Abstract
Two monkeys trained for >5 years to perform 12 finger and wrist movements had both a greater prevalence of motor cortex neurons with significant effects in spike-triggered averages and a greater ratio of synchrony effects to pure postspike effects than a monkey trained <1 year to perform six movements. By comparison, stimulus-triggered averages were generally similar in all three monkeys, indicating that the increased prevalence of synchrony in spike-triggered averages was a feature of voluntary motor system activity in the monkeys trained for a longer period of time. Synchronization among neurons with relatively direct connections to spinal α-motoneuron pools, including motor cortex neurons, may increase as a repertoire of skilled movements is acquired and practiced during long-term training.
Keywords: electromyographic activity, finger, learning, motor cortex, movement, muscle, skill, spike-triggered averaging, training
As normal subjects train at skilled movement tasks over periods ranging from hours to years, use-dependent changes occur in the output of the primary motor cortex (M1) (Pascual-Leone et al., 1993, 1995; Karni et al., 1995; Classen et al., 1998; Plautz et al., 2000). Underlying mechanisms may include long-term potentiation (LTP) of existing synapses (Aroniadou and Keller, 1995; Asanuma and Pavlides, 1997;Rioult-Pedotti et al., 1998), decreases in intracortical inhibition (Jacobs and Donoghue, 1991; Ziemann et al., 2001), and formation of new synaptic contacts (Kleim et al., 1996). Although these mechanisms might be expected to increase discharge synchrony among neurons, synchronization has not previously been associated with motor skill training. Neuronal synchrony in M1 has been demonstrated in the pairwise cross-correlation of simultaneously recorded spike trains (Smith and Fetz, 1989; Riehle et al., 1997; Baker et al., 2001), and rhythmic synchrony among ensembles of M1 neurons is indicated by local field potential oscillations, which may show coherence with oscillations in electromyographic (EMG) activity (Murthy and Fetz, 1996; Donoghue et al., 1998; Baker et al., 1999; Gross et al., 2000;Ohara et al., 2000). Synchrony among neurons that provide input to a pool of spinal α-motoneurons also can be detected in the spike-triggered average (SpikeTA) of rectified EMG activity (Smith and Fetz, 1989; Flament et al., 1992; Baker and Lemon, 1998; McKiernan et al., 1998; Perlmutter et al., 1998). Here, spike-triggered averaging revealed that long-term training at a large repertoire of skilled finger movements is associated with increased synchrony of M1 neurons.
MATERIALS AND METHODS
Animals and behavioral procedures. All care and use of these purpose-bred monkeys complied with United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and was approved by the University Committee on Animal Resources at the University of Rochester. Each monkey was trained to perform visually cued individuated flexion and extension movements of the right-hand fingers and/or wrist (Schieber, 1991). As the monkey sat in a primate chair, the right elbow was restrained in a molded cast, and the right hand was placed in a pistol-grip manipulandum that separated each finger into a different slot. At the end of each slot, the fingertip lay between two microswitches. By flexing or extending the digit a few millimeters, the monkey closed the ventral or dorsal switch, respectively. The manipulandum, in turn, was mounted on an axis that permitted flexion and extension wrist movement for monkeys C and G. Each monkey viewed a display on which each digit (and the wrist) was represented by a row of five light-emitting diodes (LEDs). When the monkey flexed or extended a digit, closing a microswitch, the central yellow LED went out and a green LED to the left or right, respectively, came on. Red LEDs to the far left or right were illuminated one at a time, under microprocessor control, instructing the monkey to close that one switch (or move the wrist). If the monkey closed the instructed switch within the 700 msec allowed after illumination of the red instruction LED, and held it closed for a 500 msec final hold period without closing any other switches, he received a water reward. After each rewarded trial, the finger movement to be instructed for the next trial was rotated in a pseudorandom order. Whereas monkeys C and G performed all 12 individuated flexion and extension movements of the five digits and wrist, monkey A performed only six. With each movement denoted by the number of the instructed digit (1 = thumb through 5 = little finger; W = wrist), and the first letter of the instructed direction (f, flexion; e, extension), monkey A performed instructed movements 1f, 2f, 3f, 4f, 2e, and 3e. Furthermore, monkey A's wrist was stabilized mechanically by locking the wrist axis.
