Abstract
Heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) is found in cerebral neurons, and its expression is increased after hypoxic or ischemic injury, which also stimulates neurogenesis. To investigate the possible role of HB-EGF in hypoxic–ischemic induction of neurogenesis, we measured its expression, effects, and target receptors in embryonic murine cerebral cortical cultures and in adult rat brain. Hypoxia increased HB-EGF expression by ∼50% in cortical cultures, where expression was associated with mature and immature neurons. HB-EGF (5–100 ng/ml) stimulated by ∼80% the incorporation of bromodeoxyuridine (BrdU) into cultured cells that expressed the HB-EGF receptors epidermal growth factor receptor (EGFR)/avian erythroblastic leukemia viral oncogene homolog 1 (ErbB1) and N-arginine dibasic convertase (NRDc). Intracerebroventricular administration of HB-EGF in adult rats increased BrdU labeling in the subventricular zone and in the subgranular zone of dentate gyrus, where EGFR/ErbB1 and NRDc were also expressed and where ischemia-induced neurogenesis is observed. We conclude that HB-EGF stimulates neurogenesis in proliferative zones of the adult brain that are also affected in ischemia and that it does so by interacting with EGFR/ErbB1 and possibly NRDc. Therefore, HB-EGF may help to trigger proliferation of neuronal precursors in brain after hypoxic or ischemic injury.
Keywords: neurogenesis, hypoxia, ischemia, heparin-binding epidermal growth factor-like growth factor (HB-EGF), epidermal growth factor receptor (EGFR/ErbB1), N-arginine dibasic convertase (NRDc)
Heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) is an 87 amino acid mitogenic and chemotactic glycoprotein containing an EGF-like domain with six conserved cysteine residues (Higashiyama et al., 1991). HB-EGF is released from cells (Gechtman et al., 1999; Dong and Wiley, 2000) and acts through the tyrosine kinase EGF receptor (EGFR)/avian erythroblastic leukemia viral oncogene homolog 1 (ErbB1) (Raab and Klagsbrun, 1997; Davis-Fleischer and Besner, 1998; Jost et al., 2000), but it also acts through EGF-insensitive receptors, including ErbB4, heparan sulfate proteoglycan (HSPG) (Raab and Klagsbrun, 1997), and the metalloendopeptidase, N-arginine dibasic convertase (NRDc) (Nishi et al., 2001). Downstream signaling by EGF family members involves the activation of protein kinase cascades (Jost et al., 2000) and nuclear translocation of EGF receptors, which may act as transcription factors (Lin et al., 2001b). The membrane-bound HB-EGF precursor, pro-HB-EGF, also binds diphtheria toxin (Naglich et al., 1992) and the anti-apoptotic protein Bag1 (Lin et al., 2001a).
HB-EGF is widely distributed in neurons and neuroglia throughout the brain (Mishima et al., 1996; Hayase et al., 1998), where it is expressed in both proliferating and post-mitotic cells of prenatal and postnatal rats (Nakagawa et al., 1998). HB-EGF mRNA and protein are induced in hippocampal CA3 and dentate gyrus (DG) neurons after global cerebral ischemia (Kawahara et al., 1999) and neonatal hypoxic–ischemic injury (Tanaka et al., 1999) in the rat. HB-EGF mRNA and protein are also induced in rat hippocampus by kainic acid, and HB-EGF protects cultured hippocampal neurons from kainate toxicity (Opanashuk et al., 1999). These findings are consistent with a neuroprotective role for HB-EGF in ischemia and related insults.
Ischemia and other cerebral injuries stimulate neurogenesis in neuroproliferative regions of the adult brain, including the rostral subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal DG (Gould and Tanapat, 1997; Parent et al., 1997; Liu et al., 1998; Takagi et al., 1999; Gu et al., 2000; Magavi et al., 2000;Jin et al., 2001; Yoshimura et al., 2001; Zhang et al., 2001). Growth factors such as fibroblast growth factor-2 (FGF-2) seem to be important mediators of this phenomenon because post-ischemic neurogenesis is attenuated in FGF-2 knock-out mice and restored when exogenous FGF-2 is supplied (Yoshimura et al., 2001). Some evidence also points to a role for HB-EGF in neurogenesis, because HB-EGF increases the proliferation of neuroblasts from the external granular layer of the cerebellum, as measured by the incorporation of [3H]thymidine in vitro(Opanashuk and Hauser, 1998).
The observations that (1) HB-EGF is induced by cerebral hypoxia or ischemia (Kawahara et al., 1999; Tanaka et al., 1999) and (2) HB-EGF stimulates neurogenesis in neonatal cerebellar neuroblast cultures (Opanashuk and Hauser, 1998) raise the possibility that HB-EGF might be involved in hypoxia- and ischemia-induced neurogenesis in the adult brain. Therefore, we investigated whether HB-EGF protein expression is induced by neuronal hypoxia in vitro and whether HB-EGF reproduces the effect of neuronal hypoxia or ischemia on neurogenesis both in vitro and in vivo.
MATERIALS AND METHODS
Animal experiments were approved by local committee review and conducted according to policies on the use of animals of The Society for Neuroscience.
Cell culture. Cortical neuron cultures were prepared from 16 d Charles River CD1 mouse embryos as described (Sun et al., 2001), except that Neurobasal medium containing 2% B27 supplement, 2 mm glutamate, and 1% penicillin and streptomycin (Invitrogen, Rockville, MD) was used (Brewer et al., 1993). After 4 d, one-half of the medium was replaced with Neurobasal medium containing 2% B27. Experiments were conducted at 7 d in vitro.
Hypoxia. To induce hypoxia, cultures were placed in modular incubator chambers (Billups-Rothenberg, Del Mar, CA) for 0–24 hr at 37°C in humidified 95% air/5% CO2 (control) or humidified 95% N2/5% CO2(hypoxia) (Goldberg and Choi, 1993; Koretz et al., 1994). Cultures were then returned to normoxic conditions for the remainder, if any, of 24 hr.
Western blotting. Cell lysates were extracted in PBS, pH 7.5, containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 μg/ml of aprotinin, and 100 μg/ml of PMSF, and protein concentration was determined using a Bio-Rad protein assay (Bio-Rad, Hercules, CA). Protein (100 μg per lane) was boiled at 100°C in SDS sample buffer for 5 min, electrophoresed by 15% SDS-PAGE, and transferred to polyvinylidene difluoride membranes. These were incubated overnight at 4°C with a goat polyclonal anti-HB-EGF antibody directed against an epitope mapping at the C terminus of mouse HB-EGF (Santa Cruz Biotechnology, Santa Cruz, CA; 1:250), which does not cross-react with EGF. Membranes were washed with PBS containing 0.1% Tween-20, incubated at room temperature for 60 min with horseradish peroxidase-conjugated anti-goat secondary antibody (Santa Cruz Biotechnology; 1:3000), and washed three times (15 min each) with PBS/Tween-20. Peroxidase activity was visualized with a chemoluminescence substrate system (NEN Life Science Products, Boston, MA). Differences in protein expression on Western blots were quantified using a GS-710 calibrated imaging densitometer and Quantity One software (Bio-Rad).
