Abstract
Mammalian cells express a transport system known as system xc−, which is an exchange agency specific for anionic forms of cystine and glutamate. System xc− activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. We have shown that the cloned cDNAs encoding the transporter for system xc− consist of two components, xCT and the heavy chain of 4F2 antigen. In the present study, we have investigated the mRNA distribution for these components in the mouse brain by in situ hybridization. The xCT mRNA was expressed in the area postrema, subfornical organ, habenular nucleus, hypothalamic area, and ependymal cells of the lateral wall of the third ventricle in the adult mouse brain. A strong signal was also detected in the meninges in both adult and fetal mouse brains. The mRNA expression of the heavy chain of 4F2 antigen was detected in a more broad area, including all of the regions in which xCT mRNA was detected. These data are compatible with our biochemical evidence that xCT functions in combination with the heavy chain of 4F2 antigen to elicit system xc− activity. The expression of system xc− in meninges and some circumventricular organs may suggest that this transporter contributes to the maintenance of the redox state (i.e., cysteine/cystine ratio) in the CSF.
Keywords: system xc−, cystine, glutamate, glutathione, amino acid transport, CSF, circumventricular organ
Transport of amino acids across plasma membrane is mediated by several transport systems in mammalian cells (Christensen, 1990). We have described an Na+-independent, anionic amino acid transport system that is highly specific for cystine and glutamate in cultured human fibroblasts and mouse peritoneal macrophages (Bannai and Kitamura, 1980; Watanabe and Bannai, 1987). This system, designated as system xc−, transports an anionic form of cystine in exchange for glutamate (Bannai, 1986). The activity of system xc− is drastically induced by electrophilic agents and oxygen in various cultured cells (Bannai, 1984a; Bannai et al., 1986, 1989; Miura et al., 1992). The cells expressing system xc− take up cystine in the medium and reduce it to cysteine (sulfhydryl form), which is in turn used for the synthesis of glutathione and proteins. A part of cysteine is released back into the medium via neutral amino acid transport systems; then cysteine is rapidly oxidized to cystine by oxygen in the medium (Bannai et al., 1989). Thus, the activity of system xc− contributes to driving the cystine/cysteine cycle and to maintaining the balance between cystine and cysteine in the culture medium. In these cultured cells, the activity of system xc− is also demonstrated to be essential for maintaining the intracellular glutathione levels (Bannai and Tateishi, 1986). Because glutathione is one of the cellular antioxidant systems and plays a central role in eliminating oxidative stress, system xc− is categorized as the antioxidant defense system, at least in cultured cells. However, it is unknown whether system xc− functions as an antioxidant defense system in vivo.
We have recently isolated cDNAs for this transporter and found that it is composed of two protein components, the surface antigen of 4F2 heavy chain (4F2hc) and a novel protein named xCT (Sato et al., 1999). xCT has 12 putative transmembrane domains, whereas 4F2hc is predicted to have a single transmembrane domain. It has been shown that in some amino acid transporters, such as systems L and y+L, 4F2hc brings the counterpart light-chain proteins to the surface of the plasma membrane (Mastroberardino et al., 1998; Torrents et al., 1998). We have shown that the combined expression of xCT and 4F2hc is indispensable for eliciting xc− activity in Xenopus oocytes (Sato et al., 1999). Thus, it is likely that 4F2hc functions similarly in system xc−. In Northern blot analysis, the mRNA for xCT was detected in the mouse brain, but not in the heart, liver, lung, or kidney (Sato et al., 1999). The activity of system xc− has been found in primary cultures of rat cortical neurons (Murphy et al., 1990;Ishige et al., 2001), isolated brain cells from fetal rats (Sagara et al., 1993), and human glioma cells (Ye et al., 1999). In the present study, we have investigated the distribution of xCT and 4F2hc mRNAs in the mouse brain by in situ hybridization. The data indicate that system xc− is robustly expressed in some specific brain regions facing the cerebral ventricles and in meninges, suggesting that this transporter is involved in maintaining the redox state of the CSF in the brain.
MATERIALS AND METHODS
Mouse xCT cDNA digested with PstI (34–369 bp), mouse xCT cDNA digested with BamHI and HindIII (886–1843 bp), and mouse 4F2hc cDNA digested with PstI andEcoRI (948–1793 bp) were subcloned into pBluescript. Each cRNA probe was digoxigenin (DIG)-labeled by transcription from the linearized plasmid using RNA-labeling mix (Roche Diagnostics GmbH, Mannheim, Germany) and T3/T7 RNA polymerase (Stratagene, La Jolla, CA). The in situ hybridization was performed as described previously (Ohto et al., 2002). Female C57BL/6Cr mice weighing 18–21 gm were deeply anesthetized with sodium pentobarbital (20 mg/kg, i.p.), perfused, and fixed with 4% paraformaldehyde in PBS, pH 7.4. The brains were excised and postfixed in the same fixative overnight. Then the brains were incubated in 30% sucrose in PBS overnight and embedded with optimal cutting temperature (OCT) compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan). Sections (10 μm) were cut in a cryostat and picked up on Matsunami-aminosilane-coated microscope slides (Matsunami, Osaka, Japan). To remove the OCT compound, the slides were placed in PBS containing 0.1% Tween 20 (PBT) at room temperature twice for 5 min and then incubated in PBT containing 1 gm/ml proteinase K at 37°C for 5 min. The slides were rinsed in PBT three times, fixed with 4% paraformaldehyde, rinsed in PBT three times, and incubated with hybridization buffer (50% formamide, 5× SSC, pH 4.5, 1% SDS, 50 μg/ml heparin, and 50 μg/ml yeast RNA). After the addition of probe (1000 ng/ml), slides were hybridized at 65°C overnight. Slides were rinsed in 50% formamide, 5× SSC, pH 4.5, 1% SDS at 65°C for 30 min; in 50% formamide, 2× SSC at 65°C three times for 30 min each; and with 25 mm Tris-HCl, pH 7.5, 0.8% NaCl, 0.02% KCl, 0.1% Tween 20 (TBST) at room temperature three times for 5 min each. Slides were submerged in the blocking buffer [0.5% blocking reagent (Roche) in TBST] at room temperature for 1 hr and then incubated in sheep anti-DIG antibody conjugated to alkaline phosphatase in the blocking buffer at 4°C overnight. Slides were rinsed with TBST containing 2 mm levamisole at room temperature three times for 20 min each. After a 5 min rinse in 0.1m NaCl, 50 mm Tris, pH 9.5, 50 mm MgCl2, 0.1% Tween 20, and 2 mm levamisole, slides were developed in the dark in BM purple alkaline phosphatase substrate solution (Roche) containing 2 mm levamisole for 2 d. The development was stopped by rinsing several times with 10 mm Tris and 1 mm EDTA.
