Abstract
Interactions between AMPA receptor subunits and proteins containing postsynaptic density 95/disc large/zonula occludens-1 (PDZ) domains have been shown to play critical roles in the proper trafficking of receptors to excitatory synapses. Synaptic accumulation of AMPA receptors containing the glutamate receptor 1 (GluR1) subunit can be driven by calcium/calmodulin-dependent protein kinase II activity or long-term potentiation and requires an interaction between GluR1 and a type I PDZ domain-containing protein. Synaptic incorporation of AMPA receptors with only GluR2 occurs continuously, and this requires an interaction between GluR2 and a type II PDZ domain-containing protein. We used dual-channel, two-photon laser scanning microscopy to provide high-resolution visualization and quantification of green fluorescent protein-tagged AMPA receptors in different subcellular compartments. We showed that mutations on GluR1 or GluR2 AMPA subunit that perturb interactions with PDZ domain proteins lead to the accumulation of these receptors at different subcellular sites. GluR1 mutants accumulate in the dendrite, whereas GluR2 mutants accumulate in dendritic spines. This suggests that the critical PDZ domain interactions are required for entry into spines for GluR1 and for entry into synapses for GluR2.
Keywords: GluR1, GluR2, PDZ domain protein, two-photon laser scanning imaging, dendrite, dendritic spine, organotypic slice culture
Excitatory synapses in the CNS are found predominantly on dendritic spines, specialized postsynaptic structures that protrude from dendritic shafts (Harris and Kater, 1994). AMPA-type receptors (AMPA-Rs) mediate most of the fast excitatory synaptic transmission in the CNS, and a change in AMPA-R-mediated transmission underlies several developmental and adult forms of synaptic plasticity (Bliss and Collingridge, 1993; Linden and Connor, 1995; Nicoll and Malenka, 1995; Wu et al., 1996; Bear and Rittenhouse, 1999). Recent studies on the mechanisms controlling synaptic plasticity have led to models that include rapid redistribution of AMPA-Rs to or from synaptic sites (Carroll et al., 1999; Luscher et al., 1999; Shi et al., 1999; Passafaro et al., 2001;Malinow and Malenka, 2002). In these models, regulated insertion of AMPA-Rs into synapses accounts for increased synaptic efficacy, whereas regulated removal of synaptic AMPA-Rs accounts for decreased synaptic efficacy.
AMPA-Rs are hetero-oligomers composed of variable combinations of four subunits, glutamate receptor 1 (GluR1)–GluR4 (also referred to as GluRA–GluRD) (Seeburg, 1993; Hollmann and Heinemann, 1994;Dingledine et al., 1999). In the hippocampus, GluR1, GluR2, and GluR3 predominate in adults, whereas GluR4 is primarily expressed early in development (Zhu et al., 2000). In the adult hippocampus, the majority of AMPA-Rs contain either GluR2 and GluR1 or GluR2 and GluR3 (Wenthold et al., 1996) and the mature receptors are likely to contain four AMPA subunits (Rosenmund et al., 1998). AMPA-R subunits have four transmembrane domains, with a large extracellular N-terminal domain and an intracellular C terminus. The extracellular domain and the four membrane-associated domains show considerable homology among different subunits. In contrast, the cytoplasmic C termini of these subunits are either long (e.g., GluR1 and GluR4) or short (e.g., GluR2 and GluR3) (Fig. 1). Receptors with long cytoplasmic C termini are driven to synapses in a manner that requires synaptic NMDA receptor activity (Shi et al., 1999; Hayashi et al., 2000; Zhu et al., 2000; Passafaro et al., 2001). Significantly, the synaptic delivery of GluR1 and GluR4 is governed by different processes, because synaptic delivery of GluR1 is driven by CaMKII activity (Hayashi et al., 2000), whereas synaptic delivery of GluR4 is driven by PKA activity (Esteban and Malinow, 2001). In contrast to GluR1 and GluR4, receptors with only short cytoplasmic tails incorporate into synapses in a manner independent of synaptic activity (Shi et al., 2001). A number of protein–protein interactions between cytosolic proteins and the C termini of specific glutamate receptor subunits have been identified. Among these, interactions with proteins containing postsynaptic density 95/disc large/zonula occludens-1 (PDZ) motifs appear to play a central role in scaffolding receptors and signaling elements and have been shown to be important in the anchoring and delivery of the receptors to synapses (Ziff, 1997; Garner et al., 2000; Sheng and Sala, 2001; Malinow and Malenka, 2002). Previous electrophysiological studies with recombinant receptors composed of GluR1 or GluR2 have shown that PDZ domain interactions are critical for their incorporation into synapses. However, it is not known at what subcellular sites these PDZ domain interactions are required.
