Abstract
GABA type A (GABAA) receptors are functionally regulated by external protons in a manner dependent on the receptor subunit composition. Although H+ can regulate the open probability of single GABA ion channels, exactly what residues and receptor subunits are responsible for proton-induced modulation remain unknown. This study resolves this issue by using recombinant α1βi subunit GABAA receptors expressed in human embryonic kidney cells. The potentiating effect of low external pH on GABA responses exhibited pKa in accord with the involvement of histidine and/or cysteine residues. The exposure of GABAA receptors to the histidine-modifying reagent DEPC ablated regulation by H+, implicating the involvement of histidine residues rather than cysteines in proton regulation. Site-specific substitution of all conserved external histidines to alanine on the β subunits revealed that H267 alone, in the TM2 domain, is important for H+ regulation. These results are interpreted as a direct protonation of H267 on α1βi receptors rather than an involvement in signal transduction. The opposing functional effects induced by Zn2+ and H+ at this single histidine residue most likely reflect differences in charge delocalization on the imidazole rings in the mouth of the GABAA receptor ion channel. Additional substitutions of H267 in β subunits with other residues possessing charged side chains (glutamate and lysine) reveal that this area of the ion channel can profoundly influence the functional properties of GABAA receptors.
Keywords: GABAA receptor; pH modulation; β subunit; histidine, H+; ion channel
GABA type A (GABAA) receptors are the major mediators of inhibitory neurotransmission in the brain. Fast inhibition occurs via the synaptic GABAA receptors (Smart, 1998), whereas extrasynaptic isoforms have been demonstrated to underlay continuous tonic inhibition (Otis et al., 1991). Those receptors expressed at inhibitory synapses will be regulated by endogenous processes, including trafficking, endocytosis, phosphorylation, redox reagents, and ions normally present in vivo (e.g., Zn2+ and H+) (Kaila, 1994; Sieghart, 1995; Rabow et al., 1996; Moss and Smart, 2001). Variations in the level of endogenous ions in the CNS represent a rapid and effective method for regulating receptor function (Kaila, 1994; Smart et al., 1994). Previous studies have indicated that protons can differentially affect neuronal GABAAreceptors, resulting in potentiation, inhibition, or no effect on GABA-activated responses (Kaila, 1994; Robello et al., 1994; Pasternack et al., 1996; Zhai et al., 1998; Krishek and Smart, 2001). This variability to pH has been ascribed to differences in the neuronal receptor subunit composition that can be reproduced to some extent using recombinant GABAA receptors (Krishek et al., 1996).
GABAA receptors are presumed to be pentameric proteins (Nayeem et al., 1994) composed of combinations of the following subunits: α(1–6), β(1–3), γ(1–3), δ, ε, π, and θ (Rabow et al., 1996; Mehta and Ticku, 1999). Notably, receptors composed of α1β1, α1β1δ, and α1β1γ2δ subunits were differentially sensitive to external pH, whereas the α1βiγ2 subunit combinations (where i = 1,2) were primarily unaffected (Krishek et al., 1996). Subsequent single-channel studies in neurons indicated that raised H+ concentrations caused inhibition of GABA-activated responses by reducing the single-channel open probability with no effect on channel conductance (Huang and Dillon, 1999; Krishek and Smart, 2001). Furthermore, for cerebellar granule neurons, developmental changes in GABAA receptor α1 and α6 subunit expression (Laurie et al., 1992) occurred concurrently with changes in GABAA receptor sensitivity to external pH, suggesting that protons may be a useful probe for detecting changes in receptor subunit composition (Krishek and Smart, 2001).
Modulating GABAA receptor function with external pH may be important during CNS trauma and neurological pathologies by causing changes in neuronal excitability (Xiong and Stringer, 2000). However, the underlying amino acid residues on GABAA receptors that are responsible for the proton effects remain unidentified. The present study investigates this question using heterologous expression of GABAAreceptors and resolves that a histidine residue at position 267 in the ion channel domain of the β subunit is not only completely responsible for proton regulation of α1βi receptor function, but also that after substitution, this location in the ion channel can profoundly influence the functional properties of the GABAA receptor. To date, this histidine residue plays a unique role in the function of the GABAAreceptor's forming a potential ion binding site and significant transduction pathway. Some of these results have been reported previously in abstract form (Wilkins et al., 2001).