Immunocytochemistry. Cultures were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and incubated with blocking buffer (2% horse serum, 1% BSA, and 0.1% Triton X-100 in PBS, pH 7.5). Primary antibodies were added at 4°C overnight and included the following: goat polyclonal anti-HB-EGF (Santa Cruz Biotechnology; 1:100), mouse monoclonal anti-BrdU (Roche, Indianapolis, IN; 2 μg/ml), sheep polyclonal anti-BrdU (Biodesign, Saco, ME; 25 μg/ml), mouse monoclonal anti-neuronal nuclear antigen (NeuN) (Chemicon, Temecula, CA; 1:200), mouse monoclonal anti-MAP2 (Sigma, St. Louis, MO; 1:100), mouse monoclonal anti-rat nestin (BD PharMingen, San Diego, CA; 1:400), mouse monoclonal anti-βIII-tubulin (Caltag Laboratories, Burlingame, CA; 1:400), rabbit polyclonal anti-EGFR/erbB1 (Santa Cruz Biotechnology; 1:100), mouse monoclonal and goat polyclonal anti-ErbB4 (Santa Cruz Biotechnology; 1:100), and rabbit polyclonal anti-NRDc raised against a fusion protein of the acidic stretch of rat NRDc (amino acids 127–214) and glutathione S-transferase (1:300). The secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG, FITC-conjugated goat anti-rabbit IgG (Vector, Burlingame, CA; 1:200), and FITC-conjugated pig anti-goat IgG, rhodamine-conjugated rat-absorbed donkey anti-mouse IgG, rhodamine-conjugated rat-absorbed donkey anti-rabbit IgG, and rhodamine-conjugated rat-absorbed donkey anti-sheep IgG (Jackson ImmunoResearch, West Grove, PA; 1:200). DAPI (Vector) was used to counterstain nuclei, and fluorescence signals were detected with a Nikon E300 microscope at excitation/emission wavelengths of 535/565 nm (rhodamine, red), 470/505 (FITC, green), and 360/400 (DAPI, blue). Results were recorded with a Magnifire digital camera (ChipCoolers, Warwick, RI). Controls included omitting or preabsorbing the primary antibody and omitting the secondary antibody.
BrdU labeling in vitro. For immunocytochemical detection, BrdU (Sigma; 50 μg/ml) was added to cultures for 24 hr. Cultures were fixed in 4% paraformaldehyde in PBS for 40 min, washed with PBS, incubated for 2 hr with blocking buffer (2% horse serum, 1% BSA, and 0.1% Triton X-100 in PBS, pH 7.5), incubated overnight at 4°C with mouse monoclonal anti-BrdU primary antibody (Roche; 2 μg/ml), and then incubated for 1 hr at room temperature with rhodamine-conjugated goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch; 1:200). BrdU-immunoreactive cells were counted in five microscope fields per well (center and 3, 6, 9, and 12 o'clock). In some experiments, BrdU incorporation was measured using a commercially available enzyme-linked immunosorbent assay kit (Roche) (Law et al., 1996). In these experiments, growth factors were added to cultures for 24 hr and then BrdU was added for 4 hr; in some cases, HB-EGF was added together with a neutralizing mouse monoclonal antibody against EGFR/ErbB1 (clone 225; NeoMarkers, Fremont, CA).
Cell viability. Cell viability was assayed by incubating cells with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 5 mg/ml; Sigma) at 37°C for 2 hr. Medium was removed, and cells were solubilized with dimethylsulfoxide and transferred to 96-well plates. Absorbance at 570 nm was measured in a Cytofluor 4000 plate-reader (PerSeptive Biosystems, Framingham, MA). Results were expressed as a percentage of control absorbance, measured in normoxic cultures, after subtracting background absorbance measured in freeze-thawed cultures.
Retroviral infection. Humanized Renilla reniformis green fluorescent protein (hrGFP) was cloned downstream of the viral promoter of pFB retroviral vector (Stratagene, La Jolla, CA). The vector was produced by transiently transfecting NIH 3T3 cells with two additional vectors expressing gag-pol and vesicular stomatitis virus G envelope protein. pFB-hrGFP supernatant containing ≥4.9 × 10−7 infectious virus particles was filtered through a 0.45 μm filter and frozen at −80°C. Ten microliters of retroviral supernatant were added to cultures in four-well plates containing 500 μl of medium and incubated at 37°C for 2 d and then for 24 hr in normoxic or hypoxic conditions. GFP-expressing cells were detected with a Nikon E300 microscope with excitation at 470 and emission at 505 nm.
HB-EGF and BrdU administration in vivo. Adult (8–10 week) male Sprague Dawley rats weighing 280–310 gm were anesthetized with 4% isoflurane in 70% N2O/30% O2 and implanted with an osmotic minipump (Alzet 1003D; Alza Scientific Products, Mountain View, CA). The cannula was placed in the right lateral ventricle 4.0 mm deep to the pial surface, +0.8 mm anteroposterior relative to bregma, and 1.3 mm lateral to the midline. Each rat was infused for 3 d with 1 μl/hr of either recombinant human HB-EGF (1 μg/ml, R&D Systems, Minneapolis, MN) in artificial CSF (aCSF) containing (in mm): 128 NaCl, 2.5 KCl, 0.95 CaCl2, 1.99 MgCl2(n = 4) or aCSF alone (n = 6). BrdU (50 mg/kg, Sigma) was dissolved in saline and given intraperitoneally twice daily at 8 hr intervals, for the same 3 consecutive days, and rats were killed 1 week later.
BrdU immunohistochemistry in brain sections. Brains (four per condition) were removed after perfusion with saline and 4% paraformaldehyde in PBS. Adjacent 50 μm sections, corresponding to coronal coordinates interaural 8.7–10.2 mm, bregma −0.30 to bregma −1.2 mm (SVZ), and interaural 4.48–5.86 mm, bregma −4.52 to bregma −3.14 (DG), were cut with a cryostat and stored at −80°C. Sections were pretreated with 50% formamide, 280 mm NaCl, and 30 mm sodium citrate at 65°C for 2 hr, incubated in 2 m HCl at 37°C for 30 min, and rinsed in 0.1 m boric acid, pH 8.5, at room temperature for 10 min. Sections were incubated in 1% H2O2 in PBS for 15 min, in blocking solution (2% goat serum, 0.3% Triton X-100, and 0.1% bovine serum albumin in PBS) for 2 hr at room temperature, and with 2 μg/ml of mouse monoclonal anti-BrdU antibody (Roche) at 4°C overnight. Sections were washed with PBS, incubated with biotinylated goat anti-mouse secondary antibody (Vector, 1:200) for 2 hr at 25°C, washed, and placed in avidin–peroxidase conjugate (Vector) solution for 1 hr. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB) and 0.03% H2O2. Processing was stopped with H2O, and sections were dehydrated through graded alcohols, cleared in xylene, and coverslipped in permanent mounting medium (Vector). Sections were examined with a Nikon E300 epifluorescence microscope.
For double immunolabeling studies, sections were fixed with 4% paraformaldehyde in PBS for 1 hr at room temperature, washed twice with PBS, and incubated in 2 m HCl at 37°C for 1 hr. After washing again, sections were incubated with blocking solution, then with primary antibodies at 4°C overnight, and with secondary antibodies in blocking solution at room temperature for 2 hr. The primary antibodies used were mouse monoclonal anti-BrdU (Roche; 2 μg/ml) and affinity-purified goat polyclonal anti-NeuroD (1:100, Santa Cruz Biotechnology), and the secondary antibodies were rhodamine-conjugated rat-absorbed donkey anti-mouse IgG (Jackson ImmunoResearch; 1:200) and FITC-conjugated pig anti-goat IgG (Jackson ImmunoResearch; 1:200). Sections were mounted with Vectashield (Vector), and fluorescence signals were detected with a Nikon E800 microscope at excitation/emission wavelengths of 535/565 nm (rhodamine, red) and 470/505 (FITC, green). Results were recorded with a Magnifire digital camera (ChipCoolers). For confocal microscopy, a Nikon PCM-2000 laser-scanning confocal microscope and Simple PCI imaging software (Compix) were used.
