Abstract
During normal rostral scratching in the spinal turtle, there is rhythmic alternation between hip-flexor and hip-extensor motor activity. During rostral scratching with hip-extensor deletions, there are successive bursts of hip-flexor motor activity and no activity in hip-extensor motor neurons. We characterized the ON- and OFF-phases of 72 descending propriospinal interneurons with distinct activity bursts during normal rostral scratching. We also studied the activity of these interneurons during deletion scratching. Hip-extensor interneurons were active when hip-flexor motor neurons were quiet in normal scratching and had zero overlap with hip-flexor motor activity. This population of hip-extensor interneurons, termed the hip-extensor module or hip-extensor unit-burst generator, was mainly quiet during deletion scratching. Our observation supports the concept that a module is a neuronal population that may be active or quiet in a coordinated manner during a spinal motor rhythm. During normal scratching, hip-flexor interneurons were active during hip-flexor motor activity, and spanning interneurons were active during both hip-flexor motor activity and quiescence. Hip-flexor and spanning interneurons with intermediate overlap with hip-flexor motor activity fired in bursts during deletion scratching. Hip-flexor and spanning interneurons with large overlap with hip-flexor motor activity fired continuously during deletion scratching. Key features of hip-flexor and spanning interneuron firing during normal scratching were preserved during deletion scratching. Thus these features do not require activity in the hip-extensor module in every cycle of a motor rhythm.
Keywords: spinal cord, scratch reflex, half-center, central pattern generator, turtle, reciprocal inhibition, fictive motor patterns
Rhythmic alternation between hip flexors and hip extensors is a fundamental feature of many motor behaviors of the vertebrate hindlimb (Stein and Smith, 1997). A major goal of spinal cord research is characterization of interneuronal populations responsible for rhythm generation in hindlimb motor neurons (Stein et al., 1997). Several hypotheses of spinal cord organization postulate specific interneuronal populations that fire in a coordinated manner; each population contains neurons active during specific phases of the cycle. The hypotheses differ according to the specific composition of each population. Half-center hypotheses of hindlimb rhythm generation postulate a set of interneuronal populations that are each related to a single function at all joints of a hindlimb, e.g., the extensor half-center related to hip, knee, and ankle extension (Brown, 1911, 1914; Jankowska et al., 1967; Lundberg, 1981). Half-center hypotheses can explain “conventional” synergies in which all extensors are active at the same time; they do not explain “mixed” synergies in which hip extensors and knee extensors are active at different times, e.g., paw shake in cat (Stein and Smith, 1997). Modular hypotheses (Jordan, 1991) of hindlimb rhythm generation postulate a set of interneuronal populations that are each related to a single function at a single joint, e.g., hip-extensor module or hip-extensor unit-burst generator (Grillner, 1981; Stein et al., 1995,1998a,b; Currie and Gonsalves, 1997, 1999). Modular hypothesis have the ability to explain both conventional and mixed synergies. Analyses of interneuronal populations during variations in motor rhythms with mixed synergies allow further examinations of these hypotheses. Half-center hypotheses predict that all extensor half-center interneurons will display similar responses during a mixed-synergy variation. Modular hypotheses predict that hip-extensor interneurons will display different responses than knee-extensor interneurons during a mixed-synergy variation.
The motor rhythms of rostral scratching in the turtle display a mixed synergy (Stein and Grossman, 1980; Robertson et al., 1985; Robertson and Stein, 1988; Stein et al., 1995, 1998a,b; Earhart and Stein, 2000a,b) and allow examination of these predictions. Rhythmic alternation between hip-flexor and hip-extensor motor activity occurs during normal rostral scratching. During rostral scratching with hip-extensor deletions, a spontaneously occurring variation, there are rhythmic bursts of hip-flexor motor activity and no hip-extensor motor activity. During both types of rostral scratching, knee-extensor motor neurons are active during the latter portion of hip flexor activity. Recordings of synaptic potentials in turtle motor neurons during both types of rostral scratching support the concept that hip-extensor interneurons belong to a different population than knee-extensor interneurons (Robertson and Stein, 1988).
Berkowitz and Stein (1994a,b) characterized the activity patterns of descending propriospinal interneurons during normal scratching using single-unit recordings from descending axons at the posterior cut face of the turtle spinal cord (Currie and Stein, 1990). We use this technique to characterize unit activity of descending propriospinal interneurons with axons in the turtle dorsolateral funiculus that fire in bursts during normal rostral scratching. We also study these interneurons during rostral scratching with hip-extensor deletions.
Our data support modular hypotheses and have been presented previously in an abstract (Stein and Daniels-McQueen, 2001).
MATERIALS AND METHODS
Surgical preparation. Red-eared turtles (n = 20; Candy's Quality Reptiles, La Place, LA; William A. Lemberger Company, Oshkosh, WI; and Charles D. Sullivan Company, Nashville, TN), Trachemys scripta elegans(formerly Pseudemys scripta elegans), weighing 500–850 gm, were placed on crushed ice at least 1 hr before surgery to induce hypothermic analgesia (Melby and Altman, 1974). Each turtle was then spinalized just caudal to the forelimb enlargement by complete spinal transection midway between the D2 and D3 dorsal roots (see Fig. 1). After spinal transection, the cord was covered with Gelfoam surgical sponges (Upjohn, Kalamazoo, MI) soaked in turtle saline (Stein and Schild, 1989), and the opening was sealed with a wax plug glued to the shell.
Fig. 1.
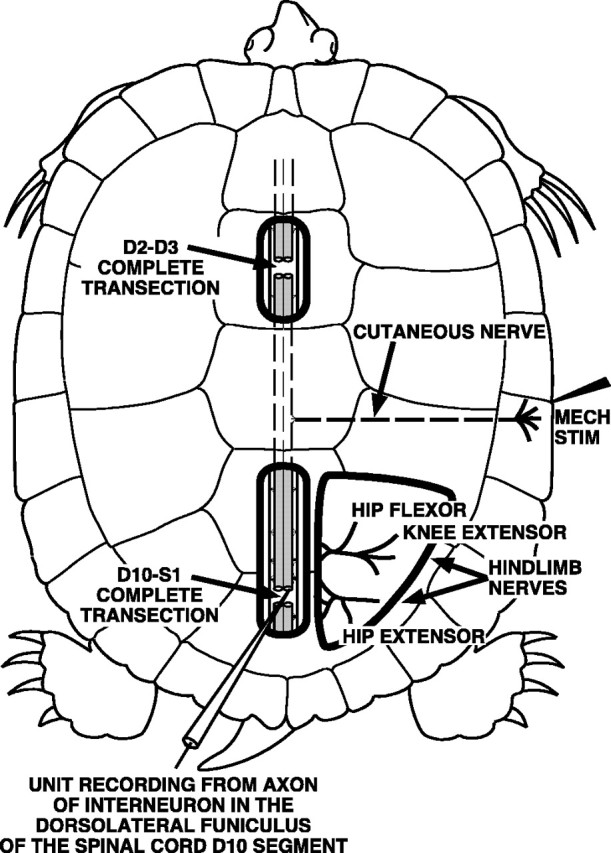
Sketch of the D3–D10 preparation. The spinal cord was completely transected in two locations: at the border of the D2 and D3 spinal segments just posterior to the forelimb enlargement and at the border of the D10 and S1 spinal segments within the hindlimb enlargement. ENG recordings were obtained from hip-flexor, knee-extensor, and hip-extensor motor nerves. Single-unit recordings from descending propriospinal interneurons were obtained from the cut ends of axons in the dorsolateral funiculus at the posterior face of the D10 spinal segment. Fictive rostral scratching was evoked by mechanical stimulation of the rostral-scratch receptive field located on the shell bridge in the midbody region of the turtle.
The spinal cord was exposed from the D7 segment to the D10 segment so that the dorsal roots of segments D7–D10 could be visualized (Mortin and Stein, 1990). At the posterior end of the D10 segment, the spinal cord was completely transected (see Fig. 1). This preparation with two spinal transections is termed the D3–D10 preparation (Mortin and Stein, 1989). The spinal cord, from the D7 segment to the D10 dorsal roots, was covered with Gelfoam soaked with turtle saline. The remainder of the exposed spinal cord, including the posterior cut face of the D10 segment, was covered with turtle saline. We visualized the boundary of the gray matter and the white lateral funiculus in the unstained cut face of the D10 segment of the spinal cord.
The right hip flexor nerve VP-HP that innervates puboischiofemoralis internus, pars anteroventralis muscle (Walker, 1973; Robertson et al., 1985) was dissected in all preparations for recordings of electroneurograms (ENGs) and is labeled HIP FLEXOR in Figures 1-7. In each preparation, one or more of the following right nerves were also dissected for ENG recordings: the right biarticular knee extensor nerve AM-KE that innervates triceps femoris, pars ambiens (labeled KNEE EXTENSOR in Figs. 1, 2, 4-7); the right monoarticular knee extensor nerve FT-KE that innervates triceps femoris, pars femorotibialis (data not shown); and the right hip extensor nerve HR-KF that innervates the flexor cruris, pars flexor tibialis internus muscle and several other muscles that extend the hip and flex the knee (labeledHIP EXTENSOR in Figs. 1, 2). The turtle remained on crushed ice throughout all of the above surgical procedures.