MATERIALS AND METHODS
cDNA constructs and site-specific mutagenesis. The murine GABAA receptor α1, β1, and β2 subunit cDNAs were cloned into the vector pRK5. Site-specific mutagenesis was achieved by using oligonucleotides in conjunction with a primer-directed PCR method (Quickchange; Stratagene, La Jolla, CA), and purified DNAs were prepared using the Plasmid Maxi Kit (Qiagen, Crawley, UK). The entire coding region of all mutants was sequenced using the BigDye ready reaction mix (PerkinElmer Life Sciences, Emeryville, CA/Applied Biosystems, Foster City, CA) and an ABI 310 automated DNA sequencer (Applied Biosystems).
Cell culture and electroporation. Human embryonic kidney (HEK) cells were cultured as described previously (Wooltorton et al., 1997b). The cells were transfected by electroporation (Gene Electropulser II, Hemel Hempstead, UK) using the following parameters: 0.4 kV, 125 μF capacitance, and infinite resistance in the presence of 12 μg of cDNA for the GABAA receptor subunits, present in equal ratios, and 3 μg of green fluorescent protein. Electroporated cells were plated onto poly-l-lysine-coated glass coverslips, which were used for electrophysiological recording 18–72 hr after transfection.
Patch-clamp electrophysiology. Membrane currents were recorded using a whole-cell patch-clamp technique from single HEK cells with an Axopatch 1-C amplifier (Axon Instruments, Foster City, CA). Patch pipettes (resistance, 3–5 MΩ) were filled with a solution containing (in mm): 120 KCl, 1 MgCl2, 11 EGTA, 30 KOH, 10 HEPES, 1 CaCl2, 2 adenosine triphosphate, and 12 creatine phosphate, pH 7.11. The cells were continuously perfused with Krebs' solution containing (in mm): 140 NaCl, 4.7 KCl, 1.2 MgCl2, 2.52 CaCl2, 11 glucose, and either 5 HEPES or 5 MES (see pH buffers). The Krebs' pH was finally adjusted to pH 5.4–8.4 with 1 or 5 N NaOH. Membrane currents were filtered at 5 kHz (−3 dB, sixth pole Bessel, 36 dB/octave) and analyzed with Clampex 8 (Axon Instruments). Any change of >10% in the membrane conductance/series resistance resulted in the cessation of recording. Drugs and solutions were rapidly applied to the cells using a modified Y-tube positioned ∼300 μm from the recorded cell. The response rise times were within 20–30 msec (Wooltorton et al., 1997b). All drugs were dissolved in external Krebs' solution at the appropriate pH. DEPC was added directly to the Krebs' solution, immediately before use, to yield final concentrations of 1 mm.
pH buffers. To ensure accurate control of the Krebs' pH over the range 5.4–8.4, two different pH buffers were used. For pH excursions between 6.8 and 8.4, HEPES (pKa 7.5) was added to the Krebs' solution, and for the pH range 5.4–7, MES was used (pKa 6.1), both at 5 mm final concentrations.
Analysis of whole-cell current data. Peak amplitude membrane currents activated by GABA (I) were determined at −50 mV holding potential. GABA equilibrium concentration–response relationships were constructed by measuring the peak GABA currents, which were normalized to the response induced by 10 μm GABA in control Krebs' solution at pH 7.4 (I10) and subsequently fitted with the Hill equation:
| Equation 1 |
where EC50 represents the concentration of GABA ([A]) inducing 50% of the maximal current evoked by a saturating concentration of GABA and n is the Hill coefficient.
When the maxima of the curves demonstrated a clear depression with incrementing GABA concentration, the data were fitted with the following equation, accounting for the reduced maximum response by assuming that the ligand binds to two distinct sites, one producing potentiation and the other producing inhibition (or desensitization) of the GABA-activated current:
| Equation 2 |
where I, I10, EC50, and n are as defined, IC50 is the GABA concentration producing a 50% inhibition of the current, and m represents the corresponding Hill coefficient. To ensure a fit to the inhibitory component, occasionally the IC50 was constrained where limited numbers of data points were available.
The GABA concentration–response curve data were analyzed using ANOVA with a Bonferroni post hoc test. Significance was determined at the p < 0.05 level.
