Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) causes calcium influx, intracellular calcium release, and elevation of cAMP in chromaffin cells. Calcium influx is required for PACAP-stimulated secretion of catecholamines and neuropeptides. The role of cAMP elevation in the action of PACAP at either sympathetic or adrenomedullary synapses, however, is unknown. Here, we show that PACAP-27-induced calcium influx through voltage-sensitive calcium channels (VSCCs), together with elevation of intracellular cAMP, was sufficient to stimulate vasoactive intestinal polypeptide (VIP) biosynthesis at least 40-fold. Combined treatment of chromaffin cells with 40 mm KCl, which elevates intracellular calcium, and 25 μm forskolin, which elevates intracellular cAMP, caused an increase in VIP peptide and mRNA much greater than that elicited by either agent alone, and comparable to the increase caused by 10–100 nm PACAP-27. Elevation of VIP mRNA by either KCl plus forskolin, or PACAP, (1) was independent of new protein synthesis, (2) was blocked by inhibition of calcium influx through voltage-sensitive calcium channels, (3) was calcineurin dependent, and (4) was dependent on MAP kinase activation but not activation of protein kinase A. The degree of activation of two different second-messenger pathways, calcium influx and cAMP elevation, appears to determine the magnitude of transcriptional activation of the VIP gene in chromaffin cells. Maximal stimulation of VIP biosynthesis by PACAP appears to require the coincident activation of both of these pathways.
Keywords: vasoactive intestinal polypeptide, VIP, pituitary adenylate cyclase-activating polypeptide, PACAP, calcium, cAMP, signal transduction, calcineurin, mitogen activated protein kinase, MAPK
Catecholamine and peptide hormone secretion and biosynthesis in the adrenal medulla are regulated in concert by preganglionic inputs from the splanchnic nerve (Sietzen et al., 1987; Fischer-Colbrie et al., 1988; Nankova and Sabban, 1999). This synapse is a well studied model for trans-synaptic regulation throughout the nervous system (Kumakura et al., 1979; Eiden et al., 1984b; Comb et al., 1987). Two transmitters are contained in splanchnic nerve terminals: the classical neurotransmitter acetylcholine (Douglas and Rubin, 1961) and the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) (Frödin et al., 1995; Dun et al., 1996; Sundler et al., 1996; Holgert et al., 1998). Both transmitters stimulate secretion and enhance production of catecholamine and neuropeptides (Douglas and Rubin, 1961; Eiden et al., 1984b; Malhotra et al., 1989; Isobe et al., 1993; Rius et al., 1994;Babinski et al., 1996; Haycock, 1996; Przywara et al., 1996; Tanaka et al., 1996; Tönshoff et al., 1997; Hahm et al., 1998; Wakade, 1998; Lamouche et al., 1999). PACAP neurotransmission may be uniquely required to maintain long-term secretion and biosynthesis of adrenomedullary hormones during periods of prolonged metabolic or psychogenic stress (Wakade, 1988; Wakade et al., 1988; Hamelink et al., 2002).
Both PACAP and acetylcholine cause calcium influx, which is required to stimulate the release and biosynthesis of neuropeptides such as vasoactive intestinal polypeptide (VIP) (Douglas, 1968; Waschek et al., 1987; Tanaka et al., 1996; Lee et al., 1999). However, PACAP elevates VIP peptide levels in chromaffin cells much more than depolarization with acetylcholine or elevated potassium (Waschek et al., 1987; Eiden et al., 1998; Lee et al., 1999). This suggests a special role for PACAP in regulating adrenomedullary neuropeptides like VIP, which mediates local vasodilatory effects and steroidogenesis under paraphysiological conditions, including prolonged stress (Lundberg et al., 1980; Bloom et al., 1988; Edwards and Jones, 1993; Bornstein et al., 1996; Nussdorfer, 1996; Lonning et al., 1997; Ehrhart-Bornstein et al., 2000). It also suggests a unique mechanism for PACAP signaling to the VIP gene in addition to increased calcium influx. PACAP, through the PAC1 receptor, also stimulates adenylate cyclase and phospholipase C, leading to elevation of intracellular cAMP, inositol 1,4,5,-trisphosphate (IP3), and diacylglycerol (DAG) (Schadlow et al., 1992; Spengler et al., 1993; Tanaka et al., 1998). The effects of PACAP on catecholamine secretion from chromaffin cells, and neuropeptide secretion from sympathetic neurons in primary culture, appear to be dependent on activation of both phospholipase C and calcium entry through voltage-sensitive channels (VSCCs), but not on the elevation of cAMP caused by PACAP (Tanaka et al., 1996, 1998; Hahm et al., 1998;Beaudet et al., 2000). The role of multiple second messengers in PACAP signaling leading to activation of gene transcription has not yet been addressed. Here, we investigated whether combinatorial actions of the multiple second messengers elevated by PACAP might provide a gene transcriptional response greater than that predicted by the action of each individual second messenger. In particular, PACAP signaling to the VIP gene might depend on the synergistic action of two or more of the second messengers activated by PACAP in chromaffin cells.
MATERIALS AND METHODS
Chromaffin cell culture. Chromaffin cells were obtained from steer adrenal medullas, according to previously published methods (Fenwick et al., 1978) as modified by this laboratory (Eiden et al., 1984a). Steer adrenal medullas were perfused multiple times through the portal vein with standard release media (SRM) containing (in mm): 143 NaCl, 4.6 KCl, 10 glucose, 25 HEPES, 2.2 CaCl2, 1.2 MgSO4 at 37°C until the gland was rinsed free of blood. Glands were perfused three times with warmed 0.1% collagenase (Worthington Biochemicals, Lakewood, NJ) and 6.7 μg/ml DNase for 15 min at 37°C. Medullary tissue was minced and collected in SRM without bovine serum albumin (BSA). Cells were rinsed briefly and centrifuged twice in SRM containing 1% BSA. Cells were filtered three times through sterile gauze, followed by filtration through 150 mesh nylon screen. Cells were washed four times in SRM/1% BSA and suspended in complete medium [DMEM high glucose 25 mm HEPES medium (Invitrogen, Rockville, MD)] with 5% heat-inactivated fetal bovine serum, 100 U/ml penicillin-streptomycin, 2 mm glutamine, 10 μg/ml cytosine β-d-arabinofuranoside, and 100 U/ml nystatin, and filtered again through 150 mesh nylon screen. Forty million cells were seeded into T150 flasks overnight for differential plating. The following day cells were replated onto poly-d-lysine (Sigma, St. Louis, MO)-coated 24-well dishes at 250,000–500,000 cells per well in 1 ml of medium. Twenty-four hours later cells were preincubated with appropriate agents by addition of 100 μl of a 10× concentrated stock solution to each well. Thirty minutes later, medium was removed and replaced with complete medium without nystatin, containing vehicle or PACAP-27, with or without the agents added during the preincubation period.
For measurement of total VIP content per well, medium was removed for direct measurement of VIP peptide by radioimmunoassay, and cells were extracted for VIP peptide radioimmunoassay after the addition of 500 μl of 0.1N HCl to each well, as described previously (Eiden et al., 1984a). For measurement of VIP mRNA, cells were harvested 18–24 hr after drug additions into SDS-EDTA-Tris buffer containing 100 μg/ml of proteinase K (Hahm et al., 1998). Drugs were initially dissolved in ethanol, water, or complete medium such that the final concentration of vehicle in incubation media was 0.1% (v/v).
Chromaffin cells were counted after plating, and after various treatments, in a Coulter Model Z1 Cell Counter, with lower and upper limits of 9 and 19 μm, respectively, after rinsing in PBS and suspension in Accutase (Intermountain Scientific Corp., Kaysville, UT). There was no change in cell number 72 hr after PACAP treatment (control, 287,000 ± 6,500 cells per well; 10 nmPACAP, 288,000 ± 4,100 cells per well; 100 nm PACAP, 293,000 ± 3,400 cells per well), and no increase in cell number of untreated cells 48 and 72 hr after initial plating, confirming the lack of proliferative activity of bovine chromaffin cells in the presence of cytosine arabinofuranoside in these experiments. Thus changes in VIP content reflect increased VIP peptide or mRNA per chromaffin cell, rather than an increase in cell number without a change in peptide or peptide-encoding mRNA content per cell. Data are expressed here as levels per well (250,000–500,000 cells).
NBFL cell culture. A human neuroblastoma cell line, NBFL (Symes et al., 1997), was cultured in DMEM with 4.5 gm of glucose/l, containing 5% fetal bovine serum (heat inactivated) and 5% horse serum supplemented with glutamine (0.03%), penicillin (100 U/ml), and streptomycin (100 μg/ml). For mRNA analysis, cells were grown in six-well plates to ∼70% confluence and treated with vehicle (0.1% DMSO) or 100 nm PACAP for 6 hr.
Cloning of bovine VIP cDNA. Bovine VIP cDNA was cloned using total RNA isolated from bovine chromaffin cells. Total RNA was reverse transcribed using random hexamers as primers and then checked for quality by PCR using a bovine GAPDH primer pair. cDNA obtained by reverse transcription of mRNA was used as a PCR template to obtain the 168 bp product of bovine VIP cDNA, using primers corresponding to sequences within the human VIP cDNA sequence (forward primer, 5′-CATGCTGATGGAGTTTTCACCAGTGAC-3′; reverse primer, 5′-CTCAAGGTACTTTTTGGCAGAAAGTTGACCCAAGAG-3′). This PCR product (168 bp) was subcloned into the pCRII-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced. Rapid amplification of cDNA ends (RACE) (3′ and then 5′) was performed with the 168 bp bovine VIP cDNA fragment, reverse transcribed from bovine chromaffin cell total RNA, as prescribed by the manufacturer (Invitrogen). Final PCR products of 1.1 or 0.52 kb (by 3′ or 5′ RACE, respectively) were subcloned into pCRII-TOPO and sequenced. The sequences of bovine preproVIP/PHI and its cognate cDNA have been submitted to GenBank (accession numberAF503910).
Radioimmunoassay. VIP and met-enkephalin were assayed directly in aliquots of culture medium, or in lyophilized 0.1N HCl extracts of chromaffin cells, harvested 24–72 hr after exposure to various drugs, as described previously (Eiden and Hotchkiss, 1983).
cAMP was measured in chromaffin cells after 30 min or 6 hr exposure to PACAP, VIP, or forskolin, with the NEN [125I] RIA kit (NEN, Boston, MA). Lyophilized 0.1N HCl cell extracts were acetylated before addition of tracer and antibody solutions. Bound cAMP was precipitated by the addition of a secondary antibody and counted. Sensitivity of the assay is 7.8 fmol. cAMP in NBFL cells was assayed after 10 min exposure to PACAP, using 50 μl of 0.1N HCl cell extracts with the cAMP [3H] Assay System (Amersham, Piscataway, NJ). Unbound cAMP was precipitated by charcoal adsorption, and bound cAMP in the supernatant was counted. Sensitivity of the assay is 1 pmol.
Northern analysis of VIP mRNA. Northern blot analysis of VIP mRNA was performed with nick-translated human or bovine VIP cDNA probes with comparable results. Equal well-equivalents of total RNA were loaded per lane. Uniformity of total chromaffin cell RNA per lane was verified by staining of ribosomal (18 and 28 S) RNA with ethidium bromide after electrophoresis. Quantitation of VIP mRNA was performed by densitometric scanning of autoradiograms of each Northern blot.