Fig. 1.
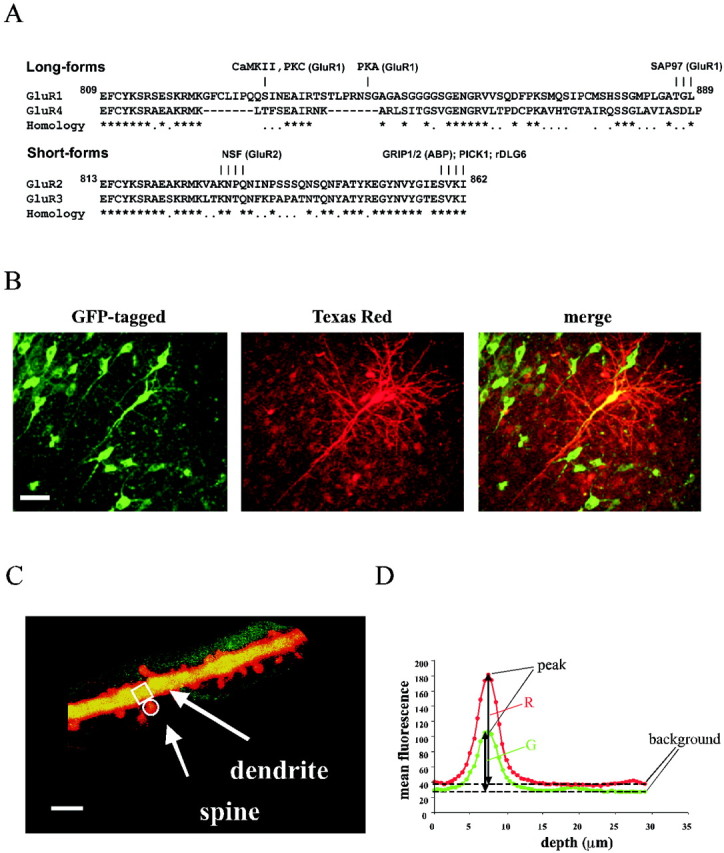
Dual-channel TPLSM imaging of CA1 pyramidal neurons reveals both channel distribution and cell morphology.A, Alignment of the cytoplasmic C termini of AMPA-R subunits. Asterisks indicate identical residues;dots indicate homologous residues.Numbers indicate the amino acid number in GluR1 and GluR2 without signal peptides. Some known sites for protein interactions or phosphorylation are shown. B, TPLSM of hippocampal CA1 pyramidal neurons expressing GluR1–GFP (left): one infected cell was injected with Texas Red dye (center), and images are merged (right). Images shown are projections of several sections acquired 1.5 μm apart. Scale bar, 50 μm. C, High-magnification image of an apical dendrite from a neuron infected with GluR1–GFP. The amount of receptor in a dendrite is compared with the amount in an adjacent spine. The regions indicated on spine and parental dendrite are typical examples of the ones selected for the analysis. The image shown is a projection of several sections acquired 0.5 μm apart. Scale bar, 5 μm. D, Graph of mean fluorescence of a typical small region (from a dendrite, in this case) for the GFP (green) channel and the Texas Red (red) channel plotted as a function of depth. The peak of mean fluorescence and the correspondent background for each channel used for image analysis are indicated.
We used dual-channel, two-photon laser scanning microscopy (TPLSM) to provide high-resolution visualization and quantification of AMPA-Rs in different subcellular compartments. This method allowed us to determine the relative amounts of AMPA-R located in the dendritic shaft or in an adjacent dendritic spine. By monitoring the localization of various mutant receptors, we showed that GluR1 mutant receptors lacking PDZ(I) domain interactions displayed reduced accumulation in spines and remained in dendritic shafts. In contrast, GluR2 mutant receptors lacking PDZ(II) domain interactions show enhanced accumulation in spines, despite their inability to incorporate stably into synapses (Osten et al., 2000; Shi et al., 2001). Thus, these results suggest that GluR1–PDZ interactions occur at the dendrite–spine border and that GluR2–PDZ interactions occur at the spine–synapse interface.