The Zn2+ inhibition concentration relationships were fitted with the following equation:
| Equation 3 |
whereIN′ andIN represent the normalized GABA-induced current in the presence and absence of Zn2+ at concentration B, respectively, and IC50 defines the concentration of Zn2+ producing 50% inhibition of the GABA-induced current.
RESULTS
External pH and recombinant α1βi GABAA receptors
To assess whether the identity of the βi subunit was important for the pH-induced regulation of GABAA receptor function, recombinant GABAA receptors composed of α1β1 and α1β2 were expressed in HEK cells and used to construct full GABA concentration–response curves at normal physiological pH 7.4 and after a pH excursion to 5.4 (Fig. 1). For either construct, increasing the total H+ concentration by 100-fold resulted in potentiated responses to GABA and an increased maxima for the concentration–response curves (p < 0.05 for the range of 5 μm to 1 mmGABA). The GABA EC50s for α1β1 (pH 7.4, 5.0 ± 0.7 μm; pH 5.4, 3.3 ± 0.6 μm; n = 3–5) and α1β2 (pH 7.4, 3.2 ± 0.4 μm; pH 5.4, 4.9 ± 0.7 μm; n = 3–5) displayed no significant change, suggesting that GABA potency was primarily unaffected by the change in external pH (Fig. 1A,B). A direct comparison between the concentration–response curves for the two GABAA receptor heteromers indicated that H+ induced a slightly larger potentiation of GABA-activated responses on α1β1 receptors compared with α1β2 receptors for 10 μm GABA-activated responses (potentiated by 197.6 ± 37.5 and 120.1 ± 15.5%, respectively; n = 5–7). An additional distinction between α1β1 and α1β2 GABAA receptors was that at high GABA concentrations (>100 μm) in pH 5.4, the peak of the GABA concentration–response curve was depressed only for the α1β1 receptors, possibly indicative of inhibition or increased desensitization. Overall, these data suggested that the identity of the β subunit in α1βi constructs, although partially influential, did not have a major effect on the extent and type of modulation by H+.
Fig. 1.
External pH modulates the GABA concentration–response relationships. For α1β1 (A) and α1β2 (B) GABAA receptors, peak amplitude responses were measured at pH 7.4 (○) and pH 5.4 (▪) and were normalized to the responses induced by 10 μm GABA at pH 7.4 (= 1). In this and comparable figures, the curves were generated according to Equations 1and/or 2, presented in Materials and Methods. All points in this and comparable figures represent the mean ± SEM fromn = 3–5 cells. The insetsillustrate sample GABA-activated currents induced by 10 μm GABA applied for the duration of the solid line at the indicated external pH. Calibration: 200 pA, 2 sec.
Identifying the residues underlying H+modulation of α1βi GABAA receptors
To establish the residue(s) that is important for the H+ modulation of α1βi GABAA receptors, we titrated the H+-induced potentiation for α1β1 and α1β2 GABAA receptors against the external Krebs' pH; this yielded pKas of 6.73 ± 0.09 and 6.91 ± 0.13, respectively (n = 5). We therefore deduced that histidines (pKa 6) are the most likely candidates to be involved in proton modulation of GABAA receptors. Although cysteine residues also have a comparable pKa (∼8) they were considered unlikely to be involved, considering that the only two external cysteine residues, located within the N terminal, are postulated to form a disulfide bridge (Barnard et al., 1987; Pan et al., 1995, 2000;Amato et al., 1999), precluding their involvement with H+-induced modulation.
To investigate a potential role for histidine residues in H+ regulation of GABAA receptor function, we used DEPC, a reagent that irreversibly converts neutral imidazole groups intoN-carbethoxyhistidyl derivatives (Miles, 1977). HEK cells expressing α1βi subunit constructs were exposed to 1 mm DEPC at pH 7.4. A brief application (4 min) of DEPC to α1βi receptors irreversibly abolished the potentiation of GABA-activated responses observed at pH 5.4 in the absence of DEPC. Inspection of GABA concentration–response curves revealed that after DEPC treatment, lowering the external pH to 5.4 induced a small lateral shift in the GABA concentration–response relationship, causing a reduction in the potency of GABA (EC50s for α1β1: pH 7.4, 5.1 ± 1.3 μm; pH 5.4, 18.8 ± 4.6 μm; p < 0.05) (Fig. 2). These data were in accord with one or more histidines underlying the H+-induced potentiation of GABA-activated responses, and this effect appeared to be masking a weak inhibitory action of H+ that was clearly manifest only when the histidines had been covalently modified.