Quantitative RT-PCR. Bovine chromaffin cells were grown in 24-well plates, and NBFL cells were grown in 6-well plates as described previously and harvested for total RNA using the RNAqueous kit essentially as prescribed by the manufacturer (Ambion, Austin, TX). DNA was removed by treatment with RNase-free DNase I, and complementary DNA was prepared with SuperScript First Strand Synthesis System for RT-PCR (Invitrogen). Real-time quantitative PCR (Q RT-PCR) (Gibson et al., 1996) was performed on cDNA obtained by reverse transcription of 0.2 μg of total RNA using the Taq-Man 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) using bVIP primers in bovine chromaffin cells (forward primer, 5′-TTGAGTCCCTTATTGGAAAACGA-3′; reverse primer, 5′-AGCATCTGAGTGGCGTTTGA-3′; probe, 6FAM-TTAGCAATAGCATCTCAGAAGACCAGGGACC-TAMRA) and hVIP primers in NBFL cells (forward primer, 5′-CCGCCTTAGAAAACAAATGGC-3′; reverse primer, 5′-CTAACTCTTCTGGAAAGTCGGGAG-3′; probe, 6FAM-ATTCTGAATGGAAAGAGGAGC-AGTGAGGGAG-TAMRA). Reactions contained 1× Mastermix (Applied Biosystems) containing preset formulations of dNTPs, MgCl2, and buffers, along with 90 nm forward and reverse primers and 20 nm probe. RNA levels were deduced by comparison of cDNA-generated signals in samples to signals generated by a standard curve constructed with known amounts of VIP cDNA, and internally corrected with the GAPDH cDNA signal for variations in amount of input mRNA.
Single cell [Ca2+]i measurement. Circular glass coverslips (Assistant) were coated overnight with poly-d-lysine (0.2 mg/ml in H2O). NBFL neuroblastoma cells or bovine chromaffin cells were seeded at 1–2 × 106 and 2–3 × 106 cells per coverslip, respectively. Twenty-four hours later, cells were washed once with Krebs'–Ringer's saline solution (KRB) containing (in mm): 125 NaCl, 5 KCl, 1 Na2HPO4, 1 MgSO4, 1 CaCl2, 5.5 glucose, 20 HEPES, pH 7.2. After washing, cells were loaded with 2 μm fura-2 AM (Molecular Probes, Eugene, OR) for 22 min under continuous gentle agitation. Loading was performed at room temperature to minimize dye penetration into organellar compartments (Roe et al., 1990). After loading, the cells were washed once with fresh KRB and kept for an additional 22 min in fura-2 AM-free KRB. Finally the coverslips were mounted in a custom-fabricated perfusion chamber and perfused with KRB at ∼800 μl/min, with drugs added to the perfusion buffer at the concentrations and times indicated in Table1 and Figure 5. Fluorescence intensity at 340 and 380 nm was measured every 2 sec, and their ratios were determined. Data were analyzed with the software MetaFluor (Universal Imaging) as described previously (Grimaldi et al., 1999). Experiments were performed at least three times on different cell preparations. Data shown represent mean values (±SEM) for all the cells studied.
Table 1.
Increase in cAMP, calcium, and VIP after treatment with increasing concentration of PACAP-27
| [PACAP-27]applied | cAMP, picomoles per well | [Ca2+]i (R/R), peak/plateau | VIP, picograms per well |
|---|---|---|---|
| Vehicle | 7.00 ± 0.57 | 0.75 ± 0.8 | 28 ± 2.8 |
| 0.1 nm | 6.87 ± 0.41 | n.d. | 30 ± 0.95 |
| 1 nm | 9.98 ± 0.06 | n.d. | 169 ± 10 |
| 10 nm | 22.40 ± 0.20 | 3.2 ± 0.6/2.0 ± 0.4 | 998 ± 60 |
| 100 nm | 22.22 ± 1.12 | 14.3 ± 1.1/5.9 ± 0.4 | 1259 ± 213 |
| 1000 nm | 21.54 ± 0.44 | n.d. | 1115 ± 124 |
PACAP-27 applied to bovine chromaffin cells stimulates intracellular cAMP, calcium, and VIP concentrations. cAMP values were obtained after 30 min exposure to PACAP. In comparison with PACAP, cAMP levels measured after a comparable exposure to VIP ranged from 5.85 ± 0.23 pmol per well at 0.1 nm VIP to 6.9 ± 0.50 pmol per well at 100 nm VIP. cAMP was increased by 25 μm forskolin to 41.85 ± 0.91 pmol per well. Calcium values given are the 340/380 nm ratio of the calcium spike, over the 340/380 nm ratio of the calcium plateau (peak/plateau). n.d., Not determined. VIP levels in cells and culture medium were measured as described in Materials and Methods, after 72 hr exposure to PACAP.
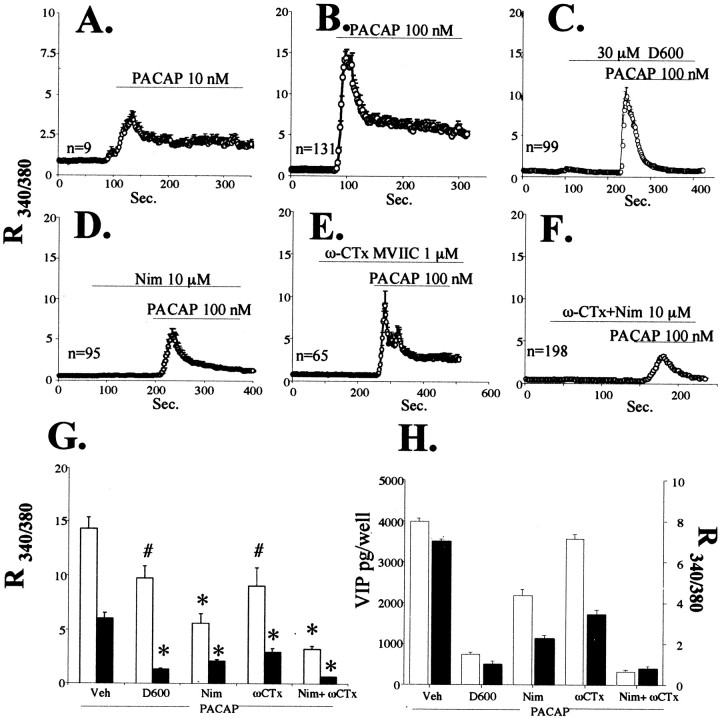
Fig. 5.
Effects of L- and P/N/Q-type voltage-sensitive calcium channel blockade on PACAP-induced calcium elevation and VIP peptide elevation.A–F, Elevation in intracellular calcium elicited by 10 or 100 nm PACAP in the presence of D600, nimodipine, ω-conotoxin MVIIC, or combined ω-conotoxin and nimodipine. Drugs were added as indicated to the perfusion chamber over glass-coverslipped bovine chromaffin cells (as described in Materials and Methods) at 10 or 100 nm PACAP-27, 30 μmD600, 10 μm nimodipine (Nim), or 1 μm ω-conotoxin MVIIC (ω-CTx).G, Comparison of peak versus plateau calcium ratios of treatments in B–F. Data represent means of peaks (white bars) or plateaus (black bars). All comparisons are relative to PACAP alone. *Different from PACAP; p < 0.0001; # different from PACAP; p < 0.01. H, Comparison between inhibition of VIP induction, and inhibition of the plateau phase of calcium elevation, by calcium channel blockade. VIP induction (picograms per well) is shown with white bars, and cytosolic calcium levels during the plateau phase (as the R340/380 ratio, taken from data depicted inA–E) are shown as black bars.
Transient expression assays. Differentially plated chromaffin cells were plated onto poly-d-lysine-coated 12-well dishes at 1 × 106 cells per well in 2 ml of medium and transfected by calcium phosphate/DNA coprecipitation using the PromegaProfection Mammalian Transfection System-Calcium Phosphate according to the manufacturer's instructions (Promega, Madison, WI) and as described previously (Anouar and Eiden, 1995). Using the PathDetect CREB Trans-Reporting System, (Stratagene, La Jolla, CA), 1.0 μg of reporter plasmid (pFR-Luc) and 50 ng of transactivator plasmid (pFA2-CREB) were cotransfected with 50 ng of positive control plasmid (pFC-PKA) or negative control plasmid (pFC2-dbd), to maintain input DNA concentration constant in all transfections. DNA was added to 10 μl of 2 m calcium chloride and adjusted to 80 μl with water. This solution was added to 80 μl of 2× HEPES-buffered saline drop-wise with gentle vortexing. The final mixture was incubated at room temperature for 30 min, vortexed again, and added drop-wise to each well, which contained 0.5 ml of complete fresh medium, with or without 10 μm H89. After overnight incubation at 37°C in 5% CO2/air, the medium was removed. Cells were washed twice, further incubated with or without 10 μm H89, and harvested 36 hr later for measurement of luciferase activity.
NBFL cells were plated in 12-well dishes at 4 × 105 cells per well in 1 ml of medium and allowed to grow to 70% confluence (∼48 hr) and transfected with 0.5 μg of VIP reporter and extracellular signal-regulated protein kinase (ERK)1/2 dominant negative (dn) DNA and 1.5 μl of FuGENE6 per well (Roche, Indianapolis, IN). Cells were treated with PACAP-27 (100 nm) 24 hr later and harvested for luciferase activity measurements 6 hr after that. Luciferase activity was determined from 20 μl of cell lysates with 50 μl of luciferase substrate (Promega) counted for 10 sec in a Berthold Lumat 9501 luminometer. VIP reporter gene constructs used are as previously described (Hahm and Eiden, 1998). dn ERK1 and ERK2 plasmids were a gift from Melanie Cobb (University of Texas Southwestern Medical Center) (Robbins et al., 1993).
Immunoblotting of phosphorylated p44/42 MAP kinase and total p44/42 MAP kinase. Immunoblotting was performed according to the protocol of Cell Signaling Technology (Beverly, MA). In brief, chromaffin cells were washed with 1× PBS, followed by addition of 300 μl of lysis buffer and 200 μl of 2× SDS sample buffer to each 10 cm dish (5 × 106 cells). Cell lysates were sonicated for 15 sec to shear DNA and reduce sample viscosity, heated at 95–100°C for 5 min, and centrifuged at 12,000 rpm for 10 min. The supernatant fraction (cell extract) was subjected to SDS-PAGE (1.5 hr at 125 V) on 14% Tris-glycine gels (Invitrogen/NOVEX, San Diego, CA) followed by transfer to polyvinylidene difluoride membranes (Invitrogen/NOVEX) by electroblotting for 1.5 hr at 25 V. Blots were incubated with a 1:1000 dilution of rabbit polyclonal antibody specific for phosphorylated p44/42 MAP kinase and total p44/42 MAP kinase (Cell Signaling Technology), followed by peroxidase-labeled anti-rabbit secondary antibody (1:4000 dilution). Immunoreactive bands were developed using an ECL kit (Western Blotting Analysis System, Amersham Biosciences, Buckinghamshire, UK).
Materials. PACAP-27 was purchased from Phoenix Pharmaceuticals (Mountain View, CA); ascomycin, cycloheximide, methoxyverapamil (D600), and phorbol myristate acetate (PMA) were from Sigma; and Gö6983, chelerythrine, nimodipine, ω-conotoxin MVIIC, forskolin, 1,9-dideoxyforksolin, U0126, U73122, isobutylmethylxanthine, dibutyryl cAMP, 8-bromocyclic AMP, and H89 were from Calbiochem (San Diego, CA).