Data collection and analysis. After training, conventional techniques were used to record single M1 neurons simultaneously with EMG activity from 8 to 16 muscles of the forearm and hand (bipolar fine-wire electrodes; amplification, 2000–100,000×; bandpass, 0.3–3 kHz; sampling, 4 kHz per channel) (McKiernan et al., 1998) as the monkey performed individuated finger movements. Using these recordings, SpikeTAs were formed for each EMG channel by averaging segments of rectified EMG activity from 30 msec before to 50 msec after each spike of the M1 neuron. As more segments are averaged, EMG activity that is not time-locked to the neuronal spike evens out toward a baseline, while EMG activity that is time-locked to the neuronal spike progressively accumulates, forming a facilitatory peak or suppression trough (Fetz and Cheney, 1980). Additional recordings were made during the same sessions as single intracortical microstimulation (sICMS) pulses (biphasic; 0.2 msec per phase; 5–20 μA) were delivered through the microelectrode at interpulse intervals varying continuously between 60 and 80 msec (Park et al., 2001). Stimulus-triggered averages (StimulusTAs) were formed for each EMG in these recordings. Because the minimal latencies of StimulusTA effects represent the earliest effects M1 neurons can have on EMG activity, and because the interval between sICMS pulses is generally too long to permit temporal summation from successive stimuli, StimulusTA effects provided an important comparison with SpikeTA effects. At many sites, sICMS was delivered at 5, 10, and/or 20 μA in two or three separate recordings. In such cases, the effects at each tested current intensity were included here for comparison with SpikeTA effects.
Significant effects in SpikeTAs and StimulusTAs were identified with multiple-fragment statistical analysis (Poliakov and Schieber, 1998). This approach divides the spike (or stimulus) train into multiple fragments, forms a triggered average using the spikes (or stimuli) in each fragment, and subtracts the mean value of the average in a test window from the mean in immediately preceding and succeeding control windows. If this difference on average is significantly different from 0 across all of the fragments, the peak (or trough) in the test window is statistically significant. Because previous studies have shown that the peaks and troughs of postspike effects in SpikeTAs typically occur at latencies from 6 to 16 msec after the M1 neuron spike (Fetz and Cheney, 1980; Cheney and Fetz, 1985; Kasser and Cheney, 1985; Lemon et al., 1986; McKiernan et al., 1998), statistically significant peaks (or troughs) were identified in that temporal window. Potentially significant effects occurring at other latencies were not examined in the present analysis.
Initial averages were formed using all spikes or sICMS pulses. If any muscle showed a peak (or trough) still significant at thep < 0.05 level after Bonferroni correction for testing multiple EMG channels, the M1 neuron or sICMS site was accepted as producing effects. Then every EMG in that recording with a peak (or trough) significant at p < 0.05 without correction was submitted to the following analysis. To eliminate contributions of sweeps containing only noise, a second, filtered average was formed, using spikes as triggers only if the rms value of the EMG from 30 msec before to 50 msec after the trigger was >0.05 V (i.e., greater than the typical noise level of 0.3–0.4 V) (McKiernan et al., 1998). If the peak (or trough) in this filtered average remained significant at p < 0.05 (without Bonferroni correction), the effect was retained for additional analysis. Any baseline ramp in the filtered average was subtracted, and the filtered average was smoothed with a flat, five point, finite impulse response filter. When significant effects were identified in multiple muscles in the same recording, one of any pair of effects potentially resulting from cross-talk between EMG recordings was eliminated (Kasser and Cheney, 1985; Buys et al., 1986). In the final data set, the EMG-filtered SpikeTAs had from 217 to 60,921 triggers [9734 ± 8326 (mean ± SD)]; StimulusTAs had from 120 to 4570 triggers (1249 ± 971).
A computer algorithm performed the following computations for each SpikeTA or StimulusTA effect. The mean and SD were calculated over a baseline period from 30 to 10 msec before the trigger. The maximum value of the peak (or minimum of the trough) was identified, and the average was followed backward until it fell within 2 SD of the baseline mean. This time was defined as the onset, and its latency was corrected for the time from spike or stimulus onset to the trigger pulse used for averaging. One-half the height of the peak above (or trough below) the baseline mean was computed, and the width of the peak (or trough) at this level was measured as the peak width at half maximum (PWHM).