MATERIALS AND METHODS
Recombinant receptors and expression. Constructs of AMPA receptor subunits tagged with green fluorescent protein (GFP) were made as described previously (Hayashi et al., 2000; Shi et al., 2001). Briefly, the GFP coding sequence (enhanced GFP; Clontech, Palo Alto, CA) was inserted after the predicted signal peptide cleavage site of the corresponding AMPA receptor subunit cDNA.
These constructs were expressed in CA1 neurons in rat organotypic hippocampal slices using the Sindbis virus expression system. Slices were prepared from postnatal 6- to 7-d-old animals, infected after 5–8 d in culture and imaged 2 d after the infection. Experiments were performed at room temperature (22–25°C) in physiological ACSF (in mm: 119 NaCl, 26 NaHCO3, 1 NaH2PO4, 11d-glucose, 2.5 KCl, 2 CaCl2, 1.3 MgCl2, and 1.25 NaHPO4) gassed with 5% CO2 and 95% O2.
Two-photon microscopy. Before (20 min) imaging, infected neurons were identified by fluorescence illumination and were fully loaded (10–15 min after break-in) with a patch recording pipette (3–6 MΩ) containing 10 μm Texas Red (sulforhodamine 101; Sigma, St. Louis, MO) in the internal solution. The internal solution consisted of (in mm): 115 K-gluconate, 10 HEPES, 2 MgCl2, 2 MgATP, 2 Na2ATP, 0.3 Na3GTP, and 20 KCl. Two-photon images were collected on a custom-built instrument based on a Fluoview laser scanning microscope (Olympus America, Melville, NY). The light source was a mode-locked Ti:sapphire laser (Mira 900F, Santa Clara, CA) running at 910 nm. We used a LUMPlanFl/IR 40× numerical aperture 0.75 dipping lens. Each optical section was resampled three times and typically was captured every 0.5 μm.
Image analysis. The borders of the locations defined as spine and dendrite were selected using only the Texas Red channel image. No tagged GFP information was used at this stage to prevent any unconscious bias. We then measured the GFP fluorescence in these predefined dendritic and spinal compartments. The diffusional equilibration of a dye between the spinal compartment and the parental dendritic shaft occurs in milliseconds (Svoboda et al., 1996). Therefore, the Texas Red filling of the two structures was equilibrated in the time scale of the experiment. We measured the mean intensity fluorescence, as a function of z-dimension, in two small areas (≤2 μm2) over the dendrite and nearby spine. Areas were drawn manually on each spine so as to enclose their fluorescent signal. In the dendritic region at the base of the spine, a region of similar size to the spine was chosen. The mean fluorescence within each region was obtained using Fluoview 3.2 software (Olympus).
The subsequent analysis was performed with a custom-written software program using the following approach: the peak of mean fluorescence per area in the GFP channel and in the Texas Red channel for a particular spine–dendrite pair was background subtracted, yielding the valuesG and R, respectively. The background value is the mean of the four values about the lowest fluorescence value obtained for a chosen region in each stack. The spine/dendrite ratio (s/d) is then calculated as follows: s/d = [G/R]spine/[G/R]dend.For assessment of the statistically significant difference between two sets of cumulative distributions, we used the Kolmogorov–Smirnov test. For clarity, in the cumulative distribution plots, only thex-axis (s/d) values <3 are shown (except see Fig.4B). Because there were some s/d values >3, the cumulative distributions shown do not always reach 100%. To examine the exit of receptor from the cell body, the GFP fluorescence of expressed GFP-tagged receptors was measured as a function of distance from the cell body. Small areas (≤2 μm2) were drawn on the cell body and along the primary dendrite every 10 μm. Values were background subtracted and normalized by the intensity at the cell body and averaged across cells. The background value was obtained for each cell by choosing an area (≤2 μm2) on the uninfected tissue nearby.
Fig. 4.