Statistics. All data were analyzed using the superANOVA software package unless indicated otherwise. Data were analyzed by one way ANOVA with Scheffe's post hoc analysis. Significance was set at p < 0.05.
RESULTS
Candidate pathways known to be activated by PAC1 receptor occupancy, including calcium, cAMP, and protein kinase C (PKC) were examined for possible roles in PACAP signaling to the VIP gene. PACAP-27 caused dose-dependent increases in cAMP, cytosolic calcium, and VIP biosynthesis that were maximal by 100 nm (Table1). To confirm that the effects of PACAP-27 on bovine chromaffin cells occur through the PACAP-preferring PAC1 receptor and not the PACAP/VIP-preferring VPAC1 and/or VPAC2 receptors (Tanaka et al., 1998), the abilities of VIP and PACAP to elevate intracellular cAMP were compared. VIP caused no measurable increase in intracellular cAMP at concentrations up to 1 μm (Table 1, see legend).
The maximal effect of PACAP on VIP biosynthesis was compared with the effects of depolarization with KCl, cAMP elevation by forskolin, and stimulation of protein kinase C by PMA (Pruss et al., 1985; Siegel et al., 1985). VIP peptide levels were increased >20-fold within 24 hr of exposure to 100 nm PACAP and >30-fold at 72 hr. Only the protein kinase activator PMA, at a concentration that elicits maximal induction of neuropeptide biosynthesis, caused an elevation in VIP peptide levels comparable to PACAP-27, with maximal elevations by 72 hr of exposure (Fig. 1A). VIP mRNA was induced within 24 hr of exposure to 100 nm PACAP, PMA, KCl, or forskolin (Fig.1B). The extent of mRNA induction by PACAP was significantly greater than that for KCl or forskolin: >1000-fold as measured using Q RT-PCR (Fig. 1C).
Fig. 1.
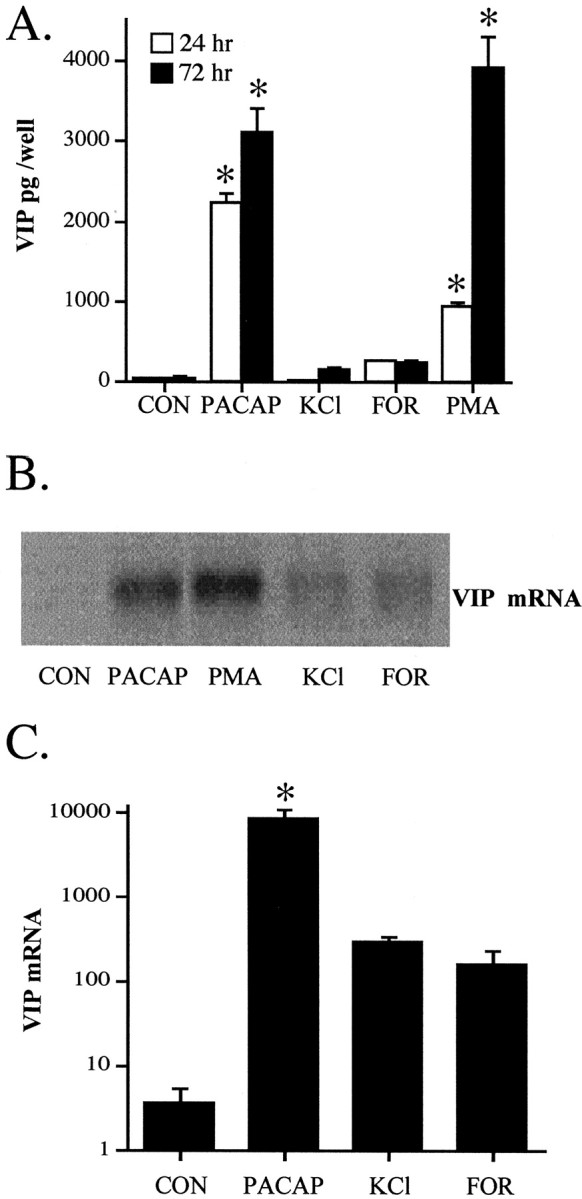
PACAP-27 upregulation of VIP peptide and mRNA levels in comparison to stimulation of protein kinase C, elevation of cAMP, and depolarization. A, VIP peptide upregulation by treatment with PACAP, PMA, KCl, or forskolin at 24 and 72 hr. Drug treatments were performed as described in Materials and Methods, with cells and medium harvested for VIP radioimmunoassay after 24 and 72 hr exposure to 100 nm PACAP, 100 nm PMA, 40 mm KCl, or 25 μm forskolin (FOR). Values represent the mean ± SEM of quadruplicate wells from a single cell dispersion, repeated at least once with similar results. *Different from control at corresponding time; p < 0.001; Scheffe's post hoc analysis. B, VIP mRNA levels after treatment with 100 nm PACAP, 100 nm PMA, 40 mm KCl, or 25 μm forskolin. Drug treatments were performed as described for peptide induction, except that cells were harvested for RNA isolation and Northern blotting as described in Materials and Methods 18 hr after drug addition. Equal well-equivalents of total RNA were added to each lane. The single experiment shown was repeated twice with similar results. C, Quantitative PCR for VIP mRNA after treatment with 100 nm PACAP, 40 mm KCl, or 25 μm forskolin. Cells were harvested for quantitative RT-PCR at 18–24 hr after treatment as described in Materials and Methods. Values represent the mean ± SEM of quadruplicate wells from a single cell dispersion, repeated at least once with similar results. *Different from control;p < 0.001; Scheffe's post hocanalysis.
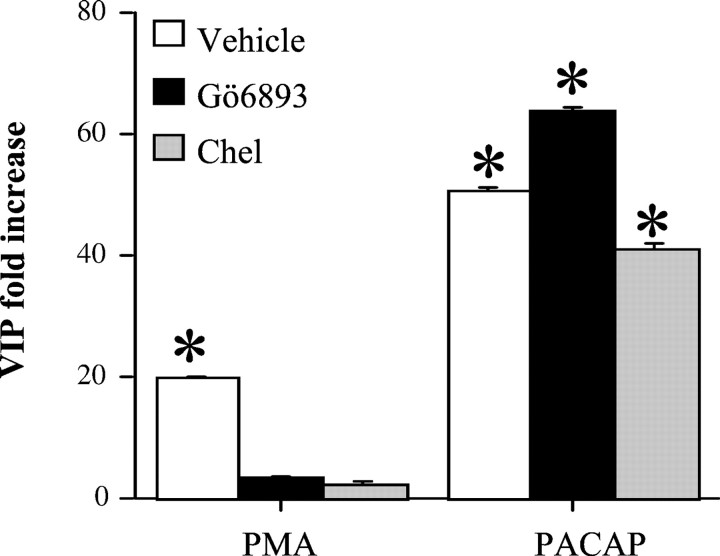
PAC1 receptor coupling to phospholipase C leads to increased liberation of DAG and increased levels of IP3 that in turn causes the release of calcium from intracellular stores in chromaffin cells (Isobe et al., 1993). Calcium and DAG together are known to activate several isoforms of PKC (Wilson, 1990; Ron and Kazanietz, 1999). Thus, activation of PKC could participate in PACAP signaling to the VIP gene, consistent with the similar magnitude of VIP mRNA and peptide elevation caused by PMA and PACAP (Fig. 1). Accordingly, VIP induction by PACAP and PMA were compared pharmacologically. PACAP induction of VIP peptide and mRNA is blocked by ascomycin, a calcineurin inhibitor (Lee et al., 1999). Ascomycin did not block VIP peptide or mRNA induction by PMA, and conversely the protein synthesis inhibitor cycloheximide blocked PMA but not PACAP induction of VIP mRNA (data not shown). Thus, the characteristics of PACAP and PMA induction of VIP were quite distinct and inconsistent with a role of PKC in PACAP signaling to the VIP gene. In addition, the protein kinase C inhibitors Gö6983 and chelerythrine blocked VIP peptide induction by PMA but not by PACAP (Fig. 2).
Fig. 2.
PACAP and PMA stimulation of VIP biosynthesis are differentially sensitive to blockade by inhibitors of protein kinase C. Chromaffin cells were pretreated for 30 min with vehicle (0.1% DMSO), the protein kinase C inhibitors Gö6983 (1 μm), or chelerythrine (Chel; 5 μm), and additionally for 24 hr with vehicle, 100 nm PMA, or 100 nm PACAP-27 with or without the PKC inhibitors. Cells and mediums were harvested for VIP radioimmunoassay as described in Materials and Methods and expressed as the fold increase in VIP immunoreactivity per well compared with the corresponding control. Values represent the mean ± SEM of three individual wells from a single cell dispersion. *Different from corresponding control atp < 0.001; Scheffe's post hocanalysis. Control values were as follows: vehicle, 41 ± 2.8 pg per well; Gö6983, 24.2 ± pg per well; chelerythrine, 27.2 ± 3.0 pg per well.
Induction of VIP mRNA by PACAP could conceivably be dependent on activation of other downstream targets besides PKC, such as IP3 and DAG. To test this hypothesis, induction of VIP by PACAP-27 was measured in the presence and absence of 10 μmU73122, a specific phospholipase C inhibitor (Jin et al., 1994). U73122 had a slight stimulatory effect on VIP biosynthesis alone, but was without effect on the induction of VIP peptide by PACAP-27 (total VIP peptide in picograms per well: mean ± SEM;n = 3; 10 μmU73122 or vehicle added 30 min before addition of 10 nm PACAP and peptide harvested at 72 hr as described in Materials and Methods: 57 ± 1.4 control; 4162 ± 216 10 nmPACAP; 205 ± 6.5 10 μmU73122; 4644 ± 782 PACAP + U73122). Stimulation of met-enkephalin biosynthesis and release by histamine is well known to involve stimulation of phospholipase C, generation of IP3, and elevation of [Ca2+]i (Noble et al., 1986; Bommer et al., 1987; Stauderman and Pruss, 1990; Bunn and Boyd, 1992; Firestone and Browning, 1994). U73122 did block histamine-induced met-enkephalin peptide elevation (total met-enkephalin peptide in picograms per well: mean ± SEM;n = 3; 5804 ± 358, control; 11062 ± 335, 10 μm histamine; 5156 ± 187, 10 μmU73122; 7312 ± 382, 10 μm histamine + 10 μmU73122), demonstrating the efficacy of this compound to block chromaffin cell phospholipase C activation at the dose used.
Because PACAP increases both intracellular cAMP levels and [Ca2+]i in chromaffin cells, we examined whether the potencies of PACAP to elevate VIP peptide levels, cAMP, and [Ca2+]i were comparable. PACAP-27 increased both intracellular cAMP and VIP peptide levels similarly, with the maximal effect reached between 1 and 10 nm PACAP-27 (Table 1). In contrast, elevation of [Ca2+]i increased sharply between 10 and 100 nm PACAP-27, suggesting that the level of [Ca2+]ielevation elicited by 10 nm PACAP-27 is sufficient for VIP gene induction.