Fig. 2.
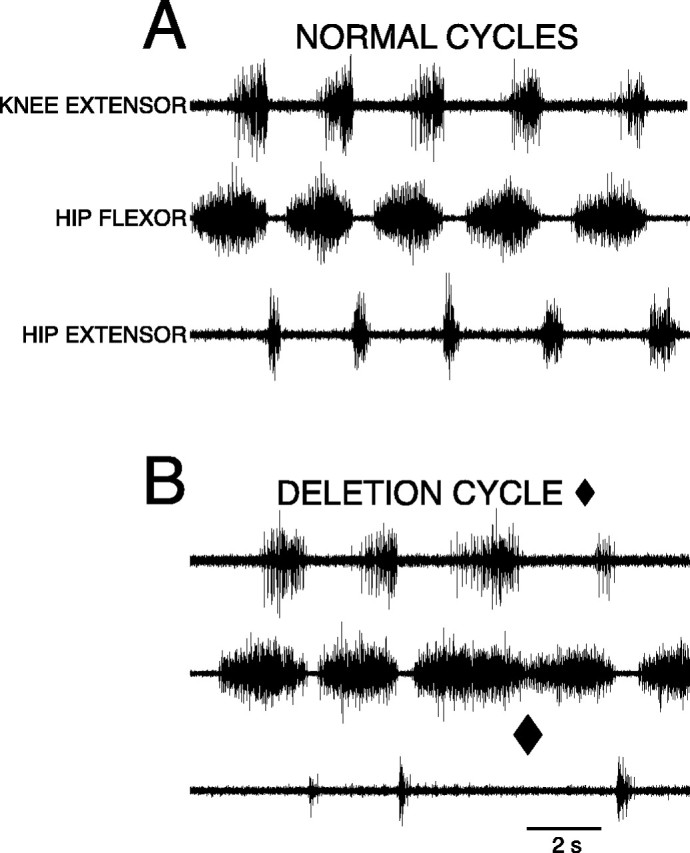
A, B, ENG recordings from right knee-extensor (top trace), right hip-flexor (middle trace), and right hip-extensor (bottom trace) motor nerves during fictive rostral scratching.A, Normal rostral scratching with rhythmic alternation between hip-flexor activity and quiescence elicited by mechanical stimulation of the right rostral-scratch receptive field at SP1. Hip-extensor activity occurred during hip-flexor quiescence.B, One cycle of rostral scratching with a hip-extensor deletion (end of cycle marked with ♦). At the ♦ there was no hip-extensor activity and no quiescent period between the end of the hip flexor burst and the start of the next hip flexor burst. The other cycles exhibited normal rostral scratching. The episode was elicited by mechanical stimulation of the right rostral-scratch receptive field at SP2.5 [see Mortin and Stein (1990) for descriptions of locations on the turtle shell].
Fig. 3.
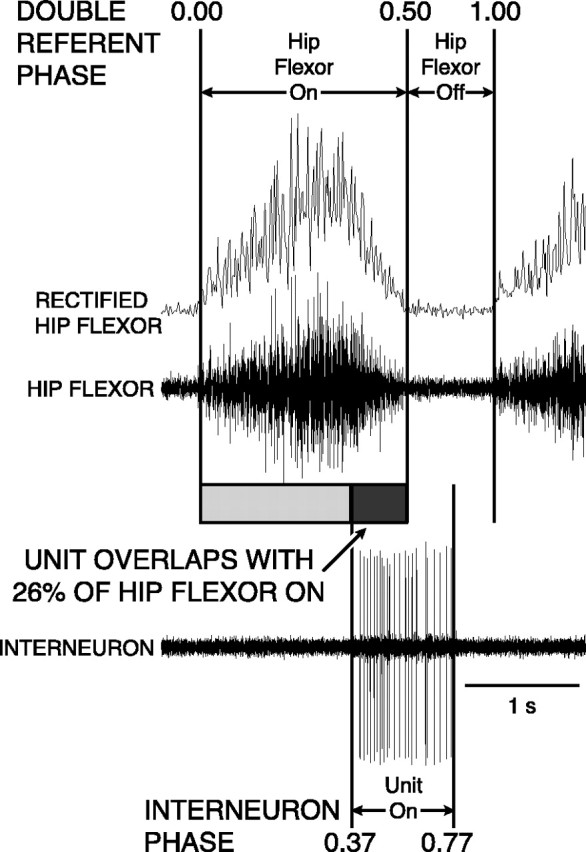
Sketch of technique used to measure double-referent phase of single-unit interneuron (bottom trace) activity during normal rostral scratching with respect to the hip-flexor ENG burst (middle trace) and the integrated full-wave rectified hip-flexor ENG (top trace). The start of the burst of the integrated full-wave rectified hip-flexor ENG was defined as 0.0 (and 1.0) phase; the end of the burst was defined as 0.5 phase. In this example, the start of the unit burst occurred when hip-flexor activity was 74% complete. This ON-phase occurred at 0.37 (= 0.74/2). The end of the unit burst occurred when hip-flexor quiescence was 54% complete. This occurred at an OFF-phase of 0.77 [= 0.5 + (0.54/2)]). See Materials and Methods for details of double-referent phase calculation (Berkowitz and Stein, 1994b). The unit was active during 26% of the hip-flexor burst; in this example of a single cycle shown for illustration, we term this 26% overlap. In the remainder of the paper, overlap percentage was calculated on the basis of mean ON- and OFF-phases for each unit.
Fig. 4.
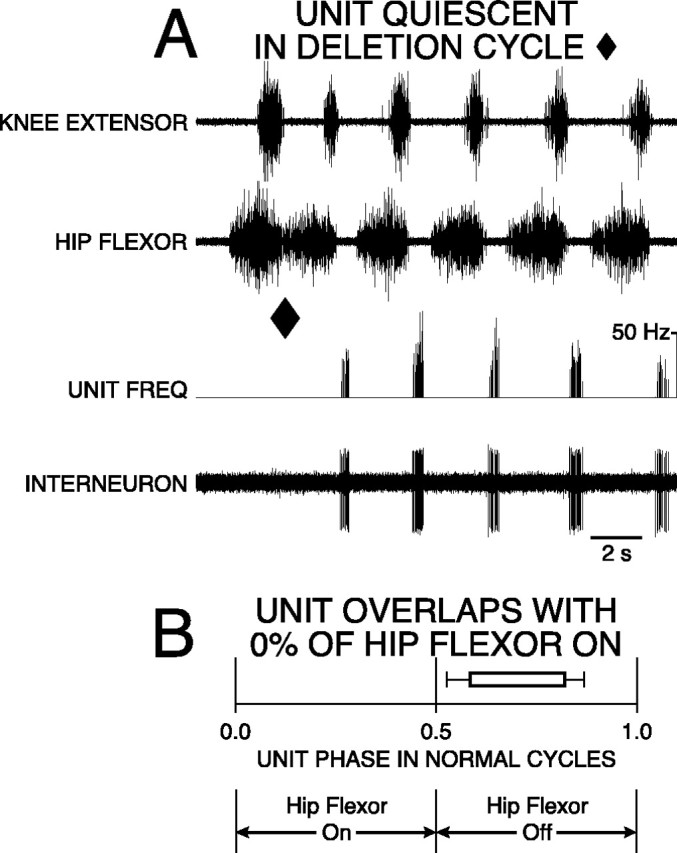
Hip-extensor interneuron with 0% overlap was active in a burst during hip-flexor quiescence of normal rostral scratching and was quiet during rostral scratching with a hip-extensor deletion. A, Recordings of knee-extensor motor nerve (top trace), hip-flexor motor nerve (second trace), instantaneous frequency of unit (third trace), and interneuron unit activity (bottom trace) during rostral scratching elicited by mechanical stimulation of SP1 in the right rostral-scratch receptive field. The first cycle is an example of rostral scratching with a hip-extensor deletion (end of cycle marked with ♦). The other cycles are examples of normal rostral scratching. B, Startand end of bar represent mean ON-phase of 0.58 (±0.06 angular deviation; p < 0.001; Rayleigh Test) and mean OFF-phase of 0.82 (±0.05 angular deviation;p < 0.001; Rayleigh Test) of unit firing during 57 cycles of normal rostral scratching. Bar isunfilled to represent unit quiescence during all nine cycles of deletion rostral scratching.
Fig. 5.