RESULTS
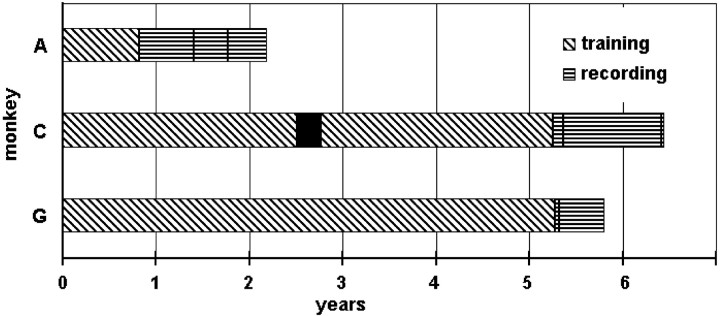
Three monkeys were studied as they performed visually cued individuated flexion and extension movements of the right-hand fingers and wrist after different periods of training (Fig.1). In monkey A, SpikeTAs from M1 neurons were obtained between 1 and 2 years after training began. This monkey performed only six relatively easy finger movements, with the wrist stabilized by external support, rendering the finger movements easier still. In monkeys C and G, SpikeTAs from M1 neurons were obtained between 5 and 6.5 years after training began. Monkeys C and G each performed 12 movements: flexion and extension of each finger and of the wrist. The wrist flexion/extension axis was continuously mobile as these monkeys performed the finger and wrist movements.
Fig. 1.
Duration of training and recording.Horizontal bar graphs show the training period (angle hatching) and recording period (horizontal hatching) for each monkey, A, C, and G, in years. Two solid vertical lines within each monkey's recording period indicate the day on which the first and last neurons with significant effects in spike-triggered averages were recorded. (Monkey G's last neuron was recorded very close to the end of the recording period.) Thesolid black box in monkey C's training period indicates a 3 month hiatus during a lab move.
SpikeTAs were obtained for 166, 318, and 136 neurons in the M1 hand representation of monkeys A, C, and G, respectively. The number of M1 neurons with versus without a significant peak or trough for at least one muscle differed across monkeys (with:without, 27:139 in A, 159:159 in C, 53:83 in G; χ2 test;p < 0.00001). Post hoc testing showed that neurons with such SpikeTA effects were significantly more frequent in monkeys C and G than in monkey A (A, 16.2%; C, 50.0%; G, 40.0%). Overall, 1649, 3561, and 2764 neuron–EMG pairs were tested for SpikeTA effects in monkeys A, C, and G, respectively. The number of neuron–EMG pairs with versus without significant effects also differed across monkeys (47:1602, 467:3094, and 212:2552 in monkeys A, C, and G, respectively, χ2 test; p< 0.00001), with monkey A having the lowest percentage of neuron–EMG pairs with significant effects (A, 2.9%; C, 13.1%; G, 7.7%). Therefore, M1 neurons with SpikeTA effects as well as neuron–EMG pairs with effects were more prevalent in monkeys C and G than in monkey A.
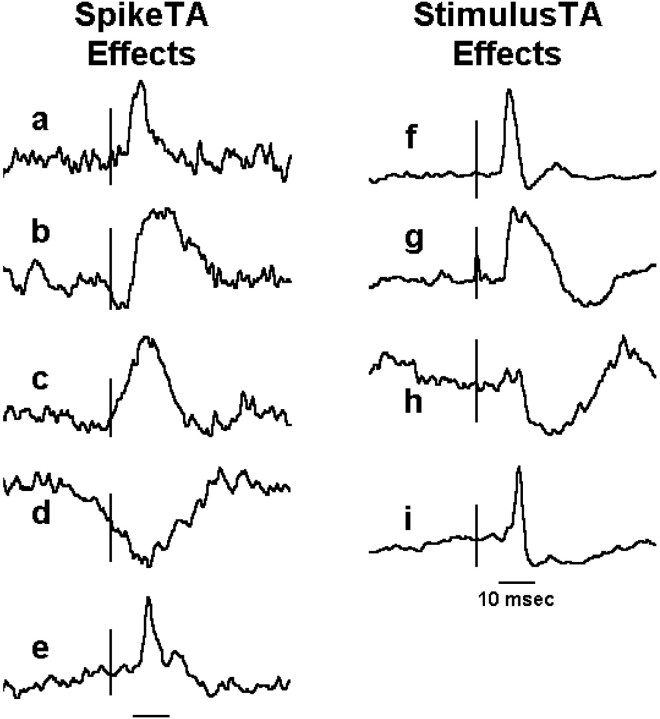
SpikeTA effects showed a variety of temporal features (Fig.2, left column) that reflect the temporal features of the inputs arriving in the motoneuron pool of the recorded muscle (Smith and Fetz, 1989; Flament et al., 1992; Baker and Lemon, 1998; McKiernan et al., 1998; Perlmutter et al., 1998). Pure postspike effects (Fig. 2a) had both (1) an onset latency after the trigger consistent with relatively direct connections from the recorded M1 neuron to the motoneuron pool, and (2) a narrow peak consistent with time-locked input to the motoneuron pool from only the recorded M1 neuron. Other peaks (Fig. 2b) had an onset latency consistent with relatively direct connections, but lasted too long to reflect input from only the M1 trigger neuron, indicating that additional synchronized inputs reached the motoneuron pool after those from the M1 neuron. Still other effects (Fig. 2c,d) had onset latencies too early and peaks too wide to be caused by input from the M1 trigger neuron, indicating that inputs from other neurons arrived at the motoneuron pool nearly synchronously with (± several milliseconds) or even before the input from the M1 neuron. Finally, some peaks (Fig. 2e) consisted of a pure postspike effect riding on an underlying synchrony effect.