To explore the possibility that both signals, calcium and cAMP, might act synergistically on VIP biosynthesis induction, the effects of depolarization with KCl and the elevation of intracellular cAMP were compared alone and in combination. The phosphodiesterase inhibitor isobutylmethylxanthine, the cAMP analogs dibutyryl cAMP and 8-bromocyclic AMP, and the adenylate cyclase stimulator forskolin all showed a marked synergism with KCl in elevation of VIP peptide levels in chromaffin cells (Fig. 3A). Effects of forskolin not related to elevation of cAMP, including inhibition of glucose transport, enhancement of nicotinic receptor desensitization, inhibition of carbachol-mediated ion flux through nicotinic receptors, and modulation of voltage-dependent potassium channels are shared by its congener 1,9-dideoxyforskolin, which does not, however, stimulate adenylate cyclase (Laurenza et al., 1989). 1,9-dideoxyforskolin had no effect on VIP peptide levels alone or in combination with KCl (Fig. 3A). The dose-dependent induction of VIP by KCl, forskolin, and both agents in combination was further compared with the effect of PACAP. As shown in Figure 3B, KCl from 10 to 40 mm and forskolin from 0.25 to 25 μm each caused a dose-related increase in VIP peptide levels reaching a maximum that was <25% of the effect of 100 nm PACAP. Only the combined KCl and forskolin treatment gave a greater effect than either KCl or forskolin alone at all dose combinations tested, stimulating VIP levels comparable to the maximal effect of PACAP with 25 μm forskolin and 40 mm KCl (Fig. 3B).
Fig. 3.
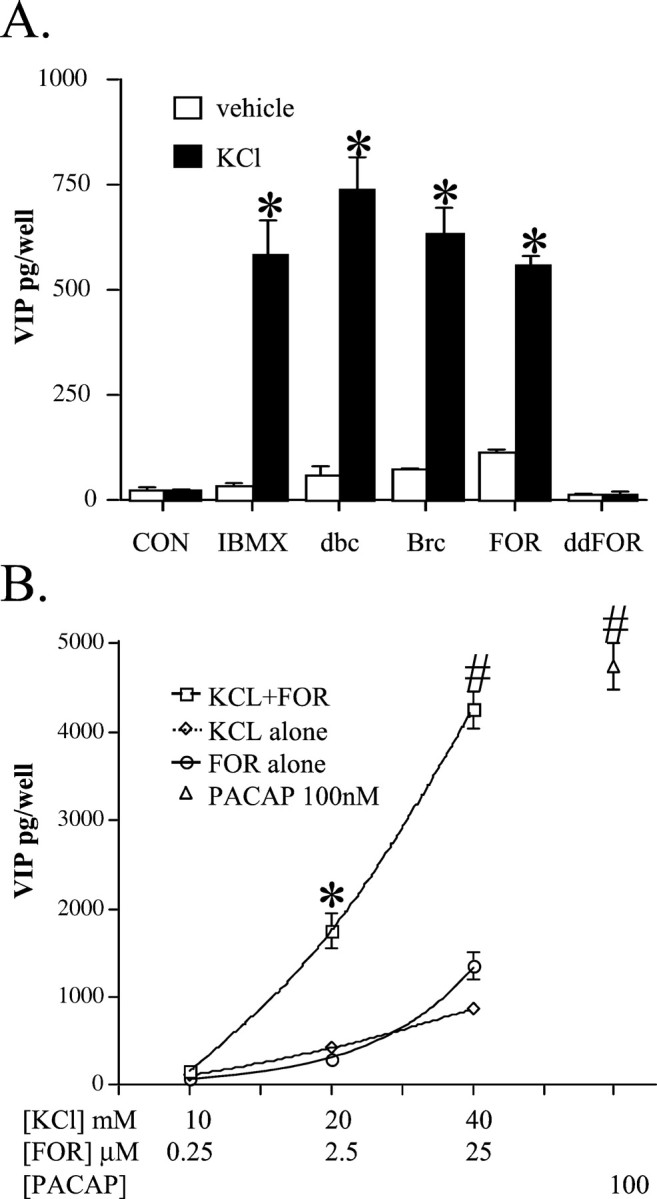
Combinatorial depolarization and cAMP elevation result in a synergistic increase in VIP biosynthesis comparable to that elicited by PACAP. A, Synergistic effects of 0.5 mm isobutylmethylxanthine (IBMX), 1 mm dibutyryl cAMP (dbc), 1 mm 8-bromocyclic AMP (Brc), or 25 μm forskolin (FOR) with 40 mm KCl on induction of VIP peptide levels. The phosphodiesterase inhibitor IBMX, cAMP analogs dbc and Brc, adenylate cyclase stimulator FOR, and non-cyclase-stimulating forskolin analog 1,9-dideoxyforskolin (ddFOR; 25 μm) were applied to cultured chromaffin cells with or without 40 mm KCl, and cells and mediums were harvested 24 hr later for peptide radioimmunoassay. Experiments were performed in triplicate. *Different from KCl; p < 0.001, Scheffe's post hoc analysis. B, Dose-dependent synergistic effects of 40 mm KCl and 25 μm forskolin (FOR) on VIP peptide levels. Experiments were performed as described above, but cells and mediums were harvested for VIP peptide radioimmunoassay 72 hr after addition of drugs. Values are the mean ± SEM of triplicate or quadruplicate wells from a single cell dispersion, repeated at least once with similar results at each dosage combination. The experiment was repeated once with similar results at each dose and at least four times at concentrations of KCl, forskolin, and PACAP of 40 mm, 25 μm, and 100 nm, respectively. ∗Different from 20 mm KCl or 2.5 μm FOR, p < 0.001; #Different from 40 mm KCl or 25 μm FOR, p < 0.001; Scheffe's post hoc analysis.
The effects of KCl plus forskolin on VIP biosynthesis and VIP mRNA were inhibited by blockade of VSCCs by D600 and by inhibition of calcineurin with ascomycin (Fig.4A,B). This was the same pharmacological profile exhibited by PACAP-27 (Lee et al., 1999). The effects of KCl alone were completely calcium influx dependent, and the effects of forskolin alone were essentially unaffected by blockade of calcium influx (Fig. 4C). It has been suggested that PACAP may act in a calcium-dependent manner in some neuroendocrine cell types via a cAMP-dependent increase in general cation channel conductance (Darvish and Russell, 1998). Blockade of the effect of forskolin by D600 would be predicted if this were the case in bovine chromaffin cells, but that was not observed here. In addition, if calcium acts downstream of PAC1 receptor stimulation solely by potentiating cAMP generation, any agent that increases cAMP to the extent that PACAP-27 does should elicit a comparable elevation in VIP peptide and mRNA levels. This is not the case: the increase in cAMP generation by 25 μm forskolin is greater than that elicited by PACAP-27 (Table 1, see Fig. 8), yet forskolin increased VIP peptide levels less than PACAP did (Fig.1B). Taken together, these data suggest that the synergistic effects of KCl and forskolin represent neither potentiation of KCl-induced calcium influx by elevation of cAMP nor calcium-dependent potentiation of cAMP elevation. The results imply that cAMP elevation and calcium influx occur independently after PAC1 receptor occupancy by PACAP-27 and exert their synergistic effects on VIP biosynthesis at a point downstream of second messenger generation.
Fig. 4.
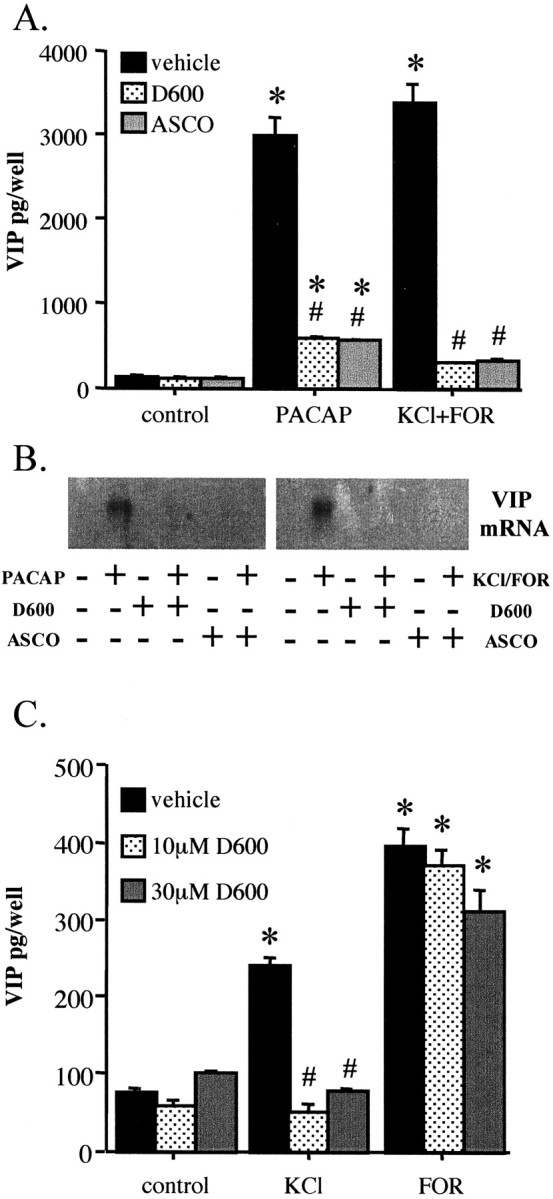
Comparison of pharmacological characteristics of VIP induction by PACAP and the combination of KCl and forskolin.A, D600 and ascomycin block VIP peptide induction by PACAP and combined KCl and forskolin. Chromaffin cells were pretreated for 30 min with 30 μm D600 or 0.1 μmascomycin (ASCO), followed by treatment with 100 nm PACAP-27 or a combination of 40 mm KCl and 25 μm forskolin (FOR). Vehicle (0.1% ethanol in culture medium) was identical for all conditions. Cells were harvested 72 hr later for VIP radioimmunoassay as described in Materials and Methods. *Different from corresponding control,p < 0.0001; #different from corresponding treatment without inhibitor, p < 0.0001; Scheffe's post hoc analysis. B, D600 and ascomycin block VIP mRNA induction by PACAP and combined KCl and forskolin. Experimental conditions are as described forA, except that RNA extracts were prepared 18 hr after addition of PACAP or KCl plus forskolin, as described in Materials and Methods. C, Effect of D600 on induction of VIP by forskolin and KCl. VIP peptide levels were measured 72 hr after addition of 25 μm forskolin or 40 mm KCl. Either 10 or 30 μm D600 or vehicle was added 30 min before addition of forskolin, KCl, or vehicle. *Different from corresponding control; p < 0.0001;# different from corresponding treatment without inhibitor; p < 0.0001; Scheffe's post hoc analysis.