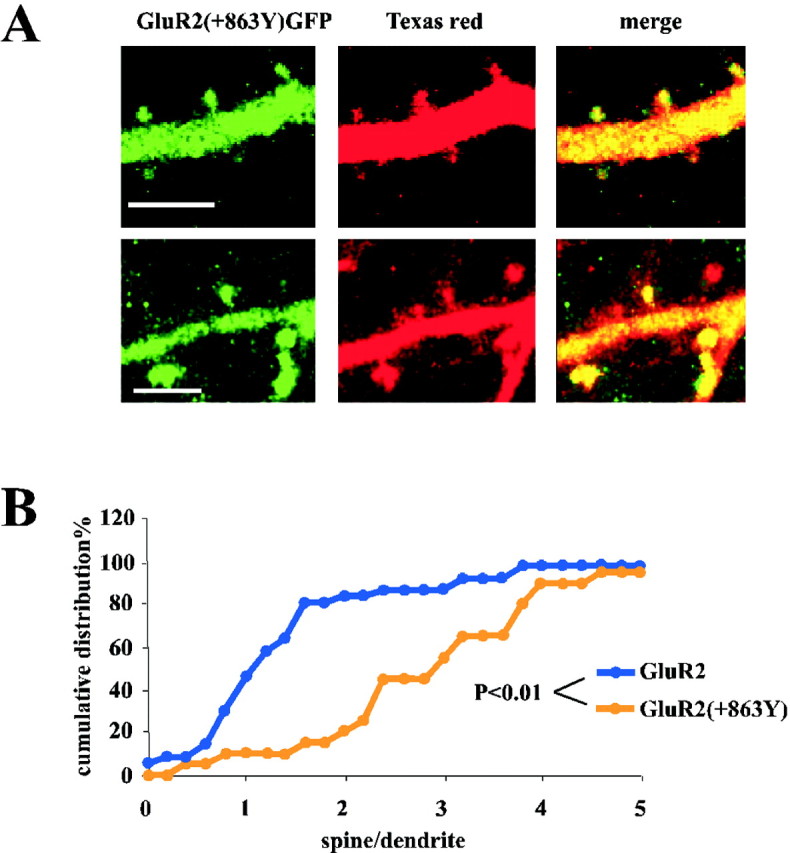
The PDZ(II) domain mutant GluR2(+863Y) is concentrated in spines. A, GluR2–GFP receptor is clearly detected in spines. B, The amount of fluorescence detected in the spines compared with the parent shaft is significantly larger for the mutant than for the wild-type GluR2–GFP. The cumulative distribution of [G/R]spine/[G/R]dendvalues from cells expressing GluR2(+863Y)–GFP is greater than the values obtained from cells expressing GluR2–GFP (spine–dendrite pairs: n = 20 for GluR2(+863Y),n = 36 for GluR2–GFP; p= Kolmogorov–Smirnov test between GluR2(+863Y) and GluR2–GFP).
RESULTS
The subcellular distribution of AMPA-Rs plays a role in determining synaptic strength and synaptic plasticity. To examine quantitatively the subcellular distribution of recombinant AMPA receptors, we injected pyramidal neurons expressing different GFP-tagged subunits with the freely diffusible marker, Texas Red (Fig.1B). Our intent was to focus on the relative distribution of AMPA-Rs at two sites: dendritic spines and dendritic shafts just below spines (Fig. 1C). The distribution of Texas Red reliably revealed neuronal morphology, as indicated by the nearly identical distribution of Texas Red and plain GFP (Fig.2C). As shown in Figure2D, the ratios of signals in the GFP channel and Texas Red channel (G/R) were nearly identical for spines and dendritic regions. We have reported previously that AMPA-Rs composed of GluR1–GFP appear to be restricted from dendritic spines (Shi et al., 2001). This was concluded from the fact that neurons expressing GluR1–GFP do not display a GFP signal that appears like spines. We demonstrated this directly by showing that spines, as revealed by filling with Texas Red, contain very little GluR1–GFP. To quantify the relative distribution of GluR1, the ratios between the green channel (GluR1–GFP) and the red channel (Texas Red) were determined in both the spine and the underlying dendrite. Significantly, the values for [G/R]spine/[G/R]dendare almost all <1 (Fig. 2D), arguing for a restriction of GluR1–GFP from entering dendritic spines. In contrast to GluR1–GFP, the signal for GluR2–GFP was clearly detectable in dendritic spines, as reported previously (Shi et al., 2001) (Fig.2B). The G/R values in spines were not significantly different from those in dendrites (Fig.2D). Importantly, the values [G/R]spine/[G/R]dendfor GluR2–GFP were variable, much more than such values for plain GFP. This indicates that GluR2–GFP was restricted from some spines and was accumulated in others.
Fig. 2.
