Abstract
A proper balance between striatal muscarinic cholinergic and dopaminergic neurotransmission is required for coordinated locomotor control. Activation of striatal muscarinic acetylcholine receptors (mAChRs) is known to modulate striatal dopamine release. To identify the mAChR subtype(s) involved in this activity, we used genetically altered mice that lacked functional M1–M5 mAChRs [knock-out (KO) mice]. In superfused striatal slices from wild-type mice, the non-subtype-selective muscarinic agonist oxotremorine led to concentration-dependent increases in potassium-stimulated [3H]dopamine release (by up to 60%). The lack of M1 or M2 receptors had no significant effect on the magnitude of these responses. Strikingly, oxotremorine-mediated potentiation of stimulated striatal [3H]dopamine release was abolished in M4 receptor KO mice, significantly increased in M3 receptor-deficient mice, and significantly reduced (but not abolished) in M5 receptor KO mice. Additional release studies performed in the presence of tetrodotoxin suggested that the dopamine release-stimulating M4receptors are probably located on neuronal cell bodies, but that the release-facilitating M5 and the release-inhibiting M3 receptors are likely to be located on nerve terminals. Studies with the GABAA receptor blocker bicuculline methochloride suggested that M3 and M4receptors mediate their dopamine release-modulatory effects via facilitation or inhibition, respectively, of striatal GABA release. These results provide unambiguous evidence that multiple mAChR subtypes are involved in the regulation of striatal dopamine release. These findings should contribute to a better understanding of the important functional roles that the muscarinic cholinergic system plays in striatal function.
Keywords: acetylcholine, dopamine release, knock-out mice, muscarinic receptors, oxotremorine, striatum
It is well documented that a proper balance between striatal muscarinic cholinergic and dopaminergic neurotransmission is required for coordinated locomotor control (Hornykiewicz, 1981; Graybiel, 1990; Di Chiara et al., 1994; Calabresi et al., 2000; Kaneko et al., 2000). Consistent with this concept, muscarinic antagonists are clinically useful in the treatment of Parkinson's disease (Fahn et al., 1990), a disorder caused by the relative lack of striatal dopamine resulting from the loss of dopaminergic neurons in the substantia nigra pars compacta (Hornykiewicz, 1981; Graybiel, 1990).
The release of striatal ACh from intrinsic cholinergic interneurons is modulated by dopamine via activation of different dopamine receptor subtypes (Di Chiara et al., 1994). Reciprocally, ACh-mediated activation of striatal muscarinic acetylcholine receptors (mAChRs) is known to facilitate striatal dopamine release, as has been shown in both in vitro (Lehmann and Langer, 1982; Raiteri et al., 1984; Schoffelmeer et al., 1986; Kemel et al., 1989) and in vivo (Xu et al., 1989; De Klippel et al., 1993; Smolders et al., 1997) studies. Stimulation of striatal mAChRs can also result in reduced striatal dopamine release, at least under certain experimental conditions (De Belleroche and Bradford, 1978; Kemel et al., 1989; Xu et al., 1989; De Klippel et al., 1993).
The mAChR family consists of five molecularly distinct subtypes (M1–M5), all of which are expressed in the striatum in a complex, overlapping manner (Weiner et al., 1990; Levey et al., 1991; Bernard et al., 1992; Yasuda et al., 1993; Hersch et al., 1994; Yan et al., 2001). From a therapeutic point of view, identification of the mAChR subtype(s) modulating striatal dopamine release should be of considerable interest. Classical pharmacological studies have led to contradictory results regarding the nature of the mAChR subtypes involved in this activity (Raiteri et al., 1984; Schoffelmeer et al., 1986; Xu et al., 1989; De Klippel et al., 1993), reflecting the limited receptor subtype selectivity of the muscarinic agonists and antagonists used in these studies (Caulfield, 1993; Wess, 1996).