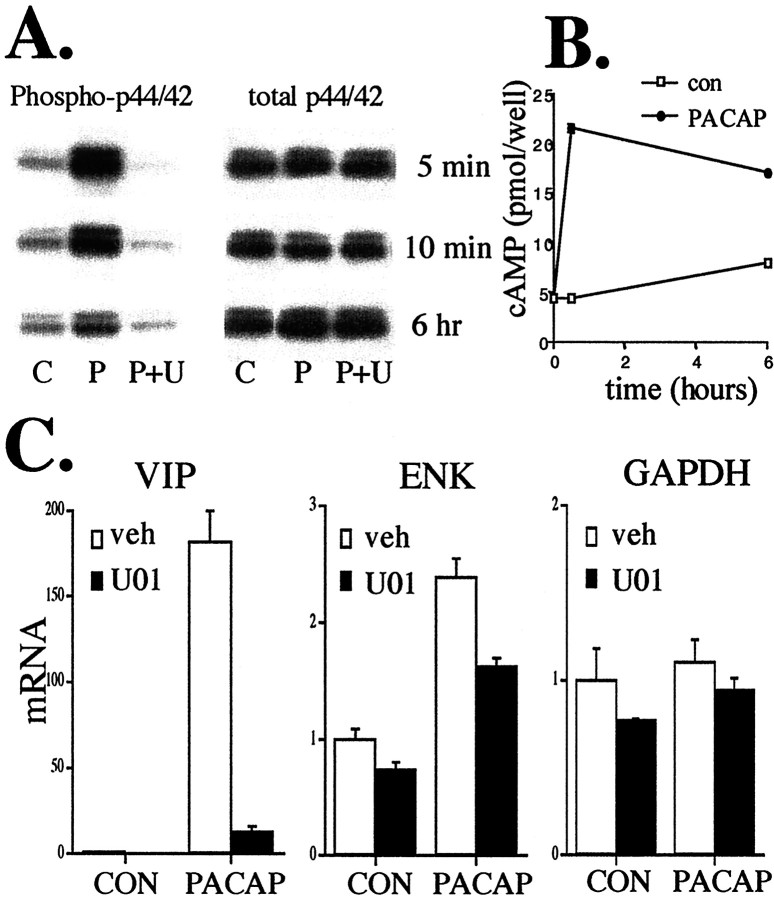
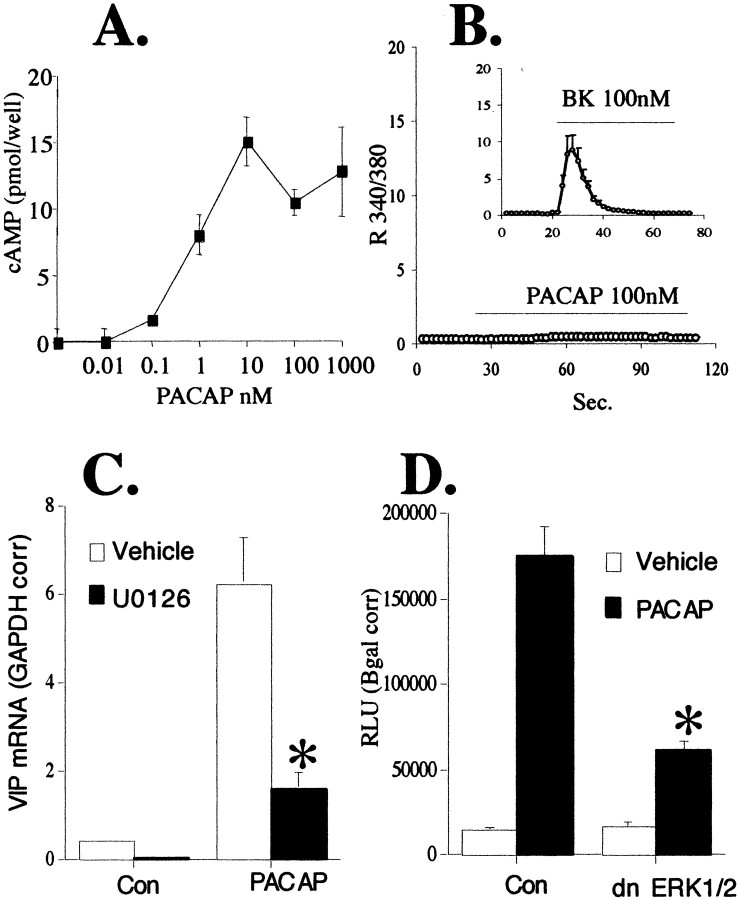
Fig. 8.
PACAP rapidly phosphorylates ERK, increases cAMP, and elevates VIP mRNA. A, PACAP (100 nm) phosphorylates ERK1/2. The ability of PACAP to phosphorylate ERK1/2 continues for up to 6 hr of PACAP exposure. By 24 hr, PACAP stimulatory effects are gone. Upregulation of phospho-ERK by PACAP (P) is completely inhibited by 30 μm U0126 (U) at all time points, whereas total ERK levels are unaffected. C, Control-treated cells. B, PACAP elevates cAMP for 6 hr. Cells were treated as described in Materials and Methods and harvested for cAMP measurements at 0 min, 30 min, and 6 hr with or without 100 nm PACAP. C, PACAP stimulation of VIP at 6 hr is blocked by U0126. Cells were treated with the MEK inhibitor 30 μm U0126 (U01) or vehicle (0.1% ethanol in culture medium) 30 min before and during exposure to 100 nm PACAP and harvested for RNA and Q RT-PCR as described in Figure7A. Values are the mean ± SEM of three separate wells from a single chromaffin cell dispersion. Values represent uncorrected data demonstrating specificity of U0126 for VIP blockade compared with ENK and GAPDH.
KCl-mediated induction of VIP and met-enkephalin biosynthesis is completely blocked by L-type calcium channel blockers. We examined the effects of L-type calcium channel blockade on induction of VIP biosynthesis by PACAP. Surprisingly, the L-type calcium channel blockers nifedipine, (−)-202–791, and nimodipine had at best only a small effect on VIP induction by 10 nm PACAP, whereas VIP peptide elevation by 40 mm KCl was completely blocked by L-type channel inhibitors as reported previously (Siegel et al.) (data not shown).
We next examined the effect of PACAP on [Ca2+]i directly. PACAP-27 showed a dose-dependent elevation of cytosolic calcium levels as measured with the calcium-sensitive dye fura-2 AM (Fig.5A,B). The effects of various calcium channel blockers on increases in [Ca2+]i in response to PACAP were compared with their ability to block PACAP-stimulated elevation of VIP peptide levels. The calcium channel blockers that were used included D600, which blocks all voltage-dependent calcium channels; nimodipine, which blocks L-type calcium channels; and ω-conotoxin MVIIC, which at 10 μm blocks P-, N-, and Q-type VSCCs (Tanaka et al., 1996, 1998; Hahm et al., 1998). PACAP-induced calcium elevation was dose dependent and characterized by a spike and a plateau phase, as reported previously by Kanno and coworkers (Tanaka et al., 1996, 1998). The plateau was maintained throughout the period of PACAP application (Fig. 5A,B). D600 abolished the plateau and reduced the spike phase of the PACAP-induced [Ca2+]i elevation <40% (Fig. 5C). Application of either nimodipine (Fig.5D) or ω-conotoxin MVIIC (Fig. 5E) substantially reduced PACAP-induced [Ca2+]i elevation, but each to a significantly lesser extent than D600 (Fig.5C). Combined exposure of both nimodipine and ω-conotoxin was required to further reduce the spike and completely inhibit the plateau (Fig. 5F). The effects of voltage-sensitive calcium channel blockers on the plateau and spike values for cytosolic calcium elevation by PACAP-27 are summarized in Figure 5G. VIP peptide elevation by PACAP was measured under the five conditions in which PACAP-induced [Ca2+]i elevation was examined and compared with [Ca2+]i measured during the plateau phase (Fig. 5H). The magnitude of cytoplasmic calcium elevation during the plateau phase paralleled the increase in VIP peptide levels. In particular, low but measurable residual calcium elevation during the plateau phase after application of PACAP-27 in the presence of nimodipine or ω-conotoxin alone was accompanied by elevation of VIP peptide levels. This calcium elevation was eliminated after exposure of chromaffin cells to PACAP-27 in the presence of nimodipine and ω-conotoxin together. The increase in VIP peptide levels seen after exposure to PACAP-27 was blocked down to the level seen with forskolin stimulation when both nimodipine and ω-conotoxin were applied (Fig. 5H). Thus, the entry of calcium through either L or P/N/Q voltage-sensitive calcium channels, but not both, during the plateau phase appears to be required for VIP peptide elevation by PACAP.
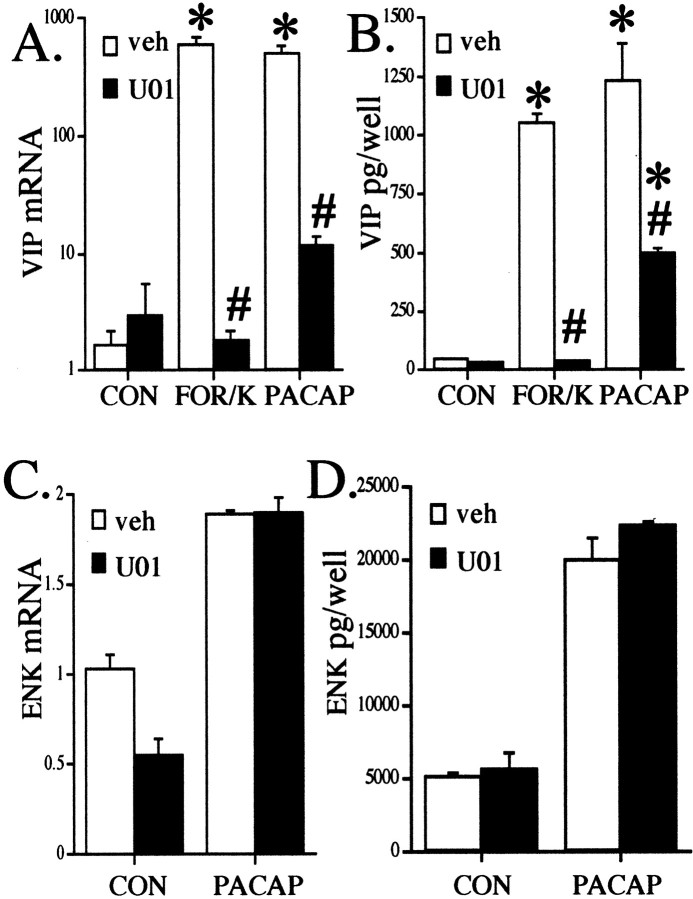
The failure of D600 to block the effect of forskolin on VIP peptide levels (Fig. 4C), as well as the residual induction of VIP biosynthesis by PACAP under conditions in which calcium influx is completely abolished (Fig. 5H), argues for a cAMP-dependent component of the action of PACAP that synergizes with calcium influx but does not contribute to it. To characterize the cAMP-dependent component of the action of PACAP, we examined the effect of the PKA inhibitor H89. To our surprise, H89 failed to inhibit the action of PACAP, suggesting a cAMP-dependent, PKA-independent pathway in chromaffin cells (Fig.6A). Consistent with the presence of such a pathway, the effect of 25 μm forskolin on VIP peptide induction was also not blocked by 10 μm H89 (data not shown). The efficacy of H89 as a PKA inhibitor in chromaffin cells was confirmed by transfecting a reporter gene responsive to Gal4/CREB fusion protein transactivation, vectors expressing a Gal4/CREB fusion protein, and the catalytic subunit of PKA, into chromaffin cells. A strong (>16-fold) PKA-dependent activation of the Gal4/CREB-responsive gene was blocked by H89, demonstrating its efficacy as a PKA inhibitor in chromaffin cells (Fig. 6B). In contrast to H89, the MEK1 inhibitor U0126 blocked the effects of both forskolin plus KCl and PACAP-27 on VIP peptide (Fig.7A) and VIP mRNA levels (Fig. 7B). To demonstrate the specificity of U0126 blockade on PACAP-stimulated VIP gene expression, we also examined the effects of U0126 on PACAP-stimulated enkephalin gene expression. U0126 did not inhibit the elevation of either enkephalin mRNA or peptide levels by PACAP (Fig. 7C,D). These data suggest that the PKA-independent elevation of VIP levels induced by both forskolin and PACAP are dependent on a MAP kinase signaling pathway that includes the MAP kinase kinase MEK1.