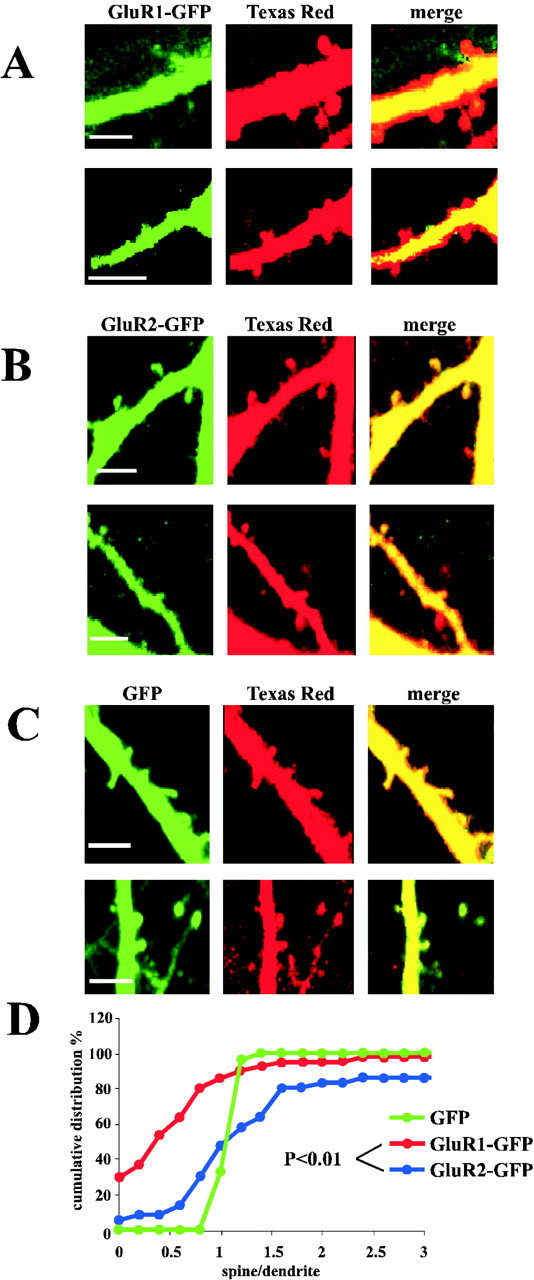
GluR1 and GluR2 show different expression levels in spines. Dual-channel images of apical dendrites show that GluR1–GFP appears restricted from dendritic spines (A), whereas GluR2–GFP is detectable in dendritic spines (B). Scale bar, 5 μm.C, Dual-channel images of apical dendrites show that the distribution of GFP and Texas Red are virtually identical. Scale bar, 5 μm. D, Cumulative distribution of the [G/R]spine/[G/R]dendfluorescence values GluR1–GFP are almostall <1. The GluR2–GFP [G/R]spine/[G/R]dendvalues are not significantly different from those in the dendrites (spine–dendrite pairs: n = 41 for GluR1–GFP,n = 36 for GluR2–GFP, and p = Kolmogorov–Smirnov test between GluR1–GFP and GluR2–GFP). Cumulative distribution of the GFP [G/R]spine/[G/R]dendfluorescence values shows no difference between the two channels (s/d ∼1; n = 24).
We have shown previously, using electrophysiological methods, that GluR1–GFP is driven into synapses by coexpression of a constitutively active form of calcium/calmodulin-dependent protein kinase II (tCaMKII) (Hayashi et al., 2000). We examined this phenomenon with imaging methods to determine the extent of GluR1 redistribution in response to increased CaMKII activity. The resulting images showed that a significant amount of GluR1–GFP can be detected in spines. Thus, although [G/R]spine is less than [G/R]dend in neurons expressing GluR1–GFP, the [G/R]spine is not different from [G/R]dendfor neurons expressing both GluR1–GFP and tCaMKII. The cumulative distribution of values for [G/R]spine/[G/R]dendfrom cells expressing GluR1–GFP was significantly different from the distribution of such values from cells expressing both GluR1–GFP and tCaMKII. Indeed, the distribution of GluR1–GFP in cells coexpressing tCaMKII became indistinguishable from the distribution of GluR2–GFP (Fig. 3C).
Fig. 3.