Fig. 2.
Modification of histidine residues by DEPC prevents proton-induced modulation of α1β1 GABAAreceptors. Normalized GABA concentration–response relationships obtained at pH 7.4 (○) and pH 5.4 (●) after exposure to DEPC (1 mm) are shown. For comparison, the GABA concentration–response curves at pH 7.4 (dotted line) and pH 5.4 (dashed line) were taken from Figure1A. Data are from n = 3–5 cells.
Site-specific mutagenesis of external histidines on α1βi receptors
Considering the ablation of H+modulation by DEPC, the exact molecular determinants of this potentiation were sought using site-specific mutagenesis. The α1βi heteromers possess numerous external histidine residues, eight on the α1 subunit and four on the βi subunits. External residues were selected because internal pH changes have little effect on GABAA receptor function (Krishek et al., 1996). The β subunit was examined primarily because of the pivotal role this subunit family plays in the expression and function of GABAA receptors (Connolly et al., 1996). The number of histidines considered to be potentially involved in the H+ modulation on the β subunit was reduced to three by including only those that are conserved between all β subunits, because H+ modulation of GABAA receptor function is not critically dependent on the identity of the β subunit. The three candidate histidines on the β2 subunit were H107 and H119, located in the N terminal, and H267, located in TM2, the ion channel domain. Each histidine in the β2 subunit was sequentially substituted, initially to alanine, and coexpressed with wild-type α1 subunits in HEK cells.
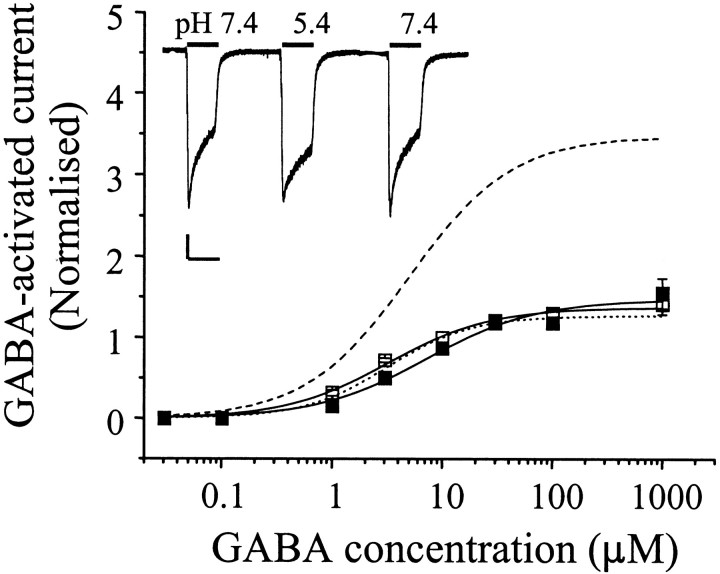
Neither the mutant α1β2H107A nor α1β2H119A GABAAreceptors differed in their response to changing the external pH to 5.4, which still potentiated responses to GABA to levels similar to those observed with the wild-type α1β2 receptors (EC50s: α1β2H107A: pH 7.4, 4.1 ± 0.6 μm; pH 5.4, 4.8 ± 0.9 μm; α1β2H119A: pH 7.4, 3.0 ± 0.4 μm; pH 5.4, 4.9 ± 0.5 μm;p > 0.05), compared with α1β2 wild type for both mutants (Fig. 3A,B). However, substituting H267 with alanine, previously identified to play a major role in Zn2+ inhibition of GABA-activated responses (Wooltorton et al., 1997a; Horenstein and Akabas, 1998) on α1βi receptors, ablated the potentiating effect of H+ (Fig. 4). Although the GABA concentration–response curve for the mutant α1β1H267A did not exhibit an enhanced maximum response at pH 5.4, it was evident for both α1β1H267A and α1β2H267A receptors that low pH caused a small reduction in GABA potency (EC50s for α1β2H267A: pH 7.4, 3.2 ± 0.4 μm; pH 5.4, 7.1 ± 1.9 μm; p > 0.05) (Fig. 4). This weak inhibitory effect of H+ was manifest between GABA concentrations of 1 and 30 μm and appeared analogous to the effect of DEPC on the GABA concentration–response curve for the wild-type α1β1 (Fig. 2) or α1β2 receptors.