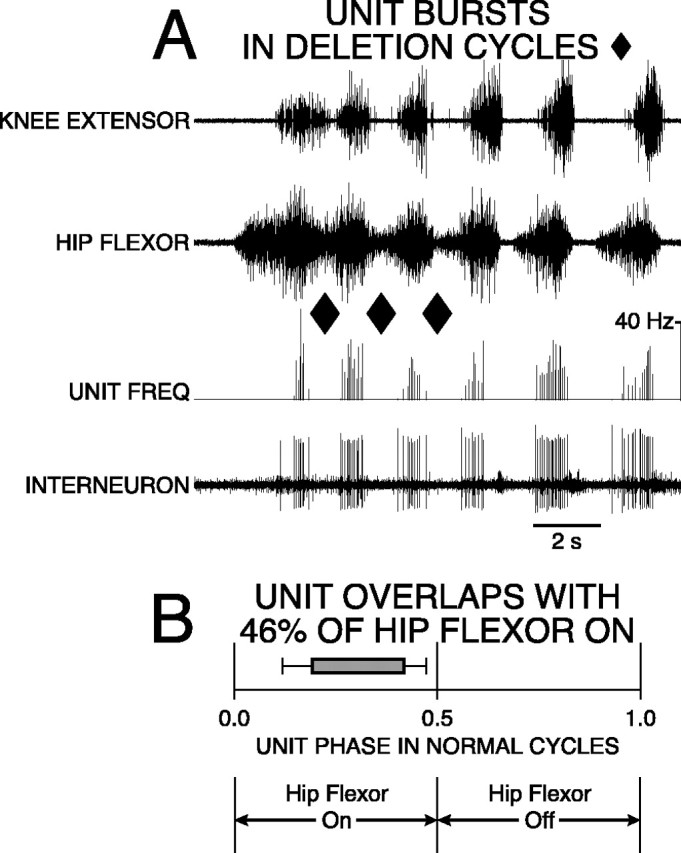
Hip-flexor interneuron with 46% overlap was active in a burst during normal and deletion rostral scratching. During normal scratching, the burst of the unit occurred during a portion of the hip-flexor motor activity. A, Labeling of traces as in Figure 4. Rostral scratching was elicited by mechanical stimulation of SP1 on the right side. The first three cycles are examples of rostral scratching with hip-extensor deletions (ends of cycles marked with ♦). The remaining cycles are examples of normal rostral scratching. B, Start andend of bar represent mean ON-phase of 0.19 (±0.07 angular deviation; p < 0.001; Rayleigh Test) and mean OFF-phase of 0.42 (±0.05 angular deviation;p < 0.001; Rayleigh Test) of unit firing during 34 cycles of normal rostral scratching. Bar isgray-filled to represent unit bursting during all 13 cycles of deletion rostral scratching.
Fig. 6.
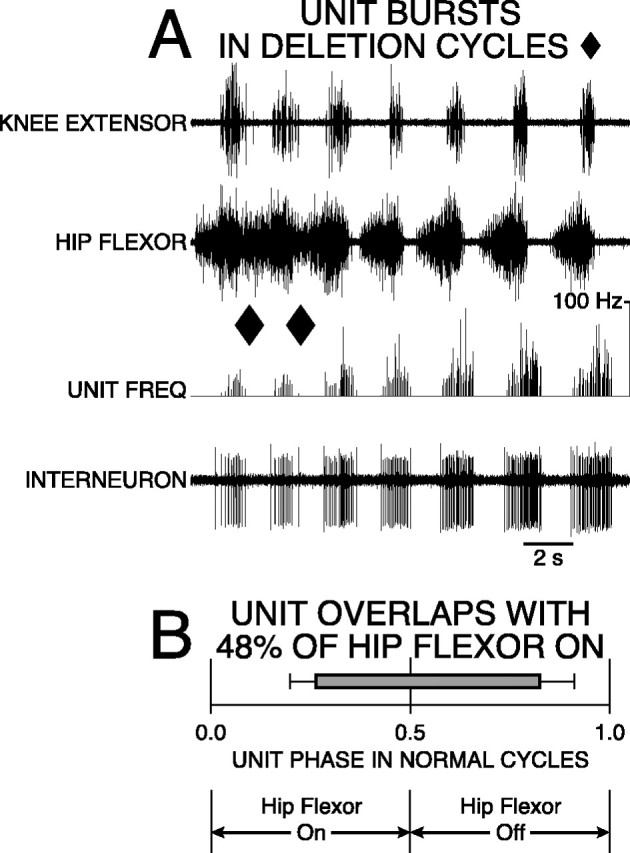
Spanning interneuron with 48% overlap was active in a burst during normal and deletion rostral scratching. During normal scratching, the burst of the unit occurred during the latter portion of hip-flexor activity and during the early portion of hip-flexor quiescence. During deletion scratching, the burst of the unit occurred during the latter portion of hip-flexor activity. A, Labeling of traces as in Figure 4. Rostral scratching was elicited by simultaneous mechanical stimulation of SP2 on the left and right sides. The first two cycles are examples of rostral scratching with hip-extensor deletions (ends of cycles marked with ♦). The remaining cycles are examples of normal rostral scratching. B,Start and end of barrepresent mean ON-phase of 0.26 (±0.06 angular deviation;p < 0.001; Rayleigh Test) and mean OFF-phase of 0.83 (±0.09 angular deviation; p < 0.001; Rayleigh Test) of unit firing during 21 cycles of normal rostral scratching. Bar is gray-filled to represent unit bursting during all 10 cycles of deletion rostral scratching.
Fig. 7.
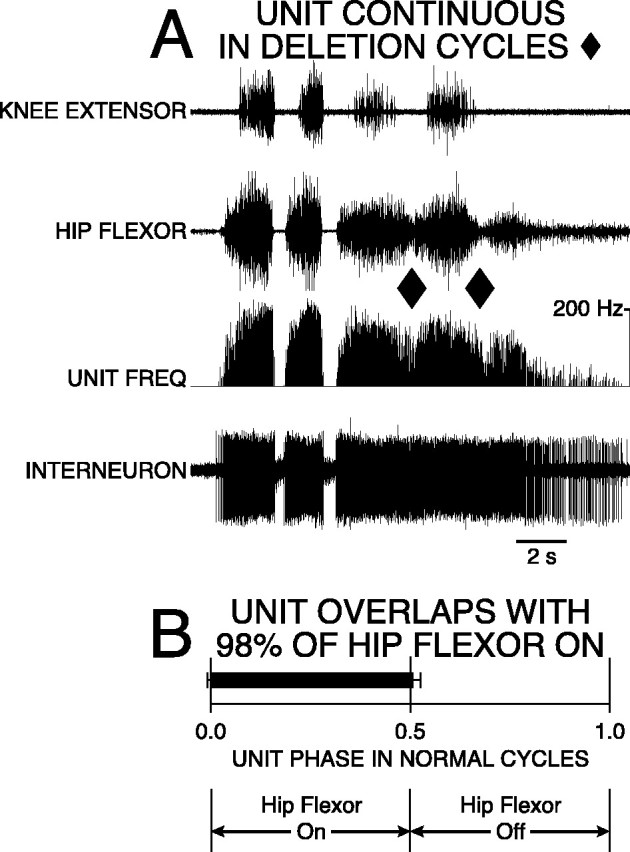
Spanning interneuron with 98% overlap was active in a frequency-modulated burst during normal rostral scratching and fired continuously with frequency modulation during rostral scratching with hip-extensor deletions. A, Labeling of traces as in Figure 4. Rostral scratching was elicited by mechanical stimulation of SP3 on the right side. The third and fourth cycles are examples of rostral scratching with hip-extensor deletions (ends of cycles marked with ♦). The first and second cycles are examples of normal rostral scratching. B, Start andend of bar represent mean ON-phase of 0.01 (±0.01 angular deviation; p < 0.001; Rayleigh Test) and a mean OFF-phase of 0.51 (±0.02 angular deviation;p < 0.001; Rayleigh Test) of unit firing during 35 cycles of normal rostral scratching. Bar isblack-filled to represent continuous firing of the unit during all five cycles of rostral scratching with hip-extensor deletions.
After the above procedures were completed, the turtle was removed from the crushed ice, allowed to warm up to room temperature, and immobilized with gallamine (Flaxedil; Rhone-Poulenc Rorer Canada, Montreal, Canada), a neuromuscular blocking agent, at a dosage of 8 mg/kg body weight. Dental wax (Modern Materials Red Utility Wax Strips, Miles, South Bend, IN) was molded into wells to surround each of the surgically exposed regions; the wells were glued to the shell with Permabond 910 adhesive (Permabond, Englewood, NJ). The peripheral-nerve well was filled with mineral oil. The well around the D7–D10 spinal-cord exposure was filled with saline. The trachea was intubated, and the turtle maintained on artificial respiration for the remainder of the experiment.
Recordings. ENGs from each right nerve were obtained using a pair of silver wire electrodes 100 μm in diameter that were immersed in the mineral-oil pool (Robertson et al., 1985). The ENGs were amplified and filtered (0.1–1 kHz bandpass). Single-unit recordings of action potentials of descending propriospinal interneurons in the right dorsolateral funiculus at the posterior cut face of the D10 segment were obtained using a microsuction electrode with an inner diameter of 5–15 μm (Currie and Stein, 1990; Berkowitz and Stein, 1994a,b). The dorsolateral funiculus was defined as the region of the lateral funiculus dorsal to the central gray commissure. The unit extracellular action potentials were amplified and filtered (0.3–3 kHz bandpass). The ENGs and unit action potentials were stored on digital audio tape (DC-5 kHz bandpass) for later analyses and hard-copy printouts.