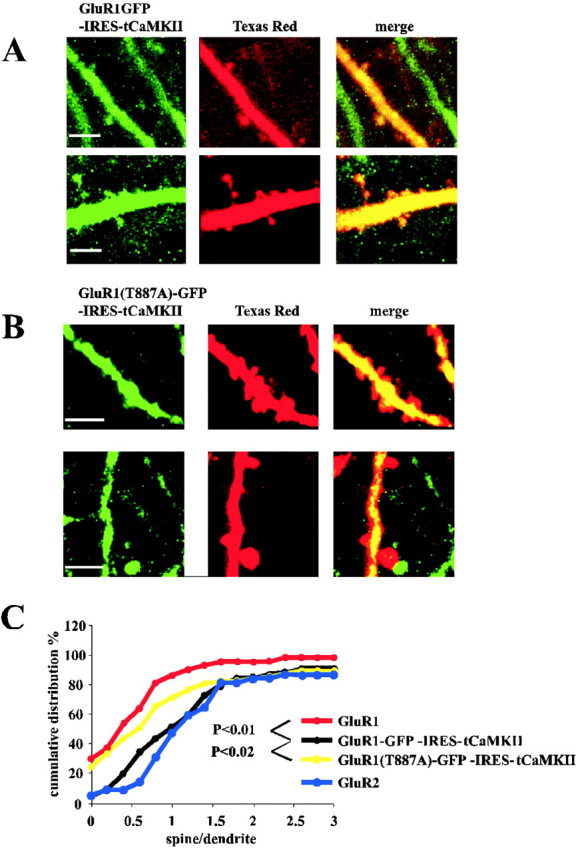
Coexpression of GluR1–GFP and active αCaMKII results in translocation of GluR1 into dendritic spines. However, expression of GluR1(T887A)–GFP–IRES–tCaMKII does not result in translocation of GluR1 into dendritic spines. A, In the presence of active CaMKII, a significant amount of GluR1–GFP can be visualized in spines. B, Little or no GluR1(T887A)–GFP appears in dendritic spines in the presence of active CaMKII. Scale bars: A, B, 5 μm. C, Cumulative distributions of indicated constructs. Spine–dendrite pairs: n = 88 for GluR1– GFP–IRES–tCaMKII,n = 41 for GluR1–GFP, and n = 71 for GluR1(T887A)–GFP–IRES–tCaMKII; p values provided for Kolmogorov–Smirnov test between indicated constructs. GluR2–GFP from Figure 2, shown for comparison.
Previous electrophysiological studies have indicated that GluR1 requires a group I PDZ domain interaction for tCaMKII to drive GluR1 into synapses (Hayashi et al., 2000). We tested whether this interaction is required to move GluR1 from dendrites into spines or from spines into synapses. We expressed GluR1–GFP(T887A), a mutant subunit that forms a functional receptor and can prevent the association with group I PDZ domain proteins, together with CaMKII (Hayashi et al., 2000). As shown in Figure 3B, this mutation appears to prevent the receptor from entering dendritic spines. The cumulative distribution of [G/R]spine/[G/R]dendvalues from cells expressing GluR1(T887A)–GFP and tCaMKII was not significantly different from the distribution of the values from cells expressing GluR1–GFP (p = 0.39) (Fig.3C). In addition, the cumulative distribution of [G/R]spine/[G/R]dendvalues from cells expressing GluR1(T887A)–GFP and tCaMKII was significantly different from the distribution of the values from cells expressing GluR1–GFP–internal ribosome entry site (IRES)–tCaMKII (p = 0.015) (Fig.3C). We conclude that GluR1–PDZ(I) interaction is required for tCaMKII to drive GluR1 from dendrite to spine.
We also investigated the trafficking of homomeric GluR2 receptors. We have found previously that a group II PDZ domain interaction is necessary for the continuous delivery of GluR2 to synapses. To localize the subcellular compartment where the interaction between GluR2 and PDZ domain II occurs, we focused on a mutant GluR2 with a tyrosine added at the end of the C terminus (+863Y). This mutation prevents the interaction between GluR2 and PDZ II domain-containing proteins (Xia et al., 1999) and prevents incorporation of the receptor into synapses (Shi et al., 2001). Surprisingly, although GluR2(+863Y)–GFP does not incorporate into synapses, this receptor was clearly detected in spines (Fig. 4A). Indeed, this receptor shows increased accumulation in spines. The cumulative distribution of [G/R]spine/[G/R]dendvalues from cells expressing GluR2(+863Y)–GFP was greater than the values obtained from cells expressing GluR2–GFP (Fig.4B). This indicates that the amount of fluorescence detected in the spines compared with the parent shaft was significantly larger for the mutant than for the wild-type GluR2–GFP (Fig.4B). This last finding suggests that GluR2(+863Y)–GFP is able to enter the spine compartment and that the interaction with the PDZ II domain required for delivery to the synapse occurs in the spine.
