In the article, “Regulation of Exocytosis through Ca2+/ATP-Dependent Binding of Autophosphorylated Ca2+/Calmodulin-Activated Protein Kinase II to Syntaxin 1A,” by Akihiro Ohyama, Kohei Hosaka, Yoshiaki Komiya, Kimio Akagawa, Emiko Yamauchi, Hisaaki Taniguchi, Nobuyuki Sasagawa, Ko- nosuke Kumakura, Sumiko Mochida, Takashi Yamauchi, and Michihiro Igarashi, which appeared on pages 3342–3351 of the May 1, 2002 issue, Figure 2B,E printed with several labels missing. A revised version of Figure 2, along with a corrected legend, is printed here.
Fig. 2.
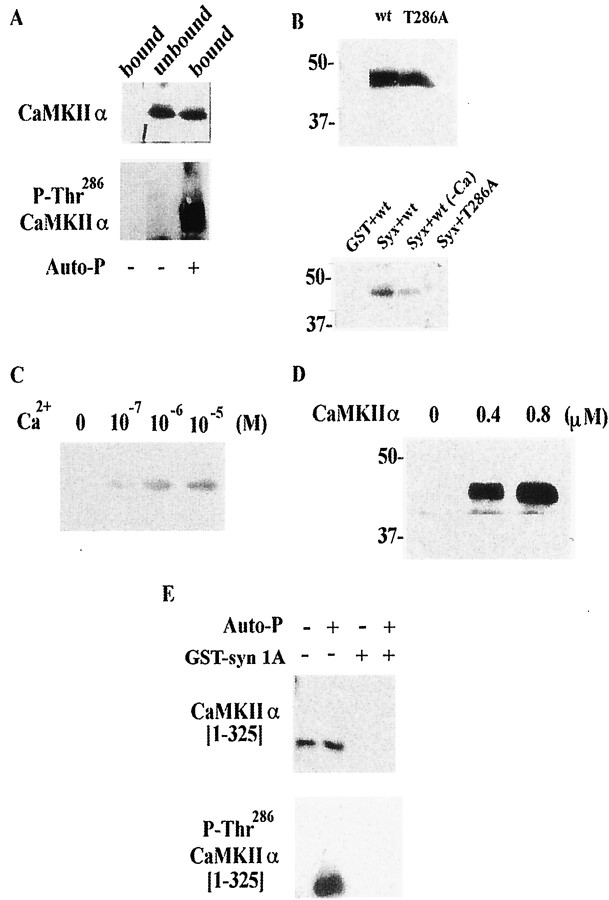
CaMKII binds to syntaxin only when autophosphorylated. A, Purified CaMKIIα also binds syntaxin after autophosphorylation. The fraction unbound to GST—syntaxin after re-autophosphorylation was not recognized by anti-autophosphorylated CaMKII antibody. CaMKIIα (5 μg) purified from rat brain was autophosphorylated (Auto-P) in buffer containing 50 mm HEPES-NaOH, pH 8.0, 8 mm Mg (CH3COO)2, 0.25 mmCaCl2, 2 μm CaM, and 0.5 mm ATP for 5 min at 30°C, and then incubated with immobilized GST or GST—syntaxin (4 nmol) for 1 hr at 4°C. After centrifugation, the supernatant was collected as the unbound fraction. The PreScission protease fraction was collected as the bound fraction. Samples were resolved by 10% SDS-PAGE and immunoblotted using anti-CaMKIIα mAb or anti-autophosphorylated CaMKII mAb. B, T286A-CaMKIIα could not bind syntaxin. Top panel (two lanes), Both wild-type CaMKII (wt) and T286A-CaMKIIα (T286A) were produced by in vitro translation. Their apparent molecular masses wre ∼48 kDa. Bottom panel (four lanes), Wild-type CaMKIIα (Syx+wt), but not T286-CaMKIIα (Syx+T286A), bound to syntaxin 1A in a pull-down study in the presence of Ca2+/ATP. The wild-type CaMKIIα did not bind to GST (GST+wt). Just as seen for the native CaMKIIα from rat brain (Fig. 1B), the wild-type CaMKIIα produced by in vitro translation could not bind to syntaxin without Ca2+ (Syx+WT−Ca). The cDNA encoding rat CaMKIIα or T286A-CaMKIIα (provided by Dr. H. Schulman) was added to the in vitro translation kit (Promega). Proteins were expressed by incubating kit components for 1.5 hr at 30°C and then by adding immobilized GST—syntaxin as described above. After elution with SDS-sample buffer, bound proteins were blotted and detected using streptavidin-conjugated alkaline phosphatase. Molecular masses (in kilodaltons) are shown to the left. C, CaMKIIα produced using in vitro translation also shows Ca2+sensitivity for binding to syntaxin after autophosphorylation. Translation in vitro proceeded as described above, and CaMKIIα was autophosphorylated in buffer containing Tris-HCl, pH 7.6, 0.5 mm CaCl2, 2 μm CaM, 2 mm MgCl2, and 0.5 mm ATP at 30°C for 15 min. The autophosphorylated CaMKIIα was incubated with immobilized GST—syntaxin (4 nmol) at 4°C for 1 hr and then with binding buffer containing various concentrations of Ca2+for an additional 1 hr. D, Dose-dependent binding of CaMKII to syntaxin. GST—syntaxin (4 nmol) was incubated with recombinant CaMKIIα at various concentrations. E, CaMKIIα lacking the association domain [1-325] (i.e., monomeric CaMKIIα) does not bind to syntaxin, even when autophosphorylated. Non-autophosphorylated [Auto-P (−), Gst-syn 1A (+)] or autophosphorylated monomeric CaMKIIα [(Auto-P (+), GST-syn 1A(+)] was incubated with GST—syntaxin 1A, and each bound fraction was eluted as described above (see A). Together with these fractions, both forms of monomeric CaMKIIα before incubation with the immobilized syntaxin [Auto-P (−), GST-syn 1A (−) or Auto-P (+), GST-syn 1A (−)] were also electrophoreses and analyzed by immonoblotting. The apparent molecular mass of CaMKIIα [1-325] was approximately 35 kDa and was recognized by anti-CaMKIIα mAb. After the autophosphorylation, the truncated CaMKIIα was also recognized by anti-P-Thr286 CaMKIIα mAb, as well as the native one (seeA).