To study the physiological and pathophysiological roles of individual mAChRs in a more direct manner, we (Gomeza et al., 1999a,b; Miyakawa et al., 2001; Yamada et al., 2001a,b; Fisahn et al., 2002) and others (Hamilton et al., 1997; Matsui et al., 2000; Gerber et al., 2001) used gene targeting techniques to generate M1–M5 mAChR-deficient mice. In the present study, we performed systematic dopamine release experiments using superfused striatal slices prepared from M1–M5 mAChR knock-out (KO) mice. Specifically, we compared the effects of oxotremorine, a non-subtype-selective muscarinic agonist, on potassium-stimulated [3H]dopamine release in wild-type (WT) and M1–M5 mAChR KO mice.
We provide evidence that multiple mAChRs are involved in modulating striatal dopamine release. Our findings are consistent with the concept that stimulation of M4 and M5 receptors facilitates stimulated striatal dopamine release, whereas activation of M3receptors inhibits this release.
MATERIALS AND METHODS
Animals. The generation of homozygous M1–M5 receptor KO mice [genetic background: 129/SvEv × CF1 (M1, M3, M4, and M5) or 129J1 × CF1 (M2)] has been described previously (Gomeza et al., 1999a,b; Yamada et al., 2001a,b;Fisahn et al., 2002). For each KO strain, the corresponding WT mice were used in parallel as controls. All experiments were performed with adult male mice that were at least 8 weeks of age. Mouse genotyping was performed by PCR analysis of mouse-tail DNA.
Dopamine release studies. Striatal slices (250 × 250 μm) prepared from one mouse were pooled and dispersed in 25 ml of oxygenated (95% O2 and 5% CO2) Krebs–Ringer buffer (in mm: 11.5 glucose, 25 NaHCO3, 1.2 MgCl2, 1.2 NaH2PO4, 118 NaCl, 4.8 KCl, 2.5 CaCl2, and 0.004 Na2EDTA, pH 7.4,) at 33°C for 20 min. Slices were incubated with [3H]dopamine (48.2 Ci/mmol; PerkinElmer Life Sciences, Boston, MA) for 30 min at a final concentration of 0.2 μm in the presence of the anti-oxidant ascorbate (5 mm) and the monoamine oxidase inhibitor pargyline (10 μm) to reduce the metabolism of [3H]dopamine. To prevent the uptake of [3H]dopamine into serotonergic and noradrenergic terminals, citalopram (1 μm) and desipramine (5 μm) were added. After rinsing, slices were transferred to a superfusion system (SF-12; Brandel, Gaithersburg, MD) and superfused at 33°C at a constant rate of 0.4 ml/min. Striatal slices prepared from one mouse were aliquoted into six superfusion chambers (∼25–35 slices per chamber), allowing the construction of complete oxotremorine concentration–response curves (Fig. 1). Fractions were collected every 4 min beginning after a 60 min superfusion. Two 2 min periods of 20 mm KCl were applied after 72 (S1) and 104 (S2) min of superfusion. Tetrodotoxin (TTX) (600 nm) was added at the beginning of superfusion when indicated. TTX is a highly selective sodium-channel blocker that blocks the conduction of action potentials along axons. This pharmacological action can be used in release assays to prevent indirect postsynaptic regulation of neurotransmitter release involving neuronal activity and can help to determine whether release-modulatory receptors are located at nerve terminals or at other cellular locations (cell bodies and/or dendrites).
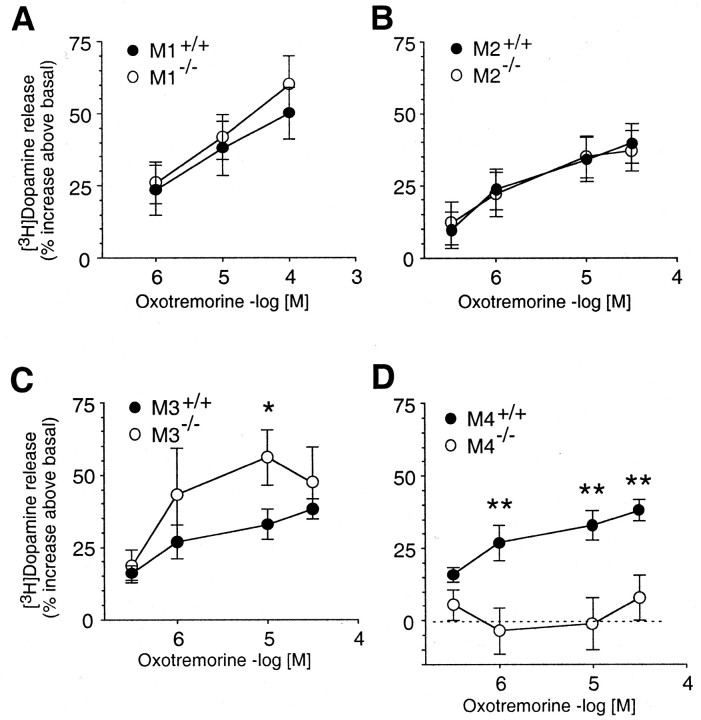
Fig. 1.