BrdU-immunopositive cell counting. BrdU-positive cells in SGZ and SVZ were counted blindly in five to seven DAB-stained, 50 μm coronal sections per animal, spaced 200 μm apart. Cells were counted under high-power (200×) on a Nikon E300 microscope with a Magnifire digital camera, and the image was displayed on a computer monitor. Results were expressed as the average number of BrdU-positive cells per section.
Data analysis. Quantitative data were expressed as mean ± SEM from at least three experiments. ANOVA and Student'st test were used for statistical analysis, withp < 0.05 considered significant.
RESULTS
Cellular composition of cultures
At 7 d in vitro, cultures contained primarily cells of neuronal lineage, as determined by the expression of cell type-specific markers (Table 1), and the expression of non-neuronal markers was not altered by hypoxia.
Table 1.
Cellular composition of cultures based on expression of cell type-specific marker proteins
| Lineage | Marker | Treatment | ||
|---|---|---|---|---|
| Control | Hypoxia | HB-EGF | ||
| Neuronal | Nestin | 23 ± 3 | ND | 30 ± 3 |
| NeuroD | 6 ± 1 | ND | 14 ± 3* | |
| βIII tubulin | 75 ± 1 | ND | 99 ± 11-160 | |
| Hu | 82 ± 4 | ND | 99 ± 11-160 | |
| Calbindin | 13 ± 1 | ND | 19 ± 2* | |
| Astroglial | GFAP | 2 ± 1 | 2 ± 1 | 2 ± 2 |
| Microglial | CD11b | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Endothelial | CD146 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Cultures were maintained for 24 hr in 95% air/5% CO2 (control), 95% N2/5% CO2(hypoxia), or 95% air/5% CO2 plus 10 ng/ml of HB-EGF (HB-EGF). Data shown are mean percentages ± SEM (n = 3–5) of cells expressing a given marker.
p < 0.05,
F1-160: p< 0.005 relative to control.
Hypoxia induces HB-EGF expression in cortical cultures
To determine the effect of hypoxia on the expression of HB-EGF protein, cortical cultures were exposed to hypoxia for 0–24 hr, and HB-EGF expression was measured by Western blotting. The polyclonal anti-HB-EGF antibody used does not cross-react with other EGF-family members, including EGF. Accordingly, as shown in Figure1, a band was labeled at ∼22 kDa, as predicted for HB-EGF [which shows anomalous, retarded migration on SDS-PAGE gels caused by heavy glycosylation and positively charged domains (Raab and Klagsbrun, 1997)] but not at ∼7 kDa, the anticipated Mr for EGF. HB-EGF expression increased over basal levels after 4–8 hr of hypoxia and declined with more prolonged hypoxic exposure. Maximal induction, observed at 4 hr, reached ∼150% of control levels. HB-EGF expression was localized to the cytoplasmic compartment by immunocytochemistry, consistent with findings in rat brain (Hayase et al., 1998). HB-EGF was present in mature neurons that expressed NeuN or MAP2, immature neurons expressing βIII tubulin, and neuroepithelial precursor cells, which express nestin (Fig. 2).
Fig. 1.
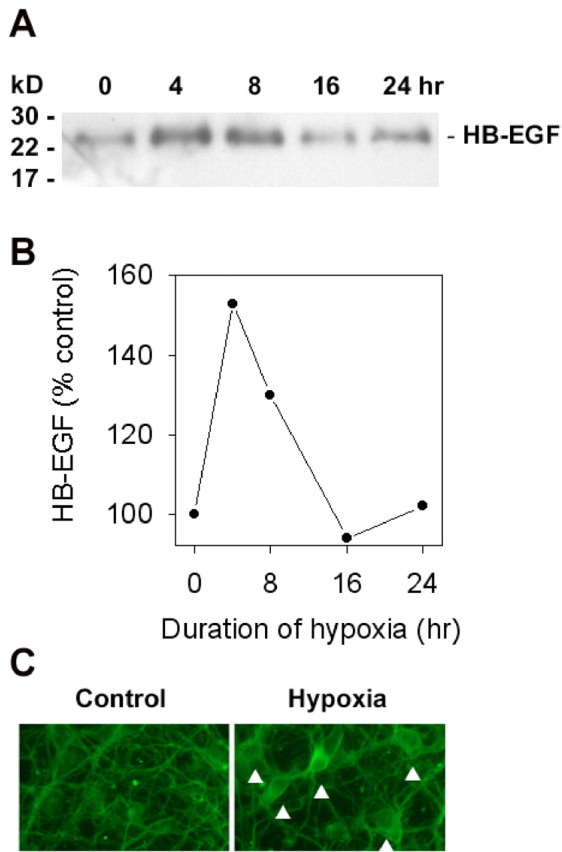
Hypoxia induces the expression of HB-EGF protein in cerebral cortical cultures. Cultures were exposed to hypoxia for up to 24 hr, and HB-EGF expression was measured by Western blotting (A), which showed a hypoxia-induced increase in the expression of HB-EGF at 4–8 hr. The increase in cellular expression of HB-EGF was quantified by computer densitometry (B), which showed that maximal induction, observed at 4 hr, reached 153 ± 13% of control levels (range, 139–179%; n = 3). HB-EGF expression was localized to the neuronal cytoplasm by immunocytochemistry (C,arrowheads; original magnification, 40×). Data are representative (A, C) or means (B) of at least three independent experiments.
Fig. 2.
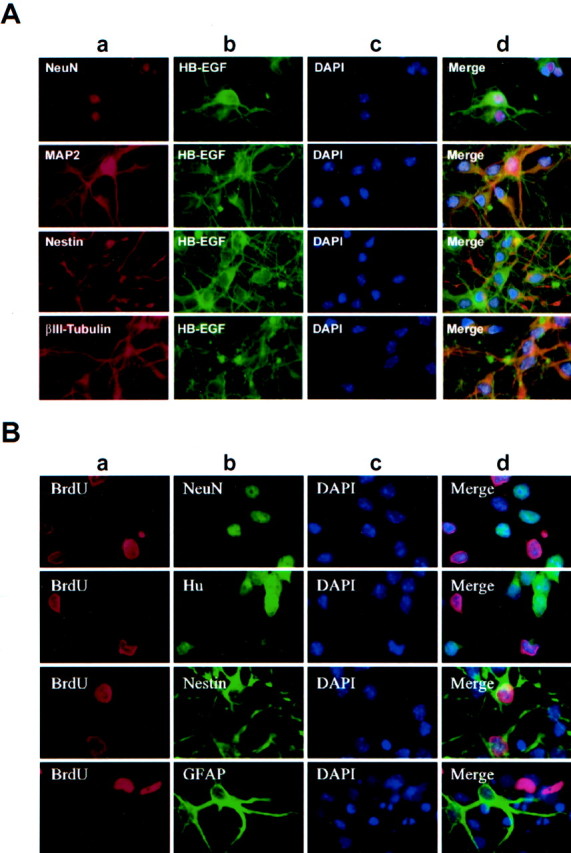
Hypoxia-inducible expression of HB-EGF is associated with both mature and immature neurons. A, Cultures were exposed to hypoxia for 8 hr and labeled with an antibody against a marker of mature neurons (NeuN,MAP2), immature neurons (βIII-tubulin), or neuroepithelial precursor cells (Nestin) (a); an antibody against HB-EGF (b); and the nuclear stain DAPI (c). Merged images are shown in d. NeuN-, MAP2-, βIII-tubulin-, and nestin-immunopositive cells all coexpressed HB-EGF. B, Cultures were treated with BrdU, exposed to hypoxia for 8 hr, and labeled with an antibody against BrdU (a); an antibody against the mature neuronal marker NeuN, the late immature to mature neuronal marker Hu, the neuroepithelial precursor cell marker nestin, or the astroglial marker GFAP (b); and the nuclear stain DAPI (c). Merged images are shown in d. Only nestin-immunopositive cells were labeled with BrdU. Data inA and B are representative fields from at least three independent experiments per row. Original magnification, 40×.