In Northern blot analysis, total RNA was isolated from several mouse brain regions, dissected under a microscope, and electrophoresed on a 1% agarose gel in the presence of 2.2 m formaldehyde. Then the RNA was transferred onto positively charged nylon membranes (Roche) and hybridized with the DIG-labeled RNA probes in DIG Easy Hyb (Roche) for 16 hr at 68°C. The membrane was washed twice for 5 min at room temperature with 2× SSC and 0.1% SDS and then washed twice for 15 min at 68°C with 0.1× SSC and 0.1% SDS. In Northern blot analysis using32P-labeled DNA probes, the RNA was transferred onto Hybond N+ membrane (Amersham Biosciences, Arlington Heights, IL) and hybridized in a solution containing 50% formamide for 16 hr at 42°C. The membrane was washed twice for 15 min at room temperature with 1× SSC and 0.1% SDS and then washed twice for 15 min at 68°C with 0.25 or 0.1× SSC and 0.1% SDS. The DNA probes were labeled using [α-32P]dCTP and the Rediprime II random prime labeling system (Amersham Biosciences). The templates of the 32P-labeled DNA probes were PCR-amplified fragments between 324 and 1461 bp of mouse xCT cDNA and 221 and 1478 bp of mouse 4F2hc cDNA, respectively.
In reverse transcription PCR (RT-PCR), the first-strand cDNA was synthesized from 1 μg of the total RNA isolated from several mouse brain regions dissected under a microscope using oligo-dT12–18 as a primer. PCR amplification of the cDNA was performed using the primer set 5′-CTCGTGACAGCTGTGGGCAT-3′ and 5′-GGCACTAGACTCAAGAACTGTG-3′, corresponding to the nucleotide sequences of mouse xCT (GenBank/European Molecular Biology Laboratory/DNA Databank of Japan accession no. AB022345).
The experimental procedures involving animals were approved by the University of Tsukuba Animal Care and Use Committee and were done in accordance with its guidelines.
RESULTS
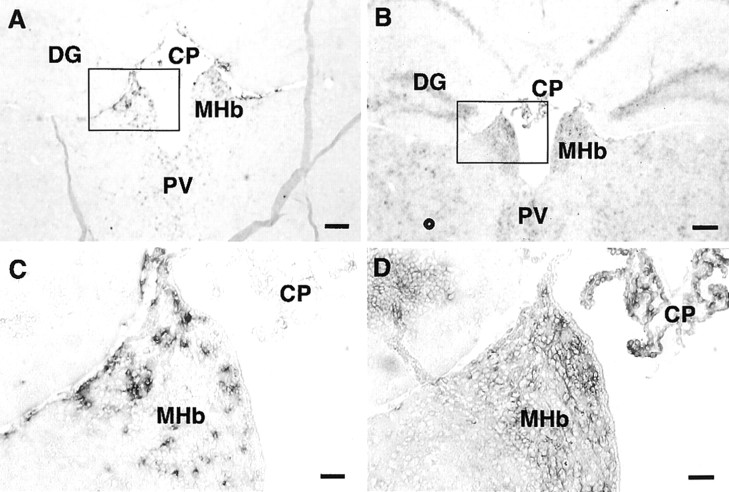
Expression of xCT and 4F2hc mRNAs in the mouse brain was investigated by nonisotopic in situ hybridization using DIG-labeled riboprobes. The antisense probes for both xCT (aBamHI–HindIII fragment of 886–1843 bp) and 4F2hc (a PstI–EcoRI fragment of 948–1793 bp) detected their mRNA expression in some brain regions, whereas the sense probes used as negative controls gave rise to no signal (Figs.1, 2). As shown in Figure 1A–D, both xCT and 4F2hc mRNAs were robustly expressed in approximately one-half of the cells in the area postrema (AP) and in one of the circumventricular organs (CVOs); weaker expression of these mRNAs was observed in the nucleus of the solitary tract. Positive signals for both xCT and 4F2hc were also detected in the subfornical organ (SFO), another CVO (Fig. 2). In addition, the 4F2hc signal was detected in the choroid plexus, cerebellum, brainstem, and thalamus, whereas the signal for xCT was hardly detected in these regions (Figs. 1, 2). Besides CVOs, strong expression of xCT mRNA was observed in the ependymal cells located in the single layer of the ventricular wall adjacent to the ventromedial hypothalamic area (Fig. 3A,B). Expression of the 4F2hc mRNA was also detected in the ependymal cells in the same region (Fig. 3D,E). In the hypothalamic area, positive signals for both xCT and 4F2hc were observed in the scattered cells (Fig. 3C,F), although it remains undetermined which cell types are expressing these mRNAs. Strong expression of xCT was detected in medial habenular nuclei and paraventricular thalamic nuclei facing the dorsal third ventricle (Fig.4A,C), whereas the 4F2hc signal was more broadly detected in these regions (Fig.4B,D).