Fig. 2.
Illustrative examples of effects in SpikeTAs and StimulusTAs. Vertical bars indicate the time of the triggering M1 neuron spike (left column) or sICMS pulse (right column). Short horizontal barsbeneath each column represent the 10 msec test window, from 6 to 16 msec after the trigger, in which each average was examined for a significant peak or trough. Each trace is 80 msec long (from 30 msec before to 50 msec after the trigger) and is scaled vertically to fill a constant height from the minimum to the maximum value. See Results for a detailed description of each example.
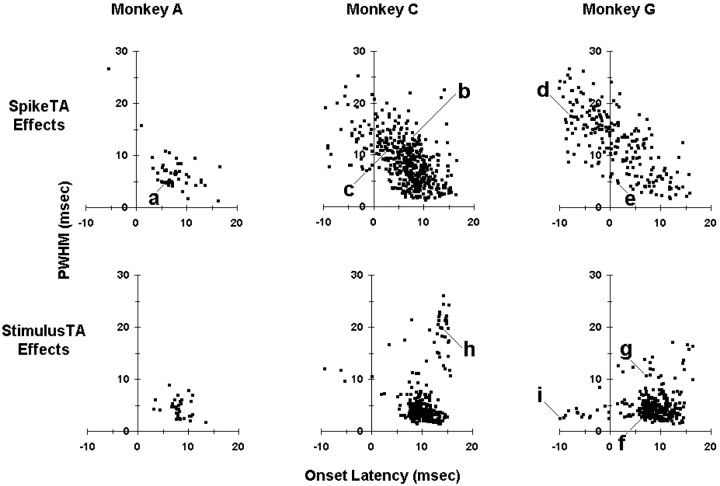
Onset latency and PWHM were measured for each significant peak or trough. Figure 3 shows scatter plots of PWHM versus onset latency for each significant effect in each of the three monkeys, A, C, and G. In all three monkeys, the majority of StimulusTA effects (Fig. 3, bottom row) had onset latency and PWHM appropriate for relatively direct inputs from stimulated M1 neurons. In monkey A, SpikeTA effects (Fig. 3, top row) showed a similar distribution, indicating that most SpikeTA effects in monkey A were relatively pure postspike effects. But in monkeys C and G, the majority of SpikeTA effects (Fig. 3, top row) had an onset latency too early and/or a PWHM too wide to be pure postspike effects from the M1 trigger neuron, indicating that other neurons also providing inputs to the motoneuron pool discharged synchronously with the recorded M1 neuron.
Fig. 3.
Scatter plots of onset latency (abscissa) versus PWHM (ordinate) for each significant SpikeTA effect (top row) or StimulusTA effect (bottom row) from each monkey: A (left), C (middle), and G (right). a–i indicate the points representing the example effects shown in Figure 2.