HB-EGF stimulates neurogenesis in mouse cerebral cortical cultures
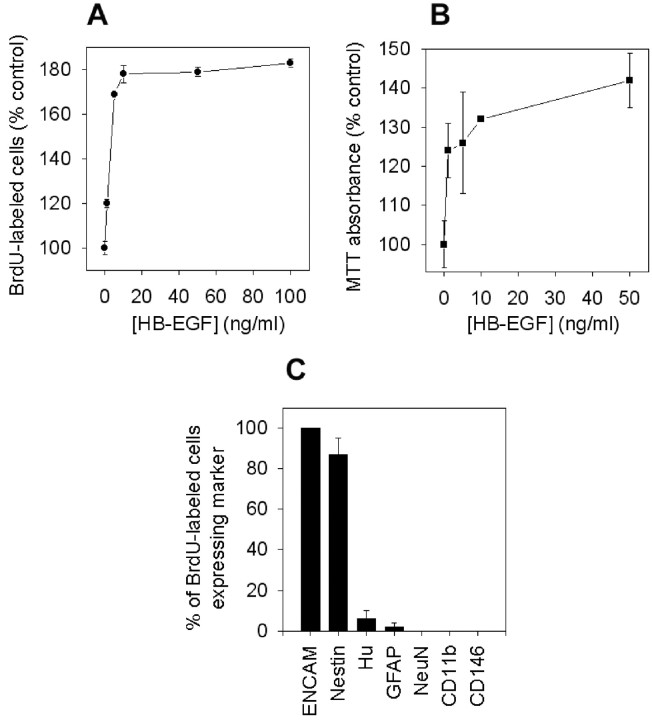
To determine whether HB-EGF stimulates neurogenesis, BrdU was added to cortical cultures to label cells undergoing mitosis (S-phase of the cell cycle), and cultures were maintained for 24 hr under normoxic conditions in the presence of 0–100 ng/ml of HB-EGF. In these cultures, BrdU incorporation colocalizes with markers of cell division, including the proliferating cell nuclear antigen (Ino and Chiba, 2000), the mitotic chromosome condensation marker phospho-histone-H3 (Hendzel et al., 1997), and the replication-licensing protein CDC47 (Fujita et al., 1996), but not with markers of cell injury (Chen et al., 1997; Jin et al., 1999), such as labeling with the Klenow fragment of DNA polymerase I, DNA polymerase I-mediated biotin-dATP nick translation labeling, or terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (our unpublished data). Figure 3A shows that HB-EGF increased BrdU labeling by ∼80% over control levels. The effect was maximal between 5 and 100 ng/ml, which is consistent with the range of HB-EGF concentrations (10–100 ng/ml) that protect cultured hippocampal neurons from kainate toxicity (Opanashuk et al., 1999). Most cells labeled by BrdU in the presence of HB-EGF expressed neuronal precursor and immature neuronal markers (Fig. 3C), and the effect of HB-EGF was associated with increases in the percentage of cells expressing markers of neuronal lineage (Table 1).
Fig. 3.
HB-EGF stimulates BrdU incorporation and increases the number of viable cells in cerebral cortical cultures. Treatment of normoxic cultures for 24 hr with HB-EGF increased both BrdU incorporation measured by ELISA (A) and the number of viable cells, measured by MTT absorbance (B). Most cells stimulated to incorporate BrdU in the presence of HB-EGF expressed markers of immature neurons (ENCAM) or neuroepithelial precursor cells (Nestin), whereas few or no BrdU-labeled cells expressed markers of more mature neurons (Hu,NeuN), astroglia (GFAP), microglia (CD11b), or endothelial cells (CD146). Data are mean ± SEM;n = 3.
HB-EGF increases the number of viable cells in cortical cultures
If HB-EGF stimulates neurogenesis, then it should increase the number of viable cells in culture. To test this hypothesis, cultures were maintained for 24 hr in the presence of 0–100 ng/ml of HB-EGF, and cell viability was measured by MTT absorbance and by cell counting. At concentrations similar to those associated with increased incorporation of BrdU, HB-EGF increased MTT absorbance by as much at 40% (Fig. 3B). Because MTT absorbance measures cell viability as a function of metabolic activity, and because it is conceivable that an increase in MTT absorbance could result from an increase in metabolic activity per cell rather than an increase in cell number, we also counted cells in control and HB-EGF-treated cultures. Cell counts increased to 125 ± 7% of control values (n = 15) in cultures exposed for 24 hr to 10 ng/ml of HB-EGF, confirming that HB-EGF stimulates cell proliferation.
Mechanism for stimulation of neurogenesis by HB-EGF
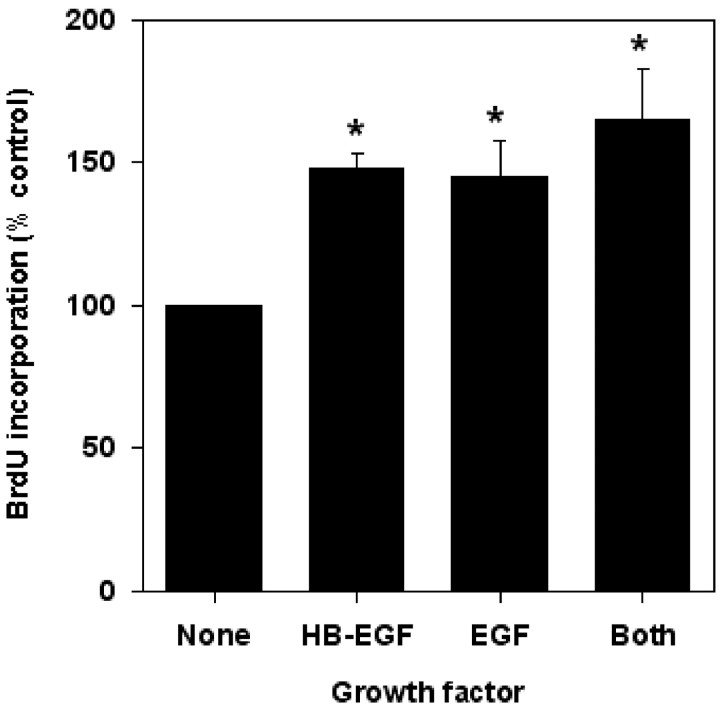
HB-EGF exerts its biological effects by interacting with various receptors, the best characterized of which are the tyrosine kinase EGF receptor EGFR/ErbB1 (Raab and Klagsbrun, 1997; Davis-Fleischer and Besner, 1998; Jost et al., 2000) and the EGF-insensitive tyrosine kinase receptor ErbB4 (Raab and Klagsbrun, 1997). To evaluate which of these receptors might mediate the neuroproliferative effect of HB-EGF, we first measured BrdU incorporation into cortical neuron cultures treated for 24 hr with 10 ng/ml of HB-EGF or 10 ng/ml of EGF or both growth factors in combination. Figure 4shows that HB-EGF and EGF stimulated BrdU incorporation to similar extents and that their effects were not additive, which is consistent with a shared mechanism of action.
Fig. 4.
