Abstract
Endothelin-1 (ET-1) causes pain through activation of nociceptors, by either direct depolarization or increased excitability. Here we examined the effect of ET-1 on nociceptor-associated tetrodotoxin-resistant (TTX-R) sodium currents using whole-cell voltage clamp of acutely dissociated rat dorsal root ganglion (DRG) neurons. DRG neurons that responded had enhanced activation gating when exposed to 10 nm ET-1, as determined by significant shifts in their average activation midpoint potentials (ΔE0.5 = −8.0 ± 0.5 mV) when compared with control (ΔE0.5 = −2.2 ± 0.4 mV; n = 6) and ET-1 unresponsive cells (ΔE0.5 = −3.2 ± 0.2 mV). ET-1 also modified the availability of TTX-R channels, as determined by negative shifts in the average midpoint potential for inactivation of ET-1 responsive cells when compared with controls. These actions of ET-1 occurred predominantly in cells with more slowly inactivating TTX-R currents. Both time-to-peak current and inactivation time constants were shortened by ET-1 in responsive cells. Previous exposure of cells to the endothelin-A (ETA) receptor antagonist BQ-123 (1 μm) prevented ET-1-induced shifts in TTX-R activation. In contrast to changes in TTX-R, ET-1 did not modify tetrodotoxin-sensitive currents recorded from DRG neurons. These results demonstrate that the algogenic peptide ET-1 induces ETA receptor-mediated, hyperpolarizing shifts in the voltage-dependent activation of TTX-R Na+ channels, a potential mechanism for selective excitation by ET-1 of nociceptors that we observed in vivo.
Keywords: nociception; excitability; G-protein-coupled receptor; sodium channel; hyperalgesia, dorsal root ganglion neurons
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide that has been implicated in the pathogenesis of tissue inflammation and pain (Ferreira et al., 1989; Piovezan et al., 1997; Davar et al., 1998; De-Melo et al., 1998; Graido-Gonzalez et al., 1998; Griswold et al., 1999; Fareed et al., 2000; Jarvis et al., 2000; Wacnik et al., 2001). Recent results from our laboratory suggest that the pain-inducing actions of ET-1, which include the induction of firing in peripheral nociceptors (Gokin et al., 2001), are attributable to direct actions on these cells rather than secondary to any vasoactive effect (Zhou et al., 2001). ET-1 might excite nociceptors by activating or potentiating an inward current or by suppressing an outward current that contributes to the resting potential. Indeed, a tetrodotoxin-resistant (TTX-R) voltage-gated Na+ current in human cardiac tissue (hH1, NaV1.5) is known to be enhanced by ET-1 (Cheng et al., 1995). In nociceptive neurons, TTX-R channels have been implicated in the increased sensitivity to pain that follows tissue inflammation and are believed to play a critical role in sensory neuronal excitability (Gold et al., 1996; England et al., 1996; Renganathan et al., 2001). Furthermore, the modulation of TTX-R currents by the injury-related mediators prostaglandin E2, adenosine, and serotonin (Gold et al., 1998; Cardenas et al., 2001) has been shown to depend on neuronal protein kinases, which produce long-lasting changes that might underlie the prolonged firing that we induce in nociceptors with ET-1.
The possible contribution of enhanced TTX-R currents to ET-1-induced nociception has not been examined. Therefore, in these experiments, we examined the effect of ET-1 on early and late TTX-R and tetrodotoxin-sensitive (TTX-S) sodium currents in acutely dissociated rat dorsal root ganglion (DRG) neurons using whole-cell voltage clamp. We also determined the receptor-dependence of the actions of ET-1 on TTX-R currents and analyzed the TTX-R activation and inactivation gating.
MATERIALS AND METHODS
Cell preparation. DRGs were harvested from male Sprague Dawley rats (150 gm; Charles River, Wilmington, MA) as described previously (Gold et al., 1998). Rats were anesthetized with Na pentobarbital (Nembutal; 60 mg/kg, i.p.; Parke-Davis Courbevoie, France). Lumbar DRGs (L1–L6) were removed and treated with 0.125–0.25% collagenase (Boehringer Mannheim, Indianapolis, IN) and 0.25% (w/v) trypsin (Worthington, Freehold, NJ) in ice-cold complete modified Eagle's medium (MEM) composed of 90% MEM (Invitrogen, Grand Island, NY) and 10% fetal bovine serum (Invitrogen). Cells were plated and incubated at 37°C with 5% CO2 and then transferred to a 27°C incubator with 5% CO2. Cells were used between 2 and 24 hr after this final plating.