Fig. 1.
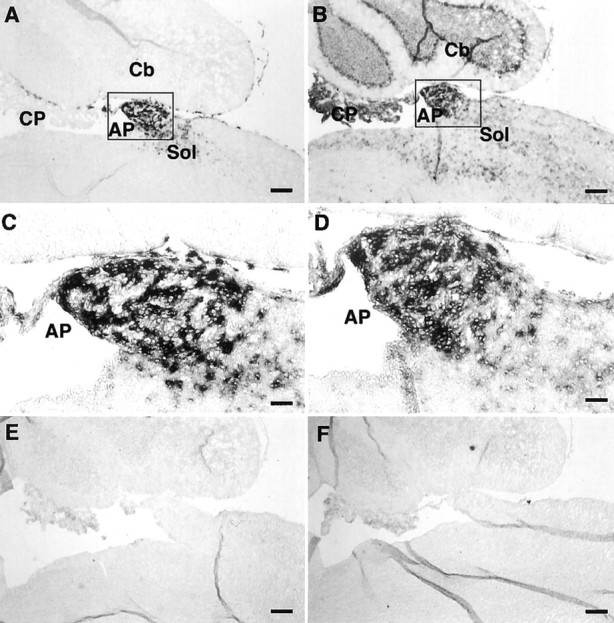
Expression of xCT and 4F2hc mRNAs in the AP of the adult mouse brain detected by nonisotopic in situhybridization. Adjacent sagittal sections were hybridized with DIG-labeled antisense riboprobes for xCT (A, C) and 4F2hc (B, D), respectively. C andD are magnifications of the boxed regionsin A and B, respectively. No signal was detected with the sense probes for xCT and 4F2hc, respectively (E, F). Cb, Cerebellum;CP, choroid plexus; Sol, nucleus of the solitary tract. Scale bars: A, B, E, F, 200 μm;C, D, 50 μm.
Fig. 2.
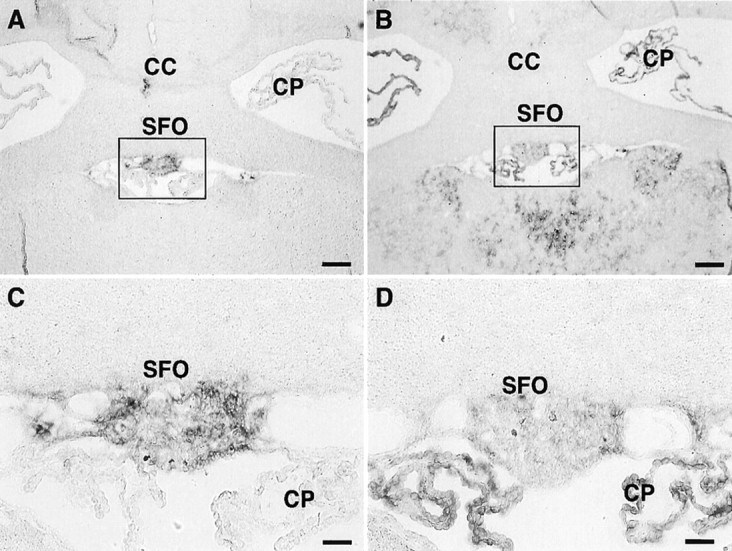
Expression of xCT and 4F2hc mRNAs in the SFO of the adult mouse brain detected by nonisotopic in situhybridization. Adjacent coronal sections were hybridized with DIG-labeled antisense riboprobes for xCT (A, C) and 4F2hc (B, D), respectively. C andD are magnifications of the boxed regionsin A and B, respectively.CC, Corpus callosum; CP, choroid plexus. Scale bars: A, B, 200 μm; C, D, 50 μm.
Fig. 3.
Expression of xCT and 4F2hc mRNAs in the ependymal cells of the lateral wall of the third ventricle (3V) and in the hypothalamic area of the adult mouse brain detected by nonisotopic in situhybridization. Adjacent coronal sections were hybridized with DIG-labeled antisense riboprobes for xCT (A–C) and 4F2hc (D–F). B, C andE, F are magnifications of the boxed regions with solid lines and broken lines in A and D, respectively. Scale bars: A, D, 200 μm; B, C, E, F, 50 μm.
Fig. 4.
Expression of xCT and 4F2hc mRNAs in the medial habenular nucleus (MHb) of the adult mouse brain detected by nonisotopic in situ hybridization. Adjacent coronal sections were hybridized with DIG-labeled antisense riboprobes for xCT (A, C) and 4F2hc (B, D).C and D are magnifications of theboxed regions in A and B, respectively. CP, Choroid plexus; DG, hippocampal dentate gyrus; PV, paraventricular thalamic nucleus. Scale bars: A, B, 200 μm; C, D, 50 μm.