Fig. 3.
Mutating histidines 107 or 119 in the β2 subunit do not alter proton-induced modulation of α1β2 GABAAreceptors. Normalized GABA concentration–response relationships for α1β2H107A (A) and α1β2H119A (B) at pH 7.4 (■) and pH 5.4 (▪). The comparative concentration–response curves for the wild-type α1β2 receptor at pH 7.4 (dotted line) and pH 5.4 (dashed line) were taken from Figure 1B. Data are from n = 3–5 cells.
Fig. 4.
Mutation of the TM2 histidine 267 in the β2 subunit ablates proton sensitivity of α1β2 GABAAreceptors. A normalized GABA concentration–response relationship for α1β2H267A GABAA receptors at pH 7.4 (■) and pH 5.4 (▪) is shown, including the comparison with the curve fits for α1β2 receptors at pH 7.4 (dotted line) and pH 5.4 (dashed line). Theinset demonstrates typical responses to 10 μm GABA recorded at pH 7.4 and pH 5.4 for α1β2H267A receptors. Calibration: 200 pA, 2 sec.
The lack of any potentiating effect of H+on the GABAA receptor incorporating the mutant H267A suggested that this histidine residue may form a key protonation site for H+ ions and is likely to be the only histidine involved. This latter point was reassessed by exposing α1β1H267A GABAAreceptors to 1 mm DEPC. This modifying agent did not further affect the GABA concentration–response curves, nor did it affect the weak inhibitory effect revealed once the H+-induced potentiating effect had been abolished (data not shown).
Charged mutations at H267 in the β subunit
To further investigate whether H+could be binding to H267, the residue that also has a pivotal role in Zn2+ inhibition, two additional substitutions were made in the β2 subunit for subsequent coexpression with wild-type α1 subunits. These substitutions introduced amino acids with charged side chains: glutamate (E, negative) and lysine (K, positive) at position H267. Glutamate was selected because it has a projected pKa for the side chain carboxyl group of ≤4 and probably less in the GABAA receptor protein and would not be protonated at pH 7.4 (100% anionic) and only minimally at pH 5.4 (96.2% anionic). Thus, we predict that H+ will be ineffective at potentiating GABA-activated responses if they do indeed bind to H267. However, for Zn2+ inhibition, the ability of the glutamate carboxyl group to act as an electron donor suggests that it will be capable of binding Zn2+, a role that it fulfills in Zn2+-containing metalloenzymes (Vallee and Auld, 1990). Thus, if Zn2+ binds to H267, we would expect the retention of some inhibitory activity at the mutant α1β2H267E receptor but possibly at a reduced level compared with the wild-type receptor containing β2H267.
Exposure of α1β2H267E receptors to low external pH 5.4 abolished the effect of H+on GABA-activated responses, as predicted if this residue forms a site for protonation (EC50: pH 7.4, 3.4 ± 0.5 μm; pH 5.4, 4.8 ± 1 μm;p > 0.05 compared with α1β2 wild type) (Fig.5A). However, the level of Zn2+ inhibition over the concentration range of 1 μm to 10 mmfor the β2H267E substitution was less than expected (Fig. 6). The mutation disrupted antagonism, but the Zn2+IC50 was 30.6 ± 7.3 μm, almost identical to that determined previously for GABAA receptors incorporating the mutation βiH267A (Wooltorton et al., 1997a) but greater than that for the α1β2 wild type (IC50: 0.65 ± 0.03 μm; n = 4) (Fig. 6).
Fig. 5.
Effect of charged mutations at position H267 in the β subunit on α1β2 GABAA receptor sensitivity to protons. Normalized GABA concentration–response relationships for α1β2H267E (A) and α1β2H267K (B) GABAA receptors at pH 7.4 (○) and pH 5.4 (●) are shown, together with curve fits for the α1β2 wild-type receptor at pH 7.4 (dotted line) and pH 5.4 (dashed line). Data are from n = 3–5 cells. Theinset illustrates 10 μm GABA-activated currents at pH 7.4 and pH 5.4 obtained from α1β2H267K receptors. Note the increased rate and extent of receptor desensitization. Calibration: 400 pA, 2 sec.