Fig. 6.
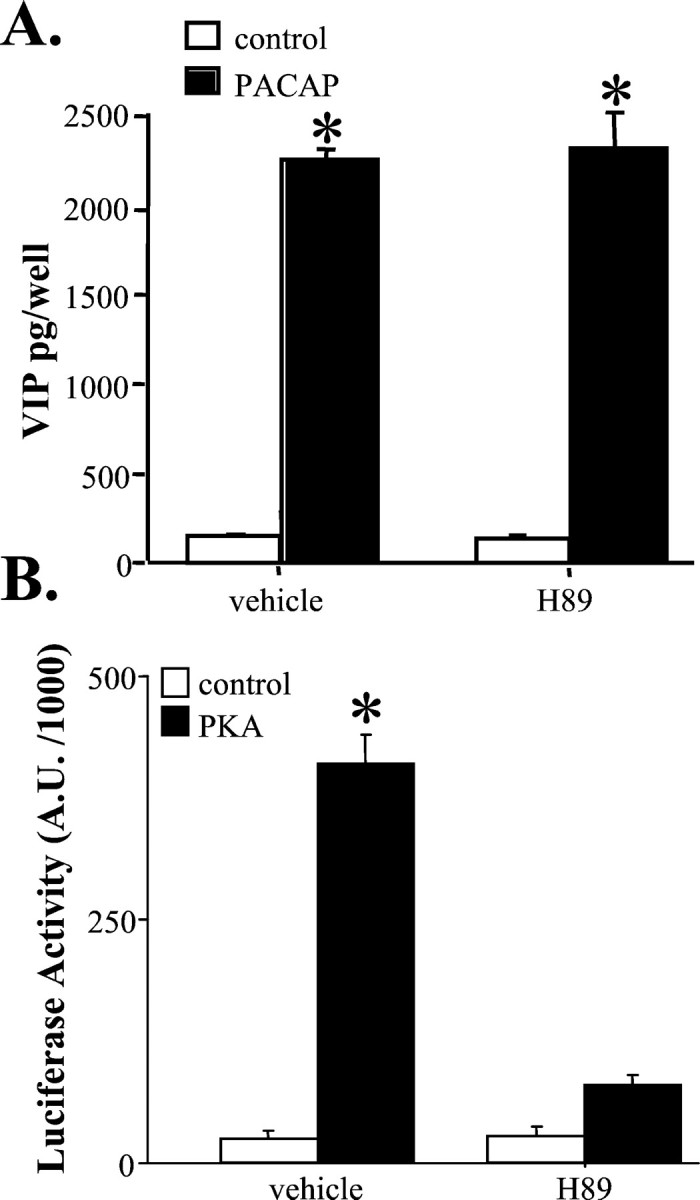
Stimulation of VIP biosynthesis by 100 nm PACAP and forskolin does not involve protein kinase A activation. A, H89 does not inhibit PACAP induction of VIP. Chromaffin cells were pretreated for 30 min with 10 μm H89 followed by treatment with 10 nmPACAP-27. Vehicle (0.1% ethanol in culture medium) was identical for all conditions. Cells were harvested 72 hr later for VIP radioimmunoassay as described in Materials and Methods. *Different from vehicle; p < 0.0001; Scheffe's post hoc analysis. B, H89 inhibits PKA activity in chromaffin cells. Chromaffin cells were cotransfected with pFR-Luc (reporter), pFA2-CREB, and pFC-PKA (PKA) or pFC2-dbl (control) in the presence or absence of 10 μmH89 as described in Materials and Methods. Cells were harvested 36 hr later for measurement of luciferase activity. *Different from PKA/H89;p < 0.0001; Scheffe's post hocanalysis.
Fig. 7.
The MEK inhibitior U0126 blocks forskolin + KCl and PACAP elevation of VIP peptide and VIP mRNA, but not enkephalin.A, Blockade of VIP mRNA induction by forskolin + KCl or PACAP by U0126. Cells were treated with the MEK inhibitor U0126 (30 μm, U01) or vehicle (0.1% ethanol in culture medium) 30 min before and during exposure to 25 μm forskolin + 40 mm KCl (FOR/K) or 100 nmPACAP and harvested for RNA, preparation of cDNA, and Q RT-PCR as described in Materials and Methods 18 hr later. Values are the mean ± SEM of three separate wells from a single chromaffin cell dispersion, corrected for total GAPDH signal per well. *Different from corresponding control; p < 0.001;# different from corresponding treatment without inhibitor; p < 0.001; Scheffe's post hoc analysis. B, Blockade of VIP peptide induction by forskolin + KCl or PACAP by U0126. Experiments were performed as described in A, except that chromaffin cells and mediums were harvested for VIP radioimmunoassay 72 hr after addition of vehicle, forskolin + KCl, or PACAP. *Different from corresponding control; p < 0.01;# different from corresponding treatment without inhibitor; p < 0.001; Scheffe's post hoc analysis. C, No blockade of enkephalin (ENK) mRNA induction by PACAP with U0126. Cells were treated with the MEK inhibitor U0126 (30 μm) or vehicle (0.1% ethanol in culture medium) 30 min before and during exposure to 100 nm PACAP and harvested for RNA, preparation of cDNA, and Q RT-PCR as described inA. Values are the mean ± SEM of three separate wells from a single chromaffin cell dispersion, corrected for total GAPDH signal per well. D, No blockade of ENK peptide induction by PACAP by U0126. Experiments were performed as described inA, except that chromaffin cells and mediums were harvested for ENK radioimmunoassay 72 hr after addition of vehicle or PACAP.
The ability of PACAP to phosphorylate ERK1/2 in bovine chromaffin cells was examined. ERK1/2 phosphorylation occurred very quickly in response to PACAP. Five minutes after treatment, chromaffin cells showed a dramatic increase in ERK1/2 phosphorylation that persisted for 6 hr. U0126 inhibited ERK1/2 phosphorylation by PACAP without affecting total ERK1/2 levels (Fig.8A). Because the cAMP signaling pathway in these cells appears not to proceed through PKA but rather through ERK1/2, we next examined the ability of PACAP to stimulate cAMP elevations at these same times. As shown in Figure8B, PACAP not only doubled intracellular cAMP levels rapidly, but elevation persisted for at least 6 hr. PACAP induction of VIP mRNA in these cells also occurred within 6 hr and was inhibited by the ERK1/2 inhibitor U0126 (Fig. 8C). In contrast, U0126 had minor effects on enkephalin mRNA after these treatments and no effects on total GAPDH mRNA levels.
NBFL neuroblastoma cell cultures were used to examine in further detail the cAMP component of PACAP signaling to the VIP gene. In these cells, PACAP stimulated VIP mRNA transcription via elevation of cAMP levels (Fig. 9A) without an increase in intracellular calcium (Fig. 9B). This lack of the calcium component of PACAP signaling accounts for the observation that VIP mRNA upregulation by forskolin was of similar magnitude to that induced by PACAP (data not shown). As in bovine chromaffin cells, PACAP signaling to the VIP gene in NBFL cells is sensitive to MAPK inhibition. U0126 significantly inhibited the effects of PACAP (Fig. 9C) on VIP mRNA levels. Transient transfections of a VIP luciferase reporter gene construct along with dominant negative ERK1 and ERK2 plasmids confirmed that ERK is used in PACAP signaling to the VIP gene (Fig.9D).
Fig. 9.
PACAP stimulates VIP mRNA elevation in NBFL cells via a MAPK pathway. A, PACAP increases cAMP levels in NBFL cells. NBFL cells were plated in 12-well dishes at 4 × 105 cells per well and allowed to grow to 70% confluency. Cells were harvested 10 min after exposure to increasing concentrations of PACAP-27 from 0.01–1000 nm, and cAMP was measured as described in Materials and Methods. B, PACAP does not increase cytosolic calcium in NBFL cells, whereas bradykinin does. PACAP 100 nm or bradykinin (BK) 100 nm were added to the perfusion chamber over glass coverslipped NBFL cells as described in Materials and Methods. Values shown represent the 340/380 nm ratios. C, PACAP stimulation of VIP mRNA in NBFL cells through a MAPK pathway. NBFL cells grown in six-well plates were harvested for VIP mRNA analysis 6 hr after the addition of 100 nm PACAP-27 with or without 30 μm U0126. Q RT-PCR was performed as described in Materials and Methods. Analysis was performed on 0.2 μg of total RNA and corrected for differences in initial RNA input by dividing by the GAPDH concentrations in the same samples. *Different from vehicle-treated PACAP; p < 0.05. D, dn ERKs inhibit PACAP stimulation of VIP mRNA. NBFL cells were transiently transfected in 12-well plates as described in Materials and Methods. Six hours after the addition of 100 nm PACAP, cells were harvested for luciferase activity. Values are corrected for transfection efficiency by normalizing for β-galactosidase gene expression. *Different from control transfected PACAP-treated cells;p < 0.05.
DISCUSSION
PACAP is an important trans-synaptic regulator of adrenomedullary catecholamine and neuropeptide biosynthesis (Malhotra et al., 1988;Wakade, 1988, 1998; Isobe et al., 1993; Rius et al., 1994; Babinski et al., 1996; Haycock, 1996; Przywara et al., 1996; Tanaka et al., 1996;Barrie et al., 1997; Tönshoff et al., 1997; Beaudet et al., 1998;Lamouche et al., 1999; Inoue et al., 2000). Its protean effects on second-messenger systems, including cAMP, calcium, and phospholipase C, potentially implicate a plethora of signal transduction pathways in the effects of PACAP on neuron-specific gene transcription. However, these multiple effects have generally been associated with single transcriptional elements within individual target genes (Schadlow et al., 1992; Monnier and Loeffler, 1998). It has not previously been apparent that PACAP-associated signal transduction pathways might actually converge on a single target gene to produce a combinatorial response unique to signaling by PACAP. Here we have demonstrated such convergence for the action of PACAP on the VIP gene. Full PACAP signaling to the VIP gene requires the simultaneous activation of calcium influx through voltage-sensitive channels and the elevation of cAMP. The latter action is mediated through a novel, PKA-independent pathway in chromaffin cells.
Combinatorial or synergistic regulation of transcription by PACAP may be unique to a group of genes including the prepro-VIP/PHI gene, compared with transcriptional regulation of other neuroendocrine genes by PACAP. For example, PACAP regulation of preproenkephalin transcription is not dependent on calcium influx, although the preproenkephalin gene does contain a cAMP response element (CRE) that may function as a calcium-responsive element (Van Nguyen et al., 1990; Hahm et al., 1998). Synergistic effects of PACAP may require that the target gene possess separate yet interacting response domains for calcium and cAMP. This appears to be the case for the VIP gene: the proximal CRE is required for cAMP-initiated VIP gene transcription in NBFL cells, whereas the CRE together with upstream sequences within the distal tissue-specifier element (TSE) are needed for calcium-initiated transcription from the VIP promoter (C. Hamelink, unpublished observations).