No bimodal separation between pure and synchrony SpikeTA effects was evident in the present data. However, previous studies have shown that latencies from cortical stimulation to the onsets of StimulusTA effects are consistently >5 msec (Cheney and Fetz, 1985), and a computer simulation has estimated that <5% of synchrony effects will have a PWHM of <9 msec (Baker and Lemon, 1998). Similarly, the vast majority of the present StimulusTA effects had an onset latency of >5 msec and a PWHM of <9 msec. Therefore, these values were used here to define pure effects; effects not meeting both criteria were considered to include synchrony. Using these criteria, the ratio of pure to synchrony effects in StimulusTAs was 31:3 in monkey A, 255:50 in monkey C, and 243:57 in monkey G; the variation among monkeys was insignificant (χ2 test; p > 0.2). But for SpikeTAs, the ratio of pure to synchrony effects was 37:10 in monkey A, 225:242 in monkey C; and 47:165 in monkey G; here the variation among monkeys was highly significant (χ2 test; p < 0.00001). Moreover, comparing the ratio of pure to synchrony effects in SpikeTAs versus StimulusTAs in each monkey revealed no significant difference in monkey A (χ2 test; p > 0.1) but highly significant differences in monkeys C and G (χ2 test; p < 0.00001). In monkey A, the majority of SpikeTA effects thus were pure postspike effects, whereas in monkeys C and G, the majority of SpikeTA effects had features of synchrony.
The striking difference in the prevalence of synchrony SpikeTA effects observed in monkeys A, C, and G, in conjunction with the overt differences in their training, suggested that prolonged training at a large movement repertoire was associated with increased synchrony. If synchrony increased with practice, then synchrony might have continued to increase as the monkeys continued to practice finger movements over the months of data collection. Therefore, the number of days from recording the first to the last neuron producing SpikeTA effects in each monkey (vertical bars in Fig. 1) was divided in half, and the numbers of pure versus synchrony SpikeTA effects in the two halves were compared. Although no significant differences were found for individual monkeys (A, first half pure:synchrony 14:4, second half pure:synchrony 23:6; C, first half pure:synchrony 154:155, second half pure:synchrony 71:87; G, first half pure:synchrony 14:43, second half pure:synchrony 33:122), pooling data from all three monkeys revealed a significantly increased percentage of synchrony effects in the second half of the data collection periods (127:215; 63% synchrony) compared with the first half (182:202; 53% synchrony; χ2test; p < 0.01), supporting the notion that synchrony increased with practice.
Whereas most StimulusTA effects in all three monkeys had an onset latency of >5 msec and a PWHM of <9 msec (Fig. 2f), indicating relatively direct inputs to the recorded motoneuron pool from stimulated M1 neurons, a noteworthy minority in monkeys C and G differed. Some had a wide peak (Fig. 2g), suggesting that the motoneuron pool received additional inputs from more indirectly (i.e., trans-synaptically) stimulated neurons, which might include both corticospinal and subcortical neurons. Other StimulusTA effects (Fig. 2h) had relatively late onsets and wide PWHMs. These were particularly common in monkey C, where they all consisted of late poststimulus suppression of EMG, with little or no preceding facilitation. Still other StimulusTA effects, found only in monkey G, had a narrow peak but a paradoxically early onset that preceded the stimulus time (Fig. 2i). Such effects consistently showed a facilitation peak followed by long-lasting suppression (>50 msec after the sICMS pulse), with recovery from this suppression producing the paradoxically early onset. All three types of StimulusTA effects that suggested relatively long-lasting effects of sICMS pulses (Fig.2g–i) were seen almost exclusively in monkeys C and G, further suggesting differences in the physiological connectivity of M1 in these two monkeys compared with monkey A.
DISCUSSION
Training and synchrony
Significant peaks and troughs 6–16 msec after the spikes of M1 neurons were significantly more prevalent in the SpikeTAs of two monkeys trained for >5 years to perform 12 finger and wrist movements than in one monkey trained for <1 year to perform six finger movements. Furthermore, after long-term training the majority of SpikeTA effects were synchrony effects rather than pure postspike effects. The increased prevalence of effects, together with the predominance of synchrony, suggests that an increased number of M1 neurons produced synchrony effects in SpikeTAs after long-term training.