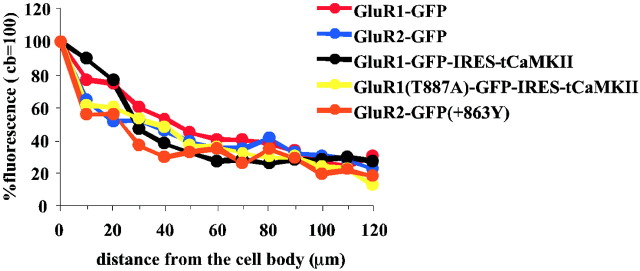
In addition to the control of receptor distribution between dendrite and spine, we also examined the general distribution of receptors within a neuron. To examine this, we measured the intensity of the GFP signal in the cell body and along the primary dendrite for each receptor examined. As shown in Figure 5, there was no detectable difference in the distribution of receptors from cell body to dendrite for any receptor examined in this study. Therefore, the exit of AMPA receptor from cell body to dendrite or the retention within the dendrite does not appear to depend on the factors considered in this study: GluR1, GluR2, CaMKII, or PDZ domain interactions.
Fig. 5.
Trafficking of receptor from/to the cell body. The intensity of the GFP signal along the primary dendrite (120 μm from the cell body), normalized over the fluorescence of the cell body (cb), was examined for each receptor.
DISCUSSION
The molecular mechanisms controlling the abundance of synaptic ionotropic glutamate receptors seem to be a major site of regulation during plasticity (Malenka and Nicoll, 1997; Malinow, 1998; Luthi et al., 1999; Garner et al., 2000; Sheng and Lee, 2001; Malinow and Malenka, 2002). In particular, AMPA-Rs can be transported to and from synapses, suggesting that receptor trafficking is a key factor in regulating synaptic strength. Two types of AMPA-R trafficking processes have been described: one is activity dependent, whereas the other is continuous (Hayashi et al., 2000; Zhu et al., 2000; Passafaro et al., 2001; Shi et al., 2001). GluR1/GluR2 receptors are added to synapses during plasticity. This requires interactions between GluR1 and type I PDZ domain proteins. In contrast, GluR2/3 receptors replace existing synaptic receptors continuously. This occurs only at the synapses that already have AMPA-Rs and requires interactions by GluR2 withN-ethylmaleimide-sensitive factor (NSF) and type II PDZ domain proteins. Interestingly, each trafficking process is distinct and each seems ruled entirely by the specific molecular modules present on the cytoplasmic C termini of a particular receptor subtype (Passafaro et al., 2001; Shi et al., 2001). Although endogenous receptors participating in activity-dependent trafficking are likely GluR1/GluR2 heteromers, this trafficking appears to be closely mimicked by recombinant homomeric GluR1 receptors (Passafaro et al., 2001; Shi et al., 2001). Similarly, endogenous receptors participating in the constitutive replacement are likely GluR2/GluR3 heteromers, and this trafficking is well modeled by homomeric GluR2 receptors (Passafaro et al., 2001; Shi et al., 2001).
In this study, we examined where, in neurons, these PDZ domain interactions are required. Two potential sites of spatial regulation were considered: from cell body to dendrite and from dendrite to dendritic spine. In none of our perturbations did we detect an effect on the general distribution within the neuron. We thus conclude that homomeric GluR2 or GluR1 receptors exit the cell body in equal amounts, and that neither CaMKII activity nor PDZ domain interactions seem to control this transition. This is in contrast to trafficking inCaenorhabditis elegans, where CaMKII regulates the transport of glutamate receptors from cell bodies to neurites (Rongo and Kaplan, 1999).