Electrophysiology. The whole-cell voltage-clamp technique was used to measure Na+ currents, using borosilicate glass electrodes (World Precision Instruments, Sarasota, FL). The resistance of electrodes pulled by a P-87 Electrode Puller (Sutter Instruments, Novato, CA) ranged from 0.8 to1.5 MΩ. Voltage clamp was controlled by an Axopatch-200 (Axon Instruments, Foster City, CA), and currents, initially filtered at 10 kHz, were collected using pClamp8 software (Axon Instruments). Series resistance (1–4 MΩ) and capacitance were partially compensated electronically. Leak and residual capacity currents were subtracted using the P/4 procedure (Armstrong and Bezanilla, 1977).
Solutions and drugs. Synthetic ET-1 (98% pure peptide content) and BQ-123 were both obtained from American Peptides (Sunnyvale, CA) and were dissolved in PBS (Invitrogen), pH 7.4, before use. TTX was obtained from Sigma (St. Louis, MO). The patch electrodes were filled with the following (in mm): 130 CsF, 3 MgATP, 0.5 LiGTP, 3 MgCl2, 5 NaCl, 1.1 EGTA, 0.1 CaCl2, and 10 HEPES, pH 7.2. The bath solution for recording TTX-R currents contained the following (in mm): 35 NaCl, 75 choline-Cl, 30 TEA-Cl, 5 KCl, 1 CaCl2, 10 glucose, 1 MgCl2, and 10 HEPES, pH 7.4. To isolate TTX-R Na currents, TTX (0.1 μm) was used in all experiments, except those used to measure TTX-S. In these latter experiments, low Na+ (5 mm) was used (with the difference substituted by equimolar choline chloride) to avoid saturation of the amplifier with the large TTX-sensitive currents found primarily in large-diameter cells.
RESULTS
ET-1 modifies peak activation of TTX-R currents in a subpopulation of sensory neurons
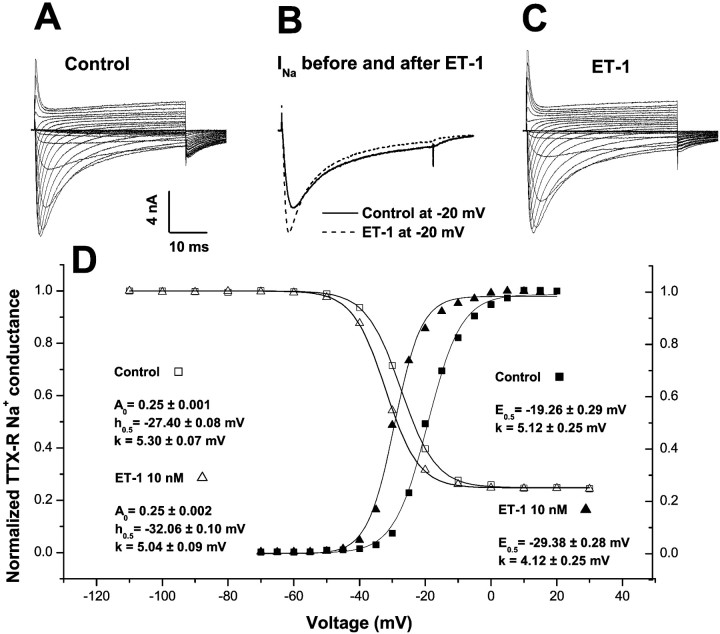
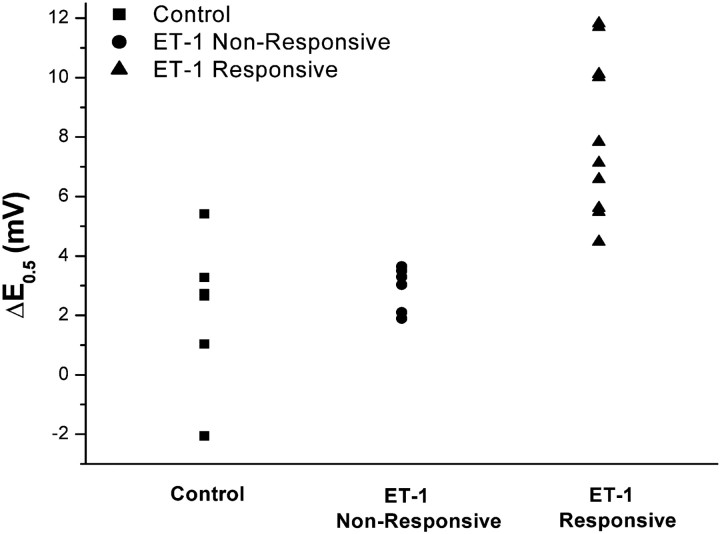
Tetrodotoxin-resistant sodium currents were recorded in 17 DRG cells exposed to 10 nm ET-1. These currents had voltage-dependent kinetics nearly identical to those reported previously for TTX-R in sensory neurons (Bossu and Feltz, 1984; Gold et al., 1996; Rush et al., 1998), and the peak current showed a reversal potential (+40.2 ± 1.7 mV) that was close to the calculated equilibrium potential for sodium ions under these conditions. In 10 of these small cells (capacitance, 23.8 ± 1.3 pF), the “activation gating” of peak Na+current was enhanced, as determined by negative shifts along the voltage axis of the peak conductance versus test potential (Fig.1). In seven cells of similar size, activation gating was unchanged compared with changes in control cells (see below; Table 1). Such gating represents the voltage-dependent probability that Na+ channels will open in response to rapid membrane depolarization and is closely linked to membrane excitability. Gating modifications developed over 2–5 min, consistent with Na+ channel modifications initiated by other G-protein-coupled receptors in DRG neurons (Gold and Levine, 1996), and reached a steady-state by 10 min, when the shifts were