Outside the brain parenchyma, the meninges showed high levels of expression of xCT and 4F2hc mRNAs (Fig.5). Figure 5A–D shows representative examples of xCT and 4F2hc mRNA expression in the meninges lining the surface of the posterior colliculus and cerebellum. Judging from the distribution of the signal, xCT and 4F2hc expression appears to be present in the arachnoid and pia matters (Fig.5C,D). In other areas, such as the one ventral to the hypothalamus, the signals for both xCT and 4F2hc were strongly detected in the meninges but not in the cells of the large blood vessels (Fig.5E,F). To investigate the ontogeny of xCT mRNA expression, we performed in situ hybridization of the embryonic mouse brain. The signal for xCT mRNA was detected primarily in the meninges surrounding the brain of the embryonic day 15 mouse, with especially high expression in the olfactory bulb (Fig.6A,B) and in the subarachnoid space of the cisterna magna (Fig. 6C,D). The signal of 4F2hc mRNA was detected throughout the brain regions, including the meninges (data not shown).
Fig. 5.
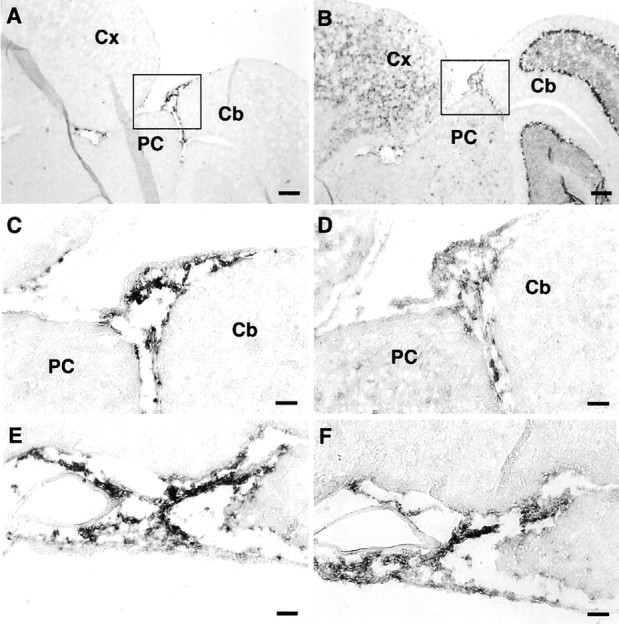
Expression of xCT and 4F2hc mRNAs in the meninges of the adult mouse brain detected by nonisotopic in situhybridization. Adjacent sagittal sections were hybridized with DIG-labeled antisense riboprobes for xCT (A, C, E) and 4F2hc (B, D, F). C andD are magnifications of the boxed regionsin A and B, respectively.E and F show the expression in the meninges underneath the hypothalamus. Cb, Cerebellum;Cx, cerebral cortex; PC, posterior colliculus. Scale bars: A, B, 200 μm;C–F, 50 μm.
Fig. 6.
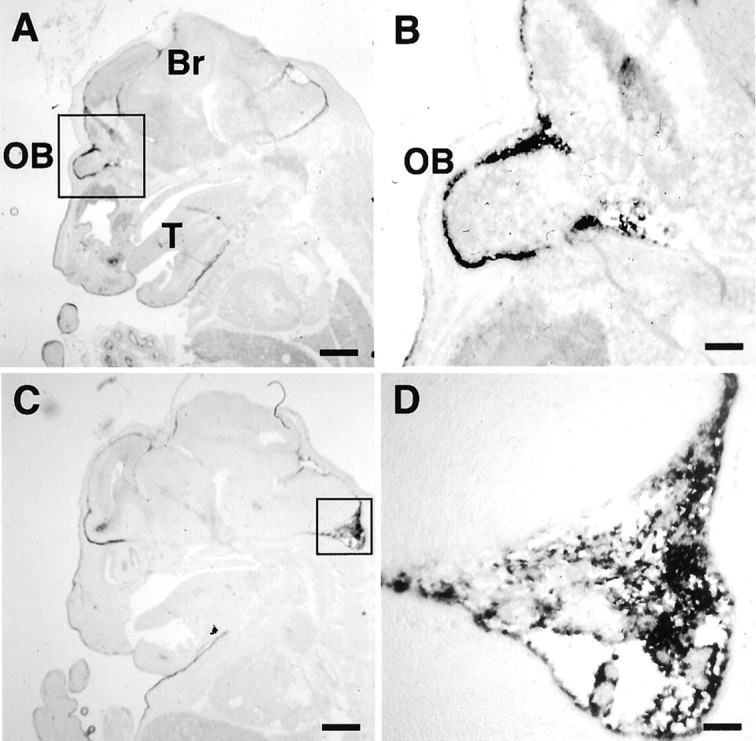
Expression of xCT and 4F2hc mRNAs in the mouse embryos detected by nonisotopic in situ hybridization. Sagittal sections of the embryonic day 15 embryos were hybridized with the DIG-labeled antisense xCT riboprobe (A–D).B and D are magnifications of theboxed regions of the olfactory lobe region inA and in the cisterna magna in C.Br, Brain; OB, olfactory bulb;T, tongue. Scale bars: A, C, 800 μm;B, 200 μm; D, 100 μm.