Stimulation. Fictive rostral scratch motor patterns were elicited by mechanical stimulation of specific sites in the right and left rostral-scratch receptive fields [see Mortin and Stein (1990) for a description of stimulation sites]. Mechanical stimulation was applied by using the smooth, fire-polished end of a glass rod. All of the scratch motor patterns in the present study were “fictive,” that is, they were obtained in the immobilized preparation in the absence of movement; when “scratching” is described in the remainder of this paper, it refers to fictive scratching. We allowed a 2–4 min recovery between stimulus presentations. In most episodes, only unilateral stimulation of a site in the right rostral-scratch receptive field was used. In other episodes, simultaneous bilateral stimulation of mirror-image sites in the left and right rostral-scratch receptive fields was used (Stein et al., 1995, 1998a,b; Currie and Gonsalves, 1997, 1999).
Rostral scratching motor patterns. The normal motor pattern of rostral scratching (see Fig. 2A) includes rhythmic alternation between hip-flexor activation and quiescence (Robertson et al., 1985). During rostral scratching with hip-extensor deletions (see Fig. 2B), hip-flexor activation occurs in bursts with no quiescence between bursts (Robertson and Stein, 1988; Stein et al., 1995, 1998a,b).
For quantitative analyses of rostral scratch motor patterns, we digitized hip flexor ENGs at 2 kHz using Cambridge Electronic Design1401plus hardware with SPIKE 2 software (Windows version 3.18, Cambridge, UK). The absolute value of the difference between each of the 2000 voltage measurements per second and the mean voltage for that channel was obtained (“full-wave rectification”); the mean of 20 successive full-wave rectified measurements was calculated (“integrated”) so that there were 100 integrated full-wave rectified data points per second (see Fig. 3). For each integrated full-wave rectified hip-flexor ENG, we measured the “baseline amplitude” of the quiescent ENG (maximum value minus minimum value) before the onset of stimulation. We defined the threshold of the hip flexor ENG burst as the sum of the value of the baseline amplitude plus the value of maximum ENG during quiescence, i.e., the threshold/noise value was set at 2:1.
We used custom software written by Dr. Gavin Perry (Washington University, St. Louis, MO) to determine the onset and offset of each hip flexor burst of integrated full-wave rectified ENG activity (Stein et al., 1995). We analyzed burst onsets and offsets during normal cycles of hip-flexor activity that displayed rhythmic alternation between hip-flexor activity and quiescence. Each cycle of normal rostral scratching had a period of hip-flexor quiescence that remained below hip-flexor ENG threshold for ≥50 msec, i.e., there were at least five successive integrated full-wave rectified data points during the hip flexor quiescence of a normal scratch cycle. We also analyzed hip-flexor burst onsets and offsets during hip-extensor deletions. Each cycle of deletion rostral scratching had at most four successive data points (40 msec) that remained below threshold.
Single-unit recognition and analyses. For quantitative analyses of interneuronal firing patterns we digitized microsuction-electrode recordings at 20 kHz using Cambridge Electronic Design hardware and software. We recognized single units (n = 72) with Spike 2 template matching software. For most interneuronal recordings, we studied only the most prominent unit; in a few cases, we discriminated between and studied the two most prominent units. During normal scratching, we identified the onset and offset of each unit's burst of two or more action potentials and measured instantaneous frequency (1/interspike interval) during each burst.
During each cycle of normal scratching, we used double-referent phase measurement techniques to calculate the phase of the onset (“ON-phase”) and the phase of the offset (“OFF-phase”) of each unit's burst (Berkowitz and Stein, 1994b). Normal scratching cycles in which the unit had zero or one action potential were not included in the phase calculation. For each unit in the study, we measured ON- and OFF-phases of bursts during at least 10 cycles of normal scratching. We defined the start of each hip-flexor burst as the 0.0 and 1.0 phases and the end of each hip-flexor burst as the 0.5 phase. The phase of an event occurring during hip-flexor activity was defined as the latency of the event from hip-flexor onset divided by twice the duration of the hip-flexor burst (see Fig. 3). The phase of an event occurring during hip-flexor quiescence was defined as 0.5 plus the latency of the event from hip-flexor offset divided by twice the duration of hip-flexor quiescence (see Fig. 3). We used vector averaging techniques appropriate for circular data (Batschelet, 1981; Berkowitz and Stein, 1994b) to calculate the mean phase and angular deviation of the ON- and OFF-phases of each unit. Angular deviation for circular data is similar to SD for linear data (Batschelet, 1981). We applied the Rayleigh Test (Batschelet, 1981) to determine whether the distribution of ON- and OFF-phases for each unit was significantly different from a random distribution.
During normal scratching, we used unit mean ON- and OFF-phases to calculate the overlap of each unit's firing with hip-flexor activity (see Fig. 3). Overlap percentage was defined as the percentage of hip-flexor activity during which the unit was active. Units with 0% overlap had mean ON- and OFF-phases during hip-flexor quiescence.
During each cycle of deletion scratching, we recognized four unit firing categories: quiescence, single spike, burst of two or more spikes, and continuous firing. For each unit in the study, we characterized at least two cycles of deletion scratching. For 70 of 72 units, we defined continuous firing as instantaneous frequency >5 Hz for an entire cycle. We needed to modify this criterion for two units that fired with low frequency. For these two units, we measured the minimum instantaneous frequency during each cycle of normal scratching: critical frequency for each unit was defined as the largest of these minima, and unit behavior was defined as continuous during a cycle of deletion scratching if instantaneous frequency always exceeded critical frequency.
For units with 0% overlap, we counted the number of action potentials during each cycle of normal scratching and during each cycle of deletion scratching. For both types of scratching, we included cycles with unit quiescence, single spike, or burst of two or more action potentials. For each unit, we used the one-tailed Mann–WhitneyU test (Siegel, 1956) to calculate the statistical significance between the number of action potentials in each cycle of normal scratching compared with the number of action potentials in each cycle of deletion scratching.
RESULTS
Fictive rostral scratching motor patterns
We studied two types of fictive rostral scratching (Robertson et al., 1985; Robertson and Stein, 1988), normal (see Fig.2A) and with hip-extensor deletions (see Fig.2B), in the D3–D10 preparation of the turtle spinal cord (Fig. 1) (Mortin and Stein, 1989). This eight-segment preparation contained the midbody spinal segments (D3–D6), the dermatomes of which included the rostral-scratch receptive field (Mortin and Stein, 1990), and the first three spinal segments (D8–D10) of the five-segment hindlimb enlargement (Ruigrok and Crowe, 1984). The D8–D10 segments contain the somata of all the hip-flexor motor neurons, all the knee-extensor motor neurons, and some of the hip-extensor motor neurons (Ruigrok and Crowe, 1984).
On some occasions, tactile stimulation in the rostral scratch receptive field elicited the normal pattern of fictive rostral scratching: there was rhythmic alternation between hip-flexor motor activity and quiescence (Fig. 2A). Hip-extensor motor activity occurred during hip-flexor quiescence. The hip-flexor burst during a rostral scratch had a characteristic shape: it began with low-level activity, gradually reached maximum activity, and then rapidly declined near the end of the burst. Knee-extensor motor activity occurred during the latter portion of hip-flexor activity.
During other occasions, tactile stimulation in the rostral scratch receptive field elicited rostral scratching with hip-extensor deletions: there was no hip-flexor quiescence and no hip-extensor activity (Fig. 2B, end of deletion cycle marked with ♦). During this variation of rostral scratching, the decline of hip-flexor amplitude at the end of one hip-flexor burst was followed immediately by the rise of hip-flexor amplitude at the beginning of the subsequent hip-flexor burst. There was a burst of knee-extensor activity in the latter portion of each hip-flexor burst.
Unit recordings of descending propriospinal interneurons
We recorded the unit activity of interneurons with axons that descended in the right dorsolateral funiculus at the posterior end of the D10 spinal segment (Fig. 1). These units were interneurons; there are no axons of sensory or motor neurons in the dorsolateral funiculus of the turtle (Currie and Stein, 1990; Berkowitz and Stein, 1994a,b,c). These units were descending propriospinal interneurons. We recorded from the cut ends of descending axons at the posterior face of an eight-segment chain of spinal cord.
We studied 72 interneurons that fired in distinct bursts of two or more action potentials during 2105 cycles of normal rostral scratching. For each cycle of normal scratching for each unit, we measured the double-referent ON- and OFF-phases of each burst with respect to the hip-flexor motor-neuron activity cycle (Fig.3). We calculated the mean ON- and OFF-phases for each unit during normal scratching. We used these mean phase values to determine the overlap of each unit with respect to hip-flexor activity during normal rostral scratching (Fig. 3). We categorized interneurons according to their overlap. Some interneurons had 0% overlap. For these interneurons, the mean ON- and OFF-phases occurred exclusively during hip-flexor quiescence (Fig.4); we term these hip-extensor interneurons (see also Discussion, Axonal recordings). Other interneurons had overlap. Some of these fired exclusively during hip-flexor activity (Fig. 5); we term these hip-flexor interneurons. Others fired during both hip-flexor activity and quiescence (Figs. 6, 7); we term these spanning interneurons. We represented the mean ON- and OFF-phases for the 72 units in the study during normal cycles of rostral scratching by the beginnings and ends, respectively, of the bars in Figure8. The distributions of each of the 72 ON-phases and 70 of the 72 OFF-phases were significantly different from random at the p < 0.001 level (Rayleigh Test) (Batschelet, 1981). Two of the OFF-phases were significantly different at the p < 0.01 level.