Effect of oxotremorine on potassium-stimulated [3H]dopamine release in striatal slices from M1–M4 mAChR KO mice and corresponding WT controls. Striatal slices that had been preincubated with [3H]dopamine were depolarized with 20 mm KCl, and the resulting [3H]dopamine outflow was quantitated in the absence and in the presence of the indicated concentrations of oxotremorine. Data are expressed as the percentage increase in [3H]dopamine release above control levels (no oxotremorine). The WT curves shown inC and D are identical. Because the M3 and M4 mAChR KO mice were both 129/SvEv × CF1 hybrids (50 of 50), only one WT strain of the same genetic background was tested in parallel with these mutant mice. Eachdata point represents the mean ± SEM from 6-11 independent experiments (mice). Asterisks indicate significant differences between responses in KO versus WT preparations (*p < 0.05; **p < 0.01; Student's t test followed by the Holm correction for multitesting adjustment).
Drugs were added to the superfusion buffer 20 min before S2. The efflux of tritium collected was calculated as a percentage of the total tritium present in the slices at the start of the fraction considered. The net efflux of tritium after S1 (fractions 5 and 6) and S2 (fractions 13 and 14) was calculated by subtracting the average of three fractions (expected basal value) before KCl stimulation (S1, fractions 2–4; S2, fractions 10–12). At the end of the [3H]dopamine release experiments, tissues from each chamber were solubilized with 200 μl of 1N NaOH, and tritium was determined in superfusate samples and tissues via liquid scintillation counting. The results were expressed as the S2/S1 ratio of release or as the percentage increase in [3H]dopamine release above control using the following equation: {[S2/S1 (drug)] − [S2/S1 (no drug)]}/[S2/S1 (no drug)] × 100.
RESULTS
To shed light on the potential roles of striatal M1–M5 mAChRs in modulating striatal dopamine release, we performed in vitro dopamine release studies using superfused striatal slices prepared from WT and M1–M5 mAChR-deficient mice. Initially, cellular dopamine pools were radioactively labeled by incubating striatal slices with [3H]dopamine. Subsequently, potassium (20 mm)-stimulated [3H]dopamine release was measured either in the absence (S1 phase) or in the presence (S2 phase) of drugs, as described in Materials and Methods. A large body of studies indicates that such measurements yield results that are physiologically relevant (for review, see Starke et al., 1989).
Basal and potassium-stimulated [3H]dopamine outflow (approximately threefold to fourfold above basal) did not differ significantly between the individual mAChR KO mice and the corresponding WT controls (data not shown).
Multiple mAChRs are involved in modulating stimulated dopamine release in the striatum
Incubation of striatal slices from WT mice with oxotremorine, a non-subtype-selective muscarinic agonist, led to concentration-dependent increases in stimulated [3H]dopamine release (maximum stimulation, ∼40–60%) (Fig. 1), which is in agreement with work published previously (Lehmann and Langer, 1982; Raiteri et al., 1984;Schoffelmeer et al., 1986). This increase in dopamine release was completely abolished in the presence of atropine (10 μm), confirming the involvement of mAChRs (data not shown).