analyzed. The criteria for a real change in such gating was based on comparison with the spontaneous changes that occurred in the absence of ET-1; activation midpoint potentials (E0.5) and slopes (k) calculated from fits of the Boltzmann equation to the conductance data (Fig. 1D) (cf. Hille, 2001) in these control cells had initial values that were not changed after 10 or 20 min (n = 4) in control medium. Thus, the seven cells with insignificantly different changes produced by ET-1 were classified as “unresponsive” (range, −1.9 to −3.9 mV; p > 0.05; unpaired t test) (Fig.2). In contrast, the 10 ET-1 “responsive” cells had highly significant average shifts ofE0.5 (range, −4.1 to −11.7 mV) when compared with baseline values (p = 0.0003; paired t test) and with the control group (p = 0.0004; unpaired Student's ttest). All 10 responsive cells had ΔE0.5 that were greater than −4mV (Fig. 2), whereas none of the unresponsive cells had changes of that magnitude (Fig. 2). Changes of the activation slope, k, that occurred spontaneously or during exposure to ET-1 were not significant (p > 0.46). ET-1 also shifted the reversal potential (Erev) slightly but significantly (from +38.7 ± 1.7 to +34.9 ± 1.9 mV;p = 0.03) in responsive but not in unresponsive or control cells, possibly consistent with actions of ET-1 on other, likely outward, ionic currents flowing at these positive voltages.
Fig. 1.
Changes in Na+ current gating induced by ET-1. A, A control family of TTX-R currents recorded during the sequential application of 40-msec-long test depolarizations, ranging from −70 to +30 mV in 5 mV steps, in a small-diameter DRG neuron. Ehold = −80 mV; ftest = 0.1 Hz;T = 20–22°C; TTX = 100 nm.B, TTX-R currents during a −20 mV depolarization recorded before and 10–12 min after exposure to 10 nm ET-1 in the same cell as that in A. C, A family of TTX-R currents recorded from the same cell and under the same conditions as in B. D, Analysis of the voltage dependence of peak conductance activation (filled symbols) and availability (inactivation; open symbols) before (squares) and 10–12 min after (triangles) exposure of one cell to ET-1 (10 nm). Current data are from the same cell described inA–C. Fits of the Boltzmann equation to the peak conductance versus test depolarization data yield values for the activation midpoint potential (E0.5, with 95% confidence limits from this single fit) that are shifted by −10 mV in this cell. Availability (inactivation) parameters were generated from Boltzmann functions fit to normalized, inactivatable peak current versus prepulse (100-msec-long) potential data, from a different cell than the one yielding the activation data. The non-inactivatable TTX-R remained at ∼0.25 of the total current after ET-1 exposure, whereas the midpoint potential for availability (h0.5) was shifted by −5 mV in this cell. The slope factors, k, for activation and for availability functions were not changed by ET-1.
Table 1.