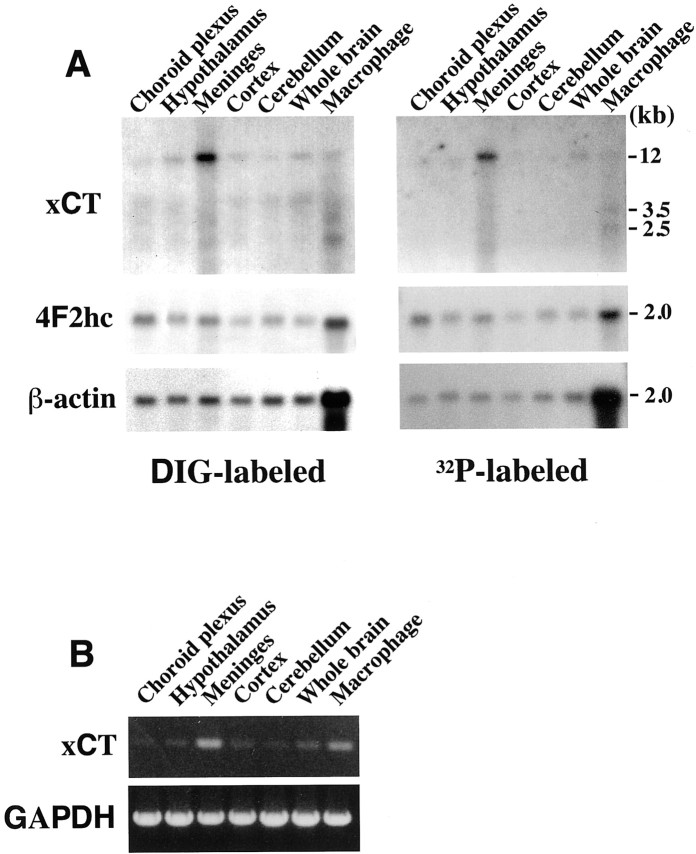
To test the specificity of the data obtained in the above experiments, we have adopted two different approaches. First, we performed in situ hybridization using another set of antisense and sense probes for xCT (a PstI fragment of 34–369 bp), which do not overlap with those used in Figures 1-6. The antisense probe for xCT detected the signal in the AP, whereas the sense probe did not give any signal (Fig. 7). This result is identical to the one obtained using the original probes. Next, we performed Northern blot analysis using the total RNA isolated from mouse brains. To this end, we carefully dissected five small regions from the adult mouse brain under a microscope, and total RNA was isolated. These RNAs were hybridized with the DIG-labeled RNA probes, which were used in thein situ hybridization experiments in this study, and also with the 32P-labeled DNA probe used previously (Sato et al., 1999). Both probes detected a strong signal in the meninges (Fig. 8A), which is in accordance with the high expression of xCT in the meninges in the in situ hybridization experiments. As shown previously, mRNA for xCT is expressed as the multiple bands, ∼12, 3.5, and 2.5 kb, which probably represent alternative splicing, alternative polyadenylation sites, or a combination of both. The prominent 12 kb band has been suggested to represent mRNA with long 3′-untranslated regions (Sato et al., 1999, 2000). The size of the band is the same as the one observed in the activated macrophage used as a positive control. Weak expression was detected in the hypothalamus and the cerebral cortex, and very faint signals were observed in the cerebellum and choroid plexus (Fig. 8A). The 4F2hc signal was detected in all of the areas examined, which is also compatible with the data obtained in the in situhybridization experiments. To confirm the results of the Northern blot analysis, we performed RT-PCR using the total RNA from the five small regions from the adult mouse brain (Fig. 8B). A strong signal was detected in the meninges; weak expression was detected in the hypothalamus and the cerebral cortex. Very faint signals were observed in the cerebellum and choroid plexus. These results are consistent with those of the Northern blot analysis. The strong signal of the macrophage sample is thought to be attributable to the relatively large amount of smaller mRNAs for xCT (3.5 and 2.5 kb) in the macrophages (Sato et al., 1999).
Fig. 7.
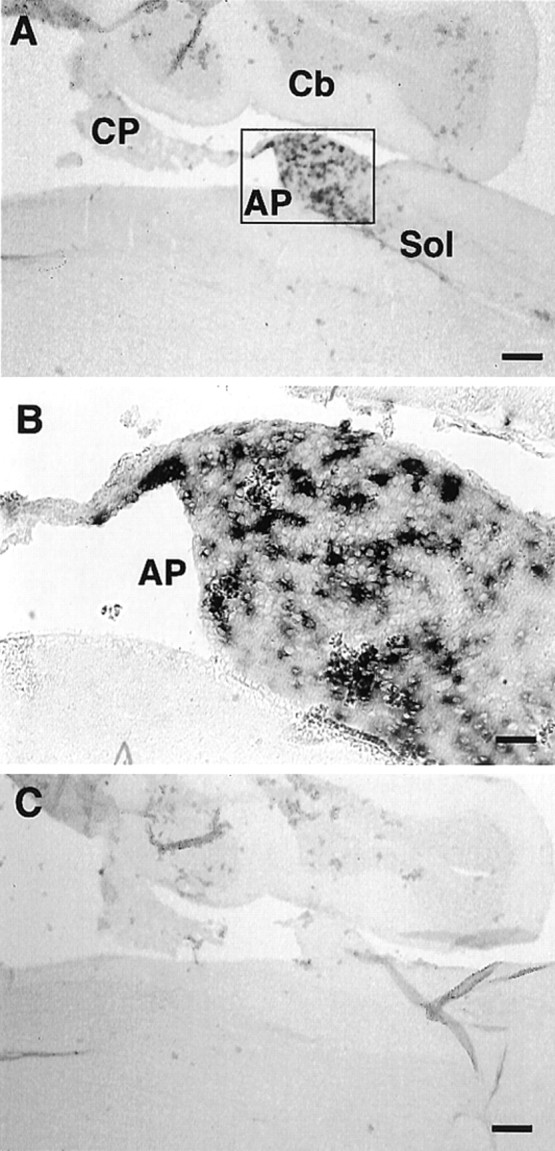
Expression of xCT mRNA in the AP of the adult mouse brain detected by nonisotopic in situhybridization. Adjacent sagittal sections were hybridized with DIG-labeled antisense (A) and sense (C) riboprobes for xCT. The probes used in this experiment do not overlap with those used in Figures 1-6.B is the magnification of the boxed region in A. Cb, Cerebellum;CP, choroid plexus; Sol, nucleus of the solitary tract. Scale bars: A, C, 200 μm;B, 50 μm.