As shown in Figure 1A,B, the lack of M1 or M2 receptors had no significant effect on oxotremorine-mediated increases in [3H]dopamine output. However, this response was significantly enhanced in striatal preparations from M3 receptor KO mice (Fig. 1C) (p < 0.05 at 10 μmoxotremorine), suggesting that the activation of striatal M3 receptors has an inhibitory effect on stimulated dopamine release in WT mice. Strikingly, the ability of oxotremorine to facilitate stimulated striatal [3H]dopamine release was totally abolished in M4 receptor KO mice (Fig.1D). Similar results were obtained with striatal slices prepared from M2/M4receptor double KO mice (data not shown). These findings indicate that M4 receptors play a key role in promoting mAChR-dependent increases in striatal dopamine output.
We have reported previously that oxotremorine-mediated enhancement of striatal dopamine release was impaired (but not abolished) in striatal slices derived from M5receptor KO mice (Yamada et al., 2001a). Together, these data clearly indicate that multiple mAChRs are involved in modulating the magnitude of stimulated dopamine release in the mouse striatum.
TTX has differential effects on oxotremorine-mediated modulation of simulated striatal dopamine release in WT and M3 and M5 receptor KO mice
We then wanted to investigate whether mAChR-mediated modulation of stimulated striatal dopamine release was dependent on the activation of mAChRs on (dopaminergic) nerve terminals or whether it required more complex neuronal circuits. To address this issue, we examined to what extent oxotremorine-mediated modulation of striatal dopamine release was altered in the presence of TTX, a selective sodium-channel blocker that prevents the propagation of action potentials along axons.
In the presence of TTX (600 nm), oxotremorine-mediated increases in potassium-stimulated [3H]dopamine release were abolished in striatal preparations from WT mice (Fig.2A). Interestingly, TTX treatment unmasked opposing modulatory effects of oxotremorine on stimulated [3H]dopamine release in striatal preparations from M3 and M5 receptor KO mice. In tissues from M3 receptor KO mice, oxotremorine (in the presence of TTX) retained the ability to induce small but significant increases in potassium-stimulated [3H]dopamine release (Fig.2B), most likely because of the presence of release-facilitating M5 receptors and the absence of release-inhibiting M3 receptors. However, in preparations from M5 receptor KO mice, oxotremorine treatment (in the presence of TTX) significantly decreased potassium-stimulated [3H]dopamine release (Fig. 3A), probably because of the presence of release-inhibiting M3and the absence of release-facilitating M5receptors.
Fig. 2.
Effect of TTX on oxotremorine-mediated modulation of potassium-stimulated [3H]dopamine release in striatal slices from WT and M3 receptor KO mice. In the presence of TTX (600 nm), oxotremorine had no significant effect on stimulated [3H]dopamine release in WT preparations (A) but induced a significant enhancement in [3H]dopamine output in striatal slices from M3 receptor KO mice (B). Each bar represents the mean ± SEM of S2/S1 values from six or seven independent experiments (mice). Micromolar concentrations are shown.Asterisks indicate significant differences from the control group (no oxotremorine) (*p < 0.05; **p < 0.01; one-way ANOVA followed by Dunnett's test).
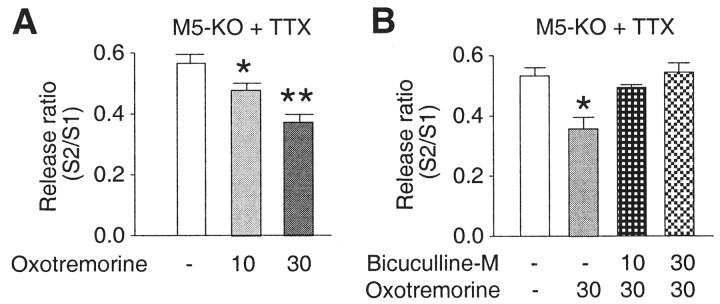
Fig. 3.
Effect of TTX on oxotremorine-mediated modulation of potassium-stimulated [3H]dopamine release in striatal slices from M5 receptor KO mice. In the presence of TTX (600 nm), oxotremorine induced a decrease in potassium-stimulated [3H]dopamine release in striatal slices from M5 receptor KO mice (A). This effect was blocked by the GABAA receptor antagonist bicuculline methochloride (B). Each bar represents the mean ± SEM of S2/S1 values from eight independent experiments (mice). Micromolar concentrations are shown. Asterisks indicate significant differences from the control group (no drug) (*p < 0.05; **p < 0.01; one-way ANOVA followed by Dunnett's test).
