be evoked by a weak solution of NaCl or a strong solution of sucrose. Instead, our results suggest that the duration of response to different chemicals provides a direct measure of a perceived quality, that of aversiveness, because the relative size of the neuronal response of spiking local interneurons and motor neurons correlates strongly with behavioral withdrawal responses (Fig. 11). Our previous experiments (Rogers and Newland, 2000) have shown that different chemicals become aversive at different concentrations, and that behavioral aversiveness is a function of both chemical identity and chemical concentration, both of which are key determinants of the size of the response in spiking local interneurons and motor neurons. Particular strengths of a neural response correspond closely with the likelihood of a locust withdrawing its leg from a chemical solution. Thus, there is a very close correspondence between behavior and neural response (Pearson correlation, 0.91; p < 0.001; n = 14). It does not appear that local circuits of the locust identify particular chemicals, assign behavioral significance to them, and then cue an appropriate response. They do, however, mediate motor responses that differentiate between acceptable and unacceptable, and a neural representation of this appears fully apparent at an early synaptic stage of chemosensory integration. An advantage of this model system is that the distance between sensory input and motor output is relatively small, and hence, it is easier to appreciate the link between behavior and neural response. Similar observations have been made recently in vertebrates. For example, studies on the amygdala of rats suggest that neural responses are not linked with stimulus identity but instead correlate well with the perceived quality of palatability of a gustatory stimulus (Scott and Giza, 2000).
It is readily understandable why locusts should be highly sensitive to and withdraw their legs from the toxic secondary plant compound NHT, but their aversion to high concentrations of nutrient chemicals is also consistent with recent models of the role of taste in dietary regulation in locusts and other animals (Simpson and Raubenheimer, 1996, 2000). These animals do not have an open-ended appetitive desire for nutrient chemicals, but instead, nutrients have to be consumed in the correct quantities and relative proportions to achieve a balanced diet. Under most circumstances, therefore, highly concentrated sources of single nutrients are not only less desirable than more balanced blends of different nutrients but may also be actively rejected at the gustatory sampling stage of feeding (or contact) depending on current nutritional requirements. This lack of desirability, regardless of whether it is elicited by a low concentration of NHT or a high concentration of sucrose, is reflected in the size of the neural response, which in turn correlates with a high probability of leg withdrawal. For the locust, similar gustatory neural responses evoke similar behaviors. Recently, a model with similar features has been developed for taste processing in vertebrates, in which interactions between the basic taste qualities provide overarching measures of just two opposing qualities, nutritional suitability or toxicity (Scott and Giza, 2000; Scott and Verhagen, 2000). Our results provide a striking corollary of this food selection process, but for the insect, the main criterion is rejection (leg withdrawal) rather than acceptance, with the consequence that high concentrations of single nutrients presented to the leg evoke large neural responses that cue withdrawal movements. Although only a single perceived quality, aversiveness, appears to be encoded in the metathoracic ganglion, in principle, a number of other qualities could be simultaneously encoded within a given population of neurons in other organisms. The detailed processing of gustatory information may be different in insects and vertebrates, although the gustatory problems all animals have to solve are similar. Even among vertebrates, however, gustatory processing varies. For example, the processing of amino acids in fish appears to be completely different from the suggested umami taste of mammals (Lindemann, 1996; Ogawa and Caprio, 1999). Our results highlight the importance of knowledge of the final output of a network, behavior, to understanding the function and utility of gustatory information. Smith and St John (1999) point out that it is not sufficient to extrapolate ideas about central coding from information gained only from the receptor cells, because synaptic processing throughout the gustatory pathway shapes and modifies gustatory signals. We believe that the system described here could provide the basis for a relatively tractable model system in which to go further and understand how gustatory signals are modified centrally in response to specific nutritional requirements or states.
Footnotes
This work was supported by an Advanced Fellowship from the Biotechnology and Biological Sciences Research Council (Swindon, UK) (P.L.N.). We thank Dr. Hans Schuppe for numerous discussions about this work and Tom Matheson, David Parker, and Hans Schuppe for their helpful comments on this manuscript.