Fig. 8.
Expression of xCT and 4F2hc mRNAs in various areas of the adult mouse brain. A, Northern blot analysis of xCT and 4F2hc. Total RNA (1.5 μg) isolated from the choroid plexus, hypothalamus, meninges, cortex, cerebellum, whole brain, and the mouse peritoneal macrophage cultured for 8 hr with 1 ng/ml bacterial lipopolysaccharide was loaded per lane. Hybridization was performed with DIG-labeled RNA probes or 32P-labeled DNA probes for xCT, 4F2hc, and β-actin. The DIG-labeled RNA probes for xCT and 4F2hc are the same as those used in Figures 1-6.B, RT-PCR of total RNA from the choroid plexus, hypothalamus, meninges, cortex, cerebellum, whole brain, and the mouse peritoneal macrophage cultured for 8 hr with 1 ng/ml bacterial lipopolysaccharide. The figure shows the ethidium bromide-stained agarose electrophoresis of the PCR products using the primer sets corresponding to the sequence of xCT (top) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH;bottom).
DISCUSSION
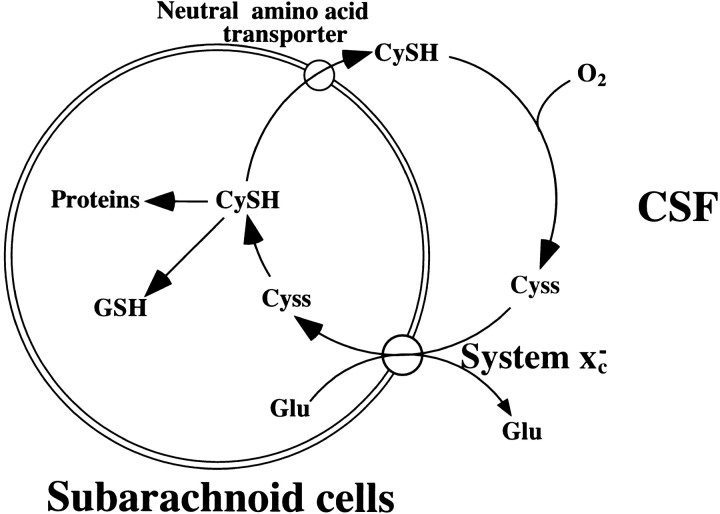
In the present study, we have demonstrated that xCT and 4F2hc mRNAs are especially abundant in several regions facing the CSF. Thus, it is likely that system xc− in these regions contributes to the clearance of cystine from the CSF. Cysteine and cystine, or glutathione and glutathione disulfide, are the critical components as cellular redox buffers in metabolism and homeostasis (Deneke, 2000). In the blood plasma, cysteine and cystine are the main redox components, and the ratio of cysteine/cystine is kept constant (Kleinman and Richie, 2000). The total concentration of free cysteine and cystine in the plasma is ∼120 μm (as half-cystine), and 10–20% of the total is present as the reduced form (Bannai, 1984b; Andersson et al., 1995), although some disturbance was observed in patients with end-stage renal failure (Wlodek et al., 2001). In contrast, the data available for the concentration of cysteine and cystine in the CSF are limited. Only total cystine (cystine plus cysteine) was determined and was reported to be 0.25–1.3 μm in human CSF (Lakke and Teelken, 1976; Araki et al., 1988). Recently, using the thiol-specific fluorogenic reagent, the concentration of cysteine in human CSF was determined to be ∼2.5 μm (Castagna et al., 1995). In rats, the concentration of cysteine in CSF has been demonstrated to be ∼4 μm(Anderson et al., 1989). Wang and Cynader (2000) have shown that the concentration of cysteine in the CSF of rats ranges from 0.68 to 1.75 μm (average, 1.12 μm), whereas cystine is not detected. Together, the ratio of cysteine to cystine in CSF is very high compared with that in plasma. Data for the auto-oxidation rate of cysteine in the CSF are not available; the stability of cysteine in the CSF remains to be investigated. Data for the glutathione level in CSF are also limited; the reported values are very variable. Concentrations of glutathione in rat and human CSF are ∼5–6 μm(Anderson et al., 1989; Wang and Cynader, 2000) and 0.2–0.3 μm (Castagna et al., 1995; Konings et al., 1999), respectively. In the brain, reactive oxygen species are produced at a higher rate because of the high consumption of oxygen. Recent studies have suggested that the reactive oxygen species are involved in the pathogenesis of various neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis (Bains and Shaw, 1997). Because cerebral ventricles, meninges, and the extracellular space in the parenchyma are filled with CSF, the antioxidant potential of CSF seems to be important. The cysteine/cystine balance may serve as a major redox buffer in the CSF. Clearance of cystine via system xc− in the cells facing the CSF and release of cysteine from the cells via neutral amino acid transporter(s) may contribute to maintaining the reduced state in the CSF against the oxidative stress. Figure9 shows the hypothetical model for system xc− in maintaining the redox state of the CSF; a similar model has long been established in the cell-culture system (Bannai et al., 1989). System xc− is an exchange agency, and the anionic form of cystine is transported in exchange for glutamate. The exchange is obligatory, with a molar ratio of 1:1. One of the glutamate transporters, GLAST (also called EAAT 1), is expressed in the AP, SFO, and meninges (Berger and Hediger, 2000). This transporter may contribute to the reabsorption of glutamate effluxed from the cells in exchange for cystine via system xc−.