In the striatum, M3 receptors are expressed by a subgroup of GABAergic projection neurons (Hersch et al., 1994; Yan et al., 2001), raising the possibility that the release-inhibiting effects of M3 receptors that persist in the presence of TTX are attributable to an increase in GABA release. Consistent with this hypothesis, the incubation of TTX-treated striatal slices from M5 receptor KO mice with the GABAA receptor antagonist bicuculline methochloride (10 and 30 μm) completely prevented oxotremorine (30 μm)-mediated inhibition of dopamine release (Fig. 3B).
As outlined above, oxotremorine lacked the ability to facilitate stimulated dopamine release in striatal slices from M4 receptor KO mice (Fig. 1D). In the striatum, M4 receptors are abundantly expressed by GABAergic projection neurons (Weiner et al., 1990; Bernard et al., 1992; Hersch et al., 1994; Santiago and Potter, 2001). Therefore, we speculated that the dopamine release-facilitating activity of M4 receptors might be attributable to M4 receptor-mediated reductions in GABA release. In agreement with this hypothesis, incubation of striatal slices from WT mice with bicuculline methochloride (100 μm) mimicked the stimulatory effect of oxotremorine (30 μm) on potassium-induced [3H]dopamine release (Fig.4). Combining bicuculline methochloride (30 or 100 μm) and oxotremorine (30 μm) did not increase the stimulated [3H]dopamine outflow from WT preparations any more than observed with either drug alone (Fig.4).
Fig. 4.
Effect of the GABAAreceptor antagonist bicuculline methochloride on oxotremorine-mediated enhancement of potassium-stimulated [3H]dopamine release in striatal slices from WT mice. When administered alone, bicuculline methochloride (100 μm) mimicked the release-facilitating effect of oxotremorine; however, when coadministered with oxotremorine, bicuculline methochloride did not further enhance [3H]dopamine output. Eachbar represents the mean ± SEM of S2/S1 values from eight independent experiments (mice). Micromolar concentrations are shown.Asterisks indicate significant differences from the control group (no drug) (** p < 0.01; one-way ANOVA followed by Dunnett's test).
DISCUSSION
Properly regulated dopamine release in the striatum is of fundamental importance for extrapyramidal locomotor control (Graybiel, 1990; Di Chiara et al.; 1994; Calabresi et al., 2000). The present study was designed to identify specific mAChR subtypes involved in modulating this process. Previous studies using classical pharmacological tools have led to conflicting results regarding the molecular identity of the mAChR subtypes involved in the regulation of striatal dopamine release, probably because of the limited degree of receptor subtype selectivity of the muscarinic agonists and antagonists used in these studies. For example, although it has been proposed that muscarinic agonist-induced enhancement of striatal dopamine release is mediated by M1 receptors (Raiteri et al., 1984;Xu et al., 1989; Smolders et al., 1997), Schoffelmeer et al. (1986)suggested that this activity is dependent on the stimulation of non-M1 mAChRs. However, it has been proposed that muscarinic agonist-induced inhibition of striatal dopamine release is mediated by M2 receptors (Xu et al., 1989; De Klippel et al., 1993).
Receptor localization studies have shown that all five mAChRs (M1–M5) are expressed in the striatum (Weiner et al., 1990; Bernard et al., 1992;Yasuda et al., 1993; Hersch et al., 1994; Yan et al., 2001), raising the possibility that multiple mAChRs play a role in the modulation of striatal dopamine release. To address this issue in a more direct manner, we performed systematic dopamine release studies using superfused striatal slices from M1–M5 mAChR-deficient mice. Previous studies have shown that disruption of specific mAChR genes has no significant effect on the expression levels of the remaining four mAChR subtypes in different regions of the brain, including the striatum (Hamilton et al., 1997; Gomeza et al., 1999a,b;Miyakawa et al., 2001; Yamada et al., 2001b; Fisahn et al., 2002; Zhang et al., 2002).