Effect of ET-1 on activation and inactivation of TTX-R and TTX-S sodium channels in rat DRG neurons
| Activation curve* | Availability curve** | |||
|---|---|---|---|---|
| E0.5(mV) | ka (mV) | h0.5(mV) | kh (mV) | |
| TTX-R | ||||
| Control cells (n = 6) | ||||
| Baseline | −14.49 ± 1.29 | 5.59 ± 0.72 | −24.75 ± 0.44a | 4.76 ± 0.02 |
| 10 min | −16.67 ± 1.35 | 6.13 ± 0.70 | −25.67 ± 0.53 | 5.29 ± 0.00 |
| 20 min | −14.11 ± 0.87 | 5.95 ± 0.67 | ||
| Δ[10 min − baseline] | −2.17 ± 0.42 | 1.03 ± 0.54 | −0.92 ± 0.09 | 0.53 ± 0.02 |
| ET-1 responsive cells (n = 10) | ||||
| Baseline | −18.94 ± 1.42 | 5.39 ± 0.25 | −27.26 ± 1.48 | 5.33 ± 0.10 |
| ET-1 | −26.98 ± 1.34 | 4.64 ± 0.27 | −32.44 ± 1.50 | 6.02 ± 0.29 |
| Δ[ET-1 − baseline] | −8.04 ± 0.54 | 0.75 ± 0.27 | −5.18 ± 0.37 | 0.69 ± 0.26 |
| ET-1 non-responsive cells (n = 7) | ||||
| Baseline | −13.33 ± 2.20 | 5.93 ± 0.45 | −20.57 ± 0.35b | 5.33 ± 0.03 |
| ET-1 | −15.58 ± 2.34 | 4.64 ± 0.27 | −22.81 ± 0.38 | 5.93 ± 0.04 |
| Δ[ET-1 − baseline] | −3.25 ± 0.18 | 0.07 ± 0.17 | −2.24 ± −0.03 | 0.60 ± 0.03 |
| BQ-123 plus ET-1-treated cells (n = 10) | ||||
| Baseline | −10.27 ± 1.85 | 6.67 ± 0.36 | −23.55 ± 1.85 | 6.41 ± 0.34 |
| BQ-123 | −12.34 ± 1.84 | 5.94 ± 0.37 | −25.12 ± 2.02 | 6.51 ± 0.56 |
| BQ-123 plus ET-1 | −13.93 ± 1.90 | 6.13 ± 0.24 | −29.05 ± 2.11 | 6.63 ± 0.38 |
| Δ[BQ-123 − baseline] | −2.07 ± 0.61 | 1.54 ± 0.45 | −1.85 ± 0.50 | 0.13 ± 0.30 |
| Δ[(BQ-123 + ET-1) − BQ-123] | −1.54 ± 0.45 | 0.19 ± 0.17 | −2.90 ± 0.35 | 0.07 ± 0.26 |
| TTX-S (n = 7) | ||||
| Baseline | −14.49 ± 1.29 | 5.59 ± 0.72 | −24.75 ± 0.44 | 4.76 ± 0.02 |
| ET-1 | −16.67 ± 1.35 | 5.95 ± 0.67 | −25.67 ± 0.53 | 5.29 ± 0.01 |
| Δ[ET-1 − baseline] | −2.17 ± 0.42 | 1.03 ± 0.54 | −0.92 ± 0.09 | 0.53 ± 0.02 |
All values listed are means ± SEM. *Boltzmann equation for fitting the activation curve is as follows:G/Gmax = {1 + exp[−(E −E0.5)/ka]}−1. **Boltzmann equation for fitting the availability curve is as follows:I/Imax = (Amax− A0)/{1 + exp[(h0.5 −E)/kh]} +A0.
n = 4.
n = 4.
Fig. 2.
Scattergram showing the distribution of changes in activation midpoint potentials (ΔE0.5) of individual DRG neurons unexposed to ET-1 (Control; changes after 10 min), exposed to ET-1 but with ΔE0.5 < −4 mV after 10 min (ET-1 Non-Responsive), and exposed to ET-1 and having ΔE0.5 > −4 mV after 10 min (ET-1 Responsive).
ET-1 also changes inactivation of TTX-R in sensory neurons
Steady-state inactivation (availability) of TTX-R Na+ channels was also modified by ET-1. This parameter indicates the fraction of Na+ channels that is poised to open in response to a rapid depolarization, a key index to the resting excitability of the membrane that is regulated through the resting potential. In 10 ET-1 responsive cells, all showing shifts of activation gating (see above), the midpoint potential for inactivation,h0.5 (determined from the amplitudes of currents during a test pulse to −10 mV after 100msec prepulses ranging from −120 to +30 mV), was negatively shifted by 5 mV after a 10 min exposure to 10 nm ET-1 (Table 1, Fig.1D). Four control cells, untreated by ET-1, had spontaneous changes over this same time course inh0.5 of −0.92 ± 0.09, significantly less than the changes with ET-1 in responsive cells (p = 0.0011). In ET-1 unresponsive cells, there was a smaller inactivation shift of −2.2 mV compared with control (p = 0.0017) and responsive (p = 0.019) cells. Treatment by ET-1 did not alter the fraction of TTX-R channels that did not inactivate in 100 msec, which remained at ∼25% (Fig. 1,A0), nor did it change the slope factor,kh, of inactivation. The kinetics of this remaining, non-inactivatable current showed the same activation rise and time-to-peak as the total current, consistent with its assignment as a non-inactivating Na+current.