The actions of PACAP on VIP gene expression in primary, fully differentiated chromaffin cells contrast with PACAP signal transduction and gene regulation in PC12 pheochromocytoma cells, in which the actions of PACAP have also been studied extensively. PACAP elevates both calcium influx and cAMP in PC12 cells, as in chromaffin cells (Barrie et al., 1997). However, PACAP stimulates gene transcription of the neurosecretory protein chromogranin A in PC12 cells through a mechanism that does not require calcium influx and proceeds via a cAMP and PKA-dependent pathway (Taupenot et al., 1998). The neuroprotective actions of PACAP after ischemic insult to the brain, on the other hand, appear to involve the activation of the MAP kinase ERK and concomitant inhibition of other MAP kinases, including JNK/SAPK and p38 (Shioda et al., 1998). Second messenger actions of PACAP in the suprachiasmatic nucleus have been demonstrated to include both elevation of cAMP and calcium influx, although these studies did not report PACAP actions on either downstream messengers or target genes in these neurons (Kopp et al., 1999). These observations, and our own, highlight the various mechanisms whereby PACAP can regulate target gene expression at synapses. It will be important to explore the involvement of dual signaling pathways in the actions of PACAP in each of these systems, which correspond to the important roles that neuronal PACAP plays in regulating circadian rhythmicity, cell division during development, and neuroprotection after ischemia (Waschek et al., 1998;Reglödi et al., 2000; Shen et al., 2000).
Our results with PACAP in chromaffin cells also have several implications for understanding the unique properties of neuropeptide signaling between neurons and at neuroeffector junctions in the peripheral nervous system, especially for peptides whose receptors are coupled to both calcium mobilization and cAMP elevation (Sun et al., 1994; Mohney and Zigmond, 1998; Beaudet et al., 2000). Specification of neuronal phenotype, e.g., tyrosine hydroxylase gene regulation, has been shown to depend on induction of specific transactivating proteins in the context of cAMP elevation during sympathetic nervous system development (Lo et al., 1999). The ability of PACAP signaling via cAMP to proceed via both PKA-dependent and -independent pathways may provide a switch that is used dynamically during development to allow PACAP signaling to relevant genes to persist as third messenger pathways develop in maturing neuronal sublineages. The synergistic action of calcium and cAMP would be expected to occur whether cAMP elevation triggers PKA-dependent or -independent signaling pathways, and whether calcium elevation results from calcium influx or intracellular calcium mobilization. The ability of PACAP to initiate combinatorial signaling through multiple sets of calcium/cAMP stimulation pathways may provide a mechanism for PACAP signaling to reach critical target genes under conditions in which voltage-dependent calcium influx is absent, as in early neuronal progenitors (Maric et al., 2000), and to support transcription of the same genes in mature neurons through VSCC coupling (Ghosh et al., 1994; Bito et al., 1996; Ginty, 1997). The same situation may occur for diverse cell types of the mature nervous system, in which either PKA-dependent or -independent cAMP-initiated signaling predominates.
Footnotes
We thank Chang-Mei Hsu for expert technical assistance with bovine chromaffin cell culture, neuropeptide radioimmunoassay, and mRNA quantitation. We thank Marty Zatz, Ted Usdin, David Vaudry, and W. Scott Young for their comments and suggestions.
Correspondence should be addressed to Lee Eiden, Building 36, Room 2A-11, 9000 Rockville Pike, Bethesda, MD 20892. E-mail:eiden@codon.nih.gov.
REFERENCES
- 1.Anouar Y, Eiden LE. Rapid and long-lasting increase in galanin mRNA levels in rat adrenal medulla following insulin-induced reflex splanchnic nerve stimulation. Neuroendocrinology. 1995;62:611–618. doi: 10.1159/000127057. [DOI] [PubMed] [Google Scholar]
- 2.Babinski K, Bodart V, Roy M, De Léan A, Ong H. Pituitary adenylate-cyclase activating polypeptide (PACAP) evokes long-lasting secretion and de novo biosynthesis of bovine adrenal medullary neuropeptides. Neuropeptides. 1996;30:572–582. doi: 10.1016/s0143-4179(96)90041-4. [DOI] [PubMed] [Google Scholar]
- 3.Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM. Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a ras-independent, mitogen-activated protein kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 1997;272:19666–19671. doi: 10.1074/jbc.272.32.19666. [DOI] [PubMed] [Google Scholar]
- 4.Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- 5.Beaudet MM, Parsons RL, Braas KM, May V. Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci. 2000;20:7353–7361. doi: 10.1523/JNEUROSCI.20-19-07353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 7.Bloom SR, Edwards AV, Jones CT. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol (Lond) 1988;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bommer M, Liebisch D, Kley N, Herz A, Noble E. Histamine affects release and biosynthesis of opioid peptides primarily via H1-receptors in bovine chromaffin cells. J Neurochem. 1987;49:1688–1696. doi: 10.1111/j.1471-4159.1987.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 9.Bornstein SR, Haidan A, Ehrhart-Bornstein M. Cellular communication in the neuroadrenocortical axis: role of vasoactive intestinal polypeptide (VIP). Endocrine Res. 1996;22:819–829. doi: 10.1080/07435809609043781. [DOI] [PubMed] [Google Scholar]
- 10.Bunn SJ, Boyd TL. Characterization of histamine-induced catecholamine secretion from bovine adrenal medullary chromaffin cells. J Neurochem. 1992;58:1602–1610. doi: 10.1111/j.1471-4159.1992.tb10031.x. [DOI] [PubMed] [Google Scholar]
- 11.Comb M, Hyman SE, Goodman HM. Mechanisms of trans-synaptic regulation of gene expression. Trends Neurosci. 1987;10:473–478. [Google Scholar]
- 12.Darvish N, Russell JT. Neurotransmitter-induced novel modulation of a nonselective cation channel by cAMP-dependent mechanisms in rat pineal cells. J Neurophysiol. 1998;79:2546–2556. doi: 10.1152/jn.1998.79.5.2546. [DOI] [PubMed] [Google Scholar]
- 13.Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas WW, Rubin RP. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol (Lond) 1961;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dun NJ, Tang H, Dun SL, Huang R, Dun EC, Wakade AR. Pituitary adenylate cyclase activating polypeptide-immunoreactive sensory neurons innervate rat adrenal medulla. Brain Res. 1996;716:11–21. doi: 10.1016/0006-8993(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 16.Edwards AV, Jones CT. Adrenal cortical and medullary responses to acetylcholine and vasoactive intestinal peptide in conscious calves. J Physiol (Lond) 1993;468:515–527. doi: 10.1113/jphysiol.1993.sp019785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrhart-Bornstein M, Haidan A, Alesci S, Bornstein SR. Neurotransmitters and neuropeptides in the differential regulation of steroidogenesis in adrenocortical-chromaffin cultures. Endocrine Res. 2000;26:833–842. doi: 10.3109/07435800009048606. [DOI] [PubMed] [Google Scholar]
- 18.Eiden LE, Hotchkiss AJ. Cyclic adenosine monophosphate regulates vasoactive intestinal polypeptide and enkephalin biosynthesis in cultured bovine chromaffin cells. Neuropeptides. 1983;4:1–9. doi: 10.1016/0143-4179(83)90002-1. [DOI] [PubMed] [Google Scholar]
- 19.Eiden LE, Giraud P, Affolter H-U, Herbert E, Hotchkiss AJ. Alternate modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc Natl Acad Sci USA. 1984a;81:3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eiden LE, Giraud P, Dave J, Hotchkiss JA, Affolter H-U. Nicotinic receptor stimulation activates both enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984b;312:661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- 21.Eiden LE, Anouar Y, Hsu C-M, MacArthur L, Hahm SH. Transcription regulation coupled to calcium and protein kinase signaling systems through TRE- and CRE-like sequences in neuropeptide genes. Adv Pharmacol. 1998;42:264–269. doi: 10.1016/s1054-3589(08)60744-9. [DOI] [PubMed] [Google Scholar]
- 22.Fenwick EM, Fajdiga PB, Howe NBS, Livett BG. Functional and morphological characterization of isolated bovine adrenal medullary cells. J Cell Biol. 1978;76:12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firestone JA, Browning MD. Calcium signalling in bovine adrenal chromaffin cells: additive effects of histamine and nicotine. Synapse. 1994;17:268–274. doi: 10.1002/syn.890170407. [DOI] [PubMed] [Google Scholar]
- 24.Fischer-Colbrie R, Iacangelo A, Eiden LE. Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci USA. 1988;85:3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frödin M, Hannibal J, Wulff BS, Gammeltoft S, Fahrenkrug J. Neuronal localization of pituitary adenylate cyclase-activating polypeptide 38 in the adrenal medulla and growth-inhibitory effect on chromaffin cells. Neuroscience. 1995;65:599–608. doi: 10.1016/0306-4522(94)00522-7. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A, Ginty DD, Bading H, Greenberg ME. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1994;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- 27.Gibson UEM, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 28.Ginty DD. Calcium regulation of gene expression: isn't that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 29.Grimaldi M, Favit A, Alkon DL. cAMP-induced cytoskeleton rearrangement increases calcium transients through the enhancement of capacitative calcium entry. J Biol Chem. 1999;274:33557–33564. doi: 10.1074/jbc.274.47.33557. [DOI] [PubMed] [Google Scholar]
- 30.Hahm SH, Eiden LE. Five discrete cis-active domains direct cell type-specific transcription of the vasoactive intestinal peptide (VIP) gene. J Biol Chem. 1998;273:17086–17094. doi: 10.1074/jbc.273.27.17086. [DOI] [PubMed] [Google Scholar]
- 31.Hahm SH, Hsu C-M, Eiden LE. PACAP activates calcium influx-dependent and -independent pathways to couple met-enkephalin secretion and biosynthesis in chromaffin cells. J Mol Neurosci. 1998;11:1–15. doi: 10.1385/JMN:11:1:43. [DOI] [PubMed] [Google Scholar]
- 32.Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haycock JW. Short- and long-term regulation of tyrosine hydroxylase in chromaffin cells by VIP and PACAP. Ann NY Acad Sci. 1996;805:219–230. doi: 10.1111/j.1749-6632.1996.tb17485.x. [DOI] [PubMed] [Google Scholar]
- 34.Holgert H, Dagerlind A, Hökfelt T. Immunohistochemical characterization of the peptidergic innervation of the rat adrenal gland. Horm Metab Res. 1998;30:315–322. doi: 10.1055/s-2007-978891. [DOI] [PubMed] [Google Scholar]
- 35.Inoue M, Fijishiro N, Ogawa K, Muroi M, Sakamoto Y, Imanaga I, Shioda S. Pituitary adenylate cyclase-activating polypeptide may function as a neuromodulator in guinea-pig adrenal medulla. J Physiol (Lond) 2000;528.3:473–487. doi: 10.1111/j.1469-7793.2000.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isobe K, Hakai T, Takuwa Y. Ca2+-dependent stimulatory effect of pituitary adenylate cyclase-activating polypeptide on catecholamine secretion from cultured porcine adrenal chromaffin cells. Endocrinology. 1993;132:1757–1765. doi: 10.1210/endo.132.4.8384995. [DOI] [PubMed] [Google Scholar]