Fig. 8.
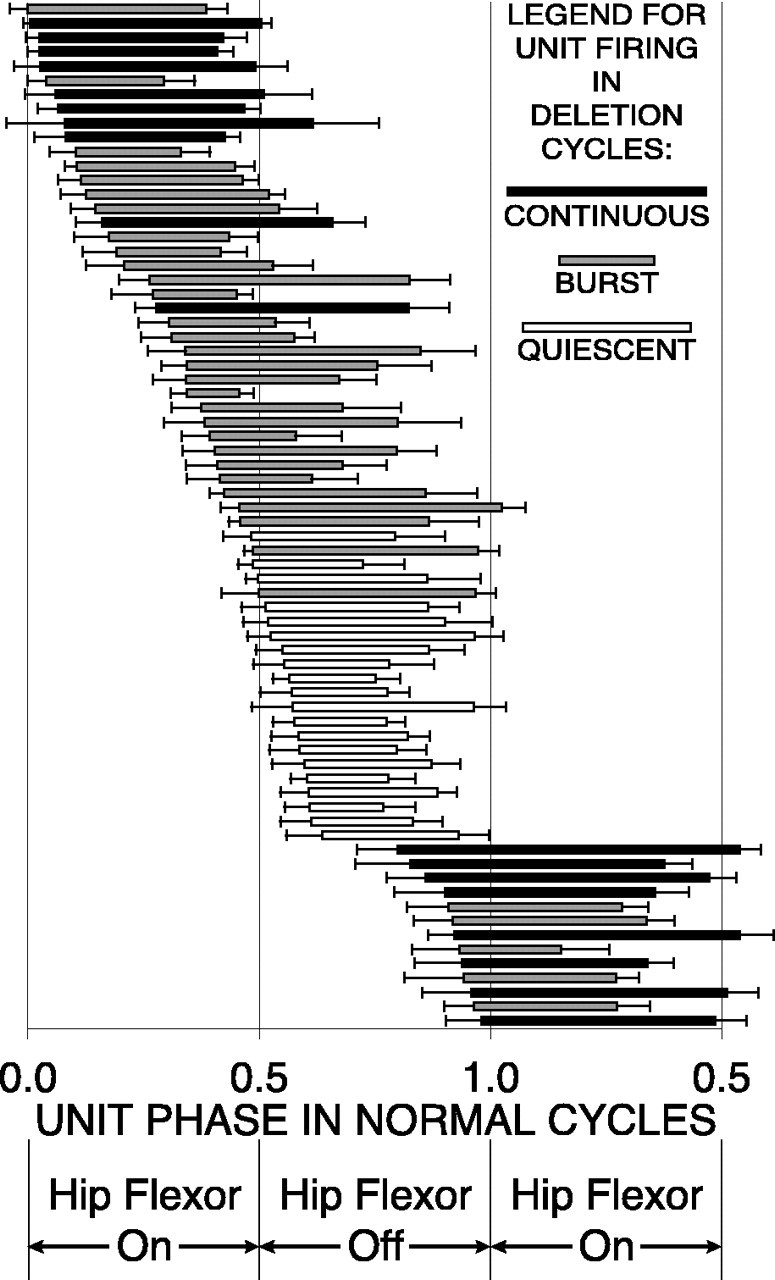
Double-referent mean ON- and OFF-phases (± angular deviation) of 72 descending propriospinal interneurons during normal rostral scratching represented by the start andend, respectively, of each bar. Unit firing during rostral scratching with hip-extensor deletions is indicated by the fill of each bar: unfilled bar for quiescence; gray-filled bar for a burst;black-filled bar for continuous firing. Nearly all units with 0% overlap with hip-flexor activity and with mean ON- and OFF-phases during hip-flexor quiescence of normal rostral scratching were quiet during deletion rostral scratching.
We also examined the firing characteristics of each of the 72 units during 495 deletion cycles with no hip-flexor quiescence, i.e., during rostral scratching with hip-extensor deletions. In Figures2B, 4A, 5A,6A, and 7A, the ends of these cycles are marked with ♦. We recognized four unit-firing categories during deletion cycles: quiescence, single spike (data not shown), burst, and continuous. For each unit, we determined which category was observed most often during deletions. We represented that category by the fill of mean ON-/OFF-phase bar of that unit (Figs. 4B,5B, 6B, 7B, and 8). Units that were quiet during deletions were represented by an unfilled bar (Figs. 4B, 8). Units that fired in a burst during deletions were represented by a gray-filled bar(Figs. 5B, 6B, 8). Units that fired continuously during deletions were represented by a black-filled bar (Figs. 7B, 8).
Hip-extensor interneurons with 0% overlap in normal cycles were quiet during deletion cycles
An example of a hip-extensor interneuron with activity bursts that had 0% overlap with hip-flexor bursts is shown in Figure4A. All but the first cycle were normal cycles with rhythmic alternation between hip-flexor activity and quiescence. The first cycle (end marked with ♦) was a deletion cycle with no hip-flexor quiescence. The hip-extensor interneuron fired a robust burst of activity during hip-flexor quiescence of normal cycles. The interneuron was quiet during the deletion cycle.
We studied a total of 19 hip-extensor interneurons during 659 cycles of normal rostral scratching. During each of these cycles, the unit was active with a burst of two or more spikes. The mean ON- and OFF-phase bars for these units in Figure 8 were located between the phases of 0.50 (onset of hip-flexor quiescence) and 1.00 (onset of hip-flexor burst). We recorded from these 19 units during 172 deletion cycles. In 89.5% of these cycles, the unit was quiet. In 4.1% of these cycles, the unit fired a single spike. In 6.4% of these cycles, the unit fired a burst of two or more spikes. These percentages are plotted in the 0% overlap bar in the bottom of Figure 9. For 18 of these interneurons, the usual firing category during deletions was quiescence. The phase bars of these units in Figure 8were unfilled to represent this characteristic.
Fig. 9.
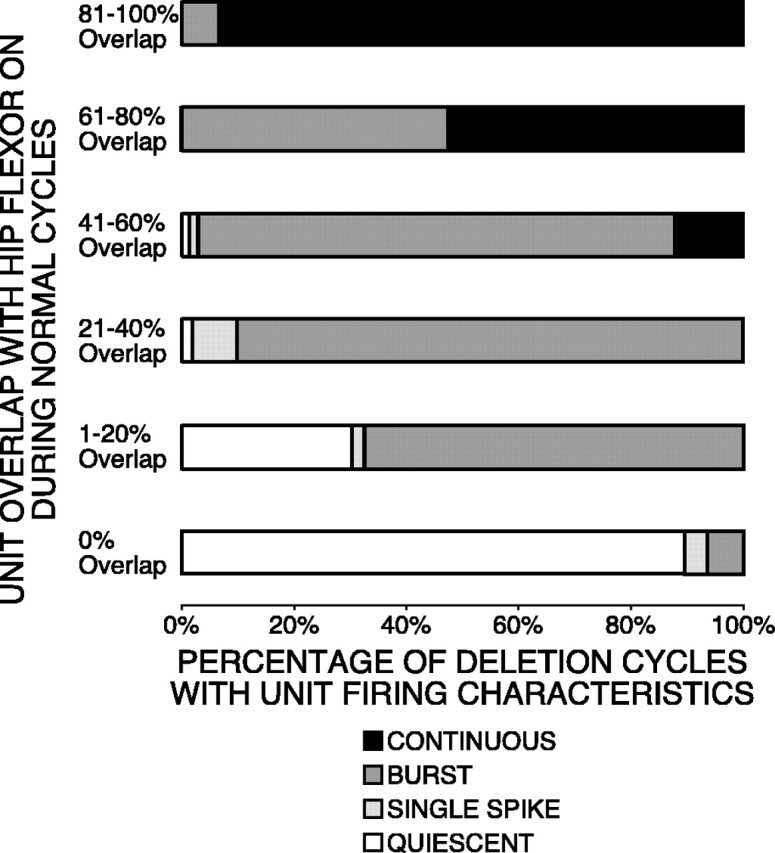
Interneuron firing behavior during deletion cycles related to overlap during normal cycles. Unit overlap with hip-flexor activity during normal cycles was divided into six groups: 0, 1–20, 21–40, 41–60, 61–80, and 81–100%. Each group is represented by abar. For units in each category, the percentage of deletion cycles is represented by the fill of a portion of each bar:unfilled portion for quiescence;light-gray-filled portion for single spike;medium-gray-filled portion for burst;black-filled portion for continuous.