Consistent with previous findings (Lehmann and Langer, 1982; Raiteri et al., 1984; Schoffelmeer et al., 1986), incubation of WT striatal slices with the non-subtype-selective muscarinic agonist oxotremorine led to concentration-dependent increases in potassium-stimulated [3H]dopamine release (Fig. 1). The magnitude of this response was not affected by the lack of M1 or M2receptors (as studied with M1 and M2 receptor KO mice) (Fig.1A,B), both of which are expressed at relatively high levels in the striatum (Weiner et al., 1990; Levey et al., 1991;Bernard et al., 1992; Hersch et al., 1994). These results clearly indicate that striatal M1 and M2 receptors do not play a significant role in the regulation of striatal dopamine output, in contrast to the conclusions drawn based on classical pharmacological studies using muscarinic ligands of limited receptor subtype selectivity.
Interestingly, in vivo microdialysis studies showed recently that M1 receptor-deficient mice have significantly elevated levels of extracellular dopamine in the striatum, probably because of increased dopamine release (Gerber et al., 2001). As discussed by Gerber et al. (2001), it is possible that the M1 receptors modulating striatal dopamine release are located on either striatal or extrastriatal (e.g., cortical) neurons projecting to the striatum. Because the lack of M1 receptors had no significant effect on basal or stimulated dopamine release in striatal slice preparations (this study), the increase in extracellular dopamine levels observed in vivo is most likely attributable to the absence of extrastriatal (e.g., cortical) M1 receptors mediating inhibition of striatal dopamine release through an indirect neuronal pathway.
Strikingly, muscarinic agonist-mediated increases in stimulated [3H]dopamine output were totally abolished in striatal slices from M4 receptor KO mice (Fig. 1D), suggesting that M4 receptors play a key role in mediating this activity. Similarly, TTX treatment (600 nm) of WT striatal slices also completely abolished the dopamine release-facilitating effects of oxotremorine (Fig.2A), suggesting that this activity requires the propagation of action potentials. In the striatum, M4 receptors are abundantly expressed by medium spiny GABAergic projection neurons (Weiner et al., 1990; Bernard et al., 1992; Hersch et al., 1994; Santiago and Potter, 2001), where they are preferentially located on cell bodies and dendritic shafts and spines (Hersch et al., 1994; Bernard et al., 1999). M4 receptors, like M2receptors, are coupled to G-proteins of the Gi/Go family, which, among other cellular effects, can reduce neuronal activity by inhibiting different classes of calcium channels (Caulfield, 1993; Hille, 1994;Howe and Surmeier, 1995). Interestingly, in WT striatal slices, the GABAA receptor antagonist bicuculline methochloride mimicked the release-facilitating effect of oxotremorine when administered alone; however, when coadministered with oxotremorine, bicuculline methochloride did not further enhance [3H]dopamine output (Fig. 4). Together, these observations are consistent with a model in which the activation of M4 receptors present on GABAergic projection neurons inhibits GABA release, resulting in reduced GABAA receptor-mediated inhibition of dopamine release from dopaminergic nerve endings. This model is supported by previous studies that indicate that muscarinic agonists inhibit GABA release in the striatum (Marchi et al., 1990; Sugita et al., 1991), that dopaminergic neurons in the substantia nigra receive synaptic input from striatal GABAergic neurons (Bolam and Smith, 1990), that dopamine release-inhibiting GABAA receptors exist on dopaminergic striatal nerve endings (Ronken et al., 1993), and that ACh-mediated enhancement of striatal [3H]dopamine release may be mediated by the collaterals of GABAergic inhibitory neurons (Kemel et al., 1989).
We have reported previously that oxotremorine-mediated potentiation of stimulated dopamine release was significantly reduced (by ∼50%) at an intermediate oxotremorine concentration (10 μm) in striatal slices derived from M5 receptor KO mice (Yamada et al., 2001a), suggesting that M5receptors also contribute to ACh-mediated enhancement of striatal dopamine release. However, maximum oxotremorine responses were not significantly affected by the lack of M5receptors (Yamada et al., 2001a), in contrast to the total lack of oxotremorine activity observed in the absence of M4 receptors (Fig. 1D).