Fig. 9.
Schematic model for the cystine/cysteine cycle in the subarachnoid space. Cystine (Cyss) in the CSF is taken up by system xc− and reduced to cysteine (CySH), which is primarily used for glutathione (GSH) synthesis; alternatively, it is taken out of the cell by neutral amino acid transporters. Although cysteine in the CSF is rapidly oxidized to cystine, it is cleared from the CSF in the subarachnoid space by the action of system xc−. Glu, Glutamate.
CVOs are highly vascularized structures; they possess unusual vascular arrangements, with many capillary loops reaching near the ventricular surface (Oldfield and McKinley, 1995). These capillaries have fenestrated endothelial cells, resulting in local disruption of the blood–brain barrier. AP and SFO contain receptors or binding sites for many peptides, including angiotensin, and participate in the general regulation of fluid balance and blood pressure (Fitzsimons, 1998). In addition, these regions contain neuronal perikarya and are referred to as the sensory CVOs (Johnson and Gross, 1993). They have extensive afferent and efferent neural networks between the CVOs and other brain structures. There can be bidirectional movement of polar molecules between the blood, the CSF, and the brain parenchyma. Thus, these regions may be allowed to detect small changes in circulating hormones, peptides, and neuroactive substances and to respond hormonally and neurally to these signals. System xc− expressed in the SFO and the AP may function as the sensor for the redox balance between cysteine and cystine in the blood and/or the CSF.
4F2hc functions as the common component not only for system xc− but also for systems L and y+L to elicit their activities (Verrey et al., 2000). In our study, 4F2hc mRNA was detected in all of the areas in the mouse brain where xCT mRNA was expressed, including the AP, SFO, lateral wall of the third ventricle, medial habenular nuclei, and meninges. 4F2hc mRNA was also detected in areas such as the hippocampus, choroid plexus, cortex, and cerebellum. Thus, 4F2hc may be coupled with other partners to elicit different transporter activities. Among the transporters that require 4F2hc for the expression of their transporter activity, L-type amino acid transporter-2 (LAT-2) mediates the transport for neutral amino acids, including cysteine; its mRNA is detected in the brain (Segawa et al., 1999). It is likely that LAT-2 mRNA is expressed in the areas where 4F2hc mRNA is detected and that the activity of system L is expressed in those areas. Although the significant signal of xCT mRNA was not detected in the cortex or cerebellum by our nonisotopic in situ hybridization, the results do not exclude the possibility that a low level of xCT mRNA is expressed in these areas. Indeed, very weak signals were detected in these brain areas by Northern blot analysis and by RT-PCR, and the activity of system xc−has been demonstrated in cortical neurons (Murphy et al., 1990; Ishige et al., 2001) and in the cerebellum (Wyatt et al., 1996; Warr et al., 1999).
Alzheimer's disease is associated with the intraparenchymal growth of plaque-like amyloid deposits (Sisodia and Price, 1995), which are composed of the amyloid β peptide (Aβ). Aβ is derived by proteolytic cleavage of the amyloid protein precursor (APP), which contains a cysteine-rich region. Both Aβ and APP are found in the CSF (Seubert et al., 1992). Huang et al. (1999) have demonstrated that Aβ is capable of generating hydrogen peroxide by metal ion reduction. The reduced state in the CSF might be important for quenching hydrogen peroxide produced by Aβ. The human xCT is localized at chromosome 4q28–31 (Sato et al., 2000). In this locus, the gene for glycophorin A is located, and a link with Alzheimer's disease has been suspected (Race, 1959), although close linkage of this gene with Alzheimer's disease has been excluded (Spence et al., 1986). In the cultured cells, the enhanced activity of system xc− increases the intracellular glutathione level. If system xc− functions similarlyin vivo, several regions expressing system xc− may contain higher levels of glutathione, which contributes to the decrease in the oxidative stress produced in the CSF and parenchyma. The involvement of system xc− in neurodegenerative diseases deserves additional exploration.
Footnotes
This work was supported by a Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank Dr. S. Hisano for helpful discussions.
Correspondence should be addressed to Dr. Hideyo Sato, Department of Biochemistry, Institute of Basic Medical Sciences, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan. E-mail:hideyo-s@md.tsukuba.ac.jp.