We counted the number of action potentials in each cycle for each of the 19 hip-extensor interneurons. For normal scratching, we included 659 cycles with unit bursts of two or more action potentials, 34 additional cycles with one action potential, and 86 additional cycles with no action potentials. For each unit, we used the one-tailed Mann–Whitney U test (Siegel, 1956) to determine the significance of the number of action potentials during each cycle of normal scratching compared with the number of action potentials during each cycle of deletion scratching. For 18 of 19 units, the number of action potentials during each cycle of normal scratching was significantly greater than the number of action potentials during each cycle of deletion scratching (12 units: p < 0.001; 5 units: p < 0.01; 1 unit: p < 0.05). During normal scratching, these 18 units fired 9465 action potentials during 767 cycles with a mean of 12.34 action potentials per cycle. During deletion scratching, these 18 units fired 24 action potentials during 167 cycles with a mean of 0.14 action potentials per cycle. Thus, in this group of 18 units, the number of action potentials per cycle during deletion scratching was 1.16% of the number of action potentials per cycle during normal scratching. This group of 18 units was mainly quiet during deletion scratching.
For one unit, there was no significant difference between the number of action potentials in each cycle of normal scratching compared with deletion scratching. The value of ON-phase (0.50) minus angular deviation (0.08) of this unit was 0.42. Thus, for some normal cycles, a portion of the firing of this unit overlapped with the hip-flexor burst. This unit was the only unit in our study with 0% overlap that usually burst during deletion cycles. Because the behavior of this unit was significantly different from that of the other 18 units with 0% overlap, it is likely that this interneuron was not a member of the same population as that of the other 18 units.
These observations of the 18 units with 0% overlap support the concept that the hip-extensor interneuron population acted as a group. During the hip-extensor phase of normal rostral scratching, each member of this population fired in a burst; during rostral scratching with hip-extensor deletions, the population was mainly quiet. The quiescence of hip-extensor interneurons during deletion scratching corresponds to the lack of EPSPs in hip-extensor motor neurons and the lack of IPSPs in hip-flexor motor neurons during deletion scratching (Robertson and Stein, 1988).
Knee-extensor motor neurons receive EPSPs during deletion scratching (Robertson and Stein, 1988). Therefore knee-extensor interneurons that synaptically excite these motor neurons are active during deletion scratching. It follows that hip-extensor interneurons and knee-extensor interneurons have different firing characteristics during rostral scratching. This supports the concept that the 18 hip-extensor interneurons recorded in the present study are members of a hip-extensor module (Grillner, 1981; Stein et al., 1995, 1998a,b).
Interneurons with intermediate overlap (21–60%) in normal cycles fired in bursts during deletion cycles
An example of a hip-flexor interneuron with intermediate overlap with hip-flexor activity during normal rostral scratching is shown in Figure 5A. The unit was active only during a portion of hip-flexor activity and was quiet during hip-flexor quiescence. The first three cycles were hip-extensor deletion cycles (ends marked with ♦); the remaining cycles were normal cycles. In both normal and deleted cycles, the hip-flexor interneuron fired with a robust burst during each burst of hip-flexor activity.
An example of a spanning interneuron with intermediate overlap with hip-flexor activity during normal rostral scratching is shown in Figure6A. The unit was active during a portion of hip-flexor activity and a portion of hip-flexor quiescence. The first two cycles were hip-extensor deletion cycles (ends marked with ♦); the remaining cycles were normal cycles. In both normal and deleted cycles, the spanning interneuron fired with a robust burst. In the normal cycles, the burst of the unit began near the middle of the hip-flexor burst and ended near the middle of hip-flexor quiescence. In deleted cycles, the burst of the unit occurred in the latter portion of the hip-flexor motor burst.
We studied a total of nine interneurons with 41–60% overlap with hip flexor bursts during 295 cycles of normal rostral scratching. For each of the nine units, the dominant firing characteristic during deletions was bursting. The phase bars of these units were gray-filledin Figure 8 to represent this characteristic. We recorded from these nine units during 66 deletion cycles. In 56 of these cycles (84.8%), the unit fired a burst. In eight of these cycles (12.1%), the unit fired continuously. In one cycle (1.5%), the unit fired a single spike; in one cycle (1.5%), the unit was quiet. These percentages are plotted in the 41–60% overlap bar in Figure 9.
Interneurons with 21–40% overlap displayed similar behavior during deletion cycles. We studied 11 units in this overlap range during 320 normal cycles. For each of the 11 units, the dominant firing characteristic during deletions was bursting; the phase bars of these units were gray-filled in Figure 8 to represent this characteristic. We recorded from these 11 units during 50 deletion cycles. In 90% of these cycles, the unit fired a burst. In 8% of these cycles, the unit fired a single spike. In 2% of these cycles, the unit was quiet. These percentages are plotted in the 21–40% overlap bar in Figure 9.
Interneurons with 21–60% overlap with hip-flexor motor activity during normal scratching are quiet during some portion of the hip-flexor burst. The observation that these units burst during deletion cycles, that is, are quiet during some portion of the hip-flexor burst in a deleted cycle, supports the concept that certain characteristics of unit firing during the hip-flexor burst present during normal scratching were preserved during deletion scratching. These preserved features during deletion scratching are therefore not dependent on the activity of neurons in the hip-extensor module.
We termed interneurons with intermediate (21–60%) overlap as hip-flexor interneurons if they fired exclusively during hip-flexor motor activity (Fig. 5) and as spanning interneurons if they fired during both hip-flexor motor activity and quiescence (Fig. 6). This classification does not address the issue of their synaptic output. Of the interneurons with intermediate overlap, some may synapse on hip motor neurons, others may synapse on knee motor neurons, some may synapse on both types of motor neurons, and others synapse on neither type of motor neuron. During the latter portion of hip-flexor motor activity during both normal and deletion rostral scratching, knee-extensor motor neurons receive EPSPs (Robertson and Stein, 1988), and some interneurons with intermediate overlap (Fig. 8) are active. We suggest that a subset of intermediate-overlap interneurons may also be knee-extensor interneurons that synaptically excite knee-extensor motor neurons. This is consistent with the idea that the population of knee-extensor interneurons (that fire in bursts during normal and deletion rostral scratching) are different from the population of hip-extensor interneurons (that fire in bursts during normal scratching and are quiet during deletion scratching).
Interneurons with large overlap (81–100%) in normal cycles fired continuously during deletion cycles
An example of a spanning interneuron with large overlap with hip-flexor activity during rostral scratching is shown in Figure7A. This unit was active during most of the hip-flexor burst. The third and fourth cycles (ends marked with ♦) were deletion cycles with no hip-flexor quiescence; the first two cycles were normal cycles with rhythmic alternation between hip-flexor activity and quiescence. The interneuron fired with frequency-modulated bursts during the normal cycles. The interneuron fired continuously with frequency-modulated bursts during the deletion cycles.
We studied a total of nine interneurons with activity bursts that had large levels (81–100%) of overlap with hip-flexor bursts during 236 cycles of normal rostral scratching. One unit was a hip-flexor interneuron that fired exclusively during the hip-flexor burst; the other eight units were spanning interneurons that either began or ended their activity during hip-flexor quiescence. For all nine of these units, the usual firing characteristic during deletions was continuous firing. The phase bars of these units in Figure 8 wereblack-filled to represent this characteristic. We recorded from these nine units during 60 deletion cycles. In 56 of these cycles (93.3%), the unit fired continuously; in four of these cycles (6.7%), the unit fired in a burst. These percentages are plotted in thetop bar of Figure 9.
Interneurons with 81–100% overlap with hip flexor activity during normal scratching were active during all or nearly all of the hip flexor burst. The continuous activity of these interneurons during deletion scratching is therefore similar to the activity of these units during normal scratching. These observations support the concept that the basic structure of firing of large-overlap units during the hip-flexor burst of a normal cycle is preserved during the hip-flexor burst of a deletion cycle. These preserved features during deletion scratching are therefore not dependent on the activity of neurons in the hip-extensor module.
DISCUSSION
Timing of interneuron activity during spinal motor rhythms
We characterized the ON- and OFF-phases of a population of spinal-cord interneurons during normal fictive rostral scratching in the turtle (Fig. 8) (Berkowitz and Stein, 1994b; Berkowitz, 2001a,b). Some interneurons were active only during hip-flexor motor activity, others were active only during hip-flexor motor quiescence, and still others displayed activity during both hip-flexor activity and quiescence. The interneuronal timings reported in turtle are similar to those reported in other limbed vertebrates: mudpuppy stepping (Wheatley et al., 1994), locomotor-like rhythms in rabbit (Viala et al., 1991) and neonatal rat (Tresch and Kiehn, 1999), cat stepping (Orlovsky and Feldman, 1972; Baev et al., 1979), and cat scratching (Berkinblit et al., 1978; Baev et al., 1981). Our findings support the hypothesis that there is a shared set of fundamental principles underlying spinal motor rhythm generation in limbed vertebrates (Grillner, 1981; Kiehn et al., 1997; Stein and Smith, 1997; Orlovsky et al., 1999).