Effects of HB-EGF, EGF, and both in combination on BrdU incorporation in cerebral cortical cultures. Normoxic cultures were exposed for 24 hr to 10 ng/ml of HB-EGF, 10 ng/ml of EGF, or a combination of the two. BrdU was added for 4 hr, and BrdU incorporation was determined by ELISA. Data are mean ± SEM (n = 3). *p < 0.05 relative toNone (ANOVA and post hocttests).
To address this issue more directly, HB-EGF- and BrdU-treated cultures were immunostained for cell proliferation markers (BrdU or GFP, expressed via a retroviral vector that infects dividing cells) and for each of three HB-EGF receptors: EGFR/ErbB1, ErbB4, and NRDc. Many BrdU- and GFP-labeled cells expressed EGFR/ErbB1 and NRDc, but few expressed ErbB4 (Fig.5A,B), suggesting that cells stimulated to proliferate by HB-EGF were primarily EGFR/ErbB1 and NRDc positive but ErbB4 negative. Expression of EGFR/ErbB1 and NRDc was associated with nestin-immunopositive neuronal precursor cells, whereas expression of ErbB4 was associated with NeuN-immunopositive neurons (Fig. 5C,D). These findings are consistent with a role for EGFR/ErbB1 or NRDc or both in HB-EGF-induced neurogenesis. A shared role for these receptors has been implicated previously in the ability of HB-EGF to induced migration of HeLa cells and keratinocytes (Nishi et al., 2001).
Fig. 5.
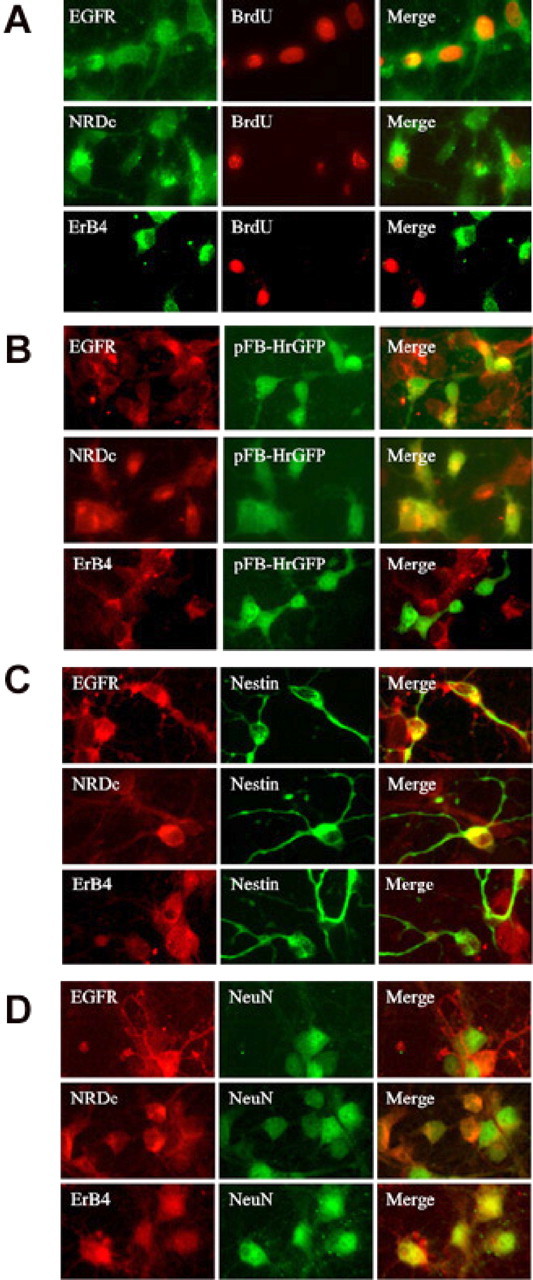
Expression of HB-EGF receptors and cell type-specific markers by cells labeled with BrdU in the presence of HB-EGF. Normoxic cultures were treated with BrdU (A) or infected with a GFP-expressing retroviral vector (pFB-hrGFP) (B) and exposed for 24 hr to 10 ng/ml of HB-EGF. Cells were immunostained for BrdU or GFP and for the HB-EGF receptors EGFR/ErbB1 (EGFR), ErbB4, and NRDc. Merged images are shown at right. BrdU and GFP immunoreactivity colocalized with EGFR/ErbB1 and NRDc but not ErbB4. Some cultures were also stained for the neuroepithelial precursor cell marker nestin (C) or the mature neuronal marker NeuN (D) and for the receptors listed above. EGFR/ErbB1 and NRDc colocalized with nestin, whereas ErbB4 colocalized with NeuN. Data are representative fields from at least three independent experiments per row. Original magnification, 40×.
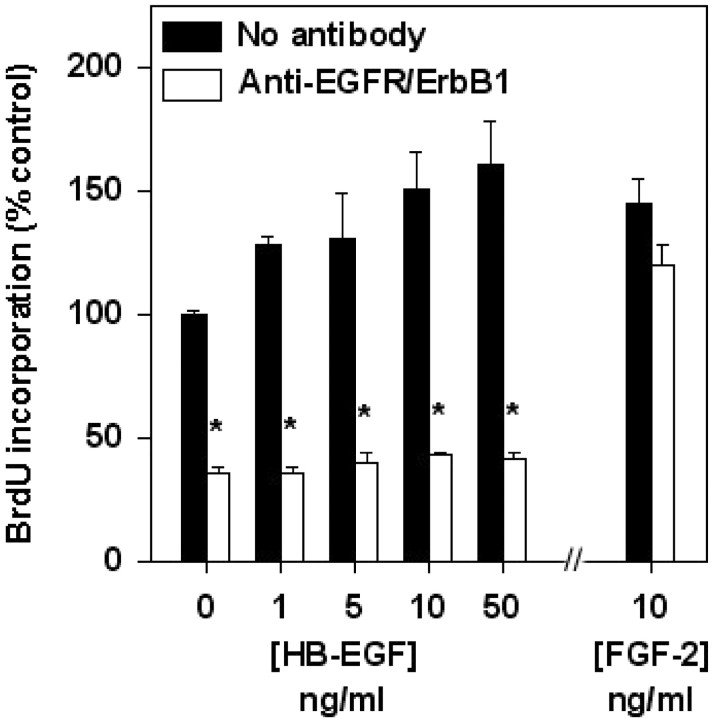
To test further the hypothesis that EGFR/ErbB1 is required for HB-EGF-induced neurogenesis, some cultures were treated with HB-EGF and BrdU in the presence of a neutralizing antibody against EGFR/ErbB1. Under these conditions, basal incorporation of BrdU was reduced, and the ability of HB-EGF to stimulate BrdU incorporation was abolished (Fig. 6). In contrast, stimulation of BrdU incorporation by FGF-2 was unaffected by the antibody. This suggests that both a component of basal BrdU incorporation (which may be stimulated by the release of endogenous EGF or HB-EGF or both) and incorporation of BrdU induced by exogenous HB-EGF are mediated through EGFR/ ErbB1. This result is also consistent with the ability of the same neutralizing antibody against EGFR/ErbB1 to block EGFR/ErbB1- and NRDc-mediated effects of HB-EGF on cell migration (Nishi et al., 2001).
Fig. 6.
Effect of a neutralizing antibody against EGFR/ErbB1 on HB-EGF-stimulated incorporation of BrdU. Normoxic cultures were treated with HB-EGF or FGF-2 in the presence of anti-EGFR/ErbB1 for 24 hr and then exposed to BrdU for 4 hr, and BrdU incorporation was determined by ELISA. Data are mean ± SEM (n = 3). *p < 0.05 relative toNo antibody (ANOVA and post hoct tests).