In contrast, oxotremorine-mediated enhancement of stimulated [3H]dopamine release was significantly increased in striatal slices from M3 receptor KO mice (Fig. 1C), indicating that M3 receptor stimulation activates a pathway that inhibits striatal dopamine release. The opposing effects of M3 versus M5 receptor stimulation on striatal dopamine release may explain the observation that oxotremorine had no net effect on stimulated dopamine release in striatal slices from M4 receptor KO mice (Fig.1D).
When release studies were performed in the presence of TTX (which is predicted to block the M4 receptor-mediated enhancement of dopamine release), oxotremorine administration led to small but significant increases in stimulated dopamine release in striatal slices from M3 receptor KO mice (Fig.2B) but to a significant decrease in dopamine output in preparations from M5 receptor KO mice (Fig.3A). These observations provide additional support for the concept that M3 and M5receptors mediate opposing effects on stimulated dopamine release in the striatum. Because M3 and M5 receptor-mediated modulation of striatal dopamine release persists in the presence of TTX, it is likely that the M3 and M5 receptors involved in this activity are located on nerve terminals.
M5 receptor mRNA is the only mAChR subtype mRNA detectable in the dopamine-containing cells of the substantial nigra pars compacta (Vilaro et al., 1990; Weiner et al., 1990), strongly suggesting that the dopamine release-facilitating M5 receptors are located on dopaminergic nerve terminals. Based on these findings (Vilaro et al., 1990; Weiner et al., 1990), together with the fact that M3 and M5 receptors (as well as M1receptors) couple to a similar set of G-proteins (Gq family) (Caulfield, 1993; Wess, 1996), it is unlikely that the dopamine release-inhibiting M3receptors are colocalized with M5 receptors on dopaminergic terminals. M3 receptors are expressed, although apparently at relatively low levels, in a subset of GABAergic projection neurons (Hersch et al., 1994; Yan et al., 2001), raising the possibility that M3 receptors located on GABAergic nerve terminals inhibit dopamine release by stimulating GABA release. Consistent with this concept, the GABAA receptor antagonist bicuculline methochloride completely blocked the oxotremorine-mediated reduction in dopamine release observed with TTX-treated striatal slices from M5 receptor KO mice (Fig. 3B). The existence of presynaptic striatal M3 receptors has also been demonstrated via immunoelectron microscopy (Hersch et al., 1994).
In summary, our data provide strong evidence that multiple mAChR subtypes are involved in the regulation of striatal dopamine release. Whereas activation of M4 and M5 receptors facilitates striatal dopamine release, stimulation of M3 receptors inhibits this process. Striatal M1 and M2 receptors do not seem to play significant roles in modulating striatal dopamine output. Our results underscore the usefulness of muscarinic receptor mutant mice to delineate the roles of individual mAChR subtypes in the regulation of striatal function. A better understanding of the role of the striatal muscarinic cholinergic system may open new perspectives for the treatment of Parkinson's disease and other extrapyramidal movement disorders.
Footnotes
This work was supported by a Cooperative Research and Development Agreement between the National Institute of Diabetes and Digestive and Kidney Diseases (J.W.) and the Eli Lilly Research Laboratories.
Correspondence should be addressed to Dr. Jürgen Wess, Molecular Signaling Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Building 8A, Room B1A-05, 8 Center Drive MSC 0810, Bethesda, MD 20892-0810. E-mail: jwess@helix.nih.gov.
M. Yamada's present address: Laboratory for Cell Culture Development, Brain Science Institute, RIKEN, Saitama 351-0198, Japan.
J. Gomeza's present address: Max-Planck-Institut für Hirnforschung, Neurochemie, Deutschordenstrasse 46, D-60528 Frankfurt/Main, Germany.