Fig. 6.
Zn2+ concentration inhibition curves for α1β2 (▪), α1β2H267E (●), and α1β2H267K (▴) receptors determined by coapplication of Zn2+ with 10 μm GABA. The ordinate describes the response amplitude to GABA in Zn2+ as a percentage of the control response to GABA in the absence of Zn2+. After each Zn2+ application, full recoveries from inhibition were obtained. The data were obtained from n = 3–5 cells and were fitted using Equation 3, presented in Materials and Methods.
The lysine substitution at position 267 was performed because the side chain, with a projected pKa of 10, is positively charged at pH 7.4 (99.7% cationic) and also at pH 5.4 (100%). Therefore, this residue would already be protonated and could not participate in the modulation of GABA-activated currents between pH 5.4 and pH 7.4. In addition, Zn2+ inhibition should be markedly reduced, as observed with the β2H267A substitution. However, the charged side chain presented by the lysine residue conferred unusual and profoundly different functional properties on the GABAA receptor. First, this mutation did not ablate the H+ sensitivity of 10 μm GABA-activated currents, with potentiation by 52 ± 7% observed at pH 5.4 compared with pH 7.4 (EC50: pH 7.4, 17.2 ± 7.4 μm; pH 5.4, 11 ± 1.9 μm; p < 0.05 compared with α1β2 wild type; n = 5) (Fig.5B). Second, the lysine mutation further reduced the sensitivity to Zn2+, with the inhibition curve then defined by an IC50 of 990 ± 116 μm (Fig. 6). Finally, the desensitization profile of the GABA-induced currents for α1β2H267K receptors was markedly affected, with increased rates of desensitization and peak currents declining back to the baseline holding current, even during GABA application (Fig. 5B, inset). These results clearly emphasized the crucial nature of the identity of the residue at position 267 in the ion channel lining of the β subunit in shaping the response profile of the GABAA receptor.
DISCUSSION
This study concludes that for α1βi GABAAreceptors, H+-induced potentiation completely relies on a single histidine residue, H267, in β subunits; this residue resides at the external portal of the ion channel. Previous site-specific mutagenesis revealed that H267 also underpinned a substantial component of Zn2+ inhibition of GABA-activated responses, suggesting that it is important for the allosteric modulation of GABAA receptor function.
The involvement of a histidine residue, conserved between the β subunit isoforms, in the regulation of GABAAreceptor function was suggested from the similar pH titration profiles for α1β1 and α1β2 receptors. This was also supported by the unequivocal effect of DEPCs completely removing any potentiation by H+ on α1β1 and α1β2 receptors. Interestingly, this action unveiled a small inhibitory effect of H+ that was observed only at low pH values with a projected pKa approaching 4, indicating the involvement of aspartate and/or glutamate residues. Mutagenesis subsequently confirmed that only H267 in the β subunits was critically involved in H+-induced potentiation. Because H+ can compete and prevent the inhibitory effect of Zn2+ on recombinant and native GABAA receptors (Krishek et al., 1998) and because H267 has already been suggested to be an important coordinating residue for Zn2+inhibition at α1βi subunit GABAA receptors (Wooltorton et al., 1997a; Horenstein and Akabas, 1998), we suggest that protonation of this residue is likely to underlie the potentiating effect of H+ on α1βi GABAA receptors. Furthermore, mutating H267 does not affect GABA activation of the receptor, thus suggesting that this residue is not part of the main agonist signal transduction process.