HB-EGF stimulates neurogenesis in vivo
The ability of HB-EGF to stimulate neurogenesis in vitro suggests that a similar effect might occur in vivo. To test whether HB-EGF can stimulate neurogenesis in vivo, HB-EGF was infused into the right lateral ventricle and BrdU was injected intraperitoneally for 3 d in adult rats, and animals were killed 1 week later. As shown in Figures 7 and 8A, the number of BrdU-immunopositive cells in DG and SVZ increased after HB-EGF administration, most prominently in SVZ, and especially on the side of the infusion. HB-EGF infusion also increased the number of cells in DG and SVZ that were colabeled with antibodies against BrdU and the immature neuronal marker, NeuroD (Lee et al., 2000) (Fig.8B), indicating that HB-EGF affected cells of neuronal lineage.
Fig. 7.
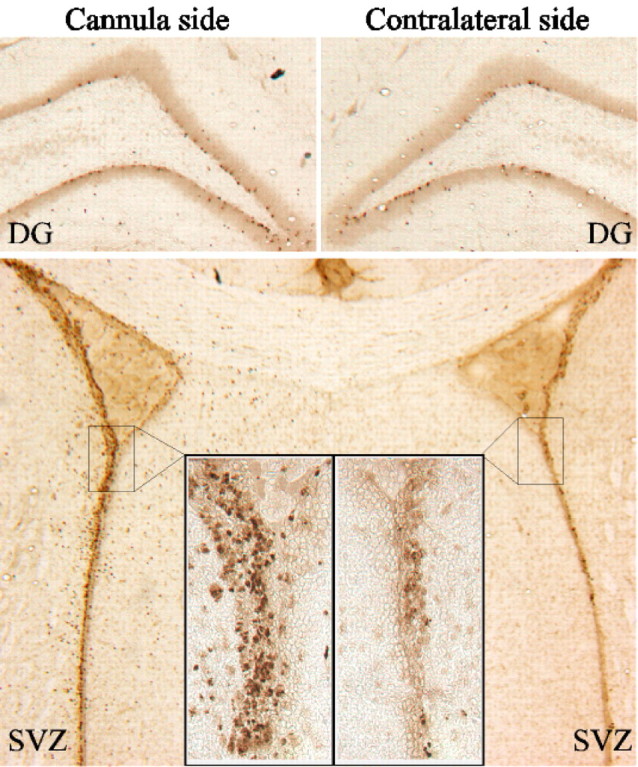
Effect of intracerebroventricular administration of HB-EGF on BrdU incorporation in DG and SVZ in vivo. Adult male rats were treated for 3 d with BrdU, given intraperitoneally, and with HB-EGF, infused into the right lateral ventricle; BrdU incorporation on the side of HB-EGF infusion (cannula side) and on the contralateral side was detected by immunohistochemistry. Sections through the DG show BrdU labeling of a similar number of cells in the SGZ on both sides, whereas sections through the SVZ show more prominent labeling on the cannula side (original magnification, 4×; insets, 40×). Data are representative fields from at least three independent experiments.
Fig. 8.
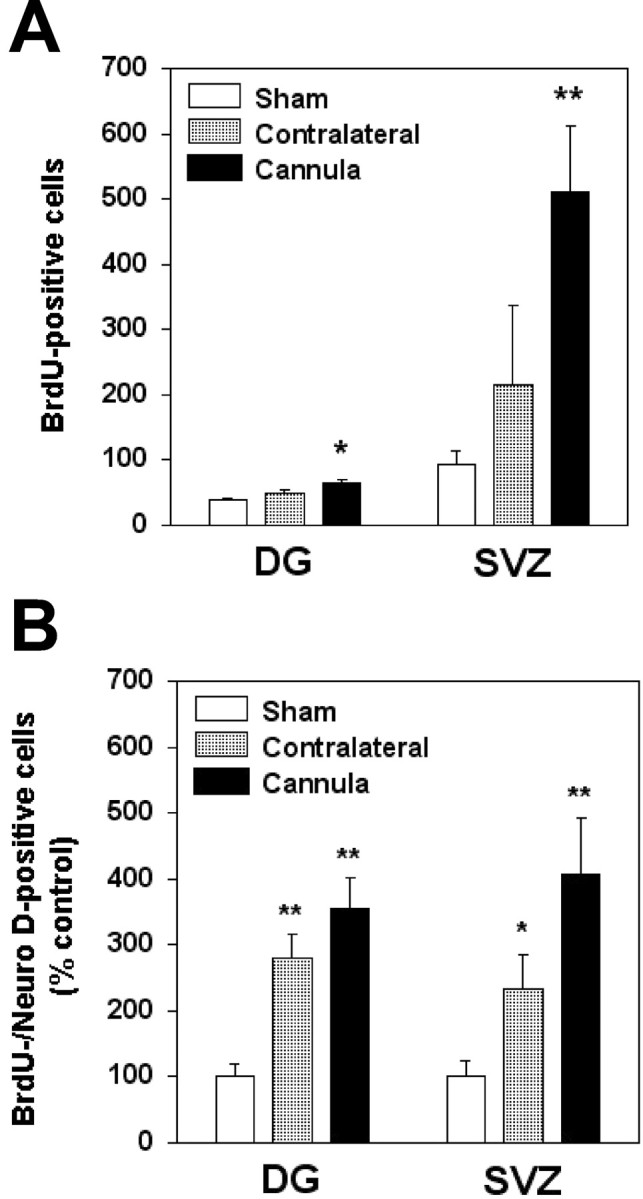
Quantitation of BrdU incorporation in DG and SVZ after intracerebroventricular administration of HB-EGF in vivo. A, Adult male rats were treated for 3 d with BrdU, given intraperitoneally, and with either HB-EGF or vehicle, infused into the right lateral ventricle. BrdU incorporation was detected by immunohistochemistry and the number of BrdU-immunopositive cells in vehicle-treated animals (Sham, white bars) and on the side contralateral (Contralateral, shaded bars) and ipsilateral (Cannula, black bars) to HB-EGF infusion was counted in DG and SVZ and expressed as mean ± SEM (n = 3). *p < 0.05 relative to Sham; **p < 0.02 relative to Sham.B, In some experiments, cells immunoreactive for both BrdU and the immature neuronal marker NeuroD were counted and expressed as a mean percentage ± SEM (n = 3) of BrdU-immunopositive/NeuroD-immunopositive cells in sham-operated controls. *p < 0.02 relative toSham; **p < 0.001 relative toSham.
To identify the cells that proliferate in response to HB-EGF in vivo, brain sections from SGZ of HB-EGF- and BrdU-treated rats 1 week after treatment were immunostained for BrdU and for markers of mature and immature neurons. These double-label studies showed that most BrdU-immunopositive cells coexpressed NeuroD (Fig.9). Therefore, HB-EGF stimulates the proliferation of cells of neuronal lineage in vivo, and most of these exhibit an immature neuronal phenotype at 1 week.
Fig. 9.