Although not associated with training, synchrony in SpikeTA effects has been observed with similar ranges of prevalence in previous studies. In monkeys performing a precision grip task, the ratio of pure to synchrony M1 neuron SpikeTA effects was 12:64 (16% synchrony effects) (Baker and Lemon, 1998); whereas in monkeys performing a reach and grasp task, the ratio was 198:275 (58% synchrony) (McKiernan et al., 1998). During flexion/extension wrist movements, the pure:synchrony ratio for the SpikeTA effects of spinal interneurons was 161:108 (40% synchrony) (Perlmutter et al., 1998), whereas for the SpikeTA effects of primary afferents, the ratio was 13:5 (28% synchrony) in one monkey but 18:56 (76% synchrony) in another (Flament et al., 1992). Given that the training duration in these studies (not specifically reported) probably was not as long, nor the movement repertoires as large, as in the present study, synchrony may become prevalent in SpikeTA effects with shorter training periods and simpler repertoires than those of monkeys C and G. Indeed, whether synchrony increases during acquisition, practice, or overtraining at a motor skill remains uncertain.
In theory, the more prevalent synchrony observed in monkeys C and G could have resulted from higher M1 neuron discharge rates. If M1 neurons discharged at higher rates during finger movements, then more spikes would have occurred synchronously by chance alone. The average firing frequency of M1 neurons differed among monkeys (15, 23, and 20 Hz in A, C, and G, respectively; ANOVA; p < 0.00001), as did the average interspike interval (20, 29, and 25 msec; ANOVA;p < 0.00001), reflecting both lower tonic discharge and more intense bursting of M1 neurons in monkey A than in monkeys C and G. These small and offsetting differences in firing frequencies and interspike intervals, which also might have resulted from the longer training and more complex repertoire of monkeys C and G, seem unlikely to account for the large differences in the prevalence of synchrony SpikeTA effects, however.
All three of the present monkeys began training at ∼1–2 years of age; therefore, monkeys C and G were ∼4 years older than monkey A at the time of data collection. Maturation of the nervous system thus might have contributed to the differences between these older monkeys and monkey A. Most measures of corticospinal development in macaques reach adult values before 2 years of age (Galea and Darian-Smith, 1995;Armand et al., 1997; Olivier et al., 1997), however, and even monkey A was at least 2 years old by the start of data collection. Although continuing intracortical development remains a potential contributing factor, maturation seems unlikely to account entirely for the greater prevalence of synchrony effects in monkeys C and G compared with monkey A.
Sources of synchrony
Synchrony effects can be observed in SpikeTAs even if the recorded neuron itself has no direct connections to motoneuron pools: the recorded neuron may simply discharge spikes synchronized with those of other neurons that do have connections to motoneurons (Baker and Lemon, 1998). Nevertheless, M1 neurons with synchrony effects may have direct connections to motoneurons as well, as evidenced by observations of pure postspike effects riding on underlying synchrony effects. As the number of synchronous inputs to the motoneuron pool increases, the pure postspike effect of an M1 neuron may become immersed among the effects of synchronously discharging neurons on the same motoneuron pool.
Although much of the synchronization observed in the present study may have occurred among M1 neurons, synchronization also may have involved many other parts of the motor system. SpikeTA effects of M1 neurons indicate that inputs that arrived in the motoneuron pool time-locked to discharges of the recorded M1 neuron. In the case of pure postspike effects, the inputs are most likely monosynaptic or disynaptic connections from the recorded M1 neuron. In the case of synchrony effects, however, the inputs may include those from other M1 neurons, neurons of the red nucleus, and even dorsal root afferents, provided that these inputs arrive in the motoneuron pool time-locked to the discharge of the M1 neuron. The sources of synchrony effects in the SpikeTAs of M1 neurons thus may be distributed widely in the motor system.
Synchrony implies that neurons are temporally coupled by shared or sequential synaptic inputs. As training progresses, LTP may strengthen existing synapses, and new synaptic connections may form between neurons that regularly participate in controlling the skilled movement. In addition to altering the M1 output map as assessed by stimulation of the cortex, these synaptic changes may increase synchronization between participating M1 neurons, and between M1 neurons and other neurons that provide inputs to motoneuron pools. As training progresses, such increased synchronization could more efficiently recruit those motoneurons required for execution of the skilled movements.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS27686. I thank Jennifer Gardinier and Lee Anne Schery for technical assistance, Marsha Hayles for editorial comments, and Andrew Poliakov for assisting in many of the recording sessions.
Correspondence should be addressed to Dr. Marc H. Schieber, Department of Neurology, University of Rochester Medical Center, 601 Elmwood Avenue, Box 673, Rochester, NY 14642. E-mail: mhs@cvs.rochester.edu.