Correspondence should be addressed to P. L. Newland, Centre for Neuroscience, School of Biological Sciences, Biomedical Sciences Building, University of Southampton, Bassett Crescent East, Southampton SO16 7PX, UK. E-mail: pln@soton.ac.uk.
S. M. Rogers' present address: Department of Zoology, University of Cambridge, Downing Street, Cambridge CB2 3EJ, UK.
REFERENCES
- 1.Barry MA. Recovery of functional response in the nucleus of the solitary tract after peripheral gustatory nerve crush and regeneration. J Neurophysiol. 1999;82:237–247. doi: 10.1152/jn.1999.82.1.237. [DOI] [PubMed] [Google Scholar]
- 2.Blaney WM. Behavioural and electrophysiological studies of taste discrimination by the maxillary palps of larvae of Locusta migratoria (L). J Exp Biol. 1975;62:555–569. doi: 10.1242/jeb.62.3.555. [DOI] [PubMed] [Google Scholar]
- 3.Burrows M. The processing of mechanosensory information by spiking local interneurons in the locust. J Neurophysiol. 1985;54:463–478. doi: 10.1152/jn.1985.54.3.463. [DOI] [PubMed] [Google Scholar]
- 4.Burrows M. The neurobiology of an insect brain. Oxford UP; Oxford: 1996. [Google Scholar]
- 5.Burrows M, Newland PL. Convergence of mechanosensory afferents from different classes of exteroceptors onto spiking local interneurons in the locust. J Neurosci. 1994;14:3341–3350. doi: 10.1523/JNEUROSCI.14-05-03341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrows M, Siegler MVS. The morphological diversity and receptive fields of spiking local interneurones in the locust metathoracic ganglion. J Comp Neurol. 1984;224:483–508. doi: 10.1002/cne.902240403. [DOI] [PubMed] [Google Scholar]
- 7.Burrows M, Watson AHD, Brunn DE. Physiological and ultrastructural characterization of a central synaptic connection between identified motor neurons in the locust. Eur J Neurosci. 1989;1:111–126. doi: 10.1111/j.1460-9568.1989.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 8.Chapman RF. Chemoreception: the significance of receptor numbers. Adv Insect Physiol. 1982;16:247–333. [Google Scholar]
- 9.Chapman RF. Chemosensory regulation of feeding. In: Chapman RF, De Boer G, editors. Regulatory mechanisms in insect feeding. Chapman and Hall; New York: 1995. pp. 101–136. [Google Scholar]
- 10.Chapman RF, Ascoli-Christensen A, White PR. Sensory coding for feeding deterrence in the grasshopper Schistocerca americana. J Exp Biol. 1991;158:241–259. [Google Scholar]
- 11.Cruz A, Green BG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 12.Dethier VG. The hungry fly. Harvard UP; Cambridge, MA: 1976. [Google Scholar]
- 13.Lindemann B. Taste reception. Physiol Rev. 1996;76:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 14.Newland PL. Morphology and somatotopic organization of the central projections of afferents from tactile hairs on the hind leg of the locust. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903120402. [DOI] [PubMed] [Google Scholar]
- 15.Newland PL. Avoidance reflexes mediated by contact chemoreceptors on the legs of locusts. J Comp Physiol [A] 1998;183:313–324. [Google Scholar]
- 16.Newland PL. Processing of gustatory information by spiking local interneurones in the locust. J Neurophysiol. 1999;82:3149–3159. doi: 10.1152/jn.1999.82.6.3149. [DOI] [PubMed] [Google Scholar]
- 17.Newland PL, Burrows M. Processing of mechanosensory information from gustatory receptors on a hind leg of the locust. J Comp Physiol [A] 1994;174:399–410. doi: 10.1007/BF00191706. [DOI] [PubMed] [Google Scholar]
- 18.Newland PL, Rogers SM, Gaaboub I, Matheson T. Parallel somatotopic maps of gustatory and mechanosensory neurons in the central nervous system of an insect. J Comp Neurol. 2000;425:82–96. [PubMed] [Google Scholar]