REFERENCES
- 1.Anderson ME, Underwood M, Bridges RJ, Meister Glutathione metabolism at the blood-cerebrospinal fluid barrier. FASEB J. 1989;3:2527–2531. doi: 10.1096/fasebj.3.13.2572501. [DOI] [PubMed] [Google Scholar]
- 2.Andersson A, Lindgren A, Hultberg B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin Chem. 1995;41:361–366. [PubMed] [Google Scholar]
- 3.Araki K, Harada M, Ueda Y, Takino T, Kuriyama K. Alteration of amino acid content of cerebrospinal fluid from patients with epilepsy. Acta Neurol Scand. 1988;78:473–479. doi: 10.1111/j.1600-0404.1988.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 4.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 5.Bannai S. Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J Biol Chem. 1984a;259:435–2440. [PubMed] [Google Scholar]
- 6.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984b;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 7.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- 8.Bannai S, Kitamura E. Transport interaction of l-cystine and l-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- 9.Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- 10.Bannai S, Takada A, Kasuga H, Tateishi N. Induction of cystine transport activity in isolated rat hepatocytes by sulfobromophthalein and other electrophilic agents. Hepatology. 1986;6:1361–1368. doi: 10.1002/hep.1840060624. [DOI] [PubMed] [Google Scholar]
- 11.Bannai S, Sato H, Ishii T, Sugita Y. Induction of cystine transport activity in human fibroblasts by oxygen. J Biol Chem. 1989;264:18480–18484. [PubMed] [Google Scholar]
- 12.Berger UV, Hediger MA. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J Comp Neurol. 2000;421:385–399. doi: 10.1002/(sici)1096-9861(20000605)421:3<385::aid-cne7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Castagna A, Le Grazie C, Accordini A, Giulidori P, Cavalli G, Bottiglieri T, Lazzarin A. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: effect of parenteral treatment with SAMe. Neurology. 1995;45:1678–1683. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- 14.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Deneke SM. Thiol-based antioxidants. In: Stadtman ER, Chock PB, editors. Current topics in cellular regulation, Vol 36. Academic; San Diego: 2000. pp. 151–180. [DOI] [PubMed] [Google Scholar]
- 16.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The Aβ peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 18.Ishige K, Chen Q, Sagara Y, Schubert D. The activation of dopamine d4 receptors inhibits oxidative stress-induced nerve cell death. J Neurosci. 2001;21:6069–6076. doi: 10.1523/JNEUROSCI.21-16-06069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 20.Kleinman WA, Richie JP., Jr Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol. 2000;60:19–29. doi: 10.1016/s0006-2952(00)00293-8. [DOI] [PubMed] [Google Scholar]
- 21.Konings CH, Kuiper MA, Teerlink T, Mulder C, Scheltens PH, Wolters ECH. Normal cerebrospinal fluid glutathione concentrations in Parkinson's disease, Alzheimer's disease, and multiple system atrophy. J Neurol Sci. 1999;168:112–115. doi: 10.1016/s0022-510x(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 22.Lakke JPWF, Teelken AW. Amino acid abnormalities in cerebrospinal fluid of patients with parkinsonism and extrapyramidal disorders. Neurology. 1976;26:489–493. doi: 10.1212/wnl.26.5.489. [DOI] [PubMed] [Google Scholar]
- 23.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 24.Miura K, Ishii T, Sugita Y, Bannai S. Cystine uptake and glutathione level in endothelial cells exposed to oxidative stress. Am J Physiol. 1992;262:C50–C58. doi: 10.1152/ajpcell.1992.262.1.C50. [DOI] [PubMed] [Google Scholar]
- 25.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- 26.Ohto T, Uchida H, Yamazaki H, Keino-Masu K, Tatsui A, Masu M. Identification of a novel nonlysosomal sulphatase expressed in the floor plate, choroids plexus, and cartilage. Genes Cells. 2002;7:173–186. doi: 10.1046/j.1356-9597.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- 27.Oldfield BJ, McKinley MJ. Circumventricular organs. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 391–403. [Google Scholar]
- 28.Race RR. Familial Alzheimer's disease: note on the linkage data. Ann Hum Genet. 1959;23:310. [Google Scholar]
- 29.Sagara J, Miura K, Bannai S. Cystine uptake and glutathione level in fetal brain cells in primary culture and in suspension. J Neurochem. 1993;61:1667–1671. doi: 10.1111/j.1471-4159.1993.tb09801.x. [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 31.Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc−. Antioxid Redox Signal. 2000;2:665–671. doi: 10.1089/ars.2000.2.4-665. [DOI] [PubMed] [Google Scholar]
- 32.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 33.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Scholossmacher M, Whaley J, Swindlehurst C, McCormark R, Wolfert R, Selkoe D, Lieberburg I, Schenk DB. Isolation and quantification of soluble Alzheimer's β-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 34.Sisodia SS, Price DL. Role of the β-amyloid protein in Alzheimer's disease. FASEB J. 1995;9:366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- 35.Spence MA, Heyman A, Marazita ML, Sparkes RS, Weinberg T. Genetic linkage studies in Alzheimer's disease. Neurology. 1986;36:581–584. doi: 10.1212/wnl.36.4.581. [DOI] [PubMed] [Google Scholar]
- 36.Torrents D, Estévez R, Pineda M, Fernández E, Lloberas J, Shi YB, Zorzano A, Palacín M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L: a candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273:32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- 37.Verrey F, Meier C, Rossier G, Kühn LC. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflügers Arch. 2000;440:503–512. doi: 10.1007/s004240000274. [DOI] [PubMed] [Google Scholar]
- 38.Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 39.Warr O, Takahashi M, Attwell D. Modulation of extracellular glutathione in rat brain slices by cystine-glutamate exchange. J Physiol (Lond) 1999;514:783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe H, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages. J Exp Med. 1987;165:628–640. doi: 10.1084/jem.165.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wlodek PJ, Iciek MB, Milkowski A, Smolenski OB. Various forms of plasma cysteine and its metabolites in patients undergoing hemodialysis. Clin Chim Acta. 2001;304:9–18. doi: 10.1016/s0009-8981(00)00369-7. [DOI] [PubMed] [Google Scholar]
- 42.Wyatt I, Gyte A, Simpson MG, Widdowson PS, Lock EB. The role of glutathione in L-2-chloropropionic acid induced cerebellar granule cell necrosis in the rat. Arch Toxicol. 1996;70:724–735. doi: 10.1007/s002040050333. [DOI] [PubMed] [Google Scholar]
- 43.Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction–mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]