REFERENCES
- 1.Armand J, Olivier E, Edgley SA, Lemon RN. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. J Neurosci. 1997;17:251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroniadou VA, Keller A. Mechanisms of LTP induction in rat motor cortex in vitro. Cereb Cortex. 1995;5:353–362. doi: 10.1093/cercor/5.4.353. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma H, Pavlides C. Neurobiological basis of motor learning in mammals. NeuroReport. 1997;8:1–6. [PubMed] [Google Scholar]
- 4.Baker SN, Lemon RN. Computer simulation of post-spike facilitation in spike-triggered averages of rectified EMG. J Neurophysiol. 1998;80:1391–1406. doi: 10.1152/jn.1998.80.3.1391. [DOI] [PubMed] [Google Scholar]
- 5.Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- 6.Baker SN, Spinks R, Jackson A, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. I. Task-dependent modulation in single-unit synchrony. J Neurophysiol. 2001;85:869–885. doi: 10.1152/jn.2001.85.2.869. [DOI] [PubMed] [Google Scholar]
- 7.Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol (Lond) 1986;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- 9.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 10.Donoghue JP, Sanes JN, Hatsopoulos NG, Gaál G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- 12.Flament D, Fortier PA, Fetz EE. Response patterns and postspike effects of peripheral afferents in dorsal root ganglia of behaving monkeys. J Neurophysiol. 1992;67:875–889. doi: 10.1152/jn.1992.67.4.875. [DOI] [PubMed] [Google Scholar]
- 13.Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cereb Cortex. 1995;5:518–540. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- 14.Gross J, Tass PA, Salenius S, Hari R, Freund HJ, Schnitzler A. Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol (Lond) 2000;527:623–631. doi: 10.1111/j.1469-7793.2000.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 16.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 17.Kasser RJ, Cheney PD. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol. 1985;53:959–978. doi: 10.1152/jn.1985.53.4.959. [DOI] [PubMed] [Google Scholar]
- 18.Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemon RN, Mantel GW, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol (Lond) 1986;381:497–527. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol. 1998;80:1961–1980. doi: 10.1152/jn.1998.80.4.1961. [DOI] [PubMed] [Google Scholar]
- 21.Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol. 1996;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- 22.Ohara S, Nagamine T, Ikeda A, Kunieda T, Matsumoto R, Taki W, Hashimoto N, Baba K, Mihara T, Salenius S, Shibasaki H. Electrocorticogram-electromyogram coherence during isometric contraction of hand muscle in human. Clin Neurophysiol. 2000;111:2014–2024. doi: 10.1016/s1388-2457(00)00448-x. [DOI] [PubMed] [Google Scholar]
- 23.Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. J Neurosci. 1997;17:267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park MC, Belhaj-Saïf A, Gordon M, Cheney PD. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci. 2001;21:2784–2792. doi: 10.1523/JNEUROSCI.21-08-02784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascual-Leone A, Cammarota A, Wassermann EM, Brasil-Neto JP, Cohen LG, Hallett M. Modulation of motor cortical outputs to the reading hand of braille readers. Ann Neurol. 1993;34:33–37. doi: 10.1002/ana.410340108. [DOI] [PubMed] [Google Scholar]
- 26.Pascual-Leone A, Dang N, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- 27.Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol. 1998;80:2475–2494. doi: 10.1152/jn.1998.80.5.2475. [DOI] [PubMed] [Google Scholar]
- 28.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 29.Poliakov AV, Schieber MH. Multiple fragment statistical analysis of post-spike effects in spike-triggered averages of rectified EMG. J Neurosci Methods. 1998;79:143–150. doi: 10.1016/s0165-0270(97)00172-6. [DOI] [PubMed] [Google Scholar]
- 30.Riehle A, Grun S, Diesmann M, Aertsen A. Spike synchronization and rate modulation differentially involved in motor cortical function. Science. 1997;278:1950–1953. doi: 10.1126/science.278.5345.1950. [DOI] [PubMed] [Google Scholar]
- 31.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 32.Schieber MH. Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. J Neurophysiol. 1991;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- 33.Smith WS, Fetz EE. Effects of synchrony between primate corticomotoneuronal cells on post-spike facilitation of muscles and motor units. Neurosci Lett. 1989;96:76–81. doi: 10.1016/0304-3940(89)90246-2. [DOI] [PubMed] [Google Scholar]
- 34.Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]