Sensitivity to ET-1 is related to Na+current kinetics
The degree of change of Na+ current by ET-1 in responsive cells depended on the baseline gating kinetics of the Na+ currents, and these kinetics, in turn, were modified by ET-1. Currents that were predominantly transient, fast inactivating (with a decay time constant, τ, of ∼10 msec or less at −20 mV) were altered less by ET-1; peak current amplitude at −20 mV was increased 1.17 ± 0.11-fold in three such cells. In contrast, currents that inactivated more slowly (τ >14 msec) were amplified more strongly by ET-1 (5.3 ± 3.5-fold). In fact, there was a significant correlation (r2 = 0.81; p< 0.002) between the control inactivation τ and the degree of ET-1-induced amplification of peak current.
The “late” Na+ currents, measured at the end of the 40-msec-long depolarization, had a reversal potential of 3.9 ± 2.5 mV, indicating that they are a mixture of Na+ currents and another outward current. The contributions from INa represent the open probability of subpopulations of channels that either close very slowly or maintain a low-level, persistent activation during sustained depolarization (Cummins et al., 1999). Activation functions for the late components of TTX-R, analyzed from currents averaged over the 35–40 msec at the end of the test depolarizations (Fig.1A,C), were fit by the Boltzmann equation with E0.5 = −27.4 ± 7.4mV and k = 3.5 ± 0.6 mV before ET-1; the same respective parameters were changed by −6.43 ± 0.04 (p = 0.002) and −0.49 ± 1.5 mV (p = 0.4) after ET-1 treatment in the responsive cells, very similar to the −8 mV shift of the peak Na+ conductance (see above). These results are consistent with a selective action of ET-1 on the non-inactivated Na+ currents at this time rather than an alteration of other “contaminating” currents.
Although there was a difference in the baseline midpoint potentials for both activation and availability gating, measured before ET-1 exposure, between the 10 responsive cells and the seven unresponsive cells, this difference did not achieve significance (Table 1) (p = 0.07; unpaired t test). These data, together with the similarity in membrane capacitances for these groups of cells, argue against any relevant phenotypic differences in these two populations of neurons.
Gating kinetics are altered by ET-1
ET-1-induced shifts in Na+conductance were accompanied by a general acceleration of gating kinetics (Fig. 1B). Currents during a −20 mV depolarization (near E0.5) in all responsive cells had a shortened time-to-peak current, to 0.70 ± 0.06 of control (p = 0.01), and a decreased τ to 0.66 ± 0.05 of control (p = 0.02). The currents in cells with faster inactivation rates at the baseline, preexposure condition (τ ∼10 msec) had this rate increased insignificantly, by a factor of 1.28 ± 0.16 after ET-1 exposure (p = 0.10), whereas in those with the slower baseline decay rate (τ >14 msec) inactivation was significantly accelerated, by a factor of 2.22 ± 0.69 (p= 0.02). Interestingly, the maximum Na+conductance, determined by the limiting slope of theINa versusEm relation at large depolarizations (analysis not shown), was not changed by ET-1; the maximum conductance ratio between treated and control cells equaled 1.043 ± 0.002 in 10 responsive cells. This implies that the maximum number of activatable Na+ channels is unchanged by ET-1, although the rapid gating kinetics are altered. In the seven unresponsive cells, the gating kinetics were unchanged by ET-1.