To further examine the role of H267, we substituted this histidine for glutamate and lysine to vary the charge in the side chain. Although inclusion of the carboxyl side chain for α1β2H267E abolished H+-induced potentiation, Zn2+ inhibition was reduced to an extent similar to that observed after alanine substitution. This suggested that the orientation of the side chain in this position must be important, because glutamate, by virtue of electron donation, would be expected to bind Zn2+ and thus substitute, at least partly, for histidine. However, it is clear that this did not occur. The lysine substitution, however, yielded unexpected results, with the H+-induced potentiation only partly reduced when we might have expected abolition; however, the level of Zn2+ inhibition was markedly reduced, far more than expected from the results with the neutral alanine substitution at position 267. We had predicted that the H+-induced potentiation would be ablated because of the positively charged lysine side chain (pKa 10); however, if H+ is protonating the lysine residue at position 267, this residue cannot be 100% charged, and the pKa must be reduced by at least 2000- to 3000-fold (2–3 pH units) to ∼7 for this to occur. Such a shift in pKa has been reported in the inward rectifier potassium channel, Kir 1.1, where an intracellular pH-sensitive lysine can be protonated at pH 5.4 (Schulte et al., 1999). Such an effect can be achieved only if other positively charged residues (e.g., arginines and lysines) electrostatically shield the proton-sensitive lysine from basal H+(Schulte et al., 1999). Indeed, both R269 and K274 (conserved throughout the GABAA receptor α, β, and γ subunit families) lie close, at least in primary sequence order, to H267K. In the β subunits, such proximity might act to shield the lysine at 267 from H+. The results with Zn2+ and H267K could be explained by the action this residue has in further disrupting, beyond that achieved with H267A, the allosteric mechanism for Zn2+ inhibition.
Although mutagenesis cannot unequivocally indicate the presence of a binding site on a receptor, the lack of any relatively comparable effects on H+ and Zn2+ modulation by E267 and K267 suggests that this position is probably not involved in a common signal transduction pathway that is “downstream” of ion binding sites. Furthermore, because H+ and Zn2+ have opposite effects on GABAA receptor function, it is also unlikely that H267 is an element in part of a common signal transduction mechanism.
The identification of H267 as a potential binding site for H+ and Zn2+presented a curious problem. If these positively charged ions are capable of binding to the same residue, how can they induce completely opposite effects of inhibition (Zn2+) and potentiation (H+) on GABAA receptor function? The key to this question may involve the different ways in which H+and Zn2+ interact with the imidazole groups of histidines. The addition of H+will cause protonation of individual imidazole rings, independent of any other residues. This protonation and subsequent potentiation of the GABA response might involve electrostatic repulsion between adjacent imidazole groups on the β subunits in the channel (two to three depending on subunit stoichiometry), causing a local conformational change in the receptor. In contrast, zinc ions will form a coordinated bond with one of the imidazole ring nitrogens and at least three other residues (which could include other imidazole rings from additional β subunits in the receptor) and an activated water molecule. This would be typical of many Zn2+ binding sites in metalloenzymes (Vallee and Auld, 1990). The coordination of Zn2+ with H267 would inhibit receptor function allosterically rather than by a physical channel block mechanism (Legendre and Westbrook, 1991; Smart, 1992; Gingrich and Burkat, 1998). This coordination, compared with protonation, would not polarize adjacent imidazole rings. Thus, mechanistically, at the level of amino acid side chains, it is conceivable that H+ and Zn2+could cause differential effects on channel function, but the precise movements of amino acids will need to await x-ray crystallographic study. A similar scenario accounts for the differential effects of Zn2+ and H+on the M2 ion channel from influenza A virus (Okada et al., 2001).
Overall, these data indicate the importance of a single TM2 residue in the ion channel lining of the β subunits for H+-induced modulation of GABAA receptor function and also suggest that H267 could be a potential binding site for at least two types of cations.
Footnotes
This work was supported by the Medical Research Council. We thank Robert Harvey for providing β1/2H267A clones.
Correspondence should be addressed to T. G. Smart, Department of Pharmacology, University College London, WC1E 6BT, UK. E-mail:t.smart@ucl.ac.uk.
M. E. Wilkin's and A. M. Hosie's present address: Department of Pharmacology, University College London, London, WC1E 6BT, UK.