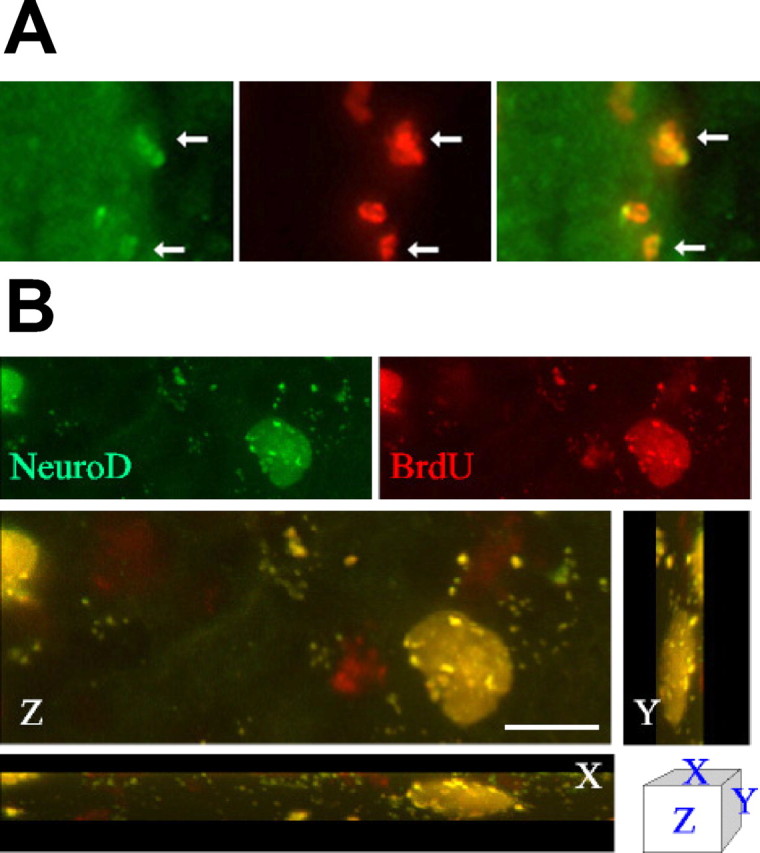
BrdU labels NeuroD-immunopositive cells after intracerebroventricular administration of HB-EGF in vivo. Adult male rats were treated for 3 d with BrdU, given intraperitoneally, and with HB-EGF, infused into the right lateral ventricle. A, Sections through SVZ were double labeled with antibodies against BrdU (red) and against the immature neuronal marker NeuroD (green), which colocalized within cell nuclei in this region (arrows). Data are representative fields from at least three independent experiments. Original magnification, 40×.B, Confocal imaging was used to confirm colocalization of BrdU and NeuroD in the same cells. Scale bar, 20 μm.
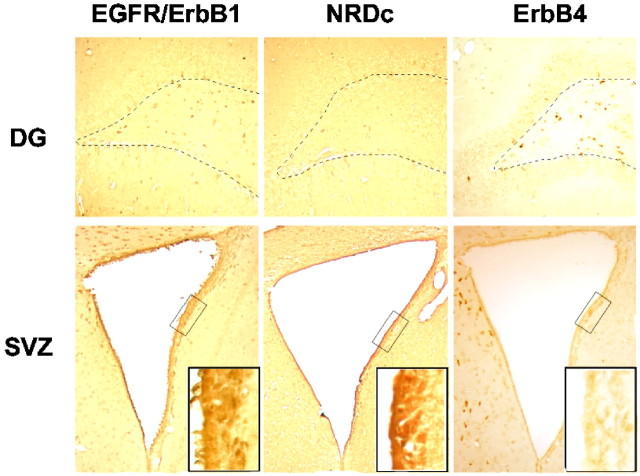
Finally, because our in vitro studies suggested that the neuroproliferative effect of HB-EGF might be mediated through the EGFR/ErbB1 receptor, we sought to determine whether the distribution of this receptor in rat brain could account for the distribution of BrdU labeling induced by HB-EGF in vivo. As shown in Figure 10, EGFR/ErbB1 and NRDc were expressed much more abundantly in SVZ than in SGZ, which is consistent with the more prominent (∼5-fold as opposed to ∼1.5-fold) stimulation of BrdU labeling by HB-EGF in SVZ. In contrast, ErbB4 was barely detectable in SVZ or in SGZ, although it was expressed in nearby cells, including those of the DG hilus.
Fig. 10.
Distribution of HB-EGF receptor expression in neuroproliferative zones of the adult rat brain. Brain sections from normal adult male rats were immunostained for each HB-EGF receptor (EGFR/ErbB1, NRDc, and ErbB4). There was little expression of these receptors in the SGZ of DG (top panels, dotted lines), whereas EGFR/ErbB1 and ErbB4 were expressed in cells of the DG hilus (between dotted lines). EGFR/ErbB1 and NRDc were expressed prominently, but ErbB4 only weakly, in SVZ (original magnification, 4×; insets, 40×).Rectangles in bottom row are 250 × 75 μm.
DISCUSSION
Ischemic brain injury triggers molecular and cellular repair mechanisms that contribute to recovery and may include ischemic activation of neurogenesis in the adult brain (Cramer and Chopp, 2000). Several studies have shown that global or focal cerebral ischemia stimulates neurogenesis, typically defined by increased incorporation of BrdU into cells that express neuronal marker proteins, in SGZ, SVZ, or cerebral cortex (Liu et al., 1998; Takagi et al., 1999; Gu et al., 2000; Jiang et al., 2001; Jin et al., 2001; Yoshimura et al., 2001; Zhang et al., 2001). Little is known about the mechanisms that couple cerebral ischemia to neurogenesis, but in one study, incorporation of BrdU into NeuN-immunopositive hippocampal cells after ischemia was reduced in FGF-2 knock-out mice and restored by intracerebroventricular administration of an FGF-2-expressing herpes simplex virus amplicon vector (Yoshimura et al., 2001).
We and others have used an in vitro model of ischemia in which cerebral cortical cultures are deprived of oxygen or glucose or both to investigate molecular and cellular mechanisms that may be involved in cerebral ischemia in vivo (Goldberg and Choi, 1993; Koretz et al., 1994). Here, we have used this system to identify factors that are released during hypoxia and can stimulate neurogenesisin vitro. We report that HB-EGF is one such factor and that it appears to act through the EGF receptor, ErbB1. Moreover, HB-EGF stimulates neurogenesis in proliferative zones of the adult brain—SVZ and, to a lesser extent, SGZ—when administered into the lateral ventricle. Thus, the effect of HB-EGF is observed in two distinct experimental systems: embryonic mouse cortical cultures in vitro and adult rat brain in vivo.
EGF has been known previously to promote the proliferation of neuronal precursors (Reynolds et al., 1992). Although EGF and HB-EGF are both members of the EGF family of growth factors, which contain 6-cysteine EGF-like domains with the structure CX7CX4CX10CXCX8C (Raab and Klagsbrun, 1997), their extent of homology is otherwise limited (∼20% at the amino acid level) (Higashiyama et al., 1991). The EGF-like domain cannot account for the full range of the biological activity of HB-EGF, which depends on binding to ErbB4, HSPG, and NRDc as well as to the EGF-sensitive receptor, EGFR/ErbB1 (Raab and Klagsbrun, 1997). Nevertheless, the neuroproliferative effect of HB-EGF in our cell culture system appeared to operate through mechanisms shared with EGF, because both growth factors increased BrdU incorporation to a similar extent, their effects were not additive, and the effect of HB-EGF involved the EGF-sensitive receptor EGFR/ErbB1.
In a previous study of ischemia-induced neurogenesis in vivo, we found that focal ischemia produced by middle cerebral artery occlusion increased BrdU labeling in SVZ and SGZ to a similar extent (Jin et al., 2001). In the present study, however, the HB-EGF-induced increase in labeling was greater in SVZ (∼400%) than in SGZ (∼65%), which may be related to the more abundant expression of the HB-EGF receptor, EGFR/ErbB1, on neuronal precursor cells of the SVZ than those in the SGZ. This result is consistent with the observation that intracerebroventricular administration of EGF (as well as of FGF-2) increased the number of progenitor cells in SVZ but not SGZ (Kuhn et al., 1997). However, it is also possible that differences in local HB-EGF levels after intraventricular infusion might account in part for the differences in BrdU incorporation observed in SVZ and SGZ.