- 19.Ogawa K, Caprio J. Facial taste responses of the channel catfish to binary mixtures of amino acids. J Neurophysiol. 1999;82:564–569. doi: 10.1152/jn.1999.82.2.564. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffmann C. The afferent code for sensory quality. Am Psychol. 1959;14:226–232. [Google Scholar]
- 21.Pfaffmann C. Specificity of the sweet receptors of the squirrel monkey. Chem Sens Flav. 1974;1:62–67. [Google Scholar]
- 22.Pfaffmann C, Frank M, Bartoshuk LM, Snell TC. Coding gustatory information in the squirrel monkey chorda tympani. In: Sprague JM, Epstein AN, editors. Progress in psychobiology and physiological psychology, Vol 6. Academic; New York: 1976. pp. 1–27. [Google Scholar]
- 23.Rogers SM, Newland PL. Local movements evoked by chemical stimulation of the hind leg in the locust Schistocerca gregaria. J Exp Biol. 2000;203:423–433. doi: 10.1242/jeb.203.3.423. [DOI] [PubMed] [Google Scholar]
- 24.Roper SD. The cell biology of vertebrate taste receptors. Annu Rev Neurosci. 1989;12:329–353. doi: 10.1146/annurev.ne.12.030189.001553. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, Beidler LM. Broad tuning of rat taste cells for four basic taste stimuli. Chem Senses. 1997;22:287–293. doi: 10.1093/chemse/22.3.287. [DOI] [PubMed] [Google Scholar]
- 26.Schoonhoven LM, Blaney WM, Simmonds MSJ. Sensory coding of feeding deterrents in phytophagous insects. In: Bernays EA, editor. Insect-plant interactions, Vol 4. CRC; Boca Raton, FL: 1992. pp. 59–79. [Google Scholar]
- 27.Scott TR, Giza BK. Issues of neural coding: where they stand today. Physiol Behav. 2000;69:65–76. doi: 10.1016/s0031-9384(00)00189-x. [DOI] [PubMed] [Google Scholar]
- 28.Scott TR, Verhagen JV. Taste as a factor in the management of nutrition. Nutrition. 2000;16:874–885. doi: 10.1016/s0899-9007(00)00423-8. [DOI] [PubMed] [Google Scholar]
- 29.Siegler MVS, Burrows M. Spiking local interneurons as primary integrators of mechanosensory information in the locust. J Neurophysiol. 1983;50:1281–1295. doi: 10.1152/jn.1983.50.6.1281. [DOI] [PubMed] [Google Scholar]
- 30.Simpson SJ. Experimental support for a model in which innate taste responses contribute to regulation of salt intake by nymphs of Locusta migratoria. J Insect Physiol. 1994;40:555–559. [Google Scholar]
- 31.Simpson SJ, Raubenheimer D. A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Philos Trans R Soc Lond B Biol Sci. 1993;342:381–402. [Google Scholar]
- 32.Simpson SJ, Raubenheimer D. Feeding behaviour, sensory physiology and nutrient feedback: a unifying model. Entomol Exp Appl. 1996;80:55–64. [Google Scholar]
- 33.Simpson SJ, Raubenheimer D. The hungry locust. Adv Study Behav. 2000;29:1–43. [Google Scholar]
- 34.Smith DV, Frank ME. Sensory coding by peripheral fibres. In: Simon SA, Roper SD, editors. Mechanisms of taste transduction. CRC; Boca Raton, FL: 1993. pp. 295–338. [Google Scholar]
- 35.Smith DV, St John SJ. Neural coding of gustatory information. Curr Opin Neurobiol. 1999;9:427–435. doi: 10.1016/S0959-4388(99)80064-6. [DOI] [PubMed] [Google Scholar]
- 36.Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Sens Flav. 1979;4:215–229. [Google Scholar]
- 37.Smith DV, St John SJ, Boughter JD., Jr Neuronal cell types and taste quality coding. Physiol Behav. 2000;68:77–85. doi: 10.1016/s0031-9384(00)00190-6. [DOI] [PubMed] [Google Scholar]
- 38.Spector AC. Linking gustatory neurobiology to behaviour in vertebrates. Neurosci Biobehav Rev. 2000;24:391–416. doi: 10.1016/s0149-7634(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 39.White PR, Chapman RF. Tarsal chemoreception in the polyphagous grasshopper Schistocerca americana: behavioural assays, sensilla distributions and electrophysiology. Physiol Entomol. 1990;15:105–121. [Google Scholar]