REFERENCES
- 1.Amato A, Connolly CN, Moss SJ, Smart TG. Modulation of neuronal and recombinant GABAA receptors by redox reagents. J Physiol (Lond) 1999;517:35–50. doi: 10.1111/j.1469-7793.1999.0035z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard EA, Darlison MG, Seeburg P. Molecular biology of GABAA receptor: the receptor/channel superfamily. Trends Neurosci. 1987;10:502–509. [Google Scholar]
- 3.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Horenstein J, Akabas MH. Location of a high affinity Zn2+ binding site in the channel of α1β1 γ-aminobutyric acidA receptors. Mol Pharmacol. 1998;53:870–877. [PubMed] [Google Scholar]
- 5.Huang RQ, Dillon GH. Effect of extracellular pH on GABA-activated current in rat recombinant receptors and thin hypothalamic slices. J Neurophysiol. 1999;82:1233–1243. doi: 10.1152/jn.1999.82.3.1233. [DOI] [PubMed] [Google Scholar]
- 6.Gingrich KJ, Burkat PM. Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the gamma-subunit. J Physiol (Lond) 1998;506:609–625. doi: 10.1111/j.1469-7793.1998.609bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 8.Krishek BJ, Smart TG. Proton sensitivity of rat cerebellar granule cell GABAA receptors: dependence on neuronal development. J Physiol (Lond) 2001;530:219–233. doi: 10.1111/j.1469-7793.2001.0219l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. J Physiol (Lond) 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishek BJ, Moss SJ, Smart TG. Interaction of H+ and Zn2+ on recombinant and native rat neuronal GABAA receptors. J Physiol (Lond) 1998;507:639–652. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legendre P, Westbrook GL. Noncompetitive inhibition of γ-aminobutyric acidA channels by Zn. Mol Pharmacol. 1991;39:267–274. [PubMed] [Google Scholar]
- 13.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 14.Miles EW. Modification of histidyl residues in proteins by dietheylpyrocarbonate. Methods Enzymol. 1977;47:431–443. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- 15.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 16.Nayeem N, Green TP, Martin IL, Barnard EA. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. J Neurochem. 1994;62:815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- 17.Okada A, Miura T, Takeuchi H. Protonation of histidine and histidine-tryptophan interaction in the activation of the M2 ion channel from influenza a virus. Biochemistry. 2001;40:6053–6060. doi: 10.1021/bi0028441. [DOI] [PubMed] [Google Scholar]
- 18.Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- 19.Pan ZH, Bahring R, Grantyn R, Lipton SA. Differential modulation by sulfhydryl redox agents and glutathione of GABA- and glycine-evoked currents in rat retinal ganglion cells. J Neurosci. 1995;15:1384–1391. doi: 10.1523/JNEUROSCI.15-02-01384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan ZH, Zhang X, Lipton SA. Redox modulation of recombinant human GABA(A) receptors. Neuroscience. 2000;98:333–338. doi: 10.1016/s0306-4522(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 21.Pasternack M, Smirnov S, Kaila K. Proton modulation of functionally distinct GABAA receptors in acutely isolated pyramidal neurons of rat hippocampus. Neuropharmacology. 1996;35:1279–1288. doi: 10.1016/s0028-3908(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 22.Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1996;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 23.Robello M, Baldelli P, Cupello A. Modulation by extracellular pH of the activity of GABAA receptors on rat cerebellum granule cells. Neuroscience. 1994;61:833–837. doi: 10.1016/0306-4522(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 24.Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, Weidemann S, Ruppersberg JP, Fakler B, Ludwig J. pH gating of ROMK (K(ir)1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci USA. 1999;96:15298–15303. doi: 10.1073/pnas.96.26.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 26.Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol (Lond) 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smart TG. Electrophysiology of GABAA receptors. In: Turner AJ, Stephenson FA, editors. Amino acid neurotransmission. Portland; London: 1998. pp. 37–63. [Google Scholar]
- 28.Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 29.Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins ME, Harvey RJ, Smart TG. Identification of the H+ modulatory site on a GABAA receptor. Br J Pharmacol. 2001;133:P166. [Google Scholar]
- 31.Wooltorton JR, McDonald BJ, Moss SJ, Smart TG. Identification of a Zn2+ binding site on the murine GABAA receptor complex: dependence on the second transmembrane domain of β subunits. J Physiol (Lond) 1997a;505:633–640. doi: 10.1111/j.1469-7793.1997.633ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci. 1997b;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 33.Xiong ZQ, Stringer JL. Extracellular pH responses in CA1 and the dentate gyrus during electrical stimulation, seizure discharges, and spreading depression. J Neurophysiol. 2000;83:3519–3524. doi: 10.1152/jn.2000.83.6.3519. [DOI] [PubMed] [Google Scholar]
- 34.Zhai J, Peoples RW, Li C. Proton inhibition of GABA-activated current in rat primary sensory neurons. Pflügers Arch. 1998;435:539–545. doi: 10.1007/s004240050550. [DOI] [PubMed] [Google Scholar]