Changes in activation gating are mediated through endothelin-A receptors
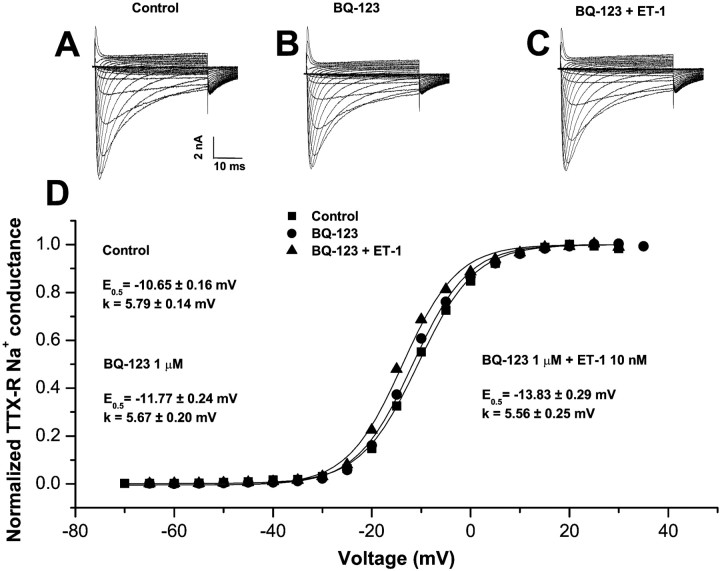
Ten minute treatment of cells modified previously by ET-1 with an antagonist of the endothelin-A (ETA) receptor BQ-123 (1 μm), which prevents ET-1-induced behavioral responses and excitation of nociceptive afferents in vivo(Davar et al., 1998; Gokin et al., 2001), did not alter the shift, which grew even more negative during the treatment period (Fig.3D) (ΔE0.5 = −11.6 ± 1.3 mV from pre-ET-1 baseline; n = 4). Subsequent washing with control media for 10 min was also ineffective (ΔE0.5 = −11.9 ± 2.5;n = 3). In contrast, pretreatment with BQ-123 (1 μm), followed by coexposure with ET-1 (10 nm), effectively prevented the shift of activation (Table 1, Fig. 3). For these procedures, ΔE0.5 after BQ-123 alone and after BQ-123 plus ET-1 were insignificantly different from spontaneous shifts (see above).
Fig. 3.
Changes in TTX-R activation induced by ET-1 are prevented by an ETA receptor antagonist. A family of control currents recorded in the same cell is shown before (A), 10 min after continuous exposure to the ETA receptor antagonist BQ-123 (1 μm) (B), and after 10 min of additional exposure to BQ-123 (1 μm) plus ET-1 (10 nm) (C). Recording conditions were identical to those described in Figure 1. D, Boltzmann functions for the normalized peak conductance versus test potential from current data shown in A–C. Neither the activation midpoint nor the slope were modified by ET-1 in the presence of BQ-123 or by BQ-123 alone.
TTX-sensitive Na+ currents are not modified by ET-1
In seven larger cells, which have almost exclusively TTX-S sodium currents and minimal TTX-R, ET-1 had no effect on gating (Table 1). Activation midpoint potentials were −17.3 ± 0.2 mV before ET-1, −18.3 ± 0.2 mV after 5 min, and −18.8 ± 0.2 mV after 20 min exposures to ET-1 (p > 0.15 for the latter two values compared with pre-ET-1). χ2analysis showed that the absence of a change in any of these seven cells is significantly different (p < 0.025) than the presence of a shift in 10 of the 17 cells expressing TTX-R current.
DISCUSSION
Relevance of this action to the pain-inducing behavior of ET-1
These results define actions of ET-1 on sensory neurons that may explain the pain behavior and selective excitation of nociceptors that we observed in vivo (Davar et al., 1998; Fareed et al., 2000; Gokin et al., 2001). The dominant action of ET-1 is an ETA receptor-dependent shift in the hyperpolarizing direction for the voltage-dependent activation of TTX-R Na+ channels, without any changes in TTX-S channels. Such shifts of TTX-R in the hyperpolarizing direction have also been described for other treatments that either sensitize nociceptors or induce pain (England et al., 1996; Gold et al., 1996;Kral et al., 1999; Cardenas et al., 2001). Because TTX-R currents represent several Na+ channel subtypes, e.g., NaV1.8 and NaV1.9 (for alternate nomenclature, see Golden, 2001), found exclusively in small-diameter soma with nociceptor properties (Elliott and Elliott, 1993; Rush et al., 1998; Amaya et al., 2000; Fjell et al., 2000), these results are consistent with the specific activation by ET-1 of nociceptive afferents in vivo (Gokin et al., 2001). Differences in the response to ET-1 probably reflect heterogeneity in the distribution or availability of ETAreceptors, or their downstream signals, rather than differences in the levels of or type of TTX-R currents (Rush et al., 1998). Although ET-1 has been shown to modulate ionic conductances in other excitable tissues (e.g., heart) (Lauer et al., 1992; Xie et al., 1996) through ETA and ETB receptors (Ono et al., 1994; Held et al., 1998; Boixel et al., 2001), this is the first report of an action of ET-1 on ion channels in the peripheral sensory nervous system.