Axonal recordings of descending propriospinal interneurons
Our recordings are from cut axons of descending propriospinal interneurons in the right dorsolateral funiculus at the posterior face of the third spinal segment of a five-segment hindlimb enlargement. Our findings support the concept that a collection of axons located on the right side of the spinal cord functions as a coordinated population. We did not identify the synaptic output of these axons. We assume that some of our recordings are from excitatory interneurons and the remainder are from inhibitory interneurons. Most likely these right-sided descending axons are presynaptic to right-sided spinal interneurons and motor neurons. We found it useful to categorize the phase of these right-sided descending axons according to the cycle times of right hip-flexor motor neurons on the basis, in part, of the assumption that right-sided spinal neurons are postsynaptic to these descending axons.
Some right-sided descending axons of propriospinal interneurons have ipsilateral cell bodies, and other right-sided descending axons have contralateral cell bodies (Berkowitz and Stein, 1994c; Eide et al., 1999). Studies using unit recordings from cell bodies (Berkowitz, 2001a,b) during normal and deletion rostral scratching are needed to examine the modular organization of spinal neurons on the basis of the location of their cell bodies; i.e., neurons with left-sided somata may be grouped into modules that differ from modules with right-sided somata.
Hip-flexor rhythms occur even when hip-extensor interneurons are quiescent
Modular hypotheses of motor pattern generation of spinal rhythms postulate the existence of a hip-flexor module and a hip-extensor module (Grillner, 1981; Stein et al., 1995, 1998a,b) [see Fig. 9 ofStein et al. (1995)]. Reciprocal inhibition between the hip-flexor module and the hip-extensor module is a major mechanism that contributes to rhythm generation (Grillner, 1981; Lundberg, 1981;Marder and Calabrese, 1996; Sharp et al., 1996; Calabrese and Feldman, 1997). Reciprocal inhibition between antagonist modules may not be the sole mechanism for spinal rhythm generation, however.
During rostral scratching with hip-extensor deletions in the turtle, there is rhythmic activation of hip-flexor motor neurons, and hip-extensor motor neurons are quiet. This supports the concept that there is an additional mechanism for rhythmogenesis: the hip-flexor module is a unit burst generator that produces a hip-flexor motor rhythm even when the antagonist hip-extensor motor neurons are quiet (Grillner, 1981; Stein et al., 1995, 1998a,b). Underlying this conceptual mechanism is the assumption that interneurons in the hip-extensor module are silent when hip-extensor motor neurons are silent. Our unit recordings from hip-extensor interneurons (Figs. 4, 8) that were quiet during rostral scratching with hip-extensor deletions provide direct support for this assumption.
Some of the hip-extensor interneurons of the present study are likely to be inhibitory. When hip-extensor inhibitory interneurons are silent, modular hypotheses predict a corresponding lack of IPSPs in hip-flexor interneurons and hip-flexor motor neurons. The predicted absence of IPSPs in hip-flexor interneurons is consistent with the concept that reciprocal inhibition between hip-flexor interneurons and hip-extensor interneurons is not the sole mechanism for rhythmogenesis. Future experiments with intracellular recordings from hip-extensor interneurons during normal and deletion rostral scratching will provide a critical test of this prediction. During normal rostral scratching, intracellular recordings from hip-flexor motor neurons demonstrate IPSPs during hip-extensor activity. During rostral scratching with hip-extensor deletions, these IPSPs in hip-flexor motor neurons are absent (Robertson and Stein, 1988). Thus our observations here with unit recordings from interneurons are consistent with previous observations of synaptic recordings from motor neurons. Both sets of observations support the concept that a population of interneurons, termed the hip-extensor module, functions as a group. This population is active during the hip-extensor phase of normal rostral scratching and quiet during rostral scratching with hip-extensor deletions.
Our observations of quiet interneurons in the hip-extensor module when hip-extensor motor neurons are quiet also support the concept that hip-extensor excitatory interneurons are quiet during rostral scratching with hip-extensor deletions. Modular hypotheses predict that hip-extensor excitatory interneurons excite each other as well as hip-extensor motor neurons. During normal rostral scratching, intracellular recordings from hip-extensor motor neurons demonstrate EPSPs during hip-extensor motor-nerve activity. During rostral scratching with hip-extensor deletions, these EPSPs in hip-extensor motor neurons are absent (Robertson and Stein, 1988). A fraction of our descending propriospinal interneurons are likely to be excitatory; therefore, our observations with unit recordings during deletions are consistent with observations of synaptic potentials from hip-extensor motor neurons.
Rostral scratching with hip-extensor deletions in the turtle is a model system to study spinal-cord rhythmic activation of agonists during antagonist quiescence. Observations of agonist rhythms during antagonist quiescence have been made in other limbed vertebrates: mudpuppy stepping (Cheng et al., 1998), neonatal rat locomotor-like rhythms (Hochman and Schmidt, 1998), and cat stepping (Grillner and Zangger, 1979; Jordan, 1991). Our inferences about the organization of the turtle spinal cord may also serve as hypotheses in studies of spinal cord in other limbed vertebrates.
Differences between a hip-extensor module and an extensor half-center
Rostral scratching in the turtle is an example of a “mixed synergy” (Stein and Smith, 1997) in which extensors at one joint fire at a different time than extensors at another joint; flexors of one joint are coactive with extensors of another joint. Another example of a mixed synergy is paw shake in cat (Smith et al., 1985; Pearson and Rossignol, 1991). In normal rostral scratching in the turtle, hip-extensor motor neurons fire at a phase of the cycle different from knee-extensor motor neurons. Knee-extensor motor neurons are active during the latter portion of each hip-flexor motor neuron burst (Fig.2A). In deletion cycles, hip-extensor motor neurons are quiet, and knee-extensor motor neurons are active during the latter portion of each hip-flexor motor neuron burst (Fig.2B).
These differences between the motor-neuron activities of hip extensors and knee extensors during rostral scratching observed previously (Stein and Grossman, 1980; Robertson and Stein, 1988) are observed at the level of interneuronal recordings in the present paper. Interneurons active during hip-extensor motor activity of normal rostral scratching were quiet during deletion rostral scratching (Figs. 4, 8). During normal rostral scratching, interneurons active during knee-extensor motor activity overlap the latter portion of hip-flexor motor activity; these interneurons fire in a burst during knee-extensor motor activity of rostral scratching with hip-extensor deletions (Figs. 5, 8). Intracellular recordings during rostral scratching with hip-extensor deletions demonstrate bursts of EPSPs in knee-extensor motor neurons during the latter portion of each hip-flexor burst (Robertson and Stein, 1988). These EPSPs are likely driven by knee-extensor excitatory interneurons active during deletion scratching. Interneurons active during knee-extensor activity belong to a different population than interneurons active during hip-extensor activity. Interneurons of the hip-extensor module are different from interneurons related to knee-extensor motor activity. This conflicts with the hypothesis that interneurons in an extensor half-center are related to extensor motor activity at all the joints of a limb. For the mixed synergy of rostral scratching in turtle, our data support the concept of a hip-extensor module and reject the concept of an extensor half-center. The half-center concept can be tested in the spinal cords of other vertebrates that generate mixed synergies, e.g., during the paw shake in the cat (Smith et al., 1985; Pearson and Rossignol, 1991). Our work with a mixed-synergy rhythm in turtle supports the prediction that the half-center concept cannot account for the full repertoire of rhythmic motor output in limbed vertebrates.
Whether interneurons related to knee-extensor activity belong to a knee-extensor module remains an open issue. Grillner (1981) postulated a distinct knee-extensor module. Robertson et al. (1985) support the utility of the concept. In contrast, Robertson and Stein (1988) andBerkowitz and Stein (1994b) failed to find strong evidence for the knee-extensor module concept. In future work, we will examine correlations between propriospinal–interneuron activity and knee-extensor activity to determine whether the activity of a subpopulation of interneurons is strongly related to knee-extensor motor activity.
Footnotes
This work was supported by National Institutes of Health Grant NS30786 to P.S.G.S. We thank Dr. Gavin Perry for software development and Drs. Ari Berkowitz and Gammon Earhart for editorial comments.
Correspondence should be addressed to Dr. Paul S. G. Stein, Department of Biology, Washington University, St. Louis, MO 63130. E-mail: stein@biology.wustl.edu.