In contrast to the lack of effects on cell body to dendrite transition, we find considerable regulation in the dendritic shaft to spine transition. We confirm that GluR1 receptors are primarily excluded from spines and that GluR2 receptors readily incorporate in spines (Shi et al., 2001). Of interest, the distribution of GluR2–GFP in spines is heterogeneous: some spines have relatively little, whereas others have considerably more. This is consistent with the view that GluR2–GFP receptors replace synaptic receptors (Shi et al., 2001) and that there is normally a wide distribution in the amount of synaptic receptors (Nusser et al., 1998; Petralia et al., 1999; Takumi et al., 1999). We also confirm an effect, described electrophysiologically, that active CaMKII can drive GluR1-containing AMPA-Rs into synapses (Hayashi et al., 2000) by showing that active CaMKII drives GluR1–GFP into spines.
The most novel findings in this study concerned disruption of PDZ domain interactions. We found that disruption of PDZ domain interaction(s) on GluR1 and GluR2 had different effects. For GluR1, the (T887A) mutation prevented accumulation of this receptor into spines, whereas for GluR2, the (+863Y) mutation enhanced accumulation of this receptor into spines. These results indicate that PDZ domain interactions by GluR1 and GluR2 are required at spatially distinct sites. It is notable that GluR2 receptors with PDZ domain mutations accumulate in spines (this study) and yet are not incorporated into synapses (Shi et al., 2001). A similar phenotype has been described for homomeric GluR3 receptors (Shi et al., 2001), which do have PDZ domains but lack NSF-binding capacity. These phenotypes are consistent with the view that the cytoplasmic tail of GluR2, NSF, and PDZ domain proteins cooperates to form functionally important complexes (Hanley et al., 2001).
A recent time-lapse study has indicated that the delivery processes controlled by GluR1 or GluR2 may track through distinct subcellular sites (Passafaro et al., 2001). In that study, GluR1 receptors were delivered to the dendritic surface and then incorporated into spines. In contrast, GluR2 receptors were delivered directly into the spine surface. In this case, if PDZ domain interactions were important in surface delivery, one would predict that mutant receptors lacking PDZ ligand domains would accumulate at different subcellular sites. In particular, GluR1 PDZ domain mutants should accumulate in dendrites and not go into spines, whereas GluR2 PDZ domain mutants should accumulate in spines. This is the distribution observed for our PDZ domain mutants, suggesting that PDZ domain interactions are critical for surface delivery of the receptors. This suggests that GluR1 and GluR2 are delivered to the surface by similar processes occurring at fundamentally different sites.
Footnotes
This work was supported by the National Institutes of Health (R.M.). We thank N. Dawkins-Pisani for technical help, B. Burbach and K. Svoboda for help with the two-photon image acquisition, M. Reigl for writing the image analysis program, Y. Hayashi and S.-H. Shi for constructs, and P. Wasling and members of the Malinow lab for helpful discussions. We thank T. Zador, S. Rumpel, and L. Van Aelst for comments on a previous version of this manuscript.
Correspondence should be addressed to Roberto Malinow, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724. E-mail:malinow@cshl.org.
REFERENCES
- 1.Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 4.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 5.Esteban JA, Malinow R. A molecular mechanism for the regulated synaptic delivery of GluR4-containing AMPA receptors. Soc Neurosci Abstr. 2001;27:20. [Google Scholar]
- 6.Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 7.Hanley JG, Khatri L, Hanson PI, Ziff EB. Regulation of AMPA receptor GluR2 subunit-PDZ protein interactions by NSF and α-/β-SNAPS. Soc Neurosci Abstr. 2001;27:403. [Google Scholar]
- 8.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 10.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 11.Linden DJ, Connor JA. Long-term synaptic depression. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 12.Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 13.Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 14.Malenka RC, Nicoll RA. Silent synapses speak up. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 15.Malinow R. Silencing the controversy in LTP? Neuron. 1998;21:1226–1227. doi: 10.1016/s0896-6273(00)80640-6. [DOI] [PubMed] [Google Scholar]
- 16.Malinow R, Malenka R (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci, in press. [DOI] [PubMed]
- 17.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 18.Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 19.Osten P, Khatri L, Perez JL, Köhr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 20.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 21.Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 22.Rongo C, Kaplan JM. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature. 1999;402:195–199. doi: 10.1038/46065. [DOI] [PubMed] [Google Scholar]
- 23.Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- 24.Seeburg PH. The TINS/TiPS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 25.Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105:825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 26.Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 28.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 29.Svoboda K, Tank DW, Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- 30.Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Ann NY Acad Sci. 1999;868:474–482. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- 31.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 33.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 35.Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]