Importance of the shifts in TTX-R activation gating for sensory neuronal excitability
These results raise the question of whether shifts in the activation gating of TTX-R produced by ET-1 are sufficient to induce impulses in responsive cells. In small-diameter DRG neurons with resting potentials of −50 to −60 mV (Wang et al., 1994; Study and Kral, 1996), the ET-1-induced changes in activation gating, approximately −8 mV, would result in a modest additional Na+ current. Given the known values of the resting resistance and capacitance of the soma, this seems unlikely to lead by itself to impulse generation. However, shifts in Na+ activation can be at least twice as large in “intact” cells versus cells with modified intracellular content, as studied by the whole-cell method used here (Gold et al., 1996). Also, the “resting” membrane potential in cell soma may oscillate by several millivolts in amplitude; oscillations that are increased as the potential becomes less negative, appear to depend on Na+ channels, and have been implicated in bursting ectopic discharges associated with pathologic pain (Amir et al., 1999). Furthermore, much higher concentrations of ET-1 than used here were applied in vivo to induce spontaneous firing, so that even larger shifts may be possible in vivo. Together, these factors could result in ET-1-induced shifts of the resting potential of 15–20 mV (Herzog et al., 2001) and possible enhanced oscillations that alone could account for ET-1-induced spike responses in cutaneous nociceptors (Gokin et al., 2001). ET-1 may also augment the responsiveness of neurons or sensory afferent terminals that are already receiving excitatory or even subthreshold stimuli.
Identification of the Na+ channel subtype involved
At least two channels carry the TTX-R Na+ current in sensory neurons. These have been characterized variously by their activation midpoint potentials (E0.5), inactivation midpoint potentials (h0.5), and inactivation rates, although there is no consensus on these gating parameters. Here the peak Na+ currents had controlE0.5 values of approximately −14 mV and h0.5 values of approximately −27 mV, identical to the values reported for so-called “TTX-R1” currents by Rush et al. (1998) and to the lumped TTX-R currents studied by others (Bossu and Feltz, 1984; England et al., 1996; Gold et al., 1996; Kral et al., 1999). Our analyses show that slowly inactivating TTX-R currents are altered much more by ET-1 than rapidly inactivating currents. This may reflect a differential sensitivity of channel isoforms, although we found no systematic difference in theE0.5 orh0.5 parameters between these more and less sensitive peak currents nor, for that matter, between currents in cells that responded to ET-1 and those that did not. Alternatively, the more rapidly inactivating currents may appear in cells with elevated pre-ET-1 exposure levels of a second messenger (e.g., cAMP), which leads to relatively faster channel inactivation as well as insensitivity to ET-1, as observed for 5-HT-induced changes in TTX-R (Cardenas et al., 2001).
Although we cannot discriminate in these experiments between the actions of ET-1 on different TTX-R channel subtypes, both of the described sensory neuron-specific TTX-R channels, Nav1.8 and Nav1.9, have been shown to play a critical role in neuronal excitability (Herzog et al., 2001; Renganathan et al., 2001). At this time, the only unequivocal assignment we can make is that more slowly inactivating TTX-R currents are the forms that respond strongly to ET-1 and that TTX-S currents are totally unaffected.
Z.Z. and G.D. contributed equally to this work.
Correspondence should be addressed to Dr. Gudarz Davar, Molecular Neurobiology of Pain Laboratory, Pain Research Center, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115. E-mail:gdavar@zeus.bwh.harvard.edu.