REFERENCES
- 1.Baev KV, Degtyarenko AM, Zavadskaya TV, Kostyuk PG. Activity of lumbar interneurons during fictitious locomotion in thalamic cats. Neurophysiology (Kiev) 1979;11:244–250. [Google Scholar]
- 2.Baev KV, Degtyarenko AM, Zavadskaya TV, Kostyuk PG. Activity of lumbosacral interneurons during fictitious scratching. Neurophysiology (Kiev) 1981;13:45–52. [Google Scholar]
- 3.Batschelet E. Circular statistics in biology. Academic; New York: 1981. [Google Scholar]
- 4.Berkinblit MB, Deliagina TG, Feldman AG, Gelfand IM, Orlovsky GN. Generation of scratching. I. Activity of spinal interneurons during scratching. J Neurophysiol. 1978;41:1040–1057. doi: 10.1152/jn.1978.41.4.1040. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz A. Broadly tuned spinal neurons for each form of fictive scratching in spinal turtles. J Neurophysiol. 2001a;86:1017–1025. doi: 10.1152/jn.2001.86.2.1017. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz A. Rhythmicity of spinal neurons activated during each form of fictive scratching in spinal turtles. J Neurophysiol. 2001b;86:1026–1036. doi: 10.1152/jn.2001.86.2.1026. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz A, Stein PSG. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: broad tuning to regions of the body surface. J Neurosci. 1994a;14:5089–5104. doi: 10.1523/JNEUROSCI.14-08-05089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz A, Stein PSG. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: phase analyses. J Neurosci. 1994b;14:5105–5119. doi: 10.1523/JNEUROSCI.14-08-05105.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkowitz A, Stein PSG. Descending propriospinal axons in the hindlimb enlargement of the red-eared turtle: cells of origin and funicular courses. J Comp Neurol. 1994c;346:321–336. doi: 10.1002/cne.903460302. [DOI] [PubMed] [Google Scholar]
- 10.Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci. 1911;84:308–319. [Google Scholar]
- 11.Brown TG. On the nature of the fundamental activity of the nervous centers, together with an analysis of the conditioning of rhythmic activity in progression, and a theory of evolution of function in the nervous system. J Physiol (Lond) 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese RL, Feldman JL. Intrinsic membrane properties and synaptic mechanisms in motor rhythm generators. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. pp. 119–130. [Google Scholar]
- 13.Cheng J, Stein RB, Jovanovic K, Yoshida K, Bennett DJ, Han Y. Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus Maculatus) spinal cord. J Neurosci. 1998;18:4295–4304. doi: 10.1523/JNEUROSCI.18-11-04295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie SN, Gonsalves GG. Right-left interactions between rostral scratch networks generate rhythmicity in the preenlargement spinal cord of the turtle. J Neurophysiol. 1997;78:3479–3483. doi: 10.1152/jn.1997.78.6.3479. [DOI] [PubMed] [Google Scholar]
- 15.Currie SN, Gonsalves GG. Reciprocal interactions in the turtle hindlimb enlargement contribute to scratch rhythmogenesis. J Neurophysiol. 1999;81:2977–2987. doi: 10.1152/jn.1999.81.6.2977. [DOI] [PubMed] [Google Scholar]
- 16.Currie SN, Stein PSG. Cutaneous stimulation evokes long-lasting excitation of spinal interneurons in the turtle. J Neurophysiol. 1990;64:1134–1148. doi: 10.1152/jn.1990.64.4.1134. [DOI] [PubMed] [Google Scholar]
- 17.Earhart GM, Stein PSG. Scratch-swim hybrids in the spinal turtle: blending of rostral scratch and forward swim. J Neurophysiol. 2000a;83:156–165. doi: 10.1152/jn.2000.83.1.156. [DOI] [PubMed] [Google Scholar]
- 18.Earhart GM, Stein PSG. Step, swim, and scratch motor patterns in the turtle. J Neurophysiol. 2000b;84:2181–2190. doi: 10.1152/jn.2000.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 19.Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol. 1999;403:332–345. [PubMed] [Google Scholar]
- 20.Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. Handbook of physiology, Sect 1, The nervous system, Vol 2, Motor control. American Physiological Society; Bethesda, MD: 1981. pp. 1179–1236. [Google Scholar]
- 21.Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- 22.Hochman S, Schmidt BJ. Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord. J Neurophysiol. 1998;79:743–752. doi: 10.1152/jn.1998.79.2.743. [DOI] [PubMed] [Google Scholar]
- 23.Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand. 1967;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 24.Jordan LM. Brainstem and spinal cord mechanisms for the initiation of locomotion. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological basis of human locomotion. Japan Scientific Societies; Tokyo: 1991. pp. 3–20. [Google Scholar]
- 25.Kiehn O, Hounsgaard J, Sillar KT. Basic building blocks of vertebrate spinal central pattern generators. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. pp. 47–59. [Google Scholar]
- 26.Lundberg A. Half-centres revisited. Adv Physiol Sci. 1981;1:155–167. [Google Scholar]
- 27.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 28.Melby ECJ, Altman NH. Handbook of laboratory animal science, Vol 1. CRC; Cleveland: 1974. [Google Scholar]
- 29.Mortin LI, Stein PSG. Spinal cord segments containing key elements of the central pattern generators for three forms of scratch reflex in the turtle. J Neurosci. 1989;9:2285–2296. doi: 10.1523/JNEUROSCI.09-07-02285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortin LI, Stein PSG. Cutaneous dermatomes for the initiation of three forms of the scratch reflex in the spinal turtle. J Comp Neurol. 1990;295:515–529. doi: 10.1002/cne.902950402. [DOI] [PubMed] [Google Scholar]
- 31.Orlovsky GN, Feldman AG. Classification of lumbosacral neurons according to their discharge patterns during evoked locomotion. Neurophysiology (Kiev) 1972;4:311–317. [Google Scholar]
- 32.Orlovsky GN, Deliagina TG, Grillner S. Neuronal control of locomotion from mollusc to man. Oxford UP; New York: 1999. [Google Scholar]
- 33.Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- 34.Robertson GA, Stein PSG. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol (Lond) 1988;404:101–128. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson GA, Mortin LI, Keifer J, Stein PSG. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. J Neurophysiol. 1985;53:1517–1534. doi: 10.1152/jn.1985.53.6.1517. [DOI] [PubMed] [Google Scholar]
- 36.Ruigrok TJH, Crowe A. The organization of motoneurons in the turtle lumbar spinal cord. J Comp Neurol. 1984;228:24–37. doi: 10.1002/cne.902280105. [DOI] [PubMed] [Google Scholar]
- 37.Sharp AA, Skinner FK, Marder E. Mechanisms of oscillation in dynamic clamp constructed two-cell half-center circuits. J Neurophysiol. 1996;76:867–883. doi: 10.1152/jn.1996.76.2.867. [DOI] [PubMed] [Google Scholar]
- 38.Siegel S. Nonparametric statistics for the behavioral sciences. McGraw-Hill; New York: 1956. [Google Scholar]
- 39.Smith JL, Hoy MG, Koshland GF, Phillips DM, Zernicke RF. Intralimb coordination of the paw-shake response: a novel mixed synergy. J Neurophysiol. 1985;54:1271–1281. doi: 10.1152/jn.1985.54.5.1271. [DOI] [PubMed] [Google Scholar]
- 40.Stein PSG, Daniels-McQueen S. Modular organization of turtle spinal interneurons during fictive rostral scratching. Soc Neurosci Abstr. 2001;27:804. doi: 10.1523/JNEUROSCI.22-15-06800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein PSG, Grossman ML. Central program for scratch reflex in turtle. J Comp Physiol. 1980;140:287–294. [Google Scholar]
- 42.Stein PSG, Schild CP. N-methyl-d-aspartate antagonist applied to the spinal cord hindlimb enlargement reduces the amplitude of flexion reflex in the turtle. Brain Res. 1989;479:379–383. doi: 10.1016/0006-8993(89)91645-4. [DOI] [PubMed] [Google Scholar]
- 43.Stein PSG, Smith JL. Neural and biomechanical control strategies for different forms of vertebrate hindlimb motor tasks. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. pp. 61–73. [Google Scholar]
- 44.Stein PSG, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci. 1995;15:4343–4355. doi: 10.1523/JNEUROSCI.15-06-04343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. [Google Scholar]
- 46.Stein PSG, McCullough ML, Currie SN. Reconstruction of flexor/extensor alternation during fictive rostral scratching by two-site stimulation in the spinal turtle with a transverse spinal hemisection. J Neurosci. 1998a;18:467–479. doi: 10.1523/JNEUROSCI.18-01-00467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein PSG, McCullough ML, Currie SN. Spinal motor patterns in the turtle. Ann NY Acad Sci. 1998b;860:142–154. doi: 10.1111/j.1749-6632.1998.tb09045.x. [DOI] [PubMed] [Google Scholar]
- 48.Tresch MC, Kiehn O. Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord. J Neurophysiol. 1999;82:3563–3574. doi: 10.1152/jn.1999.82.6.3563. [DOI] [PubMed] [Google Scholar]
- 49.Viala D, Viala G, Jordan M. Interneurons of the lumbar cord related to spontaneous locomotor activity in the rabbit. I. Rhythmically active interneurones. Exp Brain Res. 1991;84:177–186. doi: 10.1007/BF00231773. [DOI] [PubMed] [Google Scholar]
- 50.Walker WF. The locomotor apparatus of testudines. In: Gans C, Parsons TS, editors. Biology of the reptilia, Vol 4. Academic; New York: 1973. pp. 1–100. [Google Scholar]
- 51.Wheatley M, Jovanovic K, Stein RB, Lawson V. The activity of interneurons during locomotion in the in vitro necturus spinal cord. J Neurophysiol. 1994;71:2025–2032. doi: 10.1152/jn.1994.71.6.2025. [DOI] [PubMed] [Google Scholar]