- 37.Jin WZ, Lo TM, Loh HH, Thayer SA. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- 38.Kley N, Loeffler JP, Pittius CW, Höllt V. Proenkephalin A gene expression in bovine adrenal chromaffin cells is regulated by changes in electrical activity. EMBO J. 1986;5:967–970. doi: 10.1002/j.1460-2075.1986.tb04310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopp MDA, Schomerus C, Dehghhani F, Korf H-W, Meissl H. Pituitary adenylate cyclase-activating polypeptide and melatonin in the suprachiasmatic nucleus: effects on the calcium signal transduction cascade. J Neurosci. 1999;19:206–219. doi: 10.1523/JNEUROSCI.19-01-00206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumakura K, Guidotti A, Costa E. Primary cultures of chromaffin cells: molecular mechanisms for the induction of tyrosine hydroxylase mediated by 8-Br-cyclic AMP. Mol Pharmacol. 1979;16:865–876. [PubMed] [Google Scholar]
- 41.Lamouche S, Martineau D, Yamaguchi N. Modulation of adrenal catecholamine release by PACAP in vivo. Am J Physiol Regul Integr Comp Physiol. 1999;45:R162–R170. doi: 10.1152/ajpregu.1999.276.1.R162. [DOI] [PubMed] [Google Scholar]
- 42.Laurenza A, Stukowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol. 1989;10:442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee H-W, Hahm SH, Hsu C-M, Eiden LE. Pituitary adenylate cyclase-activating polypeptide regulation of vasoactive intestinal polypeptide transcription requires Ca2+ influx and activation of the serine/threonine phosphatase calcineurin. J Neurochem. 1999;73:1769–1772. doi: 10.1046/j.1471-4159.1999.731769.x. [DOI] [PubMed] [Google Scholar]
- 44.Lo L, Morin X, Brunet J-F, Anderson DJ. Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron. 1999;22:693–705. doi: 10.1016/s0896-6273(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 45.Lonning K, Carmichael SW, Helle KB. The adrenal medulla as wet sponge: a role for the intramedullary venous vasculature? Acta Physiol Scand. 1997;161:151–160. doi: 10.1046/j.1365-201X.1997.00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg JM, Anggard A, Fahrenkrug J, Hökfelt T, Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci USA. 1980;77:1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malhotra RK, Wakade TD, Wakade AR. Comparison of secretion of catecholamines from the rat adrenal medulla during continuous exposure to nicotine, muscarine or excess K. Neuroscience. 1988;26:313–320. doi: 10.1016/0306-4522(88)90147-9. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra RK, Wakade TD, Wakade AR. Cross-communication between acetylcholine and VIP in controlling catecholamine secretion by affecting cAMP, inositol triphosphate, protein kinase C, and calcium in rat adrenal medulla. J Neurosci. 1989;9:4150–4157. doi: 10.1523/JNEUROSCI.09-12-04150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maric D, Maric I, Barker JL. Developmental changes in cell calcium homeostasis during neurogenesis of the embryonic rat cerebral cortex. Cereb Cortex. 2000;10:561–573. doi: 10.1093/cercor/10.6.561. [DOI] [PubMed] [Google Scholar]
- 50.Mohney RP, Zigmond RE. Vasoactive intestinal peptide enhances its own expression in sympathetic neurons after injury. J Neurosci. 1998;18:5285–5293. doi: 10.1523/JNEUROSCI.18-14-05285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monnier D, Loeffler JP. Pituitary adenylate cyclase-activating polypeptide stimulates proenkephalin gene transcription through AP1- and CREB-dependent mechanisms. DNA Cell Biol. 1998;17:151–159. doi: 10.1089/dna.1998.17.151. [DOI] [PubMed] [Google Scholar]
- 52.Nankova BB, Sabban EL. Multiple signalling pathways exist in the stress-triggered regulation of gene expression for catecholamine biosynthetic enzymes and several neuropeptides in the rat adrenal medulla. Acta Physiol Scand. 1999;167:1–9. doi: 10.1046/j.1365-201x.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 53.Noble EP, Bommer M, Sincini E, Costa T, Herz A. H1-histaminergic activation stimulates inositol-1-phosphate accumulation in chromaffin cells. Biochem Biophys Res Commun. 1986;135:566–573. doi: 10.1016/0006-291x(86)90031-8. [DOI] [PubMed] [Google Scholar]
- 54.Nussdorfer GG. Paracrine control of adrenal cortical function by medullary chromaffin cells. Pharmacol Rev. 1996;48:495–530. [PubMed] [Google Scholar]
- 55.Pruss RM, Moskal JR, Eiden LE, Beinfeld MC. Specific regulation of vasoactive intestinal polypeptide biosynthesis by phorbol ester in bovine chromaffin cells. Endocrinology. 1985;117:1020–1026. doi: 10.1210/endo-117-3-1020. [DOI] [PubMed] [Google Scholar]
- 56.Przywara DA, Xi G, Angelilli L, Wakade TD, Wakade AR. A noncholinergic transmitter, pituitary adenylate cyclase activating polypeptide, utilizes a novel mechanism to evoke catecholamine secretion in rat adrenal chromaffin cells. J Biol Chem. 1996;271:10545–10550. doi: 10.1074/jbc.271.18.10545. [DOI] [PubMed] [Google Scholar]
- 57.Reglödi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- 58.Rius RA, Guidotti A, Costa E. Pituitary adenylate cyclase activating polypeptide (PACAP) potently enhances tyrosine hydroxylase (TH) expression in adrenal chromaffin cells. Life Sci. 1994;54:1735–1743. doi: 10.1016/0024-3205(94)00614-8. [DOI] [PubMed] [Google Scholar]
- 59.Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 60.Roe MV, Lemaster JJ, Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990;11:63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- 61.Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. [PubMed] [Google Scholar]
- 62.Schadlow VC, Barzilai N, Deutsch PJ. Regulation of gene expression in PC12 cells via an activator of dual second messengers: pituitary adenylate cyclase activating polypeptide. Mol Biol Cell. 1992;3:941–951. doi: 10.1091/mbc.3.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci USA. 2000;97:11575–11580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shioda S, Ozawa H, Dohi K, Mizushima H, Matsumoto K, Nakajo S, Takaki A, Zhou CJ, Nakai Y, Arimura A. PACAP protects hippocampal neurons against apoptosis: involvement of JNK/SAPK signaling pathway. Ann NY Acad Sci. 1998;865:111–117. doi: 10.1111/j.1749-6632.1998.tb11169.x. [DOI] [PubMed] [Google Scholar]
- 65.Siegel RE, Eiden LE, Affolter H-U. Elevated potassium stimulates enkephalin biosynthesis in bovine chromaffin cells. Neuropeptides. 1985;6:543–552. doi: 10.1016/0143-4179(85)90117-9. [DOI] [PubMed] [Google Scholar]
- 66.Sietzen M, Schober M, Fischer-Colbrie R, Scherman D, Sperk G, Winkler H. Rat adrenal medulla: levels of chromogranins, enkephalins, dopamine β-hydroxylase and of the amine transporter are changed by nervous activity and hypophysectomy. Neuroscience. 1987;22:131–139. doi: 10.1016/0306-4522(87)90203-x. [DOI] [PubMed] [Google Scholar]
- 67.Spengler D, Bweber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 68.Stauderman KA, Pruss RM. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem. 1990;54:946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y, Rao MS, Zigmond RE, Landis SC. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J Neurobiol. 1994;25:415–430. doi: 10.1002/neu.480250407. [DOI] [PubMed] [Google Scholar]
- 70.Sundler F, Ekblad E, Hannibal J, Moller K, Zhang Y-Z, Mulder H, Elsas T, Grunditz T, Danielsen N, Fahrenkrug J, Uddman R. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann NY Acad Sci. 1996;805:410–428. doi: 10.1111/j.1749-6632.1996.tb17501.x. [DOI] [PubMed] [Google Scholar]
- 71.Symes A, Gearan T, Eby J, Fink JS. Integration of Jak-Stat and AP-1 signaling pathways at the vasoactive intestinal peptide cytokine response element regulates ciliary neurotrophic factor-dependent transcription. J Biol Chem. 1997;272:9648–9654. doi: 10.1074/jbc.272.15.9648. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka K, Shibuya I, Nagamoto T, Yamasha H, Kanno T. Pituitary adenylate cyclase-activating polypeptide causes rapid Ca2+ release from intracellular stores and long lasting Ca2+ influx mediated by Na+ influx-dependent membrane depolarization in bovine adrenal chromaffin cells. Endocrinology. 1996;137:956–966. doi: 10.1210/endo.137.3.8603609. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka K, Shibuya I, Uezono Y, Ueta Y, Toyohira Y, Yanagihara N, Izumi F, Kanno T, Yamashita H. Pituitary adenylate cyclase-activating polypeptide causes Ca2+ release from ryanodine/caffeine stores through a novel pathway independent of both inositol triphosphates and cyclic AMP in bovine adrenal medullary cells. J Neurochem. 1998;70:1652–1661. doi: 10.1046/j.1471-4159.1998.70041652.x. [DOI] [PubMed] [Google Scholar]
- 74.Taupenot L, Mahata SK, Wu H, O'Connor DT. Peptidergic activation of transcription and secretion in chromaffin cells. Cis and trans signaling determinants of pituitary adenylyl cyclase-activating polypeptide (PACAP). J Clin Invest. 1998;101:863–876. doi: 10.1172/JCI1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tönshoff C, Hemmick L, Evinger MJ. Pituitary adenylate cyclase activating polypeptide (PACAP) regulates expression of catecholamine biosynthetic enzyme genes in bovine adrenal chromaffin cells. J Mol Neurosci. 1997;9:127–140. doi: 10.1007/BF02736856. [DOI] [PubMed] [Google Scholar]
- 76.Van Nguyen, Kobierski L, Comb M, Hyman SE. The effect of depolarization on expression of the human proenkephalin gene is synergistic with cAMP and dependent after a cAMP-inducible enhancer. J Neurosci. 1990;10:2825–2833. doi: 10.1523/JNEUROSCI.10-08-02825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wakade AR. Non-cholinergic transmitter(s) maintains secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons. J Neurochem. 1988;50:1302–1308. doi: 10.1111/j.1471-4159.1988.tb10608.x. [DOI] [PubMed] [Google Scholar]
- 78.Wakade AR. Multiple transmitter control of catecholamine secretion in rat adrenal medulla. Adv Pharmacol. 1998;42:595–598. doi: 10.1016/s1054-3589(08)60821-2. [DOI] [PubMed] [Google Scholar]
- 79.Wakade AR, Wakade TD, Malhotra RK. Restoration of catecholamine content of previously depleted adrenal medulla in vitro: importance of synthesis in maintaining the catecholamine stores. J Neurochem. 1988;51:820–829. doi: 10.1111/j.1471-4159.1988.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 80.Waschek JA, Pruss RM, Siegel RE, Eiden LE, Bader M-F, Aunis D. Regulation of enkephalin, VIP and chromogranin A biosynthesis in actively secreting chromaffin cells: multiple strategies for multiple peptides. Ann NY Acad Sci. 1987;493:308–323. doi: 10.1111/j.1749-6632.1987.tb27215.x. [DOI] [PubMed] [Google Scholar]
- 81.Waschek JA, Cassillas RA, Nguyen TB, DiCicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci USA. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson SP. Regulation of chromaffin cell secretion and protein kinase C activity by chronic phorbol ester treatment. J Biol Chem. 1990;265:648–651. [PubMed] [Google Scholar]