REFERENCES
- 1.Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- 2.Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boixel C, Dinanian S, Lang-Lazdunski L, Mercadier JJ, Hatem SN. Characterization of effects of endothelin-1 on the L-type Ca2+ current in human atrial myocytes. Am J Physiol Heart Circ Physiol. 2001;281:H764–H773. doi: 10.1152/ajpheart.2001.281.2.H764. [DOI] [PubMed] [Google Scholar]
- 5.Bossu JL, Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci Lett. 1984;51:241–246. doi: 10.1016/0304-3940(84)90558-5. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas LM, Cardenas CG, Scroggs RS. 5HT Increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na+ channels. J Neurophysiol. 2001;86:241–248. doi: 10.1152/jn.2001.86.1.241. [DOI] [PubMed] [Google Scholar]
- 7.Cheng TH, Chang CY, Wei J, Lin CI. Effects of endothelin 1 on calcium and sodium currents in isolated human cardiac myocytes. Can J Physiol Pharmacol. 1995;73:1774–1783. doi: 10.1139/y95-242. [DOI] [PubMed] [Google Scholar]
- 8. Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in small primary sensory neurons. J Neurosci 19 1999. RC43(1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. NeuroReport. 1998;9:2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 10.De-Melo JD, Tonussi CR, D'Orleans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998;77:261–269. doi: 10.1016/S0304-3959(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 11.Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol (Lond) 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol (Lond) 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fareed MU, Hans G, Atanda A, Strichartz G, Davar G. Pharmacological characterization of acute pain behavior produced by application of endothelin-1 to rat sciatic nerve. Pain. 2000;1:46–53. [Google Scholar]
- 14.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13 [Suppl 5]:S220–S222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 15.Fjell J, Hjelmstrom P, Hormuzdiar W, Milenkovic M, Aglieco F, Tyrrell L, Dib-Hajj S, Waxman SG, Black JA. Localization of the tetrodotoxin-resistant sodium channel NaN in nociceptors. NeuroReport. 2000;11:199–202. doi: 10.1097/00001756-200001170-00039. [DOI] [PubMed] [Google Scholar]
- 16.Gokin AP, Fareed MU, Pan H-L, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–5366. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold MS, Levine JD. DAMGO inhibits prostaglandin E2-induced potentiation of a TTX-resistant Na+ current in rat sensory neurons in vitro. Neurosci Lett. 1996;212:83–86. doi: 10.1016/0304-3940(96)12791-9. [DOI] [PubMed] [Google Scholar]
- 18.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold MS, Levine JD, Correa AM. Modulation of TTX-R/INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 21.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92:2551–2555. [PubMed] [Google Scholar]
- 22.Griswold DE, Douglas SA, Martin LD, Davis TG, Davis L, Ao Z, Luttmann MA, Pullen M, Nambi P, Hay DW, Ohlstein EH. Endothelin B receptor modulates inflammatory pain and cutaneous inflammation. Mol Pharmacol. 1999;4:807–812. [PubMed] [Google Scholar]
- 23.Held B, Pocock JM, Pearson HA. Endothelin-1 inhibits voltage-sensitive Ca2+ channels in cultured rat cerebellar granule neurones via the ET-A receptor. Pflügers Arch. 1998;436:766–775. doi: 10.1007/s004240050700. [DOI] [PubMed] [Google Scholar]
- 24.Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol. 2001;86:1351–1364. doi: 10.1152/jn.2001.86.3.1351. [DOI] [PubMed] [Google Scholar]
- 25.Hille B. Ionic channels of excitable membranes, Ed 3, p 58. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 26.Jarvis MF, Wessale JL, Zhu CZ, Lynch JJ, Dayton BD, Calzadilla SV, Padley RJ, Opgenorth TJ, Kowaluk EA. ABT-627, an endothelin ET(A) receptor-selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic pain. Eur J Pharmacol. 2000;388:29–35. doi: 10.1016/s0014-2999(99)00865-1. [DOI] [PubMed] [Google Scholar]
- 27.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. NeuroReport. 2001;12:3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 28.Kral MG, Xiong Z, Study RE. Alteration of Na+ currents in dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1999;81:15–24. doi: 10.1016/s0304-3959(98)00264-4. [DOI] [PubMed] [Google Scholar]
- 29.Lauer MR, Gunn MD, Clusin WT. Endothelin activates voltage-dependent Ca2+ current by a G protein-dependent mechanism in rabbit cardiac myocytes. J Physiol (Lond) 1992;448:729–747. doi: 10.1113/jphysiol.1992.sp019067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ono K, Tsujimoto G, Sakamoto A, Eto K, Masaki T, Ozaki Y, Satake M. Endothelin-A receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature. 1994;370:301–304. doi: 10.1038/370301a0. [DOI] [PubMed] [Google Scholar]
- 31.Piovezan AP, D'Orleans-Juste P, Tonussi CR, Rae GA. Endothelins potentiate formalin-induced nociception and paw edema in mice. Can J Physiol Pharmacol. 1997;75:596–600. [PubMed] [Google Scholar]
- 32.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 33.Rush AM, Brau ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol (Lond) 1998;511:711–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 35.Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Van den Berg RJ, Ypey DL. Resting membrane potentials and excitability at different regions of rat dorsal root ganglion neurons in culture. Neuroscience. 1994;60:245–254. doi: 10.1016/0306-4522(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 37.Xie LH, Horie M, James AF, Watanuki M, Sasayama S. Endothelin-1 inhibits L-type Ca currents enhanced by isoproterenol in guinea-pig ventricular myocytes. Pflügers Arch. 1996;431:533–539. doi: 10.1007/BF02191900. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q-L, Strichartz G, Davar G. Endothelin-1 activates ETA receptors to increase intracellular calcium in model nociceptors. NeuroReport. 2001;12:3853–3857. doi: 10.1097/00001756-200112040-00050. [DOI] [PubMed] [Google Scholar]