REFERENCES
- 1.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard V, Levey AI, Bloch B. Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation. J Neurosci. 1999;19:10237–10249. doi: 10.1523/JNEUROSCI.19-23-10237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. doi: 10.1016/0006-8993(90)90811-o. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 5.Caulfield MP. Muscarinic receptors: characterization, coupling, and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 6.De Belleroche J, Bradford HF. Biochemical evidence for the presence of presynaptic receptors on dopaminergic nerve terminals. Brain Res. 1978;142:53–68. doi: 10.1016/0006-8993(78)90176-2. [DOI] [PubMed] [Google Scholar]
- 7.De Klippel N, Sarre S, Ebinger G, Michotte Y. Effect of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism: an in vivo microdialysis study comparing normal and 6-hydroxydopamine-lesioned rats. Brain Res. 1993;630:57–64. doi: 10.1016/0006-8993(93)90642-z. [DOI] [PubMed] [Google Scholar]
- 8.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.Fahn S, Burke R, Stern Y. Antimuscarinic drugs in the treatment of movement disorders. Prog Brain Res. 1990;84:389–397. doi: 10.1016/s0079-6123(08)60922-x. [DOI] [PubMed] [Google Scholar]
- 10.Fisahn A, Yamada M, Duttaroy A, Gan J-W, Deng C-X, McBain CJ, Wess J. Muscarinic induction of hippocampal γ oscillations requires coupling of the M1 receptor to two mixed cation channels. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- 11.Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci USA. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999a;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomeza J, Zhang L, Kostenis K, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999b;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersch S, Gutekunst C-A, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 18.Hornykiewicz O. Brain neurotransmitter changes in Parkinson's disease. In: Marsden DC, Fahn S, editors. Movement disorders. Butterworth; Boston: 1981. pp. 41–58. [Google Scholar]
- 19.Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- 21.Kemel M-L, Desban M, Glowinski J, Gauchyh C. Distinct presynaptic control of dopamine release in striosomal and matrix areas of the cat caudate nucleus. Proc Natl Acad Sci USA. 1989;86:9006–9010. doi: 10.1073/pnas.86.22.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann J, Langer SZ. Muscarinic receptors on dopamine terminals in the cat caudate nucleus: neuromodulation of [3H]dopamine release in vitro by endogenous acetylcholine. Brain Res. 1982;248:61–69. doi: 10.1016/0006-8993(82)91147-7. [DOI] [PubMed] [Google Scholar]
- 23.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchi M, Sanguineti P, Raiteri M. Muscarinic receptors mediate direct inhibition of GABA release from rat striatal nerve terminals. Neurosci Lett. 1990;116:347–351. doi: 10.1016/0304-3940(90)90099-u. [DOI] [PubMed] [Google Scholar]
- 25.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raiteri M, Leardi R, Marchi M. Heterogeneity of presynaptic muscarinic receptors regulating neurotransmitter release in the rat brain. J Pharmacol Exp Ther. 1984;228:209–214. [PubMed] [Google Scholar]
- 28.Ronken E, Mulder AH, Schoffelmeer AN. Interacting presynaptic κ-opioid and GABAA receptors modulate dopamine release from rat striatal synaptosomes. J Neurochem. 1993;61:1634–1639. doi: 10.1111/j.1471-4159.1993.tb09797.x. [DOI] [PubMed] [Google Scholar]
- 29.Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- 30.Schoffelmeer AN, Van Vliet BJ, Wardeh G, Mulder AH. Muscarine receptor-mediated modulation of [3H]dopamine and [14C]acetylcholine release from rat neostriatal slices: selective antagonism by gallamine but not pirenzepine. Eur J Pharmacol. 1986;128:291–294. doi: 10.1016/0014-2999(86)90781-8. [DOI] [PubMed] [Google Scholar]
- 31.Smolders I, Bogaert L, Ebinger G, Michotte Y. Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J Neurochem. 1997;68:1942–1948. doi: 10.1046/j.1471-4159.1997.68051942.x. [DOI] [PubMed] [Google Scholar]
- 32.Starke K, Göthert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 33.Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of γ-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci USA. 1991;88:2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilaro MT, Palacios JM, Mengod G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett. 1990;114:154–159. doi: 10.1016/0304-3940(90)90064-g. [DOI] [PubMed] [Google Scholar]
- 35.Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 37.Xu M, Mizobe F, Yamamoto T, Kato T. Differential effects of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism in freely moving rats. Brain Res. 1989;495:232–242. doi: 10.1016/0006-8993(89)90217-5. [DOI] [PubMed] [Google Scholar]
- 38.Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic vasodilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 2001a;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001b;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 40.Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- 42.Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]