Both ischemia (Jin et al., 2001) and HB-EGF stimulate neurogenesis in the brain bilaterally, even when ischemia is unilateral. One explanation for this finding is that the neuroproliferative effect of ischemia might be mediated through the release of soluble growth factors that can reach distant brain regions through the cerebrospinal fluid. In support of this possibility, increased levels of some growth factors can be found in the cerebrospinal fluid after stroke (Krupinski et al., 1998). In addition, intraventricular administration of several growth factors, including EGF, FGF-2, and BDNF, can enhance neurogenesis in neuroproliferative zones of the adult brain in vivo (Kuhn et al., 1997; Zigova et al., 1998; Pencea et al., 2001).
The ability of pathological lesions to activate cytoproliferative processes that contribute to tissue repair, such as angiogenesis, is well established. Neurogenesis may be another such process, because the capacity for neurogenesis persists in the adult brain and because various neuropathological conditions, including ischemia, seizures, and trauma, trigger increased incorporation of BrdU into what appear to be proliferating neuronal precursors. Important questions about this phenomenon remain unresolved, however, including how injured brain tissue transmits proliferation signals to areas that harbor precursor cells, whether these cells achieve a fully mature neuronal phenotype, and the extent to which neurogenesis in the injured adult brain can restore cerebral function. Our results may help to address the first of these questions and indicate that the interaction between hypoxia-inducible HB-EGF and EGFR/ErbB1 may deliver a neuroproliferative signal to SVZ precursors in the ischemic brain.
Footnotes
This work was supported by United States Public Health Service Grant NS35965.
Correspondence should be addressed to Dr. David A. Greenberg, Buck Institute for Age Research, 8001 Redwood Boulevard, Novato, CA 94945. E-mail: dgreenberg@buckinstitute.org.
REFERENCES
- 1.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 3.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 4.Davis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF). Front Biosci. 1998;3:288–299. doi: 10.2741/a241. [DOI] [PubMed] [Google Scholar]
- 5.Dong J, Wiley HS. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains. J Biol Chem. 2000;275:557–564. doi: 10.1074/jbc.275.1.557. [DOI] [PubMed] [Google Scholar]
- 6.Fujita M, Kiyono T, Hayashi Y, Ishibashi M. hCDC47, a human member of the MCM family. Dissociation of the nucleus-bound form during S phase. J Biol Chem. 1996;271:4349–4354. doi: 10.1074/jbc.271.8.4349. [DOI] [PubMed] [Google Scholar]
- 7.Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 10.Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hayase Y, Higashiyama S, Sasahara M, Amano S, Nakagawa T, Taniguchi N, Hazama F. Expression of heparin-binding epidermal growth factor-like growth factor in rat brain. Brain Res. 1998;784:163–178. doi: 10.1016/s0006-8993(97)01325-5. [DOI] [PubMed] [Google Scholar]
- 12.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 13.Higashiyama S, Abraham JA, Miller JA, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 14.Ino H, Chiba T. Expression of proliferating cell nuclear antigen (PCNA) in the adult and developing mouse nervous system. Brain Res Mol Brain Res. 2000;78:163–174. doi: 10.1016/s0169-328x(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 15.Jiang W, Gu W, Brännström T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- 16.Jin K, Chen J, Nagayama T, Chen M, Sinclair J, Graham SH, Simon RP. In situ detection of neuronal DNA strand breaks using the Klenow fragment of DNA polymerase I reveals different mechanisms of neuron death after global cerebral ischemia. J Neurochem. 1999;72:1204–1214. doi: 10.1046/j.1471-4159.1999.0721204.x. [DOI] [PubMed] [Google Scholar]
- 17.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jost M, Kari C, Rodeck U. The EGF receptor—an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505–510. [PubMed] [Google Scholar]
- 19.Kawahara N, Mishima K, Higashiyama S, Taniguchi N, Tamura A, Kirino T. The gene for heparin-binding epidermal growth factor-like growth factor is stress-inducible: its role in cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:307–320. doi: 10.1097/00004647-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Koretz B, Ahern KB, Lustig HS, Greenberg DA. Pre- and postsynaptic modulators of excitatory neurotransmission: comparative effects on hypoxia/hypoglycemia in cortical cultures. Brain Res. 1994;643:334–337. doi: 10.1016/0006-8993(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 21.Krupinski J, Vodovotz Y LiC, Slowik A, Beevers D, Flanders KC, Lip G, Kumar P, Szczudlik A. Inducible nitric oxide production and expression of transforming growth factor-beta1 in serum and CSF after cerebral ischaemic stroke in man. Nitric Oxide. 1998;2:442–453. doi: 10.1006/niox.1998.0204. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law RE, Meehan WP, Xi XP, Graf K, Wuthrich DA, Coats W, Faxon D, Hsueh WA. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–1905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JK, Cho JH, Hwang WS, Lee YD, Reu DS, Suh-Kim H. Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev Dyn. 2000;217:361–367. doi: 10.1002/(SICI)1097-0177(200004)217:4<361::AID-DVDY3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Hutchinson L, Gaston SM, Raab G, Freeman MR. BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: a unique role for proHB-EGF in cell survival regulation. J Biol Chem. 2001a;276:30127–30132. doi: 10.1074/jbc.M010237200. [DOI] [PubMed] [Google Scholar]
- 26.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001b;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 29.Mishima K, Higashiyama S, Nagashima Y, Miyagi Y, Tamura A, Kawahara N, Taniguchi N, Asai A, Kuchino Y, Kirino T. Regional distribution of heparin-binding epidermal growth factor-like growth factor mRNA and protein in adult rat forebrain. Neurosci Lett. 1996;213:153–156. doi: 10.1016/0304-3940(96)12850-0. [DOI] [PubMed] [Google Scholar]
- 30.Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa T, Sasahara M, Hayase Y, Haneda M, Yasuda H, Kikkawa R, Higashiyama S, Hazama F. Neuronal and glial expression of heparin-binding EGF-like growth factor in central nervous system of prenatal and early-postnatal rat. Brain Res Dev Brain Res. 1998;108:263–272. doi: 10.1016/s0165-3806(98)00057-1. [DOI] [PubMed] [Google Scholar]
- 32.Nishi E, Prat A, Hospital V, Elenius K, Klagsbrun M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001;20:3342–3350. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opanashuk LA, Hauser KF. Opposing actions of the EGF family and opioids: heparin binding-epidermal growth factor (HB-EGF) protects mouse cerebellar neuroblasts against the antiproliferative effect of morphine. Brain Res. 1998;804:87–94. doi: 10.1016/s0006-8993(98)00647-7. [DOI] [PubMed] [Google Scholar]
- 34.Opanashuk LA, Mark RJ, Porter J, Damm D, Mattson MP, Seroogy KB. Heparin-binding epidermal growth factor-like growth factor in hippocampus: modulation of expression by seizures and anti-excitotoxic action. J Neurosci. 1999;19:133–146. doi: 10.1523/JNEUROSCI.19-01-00133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embyronic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is upregulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka N, Sasahara M, Ohno M, Higashiyama S, Hayase Y, Shimada M. Heparin-binding epidermal growth factor-like growth factor mRNA expression in neonatal rat brain with hypoxic/ischemic injury. Brain Res. 1999;827:130–138. doi: 10.1016/s0006-8993(99)01319-0. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci USA. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 44.Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]